Abstract
Tsetse fly (Diptera: Glossinidae) viviparous reproductive physiology remains to be explored at the molecular level. Adult females carry their young in utero for the duration of embryonic and larval development, all the while supplying their offspring with nutrients in the form of a “milk” substance secreted from a modified accessory gland. Flies give birth to fully developed third instar larvae that pupariate shortly after birth. Here, we describe the spatial and temporal expression dynamics of two reproduction-associated genes and their products synthesized during the first and second gonotrophic cycles. The proteins studied include a putative yolk protein, Glossina morsitans morsitans yolk protein 1 (GmmYP1) and the major protein found in tsetse “milk” secretions (Glossina morsitans morsitans milk gland protein, GmmMGP). Developmental stage and tissue-specific expression of GmmYP1 show its presence exclusively in the reproductive tract of the fly during oogenesis, suggesting that GmmYP1 acts as a vitellogenic protein. Transcripts for GmmMGP are present only in the milk gland tissue and increase in coordination with the process of larvigenesis. Similarly, GmmMGP can be detected at the onset of larvigenesis in the milk gland, and is present during the full duration of pregnancy. Expression of GmmMGP is restricted to the adult stage and is not detected in the immature developmental stages. These phenomena indicate that the protein is transferred from mother to larvae as nourishment during its development. These results demonstrate that both GmmYP1 and GmmMGP are involved in tsetse reproductive biology, the former associated with the process of oogenesis and the latter with larvigenesis.
Keywords: Viviparous reproduction, Oogenesis, Larvagenesis, Tsetse, Milk gland
1. Introduction
Sleeping sickness (African trypanosomiasis) is a deadly disease afflicting millions of people in sub-Saharan Africa. The effects of this disease are exacerbated by poor public health infrastructure, difficulty in diagnosis, lack of an effective vaccine and increasing levels of drug resistance by the parasites to the current treatments. Sleeping sickness is transmitted exclusively by tsetse flies (Glossina sp.). Control of this disease in the past has been achieved through reduction of the vector population. However, control efforts have weakened or disappeared and the disease has undergone a resurgence.
Tsetse flies are unique among insect disease vectors in that they are viviparous (bearing live young). The reproductive physiology of this fly has been investigated extensively; however, molecular biological aspects have not been studied. Among viviparous flies, tsetse is unique due to the extensive physiological (and morphological) modifications it has undergone to carry out this lifecycle. Tsetse flies typically produce 8–10 offspring in their lifespan. The tsetse reproductive tract shows extensive modifications that allow complete intrauterine larval development. These modifications include a reduced number of ovarioles per ovary (two), development of a highly tracheated and muscular uterus and modification of an accessory gland into an organ that supplies nutrients to the developing larvae (milk gland). Tsetse flies begin oogenesis before eclosion and develop a single oocyte at a time, always beginning with one of the two ovarioles in the right ovary. Oogenesis takes 6–7 days to complete and appears to be regulated by the presence of a developing embryo or larvae in the uterus. If a larva is aborted, the rate of development of the next oocyte appears to increase to minimize the time during which a larvae is not developing (Saunders, 1972).
A main process in oogenesis is vitellogenesis, the synthesis, secretion and incorporation of yolk proteins into the oocyte (Bownes et al., 1983; Raikhel, 1992). These proteins are incorporated via receptor-mediated endocytosis through the cell membrane of the oocyte (Raikhel and Dhadialla, 1992). Vitellogenic proteins in Diptera consist of molecules that are incorporated into the oocyte, and may be secreted by the fat body, the follicular epithelium of the oocyte or by both. A number of insect vitellogenic proteins have been identified, thus revealing differences between the types of proteins utilized in different species of insects and more specifically within different species of Diptera. A key difference between cyclorrhaphan Diptera (Drosophila, Glossina, Musca, Calliphora) and the rest of the Diptera is that cyclorrhaphan flies utilize yolk type proteins as the major reproductive protein, while most other insects utilize vitellogenins (Bownes, 1992). As a member of the cyclorrhaphan Diptera, one yolk protein has been identified in Glossina that shows similarity to other cyclorrhaphan yolk proteins. The yolk protein orthologue identified in Glossina appears to have an ovary-specific expression profile (Hens et al., 2004).
Upon completion of oogenesis, the oocyte is synchronously fertilized and ovulated into the uterus of the fly where it undergoes embryonic and larval development. Ovulation in tsetse flies is regulated by mating status. Laboratory reared flies prevented from mating do not ovulate, however, oogenesis of successive oocytes proceeds regardless (Gillott and Langley, 1981). In the wild, female flies mate between 3 and 8 days post-eclosion (PE) and can store sperm for their lifetime once inseminated. After the oocyte has been fertilized and ovulated, the embryo develops within the uterus and hatches into a first instar larva. The larva molts through two more instars within the mother and is deposited as a fully developed third instar larvae. The larvae then promptly burrows into the ground and pupariates (Denlinger and Ma, 1974; Buxton, 1955; Moloo, 1971).
The fly supplies nutrients to the larva in the form of a “milk” secretion from a modified accessory gland. This gland is highly specialized and extends from where it connects to the uterus throughout the fat body (Ma et al., 1975). The milk produced by this gland is thought to consist of mainly fat transferred from the fat body during early larvigenesis, and more protein during late larvigenesis (Langley and Bursell, 1980; Moloo, 1976). There are four putative milk proteins secreted by the milk gland while the larva is in its second and third instar stages in the fly. These proteins were detected by SDS–PAGE analysis of gut contents of intrauterine 2nd and 3rd instar larvae. Whether these peptides represent unique proteins is unknown. The major peptide of the four accounts for greater than 90% of the milk gland protein. This protein was isolated and partially sequenced to yield a portion of the N-terminal peptide sequence (Osir et al., 1991).
The research presented here focuses on the molecular characterization of the previously identified yolk and milk gland protein genes and their products. We describe the spatial and temporal expression profile for both gene transcripts and protein products over the initial 30-day life span of tsetse females and report on tissue localization of these protein products. Characterization of these genes will provide a molecular foundation from which the regulation of tsetse viviparous reproductive physiology can be studied.
2. Materials and methods
2.1. Biological material
The Glossina morsitans morsitans colony maintained in the insectary at Yale University was originally established from puparia from fly populations in Zimbabwe. Newly emerged flies are separated by sex and mated at 3–4 days PE. Flies are maintained at 24±1 1C with 50–55% relative humidity, and receive defibrinated bovine blood every 48 h using an artificial membrane system (Moloo, 1971).
2.2. Milk gland protein phylogenetic analysis
The full length cDNA for GmmMGP was described previously (Attardo et al., 2006). Pairwise alignment for the Glossina milk gland protein was performed using MegAlign from the DNAStar/Lasergene series of applications (Burland, 2000), in combination with PAUP4.0 for bootstrap analysis and tree generation by parsimony analysis. Bootstrap analysis was performed for 10,000 replicates. Accession numbers for sequences used can be found in Fig. 1.
Fig. 1.
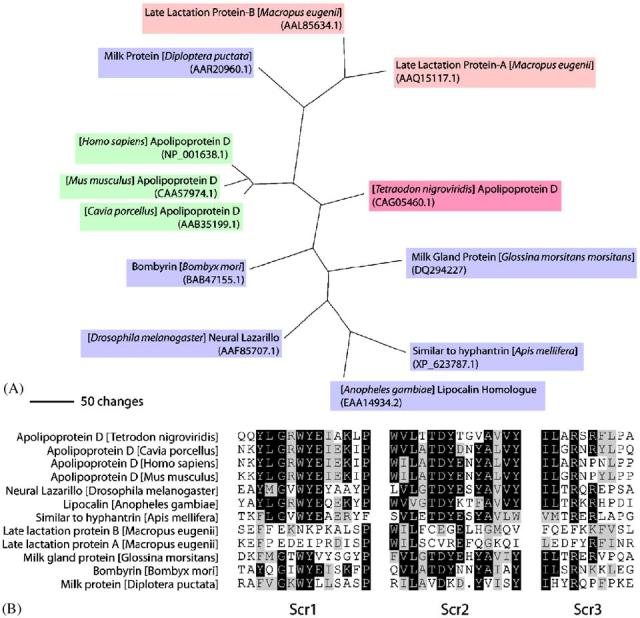
(A) Phylogenic analysis of the GmmMGP protein among other lipocalin proteins. Purple boxes represent insect sequences, green boxes represent mammalian sequences, pink boxes represent marsupial sequences and the magenta box represents a fish sequence. Sequence alignment and tree generation were done by PAUP4.0 and bootstrap analysis was performed for 10,000 replicates. (B) Alignment of the conserved SCR regions of the lipocalins family of sequences from the tree in A. Sequences were aligned in Lasergene Megalign and shaded using the BoxShade program at http://www.ch.embnet.org/software/BOX_form.html.
2.3. Northern blot analysis
Mated females were collected in groups of three per day for the 30-day time course and snap frozen in liquid nitrogen. Total RNA was isolated from individual flies using TRIzol®Reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Equal amounts of RNA from the three corresponding flies for each day were combined to provide a single sample. Ten micrograms of RNA from each sample was analyzed on a 1.5% agarose/formaldehyde gel. Northern blot analysis was performed following standard protocols (Sambrook and Russell, 2001). 32P-dATP labeled probes were generated by asymmetric PCR (Bird, 2005) utilizing gene-specific reverse primers on templates containing the full-length cDNAs for Glossina morsitans morsitans milk gland protein (GmmMGP), Glossina morsitans morsitans yolk protein 1 (GmmYP1), and Glossina morsitans morsitans tubulin (GmmTub) using the following program: 95 °C for 3 min, 95 °C for 45 s, 55 °C for 45 s, 72 °C for 5 min (Steps 2–4, 39 times) and 72 °C for 7 min. Labeling reactions were purified using Micro Bio-Spin 30 Columns (Biorad, Hercules, CA). Blots were prehybridized at 42 °C for at least 4 h and were hybridized with DNA probes overnight at 42 °C. Low stringency washes were performed with 2 × SSC with 0.1% SDS for 15 min at 42 °C. High stringency washes were performed with 0.1 SSC with 0.1% SDS at 68 °C for 15 min. Blots were exposed overnight and quantitated on a PS-1 Phosphoimager using the Imagequant analysis software (Perkin Elmer). GmmYP1 and GmmMGP expression levels were normalized according to the GmmTub level.
Tissue-specific expression was accomplished using midgut, fatbody/milk gland, reproductive tract and carcass dissected from mated flies during all stages of the reproductive cycle. Microscopically dissected tissues were collected in phosphate buffered saline (pH 7.4) and RNA was isolated using the TRIzol protocol for Northern analysis as described above.
2.4. Antibody creation
Anti-GmmMGP and GmmYP1 polyclonal antibodies were generated against bacterially-expressed 6xHis-tagged recombinant proteins. Primers were designed to amplify the mature coding region from GmmMGP and GmmYP1 cDNA clones. Primer design included restriction sites to facilitate directional, in frame cloning into the pET-28a 6xHis-tag expression vector (Novagen, Madison, WI). (Primer sequences: GmmYP1 forward: 5′-ATCAGGATCCGTCAGCGTTCCTAGATCTTTGC; GmmYP1 reverse: 5′-AGCATCTCGAGTTAATACATTCTCGTAAGTTGTTCGTC; GmmMGP forward: 5′-ATCAGGATCCATTCCCTTCCTTAGAG; GmmMGP reverse: 5′-AGCATCTCGAGTTAAAGAGTTTTGTATTGGTTAG).
The pET-28a—GmmYP1 and GmmMGP constructs were transformed into the BL21 Escherichia coli strain, and recombinant protein expression was induced by treatment of cultures with 100 μM IPTG. Bacteria were lysed by sonication and products were analyzed by SDS–PAGE. Recombinant protein was found predominantly in the insoluble fraction as inclusion bodies. Inclusion bodies were made soluble in binding buffer in the presence of 6 M urea and purified by batch protocol using nickel resin under denaturing conditions protocol (Novagen His-Bind Kit). Recombinant proteins were subsequently purified by SDS–PAGE gel, and gel slices were provided for commercial antisera production (Cocalico Biologicals). Antisera were tested by Western blot analysis using recombinant proteins and female tsetse homogenates.
2.5. Western blot analysis
Mated flies for the 30-day protein expression time course were collected in groups of three per day and snap frozen individually. Total protein extraction was performed according to previously published protocols (Park et al., 2006). Equal volumes of protein were combined from each of the three flies to generate a single sample for each day. Western blot analysis of the samples was performed following standard protocols (Sambrook and Russell, 2001). The equivalent of 1/400th of a fly was loaded in each well. Blots were prehybridized for at least four hours to overnight in 1 × PBS, 3% BSA and 0.5% Tween 20. GmmMGP and GmmYP1-specific antisera were used at a concentration of 1:20,000 in blocking buffer. The blots were also hybridized to Drosophila Actin-specific antibodies at a dilution of 1:1000 as a loading control (Abcam, Cambridge, MA). Signals were visualized using the supersignal west pico substrate (Pierce, Woburn, MA) on a Image Station 2000R (Kodak, New Haven, CT).
To detect GmmMGP in immature developmental stages, individual pregnant females were dissected in 1 × PBS (pH 7.4) to remove the developing embryo or larvae from the uterus. The carcass and immature offspring were snap frozen and homogenized in cracking buffer. The equivalent of 1/400th of a fly, embryo or larvae was loaded per well. Western blotting was performed as described above.
2.6. Immunohistochemistry
Immunohistochemical analysis of GmmMGP was performed using the antibodies generated against Rec-GmmMGP. Fat body, milk gland and reproductive tract were isolated from pregnant females. Tissues were fixed in 4% paraformaldehyde/PBS for 24 h. Tissue was subjected to serial ethanol washes (0%, 20%, 40%, 60%, 80%, 100%, 80%, 60%, 40%, 20%, 0% for at least 15 min/wash) and then bleached with 30% hydrogen peroxide for two hours to remove endogenous peroxidase activity. They were then treated with blocking solution, primary GmmMGP (1:20,000) antibodies, and horseradish peroxidase conjugated secondary antibodies (1:20,000) with appropriate washes between treatments (Sillitoe and Hawkes, 2002). Tissues were stained with the Novared Peroxidase staining kit (Vector, Burlingame, CA) according to manufactures protocols for antigen staining, and then analyzed microscopically.
3. Results
3.1. Milk gland protein structure and phylogeny
Analysis of the full length cDNA sequence of tsetse milk gland protein (GmmMGP) reveals that it codes for a member of the lipocalin protein family (Attardo et al., 2006). Lipocalins are a diverse group of small secreted proteins that have the ability to bind small hydrophobic molecules such as lipids, steroid hormones, bilins and retinoids. These proteins also form complexes with other proteins, and their main function is thought to be transport of the molecules mentioned above (Flower et al., 2000). The protein encoded by the GmmMGP gene contains a conserved lipocalin domain and loosely groups with most of the other insect lipocalins with the exception of the cockroach milk protein, which is more closely associated with marsupial lactation lipocalins (Fig. 1a). A typical feature of proteins in the lipocalin family is the presence of three structurally conserved regions (SCRs). Portions of the alignment for the phylogenic tree, including the SCR regions of the proteins, show conservation of key residues associated with lipocalin structural stability in GmmMGP (Fig. 1b) (Greene et al., 2001; Gasymov et al., 1999).
3.2. Stage specificity of GmmMGP and GmmYP1 expression in adult flies
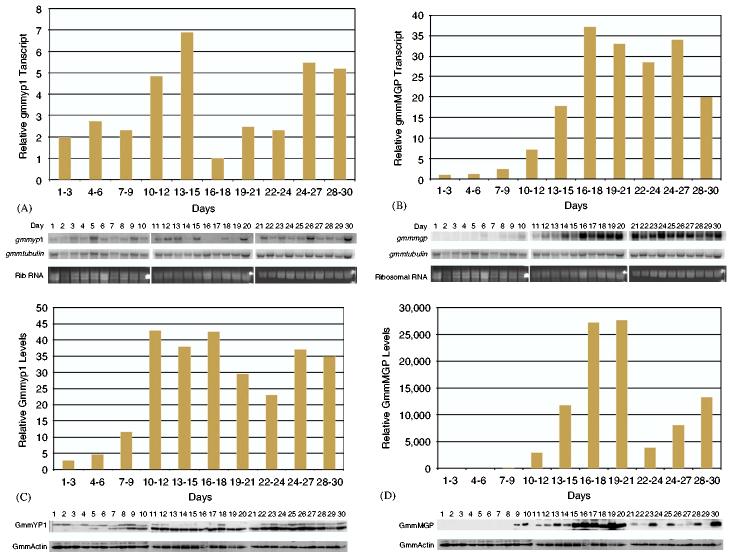
To determine the expression pattern of GmmMGP and GmmYP1 in relation to oogenesis and larvigenesis processes in pregnant females, a time course was performed over the first thirty days of adult life in tsetse. This analysis covered the first gonotrophic cycle and the beginning of the second. Developmental timing and images of the reproductive tract during the first gonotrophic cycle can be seen in Fig. 2a and b. Transcript abundance was analyzed by northern blot analysis from whole flies. Transcript levels were quantitated, normalized against GmmTub and averaged over 3-day periods.
Fig. 2.
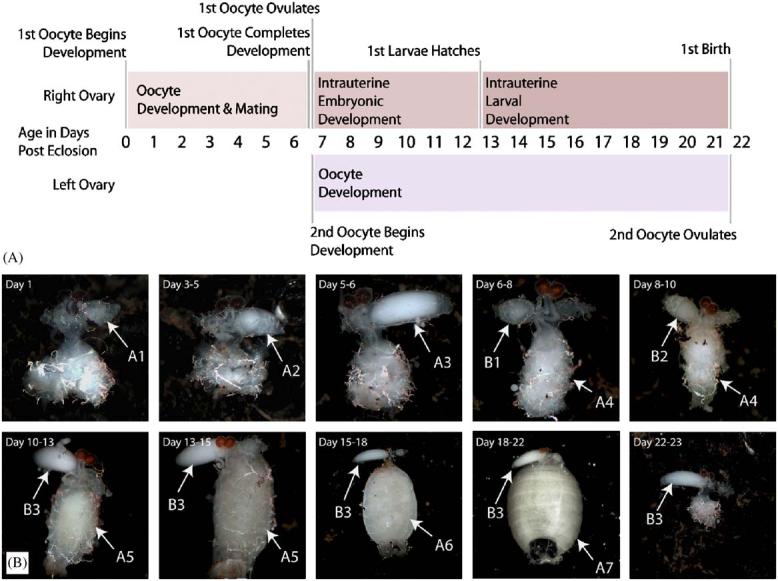
(A) Timeline of events occurring during the first gonotrophic cycle of Glossina morsitans morsitans. (B) Images of the Glossina morsitans morsitans reproductive tract at different stages during the first gonotrophic cycle. The letters A and B represent individual offspring and the numbers represent stages of development from early embryo to 3rd instar larva (1–7).
Expression of GmmYP1 is detectable immediately post eclosion (PE) and appears to increase steadily until the time the second oocyte has completed development and the first offspring is undergoing larval development in the uterus. At this point, there is a decline in transcript abundance, which then increases steadily again through the birth of the first offspring, ovulation of the second oocyte and development of the third oocyte. The general trend seen in GmmYP1 expression is that it occurs in waves, and expression appears to be strongest during the periods of active oogenesis (Fig. 3a).
Fig. 3.
(A) Northern blot analysis of GmmYp1 expression over a thirty day time course. Each time point in the course is equal to 10 μg of total RNA collected from three individual flies for that time point. β-GmmTub was used as a loading control. Data from the Northern blots was quantitated by phosphoimager and normalized against tubulin levels. Data points on the bar graphs represent the average level of expression over a three day period. Daily data points used to generate the graph are shown in the Northern blot below. (B) Northern blot analysis of GmmMGP expression over a thirty day time course. Sample treatment and analysis is the same as in A. (C) Western blot analysis of GmmYP1 levels over a 30-day time course. Each time point in the course is equal to 10 ug of total protein collected from three individual flies for that time point. Blots were probed with polyclonal antisera specific to GmmYP1. Antibodies against Drosophila actin were used as a loading control. Data from the Western blots was quantitated by Kodak imaging software and normalized against Drosophila actin levels. Data points on the bar graphs represent the average level of expression over a three day period. Daily data points used to generate the graph are shown in the Western blot below. (D) Western blot analysis of GmmMGP levels over a thirty day time course. Sample treatment and analysis is the same as in C.
Expression of GmmMGP differs from that of GmmYP1. Transcripts for GmmMGP are undetectable at the beginning of the time course in newly eclosed females. However, GmmMGP transcript levels begin to increase at the time the first larvae hatches in the uterus. Levels of GmmMGP mRNA undergo approximately 30-fold increase during the 2nd and 3rd instar larval developmental period and remain high during the second gonotrophic cycle (Fig. 3b).
3.3. Stage-specific synthesis of GmmMGP and GmmYP1 in tsetse
Protein levels for GmmMGP and GmmYP1 were also documented over the same thirty day time course. GmmYP1 levels were found to increase gradually over the first third of the time course corresponding to the process of oogenesis. Thereafter, levels of this protein remain relatively constant, with a slight decrease at the approximate time of the birth of the first offspring (Fig. 3c). The GmmYP1-specific antibodies appear to recognize two proteins, one of which is slightly larger than the other. The protein distribution appears predominantly in the larger band during the early time points, correlating with development of the first oocyte. Subsequently, the majority of the protein detected appears to shift to the lower sized band. This observation suggests possible post-transcriptional processing for GmmYP1. The larger band is likely an unprocessed version of the yolk protein and the smaller band is a processed storage form occurring within the oocyte. Post-translational processing of yolk proteins is a common event and is often used to allow the formation of yolk protein storage crystals within the oocyte (Raikhel and Dhadialla, 1992).
GmmMGP protein becomes detectable at the end of embryonic development of the first offspring. Protein levels for GmmMGP show a similar profile to those observed for its transcripts (Fig. 3d). During the larval development stage, GmmMGP protein levels increase steadily until the time of partuition (birth) of the first offspring. Immediately after the first larva is deposited, a 20-fold decrease in the level of the GmmMGP protein occurs in the flies. Its level then increases steadily throughout the remainder of the time course, during which time the second oocyte goes through embryonic development and begins larval development.
3.4. Tissue specificity of GmmMGP and GmmYP1 expression in tsetse
Northern blot analysis of GmmYP1 and GmmMGP transcripts shows that their expression is restricted to specific tissues. GmmYP1 is specific to the reproductive tract, while GmmMGP is specific to the milk gland tubule tissue (Fig. 4). The expression patterns of these genes reinforce the functional assumptions that the product of GmmYP1 is acting as a yolk protein and the product of GmmMGP is functioning as a larval milk protein.
Fig. 4.
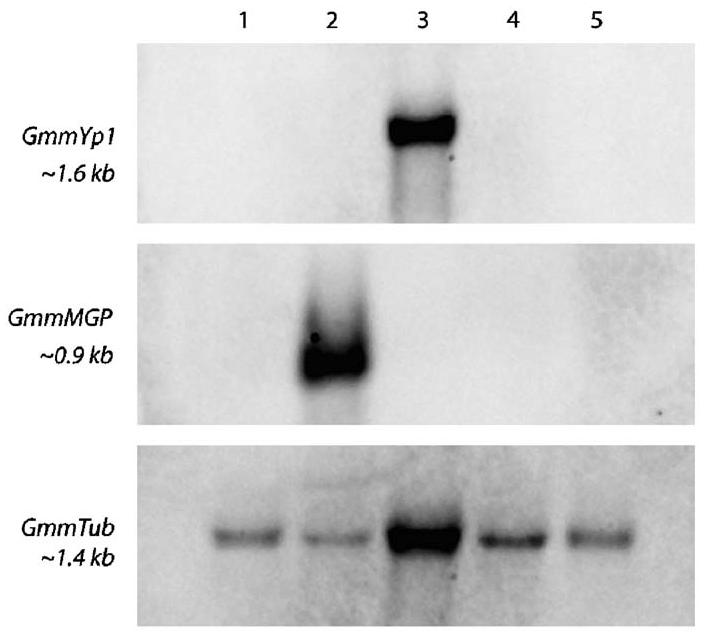
Tissue specificity analysis of GmmYp1 and GmmMGP expression by Northern blot. Specific tissues were dissected from pregnant adult flies. Ten ug of tissue-specific total RNA was loaded per lane. Lanes are labeled as follows, 1: digestive tract, 2: fat body and milk gland, 3: reproductive tract, 4: larvae, 5: carcass. β-GmmTub was used as a loading control.
3.5. Immunohistochemical localization of GmmMGP in pregnant females
We used immunohistochemical analysis to localize GmmMGP protein in the abdominal tissues of tsetse (Fig. 5). For control, flies were stained with preimmune serum and secondary antibody. Immunohistochemical staining of flies pregnant with 2nd and 3rd instar larvae reveals specific staining of the milk gland tubules (Fig. 6). Positive GmmMGP staining occurs over the entire length of the milk gland tubules traversing the fat body in the abdominal cavity. Staining is notably absent in the fat body tissue and strong staining specifically reveals the coalescence of the milk gland tubules around the uterus into a common duct on the dorsal side of the uterus, where the larval mouthparts are attached.
Fig. 5.
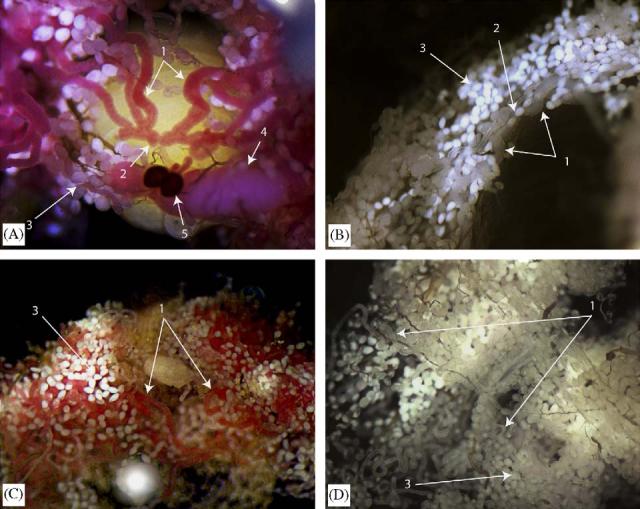
Immunohistochemical analysis of GmmMGP in the fat body, milk gland and reproductive tract of Glossina morsitans morsitans: (A) convergence of milk gland tubules into the reproductive tract (uterus) (1: milk gland tubules, 2:connection into the uterus, 3:fat body tissue, 4:developing oocyte in the ovary, 5: spermathica); (B) negative control for staining using preimmune serum (1: milk gland tubules, 2:connection into the uterus, 3: fat body tissue); (C) staining of the fat body and milk gland tissues from the abdomen (1: milk gland tubules, 3: fat body tissue) and (D) negative control for staining using preimmune serum (1: milk gland tubules, 3: fat body tissue).
Fig. 6.
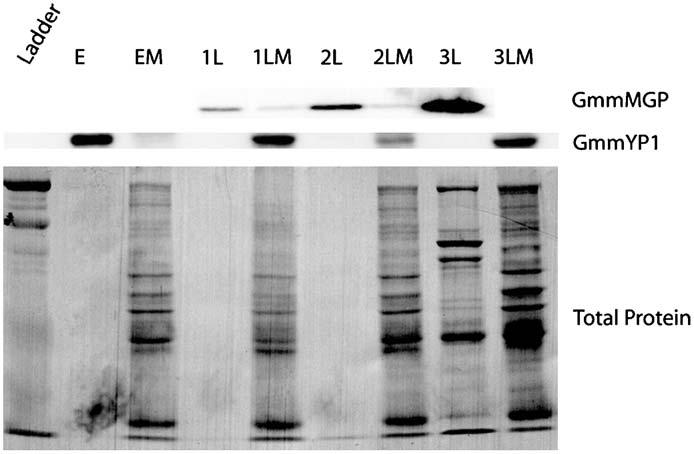
Western blot analysis of GmmMGP and GmmYP1 localization in mother and offspring. Glossina morsitans morsitans at all stages of pregnancy were dissected to separate mother from offspring. Total protein was isolated from mother and offspring for each pair. Equal relative amounts of protein were loaded for each mother/offspring pair (1/400th of either fly/embryo/larvae). Total protein levels per lane were shown by nigrosin staining of the membrane. Labels represent (E: embryo, EM: mother of embryo, 1L: 1st instar larva, 1LM: mother of 1st instart larva, 2L: 2nd instar larva, 2LM: mother of 2nd instar larva, 3L: 3rd instar larva, 3LM: mother of 3rd instar larva.
3.6. Transfer of GmmMGP from mother to larvae
To determine if the GmmMGP protein is a component of the milk transferred to the developing larvae by the mother flies, we analyzed GmmMGP protein levels in mothers and their intrauterine larvae during the course of pregnancy. Offspring were dissected from the uterus of pregnant females during the four stages of pregnancy (embryo, 1st instar larvae, 2nd instar larvae and 3rd instar larvae). GmmMGP was undetectable in the embryo and mother during embryonic development. GmmMGP became detectible in mothers and larvae during development of the 1st instar larvae. Protein levels continued to increase in both the mother and larvae during 2nd instar development. During the 3rd instar of development there appeared to be a massive shift of milk from the mother into the larva, as the 3rd instar larva contained the majority of detectable GmmMGP while the mother had relatively little (Fig. 6).
GmmYP1 was also observed over the course of this analysis in order to follow the fate of yolk protein production in the eggs. During embryonic development GmmYP1 could be detected in the embryo but not in the mother. After the embryonic development was complete, GmmYP1 was no longer detectable in any of the larval stages but was present again in the mother (Fig. 6).
4. Discussion
Vivipararous reproduction is a relatively uncommon process in insects. Tsetse flies (family Glossinidae) are one of only a few families of flies (Hippoboscidae, Nycteribiidae, Calliphoridae and Streblidae) that are capable of undergoing pseudo-placental viviparous reproduction (intrauterine larval development and nourishment) (Meier et al., 1999). This strategy results in fewer offspring per female, but a higher level of survival and fitness for the offspring. In order to accommodate this lifestyle, tsetse has undergone radical changes in its reproductive physiology and regulation of reproductive processes.
Oogenesis in tsetse is a process that has undergone major changes in relation to how it occurs in other insects. In Drosophila, a close relative of tsetse, oogenesis occurs asynchronously in multiple ovarian follicles in both ovaries (Bownes, 1982). Vitellogenesis in Drosophila occurs by the synthesis and secretion of yolk proteins from both the fat body and follicle cells of the ovaries. However, vitellogenesis in tsetse has been reduced in scale to suit their reproductive cycle. They have a small number of ovarioles per ovary (two) and only develop a single oocyte at a time. Oocyte development occurs in alternating ovaries. The first oocyte to be developed can always be found in the right ovary (Denlinger and Ma, 1974). These changes have significant implications for the regulation of genes associated with oogenesis, such as GmmYP1. Expression of GmmYP1 appears to be restricted to the reproductive tract and is under strict physiological regulation. The fact that only one oocyte develops at a time implies that expression of this gene may only occur in a subset of cells within the ovaries (most likely within the ovariole carrying the currently developing oocyte). The signals that are determining which follicles will develop and which will wait are unknown. The sequential nature of how the active follicles change between the left and right ovaries implies a mechanism of communication between the ovaries and their follicles as to their oogenic status. This mechanism may regulate the expression of GmmYP1 so that it occurs in the appropriate oocyte at the correct time. The presence of a developing embryo or larvae within the uterus also appears to have a regulatory effect upon GmmYP1 transcription. When a developing larva is present in the uterus and an oocyte is waiting to ovulate, levels of GmmYP1 transcript are minimal. Once the larva has been deposited and the next oocyte is ovulated into the uterus, GmmYP1 transcription increases again in concordance with the next round of oogenesis. The mechanisms associated with this regulatory system are not yet understood and will be the target of future research. Protein levels for GmmYP1 appear to be fairly stable after development of the initial oocyte. This is not unexpected, as different stages of oocyte are present at almost all the stages of the reproductive cycle.
The most dramatic modification of tsetse reproductive physiology is the evolution of physiology capable of sustaining an offspring throughout the duration of its larval development. A key component to this physiology is the accessory glands (milk glands), an organ consisting of tubules that intertwine throughout the fat body in the abdominal cavity and coalesce at an opening to the uterus where the developing larva feeds. This gland is lined with cells that generate large amounts of protein that is secreted along ducts running through the center of the tubules. However, tsetse is not the only insect known to produce nutrients for its offspring in the form of milk. The viviparous cockroach, Diplotera punctata, also produces milk to nourish its developing embryos (Stay and Coop, 1974). The milk is generated from secretory cells that line the walls of the brood sac where the embryos undergo development. Analysis of protein from the cockroach milk has revealed that it consists mainly of a molecule in the lipocalin family, the same family from which GmmMGP originates (Williford et al., 2004). This is an interesting observation, as proteins from the lipocalin family have also been associated with lactation in marsupials (Piotte et al., 1998). The occurrence of lipocalins in lactation products of divergent organisms suggests that lipocalins have evolved to fill the role of milk proteins multiple times. A common property of lipocalins is the ability to carry small hydrophobic molecules. These observations suggest that GmmMGP, besides being a source of raw amino acids, may also be involved in the transport of hydrophobic substances from mother to offspring.
Regulation of GmmMGP appears to be both transcriptional and translational. Transcripts for GmmMGP are undetectable until the first larva hatches in the uterus. This suggests that expression of the gene might be dependent upon signals produced by the larva, or the pregnancy state of the mother. Once transcription of GmmMGP begins, its expression is relatively constant. However, fluctuation of protein levels at the time of partuition suggests the possibility of translational control as well. The low levels of GmmMGP immediately after birth suggests that its translation may cease until embryonic development of the next offspring has been completed and the next larva hatches into the uterus. Previous work suggests that milk production may be regulated via juvenile hormone, as the corpora allata (CA) (the gland responsible for juvenile hormone synthesis) undergoes cyclical changes in volume and histological appearance in parallel with the pregnancy cycle (Ejezie and Davey, 1974). Furthermore, ablation of the CA reduces milk synthesis in tsetse flies. The phenotype caused by CA ablation is reversible by ectopic treatment of flies with juvenile hormone (Ejezie and Davey, 1976). Further analyses of GmmMGP transcriptional and translational regulation and the role of juvenile hormone in this system will be the subject of future research.
The results obtained in this work form a molecular foundation on which a functional analysis of tsetse reproductive function can be developed. These data confirm the association of the GmmYP1 and GmmMGP genes with oogenesis and larvigenesis processes, respectively. Therefore, it is possible to use the dynamics of these proteins as markers of reproductive function in experimentally compromised flies. Future work in this area will focus on the identification of signals that regulate the transcriptional and translational processes of these genes. Moreover, future experiments will include the determination of the effects of various physiological states, such as nutritional deprivation, aposymbiosis, trypanosome infection and absence of mating stimulus on reproductive gene expression. These experiments will elucidate the regulatory mechanisms controlling reproduction in tsetse, and possibly reveal new strategies that can be applied to tsetse population control.
Acknowledgments
We would like to thank Dr. Sarah Perkin and Dr. Brian Weiss for critical reading of the manuscript. This research was supported by grants from the NIH AI51584 and Robert Leet and Clara Gutherie Patterson Trust Awards to Serap Aksoy and the NIH Ruth Kirshstein Postdoctoral Training Award F32 GM077964 to Geoffrey M. Attardo.
References
- Attardo GM, Strickler-Dinglasan P, Perkin SAH, Caler E, Bonaldo MF, Soares MB, El-Sayeed N, Aksoy S. Analysis of fat body transcriptome from the adult tsetse fly (Glossina morsitans morsitans) Insect Molecular Biology. 2006;15(4):411–424. doi: 10.1111/j.1365-2583.2006.00649.x. [DOI] [PubMed] [Google Scholar]
- Bird IM. Generation of high-sensitivity antisense cDNA probes by asymmetric PCR. Methods in Molecular Medicine. 2005;108:199–213. doi: 10.1385/1-59259-850-1:199. [DOI] [PubMed] [Google Scholar]
- Bownes M. Hormonal and genetic regulation of vitellogenesis in Drosophila. Quarterly Review of Biology. 1982;57:247–274. doi: 10.1086/412802. [DOI] [PubMed] [Google Scholar]
- Bownes M. Why is there sequence similarity between insect yolk proteins and vertebrate lipases? Journal of Lipid Research. 1992;33:777–790. [PubMed] [Google Scholar]
- Bownes M, Dempster M, Blair M. The regulation of yolk protein gene expression in Drosophila melanogaster. Ciba Foundation Symposium. 1983;98:63–79. doi: 10.1002/9780470720790.ch5. [DOI] [PubMed] [Google Scholar]
- Burland TG. DNASTAR's Lasergene sequence analysis software. Methods in Molecular Biology. 2000;132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- Buxton PA. London School of Hygiene and Tropical Medicine Memoir 10: The Natural History of Tsetse Flies. H.K. Lewis and Co. Ltd.; London: 1955. [Google Scholar]
- Denlinger DL, Ma WC. Dynamics of pregnancy cycle in tsetse Glossina-morsitans. Journal of Insect Physiology. 1974;20:1015–1026. doi: 10.1016/0022-1910(74)90143-7. [DOI] [PubMed] [Google Scholar]
- Ejezie GC, Davey KG. Changes in the neurosecretory cells, corpus cardiacum and corpus allatum during pregnancy in Glossina austeni Newst. (Diptera, Glossinidae) Bulletin of Entomological Research. 1974;64:247–256. [Google Scholar]
- Ejezie GC, Davey KG. Some effects of allatectomy in female tsetse, Glossina-Austeni. Journal of Insect Physiology. 1976;22:1743–1749. doi: 10.1016/0022-1910(76)90068-8. [DOI] [PubMed] [Google Scholar]
- Flower DR, North AC, Sansom CE. The lipocalin protein family: structural and sequence overview. Biochimica et Biophysica Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Gasymov OK, Abduragimov AR, Yusifov TN, Glasgow BJ. Binding studies of tear lipocalin: the role of the conserved tryptophan in maintaining structure, stability and ligand affinity. Biochimica et Biophysica Acta. 1999;1433:307–320. doi: 10.1016/s0167-4838(99)00133-8. [DOI] [PubMed] [Google Scholar]
- Gillott C, Langley PA. The control of receptivity and ovulation in the tsetse-fly, Glossina-morsitans. Physiological Entomology. 1981;6:269–281. [Google Scholar]
- Greene LH, Chrysina ED, Irons LI, Papageorgiou AC, Acharya KR, Brew K. Role of conserved residues in structure and stability: tryptophans of human serum retinol-binding protein, a model for the lipocalin superfamily. Protein Science. 2001;10:2301–2316. doi: 10.1110/ps.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens K, Macours N, Claeys I, Francis C, Huybrechts R. Cloning and expression of the yolk protein of the tsetse fly Glossina morsitans morsitans. Insect Biochemistry and Molecular Biology. 2004;34:1281–1287. doi: 10.1016/j.ibmb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Langley PA, Bursell E. Role of fat-body and uterine gland in milk synthesis by adult female Glossina-morsitans. Insect Biochemistry. 1980;10:11–17. [Google Scholar]
- Ma WC, Denlinger DL, Jarlfors U, Smith DS. Structural modulations in the tsetse fly milk gland during a pregnancy cycle. Tissue Cell. 1975;7:319–330. doi: 10.1016/0040-8166(75)90008-7. [DOI] [PubMed] [Google Scholar]
- Meier R, Kotrba M, Ferrar P. Ovoviviparity and viviparity in the Diptera. Biological Reviews of the Cambridge Philosophical Society. 1999;74:199–258. [Google Scholar]
- Moloo SK. Oocyte differentiation and vitellogenesis in Glossina morsitans Westw. Acta Tropica. 1971;28:334–340. [PubMed] [Google Scholar]
- Moloo SK. Storage of nutriments by adult female Glossina morsitans and their transfer to the intra-uterine larva. Journal of Insect Physiology. 1976;22:111–115. doi: 10.1016/0022-1910(76)90120-7. [DOI] [PubMed] [Google Scholar]
- Osir EO, Kotengo M, Chaudhury MF, Otieno LH. Structural studies on the major milk gland protein of the tsetse fly, Glossina morsitans morsitans. Comparative Biochemistry and Physiology.B: Comparative Biochemistry. 1991;99:803–809. doi: 10.1016/0305-0491(91)90145-4. [DOI] [PubMed] [Google Scholar]
- Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. Journal of Biological Chemistry. 2006;281:11167–11176. doi: 10.1074/jbc.M601517200. [DOI] [PubMed] [Google Scholar]
- Piotte CP, Hunter AK, Marshall CJ, Grigor MR. Phylogenetic analysis of three lipocalin-like proteins present in the milk of Trichosurus vulpecula (Phalangeridae, Marsupialia) Journal of Molecular Evolution. 1998;46:361–369. doi: 10.1007/pl00006313. [DOI] [PubMed] [Google Scholar]
- Raikhel AS. Advances in Disease Vector Research. Springer; New York: 1992. Vitellogenesis in mosquitos; pp. 1–39. [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annual Review of Entomology. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Saunders DS. Effect of starvation on length of interlarval period in tsetse fly Glossina-morsitans-orientalis Vanderplank. Journal of Entomology Series A—General Entomology. 1972;46:197–202. [Google Scholar]
- Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high-throughput screen for patterning defects in the mouse cerebellum. Journal of Histochemistry and Cytochemistry. 2002;50:235–244. doi: 10.1177/002215540205000211. [DOI] [PubMed] [Google Scholar]
- Stay B, Coop AC. ‘Milk’ secretion for embryogenesis in a viviparous cockroach. Tissue and Cell. 1974;6:669–693. doi: 10.1016/0040-8166(74)90009-3. [DOI] [PubMed] [Google Scholar]
- Williford A, Stay B, Bhattacharya D. Evolution of a novel function: nutritive milk in the viviparous cockroach, Diploptera punctata. Evolution and Development. 2004;6:67–77. doi: 10.1111/j.1525-142x.2004.04012.x. [DOI] [PubMed] [Google Scholar]