Abstract
Studies of essential pathogenicity determinants in Gram-negative bacteria have revealed the conservation of type III protein secretion systems that allow delivery of virulence factors into host cells from plant and animal pathogens. Ten of 21 Hrp proteins of the plant pathogen Xanthomonas campestris pv. vesicatoria have been suggested to be part of a type III machinery. Here, we report the hrp-dependent secretion of two avirulence proteins, AvrBs3 and AvrRxv, by X. campestris pv. vesicatoria strains that constitutively express hrp genes. Secretion occurred without leakage of a cytoplasmic marker in minimal medium containing BSA, at pH 5.4. Secretion was strictly hrp-dependent because a mutant carrying a deletion in hrcV, a conserved hrp gene, did not secrete AvrBs3 and AvrRxv. Moreover, the Hrp system of X. campestris pv. vesicatoria was able to secrete proteins from two other plant pathogens: PopA, a protein secreted via the Hrp system in Ralstonia solanacearum, and AvrB, an avirulence protein from Pseudomonas syringae pv. glycinea. Interestingly, X. campestris pv. vesicatoria also secreted YopE, a type III-secreted cytotoxin of the mammalian pathogen Yersinia pseudotuberculosis in a hrp-dependent manner. YerA, a YopE-specific chaperone, was required for YopE stability but not for secretion in X. campestris pv. vesicatoria. Our results demonstrate the functional conservation of the type III system of X. campestris for secretion of proteins from both plant and mammalian pathogens and imply recognition of their respective secretion signals.
Studies of bacterial pathogens of plants and mammals have revealed common strategies to interact with their hosts. A striking example is the conservation of a protein-secretion system essential for pathogenicity in distantly related Gram-negative bacteria. Type III secretion systems serve to deliver virulence factors into host cells and have been identified in 10 pathogenic bacteria, including the mammalian pathogens Yersinia spp., Shigella flexneri, Salmonella spp., enteropathogenic Escherichia coli, Pseudomonas aeruginosa, and Chlamydia spp., and the plant pathogens Erwinia spp., Pseudomonas syringae, Ralstonia solanacearum, and Xanthomonas campestris (for a review on type III systems, see ref. 1). Type III secretion systems are encoded by approximately 20 genes clustered in 18- to 40-kb regions, either in the chromosome or on virulence plasmids. Nine genes appear to be homologous in all gene clusters sequenced so far (1). Secretion via the type III pathway is sec-independent and occurs without the cleavage of an N-terminal signal sequence. In Yersinia spp., for which this secretion system was first characterized, secretion in the culture medium occurs at 37°C in the absence of Ca2+. Type III-secreted proteins in Yersinia include YopH, a protein phosphatase, YopE and YopT, two cytotoxins, and YopJ (also called YopP), a protein that induces apoptosis in macrophages (2).
Genes encoding type III secretion systems in plant pathogens were identified by the isolation of mutants that were unable to cause disease in susceptible plants and no longer elicited the hypersensitive reaction (HR), a rapid localized plant cell death, in resistant plant tissue. The corresponding genes were designated hrp for “hypersensitive reaction and pathogenicity” (1). In our laboratory, we study X. campestris pv. vesicatoria, the causal agent of bacterial spot disease in pepper and tomato. The 25-kb hrp gene cluster is localized in the chromosome of X. campestris pv. vesicatoria and contains six transcription units, designated hrpA to hrpF (3). Based on sequence homologies and protein localization studies, 10 Hrp proteins have been suggested to be part of the core secretion apparatus (refs. 4–7; O.R. and U.B., unpublished data). Two regulators, HrpG and HrpXv, are encoded elsewhere in the genome and govern hrp gene expression in hrp gene-inducing medium and in plant leaf tissue. HrpG is homologous to response regulators of two-component regulatory systems and activates the expression of hrpA and hrpXv (8). HrpXv is an AraC-type transcriptional activator of the operons hrpB to hrpF (9). Although hrp gene induction in culture has been obtained (6), no hrp-dependent protein secretion was observed so far.
The interaction of X. campestris pv. vesicatoria with its hosts is determined by gene-for-gene relationships (10). If matching genes for resistance and avirulence are expressed in the plant and in the bacterium, respectively, the bacterium is recognized resulting in the induction of plant-defense responses, such as the HR. In X. campestris pv. vesicatoria, several avirulence genes have been cloned (10). In some cases, lack of the avr (avirulence) gene causes a reduction of bacterial growth in susceptible hosts. However, the function of most avr components remains unknown (11). The avrBs3 gene is one of the best-studied avirulence genes in X. campestris pv. vesicatoria (12). Interestingly, the recognition of the AvrBs3 protein in pepper plants carrying the corresponding resistance gene, Bs3, was shown to occur inside the plant cell (13). Moreover, the function of avrBs3 is hrp-dependent, because hrp mutants are no longer able to induce the HR on resistant pepper plants (14). Thus, AvrBs3 is a likely candidate to be delivered via the Hrp apparatus into the plant cell.
In this study, we have established conditions for hrp-dependent secretion of proteins by X. campestris pv. vesicatoria in culture. We show that the avirulence proteins AvrBs3 and AvrRxv from X. campestris pv. vesicatoria are secreted via the Hrp machinery. Furthermore, we demonstrate that the Hrp secretion system from Xanthomonas is promiscuous for the secretion of heterologous proteins from bacterial pathogens of both plants and mammals.
MATERIALS AND METHODS
Bacterial Strains, Growth Conditions, and Plasmids.
Bacterial strains used in this study were E. coli strains DH5α (Bethesda Research Laboratories), DH5α λpir (15), and HB101 (16) and P. syringae pv. maculicola M4 (17). X. campestris pv. vesicatoria strains 82–8 and 56 were described previously (10, 18). Strain 82* is a derivative of 82–8 that expresses a mutated version of hrpG. The amino acid substitution E44K leads to constitutive hrp gene expression in noninducing medium (K.W., O.R., and U.B., unpublished data). E. coli cells were cultivated at 37°C in LB and Xanthomonas strains at 30°C in NYG broth (19), in hrp-inducing medium XVM2 (6), or in minimal medium A (see ref. 20) (MA) and M9 (20) supplemented with sucrose (10 mM) and casamino acids (0.3%). Antibiotics were added to the media at the following final concentrations: 100 μg/ml ampicillin, 25 μg/ml kanamycin, 10 μg/ml tetracycline, 100 μg/ml rifampicin, and 100 μg/ml spectinomycin. Plasmids were introduced into E. coli by electroporation and into Xanthomonas by conjugation, by using pRK2013 as a helper plasmid in triparental matings as described previously (7).
Generation of a Mutation in the Secretion Apparatus.
The nonpolar deletion mutant 82*ΔhrcV was constructed as follows. First, a suicide plasmid, pRO1, was constructed by replacing the gene encoding the antibiotic resistance marker of pSB360 (21). The SmaI 6.2-kb fragment was ligated to the HindIII filled-in Ω fragment that confers spectinomycin resistance (22), resulting in pRO1. Plasmid pUCB11o, which carries a 5,900-bp insert encompassing hrcV, was linearized with SfiI, rendered blunt, and digested with EcoRV. Religation created a 562-bp deletion in hrcV (from position 752 to 1314 with respect to putative translation start; pUCBΔhrcV). The insert of this plasmid was cloned into the BamHI site of the suicide vector pRO1, giving pROΔhrcV, which was introduced into 82* as described (7). For complementation analysis of the resulting mutant 82*ΔhrcV, the Ω fragment was introduced into the SnaBI site of pUCB11o, thereby disrupting the hrpC3 gene, which is downstream of hrcV in the same operon. The insert of the resulting plasmid was cloned into the broad host-range vector pLAFR6 (12), giving pSCOB, which complemented strain 82*ΔhrcV.
Plant Material and Plant Inoculations.
Inoculation of tomato cultivar Hawaii 7998, which recognizes avrRxv (23), and the near-isogenic pepper cultivars Early Cal Wonder (ECW) and ECW-30R (which carries the resistance gene Bs3) were performed as described (12).
Secretion Experiments.
Bacteria were cultivated in MA, pH 7.0, at 30°C overnight and resuspended to an optical density of 0.1 (600 nm) in 4 ml of MA at pH 5.4 (acidified by addition of HCl) containing BSA (50 μg/ml, New England Biolabs). After 4–5 h, 0.5 ml of total culture (total protein) was precipitated for 30 min on ice with 10% trichloroacetic acid (TCA). After centrifugation at 14,000 × g for 10 min at 4°C, protein precipitates were washed with ethanol and resuspended in Laemmli buffer (1/10 vol) (24). The remaining culture was filtered with a low protein-binding filter (HT Tuffryn, 0.45 μm; Gelman). Filtrates (supernatants) were precipitated with TCA and resuspended in Laemmli buffer (1/50 to 1/200 vol). Aliquots of 5 μl were analyzed by immunoblotting as described previously (6). Antibodies used were polyclonal anti-AvrBs3 antibody (14), polyclonal anti-HrcN antiserum (U.B., unpublished data), anti-Flag M2 mAb (IBI/Kodak), monoclonal antihemagglutinin (HA) antibody (Eurogentec, Brussels), polyclonal anti-PopA antiserum (provided by C. Boucher, Toulouse, France), and specific anti-YopE and anti-YerA antisera (provided by H. Wolf-Watz, Umea, Sweden). Horseradish peroxidase-labeled goat anti-mouse or goat anti-rabbit antibodies were used as secondary antibodies. Reactions were visualized by enhanced chemiluminescence (Amersham Pharmacia).
Determination of β-Glucuronidase Activity.
To express β-glucuronidase (GUS) in X. campestris pv. vesicatoria, the uidA gene from pKEx4tr-G (25) was cloned into the EcoRI/HindIII sites of pDSK602, giving pDGUS. This plasmid was introduced into strains 82* and 82*ΔhrcV. To determine GUS activities, aliquots of supernatants and total bacterial culture (cells and supernatants) were taken before TCA precipitation. 4-Methylumbelliferyl β-d-glucuronide was used as a substrate in a modified procedure from ref. 26 in which sodium phosphate concentration of the extraction buffer was increased to 100 mM. One unit is defined as 1 nmol of 4-methylumbelliferone released per minute and per bacterium.
Epitope Tagging of the AvrRxv Protein.
The Flag epitope (IBI/Kodak) was engineered at the C terminus of AvrRxv by PCR, resulting in the replacement of the stop codon by a threonine and a serine codon, followed by the Flag-coding sequence (DYKDDDDK), a stop codon, and a HindIII restriction site. The construct was expressed under the control of the lac promoter present in the broad host-range vector pLAFR3 (27). For PCR amplification, plasmid pXV9009 (28) was used as a template by using primers 5′-GGAATTCTATGTGCGACTCC-3′ and 5′-CCCAAGCTTCACTTGTCATCGTCGTCCTTGTAGTCACTAGTGGATTCTAAGGCG-3′. After digestion of the PCR products by EcoRI and HindIII (specific sites are underlined in primer sequence), the 1,163-bp fragment was cloned into pUC118 (29). Clones were checked by sequence analysis. Subsequently, the EcoRI/HindIII insert was cloned into pLAFR3, giving pLRF.
Expression of popA, avrB, yopE, and yerA in X. campestris pv. vesicatoria.
To express popA in X. campestris pv. vesicatoria, plasmid pAZ13 (provided by C. Boucher) was digested with XbaI and SacI. The 1.6-kb insert containing popA first was cloned into the corresponding sites of pUC118 and then as an EcoRI-PstI fragment into pLAFR3, resulting in pLAZ13. This construct contains 387 bp upstream of the translation start of popA and was shown to be functional, because supernatants of R. solanacearum popA mutant strain GM1551 (pLAZ13) induced the HR on petunia and tobacco. To express yopE and yerA of Y. pseudotuberculosis in X. campestris pv. vesicatoria, both genes were cloned in pLAFR3 under the control of the lac promoter, giving pLKW1. A 1,050-bp EcoRI-HindIII fragment of pAF19 (30) containing yopE was cloned into pBluescript-KS II (Stratagene) and pLAFR3, resulting in pBKW2 and pLKW2, respectively. The yerA gene was isolated on a DpnI fragment from pAF19 and ligated into SmaI-digested pBKW2, resulting in pBKW1. The untranslated region between yerA and yopE was 375 bp long. The insert from pBKW1 was cloned as a BamHI/HindIII fragment into pLAFR3.
RESULTS
The Avirulence Protein AvrBs3 from X. campestris pv. vesicatoria Is Secreted in a hrp-Dependent Manner.
Previous studies have shown that recognition of the avirulence protein AvrBs3 from X. campestris pv. vesicatoria by Bs3-resistant pepper plants requires functional hrp genes and occurs inside the plant cell (13). We, therefore, assumed that AvrBs3 is secreted via the Hrp type III secretion system and we used it as a reporter to establish secretion conditions. Because bacteria grown in hrp gene-inducing medium do not secrete proteins in a hrp-dependent manner, we aimed for conditions that would disconnect hrp gene induction from secretion. We used strain 82*, a derivative of 82–8 (10) that contains avrBs3 and constitutively expresses hrp genes because of a mutation in the regulatory gene hrpG (K.W., O.R., and U.B., unpublished data). As a control for a cytoplasmic protein, the uidA gene, encoding GUS, was expressed in strain 82* by using plasmid pDGUS. Total protein extracts and culture supernatants were analyzed for the presence of AvrBs3 by immunoblotting. 82*(pDGUS) did not secrete AvrBs3 in solid or liquid NYG medium, in hrp-inducing medium XVM2, or in minimal medium at pH 7.0. Secretion in XVM2 and minimal medium could not be induced by changing growth temperature (in a range from 20 to 30°C), by addition of various concentrations of sodium, fructose, and sucrose, or by addition of cellulose acetate (mimicking plant cell walls) at logarithmic and stationary growth phase, respectively. Secretion was not observed in a minimal medium with a pH range of from 7.8 to 9.0. Bacterial growth in a minimal medium at pH 5.6 with different ion concentrations (Na2+, Ca2+, Fe2+, and Mg2+) or the addition of oxidized or reduced glutathione also failed to induce secretion.
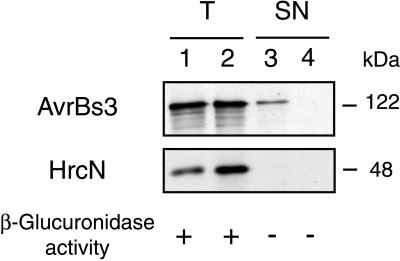
Secretion of the AvrBs3 protein, however, was achieved by incubation of bacteria for 4–5 hr in a minimal medium that was acidified by addition of HCl to pH 5.4 and supplemented with BSA (50 μg/ml) (Fig. 1, lane 3). To test whether secretion was hrp-dependent, a nonpolar mutation in hrcV, a conserved hrp gene (4, 31), was introduced into 82*. The resulting strain, 82*ΔhrcV(pDGUS), expressed AvrBs3 (Fig. 1, lane 2); however, AvrBs3 was not detected in bacterial supernatants (Fig. 1, lane 4). To rule out bacterial lysis as a reason for AvrBs3 detection in the supernatant of 82*(pDGUS), protein samples were tested for the presence of HrcN, a conserved type III system-ATPase (4, 31). Localization studies by using a polyclonal antiserum specific for HrcN indicated that approximately 60% of this intracellular protein is in the soluble fraction, most likely in the cytoplasm (O.R. and U.B., unpublished data). As shown in Fig. 1, HrcN was present in total protein extracts of both 82*(pDGUS) and 82*ΔhrcV(pDGUS) (lanes 1 and 2), but it was not detected in supernatants (lanes 3 and 4). In addition, the activity of GUS, a normally cytoplasmic protein, was determined. The GUS activity in supernatants was equally low for both strains [0.61 ± 0.13 unit × 10−10 for strain 82*(pDGUS) and 1.18 ± 0.15 unit × 10−10 for strain 82*ΔhrcV(pDGUS)]. GUS activity in total protein extracts (lysed cells plus supernatant) reached 277.41 ± 24.87 units × 10−10 for 82*(pDGUS) and 280.11 ± 47.93 units × 10−10 for 82*ΔhrcV(pDGUS). These data confirm that the presence of AvrBs3 in the supernatant of 82*(pDGUS) was not a result of bacterial lysis but of AvrBs3 secretion in a hrp-dependent manner.
Figure 1.
hrp-dependent secretion of AvrBs3. Bacteria were incubated for 4 hr in minimal medium MA at pH 5.4 with BSA (50 μg/ml). Total protein extracts (T, lanes 1 and 2) and filtered supernatants (SN, lanes 3 and 4) were precipitated with TCA and concentrated 10- and 50-fold, respectively. Five-microliter protein samples of strains 82*(pDGUS) (lanes 1 and 3) and 82*ΔhrcV(pDGUS) (lanes 2 and 4) were separated by SDS/PAGE (8% polyacrylamide) and analyzed by immunoblotting by using polyclonal antibodies directed against AvrBs3 (upper blot) and HrcN (lower blot). The molecular mass (kDa) of the proteins is indicated. For β-glucuronidase activities, see text.
Effect of pH and BSA on Secretion of AvrBs3.
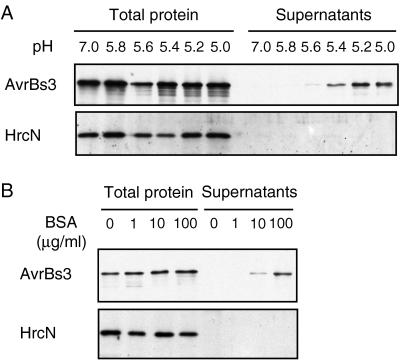
The effect of the pH on secretion of AvrBs3 by 82*(pDGUS) was tested in the presence of BSA (50 μg/ml). At pH 7.0 or 5.8, AvrBs3 was not detectable in the supernatant (Fig. 2A), but the amount of AvrBs3 in the supernatant increased as the pH of the medium was lowered from 5.6 to 5.0 (Fig. 2A). The effect of the BSA concentration on AvrBs3 secretion was determined in MA medium at pH 5.4. Amounts of AvrBs3 in the supernatant decreased as the BSA concentration was lowered from 100 to 0 μg/ml (Fig. 2B). Throughout the remainder of this study, MA medium at pH 5.4 containing 50 μg/ml BSA was used as secretion medium. Under these conditions we estimated that 15–20% of the total amount of AvrBs3 was secreted. This was done by comparing signal intensities of dilutions of total protein extracts and supernatants in immunoblotting analyses (data not shown).
Figure 2.
Effect of pH and BSA on AvrBs3 secretion. Total protein extracts and supernatants of strain 82*(pDGUS) were separated by SDS/PAGE (8% polyacrylamide) and analyzed by immunoblotting by using AvrBs3- and HrcN-specific antibodies. (A) Effect of pH on AvrBs3 secretion. pH of the MA medium was varied from 7.0 to 5.0 in the presence of BSA (50 μg/ml). (B) Effect of BSA on AvrBs3 secretion. BSA concentration was varied from 0 to 100 μg/ml in the MA medium at pH 5.4.
Secretion of AvrRxv.
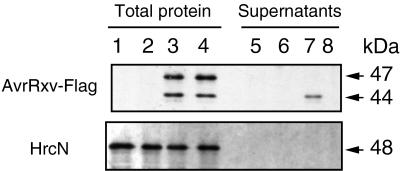
After having established conditions that allow AvrBs3 secretion in culture, we subsequently analyzed whether another avirulence protein, isolated from a different strain of X. campestris pv. vesicatoria, was secreted. AvrRxv is an avirulence protein present in strain 75–3 (23), and like AvrBs3, its recognition by resistant tomato lines depends on hrp gene function (32). AvrRxv was chosen because it is the only avirulence protein in a plant pathogen, besides its homolog AvrBsT (32), that shares similarity with virulence proteins of mammalian pathogens, i.e., YopJ from Yersinia pseudotuberculosis (33), YopP from Y. enterocolitica (34), and AvrA from Salmonella typhimurium (35) (47.6 and 45.9% similarity to AvrRxv, respectively). To allow immunodetection of AvrRxv, the Flag epitope was fused to the C terminus of the protein in plasmid pLRF. Avirulence activity of this construct was tested after conjugation into X. campestris pv. vesicatoria 56, a strain virulent on tomato cultivar Hawaii (18). Transconjugants carrying pLRF induced the HR on Hawaii. Total protein extracts of 82* and 82*ΔhrcV strains containing pLRF or the empty vector (pLAFR3) were analyzed by immunoblotting (Fig. 3). The Flag-specific antibody reacted with two proteins of 44 and 47 kDa in size. The presence of a 47-kDa protein in bacterial extracts probably is a result of an alternative translational start in the upstream region of avrRxv resulting in the addition of 25 aa. Only the 44-kDa protein was present in the supernatant of strain 82*(pLRF), and it was absent from the supernatant of the mutant 82*ΔhrcV(pLRF) (Fig. 3, lanes 7 and 8). No bacterial lysis was detectable by using the HrcN-specific antibody (Fig. 3), indicating that the 44-kDa protein was secreted in a hrp-dependent manner. Approximately 10–15% of the total amount of the 44-kDa protein was secreted.
Figure 3.
hrp-dependent secretion of AvrRxv. Total protein (lanes 1–4) and supernatants (lanes 5–8) of 82*(pLAFR3) (lanes 1 and 5), 82*ΔhrcV(pLAFR3) (lanes 2 and 6), 82*(pLRF) (lanes 3 and 7), and 82*ΔhrcV(pLRF) (lanes 4 and 8) were separated by SDS/PAGE (10% polyacrylamide) and analyzed by immunoblotting by using anti-Flag antibody (Upper) or anti-HrcN antibody (Lower). The molecular mass of the proteins is indicated by an arrow.
PopA from R. solanacearum and AvrB from P. syringae Are Secreted by X. campestris pv. vesicatoria.
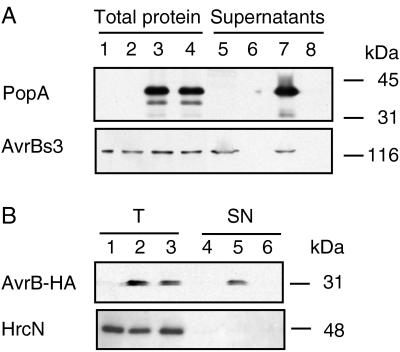
Subsequently, the ability of the Hrp secretion system from X. campestris pv. vesicatoria to export proteins from other plant pathogenic bacteria was examined. The similarity between the X. campestris pv. vesicatoria and R. solanacearum hrp gene clusters (36) prompted us to test PopA, a secreted protein from R. solanacearum (37). popA was expressed in pLAFR3 under the control of the lac promoter (pLAZ13) in strains 82* and 82*ΔhrcV. Immunoblotting analyses showed that both strains expressed PopA1 (Fig. 4A, lanes 3 and 4). However, PopA1 was detectable only in the supernatant of 82*(pLAZ13) (Fig. 4A, lane 7). This was a result of specific secretion, because HrcN was not detected in the bacterial supernatants (data not shown). Approximately 10–15% of PopA1 was secreted in these conditions. Secretion of AvrBs3 by 82*(pLAZ13) was similar to that of 82*(pLAFR3), which harbors the vector alone (Fig. 4A, lanes 5 and 7), showing that expression of popA in Xanthomonas has no negative effect on AvrBs3 secretion.
Figure 4.
PopA from R. solanacearum and AvrB from P. syringae are secreted by X. campestris pv. vesicatoria. (A) Immunoblotting analysis of total protein (lanes 1–4) and supernatants (lanes 5–8) of strains 82*(pLAFR3) (lanes 1 and 5), 82*ΔhrcV(pLAFR3) (lanes 2 and 6), 82*(pLAZ13) (lanes 3 and 7), and 82*ΔhrcV(pLAZ13) (lanes 4 and 8) by using an antibody directed against PopA (Upper) or against AvrBs3 (Lower). Total protein samples and supernatants were concentrated 10- and 100-fold, respectively. Molecular mass markers are indicated on the right. (B) Immunoblotting analysis of total protein (T, lanes 1–3) and supernatants (SN, lanes 4–6) of strains 82*(pVSP61) (lanes 1 and 4), 82*(pEM240) (lanes 2 and 5), and 82*ΔhrcV(pEM240) (lanes 3 and 6) by using anti-HA antibody (Upper) and anti-HrcN antibody (Lower). Total protein samples and supernatants were concentrated 50- and 200-fold, respectively.
In a similar experiment, we tested for secretion of AvrB, an avirulence protein from P. syringae pv. glycinea, a more distantly related plant pathogen. AvrB recognition in resistant plants depends on hrp genes (38) and has been shown to occur in the plant cell (39). A HA epitope-tagged version of AvrB was expressed in X. campestris pv. vesicatoria by using plasmid pEM240 (E. Marois and J. Dangl, unpublished data). In this construct, avrB is under the control of the promoter of avrRpm1 (17), an avirulence gene from P. syringae pv. maculicola. Although avr and hrp gene regulation in X. campestris pv. vesicatoria differs from that in P. syringae, the construct used allowed low expression of the AvrB-HA protein in Xanthomonas (Fig. 4B, lanes 2 and 3). The AvrB-HA fusion protein was detected only in the supernatant of 82*(pEM240) (lane 5) and not in the supernatant of 82*ΔhrcV(pEM240) (lane 6). Again, the intracellular marker, HrcN, was not detectable in the supernatants (Fig. 4B). X. campestris pv. vesicatoria, therefore, is able to secrete PopA and AvrB, proteins from two different plant pathogenic bacteria via the Hrp-secretion pathway.
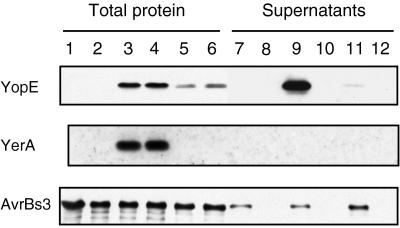
X. campestris pv. vesicatoria Secretes YopE of Y. pseudotuberculosis.
The ability of Xanthomonas to secrete PopA and AvrB prompted us to examine the secretion of a protein from a mammalian pathogen. The Y. pseudotuberculosis cytotoxin YopE, which is secreted via the Ysc/Lcr type III secretion system (40), was chosen for this study. Because secretion of YopE in Yersinia requires a specific chaperone, YerA (41), both yopE and yerA (on pLKW1) were expressed in Xanthomonas strains 82* and 82*ΔhrcV. Immunoblotting analyses showed that the bacteria expressed YopE, YerA, and the endogenous protein AvrBs3 (Fig. 5, lanes 3 and 4). Most interestingly, YopE was detected in the supernatant of 82*(pLKW1) but not in the supernatant of the mutant 82*ΔhrcV(pLKW1) (Fig. 5, lanes 9 and 10). The presence of YopE in the culture medium resulted from specific secretion, because the cytosolic YerA protein (42) was detected only in whole protein extracts and not in culture supernatants (Fig. 5). We estimated that 10–15% of the total amount of YopE was secreted under these conditions. Secretion of AvrBs3 by 82*(pLKW1) was similar to that of 82* harboring the vector alone (Fig. 5, lanes 7 and 9), showing that expression of yopE in Xanthomonas has no negative effect on AvrBs3 secretion.
Figure 5.
YopE from Y. pseudotuberculosis is secreted by X. campestris pv. vesicatoria. Immunoblotting analysis of total protein (lanes 1–6) and supernatants (lanes 7–12) of strains 82*(pLAFR3) (lanes 1 and 7), 82*ΔhrcV(pLAFR3) (lanes 2 and 8), 82*(pLKW1) (lanes 3 and 9), 82*ΔhrcV(pLKW1) (lanes 4 and 10), 82*(pLKW2) (lanes 5 and 11), and 82*ΔhrcV(pLKW2) (lanes 6 and 12) by using antibodies specific for YopE (23 kDa) (Top), YerA (14 kDa) (Middle), and AvrBs3 (122 kDa) (Bottom). Total protein samples and supernatants were concentrated 10- and 100-fold, respectively. To allow YopE detection in lane 11, lanes 7–12 were overexposed.
When yopE was expressed without its chaperone yerA (with plasmid pLKW2), the amount of YopE detectable in total protein extracts was reduced greatly in comparison with strains expressing yopE and yerA (Fig. 5, compare lanes 5 and 6 with lanes 3 and 4). This also was observed for whole-cell extracts of E. coli DH5α (data not shown). Nevertheless, small amounts of YopE were detected in the supernatant of 82*(pLKW2) (Fig. 5, lane 11). These results show that X. campestris pv. vesicatoria indeed does secrete YopE of Y. pseudotuberculosis via the Hrp secretion system.
Expression of popA, avrB, and yopE in X. campestris pv. vesicatoria Has No Effect on Pathogenicity.
Bacterial strains expressing heterologous proteins were tested for their interaction with the plant. Xanthomonas strains 82* carrying an empty vector or expressing popA, avrB, and yopE [82*(pLAFR3), 82*(pLAZ13), 82*(pEM240), and 82*(pLKW1), respectively] were inoculated into pepper. None of the strains displayed a difference in disease-symptom formation or in HR induction in susceptible ECW and resistant ECW-30R pepper plants. We also tested whether X. campestris pv. vesicatoria expressing avrB induces an HR on Arabidopsis ecotype Columbia, which carries the corresponding resistance gene RPM1. In contrast to P. syringae pv. maculicola M4 strain containing avrB on plasmid pEM240, 82*(pEM240) did not induce an HR on Arabidopsis ecotype Columbia (data not shown). Thus, X. campestris pv. vesicatoria was not able to deliver the AvrB signal in Arabidopsis.
DISCUSSION
For plant pathogenic bacteria, hrp gene function in type III protein secretion has been difficult to study either in planta or in plant tissue culture. Therefore, we established in vitro conditions for hrp-dependent secretion by X. campestris pv. vesicatoria. Two avirulence proteins of Xanthomonas, AvrRxv and AvrBs3, were found to be secreted in a Hrp-dependent manner. These endogenous avirulence proteins were good candidates for type III secretion because their function depends on hrp genes. In other plant pathogenic bacteria, secreted proteins include nonspecific elicitors of the HR such as harpins and PopA (37, 43–46), HrpA, the subunit of the P. s. pv. tomato Hrp pilus (47), and DspA (also designated DspE), an essential pathogenicity factor of Erwinia amylovora (48). However, although harpin was secreted by P. syringae in hrp-inducing medium, the avirulence protein AvrB was not detected in culture supernatants (39), indicating that secretion of some proteins in this system may be gated differentially. In contrast to other plant bacterial pathogens, Xanthomonas hrp gene expression and protein secretion appear to be even more tightly controlled. Although all known hrp loci are expressed in a synthetic medium, XVM2 (6), and some Hrp proteins apparently are localized properly in the bacterial membranes (ref. 6; O.R. and U.B., unpublished data), it has been impossible to detect any specifically secreted proteins in culture supernatants. This suggests that Xanthomonas requires an additional trigger for secretion. The breakthrough became possible by disconnecting hrp gene induction from secretion conditions by constitutive gene expression and growth of the bacteria in a minimal medium at an acidic pH. In P. syringae (38) and E. amylovora (49), pH values between 5.5 and 5.7 were shown to be important for hrp gene induction. However, for X. campestris pv. vesicatoria, hrp-gene induction in XVM1 medium is almost abolished at acidic pH and optimal at pH 6.5–7.5 (50). The acidic pH that triggered secretion in vitro might reflect the situation in natural infections, i.e., in the plant leaf apoplast. However, because our experiments were done in a synthetic medium and in the absence of plant cells, we cannot rule out that in planta the trigger for secretion is a specific plant molecule. In some experiments, in which BSA was omitted, AvrBs3 was detected occasionally in bacterial supernatants, along with a large number of degradation products. Because BSA does not induce secretion at pH 7.0 (Fig. 2) and can be replaced by a different protein, e. g., triosephosphate isomerase (data not shown), BSA most probably plays a role in stabilizing secreted proteins rather than triggering secretion.
Sequence similarities of Hrp proteins from X. campestris pv. vesicatoria with components of type III secretion systems from plant and mammalian pathogens is suggestive of functional conservation. Recently, AvrB and AvrPto from P. syringae were found to be secreted by E. coli via the cloned Hrp system from Erwinia chrysanthemi (51), showing that this heterologous system is promiscuous for secretion of proteins from other plant pathogens. The idea of promiscuity also is supported by findings in this study. The hrp gene cluster from R. solanacearum is highly similar to that of X. campestris pv. vesicatoria in terms of overall sequence similarity, operon structure, and the hrp gene regulatory system (36). It, therefore, is not surprising that Xanthomonas secretes PopA from R. solanacearum. By contrast, the hrp gene cluster of P. syringae is quite different (36), and, yet, we found AvrB secretion by Xanthomonas. Even more interesting was the finding of YopE secretion by X. campestris pv. vesicatoria, an example of type III secretion of a virulence factor from a mammalian pathogen in a plant pathogenic bacterium. YopE is a cytotoxin in Yersinia spp. that is translocated into eukaryotic cells via the ysc/lcr type III secretion pathway (2). Functional conservation of the secretion and translocation machinery has been shown previously for virulence proteins of Yersinia, Salmonella, and Shigella (30). Our study shows that the conservation of type III secretion systems can be extended to plant pathogens. The chaperone YerA appears to stabilize YopE but is dispensable for YopE secretion in Xanthomonas. Similar observations have been described for YopE and YerA in Y. pseudotuberculosis (42). There is no evidence for the existence of specific chaperones for any of the secreted proteins presented in this study. The only chaperone described in plant pathogenic bacteria is the E. amylovora DspB protein (also called DspF), which is necessary for the secretion of DspA in vitro (48).
That the type III system of X. campestris is promiscuous for secreted proteins from different origins implies recognition of their respective secretion signals. No sequence homology was found in the N terminus of the proteins secreted in this study. Type III secretion signals have been best studied for Yersinia Yop proteins and were found to reside in the N-terminal domain. Minimal regions from the N-terminal domains of Yops (as short as 15 aa for YopE) were shown to be sufficient to allow secretion of a reporter protein (2). The secretion of the 44- but not the 47-kDa AvrRxv protein might indicate that, for X. campestris pv. vesicatoria also, the signal is present in the N-terminal region of the protein. Intriguingly, experiments by Anderson and Schneewind (52) point to a secretion signal in the 5′ end of the mRNA rather than in the peptide sequence of Yop proteins. Future work on type III secretion signals of proteins from plant pathogenic bacteria is needed to fully understand the underlying mechanism.
Data presented here establish the presence of a functional type III protein secretion system in X. campestris pv. vesicatoria, encoded by the hrp genes. In mammalian pathogens, type III secretion systems have been shown to translocate specific proteins into the host cell (1). In support of communality between plant and mammalian pathogenic bacteria, previous studies have demonstrated that certain avirulence proteins induced genotype-specific cell death (HR) when they were expressed in the plant cell (11). However, transport of bacterial avirulence proteins from the pathogen into the plant cell remains to be demonstrated. It also is not known whether heterologously secreted proteins are translocated into the plant cell. Expression of AvrB or YopE in Xanthomonas did not alter pathogenicity or HR induction in plants. Our assay might not have been sensitive enough to observe some subtle effects on plant cells. Inoculation of avrB-expressing X. campestris pv. vesicatoria in Arabidopsis ecotype Columbia did not result in an HR. One possible explanation could be the low expression of AvrB in X. campestris pv. vesicatoria. Alternatively, AvrB might carry two signals, one for secretion, which is recognized by X. campestris pv. vesicatoria, and another signal for transfer into plant cells, which is not functional in Xanthomonas. The latter possibility is intriguing and is supported by studies in Yersinia. For Yop proteins, a modular structure has been reported, i.e., presence of an N-terminal secretion signal, a translocation signal located downstream, and a C-terminal effector domain (2). Whether only the secretion signal, but not the translocation signal of heterologous proteins, is conserved and recognized in the complex type III secretion system is a possibility that awaits further experimentation.
In conclusion, this study opens an important route to the identification of additional substrates of the secretion machinery of X. campestris pv. vesicatoria, especially pathogenicity factors.
Acknowledgments
We thank Christian Boucher, Hans Wolf-Watz, Jorge Galán, Eric Marois, and Jeff Dangl for kindly providing plasmids and antisera used in this study and Matthieu Arlat for testing functionality of the pLAZ13 construct in R. solanacearum. We are grateful to John Mansfield and Thomas Lahaye for critical reading of the manuscript. This work was funded in part by an Action Concertées Coordonnées–Sciences du Vivant 6 grant from the French Ministère de l’Education Nationale et de la Recherche and European Community Grant BIO4-CT97–2244 (to U.B.) O.R. was supported by a grant from the Ministère de l’Education Nationale et de la Recherche, and K.W. was supported by the Human Capital and Mobility program of the European Union.
ABBREVIATIONS
- HR
hypersensitive reaction
- avr
avirulence
- GUS
β-glucuronidase
- hrp
hypersensitive reaction and pathogenicity
- HA
hemagglutinin
Note Added in Proof
Type III-dependent in vitro secretion of another bacterial avirulence protein, AvrRpt2 from P. syringae pv. tomato, has recently been demonstrated by Mudgett and Staskawicz (53).
References
- 1.Hueck C J. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornelis G R. J Bacteriol. 1998;180:5495–5504. doi: 10.1128/jb.180.21.5495-5504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonas U, Schulte R, Fenselau S, Minsavage G V, Staskawicz B J. Mol Plant–Microbe Interact. 1991;4:81–88. [Google Scholar]
- 4.Fenselau S, Balbo I, Bonas U. Mol Plant–Microbe Interact. 1992;5:390–396. doi: 10.1094/mpmi-5-390. [DOI] [PubMed] [Google Scholar]
- 5.Fenselau S, Bonas U. Mol Plant–Microbe Interact. 1995;8:845–854. doi: 10.1094/mpmi-8-0845. [DOI] [PubMed] [Google Scholar]
- 6.Wengelnik K, Marie C, Russel M, Bonas U. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huguet E, Hahn K, Wengelnik K, Bonas U. Mol Microbiol. 1998;29:1379–1390. doi: 10.1046/j.1365-2958.1998.01019.x. [DOI] [PubMed] [Google Scholar]
- 8.Wengelnik K, Van den Ackerveken G, Bonas U. Mol Plant–Microbe Interact. 1996;9:704–712. doi: 10.1094/mpmi-9-0704. [DOI] [PubMed] [Google Scholar]
- 9.Wengelnik K, Bonas U. J Bacteriol. 1996;178:3462–3469. doi: 10.1128/jb.178.12.3462-3469.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minsavage G V, Dahlbeck D, Whalen M C, Kearney B, Bonas U, Staskawicz B J, Stall R E. Mol Plant–Microbe Interact. 1990;3:41–47. [Google Scholar]
- 11.Bonas U, Van den Ackerveken G. Plant J. 1997;12:1–7. doi: 10.1046/j.1365-313x.1997.12010001.x. [DOI] [PubMed] [Google Scholar]
- 12.Bonas U, Stall R E, Staskawicz B. Mol Gen Genet. 1989;218:127–136. doi: 10.1007/BF00330575. [DOI] [PubMed] [Google Scholar]
- 13.Van den Ackerveken G, Marois E, Bonas U. Cell. 1996;87:1307–1316. doi: 10.1016/s0092-8674(00)81825-5. [DOI] [PubMed] [Google Scholar]
- 14.Knoop V, Staskawicz B, Bonas U. J Bacteriol. 1991;173:7142–7150. doi: 10.1128/jb.173.22.7142-7150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ménard R, Sansonetti P J, Parsot C. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer H W, Roulland-Dussoix D. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 17.Ritter C, Dangl J L. Mol Plant–Microbe Interact. 1995;8:444–453. doi: 10.1094/mpmi-8-0444. [DOI] [PubMed] [Google Scholar]
- 18.Canteros B, Minsavage G, Bonas U, Pring D, Stall R. Mol Plant–Microbe Interact. 1991;4:628–632. doi: 10.1094/mpmi-4-628. [DOI] [PubMed] [Google Scholar]
- 19.Daniels M J, Barber C E, Turner P C, Sawczyc M K, Byrde R J W, Fielding A H. EMBO J. 1984;3:3323–3328. doi: 10.1002/j.1460-2075.1984.tb02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1996. [Google Scholar]
- 21.Kaniga K, Bossio J C, Galán J E. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 22.Prentki P, Krisch H M. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 23.Whalen M C, Wang J F, Carland F M, Heiskell M E, Dahlbeck D, Minsavage G V, Jones J B, Scott J W, Stall R E, Staskawicz B J. Mol Plant–Microbe Interact. 1993;6:616–627. doi: 10.1094/mpmi-6-616. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Mindrinos M, Katagiri F, Yu G L, Ausubel F M. Cell. 1994;78:1089–1099. doi: 10.1016/0092-8674(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 26.Jefferson R A, Kavanagh T A, Bevan M W. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staskawicz B J, Dahlbeck D, Keen N, Napoli C. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whalen M C, Stall R E, Staskawicz B J. Proc Natl Acad Sci USA. 1988;85:6743–6747. doi: 10.1073/pnas.85.18.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vieira J, Messing J. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 30.Rosqvist R, Håkansson S, Forsberg A, Wolf-Watz H. EMBO J. 1995;14:4187–4195. doi: 10.1002/j.1460-2075.1995.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdanove A, Beer S V, Bonas U, Boucher C A, Collmer A, Coplin D L, Cornelis G R, Huang H-C, Hutcheson S W, Panopoulos N J, et al. Mol Microbiol. 1996;20:681–683. doi: 10.1046/j.1365-2958.1996.5731077.x. [DOI] [PubMed] [Google Scholar]
- 32.Ciesiolka L D, Hwin T, Gearlds J D, Minsavage G V, Saenz R, Bravo M, Handley V, Conover S M, Zhang H, Caporgno J, et al. Mol Plant–Microbe Interact. 1999;12:34–44. doi: 10.1094/MPMI.1999.12.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Galyov E E, H†kansson S, Wolf-Watz H. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills S D, Boland A, Sory M P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardt W D, Galán J E. Proc Natl Acad Sci USA. 1997;94:9887–9892. doi: 10.1073/pnas.94.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfano J R, Collmer A. J Bacteriol. 1997;179:5655–5662. doi: 10.1128/jb.179.18.5655-5662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arlat M, Van Gijsegem F, Huet J C, Pernollet J C, Boucher C A. EMBO J. 1994;13:543–553. doi: 10.1002/j.1460-2075.1994.tb06292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huynh T V, Dahlbeck D, Staskawicz B J. Science. 1989;245:1374–1377. doi: 10.1126/science.2781284. [DOI] [PubMed] [Google Scholar]
- 39.Gopalan S, Bauer D W, Alfano J A, Loniello A O, He S Y, Collmer A. Plant Cell. 1996;8:1095–1105. doi: 10.1105/tpc.8.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosqvist R, Forsberg A, Wolf-Watz H. Infect Immunol. 1991;59:4562–4569. doi: 10.1128/iai.59.12.4562-4569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsberg A, Wolf-Watz H. J Bacteriol. 1990;172:1547–1555. doi: 10.1128/jb.172.3.1547-1555.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frithz-Lindsten E, Rosqvist R, Johansson L, Forsberg A. Mol Microbiol. 1995;16:635–647. doi: 10.1111/j.1365-2958.1995.tb02426.x. [DOI] [PubMed] [Google Scholar]
- 43.He S Y, Huang H C, Collmer A. Cell. 1993;73:1255–1266. doi: 10.1016/0092-8674(93)90354-s. [DOI] [PubMed] [Google Scholar]
- 44.Bogdanove A J, Wei Z M, Zhao L, Beer S V. J Bacteriol. 1996;178:1720–1730. doi: 10.1128/jb.178.6.1720-1730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J F, Beer S V. J Bacteriol. 1998;180:5203–5210. doi: 10.1128/jb.180.19.5203-5210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charkowski A O, Alfano J R, Preston G, Yuan J, He S Y, Collmer A. J Bacteriol. 1998;180:5211–5217. doi: 10.1128/jb.180.19.5211-5217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roine E, Wei W, Yuan J, Nurmiaho-Lassila E-L, Kalkkinen N, Romantschuk M, He S Y. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaudriault S, Malandrin L, Paulin J P, Barny M A. Mol Microbiol. 1997;26:1057–1069. doi: 10.1046/j.1365-2958.1997.6442015.x. [DOI] [PubMed] [Google Scholar]
- 49.Wei Z M, Sneath B J, Beer S V. J Bacteriol. 1992;174:1875–1882. doi: 10.1128/jb.174.6.1875-1882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulte R, Bonas U. Plant Cell. 1992;4:79–86. doi: 10.1105/tpc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ham J H, Bauer D W, Fouts D E, Collmer A. Proc Natl Acad Sci USA. 1998;95:10206–10211. doi: 10.1073/pnas.95.17.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson D M, Schneewind O. Science. 1997;278:1140–1143. doi: 10.1126/science.278.5340.1140. [DOI] [PubMed] [Google Scholar]
- 53.Mudgett M B, Staskawicz B J. Mol Microbiol. 1999;32:927–941. doi: 10.1046/j.1365-2958.1999.01403.x. [DOI] [PubMed] [Google Scholar]