Abstract
Objective
To compare the detection of clinically significant diabetic macular edema (DME) by an optical coherence tomography (OCT) grid scanning protocol and biomicroscopic examination.
Design
Retrospective case series.
Participants
Outpatients at the Doheny Eye Institute.
Methods
The clinical and imaging records of a consecutive series of 71 eyes of 40 patients referred for DME who underwent OCT using the both the Macular Grid 5 (MG5) scanning protocol (to allow a more evenly distributed sampling of points in the macula) and the standard Fast Macular Thickness Map (FMTM) pattern were reviewed. An automated algorithm was developed to generate a retinal thickness map using the MG5 data, which was then compared with a normative database to identify presumed areas of retinal edema. Clinically significant macular edema (CSME) was also identified by clinical examination and stereoscopic fundus photographs for comparison with the results of the OCT protocols.
Main Outcome Measures
Sensitivity and specificity of scanning protocols.
Results
Optical coherence tomograms were inspected visually, and automatically detected retinal boundaries were found to be correct in 69 of 71 MG5 scans and in 65 of 71 FMTM scans. Macular Grid 5 scanning was performed twice in each eye, and the repeatability (pooled standard deviation) of the total area of edema was 0.48 mm2 (coefficient of variation, 6.8%). Sensitivity and specificity of the MG5 for detection of CSME relative to the clinical examination were 89% and 86%, respectively, with κ being 0.74. Macular Grid 5 and FMTM assessment of foveal CSME also showed good agreement, with κ being 0.68.
Conclusions
The analysis algorithm for the OCT MG5 grid scan seems to be accurate and repeatable. Automated detection of CSME by the MG5 analysis correlated well with the clinical grading and standard OCT analysis (FMTM). Macular Grid 5 provides more information regarding the perifoveal macula than FMTM and may be of value to clinicians in planning treatment and in future studies of macular edema.
Macular edema is an important cause of visual loss and legal blindness in patients with diabetic retinopathy.1-5 In the Early Treatment Diabetic Retinopathy Study (ETDRS), focal laser photocoagulation was demonstrated to reduce the risk of moderate vision loss in diabetic patients with an entity termed clinically significant macular edema (CSME).6,7 As optical coherence tomography (OCT)8,9 was not available at the time of the EDTRS study, CSME was defined based on biomicroscopic observations by the examining physician.10 Three definitions of CSME were adopted by the ETDRS investigators: (1) presence of any retinal thickening within 500 μm of the foveal center, (2) lipid exudates within 500 μm of the foveal center with adjacent thickening, and (3) an area of thickening > 1 Macular Photocoagulation Study disc area (DA; 1 DA ≅ 1.767 mm2) within 1 disk diameter (1.5 mm) of the foveal center.7 To corroborate and standardize the clinical assessment, the ETDRS Fundus Photographic Reading Center reviewed color stereoscopic photographs for a number of imaging end points, including the presence and extent of macular edema.11 Because accurate methods of quantifying axial retinal thickening were not available at that time, macular edema extent, as determined by biomicroscopic examination or inspection of stereoscopic photographs, was based only on the area of thickening and not on the magnitude of the axial thickness. Variations in the amount of stereopsis present in paired stereo photographs or in the threshold for thickening adopted by the observer may further complicate the accurate and reproducible detection of areas of edema.. Thus, there is potential for considerable variability and possible lack of sensitivity in the methods for identifying macular edema used in previous clinical studies. The lack of sensitivity of the clinical examination for detection of mild edema has been demonstrated by a number of investigators, including Brown et al,12 who observed that, for eyes with a foveal center thickness between 201 and 300 μm (200 defined as the upper limit of normal), only 14% were noted to have foveal edema by contact lens biomicroscopy. They12 coined the term subclinical foveal edema to describe such cases. However, as these cases of subclinical edema were presumably not recognized in the ETDRS, the rationale for treating these lesions is presently uncertain,13-15 although it may change as data from ongoing clinical trials incorporating OCT imaging become available.
Although the system proposed by Brown et al is useful for identification of foveal edema, it is not likely to identify cases of nonfoveal CSME. As OCT has become an integral part of clinical trials and clinical practice,16-20 a system for detection of nonfoveal CSME would be valuable. Unfortunately, the commonly used macular scanning patterns on the Stratus OCT machine (Carl Zeiss Meditec, Inc., Dublin, CA) consist of radial lines that provide a high density of points near the fovea, but a relatively sparse pattern in more peripheral zones. This requires considerable interpolation to construct a thickness map. To address this limitation, we developed a concentric grid pattern (Macular Grid 5 [MG5]) and an algorithm to segment and quantify areas of retinal thickening automatically. In this report, we compare the identification of CSME by the MG5 algorithm with the clinical examination and with evaluation of the fovea using the standard Fast Macular Thickness Map (FMTM) pattern of the Stratus OCT.
Materials and Methods
Data Collection and Study Population
We retrospectively reviewed the clinical and imaging records of 71 eyes of 40 patients referred to the Doheny Ocular Imaging Unit with a diagnosis of diabetic macular edema (DME) who underwent OCT imaging using both the MG5 and FMTM. Approval for the analysis of these records was obtained from the institutional review board of the University of Southern California. For all OCT imaging studies, the Stratus OCT system with version 4.0 software was used to acquire the scan of the macula. Fast Macular Thickness Map and MG5 data from 65 normal subjects recruited from the Doheny Eye Institute and the University of Pittsburgh Medical Center were used as the reference baseline The normal subjects were recruited as part of the prospective Advanced Imaging for Glaucoma Study. The study was approved by the institutional review boards of the University of Southern California and the University of Pittsburgh School of Medicine, and all participants provided informed consent before participating in the study. All methods adhered to the Declaration of Helsinki for research involving human subjects.
Comparison of Scanning Patterns
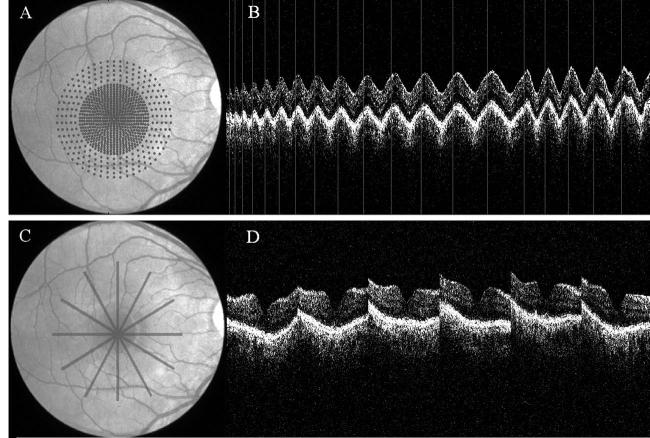
As illustrated in Figure 1A, B, the MG5 scan algorithm obtains A-scans in a relatively evenly distributed pattern (MG5, 768 A-scans within the 5-mm-diameter grid), which minimizes un-sampled gaps, particularly in the central 3 mm. In contrast, the standard Stratus FMTM (Fig 1C, D) leaves large gaps between the 6 scan meridians, with a progressive increase in the gap with increasing eccentricity, reaching a 1.3-mm gap at a distance of 2.5 mm from the center. Figure 2 compares the meridian gaps of the 2 scan patterns. As it is a newly available scanning pattern, the imaging protocols at the Doheny Ocular Imaging Unit specify that the MG5 scan be performed twice, thus allowing an assessment of the reproducibility of the scanning technique. The variation in measurements between scans was quantified using Bland–Altman21 95% limits of agreement analyses and plotted.
Figure 1.
A, Macular grid 5 (MG5) scan pattern (768 A-scans spiral from center outward; grid spacing, 0.14 mm in central 3 mm and 0.29 mm between 3 and 5 mm). B, Optical coherence tomography (OCT) image of MG5. C, Fast Macular Thickness Map scan pattern (6 radial line scans). D, Six OCT images corresponding to each of the 6 lines.
Figure 2.
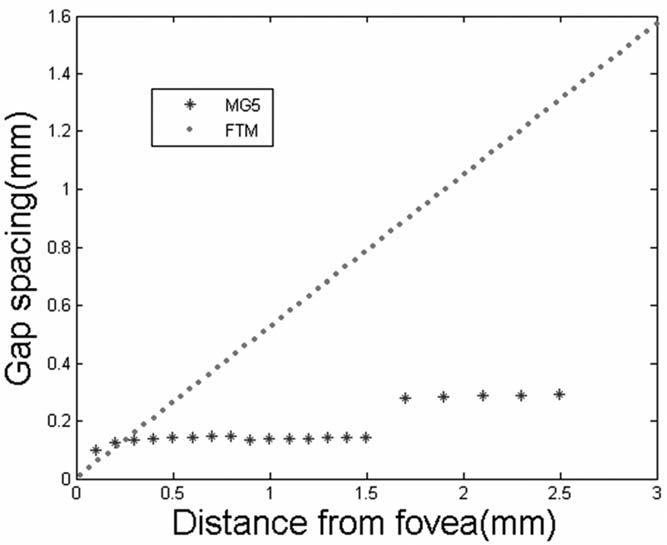
Gap between A-scan points plotted against distance from the center point (radius) for Fast Macular Thickness Map (FTM) scans and Macular Grid 5 (MG5) scans.
Detection of Clinically Significant Macular Edema by Macular Grid 5 Optical Coherence Tomography
To identify areas of potential retinal edema, the threshold for edema on the OCT map was defined arbitrarily as a retinal thickness > 2.3 standard deviations (SDs) above the normal reference mean at that location (i.e., above the 99% level). The normal reference population consisted of 68 patients (56 of whom were women), with a mean age of 51±8 years. Of the 68 normal patients, 59 were Caucasian and 9 were African American; 17 patients identified themselves as being Hispanic. Figure 3 shows the mean retinal thickness map and retinal thickness SD map of the normal reference population using the MG5 scanning pattern. An automated image processing algorithm for the MG5 scan data was developed to plot maps of retinal thickness, detect areas of edema, compute various parameters for the zones of retinal thickening (area, distance to fixation), and identify the presence of CSME. For identification of the retinal thickness, first the junction of the inner and outer segments of the photoreceptor is detected by the algorithm as the outer retinal boundary. A-scans with artifact or blocked reflectivity at the level of the retinal pigment epithelium are replaced with the results from neighboring A-scans. The inner nerve fiber layer boundary is then detected from the smoothed image as the inner retinal boundary. The distance from the inner to outer retinal boundaries is defined to be the retinal thickness. Finally, the retinal thickness map is interpolated between the sampled locations. The same method was applied to MG5 scans from the normal dataset to construct the normal reference map.
Figure 3.
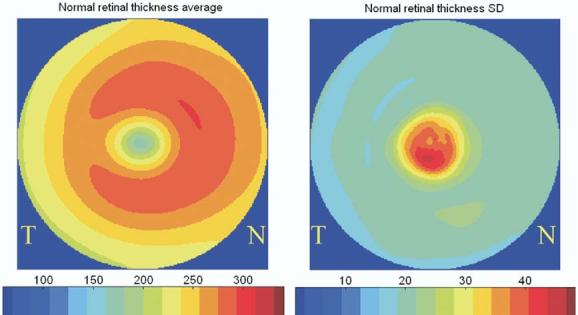
Population average and standard deviation (SD) of retinal thickness map of normal reference. N = nasal; T = temporal.
Points with a retinal thickness > 2.6 SDs (99.5% level) above the normal reference mean at that location were identified as edema kernels. The edema kernel is the seed point from which surrounding points are then assessed to determine if thickening is also present. Regions were grown from the edema kernel to include contiguous surrounding points with thickness > 2.3 SDs (99.0%) above the normal reference, to create zones of edema. The algorithm was designed, in accordance with ETDRS definitions, to identify 2 categories of CSME: CSME1, defined as retinal edema within 500 μm of the foveal center, and CSME2, defined as retinal edema > 1 Macular Photocoagulation Study DA, of which at least some portion extended to within 1500 μm of the foveal center. For identification of CSME1, to reduce spurious detection of tiny islands of thickening caused by noise, a threshold area of thickening (0.2-mm diameter, 0.126-mm2 area) was used to identify regions of true edema. The foveal zone (region affected by CSME1) is particularly susceptible to noise caused by motion artifact (due to poor fixation or transverse eye motion), given the significant normal difference in retinal thickness between the foveola and the parafoveal retina (Fig 3). If any retinal edema was present (even if not CSME), a grade of DME was assigned. Using this nonexclusive scheme, it is important to note that a case of edema within 500 μm of the foveal center and with an area > 1 Macular Photocoagulation Study DA would be classified as containing CSME1, CSME2, and DME. For the purposes of this analysis, the third ETDRS definition of CSME, the presence of lipid within 500 μm of the foveal center with adjacent thickening, was not identified due to difficulties of developing algorithms to identify lipid exudates accurately.
Clinical Diagnosis of Clinically Significant Macular Edema
The presence of CSME1 and CSME2, as identified by traditional methods of biomicroscopic examination and stereoscopic color photography, was determined by review of the clinical records. The macular drawings for each case were scrutinized first to determine if the edema was classified as CSME1 and/or CSME2 by the clinician. In cases in which the clinical record did not clearly categorize the CSME, stereoscopic color photographs obtained for the patient were reviewed by a trained member of the Doheny Image Reading Center (SRS) in an attempt to classify the edema. If macular edema was present, even when not deemed to be clinically significant, a grade of DME was assigned. In a few cases, although the patient was referred to the imaging unit with a diagnosis of macular edema, scrutiny of the clinical record did not identify any macular edema; these cases were assigned a grade of no DME. By this approach, an attempt was made to assign grades to every case. For the purpose of analysis, questionable grades were treated as if definitely present.
Stratus Optical Coherence Tomography Identification of Foveal Edema
To compare the performance of the MG5 algorithm with that of the manual interpretation of the standard FMTM algorithm for detection of CSME, an analysis was also performed of the retinal thickness maps generated by the Stratus OCT version 4.0 software. For this analysis, we adopted the criteria suggested by Brown et al12 that foveal thickening < 300 μm should be considered subclinical edema. Thus, foveal thickening ≥ 300 μm was deemed to meet the criteria for CSME1. Because CSME1 need not involve the foveal center, we chose to use the foveal subfield thickness (rather than the foveal center thickness) from the Stratus OCT output for the purpose of this analysis. In addition, because the identification of cysts (by biomicroscopy or by angiography) is frequently used by clinicians to identify the presence of thickening, we broadened the definition of CSME1 on FMTM to include cases in which retinal cysts were present in the fovea, even if the thickness was <300 μm. Attempts were not made to identify CSME2 from the FMTMs, given the large sampling gaps in the peripheral zones of this scan pattern.
Comparison of Methods for Grading Macular Edema
The MG5 assessments were compared with the clinical grade of the edema as determined by biomicroscopic examination and stereoscopic fundus photographs. The FMTM assessment for the presence of CSME1 was also compared with the clinical assessment. Sensitivities and specificities (assuming the clinical assessment to be the gold standard), κ statistics (nonweighted, Cohen),22 and agreement scores (defined as the number of cases for which the two methods agreed divided by the total number of cases) were calculated for all comparisons. κ statistics were interpreted using the scheme advocated by Landis and Koch23: 0 to 0.20, slight agreement; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, substantial; and >0.80, almost perfect agreement.
Results
The automated detection of retinal boundaries was verified visually and found to be correct in 69 of 71 MG5 scans (our software) and on 65 of 71 FMTM scans (Stratus software). There was a trend for better reliability in automated segmentation by the MG5, but the difference was not statistically significant (χ test, P = 0.145). A total of 63 cases had both MG5 and FMTM with correct boundary detection, and constituted the case set used for subsequent analysis. Figure 4 (available at http://aaojournal.org) is an illustration of the accurate detection of inner and outer retinal boundaries by the MG5 analysis algorithm. Two MG5 scans were available for each eye. The repeatability (pooled SD) of the total area of edema was 0.48 mm2 (coefficient of variation, 6.8%). Bland–Altman plots of the area-weighted average foveal thickness (diameter < 1 mm) showed an average difference of 4.7 μm between the 2 MG5 scans (Fig 5). To assess the accuracy of the MG5 relative to the existing OCT clinical practice standard, thickness maps were generated from both the FMTM and the MG5 analyses and compared. The thickness of the foveal region as measured by the FMTM and MG5 scans was substantially equivalent by Bland–Altman analysis (Fig 5).
Figure 4.

Segmentation of grid optical coherence tomography image (displayed 2-dimensionally). Upper white line, inner retinal boundary; lower white line, inner segment/outer segment junction. The retinal thickness is defined as the distance between the 2 lines.
Figure 5.
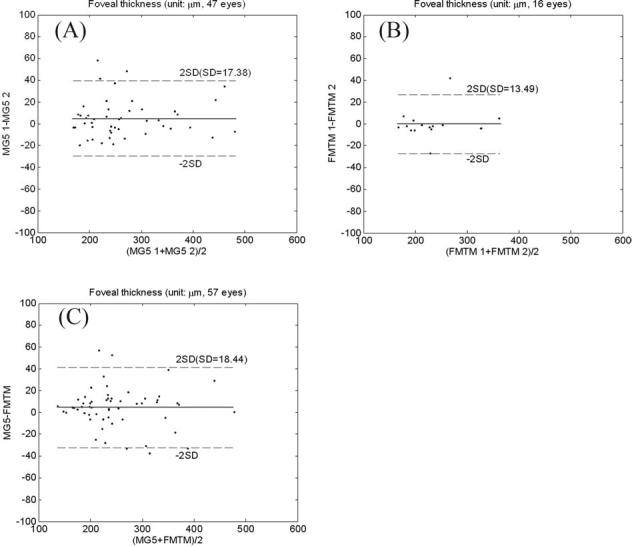
Bland–Altman plot of foveal thickness (area-weighted average in the central 1-mm-diameter circle). The solid line is the average difference (or agreement), and the dashed lines are the 95% limits of agreement. A, Comparison between 2 Macular Grid 5 (MG5) scans: average difference, 4.7 μm; standard deviation (SD) of difference, 17.4 μm. B, Comparison between 2 Fast Macular Thickness Map (FMTM) scans: average difference, 0.4 μm; SD difference, 13.5 μm. C, FMTM vs. MG5: average difference, 4.5 μm; SD difference, 18.4 μm.
Comparison of the detection of macular edema by the MG5 versus the clinical assessment is shown in Table 1. Sensitivity and specificity of MG5 for detection of any CSME compared with the clinical examination (as the gold standard) were 89% (31/35) and 86% (24/28), respectively. Substantial (κ>0.60) agreement (Table 1) was observed between the clinical assessment and the MG5 for the detection of CSME1, CSME2, and any CSME. The FMTM and MG5 also showed substantial agreement for the detection of CSME1 (Table 2 [available at http://aaojournal.org]).
Table 1.
Comparison of Clinically Significant Macular Edema (CSME) and Diabetic Macular Edema (DME) Grading by Macular Grid 5 Analysis (MG5) versus the Clinician
|
MG5 vs. Clinical Examination* |
||||
|---|---|---|---|---|
| CSME 1 | CSME 2 | Any CSME† | Any DME‡ | |
| Edema present on MG5 only | 7 | 5 | 4 | 7 |
| Edema present on clinical examination only | 3 | 3 | 4 | 4 |
| Both | 22 | 25 | 31 | 37 |
| None | 31 | 30 | 24 | 15 |
| κ (95% CI) | 0.677 (0.495–0.860) | 0.745 (0.580–0.910) | 0.743 (0.577–0.909) | 0.603 (0.393–0.814) |
| Agreement | 0.841 | 0.873 | 0.873 | 0.825 |
| Sensitivity (95% CI) | 0.880 (0.677–0.968) | 0.893 (0.706–0.972) | 0.886 (0.723–0.963) | 0.902 (0.759–0.968) |
| Specificity (95% CI) | 0.816 (0.651–0.9168) | 0.857 (0.690–0.946) | 0.857 (0.664–0.953) | 0.682 (0.451–0.853) |
CI = confidence interval; CSME 1 = any retinal thickening within 500 μm of the foveal center; CSME 2 = retinal thickening within 1 Macular Photocoagulation Study (MPS) disc diameter (1.5 mm) of the foveal center and >1 MPS disc area in size. Sensitivity and specificity for MG5 are calculated with respect to the clinical gold standard.
Grading as assessed by biomicroscopy and/or stereo photography.
CSME 1 or 2 or lipid within 500 μm of the foveal center associated with adjacent retinal thickening.
Presence of any retinal thickening within the macula.
Table 2.
Comparison of Clinically Significant Macular Edema (CSME) 1* Grading by Fast Macular Thickness Map (FMTM)† versus Other Methods
| Grading Methods Compared | FMTM vs. Clinical Examination‡ | FMTM vs. MG5 |
|---|---|---|
| CSME 1 present on FMTM only | 4 | 2 |
| CSME 1 present on clinical examination or MG5 only | 6 | 8 |
| CSME 1 present on both FMTM and clinical examination or MG5 | 19 | 21 |
| None | 34 | 32 |
| κ (95% CI) | 0.664 (0.474–0.854) | 0.676 (0.492–0.860) |
| Agreement | 0.841 | 0.841 |
| Sensitivity (95% CI) | 0.760 (0.545–0.898) | 0.724 (0.525–0.866) |
| Specificity (95% CI) | 0.895 (0.743–0.966) | 0.941 (0.789–0.990) |
CI = confidence interval; MG5 = Macular Grid 5 analysis.
Sensitivities and specificities for the FMTM are calculated with respect to either the clinical examination or the MG5 assessment as the gold standard.
Any retinal thickening within 500 μm of the foveal center.
Evaluation of foveal subfield only.
Grading as assessed by biomicroscopy and/or stereo photography.
The few cases that showed disagreement between the MG5 grade and the clinical assessment were rescrutinized to identify possible causes for disagreement. For cases in which the MG5 demonstrated CSME, cases diagnosed clinically with CSME showed a trend for a higher foveal thickness compared with cases where no CSME was evident clinically (Table 3). Cases without clinical CSME also tended to be farther from the foveal center. For cases in which MG5 did not identify CSME, the foveal thickness was slightly higher in cases clinically diagnosed with CSME compared with those without, though the difference was not statistically significant (Table 4).
Table 3.
Characteristics of Edema in Cases Graded to Have Clinically Significant Macular Edema (CSME) by Macular Grid 5 Analysis
| Clinical CSME+ | Clinical CSME− | P Value | |
|---|---|---|---|
| Mean distance to fixation* (± SD) (mm) | −0.16 (0.67) | 0.47 (0.63) | 0.14 |
| Mean edema area (± SD) (mm)2 | \#2009\\#2009\8.63 (5.79) | 8.41 (3.04) | 0.91 |
| Mean foveal thickness† (± SD) (μm) | 317 (77)\#2009\\#2009\ | 251 (47)\#2009\\#2009\ | 0.06 |
SD = standard deviation.
+, present by clinical grading; −, absent by clinical grading.
The minimum distance from edema edge to foveal center. If edema extends through the foveal center, the distance measurement will have a negative value (indicating the minimum distance from the foveal center to nonedematous retina).
Average retinal thickness in the fovea (diameter < 1 mm).
Table 4.
Characteristics of Edema in Cases Graded to Have No Clinically Significant Macular Edema (CSME) by Macular Grid 5 Analysis
| Clinical CSME+ | Clinical CSME− | P Value | |
|---|---|---|---|
| Mean distance to fixation* (± SD) | 2.09 (0.53) | 2.26 (0.45) | 0.57 |
| Mean edema area (± SD) | 0.36 (0.46) | 0.32 (0.65) | 0.90 |
| Mean foveal thickness† (± SD) | 221 (42)\#2009\\#2009\ | 204 (31)\#2009\\#2009\ | 0.50 |
SD = standard deviation.
+, present by clinical grading; −, absent by clinical grading.
The minimum distance from edema edge to foveal center. If edema extends through the foveal center, the distance measurement will have a negative value (indicating the minimum distance from the foveal center to nonedematous retina).
Average retinal thickness in fovea (diameter < 1 mm).
Representative cases of agreement and disagreement between the MG5 and the clinical assessment are shown in Figures 6, 7 (available at http://aaojournal.org), and 8 to 10. The disagreements seemed to be due to a threshold phenomenon in which retinal thickening (above the normal baseline) was apparent on the MG5 map but did not achieve the MG5 threshold definition for edema (Fig 8). In cases such as this, the clinician may have been influenced by other factors, such as the presence of lipid exudates, which may have led the clinician to adopt a lower threshold for thickening. Alternatively, the retinal thickening identified by the MG5 was just close enough to the foveal center to be considered clinically signifi-cant by the MG5 algorithm, but was deemed to be farther away by the clinician, who may not have been able to measure the distance to the foveal center as precisely (Fig 9). In other cases, the clinician may have been biased by the presence of prominent or severe thickening in one region and, as a result, may have been less impressed by or less sensitive to retinal thickening in adjacent areas (Fig 10).
Figure 6.
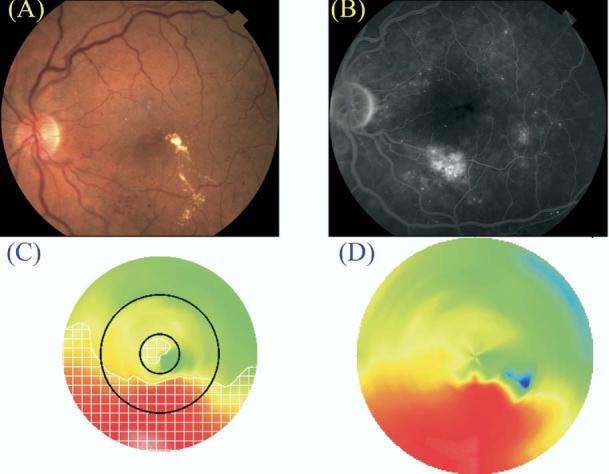
Case of clinically significant macular edema (CSME) 1 and CSME 2 diagnosed by clinical examination (A, color fundus photograph; B, late fluorescein angiogram frame) and by Macular Grid 5 (C, MG5 thickness map) and Fast Macular Thickness Map (D, FMTM) protocols. The map of edema as identified by the MG5 algorithms is delineated by the white checkered zone in C.
Figure 7.
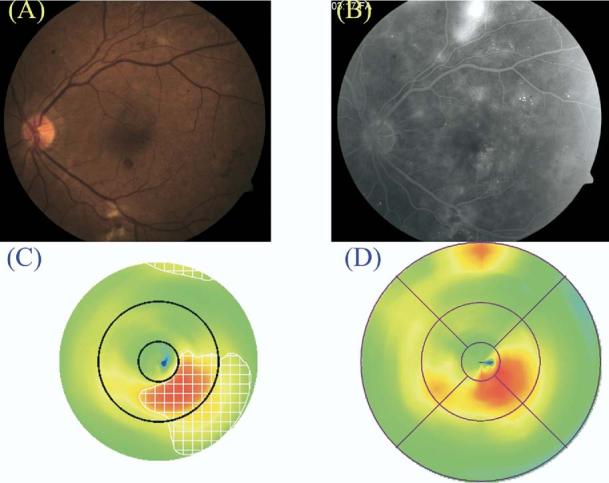
Case of clinically significant macular edema (CSME) 2 but not CSME 1 diagnosed by clinical examination (A, color fundus photograph; B, late fluorescein angiogram frame) and by Macular Grid 5 (C, MG5 thickness map) and Fast Macular Thickness Map (D, FMTM) protocols. The map of edema as identified by the MG5 algorithms is delineated by the white checkered zone in C.
Figure 8.
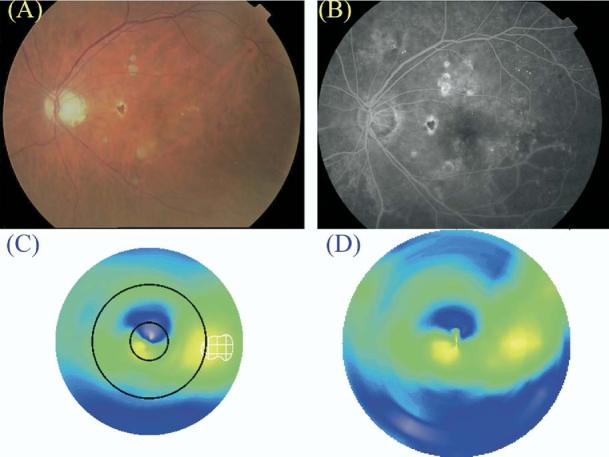
Patient diagnosed to have clinically significant macular edema (CSME) 1 by clinical examination (A, color fundus photograph; B, late fluorescein angiogram frame) but not by Macular Grid 5 (C, MG5 thickness map) or Fast Macular Thickness Map (D, FMTM) protocols. The map of edema as identified by the MG5 algorithms is delineated by the white checkered zone in C. Note that retinal thickening (compared with the normal reference) was present in the central circle of the MG5 map but did not meet the threshold level defined in this study.
Figure 10.
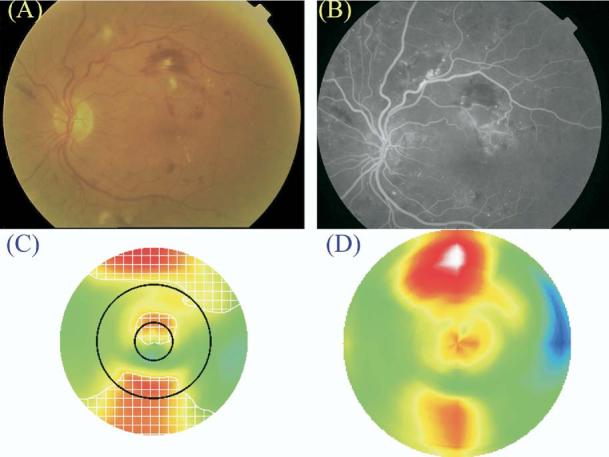
Case graded to have clinically significant macular edema (CSME) 1 by Macular Grid 5 (MG5) and Fast Macular Thickness Map (FMTM) but not identified by clinical examination. A, Color fundus photograph. B, Late fluorescein angiogram frame. C, Macular Grid 5 thickness map. D, FMTM. The map of edema as identified by the MG5 algorithms is delineated by the white checkered zone in C. Note that the retinal thickening inside the central foveal circle is above threshold on the MG5 map, but not as severe as the area of edema just outside the circle. This may explain why the clinician did not judge the edema to be within the foveal zone.
Figure 9.
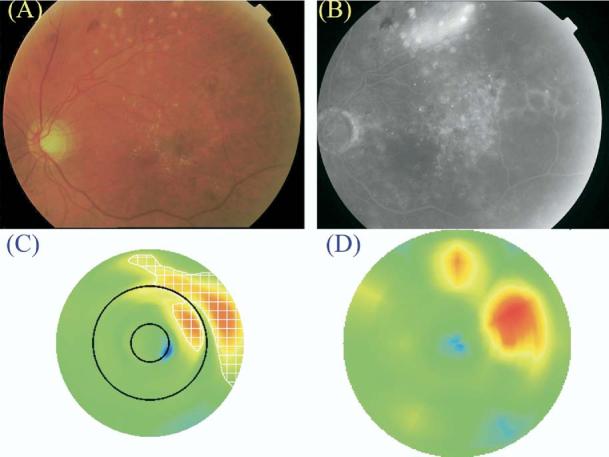
Case graded to have clinically significant macular edema (CSME) 2 by Macular Grid 5 (MG5) but not identified by clinical examination. A, Color fundus photograph. B, Late fluorescein angiogram frame. C, Macular Grid 5 thickness map. D, Fast Macular Thickness Map (FMTM). The map of edema as identified by the MG5 algorithms is delineated by the white checkered zone in C. Note that the majority of the areas of retinal thickening were in the outer circle (i.e., >1 disk diameter from the foveal center). Note also the difference in configuration of the areas of retinal thickening in the outer circle between the MG5 map and the FMTM, which relies on more interpolation between data points in the outer zone.
The minimum distance between the foveal center and the edge of the MG5-identified area of edema was compared with the measured logarithm of the minimum angle of resolution visual acuity at the time of assessment. For cases in which the edema extended into the foveal center, the distance was expressed as a negative value, indicating the minimum distance to nonedematous retina. No significant correlation between acuity and distance to edema edge was observed (data not shown).
Discussion
In this report, we describe a new macular grid scan pattern (MG5) for the Stratus OCT that provides a more evenly sampled map of the macula compared with standard radial line and fast macular thickness (FMTM) protocols commonly used by clinicians in practice for the quantification of DME. The sampling gap in the FMTM steadily rises with increasing eccentricity (Fig 2), with a gap between points that can be 5-fold greater than the MG5 at a distance of 2.5 mm from the foveal center. The large gaps (up to 1.6 mm) in the FMTM are of particular concern when considering the assessment of CSME, which, in some cases, may not extend closer than 1 mm from the foveal center.
The MG5 retinal thickness maps of the normal reference population used in this study appeared to be similar to those described previously using conventional 6–radial line patterns.24,25 Similar to these previous studies, the SD of the retinal thickness was higher in the foveal subfield than in the more eccentric fields. Although the observed foveal variability could be due to true heterogeneity within the normal population, it is likely at least in part due to differences in fixation stability and movement artifact between patients.
In this analysis, the processing program used to generate thickness measurements and assessments of edema from the MG5 scan seemed to be both accurate and repeatable for any given subject. The average difference between area-weighted average thickness was only 4.5 μm between FMTM and MG5 scans, and was only 4.7 μm between the 2 MG5 scans. Moreover, the coefficient of variation in the area of macular edema between repeat scans in this series was only 6.8%.
Reproducibility of OCT thickness measurements is of paramount importance in clinical trials and in monitoring the response of patients to therapy in clinical practice. Excellent reproducibility of thickness measurements has been demonstrated in normal subjects, with a repeatability coefficient of <7 μm in one study by Massin et al24 and an intervisit SD of only 2.4 μm in a series by Paunescu et al.25
In Massin et al's study,24 the repeatability coefficient for patients with DME was worse but still reasonably good, measuring <21 μm, in all but 1 of 10 patients. Browning,26 however, observed considerable variability in foveal zone measurements between observers in patients with DME. The variability was believed to be due to unstable fixation in patients with reduced vision as a result of foveal edema. Not surprisingly, Browning26 observed less variability in the total macular volume between observers than in the foveal center thickness, and suggested that macular volume measurements may be the preferred outcome measure for future studies. The improved repeatability of the total macular volume is likely due to the larger area sampled by the parameter, thereby rendering the measure less sensitive to fixation errors. The downside of using the total macular volume for clinical trials, however, is that it may also be less sensitive for detecting changes in edema if the area of edema is small relative to the area of the entire macula. The development of algorithms to identify the area (or volume) of edema, as described in this study, may address these limitations. Because an area (or volume) is measured, rather than the thickness at a single point (such as the foveal center), the parameter also will be less sensitive to fixation errors as long as the edematous region remains within the scanned zone. At the same time, because only the edematous region is measured, the method will retain its sensitivity to detect changes in the edema. This retinal edema area or volume may be a valuable parameter for clinicians in following their patients' response to therapy, and the edema maps (see checkered outline in Figs 6, 7 [available at http://aaojournal.org], and 8-10) produced by the MG5 grid analysis provide the clinician with a quick and tangible snapshot of the patient's retinal status.
If scanned data are to be used to quantify retinal edema, the use of a scanning pattern, such as the MG5, that provides a more uniform or evenly spaced sampling of the macula would seem to be preferred. In this study, the MG5 seemed to show good agreement with the clinical examination for the detection of CSME. The MG5 assessment of CSME 1 also showed good agreement with the FMTM. The agreement for identification of CSME 2 by the MG5 seemed to be better than that for CSME 1, possibly reflecting the improved sampling in the outer zones of the grid.
It is important to note that the thresholds for identifying edematous areas (99%–99.5% confidence interval relative to the reference mean) were arbitrarily chosen in an attempt to achieve reasonable sensitivity without a severe penalty in loss of specificity. This standard MG5 threshold, however, was applied consistently to all cases, in contrast to the subjective clinician assessments, which may have varied due to the assessment method used by the grader (e.g., contact lens vs. 90-diopter lens examination) or the level of stereopsis available in the fundus photographs. This variability in the clinical assessment may explain the few cases of disagreement observed in this study (some examples illustrated in Figs 8-10). Review of all of these cases of disagreement suggested that assessments by clinicians could be biased by context. For example, the presence of lipid exudates seemed to increase the clinician's sensitivity for identifying retinal thickening. Although this may have been an attempt by the clinician to identify the third ETDRS category of CSME (lipid within 500 [H9262]m of the foveal center with adjacent thickening), this was not supported by the OCT maps, which did not appear to show more marked retinal thickening in areas adjacent to the lipid. These observations are consistent with the mean foveal thickness measurements in Table 4, which suggest that, in some cases, clinicians can identify edema that is recognizable on the OCT map but is below the arbitrary threshold chosen in this study. On the other hand, the presence of severe thickening in some areas seemed to reduce the clinician's sensitivity for detecting milder (though well above the MG5 threshold) degrees of thickening in adjacent or contiguous regions (Fig 10, Table 3). These cases and the mean thickness measurements shown in Tables 3 and 4 highlight the variability and subjectivity of clinical assessments and the apparent value of the objective and reproducible threshold utilized by the MG5 analysis, particularly in cases of borderline CSME.
Aside from the somewhat arbitrary thresholds for identifying areas of edema, another significant limitation of the study is its retrospective design. Although the photography protocol used in the imaging unit was well standardized and consistently applied, there may be some variability in quality of the clinician's assessment of macular edema as a result of the retrospective nature of the study. Clinicians judged edema based on their prior training and experience. No protocol or specialized training was provided to clinicians to standardize their recognition and diagnosis of areas of retinal edema. In addition, in some cases stereoscopic photographs were used to assist in classifying the edema. These limitations may account for the variability in clinician assessment of edema observed in this study.
The additional information (by improved sampling) from the perifoveal macula provided by the MG5 may be of value in future clinical studies and in monitoring the response of nonfoveal edema to intervention. The MG5 data also can be used to generate other potentially useful parameters such as the distance between the edema and the foveal center. In addition, the ability of these algorithms to detect macular edema automatically and objectively may assist clinicians in identifying patients requiring treatment. The maps of the areas of retinal thickening also may be of value in planning the location and extent of focal laser treatment. Moreover, automated detection of CSME by OCT MG5 may be of value in screening programs27-33 aimed at identifying patients with sight-threatening retinopathy.
Automated classification using the MG5 scan pattern in this study generally correlated well with clinical grading and standard OCT analysis (FMTM). It is important to note that MG5 algorithms were designed to detect CSME and not subclinical macular edema evident only on OCT. MG5 provides considerably more information in the perifoveal macula than FMTM and may facilitate the diagnosis and monitoring of nonfoveal CMSE. Automated grading improves the objectivity, reproducibility, sensitivity, and precision of CSME diagnosis and may serve as a useful tool both in future clinical studies and in clinical practice.
Acknowledgments
The authors acknowledge the Advanced Imaging for Glaucoma Study Group clinical investigators for recruiting the normal patients.
Footnotes
Supported in part by National Institutes of Health, Bethesda, Maryland (grant nos.: R01 EY013516, R01-EY013178-5, P30-EY008098); Eye and Ear Foundation, Pittsburgh, Pennsylvania; National Eye Institute and National Center on Minority Health and Health Disparities (grant nos.: EY 11753, EY 03040); and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York. Dr Varma ia a Research to Prevent Blindness Sybil B. Harrington Scholar.
Drs Huang and Schuman receive research support from Carl Zeiss Meditec (Dublin, California), the manufacturer of optical coherence tomography devices. Dr Huang also receives patent royalties from optical coherence tomography.
References
- 1.Hainsworth DP. The challenge of preventing vision loss from diabetic retinopathy. Mo Med. 2005;102:41–5. [PubMed] [Google Scholar]
- 2.Fong DS, Sharza M, Chen W, et al. Vision loss among diabetics in a group model Health Maintenance Organization (HMO) Am J Ophthalmol. 2002;133:236–41. doi: 10.1016/s0002-9394(01)01364-2. [DOI] [PubMed] [Google Scholar]
- 3.Fong DS, Ferris FL, III, Davis MD, Chew EY, Early Treatment Diabetic Retinopathy Study Research Group Causes of severe visual loss in the Early Treatment Diabetic Retinopathy Study: ETDRS report no. 24. Am J Ophthalmol. 1999;127:137–41. doi: 10.1016/s0002-9394(98)00309-2. [DOI] [PubMed] [Google Scholar]
- 4.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 5.Haik GM, Jr, Terrell WL, III, Haik GM., Sr Diabetic retinopathy: a leading cause of new blindness. South Med J. 1989;82:575–9. [PubMed] [Google Scholar]
- 6.Early Treatment Diabetic Retinopathy Study Research Group Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 2. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 7.Early Treatment Diabetic Retinopathy Study Research Group Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 8.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995;113:325–32. doi: 10.1001/archopht.1995.01100030081025. [DOI] [PubMed] [Google Scholar]
- 10.Kinyoun J, Barton F, Fisher M, et al. ETDRS Research Group Detection of diabetic macular edema. Ophthalmoscopy versus photography—Early Treatment Diabetic Retinopathy Study report number 5. Ophthalmology. 1989;96:746–50. doi: 10.1016/s0161-6420(89)32814-4. discussion 750–1. [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98(suppl):786–806. [PubMed] [Google Scholar]
- 12.Brown JC, Solomon SD, Bressler SB, et al. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–5. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- 13.Panozzo G, Gusson E, Parolini B, Mercanti A. Role of OCT in the diagnosis and follow up of diabetic macular edema. Semin Ophthalmol. 2003;18:74–81. doi: 10.1076/soph.18.2.74.15854. [DOI] [PubMed] [Google Scholar]
- 14.Broecker EH, Dunbar MT. Optical coherence tomography: its clinical use for the diagnosis, pathogenesis, and management of macular conditions. Optometry. 2005;76:79–101. doi: 10.1016/s1529-1839(05)70262-1. [DOI] [PubMed] [Google Scholar]
- 15.Giovannini A, Amato G, Mariotti C, Scassellati-Sforzolini B. Optical coherence tomography findings in diabetic macular edema before and after vitrectomy. Ophthalmic Surg Lasers. 2000;31:187–91. [PubMed] [Google Scholar]
- 16.Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–77. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 17.Goebel W, Kretzchmar-Gross T. Retinal thickness in diabetic retinopathy: a study using optical coherence tomography (OCT) Retina. 2002;22:759–67. doi: 10.1097/00006982-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Moreira RO, Trujillo FR, Meirelles RM, et al. Use of optical coherence tomography (OCT) and indirect ophthalmoscopy in the diagnosis of macular edema in diabetic patients. Int Ophthalmol. 2001;24:331–6. doi: 10.1023/b:inte.0000006784.52885.e9. [DOI] [PubMed] [Google Scholar]
- 19.Micelli Ferrari T, Sborgia L, Furino C, et al. Intravitreal triamcinolone acetonide: valuation of retinal thickness changes measured by optical coherence tomography in diffuse diabetic macular edema. Eur J Ophthalmol. 2004;14:321–4. [PubMed] [Google Scholar]
- 20.Gaucher D, Tadayoni R, Erginay A, et al. Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am J Ophthalmol. 2005;139:807–13. doi: 10.1016/j.ajo.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 24.Massin P, Vicaut E, Haouchine B, et al. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–42. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 25.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45:1716–24. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browning DJ. Interobserver variability in optical coherence tomography for macular edema. Am J Ophthalmol. 2004;137:1116–7. doi: 10.1016/j.ajo.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Ruamviboonsuk P, Wongcumchang N, Surawongsin P, et al. Screening for diabetic retinopathy in rural area using single-field, digital fundus images. J Med Assoc Thai. 2005;88:176–80. [PubMed] [Google Scholar]
- 28.Cavallerano AA, Cavallerano JD, Katalinic P, et al. Joslin Vision Network Research Team A telemedicine program for diabetic retinopathy in a Veterans Affairs Medical Center—the Joslin Vision Network Eye Health Care Model. Am J Ophthalmol. 2005;139:597–604. doi: 10.1016/j.ajo.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 29.Neubauer AS, Welge-Lussen UC, Thiel MJ, et al. Tele-screening for diabetic retinopathy with the retinal thickness analyzer. Diabetes Care. 2003;26:2890–7. doi: 10.2337/diacare.26.10.2890. [DOI] [PubMed] [Google Scholar]
- 30.Olson JA, Strachan FM, Hipwell JH, et al. A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med. 2003;20:528–34. doi: 10.1046/j.1464-5491.2003.00969.x. [DOI] [PubMed] [Google Scholar]
- 31.Al Sabti K, Raizada S, Wani VB, et al. Efficacy and reliability of fundus digital camera as a screening tool for diabetic retinopathy in Kuwait. J Diabetes Complications. 2003;17:229–33. doi: 10.1016/s1056-8727(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 32.Maberley D, Cruess AF, Barile G, Slakter J. Digital photographic screening for diabetic retinopathy in the James Bay Cree. Ophthalmic Epidemiol. 2002;9:169–78. doi: 10.1076/opep.9.3.169.1517. [DOI] [PubMed] [Google Scholar]
- 33.Tennant MT, Rudnisky CJ, Hinz BJ, et al. Tele-ophthalmology via stereoscopic digital imaging: a pilot project. Diabetes Technol Ther. 2000;2:583–7. doi: 10.1089/15209150050502005. [DOI] [PubMed] [Google Scholar]