Short abstract
Serpins are protease inhibitors that use a conformational change to inhibit target enzymes and are important in many proteolytic cascades, including the mammalian coagulation and inflammatory-response pathways.
Abstract
Serpins are a broadly distributed family of protease inhibitors that use a conformational change to inhibit target enzymes. They are central in controlling many important proteolytic cascades, including the mammalian coagulation pathways. Serpins are conformationally labile and many of the disease-linked mutations of serpins result in misfolding or in pathogenic, inactive polymers.
Serpins (serine protease inhibitors or classified inhibitor family I4) are the largest and most broadly distributed superfamily of protease inhibitors [1,2]. Serpin-like genes have been identified in animals, poxviruses, plants, bacteria and archaea, and over 1,500 members of this family have been identified to date. Analysis of the available genomic data reveals that all multicellular eukaryotes have serpins: humans, Drosophila, Arabidopsis thaliana and Caenorhabditis elegans have 36, 13, 29, and about 9 serpin-like genes, respectively [1,3]. In contrast, serpins in prokaryotes are sporadically distributed and most serpin-containing prokaryotes have only a single serpin gene [4]. The majority of serpins inhibit serine proteases, but serpins that inhibit caspases [5] and papain-like cysteine proteases [6,7] have also been identified. Rarely, serpins perform a non-inhibitory function; for example, several human serpins function as hormone transporters [8] and certain serpins function as molecular chaperones [9] or tumor suppressors [10]. A phylogenetic study of the superfamily divided the eukaryotic serpins into 16 'clades' (termed A-P) [1]. The proteins are named SERPINXy, where X is the clade and y is the number within that clade; many serpins also have alternative names from before this classification was proposed.
Serpins are relatively large molecules (about 330-500 amino acids) in comparison with protease inhibitors such as basic pancreatic trypsin inhibitor (BPTI, which is about 60 amino acids) [11]. Over 70 serpin structures have been determined, and these data, along with a large amount of biochemical and biophysical information, reveal that inhibitory serpins are 'suicide' or 'single use' inhibitors that use a unique and extensive conformational change to inhibit proteases [12]. This conformational mobility renders serpins heat-labile and vulnerable to mutations that promote misfolding, spontaneous conformational change, formation of inactive serpin polymers and serpin deficiency [13]. In humans, several conformational diseases or 'serpinopathies' linked to serpin polymerization have been identified, including emphysema (SERPINA1 (antitrypsin) deficiency) [14], thrombosis (SERPINC1 (antithrombin) deficiency) [15] and angio-edema (SERPING1 (C1 esterase inhibitor) deficiency) [16]. Accumulation of serpin polymers in the endoplasmic reticulum of serpin-secreting cells can also result in disease, most notably cirrhosis (SERPINA1 polymerization) [14] and familial dementia (SERPINI1 (neuroserpin) polymerization) [17]. Other serpin-related diseases are caused by null mutations or (rarely) point mutations that alter inhibitory specificity or inhibitory function [18]. Here, we summarize the evolution, structure and mechanism of serpin function and dysfunction.
Broad organization of the serpin superfamily
Serpins appear to be ubiquitous in multicellular higher eukaryotes and in the poxviridae pathogens of mammals. In humans, the two largest clades of the 36 serpins that have been identified are the extracellular 'clade A' molecules (thirteen members found on chromosomes 1, 14 and X) and the intracellular 'clade B' serpins (thirteen members on chromosomes 18 and 6) [3].
Recent bioinformatic and structural studies have also identified inhibitory serpins in the genomes of certain primitive unicellular eukaryotes (such as Entamoeba histolytica [19]) as well as prokaryotes [4,20]. No fungal serpin has been identified to date, and the majority of prokaryotes do not contain clearly identifiable serpin-like genes. Phylogenetic analyses have found no evidence for horizontal transfer [1,21], and it is instead suggested that serpins are ancient proteins and that most prokaryotes have lost the requirement for serpin-like activity [4].
Functional diversity of serpins
Inhibitory serpins have been shown to function in processes as diverse as DNA binding and chromatin condensation in chicken erythrocytes [22,23], dorsal-ventral axis formation and immunoregulation in Drosophila and other insects [24,25], embryo development in nematodes [26], and control of apoptosis [5].
In humans, the majority (27 out of 36) of serpins are inhibitory (Table 1). Clade A serpins include inflammatory response molecules such as SERPINA1 (antitrypsin) and SERPINA3 (antichymotrypsin) as well as the non-inhibitory hormone-transport molecules SERPINA6 (corticosteroid-binding globulin) and SERPINA7 (thyroxine-binding globulin). Clade B includes inhibitory molecules that function to prevent inappropriate activity of cytotoxic apoptotic proteases (SERPINB6, also called PI6, and SERPINB9, also called PI9) and inhibit papain-like enzymes (SERPINB3, squamous cell carcinoma antigen-1) as well as the non-inhibitory molecule SERPINB5 (maspin). SERPINB5 does not undergo the characteristic serpin-like conformational change and functions to prevent metastasis in breast cancer and other cancers through an incompletely characterized mechanism [10,27]. The roles of several other well characterized human serpins are also summarized in Table 1.
Table 1.
Function and dysfunction of human serpins
| Serpin | Alternative name(s) | Protease target or function | Involvement in disease |
| SERPINA1 | Antitrypsin | Extracellular; inhibition of neutrophil elastase | Deficiency results in emphysema: polymerization and retention in the ER results in cirrhosis [14,64,65] |
| SERPINA2 | Antitrypsin-related protein | Not characterized, probable pseudogene | |
| SERPINA3 | Antichymotrypsin | Extracellular; inhibition of cathepsin G | Deficiency results in emphysema (see [61] for a review) |
| SERPINA4 | Kallistatin (PI4) | Extracellular, inhibition of kallikrein [68] | |
| SERPINA5 | Protein C inhibitor (PAI-3) | Extracellular; inhibition of active protein C (see [69] for a review) | Angioedema |
| SERPINA6 | Corticosteroid-binding globulin | Extracellular; non-inhibitory; cortisol binding | Deficiency linked to chronic fatigue [83,84] |
| SERPINA7 | Thyroxine-binding globulin | Extracellular; non-inhibitory, thyroxine binding | Deficiency results in hypothyroidism [85] |
| SERPINA8 | Angiotensinogen | Extracellular; non-inhibitory; amino-terminal cleavage by the protease renin results in release of the decapeptide angiotensin I | Certain variants linked to essential hypertension [86] |
| SERPINA9 | Centerin | Extracellular; maintenance of naive B cells [70] | |
| SERPINA10 | Protein Z-dependent proteinase inhibitor | Extracellular; inhibition of activated factor Z and XI | Deficiency linked to venous thromboembolic disease [87] |
| SERPINA11 | XP_170754.3 | Not characterized | |
| SERPINA12 | Vaspin | Extracellular; insulin-sensitizing adipocytokine [71] | |
| SERPINA13 | XM_370772 | Not characterized | |
| SERPINB1 | Monocyte neutrophil elastase inhibitor | Intracellular; inhibition of neutrophil elastase [72] | |
| SERPINB2 | Plasminogen activator inhibitor-2 (PAI2) | Intracellular; inhibition of uPA (see [73] for a review) | |
| SERPINB3 | Squamous cell carcinoma antigen-1 | Intracellular; cross-class inhibition of cathepsins L and V [6] | |
| SERPINB4 | Squamous cell carcinoma antigen-2 | Intracellular; cross-class inhibition of cathepsin G and chymase [74] | |
| SERPINB5 | Maspin | Intracellular; non-inhibitory; inhibition of metastasis through uncharacterized mechanism | Downregulation and/or intracellular location linked to tumor progression and overall prognosis [10] |
| SERPINB6 | Proteinase inhibitor-6 (PI6) | Intracellular, inhibition of cathepsin G [75] | |
| SERPINB7 | Megsin | Intracellular; megakaryocyte maturation [76] | IgA nephropathy |
| SERPINB8 | Cytoplasmic antiproteinase 8 (PI8) | Intracellular; inhibition of furin [77] | |
| SERPINB9 | Cytoplasmic antiproteinase 9 (PI9) | Intracellular, inhibition of granzyme B [78] | |
| SERPINB10 | Bomapin (PI10) | Intracellular; inhibition of thrombin and trypsin [79] | |
| SERPINB11 | Epipin | Intracellular | |
| SERPINB12 | Yukopin | Intracellular; inhibition of trypsin [80] | |
| SERPINB13 | Headpin (PI13) | Intracellular; inhibition of cathepsins L and K | |
| SERPINC1 | Antithrombin | Extracellular; thrombin and factor Xa inhibitor | Deficiency results in thrombosis (see [88] for review) |
| SERPIND1 | Heparin cofactor II | Extracellular; thrombin inhibitor | May contribute to thrombotic risk when combined with other deficiencies [89] |
| SERPINE1 | Plasminogen activator inhibitor I (PAI1) | Extracellular; inhibitor of thrombin, uPA, tPA and plasmin | Abnormal bleeding [90] |
| SERPINE2 | Protease nexin I (PI7) | Extracellular; inhibition of uPA and tPA | |
| SERPINE3 | Hs.512272 | Not characterized | |
| SERPINF1 | Pigment epithelium derived factor | Non-inhibitory; potent anti-angiogenic molecule [81] | |
| SERPINF2 | Alpha-2-antiplasmin | Extracellular; plasmin inhibitor | Unrestrained fibrinolytic activity, bleeding [91] |
| SERPING1 | C1 inhibitor | C1 esterase inhibitor | Angioedema [92] |
| SERPINH1 | 47kDa heat-shock protein | Non-inhibitory molecular Chaperone for collagens [9] | |
| SERPINI1 | Neuroserpin (PI12) | Extracellular; inhibitor of tPA, uPA and plasmin | Polymerization results in dementia [17] |
| SERPINI2 | Myoepithelium-derived serine proteinase inhibitor (PI14) | Extracellular; inhibition of cancer metastasis [82] |
Numerous important branches of the serpin superfamily remain to be functionally characterized. For example, although plants have a large number of serpin genes, the function of plant serpins remains obscure. Studies in vitro clearly show that plant serpins can function as protease inhibitors [28], but plants lack close relatives of chymotrypsin-like proteases, which would be the obvious targets for these serpins. Thus, it has been suggested that plant serpins may be involved in inhibiting proteases in plant pathogens; for example, they may be targeting digestive proteases in insects [29]. One study convincingly demonstrated a close inverse correlation between the upregulation of Cucurbita maxima (squash) phloem serpin-1 (CmPS) and aphid survival [30]. Feeding experiments in vitro showed, however, that purified CmPS did not affect insect survival [30]. Together, these data suggest that rather than directly interacting with the pathogen, plant serpins, like their insect counterparts, may have a role in the complex pathways involved in upregulating the host immune response.
Similarly, the role of serpins in prokaryotes remains to be understood; again, these molecules are capable of inhibitory activity in vitro [20], but their targets in vivo and their function remain to be characterized. Interestingly, several inhibitory prokaryote serpins are found in extremophiles that live at elevated temperatures (for example, Pyrobaculum aerophilum, which lives at 100°C); these serpins use novel strategies to function as inhibitors at elevated temperatures while resisting inappropriate conformational change [4,20,31].
Structural biology of the serpins and the mechanism of protease inhibition
Serpins are made up of three β sheets (A, B and C) and 8-9 α helices (termed hA-hI). Figure 1a shows the native structure of the archetypal serpin SERPINA1 [32]. The region responsible for interaction with target proteases, the reactive center loop (RCL), forms an extended, exposed conformation above the body of the serpin scaffold. The remarkable conformational change characteristic of inhibitory serpins is depicted in Figure 1d; the structure of SERPINA1 with its RCL cleaved [33] shows that, following proteolysis, the amino-terminal portion of the RCL inserts into the center of β-sheet A to form an additional (fourth) strand (s4A). This conformational transition is termed the 'stressed (S) to relaxed (R) transition', as the cleavage of native inhibitory serpins results in a dramatic increase in thermal stability. Native serpins are therefore trapped in an intermediate, metastable state, rather than their most stable conformation, and thus represent a rare exception to Anfinsen's conjecture, which predicts that a protein sequence will fold to a single structure that represents the lowest free-energy state [34].
Figure 1.
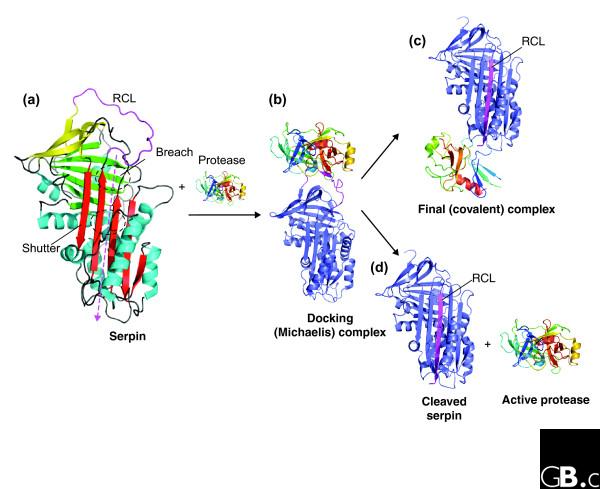
The structure and mechanism of inhibitory serpins. (a) The structure of native SERPINA1 (Protein Data Bank (PDB) code 1QLP) [32]. The A sheet is in red, the B sheet in green and the C sheet in yellow; helices (hA-hI) are in blue. The reactive center loop (RCL) is at the top of the molecule, in magenta. The position of the breach and the shutter are labeled and the path of RCL insertion indicated (magenta dashed line). Both of these regions contain several highly conserved residues, many of which are mutated in various serpinopathies. (b) The Michaelis or docking complex between SERPINA1 and inactive trypsin (PDB code 1OPH) [36], with the protease (multicolors) docked onto the RCL (magenta). Upon docking with an active protease (b), two possible pathways are apparent. (c) The final serpin enzyme complex (PDB code 1EZX [12]). The serpin has undergone the S to R transition, and the protease hangs distorted at the base of the molecule. (d) The structure of cleaved SERPINA1 is shown (PDB code 7API) [93]) with the RCL (magenta) forming the fourth strand of β-sheet A. The result of serpin substrate-like behavior can be seen where the protease has escaped the conformational trap, leaving active protease and inactive, cleaved serpin. Certain serpin mutations, particularly non-conservative substitutions within the hinge region of the RCL, result in substrate-like, rather than inhibitory, behavior [94].
Serpins use the S-to-R transition to inhibit target proteases. Figure 1b shows the structure of an initial docking complex between a serpin and a protease (SERPINA1 and trypsin [35,36]) and Figure 1c shows the final serpin-enzyme complex [12]. These structural studies [12,35,36], combined with extensive biochemical data, revealed that RCL cleavage and subsequent insertion is crucial for effective protease inhibition. In the final serpin-protease complex, the protease remains covalently linked to the serpin, the enzyme being trapped at the acyl-intermediate stage of the catalytic cycle. Structural comparisons show that the protease in the final complex is severely distorted in comparison with the native conformation, and that much of the enzyme is disordered [12]. In addition, a fluorescence study demonstrated that the protease was partially unfolded in the final complex [37]. These conformational changes lead to distortion at the active site, which prevents efficient hydrolysis of the acyl intermediate and the subsequent release of the protease. These data are consistent with the observation that buried or cryptic cleavage sites within trypsin become exposed following complex formation with a serpin [38]. It is possible that cleavage of such cryptic sites within the protease occurs in vivo and thus results in permanent enzyme inactivation. The absolute requirement for RCL cleavage, however, means that serpins are irreversible 'suicide' inhibitors.
A major advantage of the serpin fold over small protease inhibitors such as BPTI is that the inhibitory activity of serpins can be exquisitely controlled by specific cofactors. For example, human SERPINC1 (antithrombin) is a relatively poor inhibitor of the proteases thrombin and factor Xa until it is activated by the cofactor heparin [39]. Structural studies of SERPINC1 highlight the molecular basis for heparin function. Figure 2a shows the structure of native SERPINC1. Here, we use the convention of Schechter and Berger, in which residues on the amino-terminal side of the cleavage site (P1/P1') are termed P2, P3, and so on, and those carboxy-terminal are termed P2', P3', and so on; corresponding subsites in the enzyme are termed S1, S2, and so on [40]. The RCL is partially inserted into the top of the 3 sheet; the residue (P1-Arg) responsible for docking into the primary specificity pocket (S1) of the protease is relatively inaccessible to docking with thrombin, as it is pointing towards and forming interactions with the body of the serpin [41,42]. Figure 2b illustrates the ternary complex between SERPINC1, thrombin and heparin [43]. Upon interaction with a specific heparin pentasaccharide sequence present in high-affinity heparin, SERPINC1 undergoes a substantial conformational rearrangement whereby the RCL is expelled from β-sheet A and the P1 residue flips to an exposed protease-accessible conformation [44-46]]. In addition to loop expulsion and P1 exposure, long-chain heparin can bind both enzyme and inhibitor and thus provides an additional acceleration of the inhibitory interaction. Several other serpins, including SERPIND1 (heparin cofactor II), also use cofactor binding and conformational change to achieve exquisite inhibitory control [47].
Figure 2.
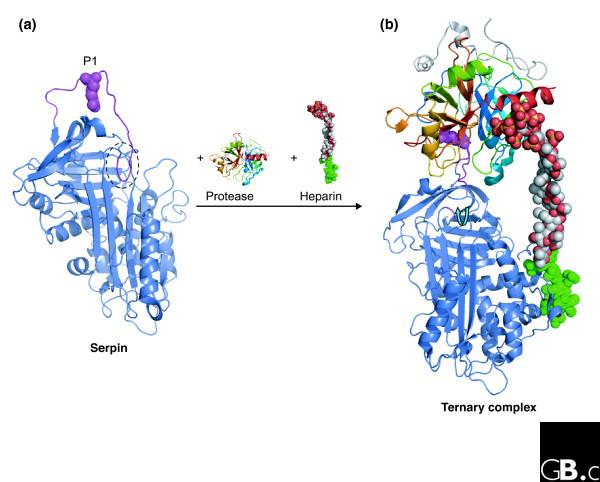
Modulation of serpin conformation by cofactors. (a) The structure of native SERPINC1 (PDB code 2ANT) [95]. The partial insertion of the RCL (two residues) into the top of β-sheet A is circled, and the position of the P1 residue is shown (magenta spheres). (b) The structure of the ternary complex between SERPINC1, inactive thrombin (the Ser195Ala mutant) and a synthetic long-chain heparin construct (PDB code 1TB6) [43]. A specific high-affinity pentasaccharide (green) on the heparin interacts with the heparin-binding site on SERPINC1 (on and around helix hD) and promotes expulsion of the RCL (blue arrow) and rearrangement of the P1 residue (magenta spheres).
Structural studies on prokaryote and viral serpins have revealed several interesting variations of the serpin scaffold. Viral proteins are often 'stripped down' to a minimal scaffold in order to minimize the size of the viral genome. Consistent with this requirement, the structure of the viral serpin crmA, one of the smallest members of the serpin superfamily [48,49], shows that it lacks helix hD. More recently, the structure of the prokaryote serpin thermopin from Thermobifida fusca revealed the absence of helix hH [20,31]. These studies also showed that thermopin contains a 4 amino-acid insertion at the carboxyl terminus that forms extensive interactions with conserved residues at the top of β-sheet A (called the 'breach'; see later); biophysical data suggest that this region is important for proper and efficient folding of this unusual serpin.
The major conformational change that occurs within both the protease and the serpin as a result of serpin-enzyme complex formation provides an elegant mechanism for cells to specifically detect and clear inactivated serpin-protease complexes. Several studies have shown that the low density lipoprotein-related protein (LRP) specifically binds to and promotes internalization of the final complexes SERPINC1-thrombin, SERPIND1-thrombin and SERPINA1-trypsin. In contrast, native or cleaved serpin alone are not internalized [50]. Additionally, recent studies on SERPINI1 show that both SERPINI1-tissue plasminogen activator complexes and native SERPINI1 are internalized in an LRP-dependent manner. However, while SERPINI1-tissue plasminogen activator complexes can bind directly to LRP, native SERPINI1 requires the presence of an (as yet unidentified) cofactor [51]. The structural basis for interaction of LRP with serpin-enzyme complexes and the subsequent intracellular signaling response remain to be fully understood. It is clear, however, that native serpins and serpin-enzyme complexes can induce powerful responses such as cell migration in an LRP-dependent manner [52].
Inactivation of serpins: latency, polymerization, deficiency and disease
The metastability of serpins and their ability to undergo controlled conformational change also renders these molecules susceptible to spontaneous conformational rearrangements. Most notably, the serpin SERPINE1 (plasminogen activator inhibitor-1) uses spontaneous conformational change to control inhibitory activity [53]. Structural and biochemical studies show that, in the absence of the cofactor vitronectin, native SERPINE1 (Figure 3a) rapidly converts to a latent inactive state (Figure 3b). The transition to latency is accompanied by insertion of the RCL into β-sheet A, where it cannot interact with the target protease. Interestingly, the structure of SERPINE1 in complex with the somatomedin B domain of vitronectin [54] shows that the cofactor-binding site on SERPINE1 is located in a similar region to the heparin-binding site of SERPINC1 (on and around helices hD and hE; Figure 3c). Whereas heparin promotes conformational change in SERPINC1, however, vitronectin prevents conformational change in SERPINE1. Several other serpins, including SERPINC1, have been shown to spontaneously undergo the transition to the latent state, and it is suggested that this may be an important control mechanism [55].
Figure 3.
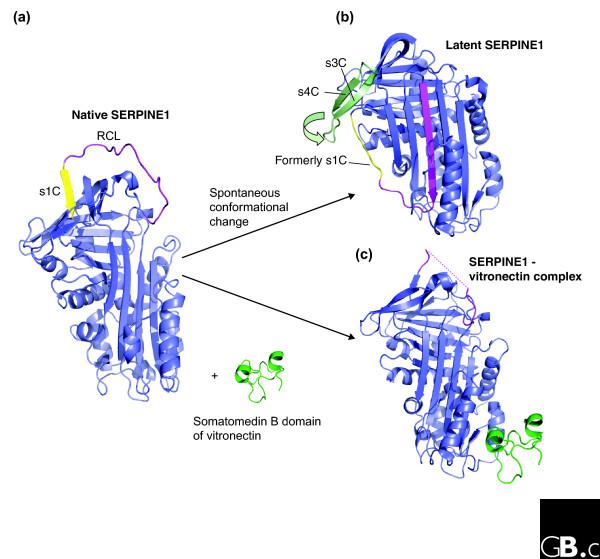
Spontaneous conformational change in serpins. (a) Structure of native SERPINE1 (PDB code 1B3K) [96]. The RCL is in magenta and strand s1c of β-sheet C is in yellow. (b) The structure of latent SERPINE1 (PDB code 1DVN) [53,97], which can form by spontaneous conversion from the native protein. The RCL (magenta) is inserted into β-sheet A. In order to enable full insertion of the RCL, s1C of β-sheet C (pale yellow) has peeled off. In addition, conformational change in the strands s3C and s4C (pale green) is indicated. (c) Structure of SERPINE1 (blue) in complex with the somatomedin B domain (green) of vitronectin (PDB code 1OC0) [54]. The interaction with vitronectin locks SERPINE1 in the native, active conformation.
Although the transition to latency could be an important control mechanism in at least one serpin, an alternative spontaneous conformational change, serpin polymerization, results in deficiency and disease (or serpinopathy) [14,56]. Serpin polymerization is postulated to occur via a domain-swapping event whereby the RCL of one molecule docks into β-sheet A of another to form an inactive long-chain serpin polymer (Figure 4a, b) [14,57-59]. Several important human serpin variants result in polymerization, the best studied and most common of which is the Z allele (Glu342Lys) of SERPINA1 [14]. Here, failure to properly control the activity of neutrophil elastase (the inhibitory target of SERPINA1) in the lung during the inflammatory response results in the destruction of lung tissue, leading to emphysema. Furthermore, in individuals homozygous for the Z-variant, the accumulation of serpin aggregates or polymers in the endoplasmic reticulum of anti-trypsin-producing cells, the hepatocytes, can eventually result in cell death and liver cirrhosis [14]. Similarly, mutation of SERPINI1 results in the formation of neural inclusion bodies and in the disease 'familial encephalopathy with neuroserpin inclusion bodies' (FENIB) [17,60,61].
Figure 4.
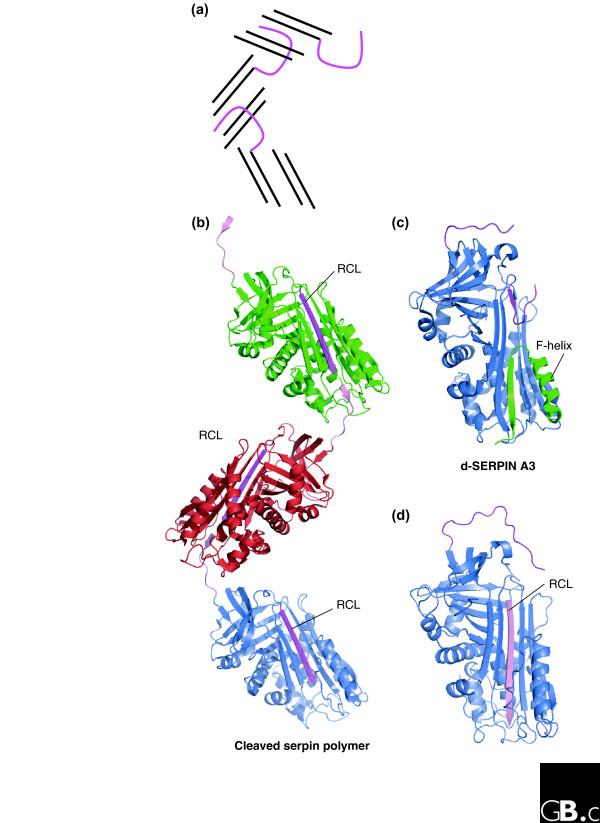
Structure of serpin polymers and other inactive conformers. (a) Schematic diagram of domain swapping in serpins; the RCL of one molecule (magenta loop), is docked into β-sheet A (black lines) of the next (only four strands of β-sheet A are shown). (b) Structure of a cleaved serpin polymer (PDB code 1D5S) [57], showing the promiscuous nature of the RCL. Cleavage at the P5/P6 position has resulted in RCL (magenta) insertion into β-sheet A; the 'gap' at the bottom of β-sheet A is filled with the P5-P1 portion (pale pink) from an RCL from another molecule. (c) The structure of an alternative confirmation of SERPINA3 -δ-SERPINA3 (PDB code 1QMN) [62]. Four residues of the RCL (magenta) are inserted into the top of β-sheet A. The F-helix (green) has partially unwound and filled the bottom half of β-sheet A. (d) Serpins can accept a peptide with the sequence of the RCL (pale pink) into β-sheet A (PDB code 1BR8) [98].
In addition to promoting polymerization, several serpin mutations have been identified that promote formation of a disease-linked latent state. Notably, a mutation in SERPINC1, the wibble variant (Thr85Met), results in formation of large amounts of circulating latent SERPINC1 (about 10% of total SERPINC1) [55]. An alternative 'half-way house' conformation of SERPINA3, termed δ, has also been identified (Figure 4c) [62]. The structure of δ-SERPINA3 also highlights the extraordinary flexibility of the serpin scaffold: in this conformation the RCL is partially inserted into β-sheet A and helix hF has partially unwound and inserted into the base of β-sheet A, completing the β-sheet hydrogen bonding (Figure 4c). Finally, the promiscuity of β-sheet A is highlighted by the ability of this region to readily accept short peptides: several structural and biochemical studies have demonstrated that peptides can bind to β-sheet A and induce the S-to-R transition (Figure 4d).
Valuable insights into the mechanism of serpin function have been gleaned from the structural location of variants that promote serpin instability [18,63]. The majority of serpinopathy-linked mutations (including antitrypsin Siiyama [64] and Mmalton [65], antithrombin wibble [55] and δ-SERPINA3 [62]) cluster in the center of the serpin molecule, underneath β-sheet A, in a region termed the shutter (marked on Figure 1a). Interestingly, Glu342, the position mutated in the Z allele of SERPINA1, is located at the breach, which is just above the shutter at the top of β-sheet A. This portion of the molecule is the point of initial RCL insertion. It is suggested that destabilization of β-sheet A in either the shutter or the breach is sufficient to favor the transition to a polymeric or latent state over maintenance of the monomeric metastable native state [14]. Interestingly, analysis of conserved residues in the serpin superfamily also reveals a striking distribution of highly conserved residues stretching down the center of β-sheet A from the breach to the base of the molecule [1].
Unsurprisingly, given the important proteolytic processes they control, simple deficiencies such as those caused by null mutations of a large number of human serpins are linked to disease (some of these are summarized in Table 1). Interestingly, however, several (rare) mutations have been identified that do not promote instability but instead interfere with the ability of the serpin to interact correctly with proteases. These include the Enschede variant of SERPINF2 [66], in which insertion of an additional alanine in the RCL results in predominantly substrate-like (rather than inhibitory) behavior upon interaction with a protease. Mutations that alter serpin specificity can also have a devastating effect. For example, the Pittsburgh variant of SERPINA1 (antitrypsin) is an effective thrombin inhibitor as a result of mutation of the P1 methionine to an arginine [67]. The carrier of this variant died of a fatal bleeding disorder in childhood.
Our knowledge of the functional biochemistry and cell biology of serpins has been shaped by extensive contributions from structural biology and genomics. The structure of six different serpin conformations, together with analysis of numerous different dysfunctional serpin variants, has allowed the characterization of a unique conformational mechanism of protease inhibition. These data highlight the intrinsic advantages as well as the dangers of structural complexity in protease inhibitors. On the one hand, conformational mobility provides an inherently controllable mechanism of inhibition. On the other, uncontrolled serpin conformational change may result in misfolding and the development of specific serpinopathies. Serpins thus join a growing number of structurally distinct molecules that can misfold and cause important degenerative diseases, such as prions, polyglutamine regions of various proteins and the amyloid proteins that form inclusions in Alzheimer's disease. While the mechanism of serpin function is now structurally well characterized, the precise role and biological target of many serpins remains to be understood.
Acknowledgments
Acknowledgements
Qingwei Zhang is a recipient of a Monash Graduate Scholarship. James Whisstock is an NHMRC Senior Research Fellow and Monash University Logan Fellow. We thank the NHMRC and the ARC for support.
References
- Irving JA, Pike RN, Lesk AM, Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.GR-1478R. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Tolle DP, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2004;32(Database):D160–D164. doi: 10.1093/nar/gkh071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Irving JA, Steenbakkers PJ, Lesk AM, Op den Camp HJ, Pike RN, Whisstock JC. Serpins in prokaryotes. Mol Biol Evol. 2002;19:1881–1890. doi: 10.1093/oxfordjournals.molbev.a004012. [DOI] [PubMed] [Google Scholar]
- Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, Pickup DJ. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69:597–604. doi: 10.1016/0092-8674(92)90223-Y. [DOI] [PubMed] [Google Scholar]
- Schick C, Pemberton PA, Shi GP, Kamachi Y, Cataltepe S, Bartuski AJ, Gornstein ER, Bromme D, Chapman HA, Silverman GA. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: a kinetic analysis. Biochemistry. 1998;37:5258–5266. doi: 10.1021/bi972521d. [DOI] [PubMed] [Google Scholar]
- Irving JA, Pike RN, Dai W, Bromme D, Worrall DM, Silverman GA, Coetzer TH, Dennison C, Bottomley SP, Whisstock JC. Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochemistry. 2002;41:4998–5004. doi: 10.1021/bi0159985. [DOI] [PubMed] [Google Scholar]
- Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. Hormone binding globulins undergo serpin conformational change in inflammation. Nature. 1988;336:257–258. doi: 10.1038/336257a0. [DOI] [PubMed] [Google Scholar]
- Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends Biochem Sci. 1996;21:22–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- Ruhlmann A, Kukla D, Schwager P, Bartels K, Huber R. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. Crystal structure determination and stereochemistry of the contact region. J Mol Biol. 1973;77:417–436. doi: 10.1016/0022-2836(73)90448-8. [DOI] [PubMed] [Google Scholar]
- Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- Carrell RW, Lomas DA. Conformational disease. Lancet. 1997;350:134–138. doi: 10.1016/S0140-6736(97)02073-4. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- Bruce D, Perry DJ, Borg JY, Carrell RW, Wardell MR. Thromboembolic disease due to thermolabile conformational changes of antithrombin Rouen-VI (187 Asn→Asp). J Clin Invest. 1994;94:2265–2274. doi: 10.1172/JCI117589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulak KS, Pemberton PA, Rosen FS, Carrell RW, Lachmann PJ, Harrison RA. Dysfunctional C1-inhibitor(At), isolated from a type II hereditary-angio-oedema plasma, contains a P1 'reactive centre' (Arg444→His) mutation. Biochem J. 1988;253:615–618. doi: 10.1042/bj2530615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Shrimpton AE, Holohan PD, Bradshaw C, Feiglin D, Collins GH, Sonderegger P, Kinter J, Becker LM, Lacbawan F, et al. Familial dementia caused by polymerization of mutant neuroserpin. Nature. 1999;401:376–379. doi: 10.1038/43897. [DOI] [PubMed] [Google Scholar]
- Stein PE, Carrell RW. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995;2:96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- Riahi Y, Siman-Tov R, Ankri S. Molecular cloning, expression and characterization of a serine proteinase inhibitor gene from Entamoeba histolytica. Mol Biochem Parasitol. 2004;133:153–162. doi: 10.1016/j.molbiopara.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Irving JA, Cabrita LD, Rossjohn J, Pike RN, Bottomley SP, Whisstock JC. The 1.5 Å crystal structure of a prokaryote serpin: controlling conformational change in a heated environment. Structure. 2003;11:387–397. doi: 10.1016/S0969-2126(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Roberts TH, Hejgaard J, Saunders NF, Cavicchioli R, Curmi PM. Serpins in unicellular Eukarya, Archaea, and Bacteria: sequence analysis and evolution. J Mol Evol. 2004;59:437–447. doi: 10.1007/s00239-004-2635-6. [DOI] [PubMed] [Google Scholar]
- Grigoryev SA, Woodcock CL. Chromatin structure in granulocytes. A link between tight compaction and accumulation of a heterochromatin-associated protein (MENT). J Biol Chem. 1998;273:3082–3089. doi: 10.1074/jbc.273.5.3082. [DOI] [PubMed] [Google Scholar]
- Irving JA, Shushanov SS, Pike RN, Popova EY, Bromme D, Coetzer TH, Bottomley SP, Boulynko IA, Grigoryev SA, Whisstock JC. Inhibitory activity of a heterochromatin-associated serpin (MENT) against papain-like cysteine proteinases affects chromatin structure and blocks cell proliferation. J Biol Chem. 2002;277:13192–13201. doi: 10.1074/jbc.M108460200. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Roth S, Reichhart JM. A serpin regulates dorsal-ventral axis formation in the Drosophila embryo. Curr Biol. 2003;13:2097–2102. doi: 10.1016/j.cub.2003.10.062. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated anti-fungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Pak SC, Kumar V, Tsu C, Luke CJ, Askew YS, Askew DJ, Mills DR, Bromme D, Silverman GA. SRP-2 is a cross-class inhibitor that participates in postembryonic development of the nematode Caenorhabditis elegans : initial characterization of the clade L serpins. J Biol Chem. 2004;279:15448–15459. doi: 10.1074/jbc.M400261200. [DOI] [PubMed] [Google Scholar]
- Law RH, Irving JA, Buckle AM, Ruzyla K, Buzza M, Bashtannyk-Puhalovich TA, Beddoe TC, Nguyen K, Worrall DM, Bottomley SP, et al. The high-resolution crystal structure of the human tumor suppressor maspin reveals a novel conformational switch in the G-helix. J Biol Chem. 2005;280:22356–22364. doi: 10.1074/jbc.M412043200. [DOI] [PubMed] [Google Scholar]
- Roberts TH, Marttila S, Rasmussen SK, Hejgaard J. Differential gene expression for suicide-substrate serine proteinase inhibitors (serpins) in vegetative and grain tissues of barley. J Exp Bot. 2003;54:2251–2263. doi: 10.1093/jxb/erg248. [DOI] [PubMed] [Google Scholar]
- Hejgaard J. Inhibitory plant serpins with a sequence of three glutamine residues in the reactive center. Biol Chem. 2005;386:1319–1323. doi: 10.1515/BC.2005.150. [DOI] [PubMed] [Google Scholar]
- Yoo BC, Aoki K, Xiang Y, Campbell LR, Hull RJ, Xoconostle-Cazares B, Monzer J, Lee JY, Ullman DE, Lucas WJ. Characterization of Cucurbita maxima phloem serpin-1 (CmPS-1). A developmentally regulated elastase inhibitor. J Biol Chem. 2000;275:35122–35128. doi: 10.1074/jbc.M006060200. [DOI] [PubMed] [Google Scholar]
- Fulton KF, Buckle AM, Cabrita LD, Irving JA, Butcher RE, Smith I, Reeve S, Lesk AM, Bottomley SP, Rossjohn J, Whisstock JC. The high-resolution crystal structure of a native thermostable serpin reveals the complex mechanism underpinning the stressed to relaxed transition. J Biol Chem. 2005;280:8435–8442. doi: 10.1074/jbc.M410206200. [DOI] [PubMed] [Google Scholar]
- Elliott PR, Lomas DA, Carrell RW, Abrahams JP. Inhibitory conformation of the reactive loop of alpha 1-antitrypsin. Nat Struct Biol. 1996;3:676–681. doi: 10.1038/nsb0896-676. [DOI] [PubMed] [Google Scholar]
- Lobermann H, Lottspeich F, Bode W, Huber R. Interaction of human alpha 1-proteinase inhibitor with chymotrypsinogen A and crystallization of a proteolytically modified alpha 1-proteinase inhibitor. Hoppe Seylers Z Physiol Chem. 1982;363:1377–1388. doi: 10.1515/bchm2.1982.363.2.1377. [DOI] [PubMed] [Google Scholar]
- Cabrita LD, Bottomley SP. How do proteins avoid becoming too stable? Biophysical studies into metastable proteins. Eur Biophys J. 2004;33:83–88. doi: 10.1007/s00249-003-0356-1. [DOI] [PubMed] [Google Scholar]
- Ye S, Cech AL, Belmares R, Bergstrom RC, Tong Y, Corey DR, Kanost MR, Goldsmith EJ. The structure of a Michaelis serpin-protease complex. Nat Struct Biol. 2001;8:979–983. doi: 10.1038/nsb1101-979. [DOI] [PubMed] [Google Scholar]
- Dementiev A, Simonovic M, Volz K, Gettins PG. Canonical inhibitor-like interactions explain reactivity of alpha1-proteinase inhibitor Pittsburgh and antithrombin with proteinases. J Biol Chem. 2003;278:37881–37887. doi: 10.1074/jbc.M305195200. [DOI] [PubMed] [Google Scholar]
- Tew DJ, Bottomley SP. Intrinsic fluorescence changes and rapid kinetics of proteinase deformation during serpin inhibition. FEBS Lett. 2001;494:30–33. doi: 10.1016/S0014-5793(01)02305-5. [DOI] [PubMed] [Google Scholar]
- Kaslik G, Patthy A, Balint M, Graf L. Trypsin complexed with alpha 1-proteinase inhibitor has an increased structural flexibility. FEBS Lett. 1995;370:179–183. doi: 10.1016/0014-5793(95)00816-R. [DOI] [PubMed] [Google Scholar]
- Rezaie AR. Calcium enhances heparin catalysis of the antithrombin-factor Xa reaction by a template mechanism. Evidence that calcium alleviates Gla domain antagonism of heparin binding to factor Xa. J Biol Chem. 1998;273:16824–16827. doi: 10.1074/jbc.273.27.16824. [DOI] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968;32:898–902. doi: 10.1016/0006-291X(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Carrell RW, Stein PE, Fermi G, Wardell MR. Biological implications of a 3 Å structure of dimeric antithrombin. Structure. 1994;2:257–270. doi: 10.1016/S0969-2126(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Schreuder H, de Boer B, Pronk S, Hol W, Dijkema R, Mulders J, The-unissen H. Crystallization and preliminary X-ray analysis of human antithrombin III. J Mol Biol. 1993;229:249–250. doi: 10.1006/jmbi.1993.1024. [DOI] [PubMed] [Google Scholar]
- Li W, Johnson DJ, Esmon CT, Huntington JA. Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin. Nat Struct Mol Biol. 2004;11:857–862. doi: 10.1038/nsmb811. [DOI] [PubMed] [Google Scholar]
- Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike RN, Potempa J, Skinner R, Fitton HL, McGraw WT, Travis J, Owen M, Jin L, Carrell RW. Heparin-dependent modification of the reactive center arginine of antithrombin and consequent increase in heparin binding affinity. J Biol Chem. 1997;272:19652–19655. doi: 10.1074/jbc.272.32.19652. [DOI] [PubMed] [Google Scholar]
- Whisstock JC, Pike RN, Jin L, Skinner R, Pei XY, Carrell RW, Lesk AM. Conformational changes in serpins: II. The mechanism of activation of antithrombin by heparin. J Mol Biol. 2000;301:1287–1305. doi: 10.1006/jmbi.2000.3982. [DOI] [PubMed] [Google Scholar]
- Baglin TP, Carrell RW, Church FC, Esmon CT, Huntington JA. Crystal structures of native and thrombin-complexed heparin cofactor II reveal a multistep allosteric mechanism. Proc Natl Acad Sci USA. 2002;99:11079–11084. doi: 10.1073/pnas.162232399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renatus M, Zhou Q, Stennicke HR, Snipas SJ, Turk D, Bankston LA, Liddington RC, Salvesen GS. Crystal structure of the apoptotic suppressor CrmA in its cleaved form. Structure. 2000;8:789–797. doi: 10.1016/S0969-2126(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Simonovic M, Gettins PG, Volz K. Crystallization and preliminary X-ray diffraction analysis of a recombinant cysteine-free mutant of crmA. Acta Crystallogr D Biol Crystallogr. 2000;56:1440–1442. doi: 10.1107/S0907444900009884. [DOI] [PubMed] [Google Scholar]
- Kounnas MZ, Church FC, Argraves WS, Strickland DK. Cellular internalization and degradation of antithrombin III-thrombin, heparin cofactor II-thrombin, and alpha 1-antitrypsin-trypsin complexes is mediated by the low density lipoprotein receptor-related protein. J Biol Chem. 1996;271:6523–6529. doi: 10.1074/jbc.271.11.6523. [DOI] [PubMed] [Google Scholar]
- Makarova A, Mikhailenko I, Bugge TH, List K, Lawrence DA, Strickland DK. The low density lipoprotein receptor-related protein modulates protease activity in the brain by mediating the cellular internalization of both neuroserpin and neu-roserpin-tissue-type plasminogen activator complexes. J Biol Chem. 2003;278:50250–50258. doi: 10.1074/jbc.M309150200. [DOI] [PubMed] [Google Scholar]
- Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- Mottonen J, Strand A, Symersky J, Sweet RM, Danley DE, Geoghegan KF, Gerard RD, Goldsmith EJ. Structural basis of latency in plasminogen activator inhibitor-1. Nature. 1992;355:270–273. doi: 10.1038/355270a0. [DOI] [PubMed] [Google Scholar]
- Zhou A, Huntington JA, Pannu NS, Carrell RW, Read RJ. How vitronectin binds PAI-1 to modulate fibrinolysis and cell migration. Nat Struct Biol. 2003;10:541–544. doi: 10.1038/nsb943. [DOI] [PubMed] [Google Scholar]
- Beauchamp NJ, Pike RN, Daly M, Butler L, Makris M, Dafforn TR, Zhou A, Fitton HL, Preston FE, Peake IR, Carrell RW. Antithrombins Wibble and Wobble (T85M/K): archetypal conformational diseases with in vivo latent-transition, thrombosis, and heparin activation. Blood. 1998;92:2696–2706. [PubMed] [Google Scholar]
- Lomas DA, Carrell RW. Serpinopathies and the conformational dementias. Nat Rev Genet. 2002;3:759–768. doi: 10.1038/nrg907. [DOI] [PubMed] [Google Scholar]
- Dunstone MA, Dai W, Whisstock JC, Rossjohn J, Pike RN, Feil SC, Le Bonniec BF, Parker MW, Bottomley SP. Cleaved antitrypsin polymers at atomic resolution. Protein Sci. 2000;9:417–420. doi: 10.1110/ps.9.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntington JA, Pannu NS, Hazes B, Read RJ, Lomas DA, Carrell RW. A 2.6 Å structure of a serpin polymer and implications for conformational disease. J Mol Biol. 1999;293:449–455. doi: 10.1006/jmbi.1999.3184. [DOI] [PubMed] [Google Scholar]
- Mast AE, Enghild JJ, Salvesen G. Conformation of the reactive site loop of alpha 1-proteinase inhibitor probed by limited proteolysis. Biochemistry. 1992;31:2720–2728. doi: 10.1021/bi00125a012. [DOI] [PubMed] [Google Scholar]
- Davis RL, Shrimpton AE, Carrell RW, Lomas DA, Gerhard L, Baumann B, Lawrence DA, Yepes M, Kim TS, Ghetti B, et al. Association between conformational mutations in neuroserpin and onset and severity of dementia. Lancet. 2002;359:2242–2247. doi: 10.1016/S0140-6736(02)09293-0. [DOI] [PubMed] [Google Scholar]
- Lomas DA. Molecular mousetraps, alpha1-antitrypsin deficiency and the serpinopathies. Clin Med. 2005;5:249–257. doi: 10.7861/clinmedicine.5-3-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooptu B, Hazes B, Chang WS, Dafforn TR, Carrell RW, Read RJ, Lomas DA. Inactive conformation of the serpin alpha(1)-antichymotrypsin indicates two-stage insertion of the reactive loop: implications for inhibitory function and conformational disease. Proc Natl Acad Sci USA. 2000;97:67–72. doi: 10.1073/pnas.97.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Stein PE. The biostructural pathology of the serpins: critical function of sheet opening mechanism. Biol Chem Hoppe Seyler. 1996;377:1–17. doi: 10.1515/bchm3.1996.377.1.1. [DOI] [PubMed] [Google Scholar]
- Lomas DA, Finch JT, Seyama K, Nukiwa T, Carrell RW. Alpha 1-antitrypsin Siiyama (Ser53→Phe). Further evidence for intracellular loop-sheet polymerization. J Biol Chem. 1993;268:15333–15335. [PubMed] [Google Scholar]
- Lomas DA, Elliott PR, Sidhar SK, Foreman RC, Finch JT, Cox DW, Whisstock JC, Carrell RW. alpha 1-Antitrypsin Mmalton (Phe52-deleted) forms loop-sheet polymers in vivo. Evidence for the C sheet mechanism of polymerization. J Biol Chem. 1995;270:16864–16870. doi: 10.1074/jbc.270.15.8393. [DOI] [PubMed] [Google Scholar]
- Holmes WE, Lijnen HR, Nelles L, Kluft C, Nieuwenhuis HK, Rijken DC, Collen D. Alpha 2-antiplasmin Enschede: alanine insertion and abolition of plasmin inhibitory activity. Science. 1987;238:209–211. doi: 10.1126/science.2958938. [DOI] [PubMed] [Google Scholar]
- Owen MC, Brennan SO, Lewis JH, Carrell RW. Mutation of anti-trypsin to antithrombin, alpha 1-antitrypsin Pittsburgh (358 Met→Arg), leads to a fatal bleeding disorder. N Engl J Med. 1983;309:694–698. doi: 10.1056/NEJM198309223091203. [DOI] [PubMed] [Google Scholar]
- Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996;127:612–620. doi: 10.1016/S0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Deyashiki Y, Nishioka J, Toma K. Protein C inhibitor: structure and function. Thromb Haemost. 1989;61:337–342. [PubMed] [Google Scholar]
- Frazer JK, Jackson DG, Gaillard JP, Lutter M, Liu YJ, Banchereau J, Capra JD, Pascual V. Identification of centerin: a novel human germinal center B cell-restricted serpin. Eur J Immunol. 2000;30:3039–3048. doi: 10.1002/1521-4141(200010)30:10<3039::AID-IMMU3039>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordula T, Dubin A, Schooltink H, Koj A, Heinrich PC, Rose-John S. Molecular cloning and expression of an intracellular serpin: an elastase inhibitor from horse leucocytes. Biochem J. 1993;293:187–193. doi: 10.1042/bj2930187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf RL, Stasinopoulos SJ. The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. FEBS J. 2005;272:4858–4867. doi: 10.1111/j.1742-4658.2005.04879.x. [DOI] [PubMed] [Google Scholar]
- Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA, Silverman GA. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem. 1997;272:1849–1855. doi: 10.1074/jbc.272.3.1849. [DOI] [PubMed] [Google Scholar]
- Scott FL, Hirst CE, Sun J, Bird CH, Bottomley SP, Bird PI. The intracellular serpin proteinase inhibitor 6 is expressed in monocytes and granulocytes and is a potent inhibitor of the azurophilic granule protease, cathepsin G. Blood. 1999;93:2089–2097. [PubMed] [Google Scholar]
- Miyata T, Nangaku M, Suzuki D, Inagi R, Uragami K, Sakai H, Okubo K, Kurokawa K. A mesangium-predominant gene, megsin, is a new serpin upregulated in IgA nephropathy. J Clin Invest. 1998;102:828–836. doi: 10.1172/JCI2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen JR, Jean F, Thomas G, Foster DC, Kisiel W. Inhibition of soluble recombinant furin by human proteinase inhibitor 8. J Biol Chem. 1998;273:1851–1854. doi: 10.1074/jbc.273.4.1851. [DOI] [PubMed] [Google Scholar]
- Sun J, Bird CH, Sutton V, McDonald L, Coughlin PB, De Jong TA, Trapani JA, Bird PI. A cytosolic granzyme B inhibitor related to the viral apoptotic regulator cytokine response modifier A is present in cytotoxic lymphocytes. J Biol Chem. 1996;271:27802–27809. doi: 10.1074/jbc.271.44.27802. [DOI] [PubMed] [Google Scholar]
- Riewald M, Schleef RR. Molecular cloning of bomapin (protease inhibitor 10), a novel human serpin that is expressed specifically in the bone marrow. J Biol Chem. 1995;270:26754–26757. doi: 10.1074/jbc.270.45.26754. [DOI] [PubMed] [Google Scholar]
- Askew YS, Pak SC, Luke CJ, Askew DJ, Cataltepe S, Mills DR, Kato H, Lehoczky J, Dewar K, Birren B, Silverman GA. SERPINB12 is a novel member of the human ov-serpin family that is widely expressed and inhibits trypsin-like serine proteinases. J Biol Chem. 2001;276:49320–49330. doi: 10.1074/jbc.M108879200. [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, Bouck NP. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- Xiao G, Liu YE, Gentz R, Sang QA, Ni J, Goldberg ID, Shi YE. Suppression of breast cancer growth and metastasis by a serpin myoepithelium-derived serine proteinase inhibitor expressed in the mammary myoepithelial cells. Proc Natl Acad Sci USA. 1999;96:3700–3705. doi: 10.1073/pnas.96.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpy DJ, Bachmann AW, Gartside M, Grice JE, Harris JM, Clifton P, Easteal S, Jackson RV, Whitworth JA. Association between chronic fatigue syndrome and the corticosteroid-binding globulin gene ALA SER224 polymorphism. Endocr Res. 2004;30:417–429. doi: 10.1081/ERC-200035599. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Bachmann AW, Grice JE, Fitzgerald SP, Phillips PJ, Whit-worth JA, Jackson RV. Familial corticosteroid-binding globulin deficiency due to a novel null mutation: association with fatigue and relative hypotension. J Clin Endocrinol Metab. 2001;86:3692–3700. doi: 10.1210/jc.86.8.3692. [DOI] [PubMed] [Google Scholar]
- Refetoff S, Murata Y, Mori Y, Janssen OE, Takeda K, Hayashi Y. Thyroxine-binding globulin: organization of the gene and variants. Horm Res. 1996;45:128–138. doi: 10.1159/000184775. [DOI] [PubMed] [Google Scholar]
- Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Water N, Tan T, Ashton F, O'Grady A, Day T, Browett P, Ockelford P, Harper P. Mutations within the protein Z-dependent protease inhibitor gene are associated with venous thromboembolic disease: a new form of thrombophilia. Br J Haematol. 2004;127:190–194. doi: 10.1111/j.1365-2141.2004.05189.x. [DOI] [PubMed] [Google Scholar]
- Perry DJ, Carrell RW. Molecular genetics of human antithrombin deficiency. Hum Mutat. 1996;7:7–22. doi: 10.1002/(SICI)1098-1004(1996)7:1<7::AID-HUMU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- He L, Vicente CP, Westrick RJ, Eitzman DT, Tollefsen DM. Heparin cofactor II inhibits arterial thrombosis after endothelial injury. J Clin Invest. 2002;109:213–219. doi: 10.1172/JCI200213432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay WP, Parker AC, Condrey LR, Shapiro AD. Human plasmino-gen activator inhibitor-1 (PAI-1) deficiency: characterization of a large kindred with a null mutation in the PAI-1 gene. Blood. 1997;90:204–208. [PubMed] [Google Scholar]
- Miles LA, Plow EF, Donnelly KJ, Hougie C, Griffin JH. A bleeding disorder due to deficiency of alpha 2-antiplasmin. Blood. 1982;59:1246–1251. [PubMed] [Google Scholar]
- De Marchi M, Jacot-Guillarmod H, Ressa TG, Carbonara AO. Hereditary angioedema: report of a large kindred with a rare genetic variant of C1-esterase inhibitor. Clin Genet. 1973;4:229–236. doi: 10.1111/j.1399-0004.1973.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Loebermann H, Tokuoka R, Deisenhofer J, Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984;177:531–557. doi: 10.1016/0022-2836(84)90298-5. [DOI] [PubMed] [Google Scholar]
- Hopkins PC, Carrell RW, Stone SR. Effects of mutations in the hinge region of serpins. Biochemistry. 1993;32:7650–7657. doi: 10.1021/bi00081a008. [DOI] [PubMed] [Google Scholar]
- Skinner R, Abrahams JP, Whisstock JC, Lesk AM, Carrell RW, Wardell MR. The 2.6 Å structure of antithrombin indicates a conformational change at the heparin binding site. J Mol Biol. 1997;266:601–609. doi: 10.1006/jmbi.1996.0798. [DOI] [PubMed] [Google Scholar]
- Sharp AM, Stein PE, Pannu NS, Carrell RW, Berkenpas MB, Ginsburg D, Lawrence DA, Read RJ. The active conformation of plasminogen activator inhibitor 1, a target for drugs to control fibrinolysis and cell adhesion. Structure. 1999;7:111–118. doi: 10.1016/S0969-2126(99)80018-5. [DOI] [PubMed] [Google Scholar]
- Stout TJ, Graham H, Buckley DI, Matthews DJ. Structures of active and latent PAI-1: a possible stabilizing role for chloride ions. Biochemistry. 2000;39:8460–8469. doi: 10.1021/bi000290w. [DOI] [PubMed] [Google Scholar]
- Skinner R, Chang WS, Jin L, Pei X, Huntington JA, Abrahams JP, Carrell RW, Lomas DA. Implications for function and therapy of a 2.9 Å structure of binary-complexed antithrombin. J Mol Biol. 1998;283:9–14. doi: 10.1006/jmbi.1998.2083. [DOI] [PubMed] [Google Scholar]


