Abstract
Introduction
It has been suggested that hormonal risk factors act predominantly on estrogen receptor and progesterone receptor (ER/PR)-positive breast cancers. However, the data have been inconsistent, especially in younger women.
Methods
We evaluated the impact of age at menarche, pregnancy history, duration of breastfeeding, body mass index, combined oral contraceptive use, and alcohol consumption on breast cancer risk by ER/PR status in 1,725 population-based case patients and 440 control subjects aged 20 to 49 years identified within neighborhoods of case patients. We used multivariable unconditional logistic regression methods to conduct case–control comparisons overall as well as by ER/PR status of the cases, and to compare ER+PR+ with ER-PR- case patients.
Results
The number of full-term pregnancies was inversely associated with the risk of ER+PR+ breast cancer (ptrend = 0.005), whereas recent average alcohol consumption was associated with an increased risk of ER+PR+ breast cancer (ptrend = 0.03). Neither of these two factors was associated with the risk of ER- PR- breast cancer. Late age at menarche and a longer duration of breastfeeding were both associated with decreased breast cancer risk, irrespective of receptor status (all ptrend≤ 0.03).
Conclusion
Our results suggest that the number of full-term pregnancies and recent alcohol consumption affect breast cancer risk in younger women predominantly through estrogen and progesterone mediated by their respective receptors. Late age at menarche and breastfeeding may act through different hormonal mechanisms.
Introduction
It has been well documented that estrogen and progesterone are important in breast tumorigenesis [1-3], and their effects on the breast are mediated by their respective receptors, the estrogen receptor (ER) and the progesterone receptor (PR) [4-7]. Furthermore, it has been hypothesized that hormone-related risk factors that reflect exposure to estrogen and progesterone may be predominantly associated with breast tumors that express ER and PR, but not with those lacking ER and PR expression [8-14]. Several epidemiological studies have examined this hypothesis by ER and PR status separately or jointly [15-17], and a review from 2004 [17] concluded that early age at menarche, nulliparity, and delayed childbearing were associated with an increased risk for receptor-positive breast cancer, but not with receptor-negative breast cancer. However, in the prospective data from the Nurses' Health Study [18], the adverse effect of nulliparity was confined to ER+PR+ breast cancer, but early age at menarche was associated with an increased risk of both ER+PR+ and ER- PR- breast cancer and the adverse effect of delayed childbearing was observed for ER- PR- but not ER+PR+ breast cancer. Results from studies of young women under the age of 50 years [19-21] or premenopausal women [12,22] are even less consistent. To help shed light on the issue, we evaluated hormone-related risk factors for breast cancer by receptor subtypes (ER+PR+ and ER- PR-) in a large study of women aged 20 to 49 years, using both case–control and case–case comparisons.
Materials and methods
Case patients
Case patients were identified through the Los Angeles County Cancer Surveillance Program (CSP), the population-based cancer registry that is part of the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) cancer registry program. Eligible case patients were US-born English-speaking, white (including Hispanic) or African-American, female residents of Los Angeles County aged 20 to 49 years when diagnosed with histologically confirmed first primary invasive breast cancer. We identified 2,882 eligible case patients (2,534 white and 348 African-American). White patients were diagnosed between February 1998 and May 2003 and African-American patients were diagnosed between January 2000 and May 2003. We were unable to interview 1,088 of the 2,882 eligible case patients (38%) because of patient refusal (n = 428), no longer living in Los Angeles County (n = 37), inability to be located (n = 88), death (n = 38), serious illness or disability (n = 18), physician refusal (n = 50), or inability to schedule the interview within 18 months of diagnosis (n = 429). We successfully interviewed 1,794 (62%) eligible case patients (1,585 white, 209 African-American).
Control subjects
Because this study was originally designed as a case–case study to examine genetic risk factors for breast cancer, we did not collect control subjects for all case patients. The control subjects who were recruited were matched by race and age (within 5 years and aged 20 to 49 years) to a subset of case patients who were diagnosed between July 2000 and March 2003. Control subjects were US-born English-speaking white or African-American women who had never been diagnosed with invasive or in situ breast cancer. They were identified by using a neighborhood walk algorithm that we had used in previous case–control studies [23,24]. Field staff conducted walks according to a predefined pattern in the neighborhoods where case patients lived at the time of their diagnoses. The houses on the immediate blocks surrounding the home of the case patients were excluded from the walk. Residences were visited sequentially and information on potentially eligible women was obtained. If no one was home at the time of the visit, we left a request for information at the door, and we sought further information from neighbors so that we could contact the residents later. If we received no response to our written request, we sent additional letters until we were able to determine whether an eligible woman lived at the address in question. Detailed records were maintained to determine the number of housing units contacted in order to identify and interview a control subject. By the end of the study we had identified 603 eligible control subjects for the 1,108 case patients (1,018 white, 90 African-American). We were unable to interview 159 of the 603 control subjects (26%) as a result of subject refusal (n = 77), no longer living in Los Angeles County (n = 18), death (n = 1), serious illness (n = 2), or inability to schedule the interview within 18 months from the date of initial household contact (n = 61). We successfully interviewed 444 (74%) of eligible control subjects (409 white, 35 African-American). On average, 20 houses were canvassed to find an eligible control subject who agreed to be interviewed.
Data collection
All participants were interviewed in person with the use of a structured questionnaire, which was a modified version of the questionnaire used for the Women's Contraceptive and Reproductive Experiences (CARE) Study [25]. Our questionnaire included reproductive history (including breastfeeding), detailed histories of oral contraceptive use and alcohol consumption, family breast cancer history, demographics, and other factors. Information was recorded up to a predetermined reference date for each participant. The reference date was the date of diagnosis for case patients and the date of initial household contact for control subjects. All participants signed informed consent documents before interview. The study protocol was approved by the federally approved Institutional Review Board at the University of Southern California Medical Center.
Information on ER and PR status for interviewed case patients was obtained from the CSP. Among the 1,794 case patients, 1,510 (84%) had information on both ER and PR status; 91 of these were reported as weakly or borderline positive (84 cases) or undecided (7 cases) for either ER or PR. Among the other 1,419 case patients, 881 (62%) were ER+PR+, 92 (6%) were ER+PR-, 41 (3%) were ER- PR+, and 405 (29%) were ER- PR- .
Data analyses
We compared demographic and hormone-related risk factors among case patients with known ER and PR information, borderline positive or undecided results, and patients without ER or PR information, with the use of F tests for differences in means and Pearson χ2 tests for differences in frequency distributions. When the two-sided p value comparing all three groups was less than 0.05, we also performed pairwise comparisons by using Bonferroni t tests or Pearson χ2 tests, imposing a Bonferroni correction to the p value, restricting the overall type I error to 5% by setting as statistically significant only two-sided p values less than 0.017 for each pairwise comparison [26].
Analyses were conducted to assess the association between breast cancer and the following factors: age at menarche, number of full-term (greater than 26-week gestation) pregnancies, age at first full-term pregnancy (defined for each woman as the age at which that pregnancy ended), duration of breastfeeding, body mass index (BMI, kg/m2) one year before the participant's reference date, duration of combined oral contraceptive (COC) use, alcohol drinking status during reference age (never, former, and current – that is, drinking alcohol during reference age), and the average number of alcoholic drinks per week in the 5-year period that ended 2 years before the reference age. One alcoholic drink was defined as 12 ounces (355 ml) of beer, 4 ounces (118 ml) of wine, or 1.5 ounces (44 ml) of liquor.
We conducted case–control comparisons for overall, ER+PR+, and ER- PR- case patients with control subjects, and also compared ER+PR+ with ER- PR- case patients. We used polytomous logistic regression [27] to compare ER+PR+ and ER- PR- case patients simultaneously with control subjects. We used a multivariable unconditional logistic regression approach [27] for the comparisons of all case patients with controls, and ER+PR+ with ER- PR- case patients. We estimated multivariable adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Tests for trend were conducted by fitting ordinal values corresponding to categories of exposure in our models and testing whether the coefficient (slope of the dose response) differed from zero.
Adjustment was made for race (white, African-American), age (less than 30, 30 to 34, 35 to 39, 40 to 44, 45 to 49 years), and education (high school or lower, technical school or some college, college graduate) in all our models. Additionally, multivariable models included variables selected a priori as potential confounders: first-degree family history of breast cancer (no first-degree family history, mother or sister with breast cancer, unknown first-degree family history), age at menarche (11 or less, 12, 13, at least 14 years), gravidity (never pregnant, ever pregnant), number of full-term pregnancies (never full-term pregnant, one, two, three, at least four full-term pregnancies), BMI 1 year before reference date (less than 25, 25 to 29, 30 to 34, at least 35 kg/m2), COC use (never, less than 1, 1 to 4, 5 to 9, at least 10 years), the average number of alcoholic drinks per week in the recent 5 years (never, less than 3, 3 to 5, 6 to 11, at least 12 drinks, drinking alcohol but not within the 5 years of interest), and a three-category variable combining menopausal status and hormone therapy use (premenopausal or – among postmenopausal women – never used hormone therapy, or had used estrogen therapy or estrogen plus progestin therapy). When estimating the effects of parity or restricting analyses to parous women, we did not include gravidity in our models. A single model was fitted to assess the joint effects of age at first full-term pregnancy (less than 22, 22 to 27, 28 to 31, at least 32 years) and breastfeeding duration (0, less than 1, 1 to 6, 7 to 23, at least 24 months) among parous women. All variables were included as categorical variables in the models. In reporting the results of trend tests, we considered a two-sided p value of less than 0.05 as statistically significant. All analyses were performed with the SAS statistical package (Version 9.0, SAS Institute, Cary, NC, USA).
To maintain a constant sample size for all analyses, we excluded 69 case patients and 4 control subjects for the following reasons: missing information on educational attainment (15 cases and 1 control), age at menarche (3 cases), parity (4 cases and 1 control), duration of breastfeeding (6 cases), BMI (4 cases), duration of COC use (17 cases and 1 control), recent alcohol consumption (14 cases); missing information on two or more of these factors (6 cases and 1 control). This resulted in 1,725 case patients and 440 control subjects available for the overall case–control analyses. Among the 1,725 case patients, 1,449 (84%) had information on both ER and PR status; 83 of these were reported as weakly, borderline positive or undecided for either ER or PR and were excluded from the analyses by receptor subtypes. Among the other 1,366 case patients, 63% were ER+PR+, 6% were ER+PR-, 3% were ER-PR+, and 28% were ER- PR- . The frequency distribution across receptor subtypes was similar to those reported by previous studies conducted within the SEER registries [21,28]. In addition, our numbers are very similar to those that we observed among the white Los Angeles cases in the Women's CARE Study. This may be the most appropriate comparison, because 88% of the cases in the present study were white. We excluded ER+PR- and ER- PR+ subtypes from the analyses by receptor status because they were rare. There were therefore 1,239 remaining for the analyses by ER and PR status (854 ER+PR+ and 385 ER- PR-).
As described above, our control subjects were identified through matching to a subset of the cases. An alternative to conditional logistic regression in matched studies with disproportionate numbers of cases and controls is to break the match and conduct unconditional logistic regression with detailed adjustment for the matching factors [29]. We conducted both detailed stratified analyses, with strata defined by race, age categories (five-year categories) and education as a proxy of socioeconomic status/neighborhood as well as standard unconditional logistic regression with adjustment for the same factors. Because the results remained the same, we chose to use multivariable unconditional logistic regression for all our analyses. We also repeated all the analyses with the subset of the cases used to identify the controls. Again, the results were essentially identical with the overall analyses; we therefore present results based on all case patients.
Results
Table 1 shows the distributions of demographic and hormone-related risk factors among case patients stratified by availability of ER/PR information and among control subjects. Age at breast cancer diagnosis (pF test = 0.0001), education level (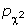 = 0.01), age at first full-term pregnancy (pF test = 0.002), and percentage of case patients who ever breastfed (
= 0.01), age at first full-term pregnancy (pF test = 0.002), and percentage of case patients who ever breastfed (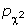 = 0.01) differed across the three case groups. Pairwise analyses revealed that the differences were between case patients with known ER/PR status and those without ER/PR information. In comparison with case patients without ER/PR information, case patients with known ER/PR status were on average 1.5 years younger at diagnosis (pt test <0.0001), better educated (
= 0.01) differed across the three case groups. Pairwise analyses revealed that the differences were between case patients with known ER/PR status and those without ER/PR information. In comparison with case patients without ER/PR information, case patients with known ER/PR status were on average 1.5 years younger at diagnosis (pt test <0.0001), better educated (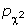 = 0.001), on average 1.8 years older at first full-term pregnancy (pt test = 0.001), and had a higher percentage of case patients who had breastfed (
= 0.001), on average 1.8 years older at first full-term pregnancy (pt test = 0.001), and had a higher percentage of case patients who had breastfed (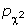 = 0.004).
= 0.004).
Table 1.
Demographic and hormone-related risk factors
| Variables | Cases by availability of ER/PR information | Controls | |||
| Cases with known receptor status | Cases with borderline positive/undecided status | Cases without information | p for comparison across the three case groups | ||
| Number of subjects | 1,366 (79.2%) | 83 (4.8%) | 276 (16.0%) | 440 | |
| Mean age at reference date (SD), years | 42.5 (5.4) | 42.8 (5.2) | 44.0 (4.6) | 0.0001a,b | 42.6 (4.9) |
| Race | |||||
| White | 1,206 (88.3%) | 77 (92.8%) | 238 (86.2%) | 405 (92.0%) | |
| African-American | 160 (11.7%) | 6 (7.2%) | 38 (13.8%) | 0.26c | 35 (8.0%) |
| Education | |||||
| High school or lower | 262 (19.2%) | 19 (22.9%) | 76 (27.5%) | 60 (13.6%) | |
| Technical school or some college | 489 (35.8%) | 28 (33.7%) | 103 (37.3%) | 150 (34.1%) | |
| College graduate | 615 (45.0%) | 36 (43.4%) | 97 (35.2%) | 0.01c,d | 230 (52.3%) |
| First-degree breast cancer family history | |||||
| No | 1,091 (79.9%) | 63 (75.9%) | 224 (81.1%) | 388 (88.2%) | |
| Yes | 229 (16.7%) | 19 (22.9%) | 46 (16.7%) | 41 (9.3%) | |
| Unknowne | 46 (3.4%) | 1 (1.2%) | 6 (2.2%) | 0.39c | 11 (2.5%) |
| Mean age at menarche (SD), years | 12.4 (1.5) | 12.6 (1.7) | 12.4 (1.4) | 0.51a | 12.7 (1.5) |
| Ever had a full-term (>26-week) pregnancy | 66.6% | 74.7% | 68.5% | 0.28c | 71.6% |
| Mean number of full-term pregnancies (SD) | 2.1 (1.0) | 2.1 (1.1) | 2.1 (0.9) | 0.92a | 2.2 (1.1) |
| Mean age at first full-term pregnancy (SD) | 26.7 (6.4) | 26.2 (6.2) | 24.9 (5.8) | 0.002a,b | 27.1 (6.2) |
| Ever breastfed among parous women | 80.4% | 77.4% | 70.9% | 0.01c,d | 87.9% |
| Mean duration of breastfeeding (all pregnancies) among those who breastfed (SD), months | 14.2 (16.3) | 13.7 (13.6) | 12.6 (14.6) | 0.56a | 16.6 (17.3) |
| Mean BMI 1 year before reference date (SD), kg/m2 | 25.8 (6.1) | 26.2 (6.4) | 26.6 (6.5) | 0.12a | 25.7 (6.4) |
| Ever used COC | 85.9% | 84.3% | 85.9% | 0.92c | 89.3% |
| Mean duration of COC use among users (SD), years | 6.9 (6.3) | 5.8 (6.6) | 6.4 (6.4) | 0.23a | 6.7 (6.2) |
| Alcohol drinking status during reference age | |||||
| Never | 428 (31.3%) | 34 (41.0%) | 96 (37.8%) | 141 (32.1%) | |
| Former | 259 (19.0%) | 16 (19.3%) | 53 (19.2%) | 90 (20.5%) | |
| Current | 679 (49.7%) | 33 (39.7%) | 127 (46.0%) | 0.30c | 209 (47.5%) |
| Alcohol drinking status in recent 5-year period | |||||
| No | 428 (31.3%) | 34 (41.0%) | 96 (34.8%) | 141 (32.1%) | |
| Yes | 759 (55.6%) | 40 (48.2%) | 137 (49.6%) | 228 (51.8%) | |
| Yes, but not in the five year period | 179 (13.1%) | 9 (10.8%) | 43 (15.6%) | 0.17c | 71 (16.1%) |
| Mean number of alcoholic drinks per week in recent 5 years for drinkers (SD) | 6.6 (20.1) | 4.9 (6.1) | 6.0 (8.4) | 0.80a | 4.6 (9.1) |
Results are for 1,725 case patients and 440 control subjects. BMI, body mass index; COC, combined oral contraceptive; ER/PR, estrogen receptor and progesterone receptor; SD, standard deviation. aF test. bBonferroni t tests for pairwise comparisons: p values for cases with complete ER/PR information versus cases without ER/PR information ≤ 0.001, and p values for the other comparisons ≥ 0.20. cPearson χ2 test. dPearson χ2 tests for pairwise comparisons: p values for cases with complete ER/PR information versus cases without ER/PR information ≤ 0.004, and p values for the other comparisons ≥ 0.32. eUnknown category was excluded from Pearson χ2 tests.
Age at menarche
Age at menarche was negatively associated with breast cancer risk regardless of ER/PR status (all ptrend≤ 0.008; Table 2). In comparison with women who had menarche before the age of 12 years, later age at menarche (at least 14 years) was associated with an approximately 40% reduced risk of breast cancer among the ER+PR+ case patients, ER- PR- case patients, and all case patients combined. The associations with age at menarche did not differ between ER+PR+ and ER- PR- case patients (ptrend = 0.85).
Table 2.
Adjusted ORs for breast cancer associated with hormone-related risk factors by ER/PR status
| Variables | No. controls | All cases versus controls | ER+PR+ cases versus controls | ER- PR- cases versus controls | ER+PR+ cases versus ER- PR- cases | |||
| Number of cases | OR (95% CI) | Number of cases | OR (95% CI) | Number of cases | OR (95% CI) | OR (95% CI) | ||
| Age at menarche, years | ||||||||
| ≤ 11 | 90 | 400 | 1.00 | 188 | 1.00 | 92 | 1.00 | 1.00 |
| 12 | 109 | 532 | 1.13 (0.83–1.55) | 278 | 1.24 (0.88–1.75) | 119 | 1.18 (0.79–1.77) | 1.04 (0.74–1.47) |
| 13 | 121 | 475 | 0.88 (0.65–1.21) | 231 | 0.87 (0.62–1.24) | 110 | 0.94 (0.62–1.41) | 0.93 (0.65–1.33) |
| ≥ 14 | 120 | 318 | 0.61 (0.44–0.85) | 157 | 0.60 (0.42–0.86) | 64 | 0.59 (0.38–0.92) | 1.02 (0.67–1.53) |
| Trend p value | 0.0007 | 0.0006 | 0.008 | 0.85 | ||||
| Number of full-term pregnanciesa | ||||||||
| None | 125 | 564 | 1.00 | 301 | 1.00 | 113 | 1.00 | 1.00 |
| 1 | 85 | 347 | 0.91 (0.66–1.25) | 157 | 0.77 (0.55–1.10) | 82 | 1.06 (0.70–1.62) | 0.70 (0.49–1.02) |
| 2 | 139 | 492 | 0.81 (0.61–1.07) | 251 | 0.77 (0.57–1.06) | 106 | 0.95 (0.64–1.39) | 0.84 (0.60–1.18) |
| 3 | 56 | 224 | 0.81 (0.56–1.18) | 100 | 0.69 (0.45–1.04) | 54 | 1.06 (0.65–1.73) | 0.68 (0.44–1.05) |
| ≥ 4 | 35 | 98 | 0.49 (0.31–0.78) | 45 | 0.47 (0.28–0.80) | 30 | 0.72 (0.39–1.33) | 0.65 (0.37–1.15) |
| Trend p value | 0.008 | 0.005 | 0.50 | 0.09 | ||||
| Age at first full-term pregnancy for parous women, yearsa,b | ||||||||
| <22 | 68 | 316 | 1.00 | 126 | 1.00 | 96 | 1.00 | 1.00 |
| 22–27 | 89 | 333 | 1.07 (0.72–1.58) | 149 | 1.14 (0.73–1.78) | 81 | 0.92 (0.56–1.50) | 1.25 (0.81–1.94) |
| 28–31 | 78 | 265 | 1.03 (0.66–1.60) | 134 | 1.17 (0.71–1.93) | 57 | 0.82 (0.46–1.45) | 1.42 (0.85–2.37) |
| ≥ 32 | 80 | 247 | 0.92 (0.57–1.48) | 144 | 1.23 (0.72–2.10) | 38 | 0.56 (0.30–1.07) | 2.22 (1.24–3.98) |
| Trend p value | 0.65 | 0.49 | 0.08 | 0.009 | ||||
| Duration of breastfeeding for parous women, monthsa,b | ||||||||
| 0 | 38 | 247 | 1.00 | 102 | 1.00 | 63 | 1.00 | 1.00 |
| <1 | 23 | 144 | 0.99 (0.56–1.77) | 65 | 1.01 (0.53–1.90) | 38 | 1.19 (0.59–2.39) | 0.82 (0.47–1.44) |
| 1–6 | 80 | 273 | 0.58 (0.37–0.91) | 131 | 0.57 (0.34–0.94) | 68 | 0.72 (0.41–1.27) | 0.77 (0.47–1.26) |
| 7–23 | 110 | 322 | 0.52 (0.33–0.82) | 165 | 0.52 (0.31–0.87) | 63 | 0.55 (0.31–0.98) | 0.97 (0.59–1.61) |
| ≥ 24 | 64 | 175 | 0.51 (0.30–0.86) | 90 | 0.49 (0.27–0.87) | 40 | 0.62 (0.32–1.21) | 0.77 (0.42–1.39) |
| Trend p value | 0.001 | 0.002 | 0.03 | 0.63 | ||||
| BMI 1 year before reference date, kg/m2 | ||||||||
| <25 | 257 | 939 | 1.00 | 495 | 1.00 | 183 | 1.00 | 1.00 |
| 25–29 | 95 | 425 | 1.18 (0.89–1.55) | 209 | 1.11 (0.82–1.50) | 101 | 1.41 (0.99–2.02) | 0.81 (0.59–1.10) |
| 30–34 | 51 | 221 | 1.01 (0.70–1.44) | 94 | 0.88 (0.59–1.30) | 61 | 1.43 (0.91–2.23) | 0.60 (0.40–0.89) |
| ≥ 35 | 37 | 140 | 0.88 (0.58–1.34) | 56 | 0.69 (0.43–1.11) | 40 | 1.18 (0.70–2.01) | 0.61 (0.38–0.99) |
| Trend p value | 0.82 | 0.20 | 0.16 | 0.005 | ||||
| Duration of COC use, years | ||||||||
| Never | 47 | 244 | 1.00 | 128 | 1.00 | 47 | 1.00 | 1.00 |
| <1 | 73 | 295 | 0.78 (0.51–1.18) | 133 | 0.70 (0.44–1.12) | 59 | 0.76 (0.43–1.33) | 0.93 (0.57–1.52) |
| 1–4 | 108 | 429 | 0.80 (0.54–1.19) | 227 | 0.81 (0.53–1.25) | 90 | 0.87 (0.52–1.46) | 0.92 (0.59–1.43) |
| 5–9 | 115 | 362 | 0.62 (0.42–0.93) | 173 | 0.57 (0.37–0.88) | 90 | 0.82 (0.49–1.37) | 0.71 (0.45–1.12) |
| ≥ 10 | 97 | 395 | 0.84 (0.56–1.26) | 193 | 0.76 (0.49–1.18) | 99 | 1.27 (0.75–2.14) | 0.61 (0.39–0.96) |
| Trend p value | 0.30 | 0.16 | 0.18 | 0.008 | ||||
| Alcohol drinking status during reference agec | ||||||||
| Never | 141 | 558 | 1.00 | 251 | 1.00 | 138 | 1.00 | 1.00 |
| Former | 90 | 328 | 0.96 (0.71–1.31) | 162 | 1.02 (0.72–1.44) | 74 | 0.88 (0.59–1.33) | 1.14 (0.79–1.64) |
| Current | 209 | 839 | 1.05 (0.81–1.36) | 441 | 1.17 (0.88–1.55) | 173 | 0.93 (0.67–1.30) | 1.25 (0.93–1.68) |
| Average number of alcoholic drinks per week in recent 5 years | ||||||||
| Never | 141 | 558 | 1.00 | 251 | 1.00 | 138 | 1.00 | 1.00 |
| <3 | 130 | 481 | 1.01 (0.76–1.35) | 248 | 1.11 (0.81–1.53) | 101 | 0.89 (0.61–1.30) | 1.22 (0.87–1.71) |
| 3–5 | 49 | 171 | 0.93 (0.63–1.37) | 90 | 1.01 (0.66–1.54) | 34 | 0.76 (0.45–1.28) | 1.31 (0.81–2.11) |
| 6–11 | 32 | 157 | 1.16 (0.75–1.81) | 82 | 1.26 (0.78–2.03) | 34 | 1.06 (0.60–1.86) | 1.20 (0.74–1.94) |
| > 12 | 17 | 127 | 1.77 (1.01–3.08) | 68 | 2.10 (1.17–3.79) | 27 | 1.71 (0.87–3.38) | 1.24 (0.73–2.09) |
| Trend p value | 0.12 | 0.03 | 0.42 | 0.23 | ||||
| Drinking alcohol but not in the recent five years | 71 | 231 | 0.88 (0.63–1.22) | 115 | 0.92 (0.63–1.34) | 51 | 0.80 (0.51–1.26) | 1.14 (0.76–1.73) |
ORs are from multivariable unconditional logistic regression models with the adjustment for race, age, education, first-degree breast cancer family history, age at menarche, gravidity, number of full-term pregnancies, BMI 1 year before reference date, COC use, the average number of alcoholic drinks per week in the recent 5 years, a variable combining menopausal status and hormone therapy use except where otherwise noted. BMI, body mass index; COC, combined oral contraceptive; CI, confidence interval; ER, estrogen receptor; OR, odds ratio; PR, progesterone receptor. aModels did not include gravidity. bAge at first full-term pregnancy and duration of breastfeeding mutually adjusted for each other. cAlcohol drinking status during reference age replaced the number of alcoholic drinks per week in the recent 5 years in the models.
Parity
A protective effect of parity was confined to women with ER+PR+ breast cancer (Table 2). The OR of ER+PR+ breast cancer decreased with increasing number of full-term pregnancies (ptrend = 0.005). Parous women who had four or more full-term pregnancies had an approximately 50% reduction in the risk of ER+PR+ breast cancer compared with women who never had a full-term pregnancy. ER+PR+ case patients were less likely to have had many full-term pregnancies than ER- PR- case patients (ptrend = 0.09).
Age at first full-term pregnancy
A slight increase in risk for ER+PR+ breast cancer and a reduced risk for ER- PR- breast cancer was observed with increasing age at first full-term pregnancy, but none of the confidence limits for the risk estimates excluded 1.0 and no linear trend in risk was observed for either cancer type (ER+PR+, ptrend = 0.49; ER- PR-, ptrend = 0.08; Table 2). ER+PR+ case patients were more likely to have had a late first full-term pregnancy than ER-PR- case patients (ptrend = 0.009).
Breastfeeding
Duration of breastfeeding was negatively associated with breast cancer risk regardless of ER/PR status (all ptrend≤ 0.03; Table 2). In the case–case analysis, duration of breastfeeding was not associated with ER/PR status (ptrend = 0.63).
Body mass index 1 year before reference date
Increasing BMI was associated with a non-statistically significant decreasing risk of ER+PR+ breast cancer (ptrend = 0.20) but was not associated with ER- PR- breast cancer (Table 2). Moreover, among premenopausal women, increasing BMI was marginally statistically significantly associated with a decreasing risk of ER+PR+ breast cancer (ptrend = 0.08) but not ER-PR- breast cancer (ptrend = 0.54). In comparison with premenopausal women who had a low BMI (less than 25 kg/m2), the OR among premenopausal women in the highest BMI category (at least 35 kg/m2) was 0.58 (95% CI 0.34 to 1.00) for ER+PR+ breast cancer and 1.07 (95% CI 0.58 to 1.97) for ER- PR- breast cancer. ER+PR+ case patients were less likely to have had a higher BMI than ER- PR- case patients (ptrend = 0.005).
Combined oral contraceptive use
COC use was not associated with risk of either ER+PR+ or ER-PR- breast cancer (Table 2). Women who had used COC for 10 years or longer had a slightly higher OR of ER- PR- breast cancer (OR 1.27, 95% CI 0.75 to 2.14) but a lower OR of ER+PR+ breast cancer (OR 0.76, 95% CI 0.49 to 1.18) compared with never users. ER+PR+ case patients were less likely to have had longer duration of COC use than ER- PR- case patients (ptrend = 0.008).
Alcohol consumption
Alcohol drinking status during reference age was not associated with breast cancer risk (Table 2). However, the average number of alcoholic drinks per week in the recent 5 years was positively associated with ER+PR+ breast cancer (ptrend = 0.03), weakly associated with all types of cancer together (ptrend = 0.12), and not associated with ER- PR- breast cancer (ptrend = 0.42). Overall, ER+PR+ case patients seemed more likely to have drunk larger quantities of alcohol than ER- PR- case patients, but the difference was not statistically significant (ptrend = 0.23).
Discussion
Overall in this study of women under age 50 years, we found associations that differed by ER/PR status for the number of full-term pregnancies, recent alcohol consumption, and possibly age at first full-term pregnancy and BMI. Risk reductions associated with late age at menarche and long duration of breastfeeding did not differ by ER/PR status. COC use was not associated with breast cancer risk in this study.
The magnitude of the protective effect from multiple full-term pregnancies for ER+PR+ breast cancer in our study is similar to that observed in a previous study of young women [21], smaller than that observed in a study of premenopausal women (OR 0.44, 95% CI 0.26 to 0.75 of ER+PR+ cancer for women having three or more live births compared with nulliparous women) [22], and greater than that in two studies of women under 45 years of age that did not find any associations with either receptor subtype [19,20]. A greater number of full-term pregnancies may protect against receptor-positive breast cancer by causing the full differentiation of breast epithelium [30], thereby reducing cyclical morphological change in breast tissue during the menstrual cycles [31].
Our results on age at first full-term pregnancy are consistent with the bulk of the evidence that early age at first full-term pregnancy is not a strong protective factor in young women. This may be due to a transient increase in breast cancer risk after pregnancy [32]. However, if early age at first full-term pregnancy protects against any subtype of breast cancer, our results suggest that it would be the receptor-positive cancer.
Five previous studies have examined the effect of BMI according to ER/PR status among young [19,20] or premenopausal [12,22,33] women. Two found that, when compared with the lowest category of BMI, the highest category of BMI was associated with a 23 to 29% reduction in risk of ER+PR+ breast cancer but not ER- PR- breast cancer [19,22]. The other three studies found no statistically significant association with any receptor subtype [12,20,33]. Our data showed a 31% reduced risk of ER+PR+ breast cancer, which did not quite reach statistical significance. A possible mechanism that would explain a protective effect of BMI on receptor-positive tumors is that high BMI results in anovulatory menstrual cycles with reduced exposure to ovarian hormones [34,35].
Among five previous epidemiological studies that examined the effect of alcohol consumption according to ER/PR status among young women [19,36] or premenopausal women [12,22,37], two found a 10 to 38% increase in risk of ER+PR+ cancer, which was not statistically significant, for women at the highest category of alcohol consumption versus women who had never drunk alcohol [22,37]; one found an increase in risk of the ER+PR- subtype [36]; and another found a non-statistically significant increase in risk of both ER+PR+ and ER- PR- receptor subtypes [19]. Our effect estimate for alcohol consumption was higher than those reported previously, possibly because our women drinkers consumed greater amounts of alcohol. Experimental [38] and cross-sectional [39] data have shown that alcohol consumption may result in an increase in blood estrogen levels among premenopausal women. Our results are consistent with the hypothesis that alcohol exerts its effect on the premenopausal breast via estrogen.
Our finding that late age at menarche is associated with a reduced risk of both ER+PR+ and ER- PR- breast cancer is consistent with two [19,20] of four previous case–control studies conducted among women under 45 years [19,20] or premenopausal women [12,22]. The magnitude of the risk reduction in the two previous studies ranged from 20 to 40% for women with the oldest versus the youngest ages at menarche [19,20]. The protective effects of breastfeeding on both receptor-positive and receptor-negative cancers in our study were also observed in previous studies among young [19,21] or premenopausal [12] parous women, although the associations were not statistically significant in two of these studies [12,19]. The magnitude of the protective effects of breastfeeding was larger in our study than was reported from previous studies. Three [12,20,22] of four previous studies also found no association between OC use and ER+PR+ or ER-PR- breast cancer among young [19,20] or premenopausal women [12,22]; the only inconsistent study found a marginally statistically significant increased risk of ER- PR- cancer among women who had ever used oral contraceptives [19].
It has been argued [10,12] that if certain hormone-related factors predominantly act through estrogen and progesterone mediated by their respective receptors, then these hormone-related factors will be associated with hormone receptor-positive, but not with receptor-negative, breast cancer. Our findings that increasing number of full-term pregnancies is associated with lower risk of ER+PR+ cancer, whereas increasing recent average weekly alcohol consumption is associated with greater risk, and that ER- PR- cancers are not affected by these risk factors, support the hypothesis that these factors act predominantly through this type of hormonal mechanism.
In contrast, it could be hypothesized that hormonal factors should affect receptor-positive and receptor-negative breast cancer similarly. It has been hypothesized that an ER- stem cell population gives rise to ER+ progenitor cells [40], which will proliferate when exposed to estrogen, but can also send paracrine signals that will cause neighboring populations of ER- cells to proliferate. Thus, late age at menarche and breastfeeding may still act through hormonal mechanisms that involve ER and PR, but our results suggest that the exact mechanism differs from that involved in the effect of parity and alcohol consumption.
One strength of our study is the large number of case patients included. Our analysis included more young breast cancer patients than six of the eight previously published studies with results for ER/PR status among women under the age of 50 years [19-21,36] or among premenopausal women [12,22,33,37]. For the only two studies with a larger sample size, one focused exclusively on alcohol consumption [36] and the other focused on reproductive factors including parity, age at first full-term pregnancy, and breastfeeding [21].
Several limitations of this study must also be considered. The number of control subjects in this study was relatively small. This could explain why we detected a similar magnitude of effect for late first full-term pregnancy on ER+PR+ cancer as the Women's CARE Study [21], but in our study it did not reach statistical significance because of our limited statistical power. This could also explain why our case–case analyses suggested that the effect of late age at first full-term pregnancy and BMI differed significantly between the ER+PR+ and the ER- PR- case patients, whereas we did not detect any statistically significant associations when comparing each of the subtypes with the control subjects.
Because of our decision not to retain the case–control match during data analyses, we used an unconditional instead of a conditional logistic regression approach, which could have biased our relative risk estimates toward the null value, as described by Rothman and Greenland [29]. However, in comparison with the data from the Women's CARE Study [21], which has so far been the largest population-based case–control study of women aged 35 to 64 years, we found similar results for parity and breastfeeding, whereas the positive association between late first full-term pregnancy and ER+PR+ cancer was statistically significant in the Women's CARE Study but not in ours. In comparison with the prospective data from the Nurses' Health Study [18] for both premenopausal and postmenopausal women, our results for age at menarche and parity were consistent with theirs, but our results for late first full-term pregnancy were not. In addition, our overall findings for all the hormone-related factors we examined are quite similar to those in the literature for young or premenopausal women.
We have no data on the methods and cutoff points for receptor status used by each laboratory because we obtained this information from the CSP, which bases its classifications on information in pathology reports from a variety of laboratories. Although we assume that most laboratories used immunohistochemistry assays and consistent cutoff points, it is possible that some laboratories used different methods or different cutoff points. However, we believe that any such inconsistencies would be unlikely to cause the observed associations and, if anything, that they would bias our relative risk estimates toward the null value.
Another limitation is that in our analyses by receptor subtypes, we excluded 21% of our case patients, because 16% of patients had no ER or PR status information and 5% were borderline positives or undecided for either ER or PR. The percentage of case patients without information from the cancer registry in this study (16%) is similar to that reported by previous studies conducted within the SEER registries [21,28]. It is unclear why some case patients do not have a known ER/PR status. We observed that, in this study, the case patients with known ER/PR status information were somewhat younger, were better educated, gave birth later, and were more likely to breastfeed than those whose ER/PR status had not been determined. These differences between case patients with known ER/PR status and those without ER/PR information from the CSP could have biased our case–control comparison by receptor subtypes and caused us to find an effect of late age at first full-term pregnancy even if none existed, but would tend to underestimate any protective effect of breastfeeding on breast cancer risk. It is unlikely that this bias would be different for ER+PR+ and ER- PR- cancer. Furthermore, this would not have influenced our case–control analysis using all case patients combined. Because our results from the case–case and the case–control analysis by receptor subtypes or using all case patients combined were generally consistent, we think it unlikely that these issues caused important bias in this study.
Conclusion
Our results suggest that the number of full-term pregnancies and recent alcohol consumption affect breast cancer risk in young women predominantly through estrogen and progesterone mediated by their respective receptors. Late age at menarche and breastfeeding may protect against breast cancer through a different hormonal mechanism.
Abbreviations
BMI = body mass index; CARE = Contraceptive and Reproductive Experiences; CI = confidence interval; COC = combination oral contraceptive; CSP = Cancer Surveillance Program; ER = estrogen receptor; OR = odds ratio; PR = progesterone receptor; SEER = Surveillance, Epidemiology, and End Results.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HM cleaned the data, performed the data analysis, and drafted the manuscript. LB and RKR participated in the design of the study and revised the manuscript. GU designed the study, supervised the data collection and data analysis, and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
The authors are deeply grateful to the interviewers of this study and to Ms Juliana Bamrick for managing the data collection. This study was supported by grants CA17054 and CA74847 from the National Cancer Institute, National Institutes of Health, 4PB-0092 from the California Breast Cancer Research Program of the University of California, and in part through contract no. N01-PC-35139. The collection of cancer incidence data used in this publication was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885. The ideas and opinions expressed herein are those of the authors, and no endorsement by the State of California, Department of Health Services is intended or should be inferred.
Contributor Information
Huiyan Ma, Email: huiyanma@usc.edu.
Leslie Bernstein, Email: lbern@usc.edu.
Ronald K Ross, Email: ross_r@ccnt.hsc.usc.edu.
Giske Ursin, Email: gursin@usc.edu.
References
- Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42:3232–3239. [PubMed] [Google Scholar]
- Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- Bernstein L, Ross RK. Endogenous hormones and breast cancer risk. Epidemiol Rev. 1993;15:48–65. doi: 10.1093/oxfordjournals.epirev.a036116. [DOI] [PubMed] [Google Scholar]
- Gorski J, Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RB, Stancel GM. Estrogen receptor-mediated processes in normal and cancer cells. J Natl Cancer Inst Monogr. 2000;27:135–145. doi: 10.1093/oxfordjournals.jncimonographs.a024237. [DOI] [PubMed] [Google Scholar]
- Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4:197–201. doi: 10.1186/bcr452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood JM, Godolphin W. Oestrogen receptors in breast tumours: associations with age, menopausal status and epidemiological and clinical features in 735 patients. Br J Cancer. 1980;42:635–644. doi: 10.1038/bjc.1980.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth NG, Kelsey JL, Eisenfeld AJ, LiVolsi VA, Holford TR, Fischer DB. Differences in breast cancer risk factors according to the estrogen receptor level of the tumor. J Natl Cancer Inst. 1983;70:1027–1031. [PubMed] [Google Scholar]
- Potter JD, Cerhan JR, Sellers TA, McGovern PG, Drinkard C, Kushi LR, Folsom AR. Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women's Health Study: how many kinds of breast cancer are there? Cancer Epidemiol Biomarkers Prev. 1995;4:319–326. [PubMed] [Google Scholar]
- Yasui Y, Potter JD. The shape of age-incidence curves of female breast cancer by hormone-receptor status. Cancer Causes Control. 1999;10:431–437. doi: 10.1023/A:1008970121595. [DOI] [PubMed] [Google Scholar]
- Huang WY, Newman B, Millikan RC, Schell MJ, Hulka BS, Moorman PG. Hormone-related factors and risk of breast cancer in relation to estrogen receptor and progesterone receptor status. Am J Epidemiol. 2000;151:703–714. doi: 10.1093/oxfordjournals.aje.a010265. [DOI] [PubMed] [Google Scholar]
- Zhu K, Beiler J, Hunter S, Payne-Wilks K, Roland CL, Forbes DS, Chinchilli VM, Bernard LJ, Jacobsen KH, Levine RS. The relationship between menstrual factors and breast cancer according to estrogen receptor status of tumor: a case-control study in African-American women. Ethn Dis. 2002;12:S3–S23-9. [PubMed] [Google Scholar]
- Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21:28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- Stanford JL, Greenberg RS. Breast cancer incidence in young women by estrogen receptor status and race. Am J Public Health. 1989;79:71–73. doi: 10.2105/ajph.79.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel LA, Stanford JL. Hormone receptors and breast cancer. Epidemiol Rev. 1993;15:209–219. doi: 10.1093/oxfordjournals.epirev.a036107. [DOI] [PubMed] [Google Scholar]
- Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- Britton JA, Gammon MD, Schoenberg JB, Stanford JL, Coates RJ, Swanson CA, Potischman N, Malone KE, Brogan DJ, Daling JR, et al. Risk of breast cancer classified by joint estrogen receptor and progesterone receptor status among women 20–44 years of age. Am J Epidemiol. 2002;156:507–516. doi: 10.1093/aje/kwf065. [DOI] [PubMed] [Google Scholar]
- McCredie MR, Dite GS, Southey MC, Venter DJ, Giles GG, Hopper JL. Risk factors for breast cancer in young women by oestrogen receptor and progesterone receptor status. Br J Cancer. 2003;89:1661–1663. doi: 10.1038/sj.bjc.6601293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin G, Bernstein L, Lord S, Karim JR, Deapen D, Press MF, Daling JR, Norman SA, Liff JM, Marchbanks PA, et al. Reproductive factors and subtypes of breast cancer defined by hormone receptor and histology. Br J Cancer. 2005;93:364–371. doi: 10.1038/sj.bjc.6602712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterchio M, Kreiger N, Theis B, Sloan M, Bahl S. Hormonal factors and the risk of breast cancer according to estrogen- and progesterone-receptor subgroup. Cancer Epidemiol Biomarkers Prev. 2003;12:1053–1060. [PubMed] [Google Scholar]
- Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Cancer Inst. 1994;86:1403–1408. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- Ursin G, Peters RK, Henderson BE, d'Ablaing G, Monroe KR, Pike MC. Oral contraceptive use and adenocarcinoma of cervix. Lancet. 1994;344:1390–1394. doi: 10.1016/S0140-6736(94)90567-3. [DOI] [PubMed] [Google Scholar]
- Marchbanks PA, McDonald JA, Wilson HG, Burnett NM, Daling JR, Bernstein L, Malone KE, Strom BL, Norman SA, Weiss LK, et al. The NICHD Women's Contraceptive and Reproductive Experiences Study: methods and operational results. Ann Epidemiol. 2002;12:213–221. doi: 10.1016/S1047-2797(01)00274-5. [DOI] [PubMed] [Google Scholar]
- Pagano M, Gauvreau K. Principles of Biostatistics. 2. Pacific Grove, CA: Duxbury Press; 2000. [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: Wiley-Interscience; 2000. [Google Scholar]
- Chu KC, Anderson WF. Rates for breast cancer characteristics by estrogen and progesterone receptor status in the major racial/ethnic groups. Breast Cancer Res Treat. 2002;74:199–211. doi: 10.1023/A:1016361932220. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- Russo J, Rivera R, Russo IH. Influence of age and parity on the development of the human breast. Breast Cancer Res Treat. 1992;23:211–218. doi: 10.1007/BF01833517. [DOI] [PubMed] [Google Scholar]
- Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;27:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wuu J, Lambe M, Hsieh S-F, Ekbom A, Hsieh C-C. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13:299–305. doi: 10.1023/A:1015287208222. [DOI] [PubMed] [Google Scholar]
- Enger SM, Ross RK, Paganini-Hill A, Carpenter CL, Bernstein L. Body size, physical activity, and breast cancer hormone receptor status: results from two case-control studies. Cancer Epidemiol Biomarkers Prev. 2000;9:681–687. [PubMed] [Google Scholar]
- Pike MC. Reducing cancer risk in women through lifestyle-mediated changes in hormone levels. Cancer Detect Prev. 1990;14:595–607. [PubMed] [Google Scholar]
- Westhoff C, Gentile G, Lee J, Zacur H, Helbig D. Predictors of ovarian steroid secretion in reproductive-age women. Am J Epidemiol. 1996;144:381–388. doi: 10.1093/oxfordjournals.aje.a008939. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Mandel MG, Marchbanks PA, Folger SG, Daling JR, Ursin G, Simon MS, Bernstein L, Strom BL, Norman SA, et al. Alcohol exposure and breast cancer: results of the women's contraceptive and reproductive experiences study. Cancer Epidemiol Biomarkers Prev. 2004;13:2106–2116. [PubMed] [Google Scholar]
- Enger SM, Ross RK, Paganini-Hill A, Longnecker MP, Bernstein L. Alcohol consumption and breast cancer oestrogen and progesterone receptor status. Br J Cancer. 1999;79:1308–1314. doi: 10.1038/sj.bjc.6690210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Lukas SE, Mello NK, Amass L, Ellingboe J, Skupny A. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacology. 1988;94:464–467. doi: 10.1007/BF00212838. [DOI] [PubMed] [Google Scholar]
- Reichman ME, Judd JT, Longcope C, Schatzkin A, Clevidence BA, Nair PP, Campbell WS, Taylor PR. Effects of alcohol consumption on plasma and urinary hormone concentrations in premenopausal women. J Natl Cancer Inst. 1993;85:722–727. doi: 10.1093/jnci/85.9.722. [DOI] [PubMed] [Google Scholar]
- Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]


