Abstract
Brain imaging and electrophysiological recording studies in humans have reported discrete cortical regions in posterior ventral temporal cortex that respond preferentially to faces, buildings, and letters. These findings suggest a category-specific anatomically segregated modular organization of the object vision pathway. Here we present data from a functional MRI study in which we found three distinct regions of ventral temporal cortex that responded preferentially to faces and two categories of other objects, namely houses and chairs, and had a highly consistent topological arrangement. Although the data could be interpreted as evidence for separate modules, we found that each category also evoked significant responses in the regions that responded maximally to other stimuli. Moreover, each category was associated with its own differential pattern of response across ventral temporal cortex. These results indicate that the representation of an object is not restricted to a region that responds maximally to that object, but rather is distributed across a broader expanse of cortex. We propose that the functional architecture of the ventral visual pathway is not a mosaic of category-specific modules but instead is a continuous representation of information about object form that has a highly consistent and orderly topological arrangement.
Keywords: face perception, functional MRI, object recognition
Motivated by the existence of category-specific deficits in brain-damaged patients, several recent imaging and electrophysiological recording studies have reported discrete cortical areas in the ventral temporal cortex of humans, specialized for the perception of faces (1–7), buildings (8, 9), and letters (3, 10). Although it may be true that there are dedicated neural mechanisms, or modules, for certain biologically relevant objects, such as faces, which emerge through evolution, it seems highly unlikely that there are modules for all object categories. An alternative possibility is that the representation of an object in ventral temporal cortex is more widely distributed. In fact, neuroimaging studies consistently have shown that the response to an object category is not restricted to the region that responds maximally to that category (5, 7–9). The fact that all objects activate a broad expanse of ventral temporal cortex, albeit to varying degrees, suggests that the representation of objects in this cortex may be feature rather than object based. Such an organization would be more in line with physiological results in the monkey (e.g., ref. 11) and computational models of object recognition (e.g., refs. 12–14).
If the representation of objects in ventral temporal cortex is feature based and widely distributed, one would predict that different categories of objects would evoke different patterns of activity across a broad expanse of this cortex. Accordingly, we investigated the patterns of response evoked in ventral temporal cortex by faces, houses, and another category of man-made objects, namely chairs. In previous studies, faces and houses have been associated with anatomically distinct ventral temporal regions (7–9). We focused our analyses on the pattern of response evoked by a category of objects outside of the region that responded maximally to that category. For example, we examined the pattern of response to houses in the regions that responded maximally to faces and chairs. As predicted, we found that each category of object was associated with a highly consistent pattern of response across the expanse of ventral temporal cortex. Based on these results, we propose that the functional architecture of the ventral temporal cortex is based on a continuous representation of object features, such that features shared by members of a category tend to cluster together.
METHODS
Subjects.
Twelve healthy right-handed volunteers with normal vision (six males, six females, age 26 ± 3 yr) participated in this study. All subjects gave written informed consent.
Task.
In Experiment 1, six subjects performed passive viewing and delayed match-to-sample tasks. In the passive viewing task, single stimuli (houses, faces, chairs, and scrambled pictures) were presented at a rate of two per second. In the delayed matching task, a single-sample stimulus (presented for 1.5 s) was followed, after a 0.5-s delay, by a pair of choice stimuli (presented for 2 s). The sample and matching choice stimuli were photographs of the same house, face, or chair taken from different viewing angles. Subjects indicated which choice stimulus matched the sample by pressing a button with the right or left thumb, and reaction time was recorded. In the control task, scrambled nonsense pictures were presented in the same configuration and sequence as the stimuli during the delayed matching tasks, and subjects responded to the presentation of a pair of scrambled patterns at the end of each control item by pressing both right and left buttons simultaneously.
In Experiment 2, six subjects performed delayed matching tasks with photographs (as in Experiment 1) and with line drawings of houses, faces, and chairs. The sample and matching choice stimuli in the line drawings condition were presented at the same angle of view.
In both experiments, tasks were presented in 21-s blocks with the same type of stimuli. All blocks with meaningful stimuli were separated by control blocks. The order of blocks with meaningful stimuli was counterbalanced across time series. Each time series contained blocks with only one task condition (matching photographs or passive photographs in Experiment 1, matching photographs or matching line drawings in Experiment 2). Each time series consisted of six blocks with meaningful stimuli, two for each category. In both experiments, six time series were obtained for each task condition, for a total of 12 time series for each subject in each experiment.
Accuracy and reaction times on the delayed match-to-sample tasks did not differ for houses, faces, and chairs (P > 0.2 in all cases). Reaction times for line drawings (mean = 814 ms across stimulus categories) were shorter (P < 0.001) than reaction times for photographs (mean = 1,111 and 1,042 ms for Experiments 1 and 2, respectively), presumably because the sample and choice stimuli were presented at different angles of view for photograph but not line drawing stimuli.
To test whether eye movements were equivalent across object categories, we recorded eye movements, using the ISCAN eye tracking system (Burlington, MA), while five subjects performed the passive viewing and delayed match-to-sample tasks outside the magnetic resonance scanner. The number and amplitude of saccadic eye movements while viewing different categories did not differ for either task (P > 0.05). During passive viewing, subjects made on average seven saccades during each 21-s block (eight for houses, six for faces, five for chairs) with an average amplitude of 1.2°. During delayed matching, subjects made on average 31 saccades during each block (35 for houses, 29 for faces, 30 for chairs) with an average amplitude of 3.5°.
Imaging.
Eighteen contiguous 5-mm-thick coronal slices were obtained in 12 time series of 91 scans each [repetition time (TR) = 3 sec]. A scan refers to a single-volume image of the brain. Gradient echo, echo planar imaging [echo time (TE) = 40 ms, field of vision (FOV) = 20 cm, 64 × 64 matrix, voxel size = 3.125 × 3.125 × 5 mm], and high-resolution spoiled gradient recalled echo structural images (28 5-mm-thick slices, TR = 13.9, TE = 5.3, FOV = 20 cm, 256 × 256 matrix) were collected by using a General Electric Signa 1.5 Tesla magnet.
Statistics.
The responses to the different object categories were analyzed by using multiple regression (15, 16) with regressors related to three orthogonal effects of interest. Three complementary models were used. The first model was based on the dual system hypothesis that the recognition of faces and the recognition of other objects are mediated by different mechanisms in the ventral object vision pathway. The three orthogonal contrasts were as follows: meaningful objects vs. control stimuli, faces vs. houses and chairs, and houses vs. chairs. The other two models tested different orthogonal contrasts (meaningful objects vs. control stimuli, houses vs. faces and chairs, and faces vs. chairs; meaningful objects vs. control stimuli, chairs vs. faces and houses, and faces vs. houses). All three models yield identical estimates for the sizes of activation for each category and identical results for the omnibus test of significant differences among the three categories, namely the combined effect of the second and third regressors.
Regions were identified that showed significantly different responses for houses, faces, and chairs, and the time series for these regions, averaged across voxels, were analyzed. Voxels were selected that showed a significant experimental effect (Z > 4) for the combined effect of the three regressors of interest in the analysis of all 12 time series, an overall increase in activity for meaningful stimuli (a positive regression weight for the contrast between meaningful and control stimuli), and a significant differential category effect (Z > 1.96, P < 0.025, for the combined effect of the second and third regressors) in the combined analysis of matching and passive time series (Experiment 1, 12 time series), and photographs (Experiment 2, six time series). Voxels were then segregated into clusters according to the category of objects that evoked the maximal response. Clusters of seven or more contiguous voxels were considered significant. A cluster of this size had a statistical significance of P < 0.05 in each subject. For each subject and each region, a mean time series, averaged across voxels in the region, was calculated. For each subject, the size of the response to each object category in each region was estimated by using multiple regression. These estimates of response magnitude were converted to percent changes above control task baseline and analyzed with four-way repeated measures ANOVAs (condition × hemisphere × region × object category) with planned comparisons for selected contrasts. Separate ANOVAs analyzed the effect of attention (matching vs. passive viewing tasks, Experiment 1), the replication of the matching with photographic stimuli condition (Experiments 1 and 2), and the effect of changing low-level visual features (photographs vs. line drawings, Experiment 2). These analyses tested contrasts that were not biased by the selection of voxels that responded maximally to one category. Voxel selection in Experiment 1 weighted the delayed matching and passive viewing tasks equally and, therefore, did not bias the analysis of the differential effects of task. Analysis of the replication of the matching-photographs condition involved only the responses to the categories that did not elicit maximal response in a region. Analysis of responses to line drawings was based on regions defined by differential responses to photographs.
RESULTS
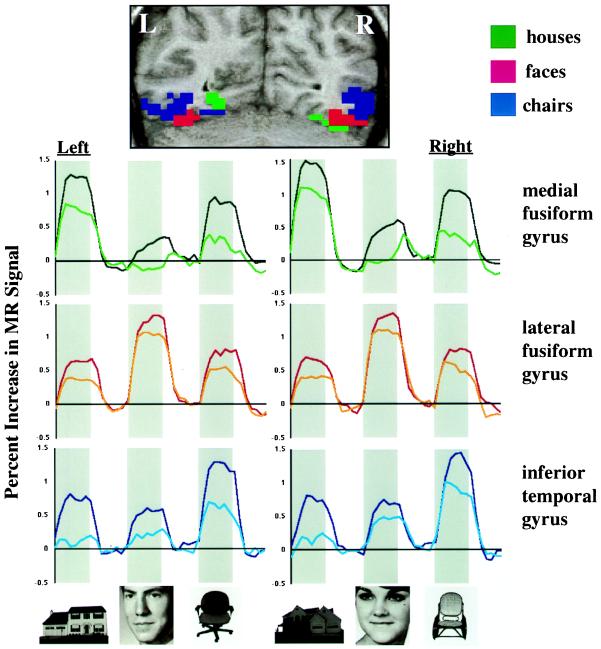
In our first experiment, cortical responses to photographs of houses, faces, and chairs were measured by using functional MRI during passive viewing and during a more attention-demanding delayed match-to-sample task. Three bilateral regions were identified in ventral temporal cortex that consistently showed significantly different responses for these three stimulus categories (Table 1 and Fig. 1). A region in the medial portion of the fusiform gyrus, including the collateral sulcus, responded most strongly to houses. An adjacent region in the lateral fusiform gyrus and occipitotemporal sulcus responded most strongly to faces. Lateral to this face-selective region, a region in the inferior temporal gyrus responded most strongly to chairs. A small sector of the medial fusiform gyrus also responded most strongly to chairs. The medial to lateral topological arrangement of these regions was consistent across all six subjects. Of the voxels in ventral temporal cortex that demonstrated category selectivity in Experiment 1, 46% responded maximally to houses, 36% responded maximally to chairs, and 18% responded maximally to faces (see Table 1).
Table 1.
Ventral temporal regions showing differential responses to houses (H), faces (F), and chairs (C)
| Region | Selectivity | Hemisphere | N | Volume, cm3 | Coordinates
|
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Experiment 1 | |||||||
| Medial fusiform | H > F and C | Left | 6 | 3.8 ± 2.0 | −26 | −57 | −14 |
| Right | 6 | 4.9 ± 2.9 | 28 | −57 | −13 | ||
| C > F and H | Left | 5 | 1.4 ± 0.8 | −27 | −51 | −19 | |
| Right | 4 | 1.1 ± 0.6 | 30 | −49 | −16 | ||
| Lateral fusiform | F > H and C | Left | 6 | 1.4 ± 0.6 | −36 | −55 | −20 |
| Right | 6 | 1.7 ± 0.5 | 40 | −52 | −19 | ||
| Inferior temporal | C > F and H | Left | 6 | 1.4 ± 0.8 | −41 | −64 | −12 |
| Right | 6 | 1.9 ± 0.7 | 48 | −62 | −11 | ||
| Experiment 2 | |||||||
| Medial fusiform | H > F and C | Left | 6 | 2.7 ± 1.5 | −25 | −58 | −16 |
| Right | 5 | 3.3 ± 1.1 | 28 | −63 | −18 | ||
| C > F and H | Left | 6 | 1.0 ± 0.6 | −29 | −56 | −22 | |
| Right | 3 | 0.9 ± 0.3 | 30 | −61 | −19 | ||
| Lateral fusiform | F > H and C | Left | 5 | 0.9 ± 0.5 | −39 | −64 | −28 |
| Right | 5 | 1.9 ± 0.4 | 41 | −60 | −20 | ||
| Inferior temporal | C > F and H | Left | 6 | 1.3 ± 1.0 | −42 | −69 | −14 |
| Right | 5 | 2.4 ± 0.7 | 48 | −73 | −8 | ||
Volumes were calculated before spatial normalization. Coordinates are in the normalized space of the Talairach and Tournoux brain atlas (17). N indicates number of subjects in whom each region was identified according to our criteria (seven or more contiguous voxels), and the mean (mean ± SD) for each region volume is calculated only for these subjects.
Figure 1.
Response topographies in ventral temporal cortex in Experiment 1. (Top) Locations of three posterior ventral temporal regions that responded differentially to houses, faces, and chairs, illustrated in a coronal section (y = −65) from a single subject. Voxels shown in color demonstrated a significant overall experimental effect (Z > 4.0) and a significant difference among responses to houses, faces, and chairs (Z > 1.96, clusters of seven or more voxels). Regions showing maximal responses to houses, faces, and chairs are shown in green, red, and blue, respectively. (Bottom) Mean time series for these three ventral temporal regions. Data are averaged across six subjects and 12 repetitions of task blocks in each subject. Gray bars indicate presentation of meaningful stimuli. The white space to the right of each gray bar indicates the presentation of control stimuli. The darker colored line in each graph is for the delayed match-to-sample task, and the lighter line is for the passive viewing task.
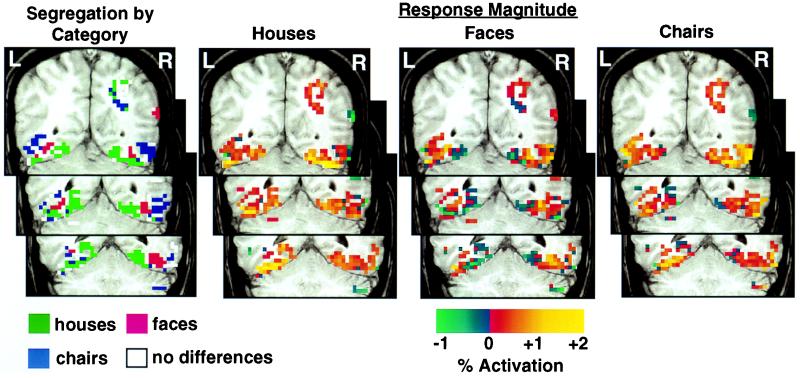
These results could be interpreted to support the existence of three modules in ventral temporal cortex, one dedicated to the perception of buildings or landmarks, one dedicated to the perception of faces, and one dedicated to the perception of chairs or some larger category that includes chairs. The data, however, can be transposed to examine the pattern of response to one stimulus category across these three regions, rather than the differential responses to categories within each region (Fig. 2). Viewed in this way, it is clear that the response to each category was not restricted to the region that responds maximally to that category but rather extended to the regions that responded maximally to other categories. The distributed nature of the response to different categories is illustrated for a single subject in Fig. 3. Note that the responses to houses and chairs extended across all three category-selective regions and that the response to faces extended into the chair-selective region and the medial portion of the house-selective region.
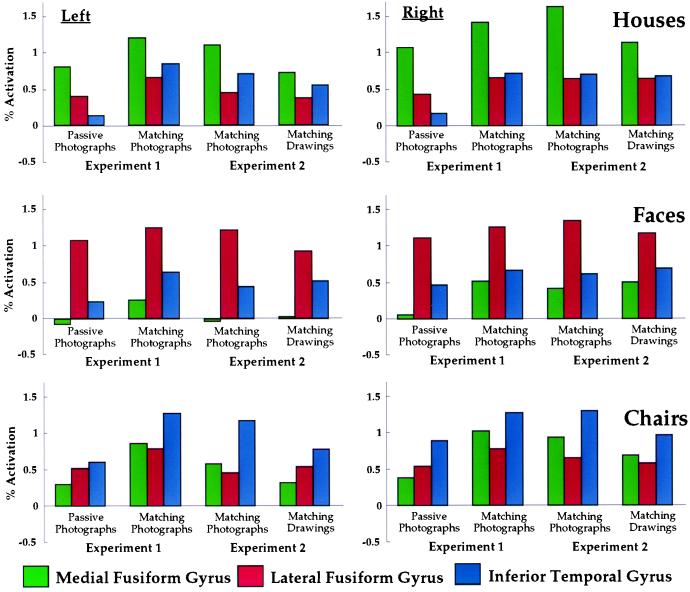
Figure 2.
Patterns of response to houses, faces, and chairs across three regions in posterior ventral temporal cortex. Results from Experiments 1 and 2 are shown in the same graphs to allow direct comparison of the patterns of response.
Figure 3.
Patterns of response to houses, faces, and chairs in three coronal sections through ventral temporal cortex in one subject. Regions showing maximal responses to houses, faces, and chairs, are shown in green, red, and blue, respectively. White voxels indicate significant activation across stimulus categories but no significant differences between categories. Strength of response to houses, faces, and chairs relative to control tasks with scrambled pictures is shown in all ventral temporal voxels that showed a significant experimental effect (Z > 4.0). (Top) y = −60; (Middle) y = −55; (Bottom) y = −50.
It is of interest to note that the response to faces was more restricted to the lateral fusiform and inferior temporal regions, whereas the responses to houses and chairs were more widely distributed across all three regions. There was no response to faces in the house-selective medial fusiform region during passive viewing. During matching, the response to faces in the medial fusiform region was significant but still much smaller than the response to chairs in the same region (P < 0.001 in both hemispheres). Moreover, the response to faces in the medial fusiform region during matching was smaller than the responses to houses and chairs in the face-selective lateral fusiform region (P < 0.001 for both houses and chairs on the left and for chairs on the right. For houses on the right, the difference was in the same direction but was a nonsignificant trend, P = 0.09).
Relative to passive viewing, the delayed matching task required more attention, and this difference in attention demand was reflected by increased responses. These increases, however, were not uniform. For example, the effect of attention on the response to houses was greater in the region maximally responsive to chairs (inferior temporal gyrus) than in the region maximally responsive to faces (lateral fusiform gyrus) (P < 0.001 on both the right and left). Similarly, the effect of attention on the response to chairs was greater in the region maximally responsive to houses (medial fusiform gyrus) than in the region maximally responsive to faces (lateral fusiform gyrus) (P < 0.001 in both hemispheres).
In our second experiment, we examined whether the differential patterns of response to houses, faces, and chairs might be caused simply by low-level visual features, such as spatial frequency and texture, by contrasting the responses evoked by photographs to the responses evoked by line drawings of the same objects during delayed matching. Regions were defined based on differential responses evoked by photographs.
The results from the photographs condition replicated those from the identical delayed matching condition in Experiment 1. The locations and volumes of these regions were equivalent (Table 1), with the same medial to lateral topological arrangement across all subjects. Moreover, the patterns of differential response across all three ventral temporal regions were equivalent for all categories and in both hemispheres (Fig. 2). The differences between responses in regions that responded maximally to other categories were significant on the left for houses (inferior temporal > lateral fusiform, P < 0.001) and bilaterally for faces (inferior temporal > medial fusiform, P < 0.001 on both sides) and chairs (medial fusiform > lateral fusiform, P < 0.001). These patterns of differential response were equivalent for Experiments 1 and 2 (replication × category × region interaction, in “secondary” regions only, F < 1).
We then measured the differential responses to line drawings within these regions. As shown in Fig. 2, the category-related patterns of response across ventral temporal regions were remarkably similar for photographs and line drawings. In all cases, the region that responded most strongly to photographs of a category of objects also responded most strongly to line drawings of the same category (P < 0.001 in all cases). The differences between responses to each category in the regions that responded maximally to other categories were also consistent for photographs and line drawings (the single exception being the left medial and lateral fusiform regions for chairs). This replication with line drawings of category-related patterns of response across ventral temporal cortex suggests that these patterns cannot be attributed to different spatial frequencies or textures in pictures of houses, faces, and chairs.
DISCUSSION
Three adjacent regions were identified in ventral temporal cortex that responded differentially to houses, faces, and chairs. The most medial region, in the medial fusiform gyrus, responded maximally to houses. This region corresponds to the proposed site for a module specialized for the perception of buildings and other objects that can serve as landmarks (8, 9). The intermediate region, in the lateral fusiform gyrus, responded maximally to faces. This region corresponds to the proposed site for a face-specific module (5, 6). The lateral region, in the inferior temporal gyrus, responded maximally to chairs. Interestingly, the ventral temporal areas of the human brain that respond to meaningful visual stimuli in our study and in many others (1–9) are confined to the posterior temporal lobe, whereas inferior temporal extrastriate cortex in the monkey brain that responds to complex visual stimuli extends to the temporal pole (18–21).
Although our data could be interpreted as evidence for separate modules, we found that the responses to a category in “secondary” regions, namely those that respond maximally to another category, were significant. Moreover, for each category the responses in its two secondary regions demonstrated consistent replicable differences. Our data thus indicate that the representations of objects are distributed across ventral temporal cortex and not restricted to category-specific anatomically segregated modules. For example, the representation of houses is not restricted to a “house module” in the medial fusiform gyrus (6.6 cm3) but is distributed across all ventral temporal regions that responded to meaningful objects (16 cm3).
Behavioral performance during viewing of houses, faces, and chairs was matched for accuracy and response time during delayed matching and for eye movements during passive viewing and delayed matching. These results indicate that the differential patterns of response that we observed in ventral temporal cortex cannot be attributed to task difficulty or oculomotor control. The responses in secondary regions also cannot be attributed to spatial blurring of the hemodynamic signal. The strength of response in secondary regions did not drop off monotonically with distance from the primary region, both in terms of continuous topography of response (see Fig. 3) and in terms of the relative strengths of responses in secondary regions that were adjacent to and distant from the primary region. For example, chairs evoked greater activity in medial fusiform gyrus than in lateral fusiform gyrus (see Fig. 2).
When we compared the response during passive viewing and delayed matching tasks, we found that the attentional modulations in secondary regions varied significantly by category. These findings suggest that secondary regions differ in the extent to which they can contribute usefully to the perception of objects in nonpreferred categories. For example, the region maximally responsive to houses is preferentially recruited to augment the perception of chairs and, conversely, the region maximally responsive to chairs is preferentially recruited to augment the perception of houses. Thus, the responses in secondary regions appear to carry information about the identity of objects. Presumably, these regions are recruited to enhance perception so that representations are sufficiently distinct to discriminate between highly similar stimuli in an attention-demanding delayed matching task.
The distributed nature of the response suggests that this expanse of cortex contains a continuous representation of information about object form (18). This representation has a consistent topological organization that reflects distinctions between categories. Such an arrangement suggests that information most characteristic of objects within a single category clusters together, resulting in a region that responds maximally to that category and giving the appearance of a module. The nature of this information is unknown, but it is not related to low-level features such as spatial frequency and texture. The information may consist of features similar to the object shape primitives described in Tanaka’s studies of single-cell responses to complex objects in monkey inferior temporal cortex (11, 18).
A continuous representation of attributes of object form could produce an unlimited variety of patterns of response for different categories (12, 13). A category-specific modular organization, on the other hand, could never provide a comprehensive account for the perception of all categories. There are simply too many categories and too little cortex. One might posit that ventral temporal cortex consists of only a limited number of modules that evolved based on the biological significance of certain classes of stimuli, such as faces, animals, tools, and possibly landmarks. We chose chairs as an additional category because of their dubious biological significance. A model based on a topologically organized continuous representation of attributes of object form would predict distinctive patterns of response to different categories of objects regardless of their biological significance (12, 13). Furthermore, it is unclear how distinctions between subordinate categories would be represented by a superordinate category module. A modular model cannot provide an account for how all categories can be distinguished without reference to an additional model of how the representation of information is distributed within a module.
Nonetheless, within this distributed representation for object form, some classes of stimuli may have a special status, the best candidate being faces (22, 23). Developmental evidence for an innate capacity for face perception (22) and physiological evidence for single cells that respond to whole faces or parts of faces and not to other objects tested (19–21) strongly suggest that there may be specialized neural mechanisms dedicated to face perception. Although the face-selective lateral fusiform region does not respond exclusively to faces, it may contain columns of face-responsive cells interdigitated with columns that respond to attributes of other objects (18). Indeed, recordings that use electrodes placed directly on the cortical surface indicate that sites do exist in the human ventral temporal cortex that appear to respond to faces and not to other objects tested (1). Our data also provide other evidence that face perception is different from object perception. The representation of faces was not as widely distributed as were the representations of houses and chairs. Moreover, attention had a greater effect on the responses to houses and chairs than on the response to faces, suggesting that face perception is more automatic.
The topological organization for information about object form in posterior ventral temporal cortex may be analogous to the well known topologies in sensory cortices. Sensory topologies are based on the arrangement of receptors in the sensory organs, such as the retina (retinotopy), the cochlear membrane (tonotopy), and the body (somatotopy). Object form topology, by contrast, reflects a consistent and orderly arrangement of information along dimensions that have no obvious point-to-point correspondence with sensory input. Instead, object form topology reflects a transformation of sensory information into attributes or primitives that are the basis of object form perception and that embody distinctions between object categories.
Acknowledgments
We thank R. Desimone for comments on an earlier draft of the manuscript; E. Hoffman for scheduling our subjects; J. Maisog, T. Ellmore, and J. Van Horn for assistance with computer programming and data analysis; and John Ingeholm for assistance with recording of eye movements.
References
- 1.Allison T, McCarthy G, Nobre A, Puce A, Belger A. Cereb Cortex. 1994;5:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- 2.Puce A, Allison T, Gore J C, McCarthy G. J Neurophysiol. 1995;74:1192–1199. doi: 10.1152/jn.1995.74.3.1192. [DOI] [PubMed] [Google Scholar]
- 3.Puce A, Allison T, Asgari M, Gore J C, McCarthy G. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark V P, Keil K, Maisog J M, Courtney S, Ungerleider L G, Haxby J V. Neuroimage. 1996;4:1–15. doi: 10.1006/nimg.1996.0025. [DOI] [PubMed] [Google Scholar]
- 5.Kanwisher N, McDermott J, Chun M M. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy G, Puce A, Gore J C, Allison T. J Cognit Neurosci. 1997;9:605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- 7.Haxby J V, Ungerleider L G, Clark V P, Schouten J L, Hoffman E A, Martin A. Neuron. 1999;22:189–199. doi: 10.1016/s0896-6273(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 8.Aguirre G K, Zarahn E, D’Esposito M. Neuron. 1998;21:1–20. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- 9.Epstein R, Kanwisher N. Nature (London) 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 10.Polk T A, Farah M J. Proc Natl Acad Sci USA. 1998;95:847–852. doi: 10.1073/pnas.95.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka K. Science. 1993;262:685–688. doi: 10.1126/science.8235589. [DOI] [PubMed] [Google Scholar]
- 12.McClelland J L, Rumelhart D E. J Exp Psychol Gen. 1985;114:159–197. doi: 10.1037//0096-3445.114.2.159. [DOI] [PubMed] [Google Scholar]
- 13.Edelman S, Grill-Spector K, Kushnir T, Malach R. Psychobiology. 1998;26:309–321. [Google Scholar]
- 14.Wallis G, Bulthoff H. Trends Cognit Neurosci. 1999;3:22–31. doi: 10.1016/s1364-6613(98)01261-3. [DOI] [PubMed] [Google Scholar]
- 15.Friston K J, Holmes A P, Poline J B, Grasby P J, Williams S C R, Frackowiak R S J, Turner R. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- 16.Haxby J V, Maisog J M, Courtney S M. In: Mapping and Modeling the Human Brain. Lancaster J, Fox P, Friston K J, editors. New York: Wiley; 1999. [Google Scholar]
- 17.Talairach J, Tournoux P. Co-Planar Stereotaxis Atlas of the Human Brain. New York: Thieme Medical; 1988. [Google Scholar]
- 18.Tanaka K. Annu Rev Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- 19.Desimone R J. Cognit Neurosci. 1991;3:1–8. doi: 10.1162/jocn.1991.3.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Rolls E T. Philos Trans R Soc London. 1992;335:11–21. doi: 10.1098/rstb.1992.0002. [DOI] [PubMed] [Google Scholar]
- 21.Perrett D J, Rolls E T, Caan W. Exp Brain Res. 1982;47:329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- 22.Farah M. Behav Brain Res. 1996;76:181–189. doi: 10.1016/0166-4328(95)00198-0. [DOI] [PubMed] [Google Scholar]
- 23.Tovee M J. Neuron. 1998;21:1239–1242. doi: 10.1016/s0896-6273(00)80644-3. [DOI] [PubMed] [Google Scholar]