Short abstract
Separate transcriptional pathways have been delineated for the maintenance of the undifferentiated state and for self-renewal in embryonic stem cells.
Abstract
Embryonic stem cells (ES cells) are powerful tools for genetic engineering and hold significant potential for regenerative medicine. Recent work provides new insights into ES cell pluripotency and delineates separate transcriptional pathways in ES cells for maintenance of the undifferentiated state and for self-renewal.
Embryonic stem cells (ES cells) are derived from the inner cell mass (ICM) of the early mammalian embryo and are distinguished by two remarkable properties. First, they can be propagated in tissue culture in an undifferentiated state for an extended period: that is, they have the property of self-renewal. Second, when introduced into a host blastocyst they contribute to all tissues and even the germ cells of the resulting chimeric animal: they have the property of pluripotency. These features have been exploited in studies of early development and for the generation of genetically engineered mice [1]. Recently, the pluripotency and self-renewal of ES cells have come under close scrutiny. An important goal is an understanding of the unique pathways used by these cells, with the intent of recreating them in somatic cells and thereby reprogramming differentiated cells to an embryonic-like state. In a recent set of experiments, Ivanova et al. [2] set out to uncover these pathways by utilizing the latest in RNAi technology on a genome-wide scale.
Mouse ES cells in culture require extrinsic factors, such as leukemia inhibitory factor (LIF), in the culture medium to maintain the undifferentiated state in vitro. LIF stimulates the LIF-STAT signaling pathway and operates predominantly through STAT3 [3,4]. This is in marked contrast to human ES cells, which do not require LIF-STAT3 signaling to maintain pluripotency [5]. Other extrinsic signals, such as bone morphogenetic protein (BMP) acting through the BMP-SMAD [6] pathways, contribute to self-renewal and pluripotency of mouse ES cells in vitro.
Transcription factors for self-renewal and differentiation
Key transcription factors have also been identified that form an intrinsic core regulatory circuit that maintains mouse ES cells in the pluripotent state in vitro. Of these, Oct4, an atypical homeodomain protein, was originally cloned on the basis of its highly restricted expression pattern; it is expressed exclusively in murine ES cells, ICM and germ cells [7]. Oct4-deficient murine ES cells differentiate into trophectoderm and fail to form all three germ layers (mesoderm, ectoderm and endoderm) [8]. Sox2 is an HMG-family protein that occupies many gene targets with Oct4 and is also required to form the ICM [9]. A recent addition to the 'pluripotency factors' is Nanog, another atypical homeodomain protein related to the Nkx subfamily. Forced expression of Nanog in ES cells lifts the requirement for LIF to maintain pluripotency, suggesting that Nanog is a major regulator of the pluripotent state [5,10,11]. Through genome-wide chromatin immunoprecipitation followed by DNA microarray analysis (ChIP-chip) [12] or ChIP-PET [13] experiments (based upon high-throughput sequencing to determine gene expression patterns), numerous target genes bound by Nanog, Oct4 and Sox2 have been identified. These factors appear to act combinatorially to regulate a limited repertoire of target genes, thereby forming a tight transcriptional regulatory circuit that maintains ES cells in a pluripotent state.
Nanog's distinguishing role in the maintenance of murine ES cells in vitro is an ability to bypass the requirement for the LIF-STAT pathway [5]. In addition, fusion experiments with mouse cells have shown the dominance of the ES cell phenotype over that of somatic cells, implying that proteins in the nucleus of ES cells are able to reprogram more differentiated cells to an embryonic-like state [14]. The full repertoire of factors involved in establishing or maintaining pluripotency, and also competent to reprogram cells, is unknown and, until recently, there had been no comprehensive effort to delineate factors necessary for the maintenance of the mouse ES cell in vitro phenotype. Ihor Lemischka and his colleagues [2] have now tackled just this issue using a functional genomics approach designed to identify novel factors required for self-renewal in mouse ES cells. They began with microarray data from mouse ES cells as they progress from an undifferentiated state into cells representing all three germ layers upon retinoic-induced differentiation [2], and hypothesized that factors required for pluripotency and self-renewal would be rapidly downregulated. Of 901 downregulated genes, 65 DNA-binding proteins or transcription factors were selected for further functional analysis.
Ivanova et al. [2] then assessed the effects of the loss of each of these proteins on the ES cells' capacity for self-renewal. To do this they devised an assay in which wild-type ES cells were mixed with ES cells transduced with lentiviruses containing short hairpin RNAs (shRNAs) to trigger RNA interference (RNAi), along with an expressed green fluorescent protein (GFP) marker. Compromised self-renewal would be reflected in a decreasing percentage of GFP-marked cells in the culture. Six genes were identified by this assay and were characterized further for their effects on mouse ES cell pluripotency. Among these six were the 'old friends' Nanog, Oct4 and Sox2, consistent with substantial previous evidence in support of their roles as core self-renewal factors. The three other genes were Esrrb, Tcl1 and Tbx3. To further characterize the possible roles of these genes, Ivanova et al. [2] carried out extensive marker-gene analysis following the shRNA inhibition. These experiments revealed that each factor appeared to repress distinct differentiation programs, although there was significant overlap.
Further characterization by microarray analysis revealed three distinct patterns of gene expression on knockdown. The expression of approximately 771 genes appeared independent of gene knockdown; expression of 474 genes was either up- or downregulated by knockdown of Nanog, Oct4 or Sox2, but unaffected by knockdown of Esrrb, Tbx3 or Tcl1; and the expression of 272 genes was upregulated by the loss of Esrrb, Tbx3 and Tcl1, but unperturbed by inhibition of Nanog, Oct4 and Sox2. These data provide the first evidence in favor of two independent transcriptional pathways in mouse ES cells; one is controlled by Nanog, Oct4 and Sox2, and may be predominantly responsible for pluripotency and suppressing differentiation. A second pathway involving Esrrb, Tbx3 and Tcl1 seems to be responsible for blocking differentiation along specific cell lineages (Figure 1). Surprisingly, modestly raised levels of Nanog compensated for the loss of Esrrb, Tbx and Tcl1, implying cross-talk between the two pathways, with Nanog perhaps serving as a master regulator.
Figure 1.
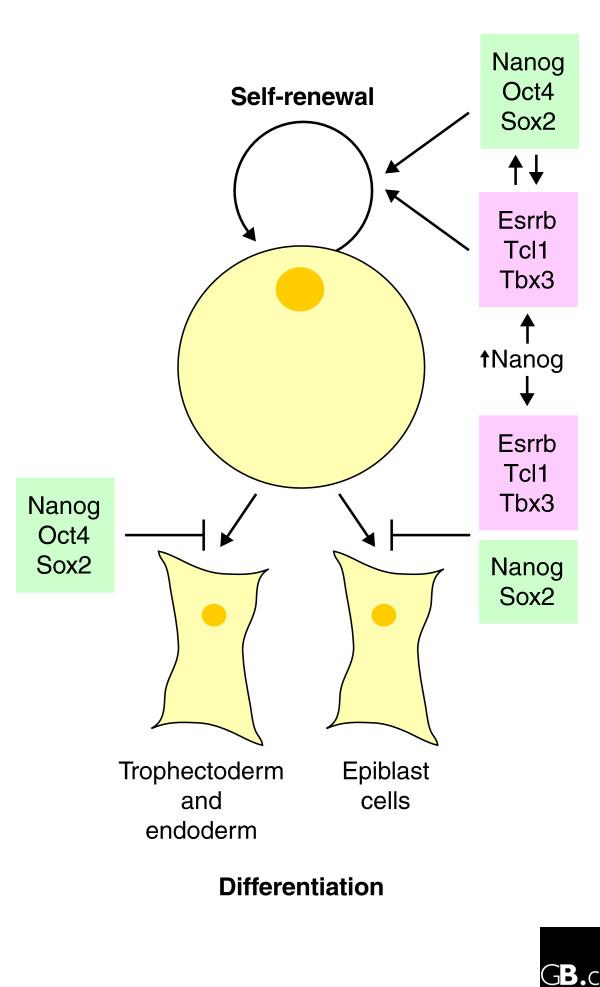
A schematic view of the transcriptional pathways involved in self-renewal and blocking differentiation of murine embryonic stem cells. Self-renewal appears to be regulated by two distinct transcriptional pathways, one involving Esrrb, Tcl1 and Tbx3 and a separate pathway involving Nanog, Oct4 and Sox2, with some degree of cross-talk. Differentiation pathways appear to be separately regulated as well. Nanog, Oct4 and Sox2 cooperate to block trophectoderm and endoderm differentiation, whereas Nanog, Sox2, Esrrb, Tcl1 and Tbx3 cooperate to prevent formation of the epiblast. Raised levels of Nanog on its own appear able to compensate for the loss of Esrrb, Tcl1 or Tbx3 for both self-renewal and blocking differentiation.
Cellular reprogramming
The elegant work of Ivanova et al. [2] is bold and a technical tour de force, in that it attempts to be both systematic and comprehensive in identification of critical factors for self-renewal and pluripotency. Only subsequent studies using other approaches will reveal the completeness of the collection of factors the authors have identified and also the significance of the different gene-expression patterns revealed upon shRNA inhibition of Nanog, Oct4 and Sox2 expression versus knockdown of Esrrb, Tbx3 and Tcl1. The unbiased genome-wide strategy contrasts with recent experiments by Austin Smith's group [15] that focus specifically on the contribution of Nanog to cellular reprogramming in the setting of mouse ES cell-somatic cell hybrids. Using the activation of a drug-selection marker under the control of Oct4 regulatory sequences, these authors [15] examined the frequency of reprogrammed, ES-like cells arising from fusions of ES cells with neural stem (NS) cells or more differentiated somatic cells. Two important observations were made. First, in ES cell-NS cell hybrids the frequency of reprogramming was markedly enhanced by modestly increasing Nanog expression. This is consistent with the view that Nanog is a major driver of pluripotency. Second, the frequency of reprogrammed cells was much lower in ES cell-fibroblast hybrids, implying that the epigenetic 'state' of the differentiated partner is a determinant of the ease with which the transcriptional program can be reset [15].
Although Smith's [15] and Lemischka's [2] groups used different approaches, some common conclusions emerge. Their findings underscore the importance of Nanog in maintaining the phenotype of mouse ES cells and in reprogramming other cell types into pluripotent cells. These studies show that the level of Nanog is critical in both settings. Indeed, dose-dependent actions of pluripotency factors appear to be a general feature of development. For example, raised levels of Oct4 promote differentiation into primitive endoderm and mesoderm, whereas reduced expression of Oct4 promotes trophectoderm development [16,17]. Such results point to the controlled interplay of transcription factor levels in determining cell fate, and these transcription factors will warrant closer scrutiny as additional factors are implicated in pluripotency.
The gradation of transcription factor levels may represent an additional level of complexity by which ES cells employ a limited number of transcription factors to regulate pluripotency. In addition, Ivanova et al. [2] are the first to suggest two distinct transcriptional pathways involved in blocking differentiation and promoting self-renewal. The work of Smith's group [15], however, clearly points to the differentiation state of a cell as critical to its propensity for be reprogrammed by nuclear factors - implying that the epigenetic state of the more differentiated nucleus needs to be considered in future studies. These two papers point to an important set of factors, all of which have a critical role in pluripotency and/or self-renewal. Nonetheless, much more will be required to understand how these factors function and the degree of cross-talk between them. Further studies will undoubtedly center on the minimum set(s) of factors required to reprogram different types of somatic cells to a mouse ES cell-like state, as demonstrated by the work of Takahashi and Yamanaka [18]. We can envisage a time in when cellular reprogramming may become routine and applied in regenerative medicine.
Acknowledgments
Acknowledgements
S.H.O. is an investigator of the Howard Hughes Medical Institute.
References
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Ivanova N, Dobrin R, Lu R, Kotenko I, Levorse J, Decoste C, Schafer X, Lun Y, Lemischka IR. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–538. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daheron L, Opitz SL, Zaehres H, Lensch WM, Andrews PW, Itskovitz-Eldor J, Daley GQ. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/S0092-8674(03)00847-X. [DOI] [PubMed] [Google Scholar]
- Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/S0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002;16:1209–1219. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]


