Abstract
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptors superfamily. The three subtypes, PPARα, PPARγ, and PPARβ/δ, are expressed in multiple organs. These transcription factors regulate different physiological functions such as energy metabolism (including lipid and carbohydrate metabolism), insulin action, and immunity and inflammation, and apparently also act as important mediators of longevity and aging. Calorie restriction (CR) is the most effective intervention known to delay aging and increase lifespan. Calorie restriction affects the same physiological functions as PPARs. This review summarizes recent findings on the effects of CR and aging on the expression of PPARγ, α, and β/δ in mice and discusses possible involvement of PPARs in mediating the effects of murine longevity genes. The levels of PPARs change with age and CR appears to prevent these alterations which make “PPARs-CR-AGING” dependence of considerable interest.
THE PPAR FAMILY
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear receptor superfamily that are ligand-dependent transcription factors. The activation of PPARs requires forming heterodimers with retinoid X receptors (RXRs), which allow binding to their specific peroxisome proliferator response elements (PPREs) [1]. By binding to specific PPREs in enhancer sites of targeted genes, PPAR/RXR heterodimers regulate their expression. PPAR genes are known to be expressed in different organs, including reproductive organs, major insulin target organs (liver, white adipose tissue, skeletal muscle), cardiac tissue, and other. PPARs have a wide spectrum of actions which include adipocyte differentiation, lipid metabolism, insulin sensitization, tissue injury and wound repair, inflammation, and immunity.
There are three known subtypes in the PPAR superfamily, each encoded by separate genes: PPARα, PPARβ/δ (also known as PPARβ or PPARδ), and PPARγ. The most explored gene of this superfamily and the most adipose-specific is PPARγ. There are two recognized isoforms of PPARγ: PPARγ1 and PPARγ2. These isoforms are generated by alternative splicing and alternate translation initiation [2–4]. Although PPARγ is the most recently cloned gene from PPARs, it quickly drew attention as a target receptor for thiazolidinediones (TZDs), the drugs used as insulin sensitizers in type 2 diabetic patients (5–8).
As its name implies, PPARα was the first gene cloned from this family. PPARα is mainly expressed in the liver, skeletal muscle, heart, and kidney. In these organs, it regulates a wide variety of target genes involved in cellular lipid catabolism. PPARα alters the expression of genes encoding enzymes involved in the fatty acid metabolic pathway, which activate the regulation of fatty acids β and ω-oxidation. These effects are mediated by the presence of PPREs that are under transcriptional control of PPARα in the promoter regions of genes coding for the enzymes involved in this metabolic pathway [5]. The activation of PPARα in the heart induces accumulation of myocardial lipids that leads to other features of diabetic cardiomyopathy [6]. PPARα-deficient mice have increased levels of total and HDL cholesterol [7].
The function of the third PPAR nuclear receptor, PPARβ/δ, is still somewhat unclear. There are some indications that PPARβ/δ is involved in lipid metabolism [8], and studies have shown that it plays an important role in epidermal maturation and skin wound healing [9, 10].
CALORIE RESTRICTION AND PPARs
Calorie restriction (CR) is of wide interest in the study of aging. There are numerous studies showing that CR can improve the health of individuals and help protect them from disease. CR is also recognized as the most effective intervention known to delay aging and increase lifespan [11]. The precise mechanisms of CR action on aging and longevity are still not well established, but CR is known to reduce body weight and the levels of plasma insulin, IGF-1, GH, glucose, and thyroid hormone.
Calorie restriction is also known to alter expression of large number of genes involved in lipid metabolism and insulin signaling. Expression of many of the same genes is regulated by PPARs acting as transcription factors. This suggests a possibility that PPARs mediate the effects of CR or that CR and PPAR/RXRs heterodimers activate the same signaling pathways.
ACTION OF PPARs ON INSULIN SIGNALING
The agonists for PPARα and PPARγ are widely used in diabetes. The study of rats fed a high-fat diet (HFD) indicated that PPARα and PPARγ agonists, WY14643 and pioglitazone, respectively, decreased glucose and leptin levels in plasma. The plasma levels of insulin and triglyceride were also reduced in rats treated with PPARs agonists in comparison to control animals; however, pioglitazone caused significantly greater reduction in comparison to PPARα-agonist-treated and control rats [12]. However, activation of PPARγ caused significant increase of body weight, which is opposite to CR action. PPARα agonist did not alter body weight and more importantly caused significant decrease of visceral fat weight in comparison to control and pioglitazone-treated rats [12]. This indicates that pioglitazone improves insulin sensitivity more effectively than WY14643. However, weight gain caused by PPARγ agonist is detrimental to the well-being of diabetic rats or humans.
DIET
The functions and characterization of PPARs suggest that these nuclear receptors are strongly connected with the diet. There is considerable evidence that various diets can affect PPARs action in different organs and that the responses to diets can be mediated by their effects on PPARs expression.
High-fat diet
High-fat diet is known to induce insulin resistance and promote type 2 diabetes in laboratory animals. In rats and mice HFD causes obesity and increases plasma insulin, glucose, and leptin levels. In HFD-fed rodents, PPARs agonists improve insulin sensitivity, presumably via activation of this nuclear receptor.
PPARα and high-fat diet
Studies of PPARα-null mice indicated that the deficiency of this nuclear receptor can protect from insulin resistance induced by HFD [13]. In this study the authors showed that HFD increases body weight and plasma insulin level but only in normal animals, with no alteration in PPARα-null mice. Moreover, insulin tolerance test (ITT), glucose tolerance test (GTT), and the calculated insulin resistance index indicated that HFD caused insulin resistance in normal animals, with no alteration of insulin signaling in PPARα deficient mice [13]. However, studies of PPARα-null mice subjected to fasting indicated that PPARα deficiency can cause severe hypoglycemia [14, 15]. Moreover, most of PPARα target genes were not altered in the liver and heart of fasted PPARα-null mice in comparison to normal controls [15]. The authors also reported that in PPARα deficient animals fasting caused hyperketonemia, hypothermia, and increase in plasma levels of free fatty acids, which reflects inhibition of fatty acid uptake and oxidation [14]. Concluding, PPARα participates in glucose homeostasis which may be important to prevent hypoglycemia under fasting condition or during exercise. However, long-term metabolic stress such as HFD could become negative for health by developing insulin resistance.
PPARγ and high-fat diet
Similarly to PPARα deficiency, PPARγ deficiency in adipose tissue (PPARγ-adiposeKO) was reported to protect from obesity and insulin resistance caused by HFD [16]. Under HFD, this tissue-specific PPARγ deficiency increased glucose tolerance in comparison to control animals on HFD. Moreover, the levels of insulin and leptin were significantly decreased in HFD-treated, PPARγ-adiposeKO mice in comparison to normal animals subjected to the same diet. Interestingly, the deficiency of PPARγ in adipose tissue resulted in increased PPARγ mRNA levels in the liver when compared to normal controls [16]. As stated by the authors, this model suggests that improved insulin sensitivity under HFD in PPARγ-adiposeKO mice can coexist with increased expression of PPARγ in the liver.
Calorie restriction diet
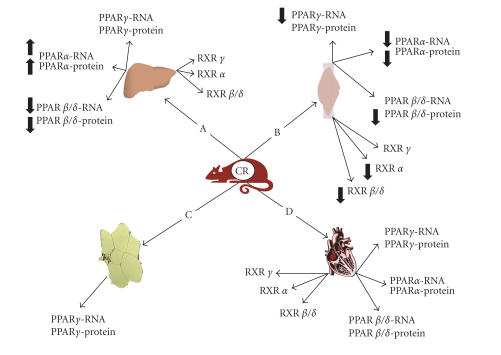
Calorie restriction is known to improve insulin sensitivity, lipid metabolism, health, and longevity. Calorie restriction is known to act on PPARs [17]; however, the effects are strikingly organ dependent. Depending on the organ, we observed a lack of changes, decrease or increase of PPARs expression in response to CR (Figure 1).
Figure 1.
Effects of calorie restriction (CR) on the expression of PPARs family genes in mouse: (A) liver, (B) skeletal muscles, (C) white adipose tissue, and (D) heart. Arrows pointing up or down indicate statistically significant increases or decreases (P < .05). Lack of arrows means no alteration.
PPARs, CR, and the liver
Data reported by our laboratory indicated that 30% CR did not alter mRNA or protein levels of hepatic PPARγ in mice. This finding suggested that improvement of insulin sensitivity in mice by CR is not mediated by PPARγ in the liver [18, 19]. However, hepatic PPARα mRNA and protein levels were significantly increased by CR in comparison to mice fed with unlimited (ad libitum; AL) access to food. This finding appears counterintuitive in view of the evidence that PPARα deficiency prevents insulin resistance in mice subjected to HFD [13]. However, the suggested involvement of PPARα in glucose homeostasis could imply that the increase of PPARα in mice subjected to CR is a mechanism protecting these animals from hypoglycemia [14, 15]. Perhaps under conditions of HFD the decrease of PPARα is adaptive, but when the animals are subjected to CR, PPARα increases to facilitate maintenance of normal glucose levels during the periods when food is not available. Additionally, a recent study conducted by Corton et al indicated that 19% of hepatic genes involved in lipid metabolism, inflammation, and cell growth which were altered by CR were dependent on PPARα. Interestingly, some of these genes were altered by CR only in normal mice but not in PPARα deficient animals. Results obtained in animals treated with a PPARα agonist indicated overlap of genes influenced by CR and by a compound activating PPARα [20]. These important findings indicated that PPARα plays an important role in mediating the action of CR [13, 20]. Corton et al also suggested that drugs activating the PPARα-RXR-LXR axis can be potential CR mimetics [20].
The expression of the remaining member of the PPAR family, PPARβ/δ, in the liver was significantly decreased by CR at both mRNA and protein levels [19]. Thus, the hepatic expression of three genes from the PPAR family is differentially altered by CR. However, CR did not alter hepatic RXRα, RXRγ, and RXRβ/δ mRNA (Figure 1A) [19].
PPARs, CR, and skeletal muscle
Similarly to the liver, the skeletal muscle is a major insulin target organ. In this tissue, the expression of PPARs and RXRs is altered differently by CR than in the liver [19, 21]. It was reported that 30% calorie restriction in mouse skeletal muscle decreased the level of PPARγ mRNA and the PPARγ protein level appeared to also be decreased [21]. We could speculate that the decrease of PPARγ in the muscle as seen in the adipose specific knockout for PPARγ is beneficial for insulin sensitivity [16]. However, muscle-specific knockout of PPARγ caused whole-body insulin resistance [22]. Interestingly, treating these knockout mice with TZD improved insulin sensitivity [22], suggesting the effect was due to PPARγ agonism in other tissues. This suggests that CR can increase insulin sensitivity through effects on PPARγ expression in tissues other than the muscle, and speculating further we could suggest that under the conditions of CR, a decreased rather than elevated PPARγ expression is beneficial.
PPARα mRNA and proteins were decreased by CR in skeletal muscle, an effect opposite to that observed in the liver [19, 21]. It was speculated that a decrease of PPARα in the muscle under CR slowed fatty acid oxidation, thus increasing the reliance on carbohydrates as the energy source. More importantly, consequences of reduced PPARα expression could prevent the muscle from using all of the FFA immediately after food intake and thus maintain a balance between energy availability and energy usage during the fasting period. The protein level of PPARβ/δ was also decreased in the skeletal muscle of CR mice [21]. It is well known that CR promotes fat depletion and prevents obesity. Studies in PPARδ-deficient mice on HFD revealed reduced energy uncoupling and obesity [21]. This would predict that reduced levels of PPARδ in the muscles of CR mice may lead to increased lipid accumulation and promote obesity. However, reduced dietary fat intake in CR animals may alter these relationships. It was suggested that CR down-regulates the pathway of lipid metabolism and accommodates it to the circumstances of restricted food intake [21]. This may serve to prevent disruption of fatty acid homeostasis in CR animals. In addition to its effects on PPARs expression, CR reduced mRNA levels of RXRα and RXRβ/δ [21]. Altered expression of these genes important to PPARs activation correlated with the changes in the expression of the corresponding members of the PPAR family (Figure 1B) [21].
PPARs, CR, and the white adipose tissue
PPARγ is mainly expressed in white adipose tissue (WAT). As mentioned previously, the deficiency of PPARγ in adipose tissue is protective against obesity and insulin resistance caused by HFD [16, 23]. However, CR increases insulin sensitivity in mice, without altering PPARγ mRNA levels in WAT (Figure 1C) [24]. It can be speculated that under conditions of reduced calorie intake diminished PPARγ would not be beneficial, or that limited fat storage does not allow increased PPARγ activation in this tissue. At the time of this writing no data are available on the effects of CR on PPARα and PPARβ/δ in WAT.
PPARs IN GENETICALLY LONG-LIVED AND SHORT-LIVED MICE
Growth hormone receptor/binding protein knockout (GHR-KO) mice
GHR-KO (Laron dwarf) mice have their GH receptor/binding protein gene disrupted and thus are deficient of GHR. Consequently these mice are GH resistant or insensitive and have greatly reduced plasma IGF-1 and insulin levels, and low glucose level [25, 26]. GHR-KO mice are also characterized by markedly extended lifespan in comparison to normal controls [27, 28].
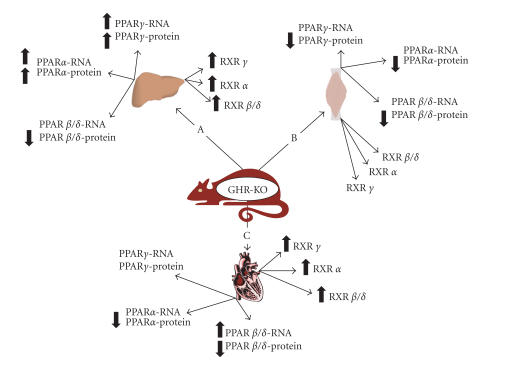
In comparison to normal animals, GHR-KO mice also have significantly elevated PPARγ mRNA and protein level in the liver (Figure 2A) [19]. We speculated that increased level of PPARγ in the liver of those long-lived animals may be responsible for or contribute to their exceptionally high insulin sensitivity. This correlates with findings in PPARγ-adiposeKO mice, which indicated that PPARγ deficiency in WAT is compensated for by increased expression of this nuclear receptor in the liver to promote insulin sensitivity [16, 19]. The findings in the muscle also suggest that in GHR-KO mice PPARγ in the liver contributes to high insulin sensitivity, because the level of PPARγ mRNA in skeletal muscle of KO mice was not altered, while the PPARγ protein level was decreased in comparison to normal controls (Figure 2B) [21].
Figure 2.
Effects of growth hormone receptor/binding protein knockout (GHR-KO) on the expression of PPARs family genes in mouse: (A) liver, (B) skeletal muscle, and (C) heart. Arrows pointing up or down indicate statistically significant increases or decreases (P < .05). Lack of arrow means no alteration.
The increased level of PPARα measured in the liver of KO mice [19] could be suspect of exerting to negative effect on insulin action and obesity. However, the higher level of PPARα suggests an increased usage of fatty acids, which could be beneficial for insulin sensitivity. Increased PPARα levels could also be correlated with decreased total cholesterol level in GHR-KO animals [21]. It is interesting that the level of PPARα in GHR-KO mice fed AL is maintained at the same level as in the normal animals subjected to CR. However, similarly to PPARγ, expression of PPARα was not altered in the muscle at the PPARα mRNA level, while the PPARα protein level was decreased in KO animals, again resembling the findings in normal mice under a CR regimen [21].
PPARβ/δ proteins were down-regulated in the liver and skeletal muscle of GHR-KO mice, which in both cases mimics the alterations of the expression of this gene caused by CR in the liver and muscle of normal mice [21].
These data indicate that CR alters PPARα and PPARβ/δ proteins and/or mRNA levels in the liver and skeletal muscle to the levels maintained in GHR-KO animals. Since GHR-KO mice are long-lived and CR increases longevity, it can be suggested that PPARα and PPARβ/δ play an important role in mediating the effects of both GHR-KO and/or CR on longevity. The RXRγ, RXRα, and RXRβ/δ mRNA were increased in the liver and not changed in the muscle of GHR-KO mice which corresponds to alterations of PPARγ and PPARα in these two organs (Figure 2A, B) [19, 21].
Dwarf mice
Snell dwarf, Ames dwarf, and “Little” mice live markedly longer than their normal siblings. Snell dwarf mice carry a mutation in the Pit1 gene (Pit1dw) and Ames dwarf mice are homozygous for recessive loss-of-function mutation at the Prop1 locus (Prop1df). These dwarf mice are deficient with GH, prolactin, and tyrotropin. Little mice have severely reduced GH levels caused by the mutation of growth hormone-releasing hormone receptor (Ghrhr). Studies of Snell dwarf mice indicated increased hepatic PPARα mRNA and protein levels in comparison to heterozygous controls [29]. The expression of PPARα mRNA and protein in the liver of Ames dwarf mice was not altered in comparison to their normal controls [18]. However, gene array studies indicated that the genes regulated by PPARα were either up-regulated in Snell dwarf, Ames dwarf, and Little mice or their expression increased in response to PPARα-agonist treatment, which was interpreted as evidence that GH action is involved in the regulation of PPARα-dependent gene products [29].
Phosphoenolpyruvate carboxykinase bovine-GH transgenic mice
PEPCK-bGH transgenic (TG) mice over-expressing the bGH gene fused to control sequences of the rat phosphoenolpyruvate carboxykinase (PEPCK) are characterized by markedly shortened lifespan in comparison to their normal siblings [30]. The findings in the liver did not indicate any alteration of PPARγ mRNA in these TG mice. However, hepatic PPARα mRNA was down-regulated in the liver of these short-living giant mice in comparison to their normal siblings [30]. This finding is very important in elucidating the potential role of PPARα in the control of longevity. As mentioned above, PPARα is increased in the liver of GHR-KO and CR mice. This contrasts with the decreased PPARα level in the liver of short-lived b-GH transgenic mice. These findings are consistent with our suggestion that PPARα can be an important mediator of genetic and dietary effects on longevity.
AGING AND CALORIE RESTRICTION
As mentioned above, the study of PPARα-null mice indicated that the deficiency of this nuclear receptor can protect from insulin resistance induced by HFD [13]. Furthermore, we speculated that PPARα can be influential in the control of longevity, and we suggested that an elevated level of this nuclear receptor is beneficial and promotes longer life. Although PPARα deficiency was useful in controlling glucose levels in HFD-fed mice, it was reported that these mice had age-dependent defects in heart, liver, and kidney, which correlated with decreased longevity in comparison to wild-type controls [31, 32]. PPARα expression is also known to decrease with age in the liver, kidney, and heart. The study of GHR-KO and normal mice fed AL and subjected to CR indicated that PPARα mRNA level in the heart is not affected by phenotype and CR (Figures 1D and 2C) [33]. CR increases the mRNA and protein level of PPARα only in GHR-KO mice [33]. Interestingly, protein level of PPARα was decreased in the heart of long-lived GHR-KO animals (Figure 2C) [33]. Moreover, earlier study [34] indicated that CR increased the cardiac level of PPARα mRNA in mice, which would support antiaging action. Analysis of gene expression in mouse heart by Lee et al indicated that CR preserved fatty acid metabolism [35]. Additionally, the study of GHR-KO mice indicated that at the age of 3 months PPARα was elevated in KO in comparison to the normal mice. When the animals reached 21 months of age this difference was no longer present [33]. The short-lived b-GH TG mice showed down regulation of this nuclear receptor in the heart at 9 months of age [33].
Sung et al reported that in rats the levels of PPARγ and PPARα in the kidney decreased with age when comparing 13- and 25-month-old animals [36]. Calorie restriction prevented these aging effects and maintained the levels of these nuclear receptors in 25-month-old rats at the same levels as in the 13-month-old animals [36]. To investigate the possible role of PPARα and PPARγ in inflammation, these authors also performed lipopolysaccharide (LPS) tests in young and old rats. Treatment with LPS decreased the level of these PPARs, to greater extent in old than in middle-age rats [36]. The authors concluded that down-regulation of PPARs in the rat's kidneys might be correlated with age-related oxidative stress, which could be reversed by antioxidative action of CR [36].
SUMMARY
Different members of the PPARs family are expressed in many tissues. Various dietary regimens such as HFD and calorie restriction can affect expression of PPARs. However, the presence and direction of these changes depend on the organ being analyzed. Additionally, the effects of the diet on the animal depend on the actions of PPARs. For example, PPARα-null or PPARγ-adiposeKO mice are protected form insulin resistance and obesity caused by HFD. Studies in genetically long- or short-lived mice together with the studies involving CR suggest that PPARs play an important role in insulin action, lipid metabolism, immunity, and inflammation as well as regulation of aging and longevity.
ACKNOWLEDGMENTS
This paper is supported by the NIA (AG 19899 and U19 AG023122), The Ellison Medical Foundation, SIU Geriatrics Medicine and Research Initiative, and Central Research Committee of Southern Illinois University School of Medicine. We would also like to thank Steve Sandstrom for helping with the editing of the manuscript.
References
- 1.Moller DE, Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. International Journal of Obesity. 2003;27(suppl 3):S17–S21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- 2.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes and Development. 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Y, Qi C, Korenberg JR, et al. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promoter use and different splicing yield two mPPARγ isoforms. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(17):7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue J-C, Schwarz EJ, Chawla A, Lazar MA. Distinct stages in adipogenesis revealed by retinoid inhibition of differentiation after induction of PPARγ . Molecular and Cellular Biology. 1996;16(4):1567–1575. doi: 10.1128/mcb.16.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Current Opinion in Lipidology. 1997;8(3):159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DP. PPARs of the heart: three is a crowd. Circulation Research. 2003;92(5):482–484. doi: 10.1161/01.RES.0000064382.46274.95. [DOI] [PubMed] [Google Scholar]
- 7.Peters JM, Hennuyer N, Staels B, et al. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. Journal of Biological Chemistry. 1997;272(43):27307–27312. doi: 10.1074/jbc.272.43.27307. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 9.Michalik L, Desvergne B, Tan NS, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. The Journal of Cell Biology. 2001;154(4):799–814. doi: 10.1083/jcb.200011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan NS, Michalik L, Noy N, et al. Critical roles of PPARβ/δ in keratinocyte response to inflammation. Genes and Development. 2001;15(24):3263–3277. doi: 10.1101/gad.207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. New England Journal of Medicine. 1997;337(14):986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye J-M, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats. Comparison with PPAR-γ activation. Diabetes. 2001;50(2):411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 13.Guerre-Millo M, Rouault C, Poulain P, et al. PPAR-α-null mice are protected from high-fat diet-induced insulin resistance. Diabetes. 2001;50(12):2809–2814. doi: 10.2337/diabetes.50.12.2809. [DOI] [PubMed] [Google Scholar]
- 14.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. Journal of Clinical Investigation. 1999;103(11):1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones JR, Barrick C, Kim K-A, et al. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor γ coactivator 1 in caloric restriction and other models of longevity. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2005;60(12):1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 18.Masternak MM, Al-Regaiey K, Bonkowski MS, et al. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2004;59(8):784–788. doi: 10.1093/gerona/59.8.b784. [DOI] [PubMed] [Google Scholar]
- 19.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Effects of caloric restriction and growth hormone resistance on the expression level of peroxisome proliferator-activated receptors superfamily in liver of normal and long-lived growth hormone receptor/binding protein knockout mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2005;60(11):1394–1398. doi: 10.1093/gerona/60.11.1394. [DOI] [PubMed] [Google Scholar]
- 20.Corton JC, Apte U, Anderson SP, et al. Mimetics of caloric restriction include agonists of lipid-activated nuclear receptors. Journal of Biological Chemistry. 2004;279(44):46204–46212. doi: 10.1074/jbc.M406739200. [DOI] [PubMed] [Google Scholar]
- 21.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2005;60(10):1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- 22.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. Journal of Clinical Investigation. 2003;112(4):608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2006;61(4):323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J-L, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. American Journal of Physiology - Endocrinology and Metabolism. 2004;287:E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- 27.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Hormone and IGF Research. 2004;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 29.Stauber AJ, Brown-Borg H, Liu J, et al. Constitutive expression of peroxisome proliferator-activated receptor α-regulated genes in dwarf mice. Molecular Pharmacology. 2005;67(3):681–694. doi: 10.1124/mol.104.007278. [DOI] [PubMed] [Google Scholar]
- 30.Masternak MM, Panici J, Wilson M, et al. The effect of calorie restriction and Metformin on insulin pathway genes in growth hormone (GH) transgenic mice. In: Proceedings of 34th American Aging Association Annual Meeting; June 2005; Oakland, Calif. [Google Scholar]
- 31.Watanabe K, Fujii H, Takahashi T, et al. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor α associated with age-dependent cardiac toxicity. Journal of Biological Chemistry. 2000;275(29):22293–22299. doi: 10.1074/jbc.M000248200. [DOI] [PubMed] [Google Scholar]
- 32.Howroyd P, Swanson C, Dunn C, Cattley RC, Corton JC. Decreased longevity and enhancement of age-dependent lesions in mice lacking the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) Toxicologic Pathology. 2004;32(5):591–599. doi: 10.1080/01926230490515283. [DOI] [PubMed] [Google Scholar]
- 33.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Experimental Gerontology. 2006;41(4):417–429. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhahbi JM, Tsuchiya T, Kim H-J, Mote PL, Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2006;61(3):218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- 35.Lee C-K, Allison DB, Brand J, Weindruch R, Prolla TA. Transcriptional profiles associated with aging and middle age-onset caloric restriction in mouse hearts. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):14988–14993. doi: 10.1073/pnas.232308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sung B, Park S, Yu BP, Chung HY. Modulation of PPAR in aging, inflammation, and calorie restriction. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2004;59(10):997–1006. doi: 10.1093/gerona/59.10.b997. [DOI] [PubMed] [Google Scholar]