Abstract
Honey bees communicate the location and desirability of valuable forage sites to their nestmates through an elaborate, symbolic “dance language.” The dance language is a uniquely complex communication system in invertebrates, and the neural mechanisms that generate dances are largely unknown. Here we show that treatments with controlled doses of the biogenic amine neuromodulator octopamine selectively increased the reporting of resource value in dances by forager bees. Oral and topical octopamine treatments modulated aspects of dances related to resource profitability in a dose-dependent manner. Dances for pollen and sucrose responded similarly to octopamine treatment, and these effects were eliminated by treatment with the octopamine antagonist mianserin. We propose that octopamine modulates the representation of floral rewards in dances by changing the processing of reward in the honey bee brain. Octopamine is known to modulate appetitive behavior in a range of solitary insects; the role of octopamine in dance provides an example of how neural substrates can be adapted for new behavioral innovations in the process of social evolution.
Keywords: Apis mellifera, biogenic amine, foraging, reward, social behavior
A key mechanism facilitating efficient coordinated foraging in honey bees (Apis mellifera) is their symbolic dance language (1–4). When they return to the hive, successful foragers may perform dances to advertise the location and relative profitability of a resource to nestmates (1, 5). Additional foragers are then recruited by dancers to profitable resources. The dance language is a classic study in ethology (5), but the neural mechanisms underlying dance remain largely unexplored.
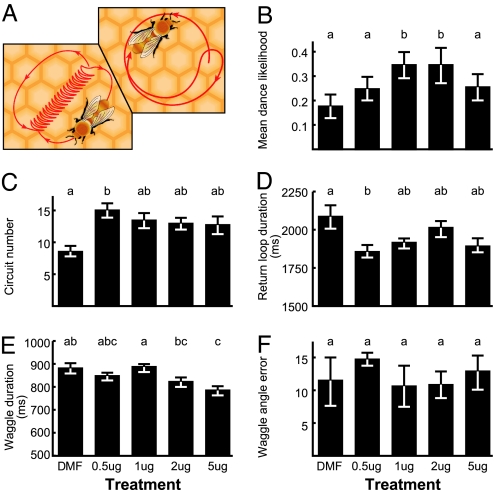
Dances represent the forager's assessment of the most direct route to the resource (6–8). Bees returning from valuable resources perform waggle dances (2), and the duration and angle of the waggling phase of the dance (Fig. 1A) communicate distance to and direction of the resource (5). For resources close to the hive, the waggle phase is extremely brief and not obvious to the naked eye, as a result of which dances for resources close to the hive are often described (9) as round dances (Fig. 1A). Dances also encode an assessment of the value of the resource; the likelihood, duration, and vigor of dancing are related to the profitability of the dancer's foraging trip (1, 10–12).
Fig. 1.
Effect of OA on honey bee waggle dances. (A) Schematic of round and waggle dances. In waggle dances, the duration of the waggle phase corresponds to the distance to a resource. The angle of the waggle phase of a dance relative to vertical corresponds to the angle between the food source and the sun on departure from the hive. A single waggle phase and return loop is one dance circuit. In round dances, the dancer runs in a tight loop reversing direction at the end of each circuit. (B–F) Effect of topical OA treatment on different elements of the waggle dance (Experiment 2). Bees were treated topically with one of four different doses of OA or dimethylformamide (DMF) as a control. More than 20 bees were analyzed per group. Bars present mean values ± SE. Superscripts refer to nonparametric statistical analyses. Columns marked by different superscripts differ at the 5% confidence level. (B) Dance likelihood: mean and S.E. calculated from arcsin transformed values; groups that differ statistically from DMF are marked by different superscripts (Mann–Whitney tests with Bonferroni correction). (C–F) Differences between treatment groups were tested with Kruskal–Wallis tests, and comparisons between specific groups were made with Dunn's post hoc tests.
Bees modulate their dance behavior by using cues that reflect their colony's foraging state. When forage is plentiful, the threshold forage profitability that triggers dancing increases, but when forage is scarce, bees dance for less profitable resources (1, 12). This mechanism ensures that a colony exploits all available forage sites when food is scarce but focuses on the most profitable sites only when food is plentiful. Dance is therefore an expression of the total integrated information gathered by a forager about her foraging trip and the current status of her colony.
Here we explored a neurochemical mechanism contributing to this integrative communication system. We focused on the biogenic amine octopamine (OA) because we had previously identified a role for OA as a modulator of the age-related transition from working in the hive to foraging (13, 14). Because dances stimulate foraging, there is an obvious link between dance and foraging. We report that OA increases the likelihood of dancing and modulates aspects of dances related to resource profitability, which we suggest reflects selective modulation by OA of the reporting of floral resource value during dances.
Results
Experiment 1: Oral OA Treatment Increases the Likelihood of Dancing for Pollen and Nectar.
We explored the effect of an established oral OA treatment method, known to modulate foraging behavior (13, 14), on the likelihood that a returning forager will dance. We repeated the experiment eight times, comparing dance behavior in eight pairs of orally OA-treated and control colonies maintained in a large flight enclosure and analyzing the behavior of pollen and sucrose foragers performing round and waggle dances.
Oral OA treatment significantly increased the probability of a returning forager dancing in five of eight trials, and this effect was significant overall (Table 1). We believe that the lack of statistical significance for sucrose foragers in Experiment 1a reflects the low overall level of dancing observed in this group. Consequently, in Experiments 1b and 1c, we increased the concentration of the sucrose feeder to 1.5 M to stimulate additional dancing. In Experiment 1c, we also measured the number of waggle dance circuits performed by OA-treated and control bees. The number of waggle dance circuits was found to be greater in OA-treated bees (mean circuit no. ± SE: OA-treated, 17.68 ± 0.65; control, 11.49 ± 0.89; t = 2.987, df = 64, P = 0.004; data from both trials pooled).
Table 1.
Proportion of foragers observed dancing in paired OA-treated and control colonies (Experiment 1)
| Experiment | Trial | Resource | Foragers observed dancing, % (n) |
P (χ2 test) | ||
|---|---|---|---|---|---|---|
| OA-treated | Control | |||||
| 1a: Bees trained to 1.25 M sucrose feeder and pollen feeder 10 m from the hive in a flight enclosure. Round dances analyzed. | 1 | |||||
| Pollen | 36.66 (25) | 0 (19) | 0.011 | |||
| Sucrose | 16.66 (18) | 4 (24) | 0.404 | |||
| Combined | 27.91 (43) | 2.32 (43) | 0.003 | |||
| 2 | Pollen | 29.62 (27) | 6.66 (33) | 0.037 | ||
| Sucrose | 10 (20) | 0 (40) | 0.207 | |||
| Combined | 21.27 (47) | 2.7 (73) | 0.003 | |||
| 3 | Pollen | 11.11 (27) | 3.37 (27) | 0.603 | ||
| Sucrose | 13.63 (22) | 0 (23) | 0.217 | |||
| Combined | 12.21 (49) | 2 (50) | 0.111 | |||
| 1b: Bees trained to 1.5 M sucrose feeder at the end of a 6-m flight tunnel to induce waggle dances. | 1 | Sucrose | 35.71 (28) | 13.79 (29) | 0.106 | |
| 2 | Sucrose | 56.52 (23) | 13.33 (30) | 0.002 | ||
| 3 | Sucrose | 68 (25) | 6 (20) | <0.001 | ||
| 1c: Bees trained to 1.5 M sucrose feeder at the end of a 6-m flight tunnel to induce waggle dances. | 1 | Sucrose | 93.75 (16) | 50 (30) | 0.008 | |
| 2 | Sucrose | 59.25 (27) | 64.51 (31) | 0.888 | ||
| Across-trials analysis. | 0.007 | |||||
Individually marked foragers that danced at least once in three observations during their first 48 h as a forager were scored as dancers. Proportions compared with χ2 tests with Yates correction. P values < 0.05 are in bold.
A limitation of the oral treatment method is that because all bees in a colony are treated with OA, the observed effects on dance behavior could perhaps be the result of changed social interactions between returning foragers and OA-treated hive bees. The “queuing time” for nectar foragers to find a receiver bee to offload their nectar to is the most important mechanism of social feedback that influences dance behavior (1). We measured this variable in Experiment 1c, and there were no differences between OA-treated and control colonies in the time it took foragers to begin to transfer their loads to receiver bees (OA-treated, 23.5 ± 3.4 s vs. control, 16 ± 2.1; t = 1.48, df = 56, P = 0.14). This finding suggests that the observed effects of OA treatment on dance behavior were not caused by changed interactions with receiver bees.
The amount of sucrose collected in a foraging trip did not differ between OA-treated and control bees. In Experiment 1c, bees were weighed on arrival at and departure from the sucrose feeder. Weights of OA-treated and control foragers, with or without a sucrose load, did not differ (t tests: Trial 1: unloaded OA-treated vs. control, 83.7 mg ± 1.53 vs. 83.1 ± 2.2; P = 0.8, df = 26; loaded, 131.6 ± 1.8 vs. 132.6 ± 3.2; P = 0.8, df = 65; Trial 2: unloaded, 85.6 ± 2.43 vs. 86.3 ± 2.2; P = 0.7, df = 24; loaded, 136.6 ± 3.31 vs. 138.5 ± 4.5; P = 0.6, df = 40). From this finding, we conclude that the mass of sucrose carried by OA-treated and control bees was similar.
Experiment 2: Topical OA Treatment Modulates Reporting of Resource Value in Waggle Dances for Sucrose.
To examine further the effect of OA on dancing, we analyzed all aspects of the waggle dances performed by bees individually topically treated with one of four different doses of OA, dissolved in 1 μl of DMF.
Different doses of OA affected dance behavior in different ways. The effect of OA treatment on waggle dance likelihood appeared to be dose-dependent (Fig. 1B), with intermediate OA doses elevating dance likelihood the most. A low dose of OA (0.5 μg) was most effective in increasing the number of waggle dance circuits performed (Fig. 1C) and reducing the duration of the return loop of the dance (increasing dance “vigor”; Fig. 1D). Duration of the waggle phase (distance reporting) was significantly affected by the highest OA dose only (5 μg; Fig. 1E). With the current data, it is not clear whether this result represents a general acceleration of dances at high doses of OA (perhaps suggesting the onset of hyperactivity at high OA doses) or whether neural mechanisms that underlie distance reporting are specifically affected by OA but less sensitive to treatment than mechanisms underlying the reporting of resource value. No OA treatment affected the accuracy of the angle signaled by dancers (Fig. 1F).
These results confirmed the effects on dance likelihood seen in Experiment 1 and also demonstrated OA modulation of dance components related to resource profitability. These findings suggest that OA affects dance behavior by influencing the reporting of resource profitability without altering directional information.
Experiment 3: Effects of OA on Dance Behavior Are Blocked by the OA Antagonist Mianserin.
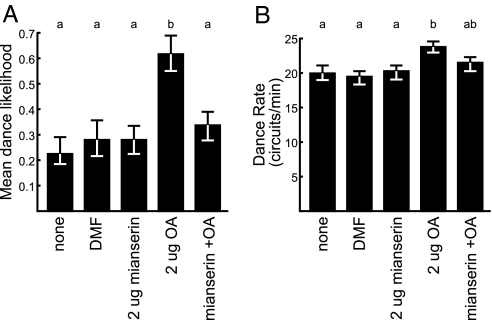
To explore whether OA influences dance behavior by acting directly on OA receptors, we examined whether the effect of OA treatment on dance could be eliminated by the OA antagonist mianserin (15, 16). Bees were trained to a sucrose feeder close to the hive and treated with OA and/or mianserin, and their round dance behavior was analyzed (Fig. 2). In round dances, the likelihood and rate of dancing both correlate with feeder profitability (11). We used 2 μg of OA because in Experiment 2 this dose had the greatest effect on dance likelihood.
Fig. 2.
Effect of OA and OA antagonist on round dances for sucrose (Experiment 3). Bees were treated topically with 2 μg of OA, 2 μg of mianserin, or both, in 1 μl of DMF. There were >30 bees per treatment group. Statistical analyses and notations are as in Fig. 1.
OA significantly increased the likelihood and rate of dancing relative to DMF- and sham-treated control groups (Fig. 2). In contrast, no such effects were seen in bees treated with 2 μg of OA in combination with 2 μg of mianserin. Mianserin on its own had no effect on dance behavior.
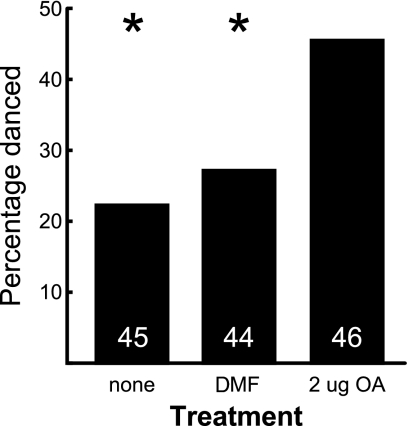
Experiment 4: OA Treatment Increases Reporting of Resource Value in Round Dances for Pollen.
OA is known to modulate responsiveness to sucrose in the proboscis-extension response assay (17). To test whether the effects of OA on dance behavior were simply the result of increased sensitivity to sucrose, we examined the effect of topical OA treatment on the round dances of bees collecting freeze-dried pollen from a dish in the absence of a sugar reward. OA treatment increased the percentage of pollen-foraging bees observed dancing (Fig. 3). This experiment reinforced the finding in Experiment 1 that OA treatment influenced the reporting of the profitability of pollen as well as sucrose foraging. Because bees collect pollen in external pollen baskets on their hindlegs rather than by ingestion as with nectar, it is not likely that OA influences dance behavior by increasing sensory sensitivity to sucrose alone.
Fig. 3.
Effect of topical OA treatment on round dances for pollen (Experiment 4). The percentage of bees that danced at least once during the 1-h observation period is shown. The sample size is shown above bars; groups that differed from OA-treated at the 5% confidence interval (χ2 test) are marked with an asterisk (∗).
Together, our data show a robust effect of OA treatment on the reporting of the value of floral resources by dancing bees. Our experiments covered 11 separate comparisons of OA-treated and control bees over a variety of environmental conditions with two different bee populations (North American and Australian).
Discussion
The dance language of the honey bee is a highly integrative form of behavior that communicates to nestmates a summary of the information gathered by a forager on the location and desirability of floral resources. Despite the complex nature of this behavior, our results indicate that treatment with OA allows a pharmacological dissection of the different components of dance communication.
Different doses of OA had different effects on dance behavior. Dance vigor and duration appear to be more sensitive to OA treatment than dance likelihood, which may indicate that different neural mechanisms, with differing sensitivities to OA treatment, are involved in the processes that control whether and how to dance.
At the highest dose of OA tested, we saw an additional effect on the duration of the waggle phase of the dance, which communicates distance. Could it be that this high dose was an overdose and accelerated dancing indiscriminately? High OA doses are known to cause hyperactivity in insects (18, 19), inhibit honey bee foraging (13), and cause aberrant honey bee behavior (D. J. Schulz and A.B.B., personal observation). We are confident that hyperactivity alone cannot explain all of our observations because dance behavior remained within an appropriate social context; even when whole colonies were treated orally with OA, only returning foragers danced. Further, not all OA-treated foragers danced all of the time, and those foragers that were treated with low or intermediate doses of OA and went on to dance produced dances that accurately communicated distance and direction to the sucrose feeder. This observation argues against OA treatment causing aberrant behavior.
The effects of OA on dance behavior were blocked by the OA antagonist mianserin, suggesting that OA modulated dance performance by direct interaction with OA receptors. Future studies need to explore the potential for further neurochemical dissection of dance communication and to identify other neural systems that contribute to different dance elements. Schricker and Stephen (20) observed that a sublethal dose of the insecticide parathion (a cholinesterase inhibitor) distorted distance communication. Bozic and Woodring (21) reported higher levels of both OA and dopamine in the brains of dancers compared with dance followers and resting bees and more dancing in two colonies fed the dopamine precursor dihydroxyphenylalanine. Because dihydroxyphenylalanine is a precursor of dopamine and OA (22), it is not clear whether this treatment affected both dopamine and OA levels.
Dopamine affects laboratory-based learning assays differently than OA (23, 24), in some cases reducing responsiveness to sucrose, in opposition to OA (17). Consequently, it will be interesting to clarify whether dopamine and OA indeed have similar effects on dance behavior. Serotonin has also been shown to act in opposition to OA in sensory systems (25–27), and it is an important modulator of the visual system (26); however, whether it modulates dance behavior is presently unknown.
OA treatments changed the dance parameters reporting resource value for sucrose and pollen, even though they did not change the amount of resource collected or behavioral interactions within the hive. Therefore, we propose that OA modulates how the brain processes floral resources and assesses their value rather than the amount of, and colony demand for, returned floral resources.
How might OA cause these changes in dance communication? OA is known to increase sensitivity and responsiveness to sucrose (17, 23, 25). In studies of associative learning, OA modulates the learning of sucrose reward in honey bees and Drosophila melanogaster (22, 24, 28) and may represent the sucrose unconditioned stimulus in the bee brain (29), i.e., the neurochemical released by the perception of sucrose that modulates downstream behavioral responses. Clearly, OA is involved in the processing of sucrose reward, but in the present study, dances for pollen were affected by OA treatment as much as dances for sucrose, and pollen is not ingested during foraging. In our study, freeze-dried pollen was collected from dishes without any additional sucrose or nectar reward. Therefore, we propose that OA does not just modulate the processing of sucrose reward, but it may be a general modulator of reward (at least floral reward) responses in the honey bee brain.
In mammals, responses to all rewarding stimuli, such as food, safety, and sexual gratification, share common neural circuitry and mechanisms (30, 31). These generalized reward-responsive circuits ramify extensively through the mammalian forebrain, and when stimulated, they release dopamine, a biogenic amine with structural similarities to OA (32, 33). In mammals, dopaminergic systems mediate the learning of reward, the motivation to seek reward, and the subjective pleasurable sensations triggered by the perception of rewarding stimuli (34). Perhaps a role of OA in the insect brain is analogous to that of dopaminergic circuits in the mammalian forebrain.
Perhaps there exist octopaminergic generalized reward-responsive circuits in the honey bee brain that mediate reward perception, learning, and reward-seeking motivation. Although there are relatively few octopaminergic neurons in the honey bee brain, these neurons ramify extensively through regions of the insect brain known to be involved in reward learning (35). If this hypothesis is correct, it would suggest a framework for unifying the diverse roles of OA in the learning of rewarding stimuli (24), motivation to forage for floral rewards (36, 37), arousal (19), and the evaluation of floral rewards communicated by dances (this work).
The role of OA in insects has previously been likened to the general arousing role of adrenaline in vertebrates (18, 38), but this notion does not contradict the suggestion that some OA-modulated circuits may be reward-responsive (39). A test of the generality of OA as a mediator of reward responses in honey bees would be to assess the role of OA in dance responses for nonnutritional rewards such as nest sites, water, or propolis (resins used in hive maintenance).
There has been much speculation about how dance behavior in honey bees might have evolved from the simpler behavioral patterns involved in food searching. Esch (40) proposed that the waggle dance evolved as a ritualization of simpler intention movements that partly reenacted flying to flowers. OA modulation of honey bee dance behavior supports this hypothesis by identifying a commonality between the neurochemical mechanisms motivating personal appetitive behavior and the social dance response.
Forager honey bees do not directly benefit from their foraging efforts; they forage for the benefit of the whole colony. OA modulation of dance communication demonstrates that a common neurochemical mechanism can motivate both self-feeding and altruistic behavior, providing an example of how social evolution can shape a neural system for a novel function.
Methods
Bees.
Bees were the typical North American (Experiments 1a and 1c) and Australian (Experiments 1b and 2–4) populations of A. mellifera, which are hybrids of various European-derived subspecies. Experiments 1a and 1c were performed at the University of Illinois Bee Research Facility (Urbana, IL). The remaining experiments were performed at the Research School of Biological Sciences (Canberra, Australia).
Experimental Colonies.
Experimental colonies were housed in glass-walled observation hives with a baffle at the entrance to direct returning foragers to run onto one side of the comb (1). For Experiment 1a, each colony contained 1,000 paint-marked 1-day-old adult bees and was provided with 500 ml of 1.5 M sucrose solution and 50 g of pollen. Each colony was housed in a single-frame observation hive. Dance observations began once colonies had established foraging forces of >50 bees. For Experiments 1b and 1c, colonies (each containing ≈5,000 bees of mixed age) were housed in two-frame observation hives. Each colony was provided with 80 g of pollen and 800 ml of 1.5 M sucrose in two frames of honeycomb. Experiments 2–4 used four-frame observation hives containing ≈10,000 bees.
Oral OA Treatment.
For Experiment 1, we adapted the oral OA treatment described in ref. 13. OA was dissolved in 2 M sucrose solution at a concentration of 10.5 mM. Whole colonies were treated with OA by loading an empty honeycomb with OA-treated sucrose solution. OA treatments were refreshed regularly. The control was a paired colony treated in the same way with plain 2 M sucrose. Great care was taken to match colonies within a pair as closely as possible. Paired experimental colonies were established at the same time with similar populations of bees from the same colony source and headed by sister queens. Food stores were matched at the start of the experiment and, if necessary, were adjusted throughout the experiment so that both OA-treated and control colonies were maintained in the same nutritional state. OA treatment was assigned randomly within each colony pair.
Experiment 1 was performed in an outdoor flight cage that was divided into two sections (each section was 3.1 m × 18.6 m) to house either the OA-treated or control colony and their respective sucrose and pollen feeders. Confinement in the flight cage controlled the resources available to foragers and their foraging experience. OA-treated colonies also collected OA-treated sucrose from their sucrose feeder (1.25 M sucrose in Experiment 1a, 1.5 M sucrose in Experiments 1b and 1c).
Previous studies have shown that the oral OA treatment method selectively elevates OA brain levels without affecting serotonin and dopamine levels (13, 36). After each trial in Experiment 1, bees were collected for HPLC analysis of brain OA levels (36). As in previous studies, treatment significantly increased brain levels of OA by 2- to 3-fold in every trial of every experiment, with no effects on serotonin or dopamine (data not shown).
Topical OA Treatment.
Experiments 2–4 used a topical treatment method to treat individual bees with controlled doses of OA or mianserin dissolved in DMF. One microliter of solution was applied to the thorax by using a Drummond glass microcapillary pipette. The 1-μl drop was applied to the center of the dorsal thorax so that it did not spread into the neck, petiole, or around the wing hinges. Sucrose foragers were treated while they sat immobile, feeding at a sucrose feeder. Pollen foragers were caught at the pollen dish and held without anesthesia for 20 sec to allow treatment. DMF- and sham-treated bees (bees restrained and touched on the thorax with an empty glass capillary while feeding) were control groups. Experiments using radiolabeled OA have shown that topical treatment to the thorax is effective in elevating brain levels of OA (41). Dance observations began 30–60 min after treatment. This method allowed individual bees to be treated with controlled doses of OA as they foraged in the open environment.
Flight Tunnels.
Experiments 1b and 1c studied waggle dances in bees housed within a 20-m outdoor flight cage. To generate a waggle dance response in the flight cage, we used a technique that manipulated the bees' perception of flight distance during a foraging trip (7). Bees were trained to a 1.5 M sucrose feeder at the end of a flight tunnel. The tunnel was lined with a contrasting checker pattern (squares 4 × 4 cm) to increase the optic flow experienced during a foraging trip, which has been shown to increase the estimate of flight distance (7). Tunnel entrances were 1 m from the entrance of the observation hives; tunnel dimensions were 6 m × 0.12 m × 0.12 m.
Dance Analyses.
In Experiment 1, individually marked bees were followed back to the colony on departure from sucrose and pollen feeders and were observed inside the hive until they left again to see whether they danced. Bees that had not danced after three observations were classed as nondancers. We compared the proportions of pollen and sucrose foragers classed as dancers in OA-treated and control colonies. For Experiments 1c-4, dances were also recorded with a digital camcorder for more detailed analyses.
Waggle Dance.
In Experiment 2, bees were trained to a 2 M sucrose feeder placed 455 m from the hive to stimulate waggle dances in the returning foragers. Dance video footage was analyzed frame by frame. We counted the number of circuits for each dance, and for each dance circuit we recorded the duration of the waggle phase and the duration of the return loop between waggle phases by counting frames, giving a measure accurate to 40 ms. We also determined the angle of the waggle phase relative to a vertical reference on the comb by digitally marking the position of the median ocellus of the dancer at the start and end of the waggle phase. The angle of the waggle phase of a dance relative to vertical corresponds to the angle between the food source and the sun on departure from the hive (5). For each dance, we calculated the mean difference between the dance angle communicated by the dancing bee and the true solar angle (determined by measuring the bearing to the feeder station and estimating solar azimuth from published tables corrected for time and the longitude and latitude of Canberra) to give a measure of the solar angle error of the dance. Values of waggle duration, return loop duration, and solar angle error for each circuit of a dance were averaged to give mean values for each dance. Finally, for each individual bee, dance likelihood was calculated as the proportion of visits to the feeder that were followed by a dance during the 90-min observation period.
Round Dance.
In Experiments 3 and 4, bees were trained to a nearby feeder, and round dances were recorded and analyzed. Dance likelihood was measured as the proportion of foraging trips in the observation period that resulted in a dance. In Experiment 4, dances for pollen were rare, and the majority of observed bees did not dance. Therefore, we analyzed the percentage of bees that danced once or more in the different treatment groups. For each round dance of >5 circuits, we counted the number of complete dance circuits that ended in a reversal of direction, and we measured the duration of the dance by counting the total number of frames of the dance. From these values, we calculated a rate of dancing (dance circuits per min), which is a parameter of this dance that correlates with foraging profitability (11).
Statistical Analysis.
All analyses were performed with the Prism 4 statistical package (GraphPad, San Diego, CA).
Acknowledgments
We are indebted to M. Srinivasan and S. Zhang for allowing use of the facilities at the Research School of Biological Sciences, Canberra, and for guidance in using the flight tunnels; T. D. Seeley and M. Sen Sarma for advice; K. Pruiett (Illinois) and P. Helliwell (Canberra) for expert beekeeping assistance and technical support; S. Beard, C. Cameron, A. Cash, P. Cziko, and S. Gallo for field assistance in Illinois; S. Foret for developing a computer program to semiautomate waggle dance analysis; and members of the Robinson laboratory and T. D. Seeley for reviewing the manuscript. This work was supported by National Science Foundation Grant IBN-0212371 (to G.E.R.) and National Institutes of Health Cutting Edge Biological Research Award DA-019864 (to G.E.R.).
Abbreviations
- DMF
dimethylformamide
- OA
octopamine.
Footnotes
The authors declare no conflict of interest.
References
- 1.Seeley TD. The Wisdom of the Hive. Cambridge, MA: Harvard Univ Press; 1995. [Google Scholar]
- 2.Dyer FC. Annu Rev Entomol. 2002;47:917–949. doi: 10.1146/annurev.ento.47.091201.145306. [DOI] [PubMed] [Google Scholar]
- 3.Sherman G, Visscher PK. Nature. 2002;419:920–922. doi: 10.1038/nature01127. [DOI] [PubMed] [Google Scholar]
- 4.Dornhaus A, Chittka L. Behav Ecol Sociobiol. 2004;55:395–401. [Google Scholar]
- 5.Frisch KV. The Dance Language and Orientation of Honeybees. Cambridge, MA: Harvard Univ Press; 1967. [Google Scholar]
- 6.Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R. Nature. 2005;435:205–207. doi: 10.1038/nature03526. [DOI] [PubMed] [Google Scholar]
- 7.Srinivasan MV, Zhang S, Altwein M, Tautz J. Science. 2000;287:851–853. doi: 10.1126/science.287.5454.851. [DOI] [PubMed] [Google Scholar]
- 8.Gould JL, Gould CG. The Honey Bee. New York: Freeman; 1988. [Google Scholar]
- 9.Kirchner WH, Lindauer M, Michelsen A. Naturwissen. 1988;75:629–630. [Google Scholar]
- 10.Seeley TD, Mikheyev AS, Pagano GJ. J Comp Physiol A. 2000;186:813–819. doi: 10.1007/s003590000134. [DOI] [PubMed] [Google Scholar]
- 11.Waddington KD. J Comp Physiol. 1982;148:297–301. [Google Scholar]
- 12.Seeley TD. Behav Ecol Sociobiol. 1994;34:51–62. [Google Scholar]
- 13.Schulz DJ, Robinson GE. J Comp Physiol A. 2001;187:53–61. doi: 10.1007/s003590000177. [DOI] [PubMed] [Google Scholar]
- 14.Schulz DJ, Barron AB, Robinson GE. Brain Behav Evol. 2002;60:350–359. doi: 10.1159/000067788. [DOI] [PubMed] [Google Scholar]
- 15.Burrell BD, Smith BH. J Insect Physiol. 1995;41:671–680. [Google Scholar]
- 16.Orr N, Orr GL, Hollingworth RM. Insect Biochem. 1991;21:335–340. doi: 10.1002/arch.940160204. [DOI] [PubMed] [Google Scholar]
- 17.Scheiner R, Plückhahn S, Öney B, Blenau W, Erber J. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 18.Adamo SA, Linn CE, Hoy RR. J Exp Biol. 1995;198:1691–1700. doi: 10.1242/jeb.198.8.1691. [DOI] [PubMed] [Google Scholar]
- 19.Corbet SA. Adv Insect Physiol. 1991;23:81–116. [Google Scholar]
- 20.Schricker B, Stephen WP. J Apic Res. 1970;9:141–153. [Google Scholar]
- 21.Bozic J, Woodring J. Comp Biochem Physiol A. 1998;120:737–744. [Google Scholar]
- 22.Roeder T. Prog Neurobiol. 1999;59:533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- 23.Mercer AR, Menzel R. J Comp Physiol A. 1982;145:363–368. [Google Scholar]
- 24.Schwaerzel M, Monasterioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erber J, Kloppenburg P, Scheidler A. Experientia. 1993;49:1073–1083. [Google Scholar]
- 26.Erber J, Kloppenburg P. J Comp Physiol A. 1995;176:111–118. [Google Scholar]
- 27.Pribbenow B, Erber J. Neurobiol Learn Mem. 1996;66:109–120. doi: 10.1006/nlme.1996.0052. [DOI] [PubMed] [Google Scholar]
- 28.Hammer M. Trends Neurosci. 1997;20:245–252. doi: 10.1016/s0166-2236(96)01019-3. [DOI] [PubMed] [Google Scholar]
- 29.Hammer M, Menzel R. Learn Mem. 1998;5:146–156. [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson TE, Berridge KC. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 31.Berridge KC, Robinson TE. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 32.Wise R, Rompre P. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 33.Schultz W. Neuroscientist. 2001;7:293–302. doi: 10.1177/107385840100700406. [DOI] [PubMed] [Google Scholar]
- 34.Wise RA. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 35.Sinakevitch I, Niwa M, Strausfeld NJ. J Comp Neurol. 2005;488:233–254. doi: 10.1002/cne.20572. [DOI] [PubMed] [Google Scholar]
- 36.Barron AB, Schulz DJ, Robinson GE. J Comp Physiol A. 2002;188:603–610. doi: 10.1007/s00359-002-0335-5. [DOI] [PubMed] [Google Scholar]
- 37.Barron AB, Robinson GE. J Comp Physiol A. 2005;191:659–668. doi: 10.1007/s00359-005-0619-7. [DOI] [PubMed] [Google Scholar]
- 38.Roeder T. Annu Rev Entomol. 2005;50:447–477. doi: 10.1146/annurev.ento.50.071803.130404. [DOI] [PubMed] [Google Scholar]
- 39.Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Behav Neurosci. 1999;113:744–754. [PubMed] [Google Scholar]
- 40.Esch H. Sci Amer. 1967;216:96–104. [Google Scholar]
- 41.Barron AB, Maleszka J, Vander Meer RK, Robinson GE, Maleszka R. J Insect Physiol. 2007 doi: 10.1016/j.jinsphys.2006.11.009. [DOI] [PubMed] [Google Scholar]