Abstract
Leptin is produced primarily by adipocytes and functions in a feedback loop regulating body weight. Leptin deficiency results in severe obesity and a variety of endocrine abnormalities in animals and humans. Several studies indicated that leptin plays an important role in immune responses. It exerts protective anti-inflammatory effects in models of acute inflammation and during activation of innate immune responses. In contrast, leptin stimulates T lymphocyte responses, thus having rather a proinflammatory role in experimental models of autoimmune diseases. Clinical studies have so far yielded inconsistent results, suggesting a rather complex role for leptin in immune-mediated inflammatory conditions in humans.
Introduction
Leptin is a 16 kDa peptide hormone with the tertiary structure of a cytokine that is highly conserved among mammalian species [1]. It is structurally and functionally related to the IL-6 cytokine family. Leptin functions as a signal in a feedback loop regulating food intake and body weight [2]. The leptin receptor Ob-R (or Lepr), is a member of the class I cytokine receptor family, which includes gp-130, the common signal transducing receptor for the IL-6 related family of cytokines [3]. Alternative splicing of the leptin receptor gene produces at least six transcripts designated Ob-Ra through Ob-Rf (Figure 1) [4]. Two of the isoforms have been described in only one species each, Ob-Rd in mice and Ob-Rf in rats [5]. In humans, only expression of Ob-Ra, Ob-Rb and Ob-Rc mRNA has been reported [5]. Ob-Re is a secreted isoform of the receptor, lacking transmembrane and cytoplasmic domains. In humans, transcripts corresponding to Ob-Re have not been described, but soluble leptin receptor protein can be generated by proteolytic cleavage of the Ob-Rb and Ob-Ra isoforms [6].
Figure 1.

Structure and isoforms of mouse leptin receptor. Ob-Rb contains the longest intracellular domain, which is crucial for leptin signaling. Ob-Ra, Ob-Rc and Ob-Rd contain only short cytoplasmic domains. Ob-Re is a secreted isoform of the leptin receptor, lacking transmembrane and cytoplasmic parts. Cytokine receptor homology module (CRH)2 is the main binding site for leptin on the Ob-R. The Ig-like and the FN-III domains are critically involved in Ob-R activation. The role of CRH1 remains to be determined [111, 112]. FNIII, fibronectin type III domain; Ig-like, immunoglobulin-like fold.
Ob-Rb is abundantly expressed in the hypothalamus, an area in the brain involved in the control of food intake. The anorexigenic effect of leptin is dependent on binding to the long form of its receptor, Ob-Rb [7]. Both leptin-deficient (ob/ob) and leptin receptor (Ob-Rb)-deficient (db/db) mice display a severe hereditary obesity phenotype, characterized by increased food intake and body weight, associated with decreased energy expenditure [8]. Administration of leptin reverses the obese phenotype in ob/ob mice, but not in db/dbmice, and decreases food intake in normal mice. Lack of response to leptin is also well described in obese Zucker rats, which bear a mutation (fa) in the leptin receptor gene [9]. Mutations in leptin and Ob-R genes associated with obesity have also been described in humans [10,11]. Leptin is produced predominantly by adipocytes, although low levels have been detected in the hypothalamus, pituitary [12], stomach [13], skeletal muscle [14], mammary epithelia [15], chondrocytes [16] and a variety of other tissues [17]. Plasma leptin concentrations correlate with the amount of fat tissue and, thus, obese individuals produce higher levels of leptin than do lean ones [18]. The correlation between serum leptin concentrations and the percentage of body fat suggests that most obese people are insensitive to endogenously produced leptin [18].
In addition to the regulation of appetite and energy expenditure, leptin exhibits a variety of other effects [19-22]. Consistently, ob/ob and db/db mice are not only severely obese, but display also several hormonal imbalances, abnormalities in thermoregulation, increased bone mass, infertility, and evidence of immune and hematopoietic defects [17,19,20,22-25]. In humans, congenital leptin deficiency is associated with hypogonadotropic hypogonadism, morbid obesity and frequent deaths due to infections [11,26].
The role of leptin in immunity and inflammation
In addition to the central role of lipid storage, adipose tissue has major endocrine functions and releases a variety of pro-inflammatory and anti-inflammatory factors, including adipo-cytokines, such as leptin, adiponectin and resistin, as well as cytokines and chemokines. Altered levels of different adipo-cytokines have been observed in a variety of inflammatory conditions (reviewed in [27]) and, in particular, the role of leptin in immune responses and inflammation has lately become increasingly evident. Altered leptin production during infection and inflammation strongly suggests that leptin is a part of the cytokine cascade, which orchestrates the innate immune response and host defense mechanisms [28,29]. Like other members of the IL-6 family, leptin was shown to activate the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway (Figure 2) [3]. Leptin also induces the expression of the suppressor-of-cytokine signaling (SOCS)-3, which inhibits STAT signaling [30]. In addition, stimulation of leptin receptor triggers activation of phos-phatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) [31]. Activation of these pathways is also characteristic for the signaling of other cytokines belonging to the IL-6 family [32]. Physiological levels of leptin can modulate the response to an inflammatory challenge by altering production of proinflammatory and anti-inflammatory cytokines and may also affect cytokine signaling by a variety of mechanisms, including induction of SOCS-3 [33].
Figure 2.
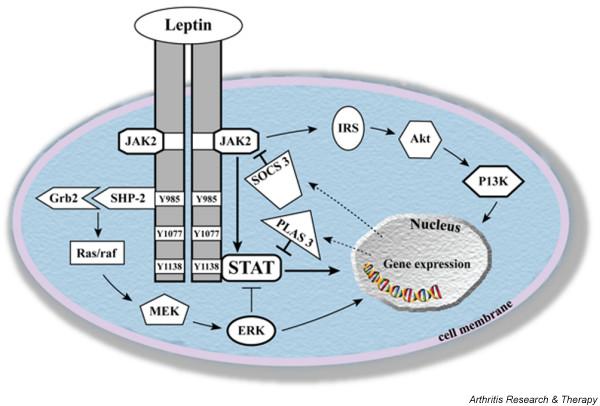
Mechanisms of leptin signaling. Upon leptin binding to Ob-Rb, the Janus kinase/signal transducer and activator of transcription (JAK/STAT), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K) pathways are activated. Akt, protein kinase B; Grb-2, growth receptor-bound-2; IRS, insulin receptor substrate; MEK, mitogen-activated protein kinase kinase; PIAS 3, protein inhibitor of activated STAT3; Raf, MEK-kinase; Ras, G-protein; SHP-2, SH2-domain containing protein tyrosine phosphatase; SOCS3, suppressor of cytokine signalling-3.
In vitro studies revealed that Ob-Rb is expressed in T and B cells, macrophages and hematopoietic cells and direct effects of leptin on those cells have been demonstrated [34-41]. Moreover, activated T cells themselves have been shown to express and secrete leptin, which sustained their proliferation in an autocrine loop [42]. However, a recent study indicated that T cell-derived leptin does not play a major role in the regulation of the inflammatory process in experimental models of hepatitis and colitis in mice, emphasizing the critical role of adipose tissue-derived leptin in immune modulation [43].
Regulation of leptin production during inflammatory conditions
Some studies report increased levels of leptin during infectious and inflammatory processes. Leptin expression in adipose tissue and circulating leptin levels are increased after administration of inflammatory stimuli such as lipopolysaccha-ride (LPS) or turpentine to hamsters [44,45]. Endotoxin has also been shown to stimulate the release of leptin into peripheral blood in human and nonhuman primates [46]. LPS, as well as proinflammatory mediators such as tumor necrosis factor (TNF)-α and IL-1, increase the expression of leptin mRNA in adipose tissue [45] and a statistically significant elevation of plasma leptin concentrations has been demonstrated in adult septic patients compared with healthy subjects [47-50]. However, other studies have not found increased leptin levels in inflammatory conditions, including acute experimental endotoxemia in humans, HIV infection and newborn sepsis [51-53]. Moreover, in tuberculosis patients, plasma leptin concentrations were significantly reduced [54]. Similarly, decreased circulating levels of leptin were observed in mice following intravenous injection of Staphylococcus aureus [55]. Increased leptin production is thus observed in inflammatory conditions in many, although not all, animal models and diseases examined.
Effects of leptin on innate immune responses
The increased sensitivity of leptin-deficient rodents to pro-inflammatory, monocyte/macrophage-activating stimuli, suggests a role for leptin in the regulation of inflammatory responses (Table 1) [56]. Ob/ob, as well as fasted wild-type mice, which display decreased leptin levels, are significantly more susceptible to LPS-induced lethality, and this phenotype was partly reversed by the administration of leptin [57,58]. Similarly, ob/ob, db/db and fasted wild-type mice are more likely to succumb after the administration of TNF-α. This phenotype was again reversed by leptin treatment in ob/ob and wild-type, but not in db/db, mice [58,59]. The protective role of leptin against TNF-α-induced toxicity was further supported by the deleterious effect of neutralizing anti-leptin antibodies administered to TNF-α-injected mice [59]. Ob-R-deficient fa/fa rats also displayed enhanced sensitivity to LPS-induced hepatotoxicity [60]. Dysregulation in cytokine induction after LPS stimulation may contribute to the increased susceptibility to LPS toxicity, as demonstrated in a number of experimental studies in transgenic and gene knockout animals. Lower levels of anti-inflammatory cytokines, such as IL-10, and IL-1Ra, and higher levels of the proinflammatory cytokines IL-12, IL-18 and interferon (IFN)-γ have been detected after LPS injection in ob/ob mice [57,60,61]. Consistently, protective effects of leptin demonstrated in a model of experimental pancreatitis were attributed to increased IL-4 production and to reduced serum TNF-α or IL-1β [62,63]. Anti-inflammatory effects of leptin were further demonstrated by reduced TNF-α and IL-6 responses in endotoxin treated primates [33]. Taken together, these different observations are mostly consistent with the notion that leptin deficiency constitutes a proinflammatory state.
Table 1.
Effects of leptin or leptin receptor deficiency and leptin administration in experimental models of innate immune response in rodents
| Model | WT mice/rats | ob/ob mice | Ob-R-deficient mice/rats | Leptin administration | References |
| LPS-induced lethality | Fasted mice: | ↑ Susceptibility | Fasted WT mice: effect reversed | [57] | |
| ↑ Susceptibility | ↓ IL-10 | ||||
| ↑ TNF-α | ↓ IL-1Ra | ob/ob mice: effect partly reversed | |||
| ↓ Interferon-γ | |||||
| LPS ip | ↓ TNF-α | fa/fa rats: | [64] | ||
| ↓ IL-6 | ↓ TNF-α | ||||
| ↓ IL-6 | |||||
| LPS-induced hepatotoxicity | ↑ Sensitivity | fa/fa rats: | [60] | ||
| ↓ Hepatic CD4+NK | ↑ Sensitivity | ||||
| T cells | ↑ IFN-γ mRNA | ||||
| ↑ Serum IL-18 | ↓ IL-12 mRNA | ||||
| ↑ Hepatic IL-18 and IL-12 | |||||
| ↓ Hepatic IL-10 | |||||
| ↑ IFN-γ | |||||
| TNF-α-induced lethality | Fasted mice: | ↑ Susceptibility | ↑ Susceptibility | Fasted WT mice: effect not reversed | [58] |
| ↑ Susceptibility | |||||
| Leptin antagonist: | Leptin antagonist: effect partly reversed | ||||
| ↑ Susceptibility | |||||
| ob/ob mice: effect reversed | |||||
| Pancreatitis | WT rats: protective effects | [62] | |||
| ↑ IL-4 | |||||
| ↓ TNF-α and IL-1β | |||||
| Escherichia coli iv infusion | ↓ Clearance | [64] | |||
| Smaller fraction of E. coli killed | |||||
| Klebsiella pneumoniae intratracheal challenge | ↑ Leptin after infection | ↑ Mortality | [65] | ||
| ↑ Bacterial counts in lungs and blood | |||||
| Candida albicans iv infusion | fa/fa rats: | [66] | |||
| ↑ Yeast/g organ | |||||
| Staphylococcus aureus-induced arthritis | ↓ Leptin production | WT mice: | [55] | ||
| ↓ Severity | |||||
| ↓ IL-6 | |||||
| Zymosan-induced arthritis | ↑ Joint inflammation | ↑ Joint inflammation | [90] | ||
| ↑ SAA and IL-6 | ↑ SAA and IL-6 |
Up and down arrows indicate increase and decrease, respectively. ip, intraperitoneal; iv, intravenous; LPS, lipopolysaccharide; ob/ob, leptin deficient mice; Ob-R, leptin receptor; SAA, serum amyloid A; TNF, tumor necrosis factor; WT, wild-type.
Effects of leptin on phagocytes
The role of leptin in the regulation of important macrophage functions is further emphasized by alterations in the phenotype of those cells during chronic leptin deficiency. Impaired phagocytic functions resulting in reduced bacterial elimination have been described for macrophages from leptin-deficient mice during infections with Escherichia coli, Candida albicans and Klebsiella pneumoniae (Table 1) [64-66]. In addition to modulating phagocytosis and cytokine production by macrophages, leptin has recently been shown to regulate other aspects of the innate immune response. Leptin was indeed reported to enhance oxidative species production by stimulated polymorphonuclear leukocytes (PMNs) [36], whereas another study provides evidence that leptin inhibits neutrophil migration in response to classical chemoattractants [67]. These findings, as well as an increased rate of death due to infections among leptin-deficient individuals [26], suggest that leptin contributes to host defense against microorganisms. Several recent studies demonstrated that PMNs express the short (Ob-Ra), but not the long isoform Ob-Rb. Whether Ob-Ra can deliver intracellular signals or not remains a matter of debate [67-69]. For instance, the effect of leptin on CD11b expression in neutrophils is likely to be indirect and mediated by the induction of TNF-α production by monocytes [69]. In contrast, it was reported that leptin directly activates neutro-phils and delays spontaneous apoptosis of these cells by inhibiting proapoptotic events proximal to mitochondria, the effect being mediated via PI3K and p38 MAPK signaling pathways [68]. In general, leptin thus appears to increase the activity of phagocytes and may thereby contribute to efficient host defense.
Effects of leptin on adaptive immune responses
Leptin was reported to stimulate the proliferation of T cells in vitro, to promote T helper (Th)1 responses and to protect T cells from corticosteroid-induced apoptosis [38,39]. Ob/ob mice display a higher level of thymocyte apoptosis and reduced thymic cellularity compared to control mice and these effects were reversed by peripheral administration of recombinant leptin [38]. In the same study, wild-type mice treated with leptin during a 48 hour fast were completely protected against the profound thymic atrophy observed in non-treated fasted mice [38]. Ob/ob mice also exhibit defective cellular and humoral immune responses and are protected from immune-mediated inflammation in various models, such as experimental colitis, T-cell mediated hepatitis, glomeru-lonephritis and experimental autoimmune encephalomyelitis (EAE), an experimental model for multiple sclerosis (Table 2) [19,28,70-74]. Leptin replacement in ob/ob mice converted resistance to EAE into susceptibility and this effect was accompanied by a switch from a Th2 to a Th1 pattern of cytokine release and consequent reversal of Ig subclass production [72]. Likewise, administration of leptin to EAE susceptible mice after disease onset increased the severity of the symptoms and leptin administration accelerated type 1 diabetes development in NOD mice [73,75]. Conversely, blockade of leptin with anti-leptin antibodies or with a soluble mouse leptin receptor chimera, either before or after onset of EAE, ameliorated the clinical symptoms, inhibited antigen-specific T cell proliferation, and switched cytokine secretion toward a Th2 and T regulatory profile [76].
Table 2.
Effects of leptin or leptin receptor deficiency and leptin administration in disease models mediated by adaptive immune responses in mice
| Models | WT mice | ob/ob mice | db/db mice | Leptin injection | References |
| Non-obese diabetic mice | ↑ Serum leptin before onset of diabetes | ↑ Destruction of insulin-producing β-cells | [75] | ||
| ↑ IFN-γ production by T lymphocytes | |||||
| AIA | ↓ Arthritis severity | ↓ Arthritis severity | [35] | ||
| ↓ Anti-mBSA Abs | ↓ Anti-mBSA Abs | ||||
| ↓ Ex vivo T-cell proliferation | ↓ Ex vivo T-cell proliferation | ||||
| ↓ IFN-γ and | ↓ IFN-γ and | ||||
| ↑ IL-10 production | ↑ IL-10 production | ||||
| EAE | ↑ Serum leptin before onset of EAE Serum leptin correlated with EAE susceptibility | ↓ Susceptibility | ↑ Severity in SJL females SJL males: become susceptible Restored susceptibility in ob/ob mice associated to Th2 to Th1 switch | [42] | |
| Administration of anti-leptin Abs or soluble leptin receptors: | |||||
| ↓ Disease severity | |||||
| T-cell mediated hepatitis | Protected from liver damage | ob/ob mice: restored susceptibility | [70, 110] | ||
| ↓ TNF-α and IL-18 | |||||
| Colitis | ↓ Severity | [71] | |||
| ↓ Local release of proinflammatory cytokines | |||||
| Immune-mediated glomerulonephritis | Protected | [74] |
Up and down arrows indicate increase and decrease, respectively. Abs, antibodies; AIA, antigen-induced arthritis; db/db, leptin receptor deficient mice; EAE, autoimmune encephalomyelitis; ob/ob, leptin deficient mice; Th, T helper; TNF, tumor necrosis factor; WT, wild-type.
Starvation and malnutrition are associated with reduced leptin levels and alterations of the immune response, which can be reversed by leptin administration [39,40,77]. Acute starvation, which is able to prevent increases in serum leptin, delayed EAE onset and attenuated clinical symptoms [42]. Furthermore, in humans, leptin deficiency was associated with reduced numbers of circulating CD4+ T cells and impaired T cell proliferation and cytokine release, all of which were reversed by recombinant human leptin administration [78]. In vitro, leptin dose-dependently enhances proliferation and activation of human circulating T lymphocytes when they are costimulated by phytohemagglutinin or concanavalin A and modulates CD4(+) T lymphocyte activation toward a Th1 phenotype by stimulating the synthesis of IL-2 and IFN-γ [79]. Finally, human dendritic cells express leptin receptors and leptin down-regulates their IL-10 production and drives naive T cell polarization towards a Th1 phenotype [80]. In view of these different observations, leptin thus seems to display a stimulatory effect on adaptive immune responses and to favor Th1 polarization.
Taken together, the experimental data collected suggest that chronic leptin deficiency differently affects adaptive versus innate immune responses: adaptive immune-mediated responses are attenuated whereas, in experimental models involving the innate immune response, leptin deficiency causes inadequate control of the inflammatory response. As already mentioned, leptin and its receptor share some homologies with the IL-6 and IL-6 receptor families, respectively [3]. Interestingly, many similarities can be observed also in the pattern of leptin and IL-6 effects during adaptive or innate immune response-mediated inflammation. IL-6 exerts deleterious actions in many models of chronic immune mediated inflammation, whereas it has been shown to possess protective effects in some models of innate immune response-mediated inflammation [81].
Direct and indirect effects of leptin during immune response and inflammation
As mentioned above, leptin exerts various direct effects on cells involved in the immune and inflammatory responses. However, the connection between leptin, immune responses and inflammation in vivo is complex. Indeed, leptin/leptin receptor deficiency causes multiple neuroendocrine and metabolic modifications in ob/ob or db/db mice, including the activation of the hypothalamic-pituitary-adrenal axis and hypercorticosteronemia, hyperglycemia and diabetes, which may also indirectly affect the immune system. Similarly, leptin deficiency after starvation in rodents is linked to increased glucocorticoid levels, and decreased levels of thyroid and growth hormone, each of which may mediate immune suppression [77,82-84]. Numerous neuroendocrine defects have been also reported in human leptin-deficient patients. These include decreased symphathetic tone, elevated thyroid stimulating hormone, parathyroid hormone, cortisol and adrenocorticotropic hormone (ACTH) levels, abnormal growth hormone stimulation, thyroid function, and others [26], which could indirectly contribute to the development of immune system dysfunction in those patients. All these data underscore the potential importance of both direct and indirect effects of leptin or leptin deficiency during immune response and inflammation. In addition, leptin deficiency results in morbid obesity and multiple immunomodulatory functions have been recently described for adipose tissue [85-87]. In fact, obesity itself may represent a low grade systemic inflammatory state and could thus favor different immune and inflammatory responses.
To investigate the relative contributions of direct and indirect effects of leptin on the immune system in a normal environment, we recently generated bone marrow chimeras by transplantation of leptin receptor-deficient db/db bone marrow cells into wild-type recipients (GP and CG, manuscript submitted). The size and cellularity of the thymus, as well as cellular and humoral immune responses were normal when db/db bone marrow was grafted into wild-type mice. Direct effects of leptin on lymphocytes are thus not necessary for T cell maturation and immune response in a normal environment. Conversely, thymus weight and cell number were decreased in the reverse graft setting when wild-type bone marrow was transferred into db/db mice, indicating that expression of the leptin receptor in the systemic and/or local environment is mandatory for T cell development. Based on these observations, it appears that in mice major effects of leptin receptor-deficiency on the immune system are indirect.
Interestingly, in contrast to leptin or leptin receptor-deficient rodents, in human patients, gradual compensations of several endocrine functions that were initially impaired due to a mutated leptin molecule were observed, possibly due to the longevity of humans [26]. The authors suggest that, over a time span of several decades, other factors seem to bring back to normal functions that were initially dysregulated in the absence of leptin, such as thyroid axis activity, reproduction, and possibly immunity. These observations further emphasize the complexity of the neuroendocrine regulatory and compensatory mechanisms in leptin-deficiency.
The role of leptin in experimental models of arthritis
A potential role of leptin has been recently investigated in several models of arthritis depending on acquired or innate immune responses. Antigen-induced arthritis (AIA) is an experimental model of rheumatoid arthritis (RA), which is based on the induction of a local Arthus reaction by intra-articular injection of methylated bovine serum albumin (mBSA) into the knee joint of mBSA-immunized mice. Ob/ob and db/db mice had a milder form of AIA than their lean littermates [35]. In addition, ex vivo proliferation and IFN-γ production following the stimulation of lymph node cells by mBSA were significantly reduced in ob/ob and db/db mice. In contrast, IL-10 production by lymph node cells from ob/ob and db/db mice was increased [35]. The levels of anti-mBSA antibodies were also decreased in immunized ob/ob and db/db mice compared to their controls. These results indicate that leptin contributes to joint inflammation in AIA by regulating both humoral and cell-mediated immune responses.
To investigate a potential effect of leptin on inflammatory events in the joint, we explored the role of leptin in zymosan-induced arthritis, a mouse model of arthritis that is not dependent on the adaptive immune response. This model relies on intra-articular injection of zymosan A, which is a ligand for toll-like receptor 2, as well as an activator of the alternative complement pathway, and which triggers a local activation of the innate immune system, causing inflammation of the injected joint [88,89]. We observed that both ob/ob and db/db mice exhibited a delayed resolution of the inflammatory process and an increased acute-phase response during zymosan-induced arthritis compared to their lean littermates [90]. It is noteworthy that this increased inflammatory response was observed in ob/ob and db/db mice, despite the presence of elevated glucocorticoid levels. This observation is in agreement with data obtained in another study, where treatment of wild-type mice with leptin caused a significant decrease in the severity of septic arthritis induced by S. aureus, which also strongly depends on innate immunity [55].
Overall, the data obtained in experimental models of arthritis suggest that, like in other experimental disease models, chronic leptin deficiency differently affects acquired versus innate immune responses: adaptive immune-mediated responses are attenuated, whereas in models involving the innate immune response, leptin deficiency causes inadequate control of inflammation.
Role of leptin in rheumatoid arthritis
As described above, leptin contributes to adaptive immunity-mediated inflammation in different models in rodents (Table 2). However, studies in humans show more controversial results. A potential role of leptin in RA, one of the most frequent immune-mediated inflammatory diseases in humans, has been investigated in several studies (Table 3). Only a couple of studies so far demonstrated elevated leptin concentrations in RA patients [91]. One of those studies showed increased plasma levels of leptin in RA patients compared to healthy controls, associated with significantly lower leptin levels in matched synovial fluid samples [91]. However, the lack of data concerning the body mass index (BMI) limits interpretation of the results of this study [56]. In another study, gender distribution differs between the groups (male:female ratio in RA patient group is 9:22, whereas in healthy controls 8:10) [92]. Plasma leptin levels are more than twice as high in healthy females than in males of corresponding weight status [93]; therefore, interpretation of these data is also limited. In addition, the only indication regarding disease activity in both these studies is measurement of C-reactive protein (CRP) levels, which either correlates [92] or not [91] with serum leptin levels.
Table 3.
Circulating leptin levels in patients with immune-mediated inflammatory diseases
| Diseases | Leptin levels: patients versus healthy controls | Correlation of leptin levels with disease activity | Comments | References |
| RA | Elevated | No correlation with CRP | No data on BMI | [91] |
| RA | Elevated | Correlation with CRP | Different gender distribution in the groups | [92] |
| RA | Similar | No correlation | Correlated with BMI and percentage of body fat | [94] |
| RA | Similar | No correlation | Correlated with BMI | [95] |
| RA | Similar | Negative correlation with CRP | No effect of short course anti-TNF-α therapy | [97] |
| and IL-6 | on leptin levels | |||
| RA | Reduced | No correlation | No correlation with BMI, CRP or total fat mass | [96] |
| SLE | Elevated | No correlation | Correlated with BMI | [102] |
| Systemic sclerosis | Reduced | No correlation | Correlated with BMI | [103] |
| Behçet's disease | Elevated | Positive correlation | Gender ratio, age and BMI similar in patient and control groups | [104] |
| Multiple sclerosis | Similar | Positive correlation | Leptin levels increased before exacerbation and decreased after treatment with IFN-β | [100] |
| Multiple sclerosis | Similar | No correlation | Leptin levels increased in IFN-β treated patients during active disease and remission | [101] |
BMI, body mass index; CRP, C-reactive protein; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TNF, tumor necrosis factor.
Moreover, two other studies showed that serum levels of leptin were not increased in RA patients compared with controls and the only correlations observed were between leptin and BMI or the percentage of body fat [94,95]. Yet another study showed even lower plasma leptin levels in RA patients than in controls and leptin did not correlate with BMI, CRP, total fat mass or disease activity score [96]. Finally, a significant inverse correlation was found between inflammation and leptin concentrations in one study on patients with active RA, although plasma leptin concentrations did not significantly differ from those in healthy controls [97]. Short course anti-TNF-α treatment did not modify leptin concentrations, despite significant reductions of CRP and IL-6. It was reported that fasting leads to an improvement of RA activity associated with a marked decrease in serum leptin and a shift toward Th2 cytokine production [98], reminiscent of the features observed during antigen-induced arthritis in ob/ob mice (Table 2). However, after a seven day ketogenic diet in RA patients, there were no significant changes in any clinical or biological measurements of disease activity, despite a significant decrease in serum leptin concentrations [99]. In conclusion, in the light of the present controversial data, it seems difficult to make an unambiguous conclusion about a potential role of leptin in RA.
Role of leptin in other immune-mediated inflammatory conditions
Several studies suggest a potential implication of leptin in the pathogenesis of other autoimmune inflammatory conditions in humans. However, the results of these studies do not consistently show a correlation between leptin levels and activity of immune-mediated diseases (Table 3). In patients with multiple sclerosis, serum levels of leptin were comparable to those of healthy controls [100,101]. Nonetheless, variable effects of IFN-β treatment on leptin levels were reported in two studies. In the first study, circulating leptin levels were increased before clinical exacerbation in relapsing patients and significantly decreased after IFN-β treatment [100]. In another study, leptin levels were increased in IFN-β treated patients compared to untreated controls during both active disease and remission [101]. Moreover, leptin induced secretion of IL-10, an anti-inflammatory cytokine, by peripheral blood mononuclear cells from multiple sclerosis patients in culture.
Elevated serum levels of leptin were found in women with systemic lupus erythematosus [102]. However, leptin levels correlated with BMI, but not with disease activity, as assessed by the Mexican SLE disease activity index. In contrast, in systemic sclerosis patients, decreased serum leptin levels were found [103]. There was no correlation between serum leptin levels and the duration of the symptoms of systemic sclerosis, while serum leptin levels correlated with BMI. In 35 patients with Behçet's syndrome, leptin levels were significantly higher than in healthy controls and correlated positively with disease activity [104]. Finally, some investigations suggest an association of leptin levels with several inflammatory markers, such as soluble TNF receptors [105,106] or CRP in healthy humans [107]. However, several recent clinical studies failed to demonstrate an effect of leptin administration on proinflammatory markers in healthy lean or obese humans [105,108,109].
Taken together, the results of these different studies do not consistently show a correlation between leptin levels and activity of immune-mediated disease. In addition, although circulating leptin levels correlated with inflammatory markers in some studies, there is no evidence for pro-inflammatory effects induced by leptin administration.
Conclusion
Taken together, results of in vitro and experimental animal studies suggest that leptin acts mostly as a proinflammatory agent during adaptive immune responses, whereas in processes involving innate immunity, anti-inflammatory effects of leptin are prevalent. However, it is difficult to elucidate the role, if any, of leptin during inflammatory conditions in human patients as different clinical studies have so far yielded inconsistent results, suggesting that leptin has a rather complex role in immune response and inflammation in humans. In particular, indirect effects of leptin or leptin deficiency are likely to considerably influence immune responses and inflammatory processes, and potentially opposite direct and indirect effects of leptin might thus partly account for some controversies observed in different investigations.
Abbreviations
AIA = antigen-induced arthritis; BMI = body mass index; BSA = bovine serum albumin; CRP = C-reactive protein; EAE = experimental autoimmune encephalomyelitis; IFN = interferon; IL = interleukin; JAK/STAT = Janus kinase/signal transducer and activator of transcription; LPS = lipopolysac-charide; MAPK = mitogen-activated protein kinase; PI3K = phosphatidylinositol 3-kinase; PMN = polymorphonuclear leukocyte; RA = rheumatoid arthritis; SOCS = suppressor-of-cytokine signaling; Th = T helper; TNF = tumor necrosis factor.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EB drafted the manuscript. GP and CG participated in discussions and manuscript revisions.
Acknowledgments
Acknowledgements
CG is supported by a Swiss National Science Foundation grant (320000-107592) and GP is supported by grants from the De Reuter and the Academic Society Foundations (Geneva, Switzerland).
References
- Gaucher EA, Miyamoto MM, Benner SA. Evolutionary, structural and biochemical evidence for a new interaction site of the leptin obesity protein. Genetics. 2003;163:1549–1553. doi: 10.1093/genetics/163.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SC, Jr, Koutras IK, Han L, Liu SM, Kay J, Young SJ, Chung WK, Leibel RL. Fine structure of the murine leptin receptor gene:splice site suppression is required to form two alternatively splicedtranscripts. Genomics. 1997;45:264–270. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- Ge H, Huang L, Pourbahrami T, Li C. Generation of soluble leptin receptor by ectodomain shedding of membrane-spanning receptors in vitro and in vivo. J Biol Chem. 2002;277:45898–45903. doi: 10.1074/jbc.M205825200. [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;84:2903–2911. doi: 10.1210/jc.84.8.2903. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscleand fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Delavaud C, Laud K, Gourdou I, Leroux C, Djiane J, Chilliard Y. Mammary leptin synthesis, milk leptin and their putative physiological roles. Reprod Nutr Dev. 2002;42:399–413. doi: 10.1051/rnd:2002034. [DOI] [PubMed] [Google Scholar]
- Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–1540. doi: 10.1172/JCI200113143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Holness MJ, Munns MJ, Sugden MC. Current conceptsconcerning the role of leptin in reproductive function. Mol Cell Endocrinol. 1999;157:11–20. doi: 10.1016/S0303-7207(99)00126-4. [DOI] [PubMed] [Google Scholar]
- Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–444. doi: 10.1016/S0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Thermoregulation in the diabetic-obese (db/db) mouse. The role of non-shivering thermogenesis in energy balance. Pflugers Arch. 1979;380:227–232. doi: 10.1007/BF00582901. [DOI] [PubMed] [Google Scholar]
- Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–3695. doi: 10.1210/jc.84.10.3686. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. Faseb J. 2001;15:2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- Pecoits-Filho R, Nordfors L, Heimburger O, Lindholm B, Anderstam B, Marchlewska A, Stenvinkel P. Soluble leptin receptors and serum leptin in end-stage renal disease: relationship with inflammation and body composition. Eur J Clin Invest. 2002;32:811–817. doi: 10.1046/j.1365-2362.2002.01063.x. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/S1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Martin-Romero C, Sanchez-Margalet V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam68. Cell Immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao E, Xia-Zhang L, Vulliemoz NR, Ferin M, Wardlaw SL. Leptin modulates inflammatory cytokine and neuroendocrine responses to endotoxin in the primate. Endocrinology. 2003;144:4350–4353. doi: 10.1210/en.2003-0532. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–1180. doi: 10.1016/S0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, Gabay C. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168:875–882. doi: 10.4049/jimmunol.168.2.875. [DOI] [PubMed] [Google Scholar]
- Caldefie-Chezet F, Poulin A, Tridon A, Sion B, Vasson MP. Leptin: a potential regulator of polymorphonuclear neutrophil bactericidal action? J Leukoc Biol. 2001;69:414–418. [PubMed] [Google Scholar]
- Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. 2000;11:7–14. [PubMed] [Google Scholar]
- Mikhail AA, Beck EX, Shafer A, Barut B, Gbur JS, Zupancic TJ, Schweitzer AC, Cioffi JA, Lacaud G, Ouyang B, et al. Leptin stimulates fetal and adult erythroid and myeloid development. Blood. 1997;89:1507–1512. [PubMed] [Google Scholar]
- Sanna V, Di Giacomo A, La Cava A, Lechler RI, Fontana S, Zappacosta S, Matarese G. Leptin surge precedes onset of autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J Clin Invest. 2003;111:241–250. doi: 10.1172/JCI200316721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, Sennello JA, Batra A, Fedke I, Lehr HA, Zeitz M, Siegmund B. Defining the role of T cell-derived leptin in the modulation of hepatic or intestinal inflammation in mice. Clin Exp Immunol. 2005;142:31–38. doi: 10.1111/j.1365-2249.2005.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1 beta mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Wardlaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab. 2003;88:1285–1291. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- Arnalich F, Lopez J, Codoceo R, Jim nez M, Madero R, Montiel C. Relationship of plasma leptin to plasma cytokines and human survivalin sepsis and septic shock. J Infect Dis. 1999;180:908–911. doi: 10.1086/314963. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Licinio J, Tauchnitz R, Engelmann L, Negrao AB, Gold P, Chrousos GP. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280–283. doi: 10.1210/jc.83.1.280. [DOI] [PubMed] [Google Scholar]
- Maruna P, Gurlich R, Frasko R, Haluzik M. Serum leptin levels in septic men correlate well with C-reactive protein (CRP) and TNF-alpha but not with BMI. Physiol Res. 2001;50:589–594. [PubMed] [Google Scholar]
- Torpy DJ, Bornstein SR, Chrousos GP. Leptin and interleukin-6 in sepsis. Horm Metab Res. 1998;30:726–729. doi: 10.1055/s-2007-978967. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Preas HL, Chrousos GP, Suffredini AF. Circulating leptin levels during acute experimental endotoxemia and anti-inflammatory therapy in humans. J Infect Dis. 1998;178:887–890. doi: 10.1086/515349. [DOI] [PubMed] [Google Scholar]
- Koc E, Ustundag G, Aliefendioglu D, Ergenekon E, Bideci A, Atalay Y. Serum leptin levels and their relationship to tumor necrosis factor-alpha and interleukin-6 in neonatal sepsis. J Pediatr Endocrinol Metab. 2003;16:1283–1287. doi: 10.1515/jpem.2003.16.9.1283. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Horgan MM, Powderly WG, Santiago JV, Landt M. Serum leptin concentrations in human immunodeficiency virus-infected men with low adiposity. Metabolism. 1997;46:303–305. doi: 10.1016/S0026-0495(97)90258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Crevel R, Karyadi E, Netea MG, Verhoef H, Nelwan RH, West CE, van der Meer JW. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. J Clin Endocrinol Metab. 2002;87:758–763. doi: 10.1210/jc.87.2.758. [DOI] [PubMed] [Google Scholar]
- Hultgren OH, Tarkowski A. Leptin in septic arthritis: decreased levels during infection and amelioration of disease activity upon its administration. Arthritis Res. 2001;3:389–394. doi: 10.1186/ar332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G, Gabay C. A role for leptin in rheumatic diseases? Ann Rheum Dis. 2003;62:913–915. doi: 10.1136/ard.62.10.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am J Pathol. 2000;156:1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Waelput W, Guisez Y. Leptin is an endogenous protective protein against the toxicity exerted by tumor necrosis factor. J Exp Med. 1999;189:207–212. doi: 10.1084/jem.189.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guebre-Xabier M, Yang S, Lin HZ, Schwenk R, Krzych U, Diehl AM. Altered hepatic lymphocyte subpopulations in obesity-related murine fatty livers: potential mechanism for sensitization to liver damage. Hepatology. 2000;31:633–640. doi: 10.1002/hep.510310313. [DOI] [PubMed] [Google Scholar]
- Jaworek J, Bonior J, Pierzchalski P, Tomaszewska R, Stachura J, Sendur R, Leja A, Jachimczak B, Konturek PC, Bielanski W, et al. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology. 2002;2:89–99. doi: 10.1159/000055897. [DOI] [PubMed] [Google Scholar]
- Warzecha Z, Dembinski A, Ceranowicz P, Jaworek J, Konturek PC, Dembinski M, Bilskl J, Konturek SJ. Influence of leptin administration on the course of acute ischemic pancreatitis. J Physiol Pharmacol. 2002;53:775–790. [PubMed] [Google Scholar]
- Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al. Leptin regulates proinflammatory immune responses. Faseb J. 1998;12:57–65. [PubMed] [Google Scholar]
- Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol. 2002;168:4018–4024. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- Plotkin BJ, Paulson D, Chelich A, Jurak D, Cole J, Kasimos J, Burdick JR, Casteel N. Immune responsiveness in a rat model for type II diabetes (Zucker rat, fa/fa): susceptibility to Candida albicans infection and leucocyte function. J Med Microbiol. 1996;44:277–283. doi: 10.1099/00222615-44-4-277. [DOI] [PubMed] [Google Scholar]
- Ottonello L, Gnerre P, Bertolotto M, Mancini M, Dapino P, Russo R, Garibotto G, Barreca T, Dallegri F. Leptin as a uremic toxin interferes with neutrophil chemotaxis. J Am Soc Nephrol. 2004;15:2366–2372. doi: 10.1097/01.ASN.0000139321.98029.40. [DOI] [PubMed] [Google Scholar]
- Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol. 2005;174:8090–8096. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils viainduction of TNF-alpha. J Immunol. 2004;172:1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Lehr HA, Fantuzzi G. Leptin: a pivotal mediator of intestinal inflammation in mice. Gastroenterology. 2002;122:2011–2025. doi: 10.1053/gast.2002.33631. [DOI] [PubMed] [Google Scholar]
- Matarese G, Di Giacomo A, Sanna V, Lord GM, Howard JK, Di Tuoro A, Bloom SR, Lechler RI, Zappacosta S, Fontana S. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Di Giacomo A, Lord GM, Howard JK, Bloom SR, Lechler RI, Fontana S, Zappacosta S. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol. 2001;31:1324–1332. doi: 10.1002/1521-4141(200105)31:5<1324::AID-IMMU1324>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Tarzi RM, Cook HT, Jackson I, Pusey CD, Lord GM. Leptin-deficient mice are protected from accelerated nephrotoxic nephritis. Am J Pathol. 2004;164:385–390. doi: 10.1016/S0002-9440(10)63128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Sanna V, Lechler RI, Sarvetnick N, Fontana S, Zappacosta S, La Cava A. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, La Cava A, Chieffi P, Nicoletti GF, Fontana S, Zappacosta S, Matarese G. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier JS. Lowered leptin slims immune response. Nat Med. 1998;4:1124–1125. doi: 10.1038/2619. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–1103. doi: 10.1172/JCI200215693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- de Hooge AS, van De Loo FA, Arntz OJ, van Den Berg WB. Involvement of IL-6, apart from its role in immunity, in mediating a chronic response during experimental arthritis. Am J Pathol. 2000;157:2081–2091. doi: 10.1016/S0002-9440(10)64846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DL, Burkart DL. Plasma corticosterone concentrations in diabetic (db) mice. Diabetologia. 1977;13:25–26. doi: 10.1007/BF00996323. [DOI] [PubMed] [Google Scholar]
- Guillaume-Gentil C, Rohner-Jeanrenaud F, Abramo F, Bestetti GE, Rossi GL, Jeanrenaud B. Abnormal regulation of thehypothalamo-pituitary-adrenal axis in the genetically obese fa/fa rat. Endocrinology. 1990;126:1873–1879. doi: 10.1210/endo-126-4-1873. [DOI] [PubMed] [Google Scholar]
- Takeshita N, Yoshino T, Mutoh S. Possible involvement of corticosterone in bone loss of genetically diabetic db/db mice. Horm Metab Res. 2000;32:147–151. doi: 10.1055/s-2007-978610. [DOI] [PubMed] [Google Scholar]
- Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–966. doi: 10.1016/S0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Juge-Aubry CE, Henrichot E, Meier CA. Adipose tissue: aregulator of inflammation. Best Pract Res Clin Endocrinol Metab. 2005;19:547–566. doi: 10.1016/j.beem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Wood IS. Signalling role of adipose tissue:adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST20051078. [DOI] [PubMed] [Google Scholar]
- Keystone EC, Schorlemmer HU, Pope C, Allison AC. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 1977;20:1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/S1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res Ther. 2004;6:R256–263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokarewa M, Bokarew D, Hultgren O, Tarkowski A. Leptin consumption in the inflamed joints of patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:952–956. doi: 10.1136/ard.62.10.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in fat-derived hormones plasmaconcentrations: adiponectin, leptin, resistin, and visfatin in rheumatoid arthritis subjects. Ann Rheum Dis. 2006 doi: 10.1136/ard.2005.046540. [AU: please provide the volume and page numbers of ref.92 or state if this is in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow VT, Phoon MC. Measurement of serum leptin concentrations in university undergraduates by competitive ELISA reveals correlations with body mass index and sex. Adv Physiol Educ. 2003;27:70–77. doi: 10.1152/advan.00001.2003. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Rihl M, Heufelder A, Loch O, Schattenkirchner M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48:745–748. doi: 10.1016/S0026-0495(99)90174-9. [DOI] [PubMed] [Google Scholar]
- Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K. Serum leptin levels in patients with rheumatoid arthritis are correlatedwith body mass index. Rinsho Byori. 2002;50:524–527. [PubMed] [Google Scholar]
- Tokarczyk-Knapik A, Nowicki M, Wyroslak J. The relation between plasma leptin concentration and body fat mass in patients with rheumatoid arthritis. Pol Arch Med Wewn. 2002;108:761–767. [PubMed] [Google Scholar]
- Popa C, Netea MG, Radstake TR, van Riel PL, Barrera P, van der Meer JW. Markers of inflammation are negatively correlated with serum leptin in rheumatoid arthritis. Ann Rheum Dis. 2005;64:1195–1198. doi: 10.1136/ard.2004.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser DA, Thoen J, Reseland JE, Forre O, Kjeldsen-Kragh J. Decreased CD4+ lymphocyte activation and increased interleukin-4 production in peripheral blood of rheumatoid arthritis patients after acute starvation. Clin Rheumatol. 1999;18:394–401. doi: 10.1007/s100670050125. [DOI] [PubMed] [Google Scholar]
- Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djoseland O, Forre O, Kjeldsen-Kragh J. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol. 2000;18:209–214. [PubMed] [Google Scholar]
- Batocchi AP, Rotondi M, Caggiula M, Frisullo G, Odoardi F, Nociti V, Carella C, Tonali PA, Mirabella M. Leptin as a marker of multiple sclerosis activity in patients treated with interferon-beta. J Neuroimmunol. 2003;139:150–154. doi: 10.1016/S0165-5728(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Chatzantoni K, Papathanassopoulos P, Gourzoulidou E, Mouzaki A. Leptin and its soluble receptor in plasma of patients suffering from remitting-relapsing multiple sclerosis (MS) In vitro effects of leptin on type-1 and type-2 cytokine secretion by peripheral blood mononuclear cells, T-cells and monocytes of MS patients. J Autoimmun. 2004;23:169–177. doi: 10.1016/j.jaut.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, Cardona-Munoz EG, Salazar-Paramo M, Gonzalez-Ortiz M, Martinez-Abundis E, Gamez-Nava JI. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- Kotulska A, Kucharz EJ, Brzezinska-Wcislo L, Wadas U. A decreased serum leptin level in patients with systemic sclerosis. Clin Rheumatol. 2001;20:300–302. doi: 10.1007/s100670170053. [DOI] [PubMed] [Google Scholar]
- Evereklioglu C, Inaloz HS, Kirtak N, Doganay S, Bulbul M, Ozerol E, Er H, Ozbek E. Serum leptin concentration is increased in patients with Behcet's syndrome and is correlated with disease activity. Br J Dermatol. 2002;147:331–336. doi: 10.1046/j.1365-2133.2002.04703.x. [DOI] [PubMed] [Google Scholar]
- Chan JL, Bullen J, Stoyneva V, Depaoli AM, Addy C, Mantzoros CS. Recombinant methionyl human leptin administration to achieve high physiologic or pharmacologic leptin levels does not alter circulating inflammatory marker levels in humans with leptin sufficiency or excess. J Clin Endocrinol Metab. 2005;90:1618–1624. doi: 10.1210/jc.2004-1921. [DOI] [PubMed] [Google Scholar]
- van Dielen FM, van't Veer C, Schols AM, Soeters PB, Buurman WA, Greve JW. Increased leptin concentrations correlate with increased concentrations of inflammatory markers in morbidly obese individuals. Int J Obes Relat Metab Disord. 2001;25:1759–1766. doi: 10.1038/sj.ijo.0801825. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, Berger PB, Somers VK. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, Lindeman JH, Toet KH, Saris WH, Eilers PH, Westerterp-Plantenga MS, Kooistra T. Leptin and the proinflammatory state associated with human obesity. J Clin Endocrinol Metab. 2004;89:1773–1778. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Salvador J, Silva C, Rotellar F, Gil MJ, Cienfuegos JA, Fruhbeck G. Leptin therapy does not affect inflammatory markers. J Clin Endocrinol Metab. 2005;90:3803. doi: 10.1210/jc.2005-0558. author reply 3803. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, Fantuzzi G. Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor alpha and IL-18. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau L, Defeau D, Iserentant H, Vandekerckhove J, Peelman F, Tavernier J. Leptin receptor activation depends on critical cysteine residues in its fibronectin type III subdomains. J Biol Chem. 2005;280:22632–22640. doi: 10.1074/jbc.M413308200. [DOI] [PubMed] [Google Scholar]
- Zabeau L, Defeau D, Van der Heyden J, Iserentant H, Vandekerckhove J, Tavernier J. Functional analysis of leptin receptor activation using a Janus kinase/signal transducer and activator of transcription complementation assay. Mol Endocrinol. 2004;18:150–161. doi: 10.1210/me.2003-0078. [DOI] [PubMed] [Google Scholar]


