Abstract
Polycystic ovary syndrome (PCOS) is the most frequent cause of female infertility. The treatment of PCOS patients with insulin sensitizers, such as metformin or thiazolidinediones, increases the ovulation rate and the number of successful pregnancies. The positive action of the insulin-sensitizing treatments could be explained by a decrease in the peripheral insulin resistance but also by a direct action at the ovarian level. We report in this review different hypotheses of thiazolidinediones actions to improve PCOS (steroid secretion by ovarian cells ; insulin sensitivity in muscle and adipocyte and fat redistribution).
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most frequent cause of female infertility, affecting about 5–10% of women in age of procreation [1]. Diagnostic criteria to establish PCOS are controversial [1, 2], involving two among the following three 2003 Rotterdam's criteria: first, clinical and/or signs of hyperandrogenism, second a chronic absence of ovulation and finally, third, the increase of ovarian volume and/or the presence of at least 12 follicles in the 2- to 9-mm range in each ovary, detected by ultrasonography [3]. Moreover, insulin resistance is a common metabolic feature associated with PCOS, up to 50–70% of patients in some series [4].
Yen et al described a vicious circle where several endocrine abnormalities could maintain the PCOS status [2, 5]. Three entrance points are proposed as follows.
Alteration of the hypothalamo-pituitary axis (∼ 50% cases) [6] with high circulating LH levels that can lead to excess androgens and contribute to the formation of cystic follicles, as described in the mouse model [7]. However, the importance of this hypothesis as an entrance point is critical in the PCOS syndrome [8].
Hyperandrogenism due to steroidogenic dysregulation in thecal cells. Mutations of Cyp11a1 or Cyp17 genes are detected in some patients and could lead to an hyperactivity of the steroidogenesis [9, 10].
As evidenced by Franks et al, hypersensitivity of ovarian cells to both insulin and gonadotropin leads to androgens hypersecretion [11].
The treatment of PCOS patients with insulin sensitizers of various drug families, such as thiazolidinediones (TZDs), metformin or D-chiro-inositol, increases the ovulation rate and the number of successful pregnancies (cf [14–17]). The positive action of these “insulin sensitizers” drugs could be explained by various manners. We report in this review different hypothesis of TZDs actions to improve PCOS at each level of the “Yen vicious circle” (Figure 1).
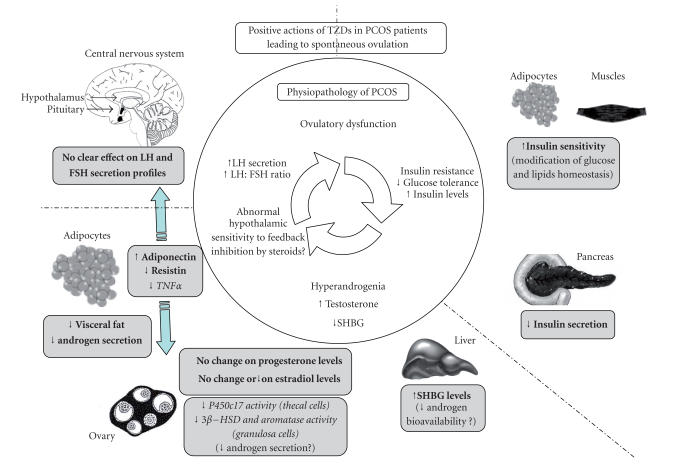
Figure 1.
Positive actions of TZDs in PCOS patients leading to spontaneous ovulation. Three entrance points/major endocrine abnormalities (hyperandrogenia, insulin resistance, or LH hypersecretion) lead or maintain the ovulatory dysfunction of PCOS as represented in the inner circle of the figure. For example, high LH concentrations or insulin increase androgen secretion by human thecal cells [12] and could contribute to impair follicular development. In addition, elevated androgens could reduce hypothalamic sensitivity to negative steroids feedback, because administration of flutamide, an antiandrogen, can restore this sensitivity [13]. The positive actions of TZDs on PCOS patients could be mainly at two levels as described in the outer part of the figure. (1) TZDs increase the insulin sensitivity and decrease the insulin secretion. (2) TZDs reduce the androgen secretion and/or activity (in ovary and/or in adipose tissue). Finally, TZDs can modulate secretion of several endocrine hormones (adiponectin, resistin, TNFα) which can reduce androgen production or improve gonadotropin secretion. The boxes show results obtained in vivo in PCOS women (in bold) or in vitro in human cell culture (in italics).
TZDs are synthetic ligands also known as glitazones (troglitazone, rosiglitazone or pioglitazone) [18], which can bind and activate the nuclear receptor, peroxysome proliferator-activated receptor gamma (PPARγ) (cf [19, 20]). PPARγ could be considered as a fuel sensor linking the energy metabolism and reproduction to inform cells on the energy status. Indeed, PPARγ can regulate the transcription and/or activity of different key regulators of energy homeostasis [19] such as glucose or lipid regulators (PPARγ upregulated expression of glucose transporters, insulin receptor, insulin receptor substrate, fatty acid-binding protein, etc) (cf [21]). Activation of PPARγ by TZDs increases insulin sensitivity mainly in adipocytes and muscle cells [22], and also stimulates the differentiation of adipose cells (cf [23, 24]).
In addition, the three PPAR isoforms (PPARα, PPARβ/δ, PPARγ) are expressed along the gonadotrope axis (central nervous system, pituitary gland and ovary) (cf [25, 26]). In the ovaries, expression of PPARγ is restricted to follicles, primarily to granulosa cells in developing follicles, slightly in theca cells and in corpus luteum (cf [25]). After the LH surge, the PPARγ expression decreases in follicle [27, 28]. In general, it is considered that TZDs activate PPARγ, nevertheless a PPARγ-independent action of TZDs cannot be excluded, as suggested by several recent studies [29, 30].
ASSESSMENT OF THE CLINICAL TRIALS OF TZDS TREATMENTS
Pioglitazone and rosiglitazone are the unique TZDs which could be used. Indeed, troglitazone was withdrawn from the worldwide market in 2000 because of its hepatotoxicity. Pioglitazone and rosiglitazone possess mainly the same properties, except that pioglitazone may have a more positive effect on lipid profile than rosiglitazone [31].
Administration of TZDs (troglitazone, rosiglitazone, pioglitazone) is able to induce ovulation, to increase the ovulation rate and pregnancy in PCOS (cf [32]). For example, a large trial performed on 305 women has shown spontaneous ovulation in over 50% of the time (600 mg troglitazone) in comparison with approximately 10% of placebo group [33]. Troglitazone [33–36], pioglitazone [37–40], and rosiglitazone [41–43], for at least 3 months of treatment, improved insulin sensitivity, decreased the insulin concentration and reduced the androgenic activity. In these studies, a decrease in total and free circulating androgen concentrations associated with an increase of sex hormone binding globulin, SHBG, levels was observed. Concentrations of progesterone in serum are equivalent [35, 37], whereas those of estradiol are equivalent or decreased [36] after TZDs treatment. The body mass index is not significantly changed with the three TZDs [35–37, 39, 43, 44].
Furthermore, in PCOS patients with a “resistance” to antiestrogens (such as clomiphene citrate), an association of clomiphene citrate with TZDs (troglitazone, rosiglitazone) can help to increase the ovulation rate [45, 46]. Thus, TZDs, by an unknown mechanism (direct or indirect actions on hypothalamo-pituitary axis in order to remove the negative feedback of estradiol) could improve the clomiphene citrate sensitivity in PCOS patients.
TZDs treatment improves the rate of spontaneous pregnancy in several trials (20–40% pregnancy success) [33, 41, 44, 45, 47]. We can note that pioglitazone and rosiglitazone are both classified by the FDA (food and drug administration) as pregnancy category C and present potential teratogenic risks. PPARγ is important for embryonic development [48] and TZDs can cause a decrease in the fetal maturation [49]. Nevertheless, two reported cases of human exposure to rosiglitazone during pregnancy have shown no malformation on babies [50, 51]. Despite this observation, women treated with TZDs will be stopped as soon as they will be pregnant. Similarly, preliminary studies have revealed that metformin, an insulin sensitizer more studied than TZDs, reduces also pregnancy losses, which are frequently observed (30–50%) in PCOS women during the first trimester [52, 53]. No risk for the fetus or teratogenicity was described after metformin administration (category B). However, it appears premature to maintain currently such treatments during pregnancy since there is no formal consensus about such indication [54].
TZDS DID NOT SEEM TO AFFECT GONADOTROPIN SECRETION
In most trials, after TZDs treatment (troglitazone, rosiglitazone or pioglitazone), basal gonadotropin levels or the luteinizing hormone (LH)/follicle stimulating hormone (FSH) ratio did not change with the therapy [33, 35, 37, 42, 43]. In addition, recently, no alteration of the LH pulse frequency and amplitude, as well as gonadotropin responses to GnRH, was observed after pioglitazone treatment, either with or without insulin infusion [55]. Nevertheless, in some trials, a decrease in the plasma luteinizing hormone concentrations was observed after troglitazone, rosiglitazone or pioglitazone treatment [36, 38, 39, 44].
DIRECT ACTION OF TZDS ON OVARY
Several studies in ruminants have shown a direct effect of glucose or fatty acids on folliculogenesis. The ovulation rate is increased without modification of gonadotropin secretion as observed in case of flushing [56]. In this perspective, we cannot reject the hypothesis for a direct action of TZDs on ovary.
PPARγ did not modify folliculogenesis or ovulation rate in rodents
Activation of PPARγ by administration of 1 mg of ciglitazone/day injected intraperitoneally during four weeks in rats [57] did not alter folliculogenesis or the number of corpus luteum. In mice, deletion of PPARγ specifically in ovaries did not change folliculogenesis or ovulation rate but decreased the number of embryos implanted, probably due to a drop in progesterone secretion by the corpus luteum [58]. Moreover, in human, linkage studies have rejected a genetic association between the PPARγ locus (3p25) and the birth of dizygotic twin [59].
PPARγ modify steroids secretion by granulosa and thecal cells
In vitro, the steroids secretions (androgens, progesterone, estradiol) are inhibited or stimulated (about 20%) by TZDs according to species or the status of the cell differentiation (follicular phase, before or after the preovulatory surge). For example, TZDs stimulated progesterone secretion by a mixture of granulosa, theca, and stroma human cells obtained from premenopausal/perimenopausal patients at the time of oophorectomy [60], and TZDs inhibited testosterone secretion (≈15% reduction, [60]), progesterone and estradiol by human granulosa cells (after hCG stimulation for in vitro fertilization) or by luteal-granulosa cells obtained from PCOS patients [61, 62]. Furthermore, TZDs inhibited in vitro, LH/insulin-stimulated androgens secretion by porcine thecal cells [63].
In any case, the inhibiting effect of TZDs, in human ovarian cells, is more due to a reduction in the activity of steroidogenic enzymes 3-beta-hydroxysteroid-dehydrogenase (3β-HSD) and aromatase, rather than an activation of PPARγ on the promoters of the genes encoding these enzymes [61, 64].
Improved insulin sensitivity in ovary induced by TZDs could also favor the restoration of steroidogenesis to a normal status. Indeed, the responsiveness to FSH in human granulosa cells obtained in PCOS patients was enhanced by insulin after improvement of the insulin sensitivity induced by the pioglitazone treatment [65]. In addition, in preliminary results, pioglitazone and rosiglitazone increased by two- to three-fold the level of insulin receptor and insulin receptor substrate-1 in human ovarian cells [66].
IMPROVEMENT OF THE METABOLIC STATUS BY TZDS INCREASES FERTILITY IN PCOS PATIENTS
Thus, TZDs can act on the ovary in order to regulate steroidogenesis by a direct action on theca and granulosa cells via PPARγ. Nevertheless, the actions of TZDs on steroidogenesis are not drastic and are varied in function by the status of the cell differentiation (and species). It will be more probable that a general improvement (redistribution of the fat tissue, increased in insulin sensitivity and inhibition of hepatic gluconeogenesis) stimulates ovulation through multiple ovary-independant mechanisms. The observations described below are in favour for this indirect action of TZDs in the treatment of PCOS.
TZDs increase insulin sensitivity
TZDs reduce insulin resistance by improving sensitivity to insulin, mainly in adipose tissue and muscle of PCOS patients [22, 67]. TZDs could stimulate glucose transporter expression and other proteins in the insulin pathway (cf [21]). Moreover, a decrease in the insulin resistance by TZDs could be explained by a redistribution of the triglycerides circulating or content in liver and skeletal muscle into the adipose tissue. These modifications are associated with a decrease in plasma free fatty acid and triglyceride concentrations [22, 68]. Free fatty acid and/or triglyceride concentrations are high in PCOS patients [69] and decrease after TZDs treatment (cf [70]).
With this improvement of the general status, spontanenous ovulation could be favored. For example, only a weight reduction by diet and exercise improved insulin sensitivity and led to restoration of normal cycles. A 10–15% weight reduction could reduce hyperandrogenism and restored ovulation in more than 75% of PCOS obese patients [71, 72].
TZDs could decrease androgen synthesis by a fat tissue redistribution
TZDs can decrease the high free androgen activity by two mechanisms: an increase in SHBG levels in serum, leading to a decrease in free circulating androgen levels [39], an adipose tissue redistribution. In contrast to metformin, a long-term TZDs treatment increases the body fat mass due to an increase in the subcutaneous adipose tissue [73] and a decrease in the amount of visceral abdominal adipose tissue associated with a decrease in free fatty acid [74]. The visceral fat mass has been associated with high serum androgen concentrations and was closely related to insulin resistance in women with PCOS [75, 76]. Thus, the reduction in the amount of profound visceral abdominal adipose tissue could contribute to explain the decrease in the testosterone and estradiol production, and consequently the improvement of the gonadotrophins pulsatility.
TZDs could restore adipokines secretion implied in reproduction
Not only adipose tissue is involved for an energy storage, but also adipocytes secrete also hormones (TNFα, leptin, adiponectin, resistin, etc) which help to maintain homeostasis. These hormones are also implied directly in the regulation of the fertility at each level of the hypothalamo-pituitary-gonads axis (cf [77]). Moreover, the increased mass of the adipose tissue in PCOS patients alters the hormonal secretion (higher circulating levels of TNFα, resistin and lower levels of adiponectin [78, 79]). However, TZDs-induced return to a “normal metabolic state” may lead to a normal hormonal secretion by adipocytes. TZDs via PPARγ stimulate adipocyte differentiation and increase the number of smaller adipocytes that are highly insulin sensitive [80–82]. These small adipocytes produce fewer free fatty acids, TNFα and leptin [81]. In addition, TZDs stimulate adiponectin secretion by adipocytes in vitro [83], and adiponectin levels were increased in PCOS women treated by rosiglitazone [84]. Adiponectin sensitizes cells to insulin and inhibits resistin secretion by adipocytes, which antagonizes the insulin action [73, 85]. Furthermore, in vitro, human thecal cells stimulated previously by insulin or forskolin, and then treated with resistin, have shown an increased activity of the p450c17 enzyme, leading to a stimulation of the androgens secretion [86].
COMPARISON BETWEEN METFORMIN AND TZDS
Metformin seems to improve fertility of PCOS patients and is commonly used as an adjuvant to general lifestyle improvements [87, 88]. Metformin acts by a decrease of peripheral insulin resistance but new results suggest that metformin can regulate directly folliculogenesis at the ovarian level. In vitro in rat hepatocytes or in vivo in the human skeletal muscle, metformin activates AMP-activated protein kinase (AMPK), a regulator of energy balance [89]. In rat granulosa cells, activation of AMPK-induced by metformin decreases progesterone secretion, and the levels of proteins implied in steroidogenesis (3β-HSD, CYP11a1, STAR, and CYP19A1) [90]. Moreover, in human granulosa cells, metformin decreases progesterone secretion [91] and androgens synthesis through a direct inhibition of the Cyp17 activity [92]. Recently, AMPK was described to be activated indirectly by TZDs and independently of PPARγ [30]. These two insulin-sensitizing agents (metformin and TZDs) cause a rapid increase in the cellular ADP : ATP ratio, probably due by the inhibition of the respiratory chain, which can lead to the phosphorylation of AMPK [93].
Interestingly, PCOS women treated with metformin present lower follicular fluid concentrations of testosterone and insulin and after gonadotropin-stimulation for in vitro fertilization, the number of mature oocytes retrieved and oocytes fertilized was increased in comparison with controls [94].
Comparative studies [47, 95–97] performed between the two treatments (metformin and TZDs) have shown similar [47, 96] or better performance [96, 97] for TZDs to improve regular menstrual cyclicity (87.8% with rosiglitazone versus 79.3% with metformin, [96]), the ovulation rate and the pregnancy rate in PCOS patients.
The different mechanism used by metformin and by TZDs to improve fertiliy induces new possibility in the treatment of PCOS. For example, one clinical trial has tested co-administration of pioglitazone and metformin to PCOS women nonoptimally responsive to metformin. The percentage of menses increased two-fold after co-administration in comparison, with only metformin-treated women [98].
CONCLUSION
Overall, insulin-sensitizing treatments for PCOS patients, such as metformin or TZDs, lead to a strong improvement of the fertility. These treatments have several sites of action (steroid secretion by ovarian cells; insulin sensitivity in muscle and adipocyte and fat redistribution). More and more clinical data are now available and encourage us to redefine our approach of insulin resistance, and the treatment of infertility in patients with PCOS.
Creation of promising ligand, for example a dual PPARα/PPARγ agonist (glitazar class) [99, 100], could be useful to treat insulin sensitivity, atherosclerotic vascular, and fertility in PCOS women. Nevertheless, before using it in routine clinical practice, several extended safety tests should be necessary to estimate the potential risk of these synthetic ligands.
It is also necessary to keep in mind that TZDs can present a risk to the fetus during pregnancy and therefore their use should be carefully monitored, especially during the first weeks of pregnancy. Finally the development of animal models, mimicking PCOS is probably mandatory in next few years to increase our knowledge on this syndrome and to better understand the molecular actions of metformin and TZDs in target organs.
References
- 1.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocrine Reviews. 1997;18(6):774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 2.Deneux C, Kuttenn F. Hyperandrogenism and fertility. Annales d'Endocrinologie. 1998;59(4):311–318. [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility. 2004;81(1):19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Dunaif A. Insulin action in the polycystic ovary syndrome. Endocrinology and Metabolism Clinics of North America. 1999;28(2):341–359. doi: 10.1016/s0889-8529(05)70073-6. [DOI] [PubMed] [Google Scholar]
- 5.Yen SSC. The polycystic ovary syndrome. Clinical Endocrinology. 1980;12(2):177–207. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinology and Metabolism Clinics of North America. 1999;28(2):295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 7.Risma KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(5):1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poretsky L. Polycystic ovary syndrome—increased or preserved ovarian sensitivity to insulin? Journal of Clinical Endocrinology and Metabolism. 2006;91(8):2859–2860. doi: 10.1210/jc.2006-0317. [DOI] [PubMed] [Google Scholar]
- 9.Wood JR, Nelson VL, Ho C, et al. The molecular phenotype of polycystic ovary syndrome (PCOS) theca cells and new candidate PCOS genes defined by microarray analysis. Journal of Biological Chemistry. 2003;278(29):26380–26390. doi: 10.1074/jbc.M300688200. [DOI] [PubMed] [Google Scholar]
- 10.Barnes RB. Pathophysiology of ovarian steroid secretion in polycystic ovary syndrome. Seminars in Reproductive Endocrinology. 1997;15(2):159–168. doi: 10.1055/s-2007-1016297. [DOI] [PubMed] [Google Scholar]
- 11.Franks S, Gilling-Smith C, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinology and Metabolism Clinics of North America. 1999;28(2):361–378. doi: 10.1016/s0889-8529(05)70074-8. [DOI] [PubMed] [Google Scholar]
- 12.Bergh C, Carlsson B, Olsson J-H, Selleskog U, Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertility and Sterility. 1993;59(2):323–331. doi: 10.1016/s0015-0282(16)55675-1. [DOI] [PubMed] [Google Scholar]
- 13.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Human Reproduction Update. 2006;12(4):351–361. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 14.De Leo V, La Marca A, Petraglia F. Insulin-lowering agents in the management of polycystic ovary syndrome. Endocrine Reviews. 2003;24(5):633–667. doi: 10.1210/er.2002-0015. [DOI] [PubMed] [Google Scholar]
- 15.Iuorno MJ, Nestler JE. Insulin-lowering drugs in polycystic ovary syndrome. Obstetrics and Gynecology Clinics of North America. 2001;28(1):153–164. doi: 10.1016/s0889-8545(05)70191-1. [DOI] [PubMed] [Google Scholar]
- 16.Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, Allan G. Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome. New England Journal of Medicine. 1999;340(17):1314–1320. doi: 10.1056/NEJM199904293401703. [DOI] [PubMed] [Google Scholar]
- 17.Seli E, Duleba AJ. Treatment of PCOS with metformin and other insulin-sensitizing agents. Current Diabetes Reports. 2004;4(1):69–75. doi: 10.1007/s11892-004-0014-8. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) Journal of Biological Chemistry. 1995;270(22):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 19.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocrine Reviews. 1999;20(5):649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 20.Sørensen HN, Treuter E, Gustafsson JA. Regulation of peroxisome proliferator-activated receptors. Vitamins and Hormones. 1998;54:121–166. doi: 10.1016/s0083-6729(08)60924-3. [DOI] [PubMed] [Google Scholar]
- 21.Picard F, Auwerx J. PPARγ and glucose homeostasis. Annual Review of Nutrition. 2002;22:167–197. doi: 10.1146/annurev.nutr.22.010402.102808. [DOI] [PubMed] [Google Scholar]
- 22.Girard J. Mechanisms of action of thiazolidinediones. Diabetes and Metabolism. 2001;27(2 pt 2):271–278. [PubMed] [Google Scholar]
- 23.Gurnell M, Savage DB, Chatterjee VKK, O'Rahilly S. The metabolic syndrome: peroxisome proliferator-activated receptor γ and its therapeutic modulation. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2412–2421. doi: 10.1210/jc.2003-030435. [DOI] [PubMed] [Google Scholar]
- 24.Staels B, Fruchart J-C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54(8):2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 25.Komar CM. Peroxisome proliferator-activated receptors (PPARs) and ovarian function—implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reproductive Biology and Endocrinology. 2005;3:41. doi: 10.1186/1477-7827-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Froment P, Gizard F, Defever D, Staels B, Dupont J, Monget P. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition. Journal of Endocrinology. 2006;189(2):199–209. doi: 10.1677/joe.1.06667. [DOI] [PubMed] [Google Scholar]
- 27.Komar CM, Braissant O, Wahli W, Curry TE., Jr Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology. 2001;142(11):4831–4838. doi: 10.1210/endo.142.11.8429. [DOI] [PubMed] [Google Scholar]
- 28.Komar CM, Curry TE., Jr Inverse relationship between the expression of messenger ribonucleic acid for peroxisome proliferator-activated receptor γ and P450 side chain cleavage in the rat ovary. Biology of Reproduction. 2003;69(2):549–555. doi: 10.1095/biolreprod.102.012831. [DOI] [PubMed] [Google Scholar]
- 29.Abe A, Kiriyama Y, Hirano M, et al. Troglitazone suppresses cell growth of KU812 cells independently of PPARγ . European Journal of Pharmacology. 2002;436(1-2):7–13. doi: 10.1016/s0014-2999(01)01577-1. [DOI] [PubMed] [Google Scholar]
- 30.LeBrasseur NK, Kelly M, Tsao T-S, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. American Journal of Physiology - Endocrinology and Metabolism. 2006;291(1):E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 31.Khan MA, St. Peter JV, Xue JL. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care. 2002;25(4):708–711. doi: 10.2337/diacare.25.4.708. [DOI] [PubMed] [Google Scholar]
- 32.Stout DL, Fugate SE. Thiazolidinediones for treatment of polycystic ovary syndrome. Pharmacotherapy. 2005;25(2):244–252. doi: 10.1592/phco.25.2.244.56943. [DOI] [PubMed] [Google Scholar]
- 33.Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. Journal of Clinical Endocrinology and Metabolism. 2001;86(4):1626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa I, Murakawa H, Suzuki M, Yamamoto Y, Kurabayashi T, Tanaka K. Effect of troglitazone on endocrine and ovulatory performance in women with insulin resistance-related polycystic ovary syndrome. Fertility and Sterility. 1999;71(2):323–327. doi: 10.1016/s0015-0282(98)00454-3. [DOI] [PubMed] [Google Scholar]
- 35.Ehrmann DA, Schneider DJ, Sobel BE, et al. Troglitazone improves defects in insulin action, insulin secretion, ovarian steroidogenesis, and fibrinolysis in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 1997;82(7):2108–2116. doi: 10.1210/jcem.82.7.4069. [DOI] [PubMed] [Google Scholar]
- 36.Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 1996;81(9):3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- 37.Glintborg D, Hermann AP, Andersen M, et al. Effect of pioglitazone on glucose metabolism and luteinizing hormone secretion in women with polycystic ovary syndrome. Fertility and Sterility. 2006;86(2):385–397. doi: 10.1016/j.fertnstert.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 38.Garmes HM, Tambascia MA, Zantut-Wittmann DE. Endocrine-metabolic effects of the treatment with pioglitazone in obese patients with polycystic ovary syndrome. Gynecological Endocrinology. 2005;21(6):317–323. doi: 10.1080/09513590500430575. [DOI] [PubMed] [Google Scholar]
- 39.Brettenthaler N, De Geyter C, Huber PR, Keller U. Effect of the insulin sensitizer pioglitazone on insulin resistance, hyperandrogenism, and ovulatory dysfunction in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89(8):3835–3840. doi: 10.1210/jc.2003-031737. [DOI] [PubMed] [Google Scholar]
- 40.Romualdi D, Guido M, Ciampelli M, et al. Selective effects of pioglitazone on insulin and androgen abnormalities in normo- and hyperinsulinaemic obese patients with polycystic ovary syndrome. Human Reproduction. 2003;18(6):1210–1218. doi: 10.1093/humrep/deg264. [DOI] [PubMed] [Google Scholar]
- 41.Cataldo NA, Abbasi F, McLaughlin TL, Lamendola C, Reaven GM. Improvement in insulin sensitivity followed by ovulation and pregnancy in a woman with polycystic ovary syndrome who was treated with rosiglitazone. Fertility and Sterility. 2001;76(5):1057–1059. doi: 10.1016/s0015-0282(01)02843-6. [DOI] [PubMed] [Google Scholar]
- 42.Cataldo NA, Abbasi F, McLaughlin TL, et al. Metabolic and ovarian effects of rosiglitazone treatment for 12 weeks in insulin-resistant women with polycystic ovary syndrome. Human Reproduction. 2006;21(1):109–120. doi: 10.1093/humrep/dei289. [DOI] [PubMed] [Google Scholar]
- 43.Sepilian V, Nagamani M. Effects of rosiglitazone in obese women with polycystic ovary syndrome and severe insulin resistance. Journal of Clinical Endocrinology and Metabolism. 2005;90(1):60–65. doi: 10.1210/jc.2004-1376. [DOI] [PubMed] [Google Scholar]
- 44.Belli SH, Graffigna MN, Oneto A, Otero P, Schurman L, Levalle OA. Effect of rosiglitazone on insulin resistance, growth factors, and reproductive disturbances in women with polycystic ovary syndrome. Fertility and Sterility. 2004;81(3):624–629. doi: 10.1016/j.fertnstert.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Mitwally MFM, Kuscu NK, Yalcinkaya TM. High ovulatory rates with use of troglitazone in clomiphene-resistant women with polycystic ovary syndrome. Human Reproduction. 1999;14(11):2700–2703. doi: 10.1093/humrep/14.11.2700. [DOI] [PubMed] [Google Scholar]
- 46.Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D, Ke RW. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertility and Sterility. 2003;79(3):562–566. doi: 10.1016/s0015-0282(02)04843-4. [DOI] [PubMed] [Google Scholar]
- 47.Ortega-González C, Luna S, Hernández L, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2005;90(3):1360–1365. doi: 10.1210/jc.2004-1965. [DOI] [PubMed] [Google Scholar]
- 48.Barak Y, Nelson MC, Ong ES, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Molecular Cell. 1999;4(4):585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 49.Sevillano J, López-Pérez IC, Herrera E, Del Pilar Ramos M, Bocos C. Englitazone administration to late pregnant rats produces delayed body growth and insulin resistance in their fetuses and neonates. Biochemical Journal. 2005;389(3):913–918. doi: 10.1042/BJ20041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yaris F, Yaris E, Kadioglu M, Ulku C, Kesim M, Kalyoncu NI. Normal pregnancy outcome following inadvertent exposure to rosiglitazone, gliclazide, and atorvastatin in a diabetic and hypertensive woman. Reproductive Toxicology. 2004;18(4):619–621. doi: 10.1016/j.reprotox.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Kalyoncu NI, Yaris F, Ulku C, et al. A case of rosiglitazone exposure in the second trimester of pregnancy. Reproductive Toxicology. 2005;19(4):563–564. doi: 10.1016/j.reprotox.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Glueck CJ, Phillips H, Cameron D, Sieve-Smith L, Wang P. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a pilot study. Fertility and Sterility. 2001;75(1):46–52. doi: 10.1016/s0015-0282(00)01666-6. [DOI] [PubMed] [Google Scholar]
- 53.Balen AH, Tan S-L, MacDougall J, Jacobs HS. Miscarriage rates following in-vitro fertilization are increased in women with polycystic ovaries and reduced by pituitary desensitization with buserelin. Human Reproduction. 1993;8(6):959–964. doi: 10.1093/oxfordjournals.humrep.a138174. [DOI] [PubMed] [Google Scholar]
- 54.Harborne L, Fleming R, Lyall H, Norman J, Sattar N. Descriptive review of the evidence for the use of metformin in polycystic ovary syndrome. Lancet. 2003;361(9372):1894–1901. doi: 10.1016/S0140-6736(03)13493-9. [DOI] [PubMed] [Google Scholar]
- 55.Mehta RV, Patel KS, Coffler MS, et al. Luteinizing hormone secretion is not influenced by insulin infusion in women with polycystic ovary syndrome despite improved insulin sensitivity during pioglitazone treatment. Journal of Clinical Endocrinology and Metabolism. 2005;90(4):2136–2141. doi: 10.1210/jc.2004-1040. [DOI] [PubMed] [Google Scholar]
- 56.Downing JA, Scaramuzzi RJ. Nutrient effects on ovulation rate, ovarian function and the secretion of gonadotrophic and metabolic hormones in sheep. Journal of Reproduction and Fertility. Supplement. 1991;43:209–227. [PubMed] [Google Scholar]
- 57.Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertility and Sterility. 2004;82(suppl 3):1008–1013. doi: 10.1016/j.fertnstert.2004.02.148. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y, Miyoshi K, Claudio E, et al. Loss of the peroxisome proliferation-activated receptor gamma (PPARγ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. Journal of Biological Chemistry. 2002;277(20):17830–17835. doi: 10.1074/jbc.M200186200. [DOI] [PubMed] [Google Scholar]
- 59.Duffy D, Montgomery G, Treloar S, et al. IBD sharing around the PPARG locus is not increased in dizygotic twins or their mothers. Nature Genetics. 2001;28(4):315. doi: 10.1038/91074. [DOI] [PubMed] [Google Scholar]
- 60.Seto-Young D, Paliou M, Schlosser J, et al. Direct thiazolidinedione action in the human ovary: insulin-independent and insulin-sensitizing effects on steroidogenesis and insulin-like growth factor binding protein-1 production. Journal of Clinical Endocrinology and Metabolism. 2005;90(11):6099–6105. doi: 10.1210/jc.2005-0469. [DOI] [PubMed] [Google Scholar]
- 61.Mu Y-M, Yanase T, Nishi Y, et al. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochemical and Biophysical Research Communications. 2000;271(3):710–713. doi: 10.1006/bbrc.2000.2701. [DOI] [PubMed] [Google Scholar]
- 62.Willis DS, White J, Brosens S, Franks S. Effect of 15-deoxy-(12,14)-prostaglandin J2(PGJ2) a peroxisome proliferator activating receptor (PPAR) ligand on human ovarian steroidogenesis. Endocrinology. 1999;(suppl 1):491. (abstract P3-247). [Google Scholar]
- 63.Veldhuis JD, Zhang G, Garmey JC. Troglitazone, an insulin-sensitizing thiazolidinedione, represses combined stimulation by LH and insulin of de novo androgen biosynthesis by thecal cells in vitro. Journal of Clinical Endocrinology and Metabolism. 2002;87(3):1129–1133. doi: 10.1210/jcem.87.3.8308. [DOI] [PubMed] [Google Scholar]
- 64.Gasic S, Nagamani M, Green A, Urban RJ. Troglitazone is a competitive inhibitor of 3β-hydroxysteroid dehydrogenase enzyme in the ovary. American Journal of Obstetrics and Gynecology. 2001;184(4):575–579. doi: 10.1067/mob.2001.111242. [DOI] [PubMed] [Google Scholar]
- 65.Coffler MS, Patel K, Dahan MH, Yoo RY, Malcom PJ, Chang RJ. Enhanced granulosa cell responsiveness to follicle-stimulating hormone during insulin infusion in women with polycystic ovary syndrome treated with pioglitazone. Journal of Clinical Endocrinology and Metabolism. 2003;88(12):5624–5631. doi: 10.1210/jc.2003-030745. [DOI] [PubMed] [Google Scholar]
- 66.Avtanski D, Kaplun J, Strizhevsky M, et al. Interactions among PPAR-, insulin signaling pathways and aromatase in human ovarian cells. In: Proceedings of the 88th Annual Meeting of the Endocrine Society (ENDO '06); June 2006; Boston, Mass. (abstract P1-397). [Google Scholar]
- 67.Dunaif A, Thomas A. Current concepts in the polycystic ovary syndrome. Annual Review of Medicine. 2001;52:401–419. doi: 10.1146/annurev.med.52.1.401. [DOI] [PubMed] [Google Scholar]
- 68.Yamauchi T, Kamon J, Waki H, et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor γ (PPARγ) deficiency and PPARγ agonist improve insulin resistance. Journal of Biological Chemistry. 2001;276(44):41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- 69.Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clinical Endocrinology. 1994;41(4):463–471. doi: 10.1111/j.1365-2265.1994.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 70.Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Annals of Internal Medicine. 2001;134(1):61–71. doi: 10.7326/0003-4819-134-1-200101020-00014. [DOI] [PubMed] [Google Scholar]
- 71.Bates GW, Whitworth NS. Effect of body weight reduction on plasma androgens in obese, infertile women. Fertility and Sterility. 1982;38(4):406–409. [PubMed] [Google Scholar]
- 72.Huber-Buchholz M-M, Carey DGP, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. Journal of Clinical Endocrinology and Metabolism. 1999;84(4):1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 73.Larsen TM, Toubro S, Astrup A. PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy? International Journal of Obesity. 2003;27(2):147–161. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 74.Arner P. The adipocyte in insulin resistance: key molecules and the impact of the thiazolidinediones. Trends in Endocrinology and Metabolism. 2003;14(3):137–145. doi: 10.1016/s1043-2760(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 75.Björntorp P. The regulation of adipose tissue distribution in humans. International Journal of Obesity. 1996;20(4):291–302. [PubMed] [Google Scholar]
- 76.Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The central issue? Visceral fat mass is a good marker of insulin resistance and metabolic disturbance in women with polycystic ovary syndrome. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113(10):1203–1209. doi: 10.1111/j.1471-0528.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 77.Budak E, Fernández Sánchez M, Bellver J, Cerveró A, Simón C, Pellicer A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3-36 with the reproductive system. Fertility and Sterility. 2006;85(6):1563–1581. doi: 10.1016/j.fertnstert.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48(4):437–441. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 79.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, et al. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Human Reproduction. 2006;21(9):2257–2265. doi: 10.1093/humrep/del146. [DOI] [PubMed] [Google Scholar]
- 80.Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Journal of Clinical Investigation. 1998;101(6):1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kahn CR, Chen L, Cohen SE. Unraveling the mechanism of action of thiazolidinediones. Journal of Clinical Investigation. 2000;106(11):1305–1307. doi: 10.1172/JCI11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duran-Sandoval D, Thomas A-C, Bailleul B, Fruchart J-C, Staels B. Pharmacology of PPARα, PPARγ and dual PPARα/γ agonists in clinical development. Medecine/Sciences. 2003;19(8-9):819–825. doi: 10.1051/medsci/20031989819. [DOI] [PubMed] [Google Scholar]
- 83.Bodles A, Banga A, Rasouli N, Ono F, Kern PA, Owens RJ. Pioglitazone increases secretion of high-molecular-weight adiponectin from adipocytes. American Journal of Physiology - Endocrinology and Metabolism. 2006;291(5):E1100–E1105. doi: 10.1152/ajpendo.00187.2006. [DOI] [PubMed] [Google Scholar]
- 84.Sepilian V, Nagamani M. Adiponectin levels in women with polycystic ovary syndrome and severe insulin resistance. Journal of the Society for Gynecologic Investigation. 2005;12(2):129–134. doi: 10.1016/j.jsgi.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Orio F, Jr, Palomba S, Cascella T, et al. Adiponectin levels in women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2003;88(6):2619–2623. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- 86.Munir I, Yen H-W, Baruth T, et al. Resistin stimulation of 17α-hydroxylase activity in ovarian theca cells in vitro: relevance to polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2005;90(8):4852–4857. doi: 10.1210/jc.2004-2152. [DOI] [PubMed] [Google Scholar]
- 87.Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. British Medical Journal. 2003;327(7421):951–953. doi: 10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norman RJ. Editorial: metformin—comparison with other therapies in ovulation induction in polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89(10):4797–4800. doi: 10.1210/jc.2004-1658. [DOI] [PubMed] [Google Scholar]
- 89.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein-kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 90.Tosca L, Solnais P, Ferré P, Foufelle F, Dupont J. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biology of Reproduction. 2006;75(3):342–351. doi: 10.1095/biolreprod.106.050831. [DOI] [PubMed] [Google Scholar]
- 91.Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertility and Sterility. 2003;79(4):956–962. doi: 10.1016/s0015-0282(02)04925-7. [DOI] [PubMed] [Google Scholar]
- 92.La Marca A, Egbe TO, Morgante G, Paglia T, Ciani A, De Leo V. Metformin treatment reduces ovarian cytochrome P-450c17α response to human chorionic gonadotrophin in women with insulin resistance-related polycystic ovary syndrome. Human Reproduction. 2000;15(1):21–23. doi: 10.1093/humrep/15.1.21. [DOI] [PubMed] [Google Scholar]
- 93.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochemical Journal. 2000;348(3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 94.Stadtmauer LA, Toma SK, Riehl RM, Talbert LM. Impact of metformin therapy on ovarian stimulation and outcome in ‘coasted’ patients with polycystic ovary syndrome undergoing in-vitro fertilization. Reproductive Biomedicine Online. 2002;5(2):112–116. doi: 10.1016/s1472-6483(10)61612-4. [DOI] [PubMed] [Google Scholar]
- 95.Baillargeon J-P, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertility and Sterility. 2004;82(4):893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 96.Rouzi AA, Ardawi MSM. A randomized controlled trial of the efficacy of rosiglitazone and clomiphene citrate versus metformin and clomiphene citrate in women with clomiphene citrate-resistant polycystic ovary syndrome. Fertility and Sterility. 2006;85(2):428–435. doi: 10.1016/j.fertnstert.2005.07.1312. [DOI] [PubMed] [Google Scholar]
- 97.Yilmaz M, Karakoç A, Törüner FB, et al. The effects of rosiglitazone and metformin on menstrual cyclicity and hirsutism in polycystic ovary syndrome. Gynecological Endocrinology. 2005;21(3):154–160. doi: 10.1080/09513590500231627. [DOI] [PubMed] [Google Scholar]
- 98.Glueck CJ, Moreira A, Goldenberg N, Sieve L, Wang P. Pioglitazone and metformin in obese women with polycystic ovary syndrome not optimally responsive to metformin. Human Reproduction. 2003;18(8):1618–1625. doi: 10.1093/humrep/deg343. [DOI] [PubMed] [Google Scholar]
- 99.Xu C, Wang L-L, Liu H-Y, et al. A novel dual peroxisome proliferator-activated receptors α and γ agonist with beneficial effects on insulin resistance and lipid metabolism. Biotechnology Letters. 2006;28(12):863–868. doi: 10.1007/s10529-006-9013-y. [DOI] [PubMed] [Google Scholar]
- 100.Fievet C, Fruchart JC, Staels B. PPARalpha and PPARgamma dual agonists for the treatment of type 2 diabetes and the metabolic syndrome. Current Opinion in Pharmacology. 2006;6(6):606–614. doi: 10.1016/j.coph.2006.06.009. [DOI] [PubMed] [Google Scholar]