Abstract
The use of cyclo-oxygenase 2 selective nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with increased risk of acute myocardial infarction (AMI). The association between the risks of AMI with nonselective NSAIDs is less clear. We reviewed the published evidence and assessed the risk of AMI with nonselective NSAIDs. We performed a meta-analysis of all studies containing data from population databases that compared the risk of AMI in NSAID users with that in non-users or remote NSAID users. The primary outcome was objectively confirmed AMI. Fourteen studies met predefined criteria for inclusion in the meta-analysis. Nonselective NSAIDs as a class was associated with increased AMI risk (relative AMI risk 1.19, 95% confidence interval [CI] 1.08 to 1.31). Similar findings were found with diclofenac (relative AMI risk 1.38, 95% CI 1.22–1.57) and ibuprofen (relative AMI risk 1.11, 95% CI 1.06 to 1.17). However, this effect was not observed with naproxen (relative AMI risk 0.99, 95% CI 0.88–1.11). In conclusion, based on current evidence, there is a general direction of effect, which suggests that at least some nonselective NSAIDs increase AMI risk. Analysis based on the limited data available for individual NSAIDs, including diclofenac and ibuprofen, supported this finding; however, this was not the case for naproxen. Nonselective NSAIDs are frequently prescribed, and so further investigation into the risk of AMI is warranted because the potential for harm can be substantial.
Introduction
One of the most revelatory issues concerning pharmaceuticals in recent years has been the relationship found between selective cyclo-oxygenase (COX)-2 inhibitors and cardiovascular thrombotic adverse events such as acute myocardial infarction (AMI) [1-5]. Received wisdom has never implicated the older class of similarly acting drugs, the nonsteroidal anti-inflammatory drugs (NSAIDs), in this association. However, new evidence suggests that there be an association between these nonselective NSAIDs and cardiovascular adverse effects, and that the risk may be similar with this class of drugs to that with COX-2 selective NSAIDs [6].
NSAIDs are among the most popular of prescribed drugs and have proven effectiveness in relieving symptoms of inflammation, including pain; they may also have a role in cancer prevention [7]. Both their benefits and adverse effects are due to the inhibition of either COX-1 or COX-2 enzymes. NSAIDs inhibit both COX-1 and COX-2, with extent of inhibition of COX-1 versus COX-2 differing between NSAIDs [8]. It is believed that the NSAID-induced inhibition of COX-1 in the gastrointestinal mucosa leads to the development of serious gastrointestinal complications such as ulcers and bleeds. The selective COX-2 inhibitors were developed to inhibit preferentially the COX-2 enzyme while sparing COX-1, with the premise that this would prevent serious gastrointestinal toxicity. However, recent studies have shown an unequivocal increase in risk of cardiovascular thrombotic events in patients treated with these drugs [1-5].
It is unclear whether the greater risk of AMI seen with selective COX-2 inhibitors is a 'class' effect of all NSAIDs. Like aspirin, nonselective NSAIDs inhibit COX-1, albeit temporarily, and they have generally been assumed to be antithrombotic or to have no cardiovascular adverse effect [8]. More recent studies suggest that many NSAIDs, both selective and nonselective, may result in an excess of AMIs [6]. In view of the large numbers of patients prescribed nonselective NSAIDs, we reviewed the available evidence and report the results of a meta-analysis conducted to determine whether nonselective NSAIDs increase AMI risk.
Materials and methods
Search strategy
We searched all major electronic databases including Medline, BIDS and EMBASE, between January 1980 and June 2005. Relevant keywords (as MeSH terms and text words) relating to NSAIDs (for instance, anti-inflammatory agents, non steroidal anti-inflammatory) and AMI (for instance, myocardial infarction, myocardial ischemia, cardiac ischemia, death) were combined to capture all potentially relevant studies. In addition, we contacted experts in the area and reviewed relevant discussions of the US Food and Drug Administration advisory panels and the UK National Institute of Clinical Excellence. Hand searching the reference lists of all relevant papers and recent topic reviews was also carried out.
Study selection
Two reviewers assessed the studies retrieved from the search independently by scanning all the titles and abstracts. Full text copies of the selected papers were obtained and scrutinised independently by both reviewers for inclusion. Studies were included if they met the following criteria. The design of included studies was required to be observational studies of data from population databases that included comparison of NSAID use and non-use or remote-NSAID use. The intervention has to be use of nonselective NSAIDs. Comparison groups were required to be current NSAID users along with non-users or remote users. Finally, the outcome was required to be objectively confirmed AMI.
Data extraction and quality assessment
Data from studies meeting the inclusion criteria were extracted into standardized data extraction forms independently by two reviewers (GS and RM), during which the quality of the studies was also assessed. This systematic review included a variety of study types. In order to maintain consistency of reporting, a validated generic checklist designed for evaluation of quantitative studies was used to assess the quality of all of the studies included in the review [9]. This checklist originally included 14 criteria, but one of these referred to random allocation of treatment and another referred to the blinding of participants. These were considered not applicable to observational studies and were excluded from the checklist; therefore, the final checklist consisted of 12 items. These items are consistent with the recommendations from the Centre for Reviews and Dissemination [10] and the consensus statement on meta-analysis reporting of observational studies in epidemiology [11]. Any disagreement relating to inclusion of studies, data extraction, or quality assessment between the reviewers was resolved by discussion.
Data synthesis
We performed a meta-analysis using the random effects model based on the generic inverse variance method on nonselective NSAIDs as a class, and where data were available meta-analyses on individual NSAIDs were also carried out. The random effects model accounts for interstudy variance and provides a more conservative estimate of effect than does the fixed effect model, whereas the generic variance method can take into account confounding by combining adjusted relative risk estimates [12]. A pooled generic measure of relative AMI risk was calculated from the individual studies' estimates of relative AMI risk (expressed in relative risks and odds ratios) and 95% confidence intervals (CIs). We used the assumption that when the outcome of interest is rare, odds ratio approximates the relative risk. Potential sources of heterogeneity were investigated and assessed using the standard χ2 test. In addition, the I2 statistic was used to evaluate inconsistencies in results reported among the studies. In addition, we assessed publication bias graphically by using a funnel plot. Sensitivity analysis was carried out to assess the robustness of the results of the meta-analysis and to explore heterogeneity. All analyses were performed using RevMan 4.2 (Cochrane Collaboration, Oxford, UK).
Results
The search strategy identified 243 potentially relevant citations for review (Figure 1). On reviewing retrieved papers, two were based on patient recall and thus were likely to be subject to reverse recall bias and were excluded [13,14]. A further two studies were identified by hand searching [15,16], of which one was excluded because it compared the effects of one NSAID with current use of three other NSAIDs but it did not have a control population of remote users or non-users [15].
Figure 1.
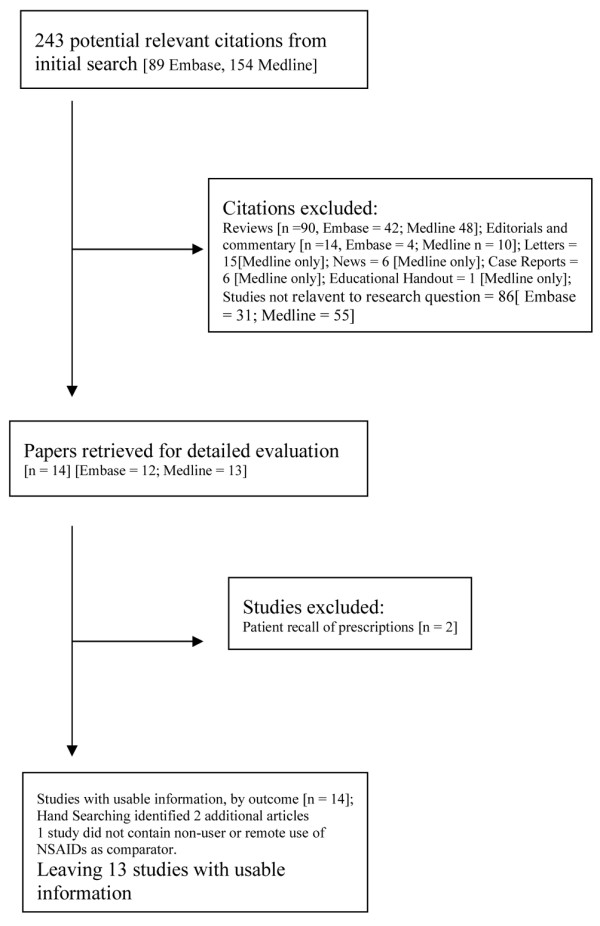
Selection of studies included in the meta-analysis.
Fourteen studies contained relevant data and were included in the analysis [4,16-28], and their characteristics are summarized in Table 1. All of the studies were based on data from validated databases from Canada, Denmark, the UK, and the USA. Most studies provided details on AMI risk with nonselective NSAIDs as a group, but some included information only on non-naproxen NSAIDs. Five studies provided information on dose: in one [19] the effect of dose was examined only in long-term users (no dose effect was seen); two studies [18,28] reported no effect of dose; one [27] reported greater risk of AMI with higher doses; and one study [24] reported a dose-dependent effect of ibuprofen but no effect with other NSAIDs. The duration of exposure in most studies was short (Table 1). Contrary to the majority of the studies included in the review, two studies were conducted in selected populations; one study [17] included only postmenopausal women and the other [16] was limited to patients with rheumatoid arthritis. In only three studies was the indication for prescription examined in detail [16,17,19].
Table 1.
Characteristics of included studies
| Ref. | Design | Data source | NSAID exposure | Study outcome | AMI events (n) | No AMI events (n) | Confounding factors |
| [19] | Nested case control | GPRD (UK) | Current NSAID users: patients (age 50–84 years) whose most recent prescription lasted until the index date or ended in the 30 days before the index date | First hospitalized AMI or death due to CHD | Diclofenac = 213 Ibuprofen = 155 Naproxen = 49 |
Diclofenac = 679 Ibuprofen = 575 Naproxen = 206 |
MI risk factors (smoking, diabetes, hypertension, hyperlipidemia, BMI, RA, OA, anemia, CHD, CVD), age, sex, calendar year, alcohol intake, and use of steroids, aspirin, anticoagulants, paracetamol, and NSAIDs |
| [17] | Nested case control | GPRD (UK) | Current NSAID users: menopausal women (age 50–74 years) who had a prescription for NSAIDs before the index date | First hospitalized AMI | Nonaspirin NSAIDs = 40 | Nonaspirin NSAIDs = 143 | Age, use of HRT, smoking, hypertension, diabetes, obesity, surgical menopause, family history of CHD, and predefined co-morbidity |
| [20] | Nested case control | Managed care database (USA) | Current NSAID users: patients (age 18–84 years) who had ≥1 prescription for a COX 2 selective or non-selective NSAID | Hospitalized AMI, sudden cardiac death | Ibuprofen = 670 Naproxen = 367 |
Ibuprofen = 2573 Naproxen = 1409 |
Sex, age, geographic location, cardiovascular risk score, admission for noncardiac-related disorders and same-day procedures, emergency room visits for noncardiovascular reasons, HRT, and high-dose prednisolone |
| [6] | Nested case control | QRESEARCH (UK) | Current NSAID users: patients (age 25–100 years) who had a prescription for selective or nonselective NSAIDs within the 3 months before the index data | First hospitalized AMI/CHD, sudden death | Ibuprofen = 460 Naproxen = 96 Other nonselective NSAIDs = 181 |
Ibuprofen = 3199 Naproxen = 677 Other nonselective NSAIDs = 1266 |
Use of aspirin, statin, tricyclic antidepressants, SSRI, ischemic heart disease, diabetes, hypertension, OA, RA, smoking obesity, and deprivation |
| [21] | Case control | Hospital discharge registry (Denmark) | Current nonaspirin NSAID users: patients (age 20–101 years) who had received a prescription within 30 days before the index date | First hospitalized AMI | Naproxen = 26 Other nonaspirin NSAIDs = 532 |
Naproxen = 175 Other nonaspirin NSAIDs = 3105 |
Discharge diagnosis of CVD, hypertension, diabetes, chronic bronchitis or emphysema, acholoism, liver cirrhosis, upper GI bleed, RA, systemic lupus erythematosus and use of high-dose aspirin, platelet inhibitors, insulin or oral hypoglycemic drugs, antihypertensive drugs, lipid-lowering drugs, oral anticoagulants, hormone therapy, nitrates, penicillamine, gold and glucocorticocoids before date of admission |
| [22] | Nested case control | Administrative health database (Québec, Canada) | New NSAID users: patients (age ≥66 years) who had a dispensed prescription with a duration that covered or overlapped with the index date | First Hospitalized AMI | Naproxen = 23 Other nonselective, nonaspirin NSAIDs = 51 |
Naproxen = 336 Other nonselective, nonaspirin NSAIDs = 962 |
Age, sex, hypertension, CAD, cerebrovascular disease, peripheral vascular disease, CCF, statin, aspirin, anticoagulants, presence of respiratory disease, GI ulcer disease, thyroid disease, depression or psychiatric illness, use of oral corticosteroids, Chronic disease score, Charlson index, health care utilization |
| [23] | Retrospective cohort | Administrative health database (Ontario, Canada) | New NSAID users: patients (age ≥66 years) who received a prescription for NSAIDs | Hospitalized AMI | Naproxen = 15 Other nonselective, non-naproxen NSAIDs = 134 |
Naproxen = 5654 Other nonselective, nonaspirin NSAIDs = 33,734 |
Hospitalization in prior year, malignancy in prior 5 years, MI, stroke, CAD or CABG in prior 5 years, age, sex, long-term care, low income, number of different drugs |
| [24] | Retrospective cohort | Tennessee Medicaid (USA) | New nonaspirin NSAID users: patients (age 50–84 years) who had a prescription of NSAIDs, with no use during the previous 365 days | Hospitalized AMI or death from CHD | Ibuprofen = 339 Naproxen = 201 Other or multiple nonaspirin NSAIDs = 301 |
Data not available | Prescribed drugs for CVS disease, hospital admissions and emergency visits for CVS and other disease, PVD, CVD, CAD, and revascularization procedures |
| [25] | Retrospective cohort | Tennessee Medicaid (USA) | Current and new NSAID users: patients (age 50–84 years) who were taking NSAIDs at enrolment were classed as current users; those who began use of an NSAID during the follow-up period were classed as new users | Hospitalized AMI or death from CHD | Current users: Ibuprofen = 190 Naproxen = 245 New users: Ibuprofen = 52 Naproxen = 72 |
Data not available | Prescribed drugs for CVS disease, hospital admissions and emergency visits for CVS and other disease, PVD, CVD, CAD, and revascularization procedures |
| [26] | Matched case control | Administrative health database (Québec, Canada) | Current NSAID users: patients (age ≥65 years) who had a dispensed prescription with a duration that covered or overlapped with the index date | First AMI | Naproxen = 255 Other NSAIDs = 1062 |
Naproxen = 212 Other NSAIDs = 722 |
Prior use of anticoagulants, nitrates, lipid lowering agents, antidiabetic agents, antihypertensive agents, prior CVD, presence of co-morbidity factors |
| [27] | Nested case control | GPRD (UK) | Current NSAID users: patients (age ≤75 years) who had their last prescription for an NSAID before the index date and which ended at or after the index date | First hospitalized AMI | Diclofenac = 97 Ibuprofen = 60 Naproxen = 19 |
Diclofenac = 277 Ibuprofen = 204 Naproxen = 105 |
Aspirin, BMI, smoking, HRT |
| [28] | Case control | Medicaid/Medicare (New Jersey, USA) | NSAID users: patients (age ≥60 years) who had use of prescribed NSAIDs during the 6 months before the index date | Hospitalized AMI | Ibuprofen = 285 Naproxen = 243 |
Ibuprofen = 1030 Naproxen = 1094 |
Hypertension, diabetes, CCF, and validated co-morbidity index |
| [16] | Case control | GPRD (UK) | Current NSAID users: patients (age 40–79 years) with RA and who had received a NSAID prescription during the 30 days before the index date | First AMI, sudden death and stroke | Data not available | Data not available | Adjusted values reported, but factors that were adjusted for were not detailed |
| [18] | Case control | GPRD (UK) | Current NSAID user: patients <89 years whose prescription overlapped with the index date | First AMI | Nonaspirin NSAID = 680 Diclofenac = 260 Ibuprofen = 176 Naproxen = 63 |
Nonaspirin NSAID = 2339 Diclofenac = 834 Ibuprofen = 656 Naproxen = 251 |
Adjusted for hypertension, hyperlipidemia, diabetes, ischemic heart disease, BMI, kidney disease, RA, and aspirin use |
AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCF, congestive cardiac failure; CHD, coronary heart disease; COX, cyclo-oxygenase; CVD, cardiovascular disease; CVS, cardiovascular system; GI, gastrointestinal; GPRD, General Practice Research Database; HRT, hormone replacement therapy; MI, myocardial infarction; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; PVD, peripheral vascular disease; RA, rheumatoid arthritis; SSRI, selective serotonin reuptake inhibitor.
All of the studies were included in the meta-analysis (Figure 2). With the exception of one study [22], which reported no effect of nonselective NSAID use and AMI risk, all studies identified a similar trend toward increased risk of AMI. The meta-analysis of nonselective NSAIDs as a class was based on 13 studies [6,16-23,25-28], because one study presented data only on specific nonselective NSAIDs as part of an evaluation of the risks of AMI with COX-2 inhibitors [24]. The results revealed an AMI risk of 1.19 (95% CI 1.08 to 1.31; P = 0.0006) in NSAID users when compared with non-users or remote users. However, there was high between-study heterogeneity (I2 = 83.8%; P < 0.00001).
Figure 2.
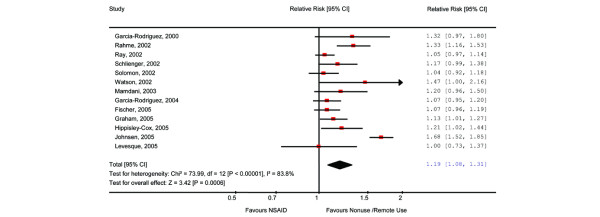
The relative AMI risk associated with use of nonselective NSAIDs versus remote use or non-use. There may be small discrepancies between the individual study values presented here and those presented in the original studies. This is due to the function of the software used for the calculation. One study did not present data on polled NSAIDs and is excluded from this figure. AMI, acute myocardial infarction; NSAID, nonsteroidal anti-inflammatory drug.
Limited data were available on individual NSAIDs (Table 2). Five studies presented data on diclofenac [6,16,18,19,27] and reported increased AMI risk with diclofenac use compared with non-use or remote use of NSAIDs; the pooled relative AMI risk was 1.38 (95% CI 1.22 to 1.57; P < 0.00001). There was no significant between-study heterogeneity (I2 = 54%; P = 0.08). Nine studies evaluated the association between ibuprofen and the risk of AMI [6,16,18-20,24,25,27,28]. Although the majority of the individual studies reported nonsignificant risk association, the pooled analysis identified a relative AMI risk of 1.11 (95% CI 1.06 to 1.17; P = 0.0001). No evidence of heterogeneity was detected (P = 0.41), and the risk estimates of individual studies were consistent (I2 = 3.2%). In contrast, naproxen use was not found to be associated with increased AMI risk (relative AMI risk 0.99, 95% CI 0.88 to 1.11; P = 0.99). The studies included in the analysis yielded conflicting results. Eight studies [16,18,19,23-25,27,28] indicated that naproxen was not associated with increased AMI risk, whereas four studies [6,20-22] suggested that naproxen was associated with increased risk of AMI. The risk estimated in two of the latter four studies [6,21] was statistically significant. Overall, significant heterogeneity (P = 0.01) and moderate inconsistency (I2 = 54%) were present among the estimates reported by the studies.
Table 2.
The relative AMI risk associated with the use individual NSAIDs compared with remote or no use.
| Drug [ref.] | Relative AMI risk | 95% CI | Test for heterogeneity | Test for inconsistency | |
| χ2 | P value | I2 | |||
| Diclofenac | 1.41 | 1.20–1.65 | 9.79 | 0.08 | 48.9% |
| [27] | 1.38 | 1.08–1.77 | |||
| [16] | 1.68 | 1.14–2.48 | |||
| [19] | 1.18 | 0.99–1.40 | |||
| [6] | 1.55 | 1.39–1.72 | |||
| [18] | 1.23 | 1.00–1.51 | |||
| Ibuprofen | 1.11 | 1.04–1.18 | 8.26 | 0.41 | 3.2% |
| [25] | 1.15 | 1.03–1.29 | |||
| [24] | 1.01 | 0.77–1.33 | |||
| [27] | 1.17 | 0.87–1.58 | |||
| [28] | 1.02 | 0.88–1.18 | |||
| [16] | 0.74 | 0.35–1.56 | |||
| [19] | 1.06 | 0.87–1.29 | |||
| [20] | 1.06 | 0.96–1.17 | |||
| [6] | 1.24 | 1.11–1.39 | |||
| [18] | 1.16 | 0.92–1.46 | |||
| Naproxen | 0.99 | 0.88–1.12 | 23.91 | 0.01 | 54% |
| [25] | 0.95 | 0.82–1.10 | |||
| [24] | 0.93 | 0.74–1.17 | |||
| [27] | 0.68 | 0.41–1.12 | |||
| [28] | 0.84 | 0.72–0.98 | |||
| [16] | 0.57 | 0.31–1.05 | |||
| [23] | 1.00 | 0.59–1.68 | |||
| [19] | 0.89 | 0.64–1.24 | |||
| [20] | 1.14 | 1.00–1.30 | |||
| [6] | 1.27 | 1.01–1.60 | |||
| [21] | 1.50 | 0.99–2.28 | |||
| [22] | 1.17 | 0.75–1.83 | |||
| [18] | 0.96 | 0.66–1.39 | |||
AMI, acute myocardial infarction; CI, confidence interval; NSAID, nonsteroidal anti-inflammatory drug.
Sensitivity analysis
Sensitivity analysis was carried out to explore the heterogeneity and inconsistencies of the results of the studies included in the meta-analysis. We first analyzed the nonselective NSAID data by excluding the two studies based on selected populations [15,25]; this had little impact on the results of the meta-analysis (relative AMI risk 1.18, 95% CI 1.05 to 1.33; P = 0.005), and significant heterogeneity remained (P < 0.00001). One of the studies [21] exhibited a higher than expected risk of AMI compared with the other studies; the analysis was therefore repeated with this study's data excluded. The results showed a relative AMI risk of 1.13 (95% CI 1.07–1.18; P < 0.00001); there was significant heterogeneity (P = 015), and a small-to-moderate degree of inconsistency remained (I2 = 30.7%). All of the analyses were also repeated using a fixed effect model, but there was little change in the results.
Discussion
Considerable scientific and media attention has been directed at reports that selective COX-2 inhibitors increase AMI risk. Two selective COX-2 inhibitors have been withdrawn, and the sales of another have plummeted. However, until recently, little attention has been focused on the risks associated with use of the nonselective NSAIDs. Because there is no randomised controlled study of nonselective NSAIDs large enough to detect an increase in a common condition such as AMI, an absence of evidence has been assumed to imply evidence of absence. Our meta-analysis shows that use of at least some nonselective NSAIDs is associated with a small but significantly increased risk of AMI compared with remote and non-use. If this small increase is indeed causally related to use of nonselective NSAIDs, then the implications for public health policy are considerable because of the large numbers of patients prescribed these drugs.
We also investigated the relative AMI risk associated with frequently prescribed nonselective NSAIDs, including diclofenac, ibuprofen, and naproxen, individually. When comparing the use of diclofenac and ibuprofen with no or remote NSAID use, the results supported the presence of increased risk of AMI, similar to that observed with NSAIDs as a class. We did not find a significant association between naproxen use and AMI, but there was significant heterogeneity and moderate inconsistency among the 12 studies. It is possible that our meta-analysis was confounded by pharmaceutical company support of several naproxen studies in the wake of the increased risk of AMI seen with rofecoxib in the VIGOR (VIOXX GI Outcomes Research) study [29,30]. The increased risk of AMI observed with rofecoxib in the VIGOR trial was explained by a purported 'cardioprotective' effect of naproxen. Several epidemiologic studies were funded by the manufacturer of rofecoxib to prove the cardioprotective effect of naproxen. These studies, included in our meta-analysis, indicate that there was a large cardioprotective effect of naproxen, unlike most other independently funded studies. This largely explains the between-study heterogeneity in our analysis, as previously established by Juni and coworkers [29]. An interim analysis of an Alzheimer's disease prevention study [31] has suggested increased risk of AMI in patients treated with naproxen, but the full data have not yet been released.
It is possible that different NSAIDs may be associated with differential increases in risk of AMI. Differences between NSAIDs, based on their pharmacologic effects, have been previously described for the risk of gastrointestinal bleeding [32]. However, there are few data available in the literature on the cardiovascular risk of NSAIDs other than ibuprofen, diclofenac, and naproxen.
There are several potential limitations to our study, many of which are inherent to all meta-analysis of observational studies. The quality of our analysis depends on the data extracted from the original publications; we may thus inherit the problems of potential bias and confounding by indication inherent to observational studies. It is possible that sicker patients may preferentially receive NSAID treatment, and these patients carry a higher baseline risk of cardiovascular complications (confounding by indication). All of the observational studies included in our meta-analysis adjusted for this confounding, but it is possible that this limitation was not completely eliminated because of unmeasured variables. However, one advantage of observational studies is that they more accurately reflect the spectrum of patients in clinical practice, and if they are large enough they can detect rare adverse events or increased occurrence of a common disorder [33]. Randomized controlled trials are more appropriate for defining efficacy and assigning causality, but their external validity or generalizability can often be low [34] and they are rarely sufficiently large or long running to identify all adverse events [35,36]. Although the randomized clinical trial remains the 'gold standard', it is unlikely that a large clinical trial to study the effect of all NSAIDs on cardiovascular risk will ever be conducted, thus emphasizing the need for critical evaluation of observational studies.
The statistical pooling of risk from observational studies is controversial because of the many biases that can arise in observational studies compared with randomized controlled trials. It has been argued that presenting a single pooled estimate without additional detail can be misleading but is justified under certain circumstances [37]. In our study the differences in risk estimates between the individual studies was small, and study designs were similar, justifying the pooling of the studies. A formal test of heterogeneity in our overall analysis showed that chance was not the explanation. In an attempt to explain this we undertook a post hoc investigation by study type and study population. One study with a much higher than expected AMI risk [21] was found to be contributing to the heterogeneity; the reasons for this was not apparent. It is possible that some of the heterogeneity may reflect the relative proportions of different NSAIDs used in the study populations.
None of the studies sought information on use of nonselective NSAIDs purchased over the counter; it is thus not possible to exclude bias arising from their use. Five of the studies were from the same database [16-19,27]. Two of the studies were restricted to either rheumatoid arthritis patients or postmenopausal women [16,17]. However, the exclusion of these studies did not change the pooled estimate of AMI risk. The three other studies [18,19,27] were undertaken over separate 5-year intervals and recorded first episode AMIs occurring during the study interval. It is thus unlikely that their inclusion altered the pooled risk estimate because of duplication. To further examine whether the inclusion of all studies was biasing the results, the analysis was repeated with the study with lowest AMI risk [18], and there was no difference in overall risk.
Another potential concern is that some of the studies relied on pharmaceutical company sponsorship and thus need to be interpreted with caution; this is particularly relevant in relation to naproxen. Furthermore, we did not include meeting abstracts in our analysis because frequently insufficient information could be extracted.
The results of our meta-analysis are largely consistent with observations from studies of selective COX-2 inhibitors. In most observational studies of COX-2 inhibitors the estimated relative risk of AMI ranged between 0.8 and 1.5, similar to the risk we found with nonselective NSAIDs [31]. Large randomized controlled trials comparing COX-2 inhibitors with nonselective NSAIDs have not invariably found an increased risk of AMI. In the VIGOR study rofecoxib 50 mg had a relative risk of 2 compared with naproxen for the composite end-point of death, stroke, and AMI [30]. Celecoxib, when compared with ibuprofen and naproxen, was not associated with any difference in the number of severe adverse cardiovascular events in the CLASS (Celecoxib Long-term Arthritis Safety Study) study [38]. Similarly, in the EDGE (Etoricoxib Diclofenac Gastrointestinal Evaluation) study [39], which compared etoricoxib with diclofenac in patients with osteoarthritis, there was no overall difference between the two drugs in serious adverse cardiovascular events. With regard to lumiracoxib, in the TARGET (Therapeutic Arthritis Research and Gastrointestinal Event Trial) study [40] the primary analysis revealed no difference when naproxen and ibuprofen were considered as a single group. In a substudy analysis lumiracoxib carried a greater risk than naproxen but less than that of ibuprofen. It has therefore been suggested cardiovascular events, including AMIs, may arise as class effect of both COX-2 selective and nonselective NSAIDS. This has resulted in the US Food and Drug Adminiatration advising manufacturers that a boxed warning of an increased risk of serious cardiovascular adverse events should accompany all NSAIDs, including those available over the counter [31].
The role of concomitant aspirin in altering the risk of thromboembloic complications with COX-2 inhibitors is controversial [31]. Although not a primary aim of our study, four studies [6,18,19,22] did report on concomitant aspirin use with NSAIDs; three found no effect and one a further reduction in events, but these observations were based on a small number of events. Larger studies are needed to study the effect of concomitant aspirin use. Similarly, there is inadequate data on the effect of dose and duration of use of NSAIDs.
The occurrence of AMI with NSAID could arise because of a number of the consequences of COX inhibition. The most frequently proposed theory regarding the excess of AMI with selective COX-2 inhibitors is one of thromboxane/prostacyclin imbalance [41,42]. Grosser and coworkers [42] postulated that nonselective NSAIDs would also increase the risk of AMI, and that this increase in risk would be dependent on the COX-2 selectivity of the NSAID, with drugs such as diclofenac carrying higher risk than naproxen; the results of our study are consistent with this. NSAID-induced hypertension is also well recognized, and even small sustained increases in blood pressure can significantly increase the risk of adverse cardiovascular events [42,43]. One estimate projects 35,700 additional events per annum from use of NSAIDS in rheumatoid arthritis and osteoarthritis patients alone [44].
Conclusion
Acknowledging the limitations of observational data, our systematic review of the only available published data indicates that several nonselective NSAIDs are associated with increased risk of AMI. Until results become available from a randomized controlled trial large enough to detect the risk we found (which, we consider, is unlikely ever to be undertaken), we endorse the pragmatic advice that the lowest possible dose should be used for the shortest possible duration [45] for all NSAIDs. Furthermore, we add that these drugs, like COX-2 inhibitors, should be used with caution in those with risk factors for atheromatous vascular disease and should be avoided in those with clinical complications. Patient selection rather than drug selectivity may thus be more important in their use.
Abbreviations
AMI = acute myocardial infarction; CI = confidence intervals; COX = cyclo-oxygenase; NSAID = nonsteroidal anti-inflammatory drug.
Competing interests
Institute of Clinical Outcomes Research and Education has received research grants from Boehringer-Ingelheim, Glaxo Smith Kline, Novartis and Pfizer, and GS has been a speaker for Pfizer. RM has received ducational grants from Abbott Laboratories, Strakan Ltd., and Merck. OW and PL declare that they have no competing interests. There was no pharmaceutical company funding in support of this study.
Authors' contributions
RM conceived the study. GS, OW, and RM collected and analyzed the data. PL advised on data analysis. All authors were involved in writing the report and approved the final manuscript.
Acknowledgments
Acknowledgements
We are grateful to Mr Robin Harbour, Director of information and Quality, Scottish Intercollegiate Guideline Network for advice on the search strategy and to Dr S Jauhar for discussion.
Contributor Information
Gurkirpal Singh, Email: gsingh@stanford.edu.
Olivia Wu, Email: o.wu@clinmed.gla.ac.uk.
Peter Langhorne, Email: P.langhorne@clinmed.gla.ac.uk.
Rajan Madhok, Email: gcl103@clinmed.gla.ac.uk.
References
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, Adenoma Prevention with Celecoxib (APC) Study Investigators Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1089. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- Maxwell SRJ, Webb DJ. COX-2 selective inhibitors: important lessons learnt. Lancet. 2005;365:449–451. doi: 10.1016/S0140-6736(05)17876-3. [DOI] [PubMed] [Google Scholar]
- Krotz F, Schiele TM, Klauss V, Sohn HY. Selective COX-2 inhibitors and risk of myocardial infarction. J Vasc Res. 2005;42:312–324. doi: 10.1159/000086459. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;330:1366. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2004;2:CD004079. doi: 10.1002/14651858.CD004079.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/S0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- Kmet LM, Lee RC, Cook LS. Standard Quality Assessment Criteria for Evaluating Primary Research Papers From a Variety of Fields. Alberta: Alberta Heritage Foundation for Medical Research; 2004. [Google Scholar]
- NHS Centre for Review and Dissemination . Undertaking Systematic Reviews of Research on Effectiveness: CRD's Guidance for Those Carrying Out or Commissioning Reviews. 2. York, UK: University of York; 2001. [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Strom BL. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142:157–164. doi: 10.7326/0003-4819-142-3-200502010-00005. [DOI] [PubMed] [Google Scholar]
- Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Storm BL. The effects of non-selective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol. 2004;43:985–990. doi: 10.1016/j.jacc.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Jick SS. The risk of gastrointestinal bleed, myocardial infarction, and newly diagnosed hypertension in users of meloxicam, diclofenac, naproxen and piroxicam. Pharmacotherapy. 2000;20:741–744. doi: 10.1592/phco.20.9.741.35209. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Rhodes T, Cai B, Guess HA. Lower risk of thromboembolic cardiovascular events with naproxen among patients with rheumatoid arthritis. Arch Intern Med. 2002;162:1105–1110. doi: 10.1001/archinte.162.10.1105. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Varas C, Patrono C. Differential effects of aspirin and non-aspirin nonsteroidal antiinflammatory drugs in the primary prevention of myocardial infarction in postmenopausal women. Epidemiology. 2000;11:382–387. doi: 10.1097/00001648-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Fischer L, Schlienger RG, Matter CM, Jick H, Meir CR. Current use of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction. Pharmacotherapy. 2005;25:503–510. doi: 10.1592/phco.25.4.503.61021. [DOI] [PubMed] [Google Scholar]
- Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, Gonzalez-Perez A. Non steroidal anti-inflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109:3000–3006. doi: 10.1161/01.CIR.0000132491.96623.04. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, Sorensen HT. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005;165:978–984. doi: 10.1001/archinte.165.9.978. [DOI] [PubMed] [Google Scholar]
- Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med. 2005;142:481–489. doi: 10.7326/0003-4819-142-7-200504050-00113. [DOI] [PubMed] [Google Scholar]
- Mamdani M, Rochon P, Juurlink DN, Anderson GM, Kopp A, Naglie G, Austin PC, Laupacis A. Effect of selective cyclooxygenase 2 inhibitors and naproxen on short-term risk of acute myocardial infarction in the elderly. Arch Intern Med. 2003;163:481–486. doi: 10.1001/archinte.163.4.481. [DOI] [PubMed] [Google Scholar]
- Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. Cox-2 selective non steroidal anti-inflammatory drugs and risk of coronary heart disease. Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non steroidal anti-inflammatory drugs and risk of serious coronary heart disease; an observational cohort. Lancet. 2002;359:118–123. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- Rhame E, Pilote L, LeLorier J. Association between naproxen use and protection against acute myocardial infarction. Arch Intern Med. 2002;162:1111–1115. doi: 10.1001/archinte.162.10.1111. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Jick H, Meier CR. Use of non steroidal anti-inflammatory drugs and the risk of first acute myocardial infarction. Br J Clin Pharmacol. 2002;54:327–332. doi: 10.1046/j.1365-2125.2002.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon DH, Glynn RJ, Levin R, Avorn J. Nonsteroidal anti-inflammatory drug use and acute myocardial infarction. Arch Intern Med. 2002;162:1099–1104. doi: 10.1001/archinte.162.10.1099. [DOI] [PubMed] [Google Scholar]
- Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger ML. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–2029. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–158. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- Decision memo: analysis and recommendations for agency action – COX-2 selective and non-selective NSAIDs http://www.fda.gov/cder/drug/infopage/cox2/NSAIDdecisionmemo.pdf
- Langman MJ, Weil J, Wainwright P, Lawson DH, Rawlins MD, Logan RF, Murphy M, Vessey MP, Colin-Jones DG. Risks of bleeding peptic ulcer associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:1075–1078. doi: 10.1016/S0140-6736(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Jick H, Garcia Rodriguez LA, Perez-Gutthann S. Principles of epidemiological research on adverse and beneficial drug effects. Lancet. 1998;352:1767–1770. doi: 10.1016/S0140-6736(98)04350-5. [DOI] [PubMed] [Google Scholar]
- Cross design synthesis: a new strategy for studying medical outcomes? [editorial] Lancet. 1992;340:944–946. doi: 10.1016/0140-6736(92)92822-W. [DOI] [PubMed] [Google Scholar]
- Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock S, Elbourne DR. Randomized trials or observational tribulations. N Engl J Med. 2000;342:1907–1909. doi: 10.1056/NEJM200006223422511. [DOI] [PubMed] [Google Scholar]
- Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, et al. Gastrointestinal toxicity with celecoxib vs non-steroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study. A randomised controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- Briefing Package for NDA 21 389 http://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4090B1_31_AA-FDA-Tab-T.htm
- Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, et al. TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364:675–684. doi: 10.1016/S0140-6736(04)16894-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. Selective cyclooxygenase inhibitors [COX-2] inhibitors and potential risk of cardiovascular events. Biochem Pharmacol. 2002;63:817–821. doi: 10.1016/S0006-2952(02)00842-0. [DOI] [PubMed] [Google Scholar]
- Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AG, Nguyen TV, Day RO. Do non-steroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Intern Med. 1994;121:289–300. doi: 10.7326/0003-4819-121-4-199408150-00011. [DOI] [PubMed] [Google Scholar]
- ALLHAT Collaborative Research Group Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone. JAMA. 2000;283:1967–1975. doi: 10.1001/jama.283.15.1967. [DOI] [PubMed] [Google Scholar]
- Singh G, Miller JD, Huse DM, Pettitt D, D'Agostino RB, Russell MW. Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol. 2003;30:714–719. [PubMed] [Google Scholar]


