Abstract
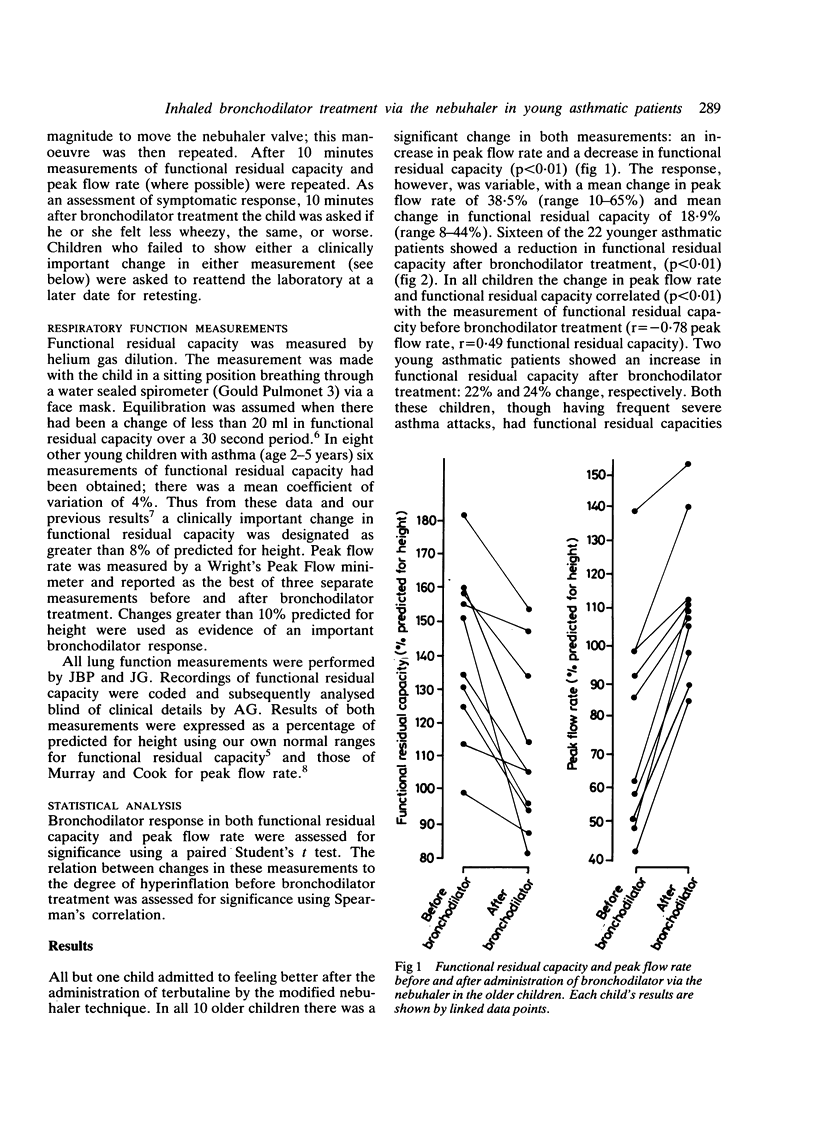
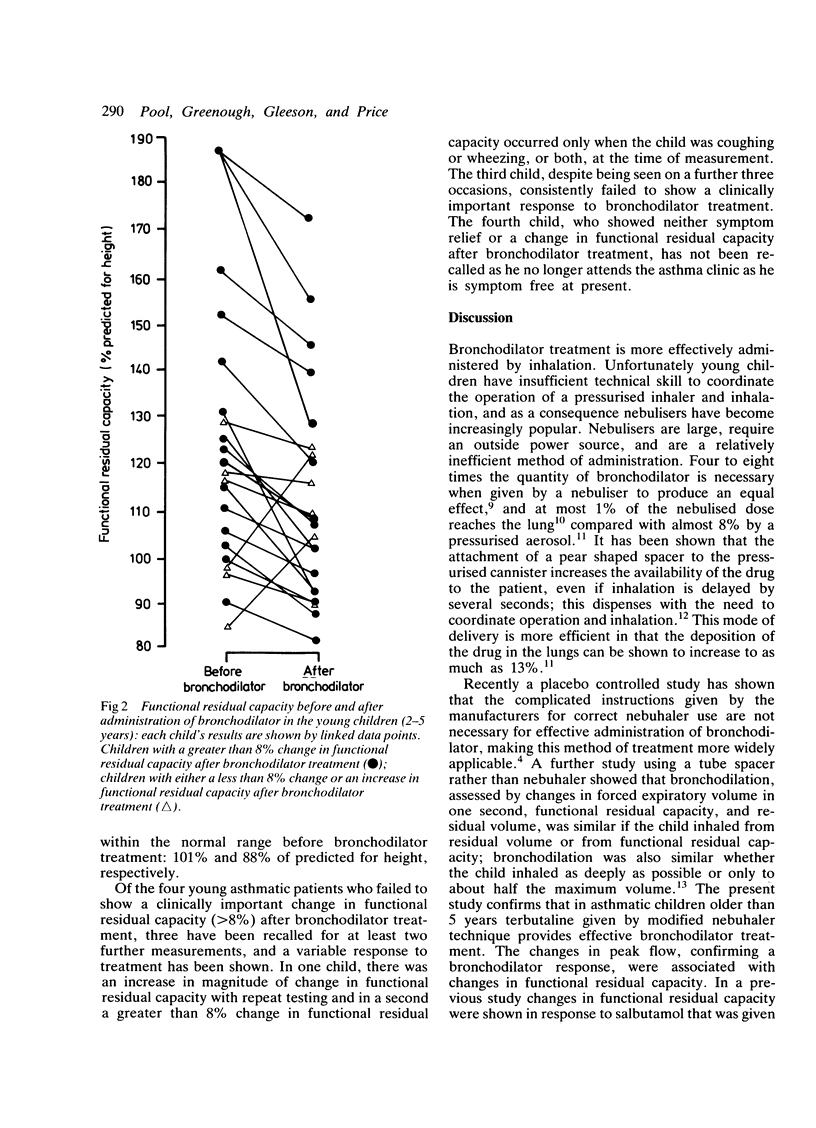
Changes in functional residual capacity and peak flow rate were measured to assess bronchodilator response to terbutaline inhaled via a nebuhaler. In 10 children with asthma, aged 5-7 years, five breaths sufficient to operate the nebuhaler valve resulted in clinically important improvement in both the functional residual capacity and the peak flow rate. In 18 of 22 children, aged 2-5 years, who were too young to have their peak flow rate measured reliably, terbutaline administered via this modified nebuhaler technique was also associated with a clinically important change in functional residual capacity. The results suggest that effective bronchodilation using a nebuhaler can be achieved even in very young children.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asmundsson T., Johnson R. F., Kilburn K. H., Goodrich J. K. Efficiency of nebulizers for depositing saline in human lung. Am Rev Respir Dis. 1973 Sep;108(3):506–512. doi: 10.1164/arrd.1973.108.3.506. [DOI] [PubMed] [Google Scholar]
- Desmond K. J., Coates A. L., Martin J. G., Beaudry P. H. Trapped gas and airflow limitation in children with cystic fibrosis and asthma. Pediatr Pulmonol. 1986 May-Jun;2(3):128–134. doi: 10.1002/ppul.1950020303. [DOI] [PubMed] [Google Scholar]
- Freelander M., Van Asperen P. P. Nebuhaler versus nebuliser in children with acute asthma. Br Med J (Clin Res Ed) 1984 Jun 23;288(6434):1873–1874. doi: 10.1136/bmj.288.6434.1873-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A., Loftus B. G., Pool J., Price J. F. Abnormalities of lung mechanics in young asthmatic children. Thorax. 1987 Jul;42(7):500–505. doi: 10.1136/thx.42.7.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A., Loftus B. G., Pool J., Price J. F. Response to bronchodilators assessed by lung mechanics. Arch Dis Child. 1986 Oct;61(10):1020–1023. doi: 10.1136/adc.61.10.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A., Stocks J., Nothen U., Helms P. Total respiratory compliance and functional residual capacity in young children. Pediatr Pulmonol. 1986 Nov-Dec;2(6):321–326. doi: 10.1002/ppul.1950020602. [DOI] [PubMed] [Google Scholar]
- MURRAY A. B., COOK C. D. Measurement of peak expiratory flow rates in 220 normal children from 4.5 to 18.5 years of age. J Pediatr. 1963 Feb;62:186–189. doi: 10.1016/s0022-3476(63)80390-x. [DOI] [PubMed] [Google Scholar]
- Madsen E. B., Bundgaard A., Hidinger K. G. Cumulative dose-response study comparing terbutaline pressurized aerosol administered via a pearshaped spacer and terbutaline in a nebulized solution. Eur J Clin Pharmacol. 1982;23(1):27–30. doi: 10.1007/BF01061373. [DOI] [PubMed] [Google Scholar]
- Newman S. P., Morén F., Pavia D., Little F., Clarke S. W. Deposition of pressurized suspension aerosols inhaled through extension devices. Am Rev Respir Dis. 1981 Sep;124(3):317–320. doi: 10.1164/arrd.1981.124.3.317. [DOI] [PubMed] [Google Scholar]
- Pedersen S. Optimal use of tube spacer aerosols in asthmatic children. Clin Allergy. 1985 Sep;15(5):473–478. doi: 10.1111/j.1365-2222.1985.tb02297.x. [DOI] [PubMed] [Google Scholar]
- Stauder J., Hidinger K. G. Terbutaline aerosol from a metered dose inhaler with a 750-ml spacer or as a nebulized solution. Comparison of two delivery systems for bronchodilator aerosol. Respiration. 1983 May-Jun;44(3):237–240. doi: 10.1159/000194554. [DOI] [PubMed] [Google Scholar]


