Abstract
The neuromodulatory peptides orexin A and B are important central nervous system regulators of appetite. We previously identified the rostral lateral portion of the hypothalamus as an area important to orexin A feeding regulation. As γ-aminobutyric-acid (GABA) within the lateral hypothalamus also mediates feeding, we sought to determine the relationship between orexin and GABA signaling within this site. Adult male Sprague Dawley rats were implanted with cannulae directed to the rostral lateral hypothalamus and saclofen (GABA-B receptor antagonist), biccuculine (GABA-A receptor antagonist), or muscimol (GABA-A receptor agonist) were injected prior to orexin A. Both GABA antagonists failed to significantly affect orexin A-induced feeding, but muscimol significantly and dose dependently inhibited orexin A-induced feeding. Using in vivo microdialysis GABA release within this region significantly dropped during the first hour following orexin A administration, coinciding with orexin A induced feeding. Together, these data indicate that orexin A may influence food intake by decreasing GABAergic tone within the rostral lateral hypothalamus.
Keywords: hypocretin, feeding behavior, bicuculline, 2-hydroxysaclofen, muscimol, microdialysis
Introduction
The neuropeptides orexin A and B (also referred to as hypocretin 1 and 2) are produced by neurons in the caudal aspect of the hypothalamus including the lateral hypothalamic area, perifornical region and the dorsomedial nucleus [4,19]. Both orexin A and orexin B are proteolytically processed to form two c-terminally amidated peptides from the same 131 amino acid residue (human) or 130 amino acid residue (rat) [19,21]. Orexin A is a 33 amino acid residue with 2 disulfide bonds whose structure is conserved across many species; while orexin B is a 28 amino acid linear residue [5,19].
Both of the identified orexin receptors belong to the g-protein-coupled super family of receptors. OX1R is Gq-coupled and has a ten-fold greater affinity for orexin A than orexin B, and OX2R is Gi- or Go-coupled and has nearly equal affinity for both peptides [19,29]. OX1R and OX2R are located on both pre- and post-synaptic processes, as well as on cell bodies [1,29]. They are distributed throughout the neuraxis, with expression particularly robust in regions important for regulation of arousal (locus coeruleus, dorsal raphe, tuberomammillary nucleus) and feeding (lateral, arcuate and paraventricular nuclei) [1,3]. Humans who lack orexin neurons suffer from narcolepsy, a condition where the sudden onset of sleep can frequently interrupt the normal wake period [26].
Food intake is stimulated after administering GABA or the GABA-A receptor agonist muscimol into the ventromedial hypothalamus and inhibited by the GABA-A receptor antagonist bicuculline [6,9-11]. While the effect of GABA administered into the lateral hypothalamic area to reduce food intake was variable, administering bicuculline into the rostrolateral hypothalamic area reliably increased food intake [9]. We have previously demonstrated that orexin A delivered just dorsal to the rostral lateral hypothalamic area results in a relatively strong feeding response in rats [24,27]. This site is 1 mm anterior to the beginning of the orexin neuron population, medial to the internal capsule and lateral to the fornix. The aim of the present set of studies was to test the hypothesis that orexin A exerts its feeding effects at this site via modulating GABAergic tone.
Methods
Animal subjects
Male Sprague-Dawley rats were used in all experiments. Rats were singly housed in hanging wire bottom cages (17.8 x 17.8 x 24.8 cm) in a light-(lights on at 0700 h and lights off at 1900 h) and climate-(21.7°C) controlled room. Rats weighing 250-275 g at the beginning of the study had rat chow (Harlan Teklad 8604) and tap water available ad libitum. All studies received local institutional animal care and use committee approval.
Cannulation surgery
Rats were implanted with a unilateral stainless steel guide cannula (Plastics One, Roanoke Virginia) directed dorsal to the rostral portion of the lateral hypothalamic area (-2.2 mm posterior, ± 1.9 mm lateral, 7.2 mm ventral, relative to bregma, with incisor bar set at - 3.3 mm). Cannulae were held in place with dental cement (Dentsply, York, Pennsylvania) and three cranially embedded anchor screws (Plastics One, Roanoke Virginia or Small parts, Miami Beach, Fl). Prior to surgery rats were anesthetized with a combination of Ketamine (90 mg/kg) and Xylazine (15 mg/kg). Flunixin (2.5 mg/kg, sc) or Banamine (1.5/mg kg, sc) was administered as a post-operative analgesic. Rats were allowed at least 7 days of recovery after surgery during which they were handled daily and adapted to the experimental procedures.
Drugs
Orexin A was obtained from Phoenix Pharmaceuticals (Sunnyvale, CA). Muscimol, bicuculline and 2-OH saclofen were obtained from Sigma (St. Louis, MO). All dose ranges were based on those that influenced behavioral outcomes in previous studies [8,15,20,23-25,27].
Drug administration
Injections were made manually via injectors (Plastics One, Roanoke Virginia; designed to extend 1 mm beyond guide cannulae) attached to polyurethane tubing (PE20) backfilled with sterile water and attached to a 2μl microsyringe (Hamilton Company, Reno, Nevada). A 2-μl air bubble separated the drug solution from sterile water. Drug (0.5 μl per injection) was infused over 30 seconds with the injector left in place for an additional 15 seconds. After surgery and between injections dummy cannulae that extended just to the tip of the indwelling guide cannulae were inserted into the guide cannulae to prevent foreign materials from clogging the indwelling cannulae.
Cannula Verification
Correct cannula placement was determined with post mortem histological verification as previously described [25] and a representative section showing the cannula tract is shown in Fig. 1. Greater than 90% of cannulae were correctly placed, and the data presented are only from animals with correctly placed cannulae (shown in Fig. 2).
Figure 1.
Representative histology of a brain section that includes the rostral lateral hypothalamic injection site targeted in experiments 1 and 2 (all studies). The cannula track ends just above the rostral LH area and the injector extends 1 mm beyond into the rostral lateral hypothalamic area. Slice is at 4x magnification under light microscope.
Fig. 2.
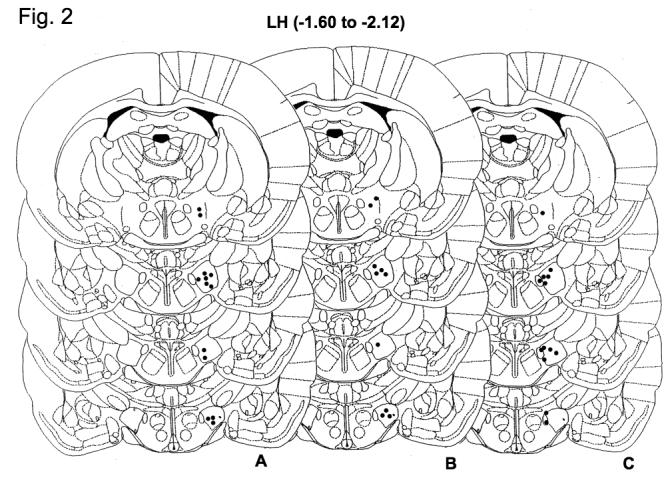
Schematic of brain atlas maps (adapted from Paxinos and Watson [16]) indicating correctly placed injection sites for all studies. The black dots represent the site at which the injection occurred (cannulae are located 1 mm above the injection site). A. Correct injection sites in studies 1a and 1b (effect of bicucculine and 2-hydroxysaclofen on orexin A induced feeding). B. Correct injection sites for study 1c (effect of muscimol on orexin A-induced feeding). C. Correct microdialysis placements in experiment 2 (effect of orexin A on extracellular GABA).
Specific Experimental designs
Experiments 1a-c: Effect of pre-administration of GABA antagonist and agonists on orexin A-induced feeding
Rats were allowed to eat chow ad libitum prior to experimental trials. Food hoppers containing standard laboratory pelleted chow (Harlan Teklad 8604) were removed from the cages and weighed just prior to injections, and returned to the cage just after injection. Food intake was measured at 1 and 2 h following injection. Injections were given in a repeated measures, counterbalanced design, and carried out between 1300 h and 1500 h, a timeframe based on our previous work demonstrating that orexin A in the rLHa significantly stimulates feeding during this time of day [27]. There were at least 72 h between injections. The same set of rats (N = 15) was used for experiments 1a and 1b. A separate set of rats (N =10) was used for experiment 1c.
Study 1a and 1b: Effect of bicuculline (1a) and 2-hydroxy-saclofen (1b) on orexin A-induced feeding
Rats were injected with vehicle (artificial cerebrospinal fluid, aCSF), bicuculline or 2-hydroxysaclofen (100, 200 and 400 pmol). Twenty minutes later rats were then injected with vehicle (aCSF) or orexin A (500 pmol). All treatments (total of 10 for this study) were given in a counterbalanced order. To limit the number of injections per animal, only the highest dose of antagonist (400 pmol) was tested alone with vehicle (aCSF) injection. This dose of antagonist was chosen based on previous data indicating efficacy within a 2 h timeframe
Study 1c: Effect of muscimol on orexin A-induced feeding
Rats were injected with vehicle (aCSF) or muscimol (20, 60 and 100 pmol). Twenty minutes later rats were then injected with vehicle (aCSF) or orexin A (500 pmol). All treatments (total of 6 for this study) were given in a counterbalanced order. To limit the number of injections, only the highest dose of muscimol (100 pmol) was tested alone with vehicle (aCSF) injection.
Experiment 2: Effect of orexin A on GABA release as measured by microdialysis
Microdialysis samples were collected from unrestrained animals in their home cages using sampling lines through a liquid swivel (Instech, Plymouth Meeting, PA) connected to a weighted counterbalance lever. Food was removed at the time of probe placement and experiments were conducted during the mid-light phase. Combination microinjection/microdialysis probes, with 0.25 x 1 mm surface area of cuprophan membrane (AKZO-Nobel, Wuppertal, Germany) and a 150 μM fused silica (Polymicron, Phoenix, AZ) injection line, were placed into the rostrolateral hypothalamic area 3 h before samples were collected. The probes were connected to a microinfusion pump (CMA/Microdialysis, North Chelmsford, MA) and continuously perfused with Kreb’s Ringer buffer (in mM: 147 NaCl, 4 KCl, 2.4 CaCl2; pH 6.4) at 1.5-μL min-1. After collecting baseline samples, 500 pmol of orexin A was injected in 0.5-μL volume of aCSF over a 5 min period. Samples were collected for an additional 2 h before food was returned and the amount consumed over the next h determined. Dialysate samples were collected at 10 min intervals into chilled microtubes and kept at -82° C until assayed for GABA. GABA was analyzed by reversed-phase HPLC using an o-phthaldialdehyde-sulfite reaction with electrochemical detection [22]. Limit of detection for the assay was 50 fmol GABA with coefficient of variations for 1 pmol GABA standard < 0.05.
Statistical analyses
Experiment 1
To determine the effect of orexin A on food intake, the veh/veh and veh/orexin data were analyzed by matched paired t-tests. To determine effectiveness of muscimol, bicuculline and 2-hydroxy-saclofen to influence food intake elicited by orexin A, data were analyzed by repeated measures one-way ANOVA for each drug tested. When a significant main effect was observed, data were analyzed post-hoc by Fishers PLSD to compare treatment means. Finally, to determine the effect of drug alone (muscimol, bicuculline or 2-hydroxy-saclofen) on food intake, the veh/veh and drug/veh data were analyzed by matched paired t-tests. A Bonferonni correction of the alpha level (.05/2 = .025) was used when performing the two paired t-tests per study. For each study, three time intervals were assessed: 0-1 h, 1-2 h and 0-2 h.
Prior to combining the data from all treatment days, data from the control injected rats (veh/veh) were analyzed by one factor ANOVA (independent variable = day of injection, dependent variables were 0-1 h, 1-2 h and 0-2 h food intake) to determine whether there was a ‘day’ effect on feeding response and none was found (P > .05). Further, to determine whether orexin A efficacy changed with repeated injections, data from only the orexin A-treated rats was analyzed by one factor ANOVA (independent variable = day of injection, dependent variables were 0-1 h, 1-2 h and 0-2 h food intake). No significant effect of day on food intake in orexin A-treated animals was found (P > .05).
Experiment 2
Data were analyzed by repeated-measures analysis of variance and Scheffe’s post-hoc comparison test. Differences from baseline were analyzed within treatments at each time point by paired t-test. Food intake was analyzed by t-test.
Results
Effect of bicuculline (1a) and 2-hydroxy-saclofen (1b) on orexin A-induced feeding
Matched paired t-tests indicate that orexin A significantly stimulated food intake at both 1 and 2 h post-injection (0-1 h: P = .0121 and 0-2 h: P = .0162, Figs. 3a and 3b) but not in the 1-2 h time interval (P > .025, Figs. 3a and 3b). Repeated measures ANOVA indicated that orexin A stimulated feeding was not significantly affected at any time-point by pretreatment with bicuculline (P > .05, Fig. 3a) or 2-hydroxy-saclofen (P > .05, Fig. 3b). Matched paired t-tests indicated that 400 pmol bicuculline alone significantly increased food intake at 1 h following injection (P = .0211, Fig. 1a) but not within the 1-2 h interval or in the 0-2 h interval (P > .025, Fig. 3a). There was no effect of 400 pmol 2-hydroxy-saclofen alone on food intake in any time interval (P > .025, Fig. 3b).
Figure 3.
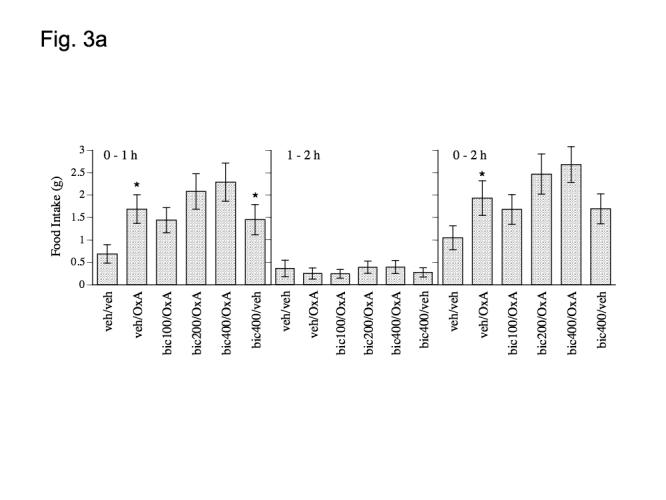
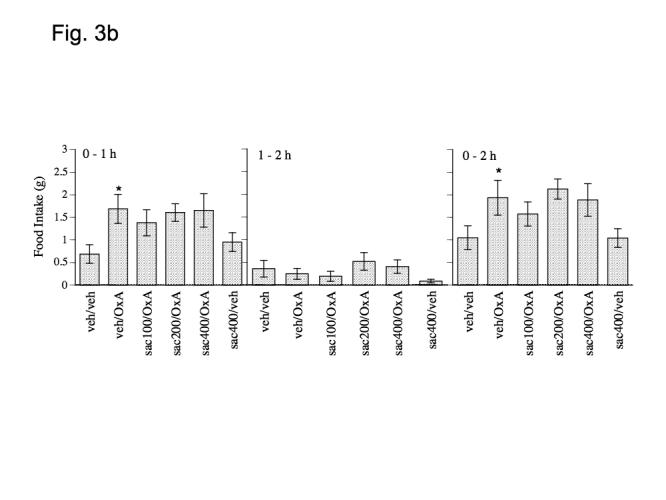
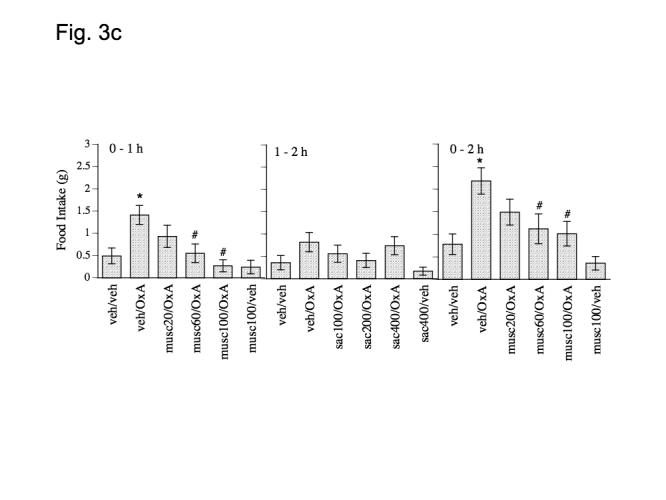
Effect of a) bicucculine (100, 200 and 400 pmol), b) 2-hydroxysaclofen (100, 200 and 400 pmol) and c) muscimol (20, 60 and 100 pmol), on orexin A (500 pmol) induced food intake at 1 and 2 h post-injection. Data represent mean values ± S.E.M. Bic = bicucculine, OxA = orexin A; musc = muscimol; sac = 2-hydroxysaclofen; veh = vehicle (aCSF). * P < .05 as compared to veh/veh group. # P < .05 as compared to veh/orexin group.
Experiment 1c: Effect of muscimol on orexin A-induced feeding
Orexin A induced feeding was inhibited by pretreatment with muscimol (Fig. 3c). Paired t-tests indicated that orexin A significantly increased feeding at 1 and 2 h following injection (0 - 1 h: P = .0020; 0-2 h: P = .0005, Fig. 3c), but not within the 1-2 h interval (P > .025, Fig. 3c). A one factor (treatment) repeated measures ANOVA with food intake (0 - 1 h and 0 - 2 h) as the dependant variable indicated a main effect of muscimol on orexin A induced feeding at the 0 - 1 h and 0-2 h time-interval (F(3,27) = 5.791, P = .0034; 0 - 2 h: F(3,27) = 53.248, P = .0373) but not within the 1-2 h time interval (P > .05, Fig. 3c). Post-hoc Fishers PLSD indicated that 60 and 100 pmol muscimol significantly reduced orexin A induced feeding at both 1 and 2 h following injection (0-1 h: 60 pmol, P = .0193 and 100 pmol, P = .0003; 0-2 h: 60 pmol, P = .0158 and 100 pmol, P = .0083, Fig. 3c). Muscimol alone was not significantly different from control treatment at any time interval (P > .025, Fig. 3c).
Experiment 2: Effect of orexin A on GABA release as measured by microdialysis
There was a significant decrease in GABA release following orexin A administration (F(1,224) = 17.87, p < 0.001), with GABA concentrations being reduced by up to 30% (1.20 ± 0.24 μM in controls vs. 1.08 ± 0.16 μM in orexin-treated rats) during the first 60 min after orexin administration (Fig. 4). We did not provide food during the dialysis experiment to avoid an experimental confound of food intake effects on GABA release [2]. Thus, as the best possible measure to verify that orexin A would have elicited it’s orexigenic effect during the experiment, had food been provided, we provided food in the cage at the end of the experiment and measured intake 1 h later (3 h after orexin A administration). Rats administered orexin A (500 pmol) consumed 2.4 ± 0.3 g during the hour following dialysis vs. 1.6 ± 0.3 g consumed by control (aCSF) rats (p = 0.11, data not shown). This insignificant increase might be predicted based on previous data showing that the efficacy of orexin A to induce a feeding response is diminished after 2 h [12,14,24,25,27,31].
Fig. 4.
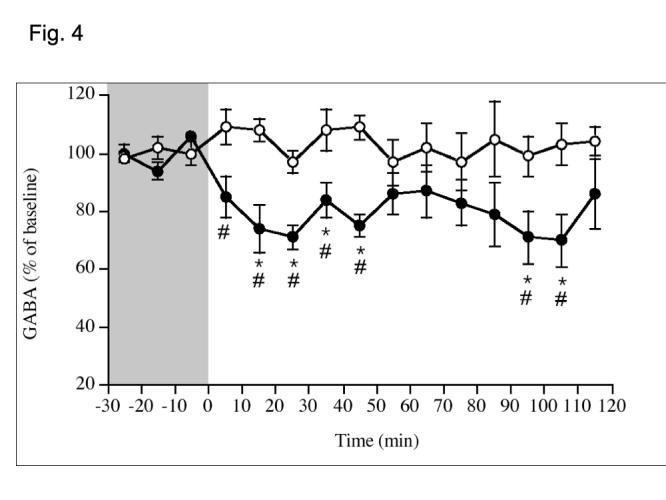
Changes in GABA concentration in rLHa after local injection of aCSF or orexin A (500 pmol/0.5 uL/5 min , starting at -5 min). Data represent mean ± S.E.M. Open circles = aCSF; filled circles = orexin A. *P < .05 different from baseline, #P < 0.05 different from aCSF. N = 7-10/group.
Discussion
The present findings support the concept that orexin A in the rostrolateral hypothalamic area affects modulates feeding behavior by influencing GABAergic tone. The feeding response induced by orexin A infusion into the rostro-lateral hypothalamic area was coincident with a decrease in GABA dialysate and inhibited by the GABA agonist muscimol. These findings support the hypothesis that orexin A facilitates feeding behavior via acting to inhibit local GABAergic tone in this region. In addition, these findings are in accord with the long established hypothesis that GABAergic tone is a critical component of the hypothalamic feeding system [10].
GABA and glutamate are the most abundant neurotransmitters in the hypothalamus [7]. The majority of fast synaptic transmission in the hypothalamus is regulated by these neurotransmitters [17,18,30]). As such, the actions of other putative orexigenic and anorexic signaling molecules within the hypothalamus undoubtedly evolved to incorporate an intricate signaling relationship with GABA and glutamate. Muscimol infusion into the paraventricular and ventromedial hypothalamic areas stimulates feeding behavior, but either has no effect [10,11] or reduces feeding behavior [10,11] following light cycle injections of muscimol (∼220 - 870 pmol) into the rostral lateral hypothalamus. In general agreement with these findings, the current data show no significant effect of 100 pmol rostro-lateral hypothalamic injected muscimol on light cycle baseline feeding behavior, but a dose-dependant reduction in orexin A-induced feeding behavior. Although we did not test pre-injections of muscimol in the microdialysis experiment, together these data suggest that muscimol abolishes orexin A-induced feeding by negating effects of reduced extracellular GABA. The time course of GABA inhibition by orexin A follows that previously observed for orexin A induced feeding [24,27]. Likewise, GABA has been shown to drop within the LH following hunger induced by 2-deoxy-D-glucose, but only when animals had access to food [2]. It is important to note that in the microdialysis study, rats were denied access to food during the microdialysis. Thus, potential feedback signals due to food consumption after orexin injection would not have occurred during the microdialysis study. Our previous and current work indicates that orexin-mediated feeding occurs primarily within the first hour after injection, with some additional feeding in the 2nd hour, and less or none in the 3rd and 4th h post-injection. Thus the sustained drop in GABA levels (Fig. 4) may have contributed to the insignificant increase in food intake we observed at 3 h following orexin A injection in the microdialysis study.
As co-injecting the GABA agonist reversed the feeding effect of orexin A, we expected that co-injection of GABA antagonists would enhance the orexigenic effect of orexin A. Although the antagonist pre-injection enhanced orexin A induced feeding slightly, it was not significant or robust. It’s possible that using a different pre-injection time or dose of the GABA antagonists, we may have observed differences. It’s also possible that there may be a minimal GABAergic tone created by orexin A administration that cannot be surpassed by the additional manipulation of GABA receptor antagonist blockade. This could explain the relative “ceiling” effect of the response. It should be noted that in other situations, including food restriction, feeding response to orexin A increases by 2-3 fold. Thus, there are situations in which feeding induced by orexin A in this region can be further enhanced above controls, but based on the present data it appears that further reducing GABAergic tone is not involved in this process. The present findings also appear to be contradictory to those of a recent report in which feeding induced by orexin A infused intraventricularly was enhanced by pretreatment with muscimol [13]. However, in that study, muscimol and orexin A were infused into the ventricles. Thus it is unclear what specific brain circuitry is involved in the effects observed, which likely accounts for the disparity between those results and our current dataset from rLH-infused orexin A and muscimol.
The observed decrease in GABA dialysate seems somewhat contradictory to the observed GABA release in arcuate hypothalamic slices in vitro [29], but the different brain area targeted in the current study and the in vivo model may account for this apparent discrepancy. Orexin A-mediated decrements in GABA are likely mediated by OX2R as OX2R has been shown to be Gi (inhibitory effector) coupled. The presence of OX2R has been reported in this region [3,28] and SB334867A (OX1R antagonist) in this region does not inhibit orexin A induced feeding (unpublished observations). Together these finding support a role for OX2R in the regulation of feeding within this region; unfortunately there is no specific OX2R antagonist yet available to test this hypothesis.
Interestingly, orexin A is thought to excite neurons [4], yet following micro-infusion of orexin A into this region there is a prolonged decrease in GABA. It may be possible that this is a compensatory rebound effect following the excitatory interaction of a bolus of exogenous orexin A with OXRs. In other words, after orexin A is injected into and excites this region, it may initiate a prolonged compensatory inhibitory signaling, which is responsible for the increased feeding seen after orexin A is injected into this site. The delayed “second” drop in extracellular GABA at 90 and 100 minutes following orexin A injection may be due to the lack of feedback control in the microdialysis study as discussed above. Significant orexin A-mediated food intake at these time-points is not usually observed [12,14,24,25,27,31].
In conclusion, we report that within the rostral lateral hypothalamus, exogenous orexin A administration is followed by a local decrease in GABAergic tone. These data agree with the numerous previously published reports asserting the importance of GABAergic modulation of feeding behavior within the hypothalamus.
Acknowledgements
We would like to thank Mary Mullett for her expert technical assistance with the microinjections, food intake measurements and histology, and Dr. Michael Kuskowski for statistical consultation. These studies were funded by the Department of Veterans Affairs, NIDDK 57573 and USDA ILLU-538-330.
Footnotes
Supported by the Department of Veterans Affairs, the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK 57573 and USDA ILLU-538-330.
References
- [1].Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–28. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- [2].Beverly JL, Beverly MF, Meguid MM. Alterations in extracellular GABA in the ventral hypothalamus of rats in response to acute glucoprivation. Am J Physiol. 1995;269:R1174–8. doi: 10.1152/ajpregu.1995.269.5.R1174. [DOI] [PubMed] [Google Scholar]
- [3].Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–44. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- [4].de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dyer CJ, Touchette KJ, Carroll JA, Allee GL, Matteri RL. Cloning of porcine prepro-orexin cDNA and effects of an intramuscular injection of synthetic porcine orexin-B on feed intake in young pigs. Domest Anim Endocrinol. 1999;16:145–8. doi: 10.1016/s0739-7240(99)00011-9. [DOI] [PubMed] [Google Scholar]
- [6].Hansen S, Ferreira A. Effects of bicuculline infusions in the ventromedial hypothalamus and amygdaloid complex on food intake and affective behavior in mother rats. Behav Neurosci. 1986;100:410–5. doi: 10.1037//0735-7044.100.3.410. [DOI] [PubMed] [Google Scholar]
- [7].Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. [DOI] [PubMed] [Google Scholar]
- [8].Kanazawa M, Sugama S, Okada J, Miura M. Pharmacological properties of the CO2/H+-sensitive area in the ventral medullary surface assessed by the effects of chemical stimulation on respiration. J Auton Nerv Syst. 1998;72:24–33. doi: 10.1016/s0165-1838(98)00085-x. [DOI] [PubMed] [Google Scholar]
- [9].Kelly J, Alheid GF, Newberg A, Grossman SP. GABA stimulation and blockade in the hypothalamus and midbrain: effects on feeding and locomotor activity. Pharmacol Biochem Behav. 1977;7:537–41. doi: 10.1016/0091-3057(77)90250-7. [DOI] [PubMed] [Google Scholar]
- [10].Kelly J, Grossman SP. GABA and hypothalamic feeding systems. II. A comparison of GABA, glycine and actylcholine agonists and their antagonists. Pharmacol Biochem Behav. 1979;11:647–52. doi: 10.1016/0091-3057(79)90257-0. [DOI] [PubMed] [Google Scholar]
- [11].Kelly J, Rothstein J, Grossman SP. GABA and hypothalamic feeding systems. I. Topographic analysis of the effects of microinjections of muscimol. Physiol Behav. 1979;23:1123–34. doi: 10.1016/0031-9384(79)90306-8. [DOI] [PubMed] [Google Scholar]
- [12].Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–9. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- [13].Kokare DM, Patole AM, Carta A, Chopde CT, Subhedar NK. GABA(A) receptors mediate orexin-A induced stimulation of food intake. Neuropharmacology. 2006;50:16–24. doi: 10.1016/j.neuropharm.2005.07.019. [DOI] [PubMed] [Google Scholar]
- [14].Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- [15].Kunos G, Varga K. The tachycardia associated with the defense reaction involves activation of both GABAA and GABAB receptors in the nucleus tractus solitarii. Clin Exp Hypertens. 1995;17:91–100. doi: 10.3109/10641969509087057. [DOI] [PubMed] [Google Scholar]
- [16].Paxinos GW,C. The Rat Brain In Stereotaxic Coordinates. Fourth Edition edn. Academic Press; 1998. [Google Scholar]
- [17].Randle JC, Renaud LP. Actions of gamma-aminobutyric acid on rat supraoptic nucleus neurosecretory neurones in vitro. J Physiol. 1987;387:629–47. doi: 10.1113/jphysiol.1987.sp016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Renaud LP, Bourque CW. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36:131–69. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- [19].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92 doi: 10.1016/s0092-8674(02)09256-5. 1 page following 696. [DOI] [PubMed] [Google Scholar]
- [20].Sandkuhler J, Herdegen T. Distinct patterns of activated neurons throughout the rat midbrain periaqueductal gray induced by chemical stimulation within its subdivisions. J Comp Neurol. 1995;357:546–53. doi: 10.1002/cne.903570406. [DOI] [PubMed] [Google Scholar]
- [21].Shibahara M, Sakurai T, Nambu T, Takenouchi T, Iwaasa H, Egashira SI, Ihara M, Goto K. Structure, tissue distribution, and pharmacological characterization of Xenopus orexins. Peptides. 1999;20:1169–76. doi: 10.1016/s0196-9781(99)00120-5. [DOI] [PubMed] [Google Scholar]
- [22].Smith S, Sharp T. Measurement of GABA in rat brain microdialysates using o-phthaldialdehyde-sulphite derivatization and high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1994;652:228–33. doi: 10.1016/0378-4347(93)e0391-3. [DOI] [PubMed] [Google Scholar]
- [23].Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–8. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- [25].Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–14. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- [26].Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thorpe AJ, Mullett MA, Wang C, Kotz CM. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1409–17. doi: 10.1152/ajpregu.00344.2002. [DOI] [PubMed] [Google Scholar]
- [28].Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–5. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- [29].van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van den Pol AN, Trombley PQ. Glutamate neurons in hypothalamus regulate excitatory transmission. J Neurosci. 1993;13:2829–36. doi: 10.1523/JNEUROSCI.13-07-02829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang C, Kotz CM. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2002;283:R358–67. doi: 10.1152/ajpregu.00558.2001. [DOI] [PubMed] [Google Scholar]


