Abstract
The Cercospora nicotianae SOR1 (singlet oxygen resistance) gene was identified previously as a gene involved in resistance of this fungus to singlet-oxygen-generating phototoxins. Although homologues to SOR1 occur in organisms in four kingdoms and encode one of the most highly conserved proteins yet identified, the precise function of this protein has, until now, remained unknown. We show that SOR1 is essential in pyridoxine (vitamin B6) synthesis in C. nicotianae and Aspergillus flavus, although it shows no homology to previously identified pyridoxine synthesis genes identified in Escherichia coli. Sequence database analysis demonstrated that organisms encode either SOR1 or E. coli pyridoxine biosynthesis genes, but not both, suggesting that there are two divergent pathways for de novo pyridoxine biosynthesis in nature. Pathway divergence appears to have occurred during the evolution of the eubacteria. We also present data showing that pyridoxine quenches singlet oxygen at a rate comparable to that of vitamins C and E, two of the most highly efficient biological antioxidants, suggesting a previously unknown role for pyridoxine in active oxygen resistance.
Keywords: SOR1, PDX1, photosensitizer, cercosporin
The filamentous, phytopathogenic fungus Cercospora nicotianae exhibits a uniquely effective, broad-spectrum resistance to potent photosensitizers of diverse chemical structure and solubility (1, 2). C. nicotianae is resistant to cercosporin, a light-activated, singlet oxygen (1O2)-generating toxin it produces in culture and during plant parasitism, and also to other potent photosensitizers including porphyrins and xanthine and thiazine dyes. Photosensitizers are highly toxic compounds that produce their deleterious effects only after activation by light. Absorbed light energy converts the photosensitizer to an excited (triplet) state molecule that may transfer an electron to oxygen to generate superoxide and/or transfer energy directly to oxygen, yielding 1O2 (3). Exposure of cells to photosensitizers plus light leads to the destruction of critical cellular components including proteins, membranes, and DNA and often results in cell death.
Studies on the mechanisms by which organisms protect themselves against reactive oxygen species have focused primarily on reduced and radical forms of oxygen, including hydrogen peroxide (H2O2), superoxide (O2⨪), and the hydroxyl radical (OH·). These active oxygen species are byproducts of normal cellular metabolism, and cells contain numerous and conserved defenses against them. By contrast, the highly reactive, but nonradical 1O2 is produced primarily via light activation of photosensitizing compounds. Most organisms do not tolerate 1O2, and few biological defenses have been identified (2). The broad-spectrum resistance expressed by Cercospora species against cercosporin and other photosensitizers of diverse structure make these organisms an excellent model for understanding the cellular basis of 1O2 resistance.
To study specific genes and proteins involved in photosensitizer and 1O2 resistance, we isolated mutants of C. nicotianae sensitive to cercosporin (1, 4) and used functional complementation to identify genes required for resistance. Our recent work has focused on the C. nicotianae SOR1 (singlet oxygen resistance) gene (5, 6). Mutant complementation studies and the production of SOR1 null mutants by targeted gene replacement suggested that SOR1 plays a role in C. nicotianae resistance both to cercosporin and other photosensitizers. SOR1 was the first cloned gene with apparent genetic and phenotypic links to resistance against compounds that generate 1O2.
Initial identification of SOR1 concomitantly uncovered an intriguing mystery. Although 1O2 and photosensitizer resistance is uncommon, SOR1 homologues are widespread, occurring in numerous organisms within four kingdoms, archaebacteria, eubacteria, plants, and fungi (5). In addition to enjoying widespread distribution, SOR1 also is one of the most highly conserved proteins yet identified (7, 8). These results suggested that the SOR1 protein is involved in an unknown but conserved metabolic function. In this paper we describe a second phenotype for SOR1 that explains its strong conservation in diverse organisms. Our data show that SOR1 is necessary for synthesis of pyridoxine (vitamin B6) in C. nicotianae and in a second filamentous fungus, Aspergillus flavus. We propose that SOR1 is part of a novel pathway for de novo biosynthesis of pyridoxine distinct from the previously described Escherichia coli de novo pathway (9–14) and that pathway divergence occurred during the evolution of the eubacteria. Finally, we also provide data demonstrating that pyridoxine quenches 1O2 and argue for a heretofore undiscovered role for pyridoxine in antioxidant defense.
MATERIALS AND METHODS
Fungal Strains and Culture Conditions.
All C. nicotianae mutants used in this study were derived from the wild-type strain ATCC 18366. Mutants used included three UV-generated sor1 mutant strains (cercosporin-sensitive CS6, CS8, and CS9) (1, 4) and three sor1 null strains generated by targeted gene replacement (5). The C. nicotianae SOR1 transformants screened for prototrophy were derived from previous studies. A. flavus strain ATCC 60045 is a pyridoxine auxotroph, kindly provided by G. A. Payne, North Carolina State University. Stock cultures of C. nicotianae were maintained in the dark, on malt medium (15) at 28°C, conditions nonconducive to cercosporin biosynthesis by Cercospora fungi. For assessment of pyridoxine auxotrophy and prototrophy, transformants and the original CS strains were grown on minimal medium (15) with and without the addition of 1 μg/ml pyridoxine-HCl.
Transformation.
Protocols for genetic transformation of A. flavus have been described (16). Two plasmids, both expressing only the SOR1 ORF, were used to transform A. flavus. The first expressed SOR1 under the control of the constitutive A. nidulans gpdA (glyceraldehyde-3-phosphate) promoter (17). The second was a previously described (5, 6) clone containing SOR1 under the control of its own promoter.
Computer Analysis of Data.
Database homology searches were performed by using the blast server at the National Center for Biotechnology Information (18). Preliminary sequence data were obtained from The Institute for Genomic Research web site at http://www.tigr.org.
Quenching of 1O2 Phosphorescence by Pyridoxine.
Singlet oxygen was detected from irradiated solutions of cercosporin or rose bengal in aerobic D2O via phosphorescence at 1,270 nm (diagnostic for 1O2). The experimental set-up used an NdYag laser (Continuum, Santa Clara, CA) for pulse excitation (532 nm), and 1O2 phosphorescence was detected by a germanium diode as described by Bilski and Chignell (19). The kinetics of decay in the presence and absence of pyridoxine, pyridoxal, pyridoxamine, pyridoxal 5-phosphate, and l-methionine were measured as described (19). Rate constants for 1O2 quenching were determined with increasing concentrations of each quencher in 50 μM rose bengal-sensitized solutions.
Chemicals.
Cercosporin was extracted and purified from mycelial cultures of Cercospora kikuchii as described (20). Pyridoxine, pyridoxal, pyridoxamine, pyridoxal 5-phosphate, and l-methionine all were purchased from Sigma.
RESULTS
SOR1 Is Required for Pyridoxine (Vitamin B6) Synthesis.
During experiments to determine whether expression of SOR1 was sufficient to confer 1O2 and photosensitizer resistance on a sensitive organism, a second phenotype for SOR1 was discovered. A construct containing the SOR1 ORF under the control of the constitutive A. nidulans gpdA promoter was transformed into an A. flavus strain auxotrophic for pyridoxine. Analysis of the transformants indicated that this isolate could be restored to pyridoxine prototrophy by SOR1 transformation, either under the control of the gpdA promoter or its own promoter (data not shown). Transformation with plasmids lacking SOR1 failed to restore prototrophy, confirming that restoration to pyridoxine prototrophy was a SOR1-specific effect. Furthermore, we also found that pyridoxine prototrophy could be used as a selective marker in A. flavus transformations with SOR1.
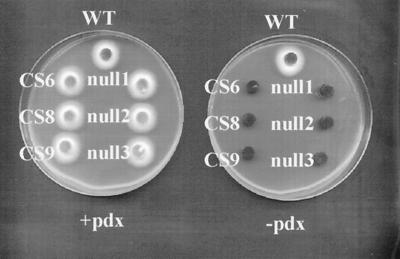
Our fungal strains are maintained on complex medium. The above results led us to test our cercosporin-sensitive strains, used in the identification of SOR1, for pyridoxine auxotrophy. Six sor1 mutant C. nicotianae strains were tested, three of which were generated by UV mutagenesis and three null strains that were generated by targeted gene replacement (1, 4–6). When tested for growth on minimal medium, all six strains required pyridoxine for growth (Fig. 1). When transformed with SOR1, all of the mutant strains were restored to pyridoxine prototrophy (data not shown). We also have learned recently that an A. nidulans gene that complements an A. nidulans pyridoxine auxotroph encodes a SOR1 homologue (A. H. Osmani, G. S. May, and S. A. Osmani, personal communication). Thus, SOR1 homologues are required for pyridoxine synthesis in both Cercospora and Aspergillus.
Figure 1.
Growth of C. nicotianae wild type (WT) and sor1 null (null 1, 2, and 3) and cercosporin-sensitive UV-mutant (CS6, CS8, and CS9) strains on minimal medium with (+pdx) and without (−pdx) 1 μg/ml pyridoxine. Six-millimeter fungal plugs were placed mycelium-side down and incubated for 4 days at 28°C.
SOR1 Encodes a Novel Pyridoxine Pathway Gene.
Studies of pyridoxine biosynthesis have centered on E. coli in which pathway genes for de novo pyridoxine biosynthesis have been identified via complementation of auxotrophic mutants (9–14, 21–23). Two of the genes (serC and gapB) encode enzymes also required in other biosynthetic pathways, but three (pdxA, pdxB, and pdxJ) are unique to pyridoxine synthesis. None of these five genes shows homology to SOR1. We performed blast searches by using SOR1 and the unique E. coli pyridoxine biosynthesis genes (pdxA, pdxB, and pdxJ) against the GenBank database as a whole as well as the genomes with separate databases. At the time of this writing, the entire genomes of 18 microorganisms and one animal were available for analysis. Without exception, organisms with SOR1 homologues lack homologues to the E. coli pyridoxine biosynthesis genes pdxA and pdxJ, whereas those with homologues to the E. coli genes lack SOR1 (Table 1). Seven genomes possess neither SOR1 nor pdxA/pdxJ. Six of these belong to obligate microbial parasites with greatly reduced coding capacity, presumably because they obtain many nutrients, such as pyridoxine, from their hosts. The seventh is the single animal representative, Caenorhabditis elegans.
Table 1.
Occurrence of SOR1 and the E. coli pyridoxine biosynthetic genes pdxA and pdxJ in organisms with completely sequenced genomes
| Organism | SOR1 | pdxA/J | Genome |
|---|---|---|---|
| Fungi | |||
| Saccharomyces cerevisiae | + | − | 16 Mb |
| Archaebacteria | |||
| Methanococcus jannaschii | + | − | 1.8 Mb |
| Pyrococcus horikoshii | + | − | 2.0 Mb |
| Methanobacterium thermoautotrophicum | + | − | 1.8 Mb |
| Archaeoglobus fulgidus | + | − | 2.2 Mb |
| Eubacteria | |||
| Haemophilus influenzae | + | − | 1.8 Mb |
| Bacillus subtilis | + | − | 4.2 Mb |
| Mycobacterium tuberculosis | + | − | 4.4 Mb |
| E. coli | − | + | 4.6 Mb |
| Synechocystis | − | + | 3.5 Mb |
| Helicobacter pylori | − | + | 1.7 Mb |
| Aquifex aeolicus | − | + | 1.5 Mb |
| Mycoplasma pneumoniae | − | − | 0.8 Mb |
| Mycoplasma genitalium | − | − | 0.6 Mb |
| Borrelia burgdorferi | − | − | 1.0 Mb |
| Treponema pallidum | − | − | 1.1 Mb |
| Chlamydia trachomatis | − | − | 1.0 Mb |
| Rickettsia prowazekii | − | − | 1.1 Mb |
| Animal | |||
| Caenorhabditis elegans | − | − | 100 Mb |
The data from completely sequenced genomes are corroborated by data from other, incompletely sequenced ones (Table 2). Here, SOR1 homologues were identified in three fungi, six plants, two archaebacteria, six eubacteria, and the Dictyostelid Dictyostelium discoidum. Thirteen additional eubacteria contain homologues to both pdxA and pdxJ (Table 2), whereas four others (Aquifex pyrophilus, Bradyrhizobium japonicum, Sphingomonas aromaticivorans, and Burkholderia cepacia) contain either pdxA or pdxJ. None of these 35 organisms encode both SOR1 and pdxA and/or pdxJ.
Table 2.
Occurrence of SOR1 and the E. coli pyridoxine biosynthetic genes pdxA and pdxJ in organisms with partially sequenced genomes
| Organism | SOR1 | pdxA/J |
|---|---|---|
| Plant | ||
| Arabidopsis thaliana | + | − |
| Oryza sativa | + | − |
| Hevea brasiliensis | + | − |
| Stellaria longipes | + | − |
| Brassica napus | + | − |
| Physcomitrella patens | + | − |
| Fungi | ||
| Schizosaccharomyces pombe | + | − |
| A. nidulans | + | − |
| Candida albicans | + | − |
| Dictyostelid | ||
| Dictyostelium discoidum | + | − |
| Archaebacteria | ||
| Methanococcus vannielli | + | − |
| Pyrococcus furiosis | + | − |
| Eubacteria | ||
| Mycobacterium leprae | + | − |
| Francisella tularensis | + | − |
| Clostridium acetobutylicum | + | − |
| Streptococcus pneumoniae | + | − |
| Thermotoga maritima | + | − |
| Deinococcus radiodurans | + | − |
| Shewanella putrefaciens | − | + |
| Caulobacter crescentus | − | + |
| Porphyromonas gingivalis | − | + |
| Chlorobium tepidum | − | + |
| Erwinia herbicola | − | + |
| Yersinia pestis | − | + |
| Pseudomonas aeruginosa | − | + |
| Neisseria meningitidis | − | + |
| Neisseria gonorrhoeae | − | + |
| Campylobacter jejuni | − | + |
| Vibrio cholerae | − | + |
| Salmonella typhi | − | + |
| Bordetella pertussis | − | + |
In contrast to pdxA and pdxJ, results with pdxB were inconclusive. Because the pdxB gene product is a dehydrogenase, there is widespread, but low, homology to the E. coli PDXB protein. Potential pdxB homologues were found in organisms with SOR1 homologues as well as in pdxA/pdxJ-containing organisms (data not shown). Further, pdxB homologues could be identified in only two (Synechocystis and Aquifex aeolicus) of the three completely sequenced pdxA/pdxJ-containing organisms. However, the data with pdxA and pdxJ strongly suggest that there are two different sets of genes involved in de novo pyridoxine synthesis. One set, of which SOR1 is a part, is found in some eubacteria, in all archaebacteria so far examined, and in eukaryotes such as fungi and plants. The second set, the E. coli version, is found so far only in some, but not all, eubacteria.
Pyridoxine and Biologically Active Pyridoxine Vitamers Quench 1O2 in Vitro.
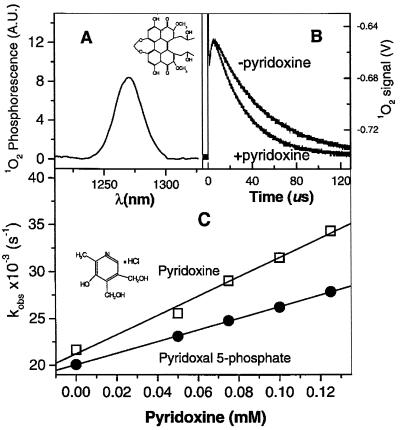
The connection in C. nicotianae between photosensitizer resistance and pyridoxine synthesis was completely unanticipated. Extensive surveys of 1O2 quenchers (24, 25) do not include pyridoxine, nor has this vitamin been implicated previously in cellular antioxidant defense. To determine whether pyridoxine could directly deactivate 1O2, we examined the interaction of these molecules in vitro. Singlet oxygen can be identified by its characteristic IR phosphorescence at 1,270 nm after irradiation of a photosensitizer-containing sample (Fig. 2A). Photosensitizer-containing samples with and without 0.1 mM pyridoxine were subjected to a laser pulse, and phosphorescence was measured at 1,270 nm. 1O2 phosphorescence decayed faster in the presence of pyridoxine (Fig. 2B), indicating that pyridoxine decreases 1O2 lifetime. Dose-response experiments confirmed that increasing concentrations of pyridoxine and pyridoxal 5-phosphate increase the rate of 1O2 decay (Fig. 2C). These data were used to calculate rate constants for pyridoxine and other biologically active forms of vitamin B6 (Table 3). All four vitamers tested exhibited quenching constants at or approaching 1 × 108 M−1⋅sec−1. They are thus more efficient quenchers than sulfur-containing antioxidants and quench 1O2 at a rate comparable to that of vitamins E and C, two of the most efficient biological antioxidants identified to date.
Figure 2.
Quenching of 1O2 phosphorescence by pyridoxine. (A) Spectrum of 1O2 phosphorescence after irradiation of a solution of cercosporin (Inset) in aerobic D2O. (B) 1O2 phosphorescence decay observed at the maximum of 1O2 emission after one-shot laser 1O2 production in the absence (−pyridoxine) and presence (+pyridoxine) of 0.1 mM pyridoxine. (C) Observed rate constant for 1O2 quenching as a function of the increasing concentration of pyridoxine (Inset) and pyridoxal 5-phosphate at pD 7.4 in D2O aerobic phosphate buffer (20 mM). The line slopes yield the quenching-rate constants (Table 3). Rose bengal (50 μM) was used to photosensitize 1O2.
Table 3.
Total quenching* of 1O2 by pyridoxine derivatives and other physiological antioxidants
| Quencher/antioxidant | kq, M−1·s−1 | Conditions, solvent | Ref. |
|---|---|---|---|
| Pyridoxine | 10.3 (± 0.5) × 107 | pD = 7.4, D2O | This work |
| Pyridoxal | 8.7 (± 0.1) × 107 | pD = 7.4, D2O | This work |
| Pyridoxamine | 9.9 (± 0.2) × 107 | pD = 7.4, D2O | This work |
| Pyridoxal 5-phosphate | 6.2 (± 0.2) × 107 | pD = 7.4, d2O | This work |
| l-Methionine | 1.1 (± 0.1) × 107 | D2O | This work |
| Vitamin C | 0.83 × 107 | H2O, pH 6.8 | (26) |
| 15 × 107 | CD3OD | (27) | |
| Vitamin E | 10 × 107 | CCl4 | (28) |
| 3 × 107 | ClCF2CCl2F | (29) | |
| Glycyl-methionine | 1.5 × 107 | 50% CH3CN, 50% H2O | (30) |
| Glutathione | 0.087 × 107 | D2O | (31) |
| Cysteine | 5 × 107 | 75% D2O, 25% EtOH | (32) |
The quenching-rate constants, kq, reported in this work were calculated from time-resolved decays of 1O2 phosphorescence recorded after a single-pulse laser irradiation (19) of rose bengal in aerobic D2O solution containing the pyridoxine quencher.
DISCUSSION
SOR1 originally was identified as a gene involved in resistance to cercosporin and other 1O2-generating photosensitizers in the fungus C. nicotianae. Although 1O2 resistance is rare in nature, sequence analysis indicated SOR1 homologues were present in widely divergent organisms not reported to be photosensitizer resistant, including fungi, plants, eubacteria, and archaebacteria (5). In addition to the gene’s widespread distribution, the predicted SOR1 protein is one of the most highly conserved proteins yet identified (7, 8). However, the precise metabolic function of the SOR1 protein remained undefined. In this study, we demonstrate that SOR1 is required for synthesis of pyridoxine, a vitamin that is a direct precursor for pyridoxal 5-phosphate, a required cofactor in enzymatic reactions such as transaminations, which are involved primarily in amino acid metabolism. The linkage of SOR1 with pyridoxine synthesis provides a singularly clear solution to the initial mystery of why SOR1 is so conserved and so much more prevalent than photosensitizer resistance. Nearly all microbes, prokaryotic and eukaryotic, as well as plants synthesize pyridoxine. Organisms that do not synthesize pyridoxine would be expected to lack genes associated with its production, clarifying why SOR1 is not present in any of the animal genomes being sequenced. Although our original analysis detected a SOR1 homologue in C. elegans (5), completion of this animal genome indicated that the supposed homologue was actually a yeast sequence.
To date, all genes involved in de novo pyridoxine synthesis are from E. coli. Biochemical and functional complementation analyses of E. coli pyridoxine auxotrophs led to the identification of the intermediate products and the genes necessary for de novo synthesis, respectively (9, 11–14). Our evidence, however, indicates that the E. coli pathway is not universal. With the exception of animals and obligate parasites, homology searches using SOR1 and the E. coli genes pdxA and pdxJ (Tables 1 and 2) clearly divide organisms into those with SOR1 homologues and those with pdxA/pdxJ homologues. The SOR1 group includes archaebacteria, eukaryotes such as fungi and plants, and some eubacteria, whereas the pdxA/pdxJ group includes only eubacteria. The evolutionary shift appears to have occurred in the gamma subdivision of the proteobacteria taxon of the eubacteria group. Two bacterial species in this subdivision encode SOR1 homologues, whereas numerous others, including E. coli, do not.
In addition to our evidence, 15N-labeling studies in yeast, which has three unlinked SOR1 homologues (7) and no homologues to either pdxA or pdxJ, determined that the nitrogen atom of yeast pyridoxine is derived from the amide moiety of glutamine (33), whereas in E. coli, glutamic acid provides the nitrogen. The 15N-labeling data, in conjunction with our database analysis, suggest that the yeast pyridoxine pathway, like the yeast thiamin synthetic pathway (34), is distinct from that of E. coli. Interestingly, there is a second gene (SNO or SNZB) found in organisms with SOR1 homologues, generally in physical proximity to SOR1. The protein encoded by this gene has been predicted to possess glutamine amidotransferase activity (8, 35), a hypothesis consistent with the 15N-labeling data.
It is unclear at this time whether the pathways in E. coli and in SOR1-containing organisms are completely disparate, or if they only partially diverge. pdxA and pdxJ encode enzymes catalyzing the final step in E. coli pyridoxine synthesis, that of ring closure (9, 21, 23). The dehydrogenation catalyzed by the pdxB gene product and the addition of the nitrogen moiety, which in E. coli is catalyzed by an aminotransferase, occur in that order and before ring closure. However, homology searches with pdxb produced far less clear results than the pdxA/pdxJ homology searches. Although SOR1-containing organisms contain putative pdxB homologues, not all pdxA/pdxJ-containing organisms do so. Furthermore, the homology between putative pdxB homologues is less pronounced than the homologies between either pdxA or pdxJ homologues. It seems feasible that SOR1 substitutes for PDXA/PDXJ and is involved in ring closure whereas SNO/SNZB are required for addition of the nitrogen moiety via an amidotransferase reaction. However, further studies need to be performed to determine the extent of conservation and/or divergence between the E. coli de novo pyridoxine synthesis pathway and the “SOR1” pathway. Interestingly, although there is divergence in the de novo synthesis of pyridoxine, the salvage pathway that interconverts and recycles the various vitamers of pyridoxine into the active coenzyme (pyridoxal 5-phosphate), appears to be conserved (12).
In this study, we also suggest a previously unrecognized role for pyridoxine in active oxygen resistance. Cellular oxidative damage has become the focus of intense interest in both the scientific and popular literature. Medical studies seek to quantify both the curative and protective effects of nutritive substances with antioxidant properties. In advertising and the popular press, numerous compounds with antioxidant properties have been touted as prophylactic or restorative agents. Although the importance of many compounds, such as vitamins C and E and β-carotene, are well known, even the highly enthusiastic lay press rarely mentions vitamin B6 in this capacity. Surveys quantifying the 1O2-quenching ability of both natural and synthesized compounds also fail to include pyridoxine or its vitamers (24, 25). Thus, the linkage of pyridoxine synthesis with photosensitizer resistance was completely unanticipated. Direct measurements of the ability of pyridoxine and its vitamers to quench 1O2 (Fig. 2 and Table 3), however, provides evidence that this vitamin may play a heretofore unforeseen biological function in addition to its known role as a cofactor in enzymatic reactions.
Corroborative evidence that pyridoxine, via expression of SOR1 and its homologues, is involved in active oxygen resistance can be found in expression studies in plants, yeast (Saccharomyces cerevisiae), and fission yeast (Schizosaccharomyces pombe). In rubbertree (Hevea brasiliensis), SOR1 homologue transcripts increase to higher amounts in response to treatment with ethylene and salicylic acid (36), two inducers of plant-defense reactions. Plant-defense reactions, including those induced by ethylene and salicylic acid, are associated with increases in production of active oxygen species that serve multiple roles as signal molecules, as substrates in the production of structural defense compounds, and as defense molecules against pathogens (37). In S. cerevisiae, one of the three SOR1 homologues (SNZ1) encodes a protein that accumulates to high levels in stationary-phase culture (7). Stationary-phase yeast cultures are subjected to increased oxidative stress, and entry into this growth stage is associated with a dramatic increase in resistance to a diversity of oxidants (38). Finally, in S. pombe, overexpression of the PAP1 transcription factor increases expression of the S. pombe SOR1 homologue (W. M. Toone, Laboratory of Gene Regulation, Imperial Cancer Research Fund, London, personal communication). PAP1 is an AP-1-like transcription factor that is a member of the basic leucine zipper superfamily of DNA-binding transcription factors (39). PAP1 is an essential part of the S. pombe cellular response to oxidative stress (40), as is the comparable S. cerevisiae YAP1 transcription factor (41, 42). We have found an AP-1 consensus region upstream of the C. nicotianae SOR1 promoter, suggesting that SOR1 may be regulated by this family of transcription factors and as a general response to cellular oxidative stress.
Ironically, our observation that sor1 mutants lack resistance to 1O2-generating photosensitizers now is complicated by our data on pyridoxine quenching. We have determined recently that pyridoxine and its vitamers quench 1O2 primarily via a chemical quenching mechanism during which the vitamin is consumed (data not shown). Measurements conducted during growth experiments indicate that pyridoxine concentrations drop rapidly in the presence of photosensitizers and light. Thus, in the process of quenching 1O2, a nutrient required by our sor1 mutants is consumed. We currently are concentrating our efforts on dissecting the relationship between photosensitizer sensitivity and auxotrophy in our mutant strains. Because the sor1 phenotype is clearly that of pyridoxine auxotrophy, we have changed the name of this gene to PDX1 (GenBank accession no. AF035619).
Acknowledgments
We thank J. D. Williamson for critical review of this manuscript, G. A. Payne for providing the A. flavus culture, and A. H. Osmani, G. S. May, S. A. Osmani, and W. M. Toone for sharing unpublished results. Preliminary sequence data were obtained from The Institute for Genomic Research web site at http://www.tigr.org. This work was supported by National Science Foundation Grant MCB-9631375 and U.S. Department of Agriculture National Research Initiative Competitive Grants Program Grant 96-35303-3204, both to M.E.D. and M.E.
References
- 1.Jenns A E, Scott D L, Bowden E F, Daub M E. Photochem Photobiol. 1995;61:488–493. [Google Scholar]
- 2.Daub M E, Ehrenshaft M, Jenns A E, Chung K R. In: Phytochemical Signals and Plant-Microbe Interactions, Recent Advances in Phytochemistry. Downum K R, Verpoorte R, editors. Vol. 32. New York: Plenum; 1998. pp. 31–56. [Google Scholar]
- 3.Spikes J D. In: The Science of Photobiology. Smith K C, editor. New York: Plenum; 1989. pp. 79–110. [Google Scholar]
- 4.Jenns A E, Daub M E. Phytopathology. 1995;85:906–912. [Google Scholar]
- 5.Ehrenshaft M, Jenns A E, Chung K R, Daub M E. Mol Cell. 1998;1:603–609. doi: 10.1016/s1097-2765(00)80060-x. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenshaft M, Chung K R, Jenns A E, Daub M E. Curr Genet. 1999;34:478–485. doi: 10.1007/s002940050423. [DOI] [PubMed] [Google Scholar]
- 7.Braun E L, Fuge E K, Padilla P A, Werner-Washburne M. J Bacteriol. 1996;178:6865–6872. doi: 10.1128/jb.178.23.6865-6872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galperin M Y, Koonin E V. Mol Microbiol. 1997;24:443–445. doi: 10.1046/j.1365-2958.1997.3671706.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill R E, Spenser I D. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt F C, Curtiss R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Vol. 1. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 695–703. [Google Scholar]
- 10.Lam H M, Winkler M E. J Bacteriol. 1990;172:6518–6528. doi: 10.1128/jb.172.11.6518-6528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao G, Winkler M E. FEMS Microbiol Lett. 1996;135:275–280. doi: 10.1111/j.1574-6968.1996.tb08001.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Zhao G, Man T K, Winkler M E. J Bacteriol. 1998;180:4294–4299. doi: 10.1128/jb.180.16.4294-4299.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hockney R C, Scott T A. J Gen Microbiol. 1979;110:275–283. doi: 10.1099/00221287-110-2-275. [DOI] [PubMed] [Google Scholar]
- 14.Drewke C, Klein M, Clade D, Arenz A, Muller R, Leistner E. FEBS Lett. 1996;390:179–182. doi: 10.1016/0014-5793(96)00652-7. [DOI] [PubMed] [Google Scholar]
- 15.Jenns A E, Daub M E, Upchurch R G. Phytopathology. 1989;79:213–219. [Google Scholar]
- 16.Woloshuk C P, Seip E R, Payne G A. Appl Environ Microbiol. 1989;55:86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punt P J, Zegers N D, Busscher M, Pouwels P H, van den Hondel C A M J J. Biotechnology. 1991;17:19–24. doi: 10.1016/0168-1656(91)90024-p. [DOI] [PubMed] [Google Scholar]
- 18.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 19.Bilski P, Chignell C F. J Biochem Biophys Methods. 1996;33:73–80. doi: 10.1016/s0165-022x(96)00012-7. [DOI] [PubMed] [Google Scholar]
- 20.Daub M E. Phytopathology. 1982;72:370–374. [Google Scholar]
- 21.Roa B B, Connolly D M, Winkler M E. J Bacteriol. 1989;171:4767–4777. doi: 10.1128/jb.171.9.4767-4777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenlein P V, Roa B B, Winkler M E. J Bacteriol. 1989;171:6084–6092. doi: 10.1128/jb.171.11.6084-6092.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam H M, Tancula E, Dempsy W B, Winkler M E. J Bacteriol. 1992;174:1554–1567. doi: 10.1128/jb.174.5.1554-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellus D. Adv Photochem. 1979;11:105–205. [Google Scholar]
- 25.Wilkinson F, Helman W P, Ross A B. J Phys Chem Ref Data. 1995;24:663–1021. [Google Scholar]
- 26.Chou P T, Khan A U. Biochem Biophys Res Commun. 1983;115:932–937. doi: 10.1016/s0006-291x(83)80024-2. [DOI] [PubMed] [Google Scholar]
- 27.Scurlock R, Rougee M, Bensasson R V. Free Radical Res Commun. 1990;8:251–258. doi: 10.3109/10715769009053358. [DOI] [PubMed] [Google Scholar]
- 28.Krasnovsky A A J. Photochem Photobiol. 1979;29:29–33. [Google Scholar]
- 29.Stevens B, Marsh K L. J Phys Chem. 1982;86:4473–4476. [Google Scholar]
- 30.Miskoski S, Garcia N A. Photochem Photobiol. 1993;57:447–452. doi: 10.1111/j.1751-1097.1993.tb02317.x. [DOI] [PubMed] [Google Scholar]
- 31.Chacon J N, Truscott T G. J Photochem Photobiol B. 1991;11:261–267. doi: 10.1016/1011-1344(91)80031-c. [DOI] [PubMed] [Google Scholar]
- 32.Michaeli A, Feitelson J. Photochem Photobiol. 1994;59:284–289. doi: 10.1111/j.1751-1097.1994.tb05035.x. [DOI] [PubMed] [Google Scholar]
- 33.Tazuya K, Adachi Y, Masuda K, Yamada K, Kumaoka H. Biochim Biophys Acta. 1995;1244:113–116. doi: 10.1016/0304-4165(94)00205-c. [DOI] [PubMed] [Google Scholar]
- 34.Tazuya K, Yamada K, Kumaoka H. Biochem Mol Biol Int. 1993;30:893–899. [PubMed] [Google Scholar]
- 35.Padilla P A, Fuge E K, Crawford M E, Errett A, Werner-Washburne M. J Bacteriol. 1998;180:5718–5726. doi: 10.1128/jb.180.21.5718-5726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sivasubramaniam S, Vanniashingham V M, Tan C T, Chua N H. Plant Mol Biol. 1995;29:173–178. doi: 10.1007/BF00019129. [DOI] [PubMed] [Google Scholar]
- 37.Mehdy M C, Sharma Y K, Sathasivan K, Bays N W. Physiol Plant. 1996;98:365–374. [Google Scholar]
- 38.Jamieson D J. Redox Rep. 1995;1:89–95. doi: 10.1080/13510002.1995.11746964. [DOI] [PubMed] [Google Scholar]
- 39.Toda T, Shimanuki M, Yanagida M. Genes Dev. 1991;5:60–73. doi: 10.1101/gad.5.1.60. [DOI] [PubMed] [Google Scholar]
- 40.Toone M W, Kuge S, Samuels M, Morgan B A, Toda T, Jones N. Genes Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephen D W, Rivers S L, Jamieson D J. Mol Microbiol. 1995;16:415–423. doi: 10.1111/j.1365-2958.1995.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuge S, Jones N. EMBO J. 1994;13:655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]