Abstract
With the advent of powerful gradient coil systems and high-resolution surface coils, magnetic resonance imaging (MRI) has recently extended its role in the staging of rectal cancer. MRI is superior to endorectal ultrasound, the most widely used staging modality in patients with rectal tumors, in that it visualizes not only the intestinal wall but also the surrounding pelvic anatomy. The crucial advantage of MRI is not that it enables exact T-staging but precise evaluation of the topographic relationship of a tumor to the mesorectal fascia. This fascia is the most important anatomic landmark for the feasibility of total mesorectal excision, which has evolved into the standard operative procedure for the resection of cancer located in the middle or lower third of the rectum. MRI is currently the only imaging modality that is highly accurate in predicting whether or not it is likely that a tumor-free margin can be achieved and thus provides important information for planning of an effective therapeutic strategy, especially in patients with advanced rectal cancer.
Keywords: Rectal cancer, Staging, MRI, Rectal carcinoma
Introduction
Colorectal cancer is the third most common cancer worldwide [1, 2]. In the United States, about 145,000 new cases and 56,000 deaths were estimated for 2005 [1]. In recent years, mortality rates have decreased due to major changes in therapeutic management, in particular the standardization of the operative procedure and the introduction of adjuvant and neoadjuvant therapy [1].
Colorectal cancer primarily develops from adenomatous polyps over a period of 10–15 years, known as the adenoma-carcinoma sequence [3]. The incidence of polyps increases with age and the risk of malignant transformation of a polyp markedly increases with its diameter. The rate of malignant transformation is about 1% for polyps less than 1 cm in diameter, but 10% for larger ones [4, 5]. Around 40–50% of colorectal cancers are located in the rectum.
Rectal cancer is defined as a tumor whose aboral margin measured with the rigid rectoscope is 16 cm or less from the anocutaneous line. This distance serves to classify rectal cancer into tumors of the upper third (12–16 cm), the middle third (6–12 cm), and the lower third (<6 cm) [6] according to the UICC.
The mesorectal fascia is an important anatomic landmark for the diagnostic evaluation of local tumor extent [7] (Fig. 1b). The fascia is a connective tissue sheath that encloses the rectum and the perirectal fatty tissue, including lymph nodes and lymphatic vessels down to the pelvic floor and acts as a natural barrier for tumor spread. The ability to visualize the mesorectal fascia on CT images has been described more than 20 years ago [8]. MRI currently is the most advanced staging modality able to depict the fascia and its relation to the tumor margins precisely. The following article will give an overview of the staging modalities currently used in rectal cancer staging, with an emphasis on the role of MRI and its significance for planning an effective therapeutical strategy for the individual patient.
Fig. 1.
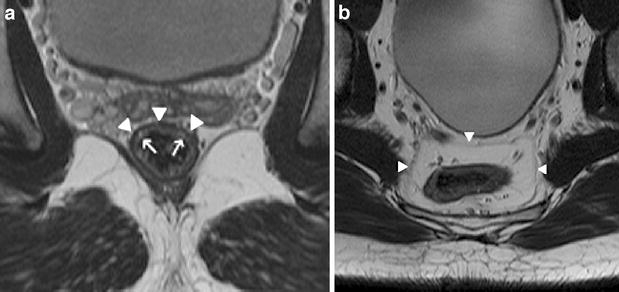
a Axial T2-weighted FSE (TSE) sequence of the pelvis depicting the layers of the rectal wall. The mucosa and submucosa can be visualized as a relatively hyperintense band (arrows). The hypointense line (arrowheads) represents the muscularis propria. b Axial T2-weighted FSE (TSE) sequence. The mesorectal fascia can be visualized as a thin line (arrowheads), enveloping the mesorectal compartment, containing the rectum, mesorectal fat, blood vessels, lymphatic vessels and nodes
Therapeutic options
Surgery
The anatomic position of the rectum in the true pelvis and its vicinity to adjacent anatomy, in particular the sphincter muscles, poses a challenge for the surgeon regardless of the surgical technique used. Surgical treatment of rectal cancer is a difficult balancing act between minimizing the risk of local recurrence and the preservation of anorectal and genitourinary function.
Total mesorectal excision (TME)
The introduction of standardized TME [9] has considerably improved prognosis in patients with cancer located in the middle or lower third of the rectum. Using this operative technique, the rectum is resected together with all surrounding lymphatic pathways, lymph nodes, mesorectal fatty tissue, and the mesorectal fascia while the parietal pelvis fascia and the pelvic splanchnic nerves (nervi erigentes) are spared. The widespread introduction of TME has markedly reduced the rate of non-continence-preserving abdominoperineal operations for rectal cancer.
Local excision is an option in patients with very small, well to moderately differentiated tumors that are confined to the mucosa and submucosa [10]. Techniques used for local excision are transanal surgical tumor removal and endoscopic microsurgical tumor ablation. Only a few patients are candidates for local excision (about 5%) and these must be selected with great care.
Circumferential resection margin (CRM) and local recurrence
The local recurrence rate after surgery performed with curative intent ranges between 3% and 32% [11]. For TME, some studies report local recurrence rates that are markedly below 10% [12–14]. Lateral circumferential tumor extent is a much more important prognostic factor for local recurrence than longitudinal tumor extent. Incomplete resection of the lateral tumor margins is now considered the most important cause of local recurrence [15–17]. In a study by Quirke et al. [15], 83% of the patients with a positive CRM had local tumor recurrence. Hence, the topographic relationship of the tumor to the mesorectal fascia that serves as a natural barrier and anatomic landmark for TME is the most important criterion in local tumor staging for therapeutic decision making.
Adjuvant/neoadjuvant therapy
The aims of adjuvant or neoadjuvant therapy are to enable or facilitate total tumor resection even in advanced disease, to prevent local tumor recurrence, and to minimize the risk of distant metastases. Adjuvant or neoadjuvant therapy leads to downstaging of the tumor [18, 19] in terms of its T and N stages, and 20% of patients even show complete tumor regression (sterilization) [20].
The timing of adjuvant or neoadjuvant therapy is still a matter of debate. Based on the results of two large studies [21, 22], preoperative radiotherapy alone or combined radiochemotherapy is the preferred option for tumors of the middle or lower rectum in Europe. One of these studies, performed in Scandinavia [21], showed that a short cycle of preoperative radiotherapy reduces the local recurrence rate from 27% to 11%. The second study showed that even patients who underwent TME, which already has a lower recurrence rate than other operative approaches, benefit from preoperative radiotherapy [22]. Preoperative irradiation significantly reduced local recurrence compared to the group treated by TME only. In the United States, adjuvant therapy consisting of combined postoperative radiochemotherapy is favored for patients with T3 and/or N1 tumors. [23].
Local tumor staging
Tumor staging is crucial for the prognosis and planning of therapy in the individual patient and aims at precisely determining the extent of the tumor as a basis for deciding whether surgery alone or surgery in combination with neoadjuvant therapy is the most suitable strategy. Of course, it is of great importance to avoid overtreatment or undertreatment of the patient. To reach a high level of accuracy in rectal cancer staging and to develop an adequate individual strategy for therapy, it is indispensable to establish a multidisciplinary team [24]. Rectal cancer staging is now mostly based on the TNM and UICC staging systems [6] (Tables 1, 2), which have largely replaced the older Dukes classification. The most important anatomic structure on which staging is based using these staging systems is the lamina muscularis propria. While T1 rectal carcinomas are confined to the mucosa and submucosa, T2 tumors invade the muscularis propria (Figs. 2a–c and 3). A T3 cancer is defined as a tumor extending beyond the lamina muscularis propria (Figs. 4, 5). However, none of the staging systems takes into account the fact that the T3 tumors are a very heterogeneous group, comprising tumors that just barely extend beyond the lamina muscularis propria as well as tumors that extend to or invade the mesorectal fascia (Figs. 4, 5) without further subclassification. The therapeutically important topographic relationship of the lateral tumor margins to the mesorectal fascia is not taken into consideration. An adequate, state-of-the-art staging classification should be able to precisely determine this relationship and to predict whether a tumor-free CRM is likely to be achieved or not. In this way one would be able to differentiate patients with minimal mesorectal infiltration in whom neoadjuvant therapy is not mandatory from patients who would definitely benefit from neoadjuvant therapy because the mesorectal fascia is infiltrated or at risk. T4 rectal cancers are defined as tumors, that reach the peritoneal surface or adjacent organs (Figs. 6a,b, 7, 8).
Table 1.
TNM classification for colorectal cancer
| Type | Description |
|---|---|
| T1 | Tumor involves submucosa |
| T2 | Tumor involves muscularis propria |
| T3 | Tumor beyond muscularis propria |
| T4 | Tumor reaches peritoneal surface or invades adjacent organ |
| N0 | No involved nodes |
| N1 | Up to three perirectal/colic nodes |
| N2 | Four or more perirectal/colic nodes |
Table 2.
UICC staging of rectal carcinoma
| Stage | Description | ||
|---|---|---|---|
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| T2 | N0 | M0 | |
| Stage IIA | T3 | N0 | M0 |
| B | T4 | N0 | M0 |
| Stage IIIA | T1, T2 | N1 | M0 |
| B | T3, T4 | N1 | M0 |
| C | Every T | N2 | M0 |
| Stage IV | Every T | Every N | M1 |
Fig. 2.

a Paraxial T2-weighted FSE (TSE) sequence. T1/2 rectal cancer. The relatively hyperintense intraluminal tumor (arrowhead) is confined to the rectal wall. Tumor invasion of the mesorectum is not visible. b Paraxial 3D-MPR and c intraluminal (virtual endoscopy) CT reconstuctions after rectal insufflation of CO2 showing the same tumor as a
Fig. 3.

Paraxial T2-weighted FSE (TSE) sequence. Tumor of the rectal wall. Fibrous strands into the mesorectum represent desmoplastic reaction (arrow). A differention between desmoplastic reaction and tumor infiltration of the mesorectum can be difficult
Fig. 4.
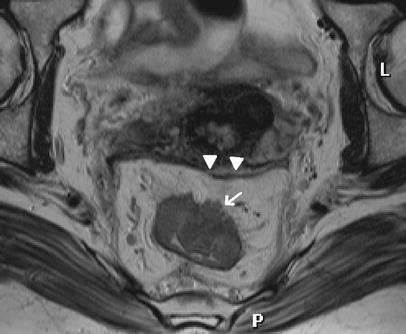
Paraxial T2 weighted FSE (TSE) sequence. A T3 rectal cancer breached through the muscularis propria (arrow) and invades the mesorectum. The tumor does not reach the mesorectal fascia (arrowheads). A tumor-free CRM can be expected
Fig. 5.

Paraxial T2-weighted FSE (TSE) sequence. A T3 rectal cancer widely invades the mesorectum. A tumor deposit (arrow) is located directly adjacent to the mesorectal fascia (arrowhead). A tumor-free resection margin cannot be predicted
Fig. 6.

a Paraxial T2-weighted FSE (TSE) sequence and b sagittal T2-weighted FSE (TSE) sequence of a T4 cancer located in the upper third of the rectum invading the uterus (arrows)
Fig. 7.
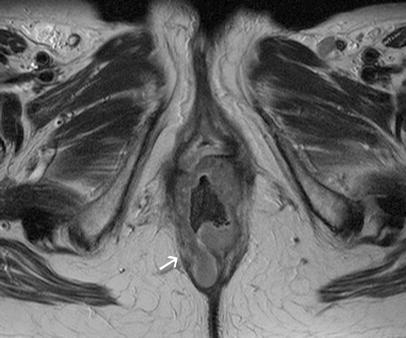
Paraxial T2-weighted FSE (TSE) sequence of a low T4 rectal cancer with infiltration of the levator ani muscle (arrow)
Fig. 8.
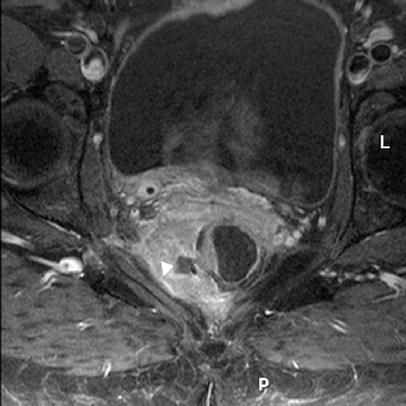
Recurrent rectal cancer. Paraxial T1-weighted SE sequence with fat suppression after i.v. apllication of gadopentetate-dimelglumine at a dosage of 0.2 mmol/kg body weight. The large extraluminal tumor shows central necrosis (arrowhead) and reaches the right pelvic wall
Staging modalities
Endorectal ultrasound (EUS)
EUS is the oldest and most widely used imaging technique for evaluating the local extent of rectal cancer. EUS depicts the anatomic layers of the rectal wall with a high degree of accuracy and thus enables precise determination of the tumor extent in relation to the different wall layers. Reported accuracy rates of transrectal ultrasound in assessing the T stage are in the range of 69–97% [25–35]. EUS is most suitable for evaluating early rectal cancer while it is limited in assessing more advanced tumors. Although EUS allows the identification of transmural tumor growth, exact determination of the circumferential tumor spread and—even more important—depiction of the relation between the edges of the tumor and the mesorectal fascia is often not possible due to the limited scan depth caused by the high frequencies used. Moreover, the accuracy varies widely with the examiner’s experience [28, 36].
Computed tomography (CT)
Most older studies report rather low accuracy rates of only 52–70% [32, 37–41] for T-staging by CT. It is remarkable that accuracy levels reported in studies including less advanced tumors were considerably lower compared with those including only advanced tumor stages. The poor accuracy of CT in the staging of superficial tumors is mainly attributable to the fact that these studies used conventional CT protocols with low spatial and contrast resolution. The accuracy has since been improved by the advent of the multislice technique (MSCT). In a study of 92 patients by Kulinna et al. [42], T-staging using MSCT was found to have an accuracy of 86%, while Filippone et al. [43] found an accuracy of 83% in a study of 41 patients. If one takes into account that four-row CT scanners were used in these studies, it is evident that further improvement is to be expected from state-of-the-art CT scanners with up to 64 detector rows that are already in use today. Hence, the role of MSCT in the local staging of rectal cancer remains to be defined. CT is superior to both EUS and MRI in that the scan typically covers the entire abdomen and pelvis and thus also allows evaluation of the liver, the most important target organ of hematogenic metastatic spread of rectal cancer.
MRI
It is undisputed that MRI is the imaging modality with the highest soft-tissue contrast. This is why MRI is also used for staging rectal cancer. However, initial results with MRI were disappointing, with accuracies in T-staging reported in older studies ranging between 58 and 74% [39, 44–46]. These rather poor results are primarily due to the poor spatial resolution achieved with the whole-body coil systems used in these studies. When endorectal coils are used, MRI has similar accuracies as EUS [31, 47–49]. MRI using endorectal coil systems is comparable to EUS in that it allows highly accurate differentiation of the layers of the intestinal wall. However, endorectal coils also have a number of disadvantages. As with EUS, the field of view (FOV) is rather small and only allows adequate evaluation of early stages of rectal cancer because the evaluation of surrounding pelvic anatomy is limited. In patients with advanced tumors, insertion of the coil system may be impossible or is very painful. Another disadvantage is the high cost of endorectal coils, which are usually disposable.
The advent of powerful gradient systems and, above all, the development of high-resolution phased array surface coil systems in recent years brought the breakthrough in the staging of rectal cancer by MRI. The use of these phased-array surface coils combines a very high spatial resolution with a large FOV that allows not only detailed evaluation of the intestinal wall but also depicts surrounding anatomy including the mesorectal fascia.
Imaging technique
Rectal cancer staging by MRI is rather fast and straightforward. No special patient preparation is required. Some authors recommend administration of a positive or negative enteral contrast medium, but this seems not to be necessary as suggested by current data in the literature. A study published only recently even indicated that rectal distension significantly reduces the distance between the rectal wall and the mesorectal fascia and that this might impact on the ability of MRI to predict accurately the distance between the tumor and the potential resection margin [50].
At our department, we administer a spasmolytic agent (butylscopolamine) at a dose of 20–40 mg to prevent artifacts caused by peristalsis of the small intestine and to distend the sigmoid and rectum. The agent has a short half-life and is therefore injected intramuscularly immediately before MRI.
For efficient planning of the pulse sequences to be employed, the radiologist performing the examination should beforehand obtain information about the approximate tumor localization (distance from anocutaneous line in cm) from the referring surgeon and ask the patient about any previous surgery or diseases of the pelvic organs.
The patient is positioned comfortably on the back and a phased-array surface coil is placed on the pelvis in such a way that the lower edge of the coil comes to lie well below the pubic bone. The coil is kept in place with belts and the patient is then advanced head-first into the bore of the magnet.
Following the usual localizer scans, a sagittal T2-weighted half-Fourier single shot turbo spin-echo (SSFSE, HASTE) sequence with a large field of view (FOV) should be acquired to obtain an overview and for planning of the subsequent sequences (e.g. TR ∞, TE 62 ms, slice thickness 5 mm, FOV 255×340 mm, matrix size 116×256, voxel size 2.2×1.3×5 mm). Precise tumor localization is then achieved with an axial T2-weighted fast spin-echo (FSE) or turbo spin-echo (TSE) sequence with a large FOV and a slice thickness of 5 mm (e.g. TR 4,170 ms, TE 98 ms, FOV 300×220 mm, matrix 282×512, voxel size 0.8×0.6×5 mm). At the core of the examination is a high-resolution T2-weighted TSE sequence with a small FOV and a slice thickness of 3 mm (e.g. TR 3,570 ms, TE 68 ms, FOV 180×180 mm, matrix 179×256, voxel size 1.0×0.7×3 mm). It is mandatory to place the slices perpendicular to the longitudinal axis of the tumor or the intestinal lumen in the vicinity of the tumor. With this sequence, it is possible to precisely evaluate the tumor and its relationship to the intestinal wall, mesorectal fascia, the pelvic organs, and possibly also to the peritoneal fold. Moreover, mesorectal lymph nodes in the immediate vicinity of the tumor can be evaluated. For visualization of more distant lymph nodes in our institution a T1 to proton-density-weighted two-dimensional (2D) TSE sequence with a short echo train length (e.g. 3 or 5) in axial orientation (e.g. TR 1,980 ms, TE 10 ms, slice thickness 5 mm, FOV 300×225 mm, matrix 219×512, voxel size 1× 0.6×5 mm), which covers the entire area up to the aortic bifurcation is used. Alternatively, a T1-weighted 3D gradient-echo sequence can be used for this purpose, allowing for the reconstruction of thinner slices. Possible infiltration of the anal sphincter muscles in patients with low tumors is evaluated using a coronal T2-weighted FSE (TSE) sequence (e.g. TR 3,570 ms, TE 68 ms, FOV 180×180, matrix 179×256, voxel size 1.0×0.7×3 mm) positioned parallel to the longitudinal axis of the anal canal. Current data in the literature suggests that intravenous contrast medium administration does not improve staging of rectal tumors by MRI [51, 52].
Since differentiation with the T2-weighted sequences is based on the contrast between the high-signal-intensity mesorectal fatty tissue and the rather low signal intensity of the tumor, spectral fat suppression techniques are not needed. The duration of the MRI protocol as just outlined is about 25–30 min, including planning.
T-staging
Although the introduction of phased-array coil systems has improved the accuracy of MRI in staging rectal cancer, even more recent studies report accuracies of only 67–86 % for T-staging [53–56]. These disappointing results are primarily due to the poor differentiation of T1/2 cancer from so-called borderline T3 cancer, where it is often not possible to distinguish true mesorectal tumor invasion from desmoplastic reactions (Fig. 3) [49, 54, 57]. Desmoplastic reactions are reactive tissue alterations which often occur in the immediate surrounding of tumors, most frequently resulting in fibrotic extensions that may contain tumor cells or not. The failure to differentiate between desmoplastic reactions and tumor growth is not specific to MRI but is also a well-known problem in rectal cancer staging with EUS [27]. Clinically and therapeutically, however, this differentiation is of minor importance. As already mentioned, it is much more important to precisely describe the relationship of the tumor to the mesorectal fascia, representing the anticipated resection plane for TME in order to assess the likelihood of a tumor-free CRM. Several recent studies have confirmed that MRI is highly suited to provide this information [54, 57–60]. In a study of 43 patients, Bissett et al. [59] found good agreement between preoperative MRI and histopathology with regard to the demonstration of tumor penetration through the mesorectal fascia (accuracy: 95%). These results are underlined by the studies of Beets-Tan et al. [54, 61], who investigated 76 patients and likewise found preoperative MRI to be highly accurate in assessment of the CRM. The agreement was 100% in T4 tumors, and 97% and 93% for both readers in tumors with a histologically determined tumor-free CRM >10 mm. Regression analysis for histologically determined margins of 1–10 mm demonstrated that a tumor-free resection margin of 2 mm was predicted with an accuracy of 97% if the distance between tumor and mesorectal fascia measured by MRI was at least 6 mm. It is noteworthy that this study likewise showed only moderate results with regard to T-staging (accuracy of 83% and 67% for the two readers) [54, 61]. In a study of 98 patients published by Brown and co-workers in 2003, the agreement between MRI and histology in assessment of the CRM was 92% [60]. These figures indicate that MRI allows accurate prediction of the CRM status after resection. The expected CRM can be described as involved if tumor invasion of the mesorectal fascia is visible or the tumor has a proximity of 1 mm or less to the mesorectal fascia. A tumor-free CRM can be assumed with a high degree of accuracy if the shortest distance from the maximum tumor extension, a mesorectal tumor deposit or a suspect lymph node in the mesorectum is more than 6 mm [54]. The role of tumors that extend towards the mesorectal fascia to a distance of less than 5 mm on MR images remains controversial.
The study by Brown et al. [60] also suggests that other important prognostic factors besides the CRM are the infiltration of extramural veins and possible infiltration of the peritoneal fold and that these can also be identified by preoperative MRI.
A study by Oberholzer and co-workers published in 2005 has shown that parallel imaging techniques do not compromise diagnostic accuracy with regard to the assessment of the CRM, but can considerable shorten the examination [62].
N-staging
Identification of metastatic lymph nodes is the greatest challenge in preoperative staging of rectal cancer, regardless of the modality used (Figs. 9, 10, 11). Exact staging is important because the number of metastatic nodes has been shown to affect the prognosis [63]. Involvement of lymph nodes in the vicinity of the mesorectal fascia is associated with a higher risk of local recurrence [16]. In patients with metastatic nodes outside the mesorectal fascia, extended lymph node resection with additional removal of the internal iliac nodes becomes necessary [64]. This lymph node group is not removed when regular TME is performed. A special problem associated with identifying lymphatic involvement in rectal cancer is that lymph node size is not a reliable criterion for metastatic involvement because micrometastasis in normal-sized lymph nodes is common [65, 66].
Fig. 9.
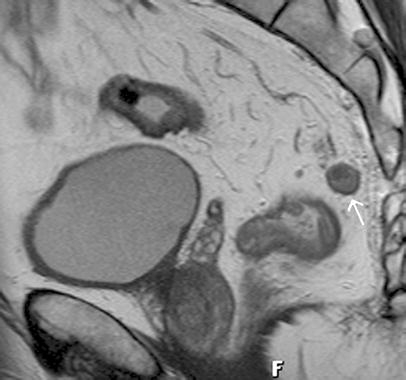
Sagittal T2-weighted FSE (TSE) sequence. The enlarged mesorectal lymph node (arrow) shows heterogenous signal intensity indicating tumor invasion
Fig. 10.
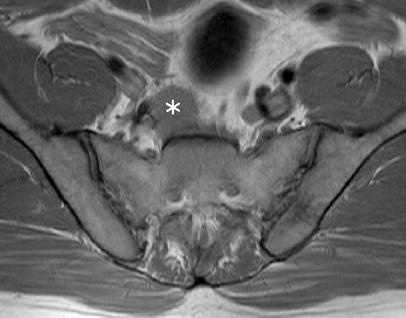
Axial PD-weighted FSE (TSE) sequence. A large lymph node metastasis (asterisk) located below the aortic bifurcation in a patient with rectal cancer
Fig. 11.

Axial T2-weighted FSE (TSE) sequence. Inguinal lymph node metastases (arrows) in a patient with low rectal cancer
The accuracy rates reported in the literature for N-staging by the different imaging modalities vary widely (EUS: 61–80% [10, 25, 26, 30–32, 34, 35, 37, 67], CT: 56–79% [32, 39, 68, 69], MRI: 57–85% [32, 39, 47, 53, 55, 60]. In a current meta-analysis including 84 studies, Lahaye et al. [70] found EUS to be slightly superior in assessing nodal status, but there were altogether no significant differences between the three staging modalities investigated. In summary, these results suggest that none of the imaging procedures currently in use enables reliable detection of metastatic lymph nodes.
In their study of MRI with histologic correlation, Brown et al. [71] identified an irregular contour and inhomogeneous signal to be the most reliable MRI criteria for lymph node metastasis (Fig. 9).
Future perspectives
USPIO
A new promising approach to detect metastatic lymph nodes by MRI is imaging in combination with ultrasmall superparamagnetic iron oxide particles (USPIO) as a contrast medium for systemic MR lymphography (Fig. 12a,b). Following intravenous administration, the particles are phagocytozed by nodal macrophages and, due to susceptibility effects, cause a signal decrease in normal or reactively changed lymph nodes on T2- and T2*-weighted images, which are usually acquired 24 h after administration of USPIO [72]. USPIO agents are currently under clinical evaluation and are not yet clinically available. Initial results of a study investigating this new approach in mesorectal lymph nodes are promising [73]. Further studies are needed to show whether USPIO can significantly improve lymph node staging by MRI.
Fig. 12.

a Axial PD-weighted sequence of the pelvis. Two small lymph nodes are visible adjacent to the iliac vessels (arrows). b Axial T2*-weighted gradient echo sequence acquired 24 hours after i.v. infusion of USPIO (Sinerem). The two lymph nodes (arrows) show homogenous signal decrease indicating normal lymphatic tissue. As USPIO agents are currently under clinical evaluation and are not yet clinically available, this image was acquired during a clinical trial
Whole-body MRI
The recent introduction of powerful whole-body MRI systems enables imaging of the whole body in a single session through repeated table movement. Several studies have already demonstrated the benefit of this approach for a variety of diagnostic queries in oncologic patients [74–77]. This technique may also be used for rectal cancer staging in the future and allow local staging and whole-body staging in a single session. In this way it would become possible to also evaluate the liver as the primary target organ of hematogenic spread of rectal cancer. The potential of parallel imaging to shorten the examination that has already been mentioned would be of particular significance in this respect [62].
Diffusion/perfusion-weighted MRI and PET
It has long been known that the pathophysiology and aggressiveness of a tumor are determined not only by the macroscopic tumor extent but also by other factors such as tumor microcirculation and angiogenesis. Several studies have shown the potential of diffusion- or perfusion-weighted imaging to indirectly determine these factors and to thus predict the response to adjuvant or neoadjuvant therapy [78–81]. However, extensive further research is necessary before the routine clinical use of these new techniques.
Recent studies have indicated that PET is able to predict response to neoadjuvant treatment of locally advanced rectal cancer with a high degree of accuracy [82].
Conclusion
The advances that have been made in the treatment of rectal cancer in recent years and that have considerably improved the prognosis of affected patients rely on differentiated pretherapeutic tumor staging. Despite its known limitations in T-staging, MRI is currently the only imaging modality that enables highly accurate evaluation of the topographic relationship between lateral tumor extent and the mesorectal fascia and to thus make a prediction about the CRM. In this way it is possible to carefully select those patients who will benefit from neoadjuvant therapy and to avoid overtreatment or undertreatment.
References
- 1.Jemal A, Murray T, Ward E et al (2005) Cancer statistics 2005. CA Cancer J Clin 55(1):10–30 [DOI] [PubMed]
- 2.Eddy DM (1990) Screening for colorectal cancer. Ann Intern Med 113(5):373–384 [DOI] [PubMed]
- 3.Vogelstein B, Fearon ER, Hamilton SR et al (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319(9):525–532 [DOI] [PubMed]
- 4.Muto T, Bussey HJ, Morson BC (1975) The evolution of cancer of the colon and rectum. Cancer 36(6):2251–2270 [DOI] [PubMed]
- 5.Winawer S, Fletcher R, Rex D et al (2003) Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 124(2):544–560 [DOI] [PubMed]
- 6.Sobin LH, Wittekind C (2002) International Union Against Cancer (UICC). TNM classification of malignant tumours: Wiley, New York
- 7.Bisset IP, Chau KY, Hill GL (2000) Extrafascial excision of the rectum: surgical anatomy of the fascia propria. Dis Colon Rectum 43(7):903–910 [DOI] [PubMed]
- 8.Grabbe E, Lierse W, Winkler R (1983) The perirectal fascia: morphology and use in staging of rectal carcinoma. Radiology 149(1):241–246 [DOI] [PubMed]
- 9.Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69(10):613–616 [DOI] [PubMed]
- 10.Akasu T, Kondo H, Moriya Y et al (2000) Endorectal ultrasonography and treatment of early stage rectal cancer. World J Surg 24(9):1061–1068 [DOI] [PubMed]
- 11.Sagar PM, Pemberton JH (1996) Surgical management of locally recurrent rectal cancer. Br J Surg 83(3):293–304 [DOI] [PubMed]
- 12.Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1(8496):1479–1482 [DOI] [PubMed]
- 13.MacFarlane JK, Ryall RD, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 341(8843):457–460 [DOI] [PubMed]
- 14.Enker WE (1992) Potency, cure, and local control in the operative treatment of rectal cancer. Arch Surg 127(12):1396–1401; discussion 1402 [DOI] [PubMed]
- 15.Quirke P, Durdey P, Dixon MF, Williams NS (1986) Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 2(8514):996–999 [DOI] [PubMed]
- 16.Adam IJ, Mohamdee MO, Martin IG et al (1994) Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 344(8924):707–711 [DOI] [PubMed]
- 17.Martling A, Holm T, Bremmer S, Lindholm J, Cedermark B, Blomqvist L (2003) Prognostic value of preoperative magnetic resonance imaging of the pelvis in rectal cancer. Br J Surg 90(11):1422–1428 [DOI] [PubMed]
- 18.Williamson PR, Hellinger MD, Larach SW, Ferrara A (1996) Endorectal ultrasound of T3 and T4 rectal cancers after preoperative chemoradiation. Dis Colon Rectum 39(1):45–49 [DOI] [PubMed]
- 19.Chari RS, Tyler DS, Anscher MS et al (1995) Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 221(6):778–786; discussion 786–777 [DOI] [PMC free article] [PubMed]
- 20.Minsky BD (1995) Conservative treatment of rectal cancer with local excision and postoperative radiation therapy. Eur J Cancer 31A(7–8):1343–1346 [DOI] [PubMed]
- 21.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336(14):980–987 [DOI] [PubMed]
- 22.Kapiteijn E, Marijnen CA, Nagtegaal ID et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345(9):638–646 [DOI] [PubMed]
- 23.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. Jama 1990;264(11):1444–1450 [PubMed]
- 24.Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D (2006) MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer 94(3):351–357 [DOI] [PMC free article] [PubMed]
- 25.Rifkin MD, Ehrlich SM, Marks G (1989) Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology 170(2):319–322 [DOI] [PubMed]
- 26.Glaser F, Schlag P, Herfarth C (1990) Endorectal ultrasonography for the assessment of invasion of rectal tumours and lymph node involvement. Br J Surg 77(8):883–887 [DOI] [PubMed]
- 27.Hulsmans FJ, Tio TL, Fockens P, Bosma A, Tytgat GN (1994) Assessment of tumor infiltration depth in rectal cancer with transrectal sonography: caution is necessary. Radiology 190(3):715–720 [DOI] [PubMed]
- 28.Herzog U, von Flue M, Tondelli P, Schuppisser JP (1993) How accurate is endorectal ultrasound in the preoperative staging of rectal cancer? Dis Colon Rectum 36(2):127–134 [DOI] [PubMed]
- 29.Milsom JW, Graffner H (1990) Intrarectal ultrasonography in rectal cancer staging and in the evaluation of pelvic disease. Clinical uses of intrarectal ultrasound. Ann Surg 212(5):602–606 [DOI] [PMC free article] [PubMed]
- 30.Garcia-Aguilar J, Pollack J, Lee SH et al (2002) Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum 45(1):10–15 [DOI] [PubMed]
- 31.Gualdi GF, Casciani E, Guadalaxara A, d’Orta C, Polettini E, Pappalardo G (2000) Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum 43(3):338–345 [DOI] [PubMed]
- 32.Kim NK, Kim MJ, Yun SH, Sohn SK, Min JS (1999) Comparative study of transrectal ultrasonography, pelvic computerized tomography, and magnetic resonance imaging in preoperative staging of rectal cancer. Dis Colon Rectum 42(6):770–775 [DOI] [PubMed]
- 33.Maldjian C, Smith R, Kilger A, Schnall M, Ginsberg G, Kochman M (2000) Endorectal surface coil MR imaging as a staging technique for rectal carcinoma: a comparison study to rectal endosonography. Abdom Imaging 25(1):75–80 [DOI] [PubMed]
- 34.Knaebel HP, Koch M, Feise T, Benner A, Kienle P (2005) Diagnostics of rectal cancer: endorectal ultrasound. Recent Results Cancer Res 165:46–57 [DOI] [PubMed]
- 35.Kauer WK, Prantl L, Dittler HJ, Siewert JR (2004) The value of endosonographic rectal carcinoma staging in routine diagnostics: a 10-year analysis. Surg Endosc 18(7):1075–1078 [DOI] [PubMed]
- 36.Solomon MJ, McLeod RS (1993) Endoluminal transrectal ultrasonography: accuracy, reliability, and validity. Dis Colon Rectum 36(2):200–205 [DOI] [PubMed]
- 37.Goldman S, Arvidsson H, Norming U, Lagerstedt U, Magnusson I, Frisell J (1991) Transrectal ultrasound and computed tomography in preoperative staging of lower rectal adenocarcinoma. Gastrointest Radiol 16(3):259–263 [DOI] [PubMed]
- 38.Shank B, Dershaw DD, Caravelli J, Barth J, Enker W (1990) A prospective study of the accuracy of preoperative computed tomographic staging of patients with biopsy-proven rectal carcinoma. Dis Colon Rectum 33(4):285–290 [DOI] [PubMed]
- 39.Zerhouni EA, Rutter C, Hamilton SR et al (1996) CT and MR imaging in the staging of colorectal carcinoma: report of the Radiology Diagnostic Oncology Group II. Radiology 200(2):443–451 [DOI] [PubMed]
- 40.Balthazar EJ, Megibow AJ, Hulnick D, Naidich DP (1988) Carcinoma of the colon: detection and preoperative staging by CT. AJR Am J Roentgenol 150(2):301–306 [DOI] [PubMed]
- 41.Thoeni RF (1997) Colorectal cancer. Radiologic staging. Radiol Clin North Am 35(2):457–485 [PubMed]
- 42.Kulinna C, Scheidler J, Strauss T et al (2004) Local staging of rectal cancer: assessment with double-contrast multislice computed tomography and transrectal ultrasound. J Comput Assist Tomogr 28(1):123–130 [DOI] [PubMed]
- 43.Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L (2004) Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography—initial experience. Radiology 231(1):83–90 [DOI] [PubMed]
- 44.Butch RJ, Stark DD, Wittenberg J et al (1986) Staging rectal cancer by MR and CT. AJR Am J Roentgenol 146(6):1155–1160 [DOI] [PubMed]
- 45.Hodgman CG, MacCarty RL, Wolff BG et al (1986) Preoperative staging of rectal carcinoma by computed tomography and 0.15T magnetic resonance imaging. Preliminary report. Dis Colon Rectum 29(7):446–450 [DOI] [PubMed]
- 46.Guinet C, Buy JN, Ghossain MA et al (1990) Comparison of magnetic resonance imaging and computed tomography in the preoperative staging of rectal cancer. Arch Surg 125(3):385–388 [DOI] [PubMed]
- 47.Chan TW, Kressel HY, Milestone B et al (1991) Rectal carcinoma: staging at MR imaging with endorectal surface coil. Work in progress. Radiology 181(2):461–467 [DOI] [PubMed]
- 48.Schnall MD, Furth EE, Rosato EF, Kressel HY (1994) Rectal tumor stage: correlation of endorectal MR imaging and pathologic findings. Radiology 190(3):709–714 [DOI] [PubMed]
- 49.Vogl TJ, Pegios W, Mack MG et al (1997) Accuracy of staging rectal tumors with contrast-enhanced transrectal MR imaging. AJR Am J Roentgenol 168(6):1427–1434 [DOI] [PubMed]
- 50.Slater A, Halligan S, Taylor SA, Marshall M (2006) Distance between the rectal wall and mesorectal fascia measured by MRI: Effect of rectal distension and implications for preoperative prediction of a tumour-free circumferential resection margin. Clin Radiol 61(1):65–70 [DOI] [PubMed]
- 51.Okizuka H, Sugimura K, Yoshizako T, Kaji Y, Wada A (1996) Rectal carcinoma: prospective comparison of conventional and gadopentetate dimeglumine enhanced fat-suppressed MR imaging. J Magn Reson Imaging 6(3):465–471 [DOI] [PubMed]
- 52.Vliegen RF, Beets GL, von Meyenfeldt MF et al (2005) Rectal cancer: MR imaging in local staging—is gadolinium-based contrast material helpful? Radiology 234(1):179–188 [DOI] [PubMed]
- 53.Blomqvist L, Holm T, Rubio C, Hindmarsh T (1997) Rectal tumours—MR imaging with endorectal and/or phased-array coils, and histopathological staging on giant sections. A comparative study. Acta Radiol 38(3):437–444 [DOI] [PubMed]
- 54.Beets-Tan RG, Beets GL, Vliegen RF et al (2001) Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 357(9255):497–504 [DOI] [PubMed]
- 55.Gagliardi G, Bayar S, Smith R, Salem RR (2002) Preoperative staging of rectal cancer using magnetic resonance imaging with external phase-arrayed coils. Arch Surg 137(4):447–451 [DOI] [PubMed]
- 56.Poon FW, McDonald A, Anderson JH et al (2005) Accuracy of thin section magnetic resonance using phased-array pelvic coil in predicting the T-staging of rectal cancer. Eur J Radiol 53(2):256–262 [DOI] [PubMed]
- 57.Brown G, Richards CJ, Newcombe RG et al (1999) Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 211(1):215–222 [DOI] [PubMed]
- 58.Blomqvist L, Rubio C, Holm T, Machado M, Hindmarsh T (1999) Rectal adenocarcinoma: assessment of tumour involvement of the lateral resection margin by MRI of resected specimen. Br J Radiol 72(853):18–23 [DOI] [PubMed]
- 59.Bissett IP, Fernando CC, Hough DM et al (2001) Identification of the fascia propria by magnetic resonance imaging and its relevance to preoperative assessment of rectal cancer. Dis Colon Rectum 44(2):259–265 [DOI] [PubMed]
- 60.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT (2003) Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 90(3):355–364 [DOI] [PubMed]
- 61.Beets-Tan RG, Beets GL (2004) Rectal cancer: review with emphasis on MR imaging. Radiology 232(2):335–346 [DOI] [PubMed]
- 62.Oberholzer K, Junginger T, Kreitner KF et al (2005) Local staging of rectal carcinoma and assessment of the circumferential resection margin with high-resolution MRI using an integrated parallel acquisition technique. J Magn Reson Imaging 22(1):101–108 [DOI] [PubMed]
- 63.Tang R, Wang JY, Chen JS et al (1995) Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg 180(6):705–712 [PubMed]
- 64.Suzuki K, Muto T, Sawada T (1995) Prevention of local recurrence by extended lymphadenectomy for rectal cancer. Surg Today 25(9):795–801 [DOI] [PubMed]
- 65.Monig SP, Baldus SE, Zirbes TK et al (1999) Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol 6(6):579–581 [DOI] [PubMed]
- 66.Bjelovic M, Kalezic V, Petrovic M et al (1998) Correlation of macroscopic and histological characteristics in the regional lymph nodes of patients with rectal and sigmoidal adenocarcinoma. Hepatogastroenterology 45(20):433–438 [PubMed]
- 67.Detry RJ, Kartheuser AH, Lagneaux G, Rahier J (1996) Preoperative lymph node staging in rectal cancer: a difficult challenge. Int J Colorectal Dis 11(5):217–221 [DOI] [PubMed]
- 68.Angelelli G, Macarini L, Lupo L, Caputi-Jambrenghi O, Pannarale O, Memeo V (1990) Rectal carcinoma: CT staging with water as contrast medium. Radiology 177(2):511–514 [DOI] [PubMed]
- 69.Chiesura-Corona M, Muzzio PC, Giust G, Zuliani M, Pucciarelli S, Toppan P (2001) Rectal cancer: CT local staging with histopathologic correlation. Abdom Imaging 26(2):134–138 [DOI] [PubMed]
- 70.Lahaye MJ, Engelen SM, Nelemans PJ et al (2005) Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 26(4):259–268 [DOI] [PubMed]
- 71.Brown G, Richards CJ, Bourne MW et al (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227(2):371–377 [DOI] [PubMed]
- 72.Taupitz M, Schmitz S, Hamm B (2003) (Superparamagnetic iron oxide particles: current state and future development). Rofo 175(6):752–765 [DOI] [PubMed]
- 73.Koh DM, Brown G, Temple L et al (2004) Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings-initial observations. Radiology 231(1):91–99 [DOI] [PubMed]
- 74.Nakanishi K, Kobayashi M, Takahashi S et al (2005) Whole body MRI for detecting metastatic bone tumor: comparison with bone scintigrams. Magn Reson Med Sci 4(1):11–17 [DOI] [PubMed]
- 75.Engelhard K, Hollenbach HP, Wohlfart K, von Imhoff E, Fellner FA (2004) Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol 14(1):99–105 [DOI] [PubMed]
- 76.Brennan DD, Gleeson T, Coate LE, Cronin C, Carney D, Eustace SJ (2005) A Comparison of Whole-Body MRI and CT for the Staging of Lymphoma. AJR Am J Roentgenol 185(3):711–716 [DOI] [PubMed]
- 77.Lauenstein TC, Freudenberg LS, Goehde SC et al (2002) Whole-body MRI using a rolling table platform for the detection of bone metastases. Eur Radiol 12(8):2091–2099 [DOI] [PubMed]
- 78.Dzik-Jurasz A, Domenig C, George M et al (2002) Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 360(9329):307–308 [DOI] [PubMed]
- 79.Devries AF, Griebel J, Kremser C et al (2001) Tumor microcirculation evaluated by dynamic magnetic resonance imaging predicts therapy outcome for primary rectal carcinoma. Cancer Res 61(6):2513–2516 [PubMed]
- 80.DeVries AF, Kremser C, Hein PA et al (2003) Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys 56(4):958–965 [DOI] [PubMed]
- 81.Hein PA, Kremser C, Judmaier W et al (2003) Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol 45(3):214–222 [DOI] [PubMed]
- 82.Denecke T, Rau B, Hoffmann KT et al (2005) Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 15(8):1658–1666 [DOI] [PubMed]


