Summary
The expression of Cyp19, the key gene of estrogen biosynthesis, in granulosa cells (GC) is essential for follicular growth and coordination of the ovulatory process. The goal of this study was to examine the effect of PGE2 and PGF2α on Cyp19 expression in undifferentiated and luteinized GC (UGC and LGC). In UGC, PGE2 increased Cyp19 mRNA and Cyp19 protein levels whereas PGF2α had no effect. In LGC, PGF2α decreased Cyp19 expression whereas PGE2 had no effect. Gene-reporter experiments demonstrated that PGE2 increases Cyp19 transcription in UGC. A protein kinase A inhibitor blocked PGE2-induced increase in Cyp19 promoter activity. PGE2 increased GATA-4 binding to the Cyp19 promoter. Mutation of the GATA binding site resulted in the loss of PGE2 stimulation. This study demonstrates that PGE2 stimulates Cyp19 expression in rat GC and suggests that GATA-4 may mediate (at least in part) the stimulatory effect of PGE2.
Keywords: Prostaglandin E2, Prostaglandin F2α, granulosa cells, cyp19 gene, aromatase
Introduction
Aromatase is highly expressed in granulosa cells of preovulatory follicles. Aromatase expression and the consequent increase in estradiol production by granulosa cells are essential for follicular growth and coordination of the ovulatory process. The follicle-stimulating hormone (FSH) is the main stimulus for the expression of the aromatase-encoding gene Cyp19. Studies performed in transgenic mice carrying progressive deletions of the human Cyp19 proximal promoter demonstrated that as little as 278 bp of this promoter are sufficient to mediate Cyp19 expression in the ovary (Hinshelwood et al., 2000). The induction of Cyp19 expression by FSH is mediated by the cyclic AMP (cAMP) pathway (Richards et al., 2002). A cAMP-response element-like binding site (CLS) and two response elements for the 5A family of nuclear receptors are present in the Cyp19 proximal promoter.Both steroidogenic factor-1 (SF-1 or NR5A1) and liver receptor homolog-1 (LRH-1 or NR5A2) have been shown to regulate Cyp19 expression via these nuclear receptor response elements (NRE) (Carlone & Richards, 1997a, Hinshelwood et al., 2003, Michael et al., 1997). The proximal Cyp19 promoter contains, in addition to CLS and NREs, two binding elements for members of the GATA family of transcription factors (Jin et al., 2000, Stocco, 2004, Tremblay & Viger, 2001). We have recently reported that only one of these GATA sites is functional and that GATA-4 binds to this element (Kwintkiewicz et al., 2006, Stocco, 2004).
In the ovary, PGE2 and PGF2α are key regulators of processes such as ovulation, luteinization, and luteolysis (Challis, 1997). Expression of cyclooxygenase-2 (COX-2), the rate-limiting step in prostaglandin synthesis, is induced by the LH surge in granulosa cells of preovulatory follicles (Sirois et al., 1992). A reduced number of ovulations is observed in animals that either lack COX-2 (Lim et al., 1997) or that received COX-2 inhibitors (Mikuni et al., 1998). Because PGE2 administration to COX-2 deficient mice restores the number of ova released, PGE2 appears to be the main COX-2 product involved in ovulation (Davis et al., 1999). PGE2 effects are mediated by four subtypes of receptors designated: EP1, EP2, EP3 and EP4. Of these receptors, EP2 and EP4 are expressed in granulosa and luteal cells (Narko et al., 2001, Segi et al., 2003). The EP2 receptor knockout mice have a severe deficiency in cumulus expansion, decreased ovulation, and reduced fertilization (Hizaki et al., 1999) suggesting an important role for PGE2 in the regulation of cumulus granulosa cells. Recent evidence suggests that PGE2 also controls the function of mural granulosa cells. For instance, GDF-9 stimulation of progesterone production by mouse mural granulosa cells requires PGE2 synthesis (Elvin et al., 2000). Furthermore, in vivo administration of EP4 agonists induces follicle development to a degree comparable to that induced by PMSG (El-Nefiawy et al., 2005a, El-Nefiawy et al., 2005b). Immunohistochemical studies indicate that EP4 receptors localize in cumulus and mural granulosa cells of secondary and preovulatory follicles but not in primordial or primary follicles (El-Nefiawy et al., 2005a). The findings that EP4 receptor knockout mice die perinatally (Nguyen et al., 1997), have precluded in vivo studies to determine the role played by PGE2/EP4 in the folliculogenesis process in vivo.
Whereas PGE2 is essential for folliculogenesis and ovulation, PGF2α is involved in the regulation of the corpus luteum, more specifically in the process of luteolysis. In mice, the absence of PGF2α receptors causes a failure in parturition due to the lack of luteal regression (Sugimoto et al., 1997). Interestingly, both PGE2 and PGF2α are implicated in the regulation of Cyp19 expression. We have recently demonstrated that PGF2α decreases Cyp19 expression in vitro in luteinized granulosa cells as well as in vivo in the corpus luteum of d-19 pregnant rats (Stocco, 2004). PGE2, in contrast, is thought to be responsible for the augmented expression of the Cyp19 gene in breast tumor adipose tissue (Zhao et al., 1996b) and in endometriosis-derived stromal cells (Noble et al., 1997). PGE2 has been shown to stimulate estradiol production in ovarian cells (Schreiber et al., 1981). The effect of PGE2 on the expression of the Cyp19 gene in granulosa and luteal cells has not been explored yet. The aim of this study was to investigate the effects of PGE2 and PGF2α on Cyp19 expression in undifferentiated and luteinized granulosa cells and to examine the intracellular mechanisms mediating PGE2 actions.
Materials and Methods
Cells Cultures:
For primary granulosa cell (GC) cultures, female Sprague-Dawley rats were obtained at 23-25 days of age (Charles River Laboratories, Inc., Wilmington, MA) and maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The following protocols were approved by the Yale Animal Resources Center. Undifferentiated GCs were obtained from rats treated subcutaneously with estradiol (1.5 mg/day) for three days, whereas luteinized GCs were obtained from rats treated with pregnant mare gonadotropin (15 IU) for 48 hours followed by treatment with hCG (15 IU) for 6 hours. In both cases, ovaries were trimmed to remove the bursa, fat, and oviducts and incubated for 15-30 min at 37°C in 6 mM EDTA in Dulbecco’s modified Eagle media/Ham’s F-12 media (DMEM/F-12). Ovaries were then incubated for 5-20 min in 0.6 M sucrose in DMEM/F-12. GCs were expressed by penetration of follicles with a 30-gauge needle. Cells were plated on laminin-coated 24-well or 12-well plates at a density of 5x104 cells/dish and 9x104 cells/well, respectively, in DMEM/F-12 serum-free medium supplemented with insulin (10 mg/ml), transferrin (5.5 mg/ml), selenium (5 mg/ml), BSA (0.5 mg/ml), penicillin G (100 units/ml), streptomycin (100 μg/ml), and Amphotericin B (250 ng/ml). Cells were treated approximately 24 h after plating.
Cloning of the rat cyp19 proximal promoter region:
The promoter region Cyp19 gene was cloned from rat genomic DNA using the following primers: forward — GCT CGA GCC ACA GAG ATC CTG ACA ACC; reverse prime — GAA GCT TTG TGG TAT TTT GCC TCA GAA GG. These primers amplify the region between -1100 to +63 of the aromatase gene, where +1 is the transcription initiation site (Fitzpatrick & Richards, 1993). Primers were designed based on a published sequence of the rat aromatase Cyp19 promoter (Young & McPhaul, 1998). No entry for this sequence was found in Genbank. PCR products were cloned into the pGL3 Basic luciferase report vector (Promega) by using XhoI and HindIII restriction sites. This construct was named -1100Cyp19pr-luc. Deletions of the -1100Cyp19pr-luc construct were generated by PCR. The following constructs were obtained: - 600Cyp19pr-luc, -245Cyp19pr-luc, -150Cyp19pr-luc, and -70Cyp19pr-luc. All deletions were confirmed by bidirectional sequencing.
Mutagenesis:
The NRE A sequence AAGGTCA (-137/-131) was mutated to AAatTCA and the GATA binding site TGATAA (-128/-123) was mutated to TtcTAA. Both mutations were performed using a Quickchange site-directed mutagenesis kit (Stratagene). The 245Cyp19pr-luc plasmid was used as a template. Mutations were confirmed by sequencing.
RNA isolation and Real-time RT-PCR:
Granulosa cells and luteinized granulosa cells were treated with PGE2, PGF2α or forskolin for the time period and concentration indicated on each figure. Treatments were terminated by aspirating medium and rinsing cells with PBS. RNA isolation was performed using Trisol reagent (Invitrogen) following the manufacturer’s instructions. Total RNA was reverse transcribed using oligo-dT primers (Invitrogen). Quantification of Cyp19 and L19 mRNA levels was performed using real time PCR (RT-PCR), as previously described (Cai & Stocco, 2005, Stocco, 2004). The sequences of the PCR primers were as follows: Cyp19: CTG CTG ATC ATG GGC CTCC and CTC CAC AGG CTC GGG TTG TT; rat L19: CTG AAG GTC AAA GGG AAT GTG and GGA CAG AGT CTT GAT GA CTC. The ratio between copies per nanogram of total RNA of Cyp19 and L19 is reported in each figure.
Transient transfection and luciferase assay:
GCs were plated on 12-well plates for transient transfection experiments. Cells were transfected with luciferase reporter plasmids (200 ng/well) using FuGene 6 transfection reagent (Roche). Transcription efficiency was normalized by co-transfection of the pCMV-β-galactosidase expression vector (20 ng/well) (Promega). Lysates were prepared using 100 μl of passive lysis buffer (Promega). Luciferase activity was determined in 50 μL of lysate/sample using the Luciferase Reporter Assay (Promega) and a TD 20/20 luminometer (Turner Designs). β-galactosidase activity was determined in 10 μl of lysate/sample using the β-galactosidase Assay System (Promega) according to the manufacturer’s protocol. Results are expressed as relative luciferase units normalized to β-galactosidase activity.
Electrophoresis mobility shift assay:
Nuclear protein extracts were prepared by extracting nuclei with buffer C (0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM dithiothreitol, 25% (v/v) glycerol, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 1 μg/ml aprotinin, pH 7.9).Protein concentration was determined using the BCA assay (Pierce). Complementary oligonucleotides spanning the regions: -164 to -143 (CLS), -145 to -125 (NRE B), -128 to -116 (GATA) and -84 to -71 (NRE A) were annealed and end-labeled by T4 kinase and [γ-32P]ATP. Numbers indicate location in relation to the transcription initiation site +1 (Fitzpatrick & Richards, 1993). Nuclear extracts (5 μg) were incubated for 20 min at room temperature in binding buffer (20 mM HEPES, pH 7.6; 60 mM KCl, 0.01 mM ZnSO4, 0.1 mM EDTA, 0.035 mM BSA, 1 mM dithiothreitol, 6% glycerol (v/v)) in the presence of 1 μg of salmon sperm DNA and 50,000 cpm of radiolabeled double-stranded oligonucleotides. Supershift assays were performed by adding an anti-GATA-4 antibody (Cruz biotechnology, catalog number sc-1237) 15 minutes before the addition of labeled probe. Following incubation, protein-DNA complexes were resolved by electrophoresis in 6% nondenaturing acrylamide gels and 0.5X TBE buffer at 300 V, 4°C for 2 hours. For quantification of band intensity, appropriate film exposures were scanned and the density of bands determined with ImageJ (NIH). Results are expressed as fold increase versus control.
Western blotting:
Undifferentiated granulosa cells were homogenized in ice-cold lysis buffer (10 mM Tris-Cl, pH 8.0; 150 mM NaCl, 1% Nonidet p-40, 0.5% sodium deoxycholate, 0.1% SDS, 40 μM PMSF, 0.3 μM aprotinin, and 1 μM leupeptin). This was followed by 30-min incubation on ice and centrifugation at 10,000 x g for 20 min at 4°C. Protein concentration was determined by the BCA assay (Pierce). Samples were denatured by adding sample 5 x buffer (62.5 mM Tris-HCL, pH6.8; 2% SDS, 10% glycerol, 0.01% bromophenol blue), followed by boiling for 10 minutes. Thirty micrograms of protein were separated on 10% SDS-PAGE gels in Tris-glycine, 0.1% SDS buffer, and transferred to nitrocellulose paper in 25mM Tris, 192 mM glycine, and 20% methanol buffer at 250 mA for 1.5 h. Blots were incubated for 2 hours at room temperature in 5% non-fat dry milk in Tris-buffered saline buffer containing Tween-20 (TBS-T), followed by incubation overnight at 4°C with monoclonal mouse anti human cytochrome p450 aromatase antibody (Serotech, UK) at a 1/4000 dilution. Blots were washed and incubated with a goat anti mouse IgG (Serotech, UK) conjugated to horseradish peroxidase (1/6000 dilution) in TBS-T plus 5% milk for 2 hours at room temperature. Protein-antibody complexes were visualized using Western Blotting Luminol Reagent following the manufacturer’s protocol (Santa Cruz Biotechnology).
Statistical analysis:
Luciferase activity values and relative Cyp19 mRNA levels showed a normal Gaussian distribution. Results are expressed as means ± S.E.M., and significances were determined by using student’s t-test for two group comparison or ANOVA followed by Tukey test for multiple group comparison. p values < 0.05 were taken as a significant difference.
Results
Effect of PGE2 and PGF2α on Cyp19 expression in undifferentiated granulosa cells
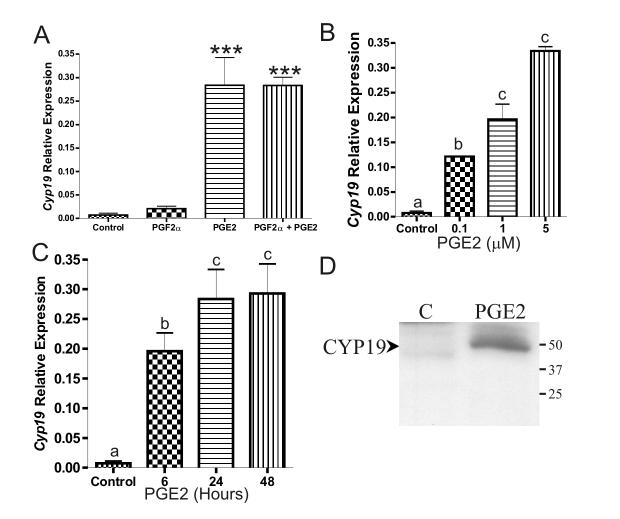
Undifferentiated granulosa cells were treated with PGF2α (1 μM), PGE2 (1 μM) or PGF2α plus PGE2 for 12 hours. Quantification of Cyp19 and L19 mRNA was performed using a quantitative RT-PCR method. The ratio between copies per nanogram of Cyp19 and that of L19 are shown. Treatment with PGF2α had no effect on Cyp19 expression (Figure 1A). In contrast, PGE2 induced a 60-fold increase in the expression of this gene. The increase of Cyp19 expression observed in the presence of PGE2 was not affected by cotreatment with PGF2α.
Figure 1.
PGE2 increases aromatase mRNA and protein levels in undifferentiated granulosa cells. A, Undifferentiated granulosa cells where treated with 1 μM PGF2α, PGE2 or both compounds for 12 hours. B, Treatment of granulosa cells with increasing concentrations of PGE2 for 8 hours. C, Treatment with 1 μM PGE2 for 6, 24 or 48 hours. cyp19 and L19 mRNA levels were quantified using real time PCR as detailed in materials and methods. Data are expressed as the ratio between the number of copies per nanogram of total RNA of cyp19 and L19. In A: *** p< 0.001 when compared to control and PGF2α (ANOVA I - Tukey test). In B and C: columns with different letters differ significantly (ANOVA I - Tukey test). All graphs represent the average ± standard error of four to six experiments. D, Western Blot analyses for CYP19 protein on granulosa cells treated with vehicle (C) or PGE2 (1 μM for 24 hours).
Over a period of eight hours, treatment of granulosa cells with increasing concentrations of PGE2 increased Cyp19 mRNA levels in a concentration-dependent manner (Figure 1B). Treatment with 1 μM of PGE2 significantly increased Cyp19 expression after 6 and 24 hours of treatment. Conversely, treatment for 48 hours caused no further increase in Cyp19 expression (Figure 1C). CYP19 protein was undetectable in non-treated undifferentiated granulosa cells (Figure 1D, lane 1), whereas this protein was readily detectable in cells treated with PGE2 (1 μM) for 24 hours (Figure 1D, lane 2).
Effect of PGE2 and PGF2α on Cyp19 expression in LG cells
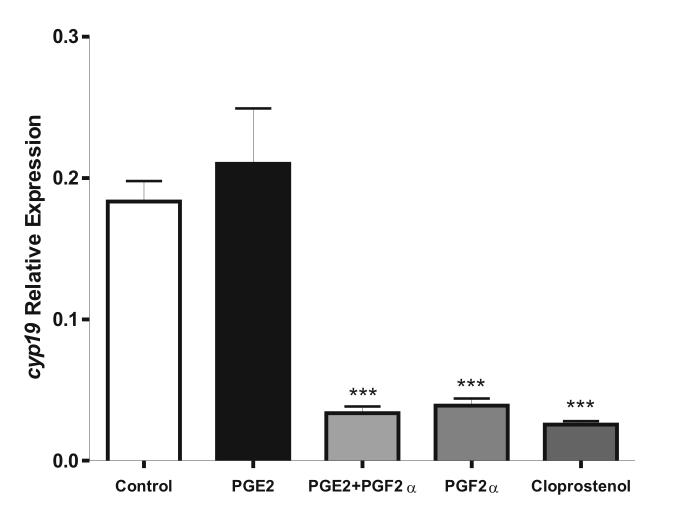
PGF2α inhibits Cyp19 expression in luteinized granulosa cells (Stocco, 2004). Because PGE2 and PGF2α have opposite effects on the regulation of CL physiology (Fitz et al., 1984), the effect of PGE2 on Cyp19 expression in luteinized granulosa cells was examined. In these cells, PGE2 had no effect on Cyp19 expression (Figure 2). As expected, PGF2α receptor activation by PGF2α or cloprostenol, a PGF2α analog, significantly reduced Cyp19 expression in LG cells. Cyp19 mRNA levels in LG cells cultured in the presence of PGE2 and PGF2α was no different from the expression levels found in LG cells cultured in the presence of PGF2α alone.
Figure 2.
PGE2 does not affect cyp19 expression in luteinized granulosa cells. Luteinized granulosa cells were treated with PGE2 (1 μM), PGF2α (1 μM), PGF2α plus PGE2 (both 1 μM) or cloprostenol (100 nM) for 8 hours. cyp19 mRNA levels were determined as in figure 1. Bars represent mean ± SEM of three independent experiments each one performed in duplicate. *** p<0.001 when compared to vehicle or PGE2 treated cells (ANOVA I - Tukey test).
Effect of PGE2 on Cyp19 promoter activity in undifferentiated GC
Next, by studying the effect of PGE2 on the activity of the Cyp19 promoter, we examined whether changes in Cyp19 mRNA levels are due to an alteration in the transcription rate of the Cyp19 gene. Undifferentiated granulosa cells were transfected with a luciferase reporter construct containing the region -1100 bp to +63 bp of the Cyp19 gene. The activity of the -1100Cyp19pr-luc construct was increased 9- to 10-fold when granulosa cells were cultured in the presence of 1 μM PGE2 for 8 hours (Figure 3A). Deletion of the aromatase promoter to -245 did not affect PGE2 induction. A marked decrease in PGE2 stimulation was observed upon deletion of the promoter region downstream of the -245 bp position (Figure 3A). Nonetheless, a significant increase in luciferase activity was still observed after PGE2 treatment in cells transfected with the -150Cyp19pr-luc construct. PGE2 had no effect on the activity of the -70Cyp19pr-luc construct or the empty vector pGL3. Treatment of granulosa cells with increasing concentrations of PGE2 for 6 hours caused a concentration-dependent increase in the activity of the -600Cyp19pr-luc reporter construct (Figure 3B) and the - 245Cyp19pr-luc reporter construct (data not shown).
Figure 3.
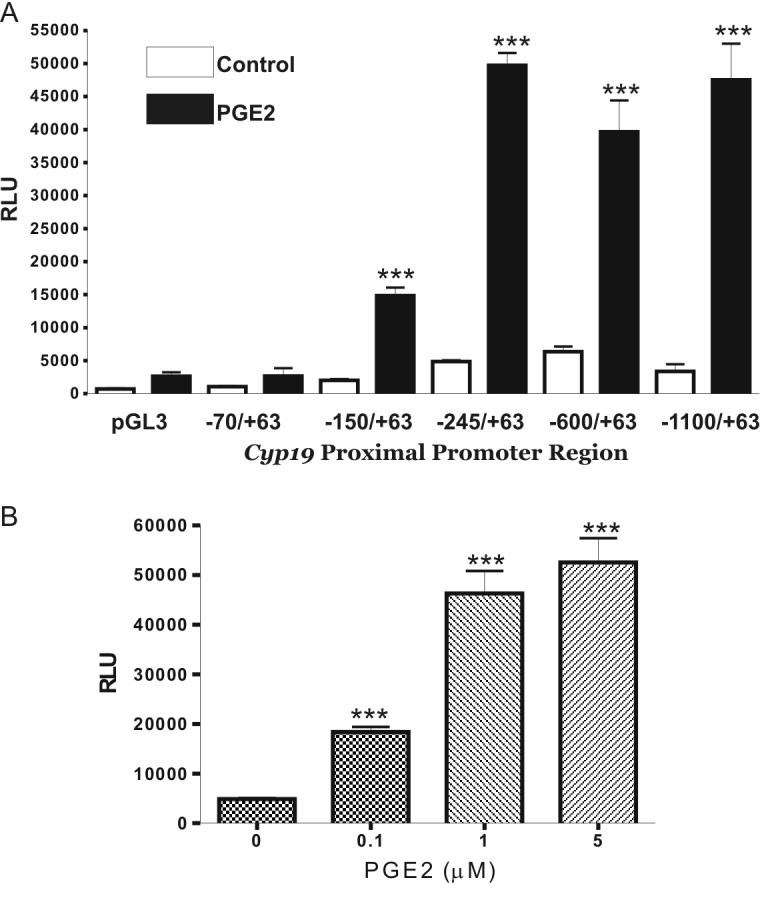
PGE2 increases cyp19 promoter activity; Effect of 5’ deletions. A, Undifferentiated granulosa cells were transfected with cyp19 promoter reporter-constructs containing 5′-serial deletions. Twenty-four hours later, cells were treated with PGE2 (1 μM) or vehicle for 8 hours. B, Cells were transfected with the -245cyp19pr-luc promoter construct and 24 hours later treated with either vehicle or increasing concentrations of PGE2 for 8 hours. Transient expression of the reporter gene was quantified by a standard luciferase bioluminescence assay and normalized against β-galactosidase. Bars represent mean ± SEM of 3 independent experiments each one performed in triplicate. *** p<0.001 vs. cells treated with vehicle (ANOVA I - Tukey test).
Participation of the cAMP/PKA signaling pathway in the induction of Cyp19 expression by PGE2
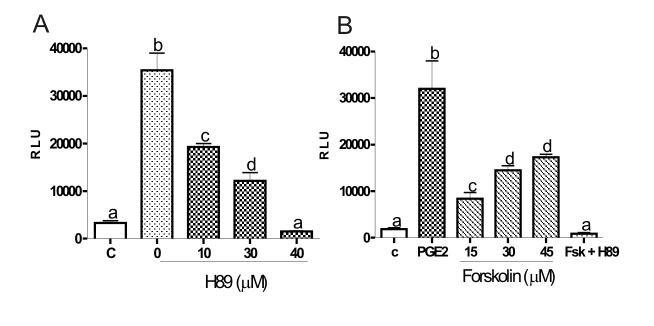
PGE2 type two and four receptors are coupled to Gs-type heterotrimeric guanine nucleotide-binding protein (G protein), the activation of which leads to increased production of cAMP (Narumiyaet al., 1999). The participation of the cAMP/PKA pathway in the stimulation of Cyp19 expression by PGE2 was studied using H89, an inhibitor of PKA. The effect of forskolin, an activator of the adenylcyclase enzyme, on Cyp19 promoter activity has been extensively studied (Fitzpatrick & Richards, 1993, Michael et al., 1997). We thought to compare the effect of forskolin and PGE2 on the activity of the Cyp19 promoter. Undifferentiated granulosa cells transfected with the -245Cyp19pr-luc promoter construct were treated with increasing concentrations of H89 (10, 30 and 40 μM) for one hour prior to the addition of PGE2 (1 μM) to the medium. Cells were harvested 8 hours after initiating PGE2 treatment. As illustrated in Figure 4A, H89 pretreatment prevented the increase in Cyp19 promoter activity induced by PGE2 in a concentration-dependent manner.
Figure 4.
Participation of protein kinase A on the stimulatory effect of PGE2. Granulosa cells were transfected with the -245cyp19pr-luc promoter construct and 24 hours later treated with: A, vehicle or increasing concentration of H89 for one hour prior to treatment with PGE2 (1 μM, 8 hours) or B, vehicle, PGE2, increasing concentrations of forskolin or with 30 μM forskolin plus H89 10 μM for 8 hours. H89 was also added one hour before addition of PGE2 or forskolin to the medium. Bars represent mean ± SEM of 3 independent experiments each one performed in quadruplicate. Columns with different letters differ significantly a-b (p<0.001), a-c (p<0.01), c-d and a-d (p<0.05).
Cells transfected with the -245Cyp19pr-luc promoter were also treated with increasing concentrations of forskolin for 8 hours. As expected, direct activation of the adenylate cyclase enzyme by treatment with foskolin (15 or 30 μM) increased Cyp19 promoter activity in a concentration-dependent manner. No further increase in Cyp19 promoter activity was observed when cells were treated with a higher concentration of forskolin (45 μM). Cotreatment of granulosa cells with forskolin (30 μM) and H89 (10 μM) completely blocked the increase in luciferase activity observed in the presence of forskolin alone (Figure 4B). The activity of the Cyp19 promoter was significantly higher in cells treated with PGE2 (1 μM) than in cells treated with forskolin (30 μM) (Figure 4B).
Effect of PGE2 on nuclear protein binding to the Cyp19 promoter region
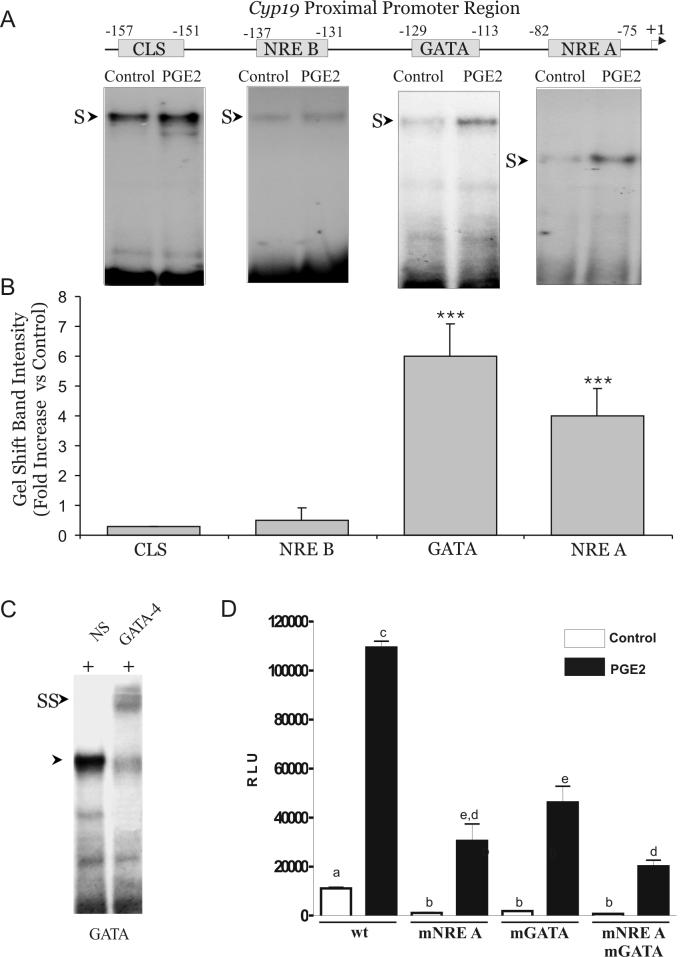
Thus far, our results suggest that PGE2 stimulates Cyp19 expression, at least in part, via the cAMP/PKA signaling pathway and that the minimal and necessary elements for PGE2 stimulation are located between the -245 bp and -70 bp regions of the Cyp19 promoter. As mentioned in the introduction, this region contains several elements that are regulated by cAMP. These elements are: a cAMP-responsive-element-like binding site (CLS), two nuclear receptor elements (NRE), and a GATA binding site (Figure 5A). Using gel shift assays, the ability of PGE2 to affect the occupancy of these binding sites was examined. As illustrated in the left panel of Figure 5A, strong binding was observed when the CLS probe was mixed with nuclear extracts of either control or PGE2 treated cells. A weak and specific binding was observed with the NRE B probe. Binding to CLS and NRE B were not affected by PGE2. Little or no binding to NRE A and GATA was detected in the control groups (Figure 5A right panels). PGE2 markedly increased binding to these two binding sites. Addition of excess unlabeled NRE A or GATA probes completely prevented the formation of their respective shifted bands, whereas 100-x excess of mutated unlabeled probes did not block their formation (data not shown). When compared to the binding observed in control groups, PGE2 increased protein binding to the GATA and the NRE A sites 6- and 4-fold respectively (Figure 5B).
Figure 5.
Effect of PGE2 on nuclear protein binding to the proximal promoter of the cyp19 gene. A, Top: Diagrammatic representation of the proximal promoter region of the rat cyp19 gene depicting location and transcription factor binding sites. Bottom: Gel shift analysis was performed using nuclear extract obtained from undifferentiated granulosa cells cultured in the presence or absence of PGE2 (1 μM) for 8 hours. This experiment was repeated four times with similar results. B, Densitometric quantification of experiments in A presented as fold increase versus control (*** p<0.001, One Sample t test). C, Supershifted analysis was performed by adding to the reaction an antibody against GATA-4. As control, a non-specific serum (NS) was added. S: bandshift; SS: supershifted bands. These experiments were repeated three times with similar results. D, Undifferentiated granulosa cells were transfected with the -245cyp19pr-LUC or the same construct carrying a mutation on the GATA site (mGATA), on the NRE A site (mNRE A) or a double mutation (mNRE A/mGATA). Thirty-six hours later, cells were treated with PGE2 (1 μM) or vehicle for 8 hours. Transient expression of the reporter gene was quantified by a standard luciferase bioluminescence assay and normalized against β-galactosidase. Bars represent mean ± SEM of 3 independent experiments each one performed in triplicate. Columns with different letters differ significantly: a-c (p<0.001), a-b (p<0.05); all other differences are p<0.01.
Addition of an antibody that recognizes the C-terminal region of the GATA-4 protein supershifted the complex formed with the GATA probe (Figure 5C). To investigate whether the NRE A and/or the GATA response elements participate in the stimulation of Cyp19 promoter activity by PGE2, these two regions were mutated separately in the -245Cyp19pr-luc construct. A double mutant construct was also produced. Mutation of either GATA or NRE A significantly reduced the basal activity of the -245Cyp19pr-luc promoter construct and decreased its stimulation by PGE2 (Figure 5D). The same effect was observed when the double mutant construct was used. PGE2 stimulation was significantly lower (p<0.05) in promoter constructs carrying a double mutation (mNRE A/mGATA) than in constructs containing a mutation in the GATA site (mGATA) only. A random mutation of this promoter had no effect on either the basal or the PGE2-stimulated activity of this promoter (data not shown).
Discussion
The aromatase encoding gene, Cyp19, is highly expressed in granulosa cells of preovulatory follicles. We show in this report that PGE2 stimulates the expression of this gene and increases the amount of aromatase protein in rat granulosa cells. The evidence also suggests that this effect of PGE2 is mediated by activation of the cAMP/PKA pathway and in part by the transcription factor GATA-4. Activation of EP4 receptors induces follicle development to a degree comparable to that induced by FSH (El-Nefiawy et al., 2005a, El-Nefiawy et al., 2005b). Our results support these findings and suggest that PGE2 may also contribute to the increase in Cyp19 expression that takes place during the differentiation of granulosa cells to the preovulatory state.
Our promoter deletion analysis showed that in rats the 245-bp region located upstream of the transcription initiation site (+1) is sufficient to mediate PGE2 stimulation. This finding is in good agreement with previous reports showing that as little as 278 bp of the human Cyp19 gene are sufficient to mediate its expression in the ovary (Hinshelwood et al., 2000). This region also mediates the increase in Cyp19 promoter activity induced by forskolin (Fitzpatrick & Richards, 1993, Michael et al., 1997). A CRE-like site (CLS), two NRE sites (Carlone & Richards, 1997b, Hinshelwood et al., 2003) and, more recently, a GATA binding site (Jin et al., 2000, Stocco, 2004, Tremblay & Viger, 2001) have been implicated in the activation of the -245/+1 region. Gel shift experiments showed that binding of nuclear proteins to the CLS element is not affected by PGE2. Although this seems surprising in view of the importance that this region has on the activity of the Cyp19 promoter, our results agree with those of Carlone and Richards (Carlone & Richards, 1997a), which clearly show that there are no differences in CRE binding (CREB) protein binding to CLS between undifferentiated and preovulatory granulosa cells. However, CLS deletion greatly reduced the stimulation of Cyp19 promoter activity by PGE2 (present results) or cAMP (Carlone & Richards, 1997a). Phosphorylation of serine 133 on CREB enhances its transcriptional activity (Johannessen et al., 2004). EP2 and EP4 receptors activation leads to CREB phosphorylation on serine 133 (Fujino et al., 2005). Consequently, although no changes in CREB binding were observed, the transcriptional activity of CREB probably increases after PGE2 treatment.
Our results suggest that in addition to CLS, the NRE and the GATA response element also participate in the induction of Cyp19 promoter activity by PGE2. Thus, although deletion of CLS greatly decreased the PGE2-induced Cyp19 promoter activity, the activity of the CLS-less - 150Cyp19pr-luc construct is stimulated approximately 5-fold by PGE2. Mutation of the GATA or the NRE binding site present in this region reduces PGE2 stimulation. Moreover, PGE2 treatment increases protein binding to these sites. Members of the GATA family of transcription factors are emerging as critical players in mammalian reproductive development and function. Two members of the GATA family, GATA-4 and GATA-6, are expressed in the vertebrate ovary. In mice, humans, and pigs, GATA-4 mRNA and protein are abundant in the granulosa cell layer of all healthy follicles (Gillio-Meina et al., 2003, Heikinheimo et al., 1997, Laitinen et al., 2000). GATA-4 transactivates the promoters for the murine Mϋllerian inhibiting substance, aromatase, steroidogenic acute regulatory (StAR) protein, or inhibin-α genes when cotransfected in a kidney cell line (Tremblay & Viger, 2001). We recently reported that GATA-4 participates in the induction of Cyp19 expression by FSH in rat granulosa cells (Kwintkiewicz et al., 2006). PGE2 stimulates binding of GATA-4 to the Cyp19 promoter and mutation of the GATA binding site prevents the stimulation of Cyp19 promoter activity by this prostaglandin, suggesting that GATA-4 is also involved in the induction of Cyp19 expression by PGE2 in granulosa cells.
Whereas only CREB binds to CLS (Fitzpatrick & Richards, 1994), several transcription factors have the potential to bind to NRE A. SF-1 was the first transcription factor proposed to bind the NRE site present in the Cyp19 promoter (Lynch et al., 1993). Recently, it was shown that aromatase co-expresses in the ovary along with LRH-1 (Hinshelwood et al., 2003, Liu et al., 2003, Mendelson et al., 2005). LRH-1, like aromatase, is selectively expressed in granulosa cells of rat and mouse ovaries, and contrary to SF-1, it is not present in theca or interstitial cells (Falender et al., 2003, Hinshelwoodet al., 2003, Mendelson et al., 2005). Transient transfection experiments have shown that the activity of the aromatase promoter is induced by LRH-1 in preadipocyte cells (Clyne et al., 2002, Hinshelwood et al., 2003). Whether SF-1 or LRH-1 is the main factor involved in the induction of aromatase expression in granulosa cells is still under debate. Independent of which protein binds to the NRE A site, both LRH-1 and SF-1 have been shown to physically interact with GATA-4 to synergistically activate the 3βHSD2 promoter (Martin et al., 2005) and the MIS promoter (Tremblay & Viger, 1999) suggesting that interactions between GATA-4 and LRH-1/SF-1 may also be important in the regulation of Cyp19 expression in granulosa cells.
Recently, Hinshelwood et al. described a second NRE, NRE B. The authors demonstrated that this region is able to bind in vitro transcribed SF-1 or LRH-1 proteins and that this response element is necessary for the stimulation of the human Cyp19 promoter by forskolin in bovine granulosa cells (Hinshelwood et al., 2003). We found that this region binds to nuclear proteins; however, this binding was not affected by PGE2. Further studies are needed to determine the role of NRE B on Cyp19 expression.
The effects of forskolin, an activator of adenylate cyclase, on Cyp19 promoter activity have been studied extensively (Carlone & Richards, 1997b, 1997a, Fitzpatrick & Richards, 1994, Orly et al., 1996). As expected, forskolin increased the activity of the Cyp19 promoter; however, PGE2-stimulated promoter activity was significantly higher than that observed in the presence of the maximal stimulatory dose of forskolin (30μM). Cotreatment with forskolin and H89 (10 μM) completely inhibited the forskolin effect. However, this concentration of H89 only partially prevented PGE2 stimulation. Higher concentration of H89 in the medium (40 μM) effectively inhibited the effect of PGE2. The possibility that H89 affects other signaling pathways cannot be ruled out. H89 inhibits Rho kinases at concentrations that are slightly higher than those needed to inhibit PKA (Leemhuis et al., 2002). Whether PGE2 activates this kinase in granulosa cells is unknown. It is also possible that treatment with PGE2 resulted in stronger PKA activation than treatment with foskolin. However, this is unlikely because forskolin is a powerful activator of adenylate cyclase (Seamon et al., 1981) and induces high levels of CREB phosphorylation in granulosa cells (Carlone & Richards, 1997a). These findings suggest that PGE2 stimulation of the Cyp19 gene may involve other mechanisms besides activation of PKA.
PGE2 and PGF2α have opposite effects on the regulation of the CL function. Whereas PGE2 increases progesterone production (Vijayakumar & Walters, 1987, Weems et al., 1985), PGF2α rapidly decreases the luteal secretion of this steroid (Behrman et al., 1976, Behrman et al., 1979). In undifferentiated granulosa cells, PGE2 increased Cyp19 expression, but PGF2α had no effect on the expression of this gene. This is not surprising since PGF2α receptor mRNA is low in theca and granulosa cells but is expressed at high levels in the corpus luteum (Anderson et al., 2001, Hasumoto et al., 1997). Conversely, although luteinized granulosa cells express EP receptors (Narko et al., 2001, Segi et al., 2003), PGE2 have no effect on Cyp19 expression in these cells; whereas, PGF2α and cloprostenol, an agonist of the PGF2α receptor, decrease Cyp19 expression. In luteinized granulosa cells, forskolin has no effect on the expression of Cyp19 (Gonzalez-Robayna et al., 1999) or on the activity of the aromatase promoter (Orly et al., 1996). These results illustrate the cell-specific actions of PGE2 and PGF2α.
Aromatase is expressed in pathophysiological conditions such as breast cancer and endometriosis. In those tissues, PGE2 increases the expression of aromatase by stimulating the proximal promoter of the Cyp19 gene (Noble et al., 1997, Zhao et al., 1996a). This study demonstrates that PGE2 also stimulates aromatase expression in ovarian cells. An ovarian granulosa-like tumor cell line expresses aromatase (Nishi et al., 2001). Whether PGE2 is involved in the regulation of the expression of Cyp19 in granulosa cancer cells remains to be determined.
Acknowledgments
This work was supported by the National Institutes of Health grant 047427 and in part by Tuner Designs through its luminometer program.
References
- Anderson LE, Wu YL, Tsai SJ, Wiltbank MC. Prostaglandin F2α receptor in the corpus luteum: recent information on the gene, messenger ribonucleic acid, and protein. Biol Reprod. 2001;64:1041–1047. doi: 10.1095/biolreprod64.4.1041. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Grinwich DL, Hichens M. Studies on the mechanism of PGF2α and gonadotropin interactions on LH receptor function in corpora lutea during luteolysis. Adv Prostaglandin Thromboxane Res. 1976;2:655–666. [PubMed] [Google Scholar]
- Behrman HR, Luborsky-Moore JL, Pang CY, Wright K, Dorflinger LJ. Mechanisms of PGF2α action in functional luteolysis. Adv Exp Med Biol. 1979;112:557–575. [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Carlone DL, Richards JS. Functional interactions, phosphorylation, and levels of 3′,5′-cyclic adenosine monophosphate-regulatory element binding protein and steroidogenic factor-1 mediate hormone-regulated and constitutive expression of aromatase in gonadal cells. Mol Endocrinol. 1997a;11:292–304. doi: 10.1210/mend.11.3.9900. [DOI] [PubMed] [Google Scholar]
- Carlone DL, Richards JS. Evidence that functional interactions of CREB and SF-1 mediate hormone regulated expression of the aromatase gene in granulosa cells and constitutive expression in R2C cells. J Steroid Biochem Mol Biol. 1997b;61:223–231. [PubMed] [Google Scholar]
- Challis JR. Prostaglandins and reproduction--what do knockouts really tell us? Nat Med. 1997;3:1326–1327. doi: 10.1038/nm1297-1326. [DOI] [PubMed] [Google Scholar]
- Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem. 2002;277:20591–20597. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology. 1999;140:2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- El-Nefiawy N, Abdel-Hakim K, Kanayama N. The selective prostaglandin EP4 agonist, APS-999 Na, induces follicular growth and maturation in the rat ovary. Eur J Endocrinol. 2005a;152:315–323. doi: 10.1530/eje.1.01837. [DOI] [PubMed] [Google Scholar]
- El-Nefiawy N, Abdel-Hakim K, Kanayama N, Terao T. Role of prostaglandin E2 receptor subtypes in ovarian follicle growth in the rat in vivo. Correlation with interleukin-8 and neutrophils. Histol Histopathol. 2005b;20:825–831. doi: 10.14670/HH-20.825. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan C, Matzuk MM. Growth differentiation factor-9 stimulates progesterone synthesis in granulosa cells via a prostaglandin E2/EP2 receptor pathway. Proc Natl Acad Sci U S A. 2000;97:10288–10293. doi: 10.1073/pnas.180295197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144:3598–3610. doi: 10.1210/en.2002-0137. [DOI] [PubMed] [Google Scholar]
- Fitz TA, Mock EJ, Mayan MH, Niswender GD. Interactions of prostaglandins with subpopulations of ovine luteal cells. II. Inhibitory effects of PGF2α and protection by PGE2. Prostaglandins. 1984;28:127–138. doi: 10.1016/0090-6980(84)90120-5. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS. cis-acting elements of the rat aromatase promoter required for cyclic adenosine 3′,5′-monophosphate induction in ovarian granulosa cells and constitutive expression in R2C Leydig cells. Mol Endocrinol. 1993;7:341–354. doi: 10.1210/mend.7.3.8387157. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS. Identification of a cyclic adenosine 3′,5′-monophosphate-response element in the rat aromatase promoter that is required for transcriptional activation in rat granulosa cells and R2C leydig cells. Mol Endocrinol. 1994;8:1309–1319. doi: 10.1210/mend.8.10.7854348. [DOI] [PubMed] [Google Scholar]
- Fujino H, Salvi S, Regan JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol Pharmacol. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- Gillio-Meina C, Hui YY, LaVoie HA. GATA-4 and GATA-6 Transcription Factors: Expression, Immunohistochemical Localization, and Possible Function in the Porcine Ovary. Biol Reprod. 2003;68:412–422. doi: 10.1095/biolreprod.102.009092. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Alliston TN, Buse P, Firestone GL, Richards JS. Functional and subcellular changes in the A-kinase-signaling pathway: relation to aromatase and Sgk expression during the transition of granulosa cells to luteal cells. Mol Endocrinol. 1999;13:1318–1337. doi: 10.1210/mend.13.8.0334. [DOI] [PubMed] [Google Scholar]
- Hasumoto K, Sugimoto Y, Yamasaki A, Morimoto K, Kakizuka A, Negishi M, Ichikawa A. Association of expression of mRNA encoding the PGF2α receptor with luteal cell apoptosis in ovaries of pseudopregnant mice. J Reprod Fertil. 1997;109:45–51. doi: 10.1530/jrf.0.1090045. [DOI] [PubMed] [Google Scholar]
- Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138:3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- Hinshelwood MM, Smith ME, Murry BA, Mendelson CR. A 278 bp region just upstream of the human CYP19 (aromatase) gene mediates ovary-specific expression in transgenic mice. Endocrinology. 2000;141:2050–2053. doi: 10.1210/endo.141.6.7611. [DOI] [PubMed] [Google Scholar]
- Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207:39–45. doi: 10.1016/s0303-7207(03)00257-0. [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Zhang X, Li H, Goss PE. Characterization of a novel silencer element in the human aromatase gene PII promoter. Breast Cancer Res Treat. 2000;62:151–159. doi: 10.1023/a:1006481228794. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211–1227. doi: 10.1016/j.cellsig.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Cai Z, Stocco C. GATA-4 participates in the regulation of cyp19 expression in granulosa cells; The EndocrineSociety’s 88th Annual Meeting, Boston USA (Abstract)..2006. [Google Scholar]
- Laitinen MP, Anttonen M, Ketola I, Wilson DB, Ritvos O, Butzow R, Heikinheimo M. Transcription factors GATA-4 and GATA-6 and a GATA family cofactor, FOG-2, are expressed in human ovary and sex cord-derived ovarian tumors. J Clin Endocrinol Metab. 2000;85:3476–3483. doi: 10.1210/jcem.85.9.6828. [DOI] [PubMed] [Google Scholar]
- Leemhuis J, Boutillier S, Schmidt G, Meyer DK. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J Pharmacol Exp Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- Liu DL, Liu WZ, Li QL, Wang HM, Qian D, Treuter E, Zhu C. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol Reprod. 2003;69:508–517. doi: 10.1095/biolreprod.102.011767. [DOI] [PubMed] [Google Scholar]
- Lynch JP, Lala DS, Peluso JJ, Luo W, Parker KL, White BA. Steroidogenic factor 1, an orphan nuclear receptor, regulates the expression of the rat aromatase gene in gonadal tissues. Mol Endocrinol. 1993;7:776–786. doi: 10.1210/mend.7.6.8395654. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA factors and the nuclear receptors, steroidogenic factor 1/liver receptor homolog 1, are key mutual partners in the regulation of the human 3β-hydroxysteroid dehydrogenase type 2 promoter. Mol Endocrinol. 2005;19:2358–2370. doi: 10.1210/me.2004-0257. [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Jiang B, Shelton JM, Richardson JA, Hinshelwood MM. Transcriptional regulation of aromatase in placenta and ovary. J Steroid Biochem Mol Biol. 2005;95:25–33. doi: 10.1016/j.jsbmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Michael MD, Michael LF, Simpson ER. A CRE-like sequence that binds CREB and contributes to cAMP-dependent regulation of the proximal promoter of the human aromatase P450 (CYP19) gene. Mol Cell Endocrinol. 1997;134:147–156. doi: 10.1016/s0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- Mikuni M, Pall M, Peterson CM, Peterson CA, Hellberg P, Brannstrom M, Richards JS, Hedin L. The selective prostaglandin endoperoxide synthase-2 inhibitor, NS-398,reduces prostaglandin production and ovulation in vivo and in vitro in the rat. Biol Reprod. 1998;59:1077–1083. doi: 10.1095/biolreprod59.5.1077. [DOI] [PubMed] [Google Scholar]
- Narko K, Saukkonen K, Ketola I, Butzow R, Heikinheimo M, Ristimaki A. Regulated expression of prostaglandin E(2) receptors EP2 and EP4 in human ovarian granulosa-luteal cells. J Clin Endocrinol Metab. 2001;86:1765–1768. doi: 10.1210/jcem.86.4.7535. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Camenisch T, Snouwaert JN, Hicks E, Coffman TM, Anderson PA, Malouf NN, Koller BH. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/endo.142.1.7862. [DOI] [PubMed] [Google Scholar]
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, Agarwal VR, Zhao Y, Carr BR, Bulun SE. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- Orly J, Clemens JW, Singer O, Richards JS. Effects of hormones and protein kinase inhibitors on expression of steroidogenic enzyme promoters in electroporated primary rat granulosa cells. Biol Reprod. 1996;54:208–218. doi: 10.1095/biolreprod54.1.208. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Schreiber JR, Nakamura K, Erickson GF. Progestins inhibit FSH-stimulated granulosa estrogen production at a post-cAMP site. Mol Cell Endocrinol. 1981;21:161–170. doi: 10.1016/0303-7207(81)90053-8. [DOI] [PubMed] [Google Scholar]
- Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi E, Haraguchi K, Sugimoto Y, Tsuji M, Tsunekawa H, Tamba S, Tsuboi K, Tanaka S, Ichikawa A. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol Reprod. 2003;68:804–811. doi: 10.1095/biolreprod.102.003590. [DOI] [PubMed] [Google Scholar]
- Sirois J, Simmons DL, Richards JS. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J Biol Chem. 1992;267:11586–11592. [PubMed] [Google Scholar]
- Stocco C. In Vivo and In Vitro Inhibition of cyp19 Gene Expression by Prostaglandin F2α in Murine Luteal Cells: Implication of GATA-4. Endocrinology. 2004;145:4957–4966. doi: 10.1210/en.2004-0625. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683. doi: 10.1126/science.277.5326.681. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol. 1999;13:1388–1401. doi: 10.1210/mend.13.8.0330. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142:977–986. doi: 10.1210/endo.142.3.7995. [DOI] [PubMed] [Google Scholar]
- Vijayakumar R, Walters WA. Ovarian stromal and luteal tissue prostaglandins, 17β-estradiol, and progesterone in relation to the phases of the menstrual cycle in women. Am J Obstet Gynecol. 1987;156:947–951. doi: 10.1016/0002-9378(87)90363-2. [DOI] [PubMed] [Google Scholar]
- Weems CW, Reynolds LP, Huie JM, Hoyer GL, Behrman HR. Effects of prostaglandin E1 or E2 (PGE1; PGE2) on luteal function and binding of luteinizing hormone in nonpregnant ewes. Prostaglandins. 1985;29:161–173. doi: 10.1016/0090-6980(85)90199-6. [DOI] [PubMed] [Google Scholar]
- Young M, McPhaul MJ. A steroidogenic factor-1-binding site and cyclic adenosine 3′,5′-monophosphate response element-like elements are required for the activity of the rat aromatase promoter in rat Leydig tumor cell lines. Endocrinology. 1998;139:5082–5093. doi: 10.1210/endo.139.12.6377. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996a;137:5739–5742. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. Tumor necrosis factor-α stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol. 1996b;10:1350–1357. doi: 10.1210/mend.10.11.8923461. [DOI] [PubMed] [Google Scholar]