Abstract
The agonist-binding domain of ionotropic glutamate receptors (iGluRs) has recently been crystallized as two polypeptide chains with a linker region. Although work on the structure of this isolated ligand-binding core has been invaluable, there is debate over how it relates to conformations adopted by intact receptors. iGluR crystals are proposed to represent the activated state as their degree of domain closure correlates well with agonist efficacy. However, iGluR crystals exhibit high agonist affinity that more closely matches that of desensitized receptors. Consequently, conformations adopted by iGluR crystals may represent this state. To test this, we have employed the plant lectin, concanavalin-A (Con-A) to report conformational changes elicited by kainate (KA) iGluR agonists during desensitization. When GluR6 KA receptors (KARs) were pre-incubated with Con-A, equilibrium responses evoked by the full agonist, l-glutamate (l-Glu), increased almost 30-fold. However, in the continued presence of l-Glu, Con-A exerted no effect suggesting that it has restricted access to its binding sites when the agonist is bound. However, Con-A does not discriminate well between agonist-bound or -unbound states with the weak partial agonist, domoate. Accessibility experiments with KA were intermediate in nature consistent with its equilibrium efficacy at GluR6 KARs. Our results suggest that full and partial agonists elicit distinct conformational changes in KARs during desensitization. This finding can be reconciled with crystallographic data if the agonist-binding domain adopts the same conformation in the activated and desensitized states. However, other interpretations are possible suggesting future work is required if this issue is to be resolved.
The concept that agonist molecules act on allosteric proteins such as ligand-gated ion channels with different efficacy was first recognized almost 50 years ago (Ariens, 1954; Stephenson, 1956; del Castillo & Katz, 1957). At fully occupied receptors, agonists that elicit the maximum response are referred to as full agonists whereas partial agonists evoke submaximal responses. Two distinct models have been developed to account for agonist behaviour: the concerted (Monod et al. 1965) and multi-state (Koshland et al. 1958, 1966) models. In the concerted model, full and partial agonists evoke identical conformational changes in protein structure, but differ in their ability to activate channel openings. Nicotinic acetylcholine receptors (nAChRs) exemplify this behaviour since membrane currents elicited by full and partial nAChR agonists have identical single-channel conductance but differ in open-channel probability (Gardner et al. 1984). Moreover, this gating behaviour is widespread amongst other signalling proteins such as glycine, GABAA and NMDA receptors, as well as cyclic-nucleotide-gated channels (Zagotta & Siegelbaum, 1996; Colquhoun & Sivilotti, 2004; Lynch, 2004; Auerbach & Zhou, 2005). In the multi-state model, full and partial agonists elicit distinct conformational changes in protein structure. Contrary to the concerted model, single-channel recordings reveal that conformations in protein structure are governed by agonist concentration (Rosenmund et al. 1998; Smith & Howe, 2000) as well as agonist type (Swanson et al. 1997; Jin et al. 2003). Although few ligand-gated ion channels operate by this mechanism, recent work on the agonist-binding domain of AMPA and KA iGluRs has suggested that their agonist behaviour is best described by this model.
Detailed X-ray analysis of iGluR subtypes has been possible since their agonist-binding domains can be reconstituted as two polypeptide chains using a linker peptide to replace transmembrane regions (Armstrong et al. 1998; Furukawa & Gouaux, 2003; Mayer, 2005). From work on AMPA iGluRs, it is proposed that agonist binding promotes closure of the isolated ligand-binding core which in the intact receptor would lead to channel opening (Armstrong et al. 1998; Armstrong & Gouaux, 2000). Therefore, conformations adopted by the isolated ligand-binding core are understood to represent the activated state. In support of this, full and partial AMPAR agonists elicit complete and partial cleft closure, respectively, correlating well with agonist efficacy (Armstrong et al. 2003; Jin et al. 2003). Ligand-binding constructs of KAR iGluRs apparently behave similarly since full and partial agonists also promote distinct conformations (Mayer, 2005) consistent with the multi-state model already proposed from functional analysis of intact KARs (Bowie & Lange, 2002; Swanson et al. 2002). However, a potential caveat is that unitary current measurements indicate that single AMPA and KAR activations are short-lived, lasting only a few milliseconds (Swanson et al. 1996, 1997; Howe, 1996). Consequently, X-ray crystal structures may represent another protein conformation that is more thermodynamically stable, such as the desensitized state(s).
Here we have characterized the state-dependent modulation of GluR6 KARs by Con-A. Previous work from our laboratory has established that this plant lectin selectively regulates desensitized GluR6 receptors (Bowie et al. 2003). We have used this property of Con-A to test if full and partial agonists elicit distinct conformations in the extracellular domain of intact GluR6 KARs during desensitization. In agreement with recent work on GluR6 crystal structures, we show that different agonists evoke distinct conformations in intact receptors. This finding further establishes that agonist efficacy at KARs is best explained by a multi-state model. Our observations on desensitized channels can be reconciled with crystallographical data if the activated and desensitized states adopt comparable conformations. However, as discussed below, alternative interpretations are possible suggesting that future structure–function analysis of KA iGluRs must address this issue.
Methods
Cell culture and transfection
Techniques used to culture and transfect mammalian cells to express GluR6 KARs have already been described in detail elsewhere (Bowie, 2002, 2003; Bowie & Lange, 2002). Briefly, tsA201 cells, a transformed human kidney (HEK 293) cell line stably expressing on SV40 temperature sensitive T antigen (provided by R. Horn, Jefferson Medical College, PA, USA) were maintained at a confluency of 70–80% in minimal essential medium with Earle's salts, 2 mm glutamine and 10% fetal bovine serum supplemented with penicillin (100 units ml−1) and streptomycin (100 μg ml−1). After plating at low density (2 × 104 cells ml−1) on plastic dishes, cells were transfected with cDNA encoding unedited wild-type glutamate receptor subunit 6 (GluR6Q) or mutant GluR6Q receptor subunits using the calcium phosphate technique as previously described (Bowie et al. 1998). The cDNA for enhanced green fluorescent protein (EGFP S65T mutant) was routinely cotransfected to help identify transfected cells.
Site-directed mutagenesis
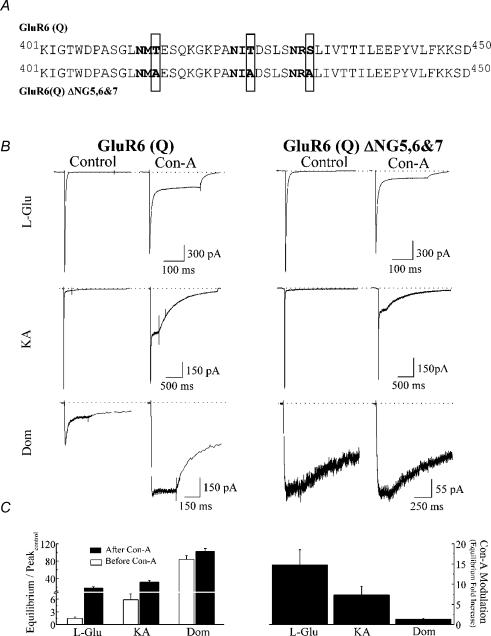
Mutation of N-glycosylated residues located in close proximity to the agonist-binding domain of GluR6 KARs was performed to disrupt lectin modulation (Fig. 4). To generate mutants, three of the N-glycosylated consensus sites (N-X-S/T, where X ≠ P) in the GluR6 sequence were changed from an S/T to an A and will be referred to as GluR6(Q)ΔNG5,6,7 according to the nomenclature of Everts et al. (1999) (Fig. 4A). Alanine substitutions of T414 (NG5), T425 (NG6) and S432 (NG7) were performed in two steps using the Quickchange II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) using PfuUltra DNA polymerase and custom primers (Alpha DNA, Montreal, Quebec, Canada). Mutant cDNAs were amplified using XL10-Gold ultra-competent cells (Stratagene), purified with the QIAprep Spin Miniprep kit (Qiagen Inc., Mississauga, Ontario, Canada), initially identified by restriction digest using BamH I or Sac I (New England Biolabs, Beverly, MA, USA) and later confirmed by automated sequencing (McGill University and Genome Quebec Innovation Center, Montreal, Quebec, Canada). To obtain larger quantities of mutant cDNA, GluR6 mutants were amplified in bacterial cultures (Top10 cells, Invitrogen) and the cDNA purified using QIAfilter Maxiprep kits (Qiagen Inc.).
Figure 4. Disruption of Con-A binding sites interferes with Con-A modulation.
A, amino acid sequence alignment of wild-type GluR6 and GluR6 (Q) ΔNG5,6 & 7 showing three N-glycosylation consensus sites (N-X-S/T, X ≠ P) highlighted in bold. Disruption of Con-A binding was achieved by replacing threonine (T) or serine (S) residues at these sites with alanines (A), as highlighted by grey boxes. B, comparison of the membrane currents evoked by l-Glu, KA and Dom at wild-type (patch number, 030724p2) and mutant (patch numbers, 041015p2 and 041008p2) GluR6 receptors before and after treatment with 10 μm Con-A. Although agonist responses evoked by wild-type and GluR6 ΔNG5,6,7 channels were comparable, the degree of modulation by Con-A was different. C: left panel, summary plot comparing the amplitude of the equilibrium response for GluR6 ΔNG5,6,7 channels with each agonist before and after treatment with Con-A; right panel, bar graph showing that the degree of modulation by Con-A of GluR6 ΔNG5,6,7 is agonist dependent but less than observed with wild-type receptors (cf. Fig. 3D).
Electrophysiological solutions and techniques
Excitatory amino acid agonists were dissolved in external solutions containing 150 mm NaCl, 5 mm Hepes and 0.1 mm each of CaCl2 and MgCl2. All concentrated agonist stocks were adjusted to pH 7.3 with NaOH before being stored at −20°C. Saturating agonist concentrations chosen for l-glutamate (10 mm), KA (1 mm) and domoate (50 μm) were at least 5-fold larger than published EC50 values at GluR6 receptors (Köhler et al. 1993; Tygesen et al. 1994; Jones et al. 1997; Donevan et al. 1998; Bowie, 2002; Alt et al. 2004). We empirically confirmed that these concentrations were saturating by doubling the agonist concentration in each case and observing that peak response amplitudes were unchanged. The internal solution was composed of 115 mm NaCl, 10 mm NaF, 5 mm Hepes, 5 mm Na4BAPTA, 0.5 mm CaCl2, 1 mm MgCl2 and 10 mm Na2ATP to chelate endogenous polyamines (Bähring et al. 1997; Bowie et al. 1998). The pH and osmolarity of internal and external solutions were adjusted to 7.3 and 295 mosmol l−1, respectively. Con-A and succinyl Con-A (Sigma, St Louis, MO, USA) were prepared in glucose-free saline solution and filtered (0.2 μm filter, Corning) immediately before use as previously described (Bowie et al. 2003). All recordings were performed with an Axopatch 200B amplifier (Axon Instruments Inc., CA, USA) using thin-walled borosilicate glass pipettes (2–5 MΩ) coated with dental wax to reduce electrical noise. Control and agonist solutions were rapidly applied to outside-out patches excised from transfected tsA201 cells as previously described (Bowie et al. 1998, 2002; Bowie & Lange, 2002). Solution exchange (10–90% rise time = 25–50 μs) was determined routinely at the end of the experiment by measuring the liquid junction current (or exchange current) between the control and agonist-containing solution in which total Na+ content was reduced by 5%. Current records were filtered at 5 kHz, digitized at 25–50 kHz and series resistances (3–10 MΩ) compensated by 95%. Recordings were performed at −20 mV membrane potential to ensure adequate voltage clamp control of peak currents. Data acquisition was performed using pCLAMP9 software (Axon Instruments Inc.). All experiments were performed at room temperature.
Results
iGluR molecular rearrangements and structural information has been inferred from the state-dependent behaviour of a number of pharmacological agents including channel blockers (Benveniste & Mayer, 1995; Bähring & Mayer, 1998; Bowie et al. 1998) and the accessibility of substituted cysteine residues (Kuner et al. 1996, 2001). At KARs, the binding and modulatory effect of Con-A is also state dependent (Everts et al. 1999). We speculated that this property may be useful in probing gating conformations elicited by full and partial KAR agonists. Therefore, our initial experiments were designed to further characterize the nature of state-dependent modulation of KARs by Con-A.
Con-A modulation of GluR6 KARs is state dependent
Previous work on invertebrate iGluRs has suggested that Con-A binding sites are masked during desensitization (Evans & Usherwood, 1985) whereas more recent studies on mammalian GluR6 receptors has proposed that binding can occur (Everts et al. 1999). However, in the latter study the authors did not exclude the possibility that incubation with desensitizing concentrations of agonists still permit Con-A to bind to GluR6 receptors recycling through the open state (Everts et al. 1999). In such conditions, recycling through the open state would occur with low probability and the onset of Con-A's effects would develop slowly. Since the authors did not determine the time course of modulation (Everts et al. 1999), it is possible that their observations reflect binding to open rather than desensitized channels.
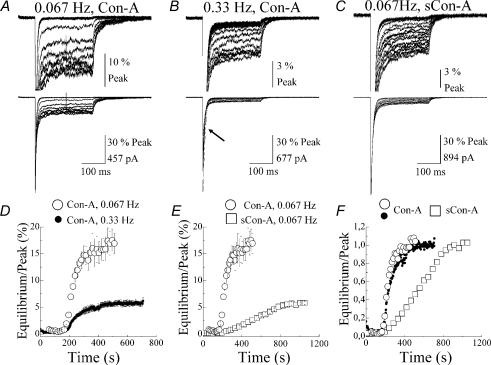
To determine if Con-A is able to bind to desensitized states, GluR6 receptors were stimulated at two frequencies, 0.067 (every 15 s) and 0.33 (every 3 s) Hz, to vary the fraction of desensitized receptors. The time course of Con-A modulation was then compared at each frequency. Figure 1A and B shows typical patch recordings where the development of Con-A effects was compared using multiple applications of 10 mm l-glutamate (l-Glu, 250 ms duration, holding potential (Vh) = −20 mV) every 15 s or 3 s, respectively. In each case, l-Glu evoked a rapidly rising inward membrane current that desensitized in the continued presence of the agonist to reach a steady-state level. At 0.067 Hz, GluR6 channels recover fully from desensitization between agonist applications (Bowie & Lange, 2002) whereas at 0.33 Hz, 50–60% of the peak response is desensitized. Consequently, the peak agonist response at 0.067 Hz was unchanged (Fig. 1A), whereas at 0.33 Hz, the peak amplitude initially declined by almost 60% before a new peak level was established (Fig. 1B, see arrow). When peak amplitudes stabilized during a recording, the outside-out patch was treated with 10 μm Con-A as previously described (Bowie et al. 2003).
Figure 1. Determining the time course for lectin modulation of GluR6 receptors.
A–C, time course for the onset of lectin modulation was determined by stimulating GluR6 receptors with 10 mm Glu (250 ms, Vh = −20 mV) every 15 s (A and C, 0.067 Hz patch numbers, 010327p2 and 010712p1) or 3 s (B, 0.33 Hz patch number, 010816p6) in the continuous presence of Con-A or succinyl Con-A (sCon-A). In each case, baseline control responses were first established before each patch was treated until a maximal effect on the equilibrium response was observed. Note, peak responses shown in B initially declined in amplitude when GluR6 receptors were stimulated at 0.33 Hz (see arrow). This effect, due to the onset of desensitization, was permitted to reach equilibrium before the patch was treated with Con-A. D, summary plot showing the development of modulation by Con-A of GluR6 receptors activated every 15 s (○, n = 8) or 3 s (•, n = 10). In each case, the rate of onset was similar, but the degree of modulation differed by more than 3-fold. E, plot comparing the time course for the onset of modulation by sCon-A (□, n = 4) and Con-A (○, n = 10) on GluR6 responses stimulated at 0.067 Hz. F, plot summarizing the data shown in D and E. In each case, the data were normalized to allow comparison between the onset of modulation at different stimulation frequencies and between different lectins. All data are expressed as mean ± s.e.m.
At both stimulation frequencies, Con-A did not significantly affect the peak amplitude but irreversibly potentiated the level of the equilibrium response (Fig. 1A and B). Since Con-A binding is irreversible, binding sites are saturated at any concentration where the total number of Con-A molecules is greater than or equal to the number of binding sites. In view of this, Con-A treatment modifies all GluR6 channels in each patch recording. At both stimulation frequencies, the time course for the onset of Con-A modulation reached a maximal effect after approximately 2–3 min of treatment (Fig. 1D–F). However, the effectiveness of Con-A on the equilibrium response was dependent on the stimulation frequency (Fig. 1D). The equilibrium responses observed at 0.067 and 0.33 Hz were 17.2 ± 2.1% (○, n = 8) and 5.7 ± 0.5% (•, n = 10) of the peak, respectively, representing a 3-fold difference in the effectiveness of Con-A (Fig. 1D). Taken together, these observations are not consistent with Con-A binding sites being masked by desensitization (Evans & Usherwood, 1985) since this mechanism would predict equi-effectiveness of Con-A at both stimulation rates but with a slower time course at 0.33 Hz. To account for the different degree of modulation, we propose that the number of glycosylated residues available for Con-A binding is restricted by desensitization.
Consistent with this, when we compared the rate and degree of modulation of GluR6 receptors with the lectin dimer, succinyl Con-A (sCon-A) (Gunther et al. 1973), the rate of onset was slower and the degree of modulation was less (□, 5.7 ± 0.5% Peak, n = 5) (Fig 1C, E and F). Since Con-A and sCon-A possess a different number of carbohydrate binding sites, it is likely that differences in stoichiometry sterically hinder binding and/or cross-linking events essential for modulating GluR6 receptors. However, these initial experiments do not exclude the possibility that different modulatory effects of Con-A at 0.067 and 0.33 Hz reflect binding to the open state rather than the desensitized state. Experiments described below and illustrated in Fig. 2 resolve this issue.
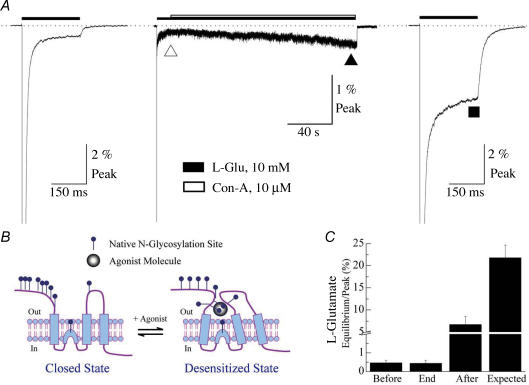
Figure 2. Con-A modulation of GluR6 receptors is a multi-step process.
A, typical experiment showing the effect of Con-A when applied to predominantly desensitized channels. l-Glu (10 mm) was applied before (▿), during (▴) and after (▪) extensive Con-A treatment (200 s, Vh = −20 mV) to monitor changes in the equilibrium response amplitude (patch number, 010817p6). The filled and open bars indicate the application period of 10 mm Glu and 10 μm Con-A, respectively. The dotted line denotes the zero current level. The first and third applications of 10 mm Glu had a duration of 250 ms. B, schematic diagram illustrating how agonist-binding may prevent access of Con-A to a subset of N-glycosylated residues in the vicinity of the agonist-binding domain. C, summary plot of data from several patches (n = 6) where the amplitude of the equilibrium response was compared at various time points as exemplified by the experiment shown in A. The values for ‘Expected’ were taken from data shown in Fig. 3C. All data are expressed as the mean ± s.e.m.
Con-A modulation of GluR6 KARs is a multi-step process
Figure 2 shows the experimental protocol used to determine if Con-A binds to desensitized GluR6 receptors. In each experiment, control responses to 10 mm l-Glu (250 ms duration) were measured to establish the baseline amplitude of the equilibrium response (Fig. 2A, left panel). During the second, longer application of l-Glu (2–3 min), Con-A was continuously co-applied to the equilibrium response for a period previously shown to fully modulate GluR6 receptors (Bowie et al. 2003) (Fig. 2A, middle panel). The effect of Con-A was then determined by comparing the amplitude of the equilibrium responses at the beginning (Fig. 2A, ▿) and end of the treatment period (Fig. 2A, ▴). Interestingly, measurement of the equilibrium response at these two time points revealed that Con-A had almost no effect on equilibrium desensitization (End (▴): 0.44 ± 0.14% Peak, n = 5) when compared to control levels (Before (▿): 0.47 ± 0.17% Peak, n = 6) (Fig. 2C). Similar results were also observed when patches were co-treated with l-Glu and Con-A for longer periods (e.g. > 5 min).
The lack of effect of Con-A on desensitized GluR6 receptors suggests one of two possibilities. Firstly, lectin binding sites are masked by conformational events that occur during desensitization as suggested from work on invertebrate iGluRs (Evans & Usherwood, 1985). Alternatively, Con-A binding may have occurred but modulation requires an additional conformational step not permissible whilst receptors are desensitized (Fig. 2B). To distinguish between these two possibilities, co-treatment of the patch with 10 μm Con-A and agonist was terminated. The receptors were then allowed to fully recover from desensitization and a third 250 ms application of only 10 mm l-Glu was applied (Fig. 2A, right panel). Surprisingly, without subsequent Con-A treatment, the equilibrium response increased 14- to 15-fold to 6.74 ± 1.76% of the peak (n = 4) (Fig. 2A, ▪). The increase in the equilibrium response represents only 30% of the modulation observed when GluR6 receptors were treated in the absence of agonist (Fig. 2C, Expected: 21.8 ± 2.9% Peak, n = 29). This experiment suggests that Con-A binds to desensitized channels but requires an additional step, involving agonist dissociation, before modulation is observed. It is unlikely that Con-A binds to GluR6 receptors recycling through the open state since the degree of modulation observed with the third l-Glu application is too large. Having established that Con-A can report agonist-induced conformations, we hypothesized that this behaviour may be useful in comparing structural changes evoked by full and partial agonists.
Con-A modulation of GluR6 KARs is agonist dependent
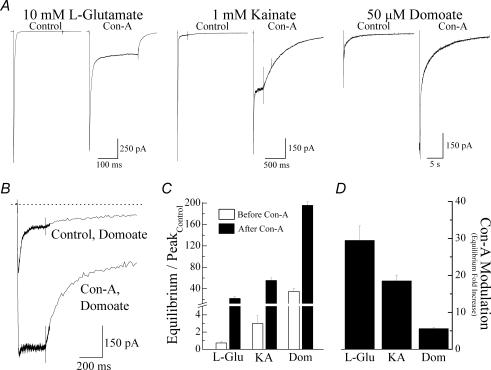
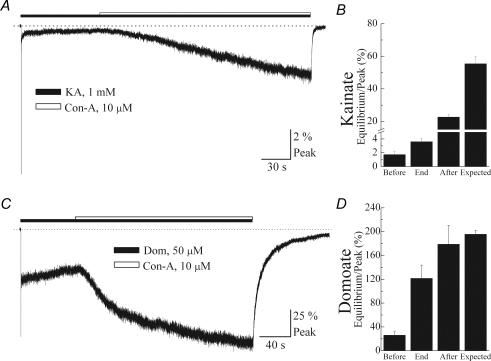
We initially compared the response profile of three structurally related agonist molecules recently crystallized in complex with the GluR6 KAR ligand-binding core (Mayer, 2005; Nanao et al. 2005). Figure 3A and B shows typical membrane currents evoked by rapid application of saturating concentrations of l-Glu (10 mm), KA (1 mm) and domoate (Dom, 50 μm) in the same patch recording before and after treatment with Con-A. Prior to Con-A treatment, peak responses to KA and Dom were 44.9 ± 2.2% (n = 13) and 12.6 ± 3.8% (n = 8), respectively, of the l-Glu response (n = 13) (Fig. 3A) confirming that KA and Dom are partial agonists at GluR6 KARs. Although, Con-A increased the amplitude of the equilibrium response for all three agonists, the degree of modulation was agonist specific (Fig. 3A–C). For example, the equilibrium response elicited by l-Glu increased 30-fold from an equilibrium/peak ratio of 0.74 ± 0.16% in control conditions to 21.8 ± 2.9% (n = 29) following Con-A treatment (Fig. 3C). In contrast, equilibrium/peak ratio for Dom was 34.8 ± 5.4% (n = 15) in the control response compared to 195.7 ± 6.8% (n = 3) after Con-A treatment, representing a 5-fold change. Finally, consistent with the rank order of agonist efficacy observed in control conditions, modulation by Con-A of equilibrium KA responses was intermediate (Fig. 3D).
Figure 3. Modulation of GluR6 receptors by Con-A is agonist dependent.
A, typical membrane currents (250 ms duration, Vh = −20 mV) elicited in the same patch by 10 mm l-Glu, 1 mm KA and 50 μm Dom before and after treatment with 10 μm Con-A (patch number, 030724p2). B, to show the early phase of the Dom response in more detail, agonist-evoked membrane currents prior to and after incubation with Con-A were superimposed. C and D, bar graphs summarizing the effect of Con-A treatment on equilibrium responses (C) and comparing its effects on different KA receptor agonists (D). All data are expressed as the mean ± s.e.m.
GluR6 equilibrium responses depend on the summed contribution of several subconductance states (Swanson et al. 1996; Howe, 1996) whose relative proportions may vary with full and partial agonists as recently proposed for AMPA receptors (Jin et al. 2003). We have shown that Con-A's effect on l-Glu responses is due to the up-regulation of a subset of conductance states (Bowie et al. 2003). Consequently, it is likely that Con-A affects KA and Dom equilibrium responses by modulating a different combination of subconductance levels. From a structural standpoint, irreversible binding of Con-A to N-glycosylated residues (Everts et al. 1997, 1999) may restrict conformational changes to a number of regions in the mature protein including the dimer interface, pore region or agonist-binding domain. Movement of the dimer interface governs the rate at which GluR6 receptors desensitize (Bowie & Lange, 2002; Horning & Mayer, 2004). Since Con-A does not affect GluR6 desensitization kinetics (Bowie et al. 2003) it is unlikely that lectin binding influences dimer–dimer interactions. Furthermore, Everts et al. (1999) have shown that N-glycosylated residues important for lectin binding are distant from the pore region (see Discussion) but located in and around the agonist-binding domain. Consequently, Con-A is unlikely to influence the pore region directly but may restrict conformations within the agonist-binding domain. To provide further experimental support for this, we performed mutational analysis of three N-glycosylated amino acid residues in close proximity to the agonist-binding domain.
Mutation of Con-A binding sites in close proximity to the agonist-binding domain
Alanine substitution of three amino acid residues, T414A, T425A and S432A (Fig. 4A) was made since previous work had established that each residue was critical for Con-A modulation (Everts et al. 1999). The triple mutant will be referred to as GluR6(Q)ΔNG5,6,7 according to the nomenclature of Everts et al. (1999). We hypothesized that if full and partial agonists elicit distinct conformational changes during desensitization, the disruption of Con-A modulation by Con-A would be agonist specific.
Figure 4 compares experiments where wild-type and mutant GluR6 receptors were modulated by Con-A. As expected from previous work (Everts et al. 1997), removal of N-glycosylated residues did not significantly affect surface expression or the response profile of GluR6 receptor agonists (Fig. 4B). However, we did observe some variation in the Dom response. The majority of patches containing wild-type or GluR6(Q)ΔNG5,6,7 receptors exhibited a sustained response to Dom (Fig. 4B, right panel), but in some cases, the onset of desensitization was evident (Fig. 4B, left panel). This observation was labile in nature only appearing during the first but not subsequent applications of Dom making it difficult to study. Here, we have included both response types in our dataset since modulation by Con-A was indistinguishable. Compared to wild-type receptors, Con-A was less effective in modulating responses elicited by agonists acting on GluR6(Q)ΔNG5,6,7 receptors (Fig. 4B and C). Moreover, this disruption was agonist dependent. For example, Dom responses were rendered almost insensitive to treatment by Con-A in the triple mutant. The equilibrium/peak ratio observed after lectin treatment was only modestly increased compared to the equilibrium/peak ratio prior to Con-A (102.4 ± 6.8%, n = 5 and 84.2 ± 8.6%, n = 5, respectively). With l-Glu, equilibrium responses elicited by mutant receptors increased 15-fold (Fig. 4C) after Con-A treatment compared to the 30-fold increase observed in wild-type GluR6 (Fig. 3D). Finally, disruption of the modulation of KA responses was intermediate (Fig. 4C) consistent with the hypothesis that Con-A can be used to compare conformations elicited by agonists with different efficacies.
GluR6 agonists promote distinct conformational changes to intact KARs
To test if agonists cause distinct conformational changes during desensitization, we repeated experiments shown in Fig. 2 using prolonged applications of the partial agonists, KA and Dom (Fig. 5). Figure 5A and C shows representative experiments where treatment with Con-A was initiated only after responses evoked by 1 mm KA or 50 μm Dom reached equilibrium levels. As previously described (cf. Fig. 2), the amplitude of the equilibrium response before and at the end of treatment with Con-A was compared to assess lectin accessibility to the N-glycosylated sites (Fig. 5B and D). Unlike the full agonist l-Glu, Con-A modulated the equilibrium response elicited by partial agonists, KA and Dom (Fig. 5A and C). Moreover, it was possible to distinguish between partial agonists since the degree of Con-A modulation observed with Dom was greater than with KA. For example, Con-A treatment increased the equilibrium response (Before: 1.73 ± 0.47% Peak) evoked by KA approximately 2-fold when GluR6 receptors were pre-desensitized with the agonist (End: 3.60 ± 0.51% Peak) compared to an increase of 20- to 30-fold (Expected: 55.4 ± 4.6% Peak) when GluR6 receptors were treated in the absence of agonist (Figs 3D and 5B). In comparison, conformational events elicited by Dom only moderately restricted Con-A's accessibility. Here, Con-A increased the equilibrium response 5-fold on Dom-bound GluR6 receptors and approximately 6- to 7-fold when the agonist was absent (cf. Figs 3D and 5D). It is unlikely that these observations reflect Con-A modulating channels recycling through the open state since this mechanism would predict a greater effect on l-Glu responses than on KA or Dom responses. Indeed, our observations report the opposite effect where Con-A has a greater effect on equilibrium responses elicited by Dom or KA when compared to l-Glu (cf. Figs 2 and 5). It is also improbable that Con-A binds to resting channels since GluR6 receptors would be fully bound due to the saturating agonist concentrations used in these experiments. Taken together, these observations are in agreement with recent crystallographic data (Mayer, 2005) showing that partial agonists promote less closure of the agonist-binding domain than full agonists.
Figure 5. Accessibility of Con-A to its binding sites is increased by partial agonists.
A and C, typical patch experiments where the effect of Con-A (10 μm) on the equilibrium response evoked by 1 mm KA (patch number, 031118p2) or 50 μm Dom (patch number, 031111p2) was tested. Filled and open bars indicate the application period of agonist and Con-A, respectively, and the dotted line denotes zero current level. Note that, unlike l-Glu, Con-A was able to modulate equilibrium responses elicited by each partial agonist. B and D, summary bar graphs showing the amplitude of the equilibrium response at various time points as described in Fig. 2. All data are expressed as the mean ± s.e.m.
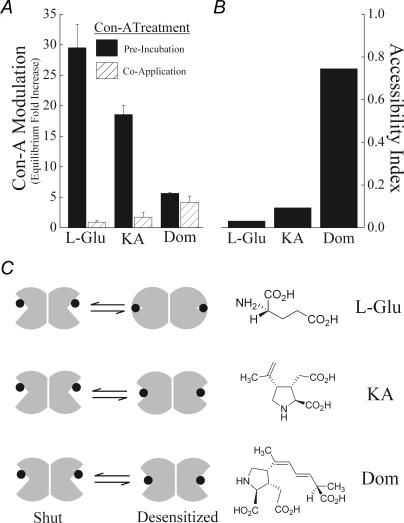
Figure 6 summarizes our results with Con-A in the presence and absence of full and partial agonists. Using the full agonist, l-Glu, Con-A's effect was strictly state dependent since the degree of modulation of the equilibrium response was dependent on whether GluR6 receptors adopted an agonist-bound (Co-Application: (0.92 ± 0.3)-fold increase) or unbound conformation (Pre-Incubation: (29.49 ± 3.9)-fold increase) (Fig. 6A). These two measurements were used to calculate an accessibility index ratio of 0.03 for l-Glu (Fig. 6B) which is consistent with crystallographic data (Mayer, 2005; Nanao et al. 2005). This finding also demonstrates that the small equilibrium response elicited by l-Glu at equilibrium (Fig. 3A) is associated with substantial conformational changes in the agonist-binding domain. In contrast, with the weak partial agonist Dom, the degree of Con-A modulation was similar whether lectin treatment occurred in the presence (Co-Application: (4.2 ± 1.0)-fold increase) or absence of agonist (Pre-Incubation: (5.62 ± 0.2)-fold increase) (Fig. 6A). In this case, the accessibility index ratio of 0.75 indicates that partial agonists promote weaker conformational changes upon binding which is associated with larger equilibrium responses (Fig. 3A and B). As expected, the accessibility index for KA (0.09) is consistent with its intermediary behaviour compared to full and weaker partial agonists (Fig. 6B). Interestingly, structural comparison revealed that Dom was the most bulky and l-Glu the most compact (Fig. 6C) suggesting that the physical nature of the agonist molecule may place constraints on the extent of domain closure. Taken together, these results suggest that the efficacy of full and partial agonists at equilibrium (Fig. 3) reflect distinct conformational changes in the agonist-binding domain of intact GluR6 KARs.
Figure 6. Multi-state model accounts for agonist behaviour at GluR6 receptors.
A, summary plot comparing the effect of Con-A on the equilibrium response evoked by each agonist, either following a period of treatment with Con-A alone (filled bars) or in the presence of agonist (hatched bars). Note that although modulation by Con-A is state dependent with l-Glu, Con-A discriminates poorly between ligand-bound or ligand-free states with Dom. The plot is constructed using data in Figs 2, 3 and 5. All data are expressed as the mean ± s.e.m. B, data from A were used to determine an accessibility index as described in Results. C, schematic diagram illustrating that full and partial agonists promote distinct conformational changes in the agonist-binding domain of GluR6 receptors. The extended molecular structure of each agonist is shown opposite revealing that l-Glu is the most compact, and domoate is the most bulky in nature.
Discussion
We show that Con-A can be employed to report agonist-induced conformational changes in the extracellular portion of intact GluR6 KARs. As discussed below, the most parsimonious explanation for our observations is that Con-A reports structural alterations in the agonist-binding domain. Crystallographical studies have not been able to provide structural information on the entire KAR due to technical considerations. Therefore, agonist behaviour has been examined by reconstituting the agonist-binding domain as two polypeptide chains with a linker domain. In agreement with reports describing KAR crystal structures, we show that GluR6 receptors adopt distinct conformations in the ligand-bound and unbound states. Moreover, the state-dependence of Con-A modulation is agonist-specific suggesting that full and partial agonists elicit distinct conformational changes in the agonist-binding domain during desensitization. Crystal structures of AMPA and KA receptors are thought to represent the activated state of the receptor since the extent of closure in the isolated ligand-binding core correlates with agonist efficacy. However, as addressed below, correlating conformational changes in this structure to functional properties of intact iGluRs remains an unresolved issue.
Comparison with previous studies
Although Con-A has been employed extensively as a pharmacological tool (Mayer & Vyklicky, 1989; Huettner, 1990; Wong & Mayer, 1993; Yue et al. 1995; Everts et al. 1997, 1999; Paternain et al. 1998), the state-dependence of its effects have not been examined in detail. State-dependent binding of Con-A was first observed in invertebrate iGluRs where Con-A-mediated effects (Mathers & Usherwood, 1976) were ineffective on desensitized channels (Evans & Usherwood, 1985). The authors concluded that structural rearrangements during desensitization masked carbohydrate moieties essential for Con-A binding (Evans & Usherwood, 1985). Since then, Con-A effects on mammalian GluR6 KARs have been documented (Yue et al. 1995; Everts et al. 1997; Paternain et al. 1998; Lerma et al. 2001) although state-dependent modulation has been described to a much lesser extent (Everts et al. 1999). This may reflect the difficulty in comparing observations with Con-A between different laboratories. For example, a significant variability in the potentiation of GluR6 KARs by Con-A has been reported in the literature ranging from 30- to 150-fold (Partin et al. 1993; Yue et al. 1995; Bowie et al. 2003) to 5000- to 6000-fold change (Everts et al. 1997, 1999). The reason for these differences is not clear but it does not reflect the electrophysiological recording techniques used (e.g. whole-cell versus patch) or the surrogate expression system (e.g. oocyte versus mammalian cell) chosen to study recombinant GluR6 receptors. In support of this, in separate experiments where we treated KARs with Con-A before or after excising patches, the degree of Con-A modulation was indistinguishable (data not shown).
Based on previous work, there are two possible explanations to account for Con-A's modulatory effect on equilibrium responses evoked by full and partial agonists (cf. Fig. 3). The first possibility is that Con-A blocks the onset of desensitization (Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993; Yue et al. 1995; Everts et al. 1997, 1999; Wilding & Huettner, 1997; Paternain et al. 1998). As a result, the potentiation of equilibrium responses evoked by strongly desensitizing agonists (e.g. l-Glu) would be expected to be greater than weakly desensitizing agonists (e.g. Dom). This explanation is unlikely, however, as there is no direct experimental evidence to support a mechanism whereby Con-A blocks entry into the desensitized state (Bowie et al. 2003). Previous studies had reached the conclusion that Con-A blocked desensitization based on the finding that lectin treatment eliminated the desensitization observed in whole-cell recordings. However, an important caveat in all of this work was that the rate of agonist perfusion used was too slow to accurately resolve the gating properties of GluR6 KARs (Bowie et al. 2003). Consequently, peak agonist responses were significantly underestimated in these studies. When experiments are performed in faster perfusion conditions, rates into and out of the desensitized state are unaffected by lectin binding (Bowie et al. 2003) demonstrating that Con-A does not block desensitization. The second possibility is based on the mechanism proposed by Bowie et al. (2003) whereby ion-conducting, desensitized states (Bowie & Lange, 2002) are up-regulated by lectin treatment. Here, the agonist-dependent nature of Con-A modulation is explained if, as proposed at AMPA receptors (Jin et al. 2003), full and partial KAR agonists activate different relative proportions of subconductance levels. As yet, analysis of single-channel currents activated by different GluR6 agonists has not been performed but would be necessary to delineate between an effect of Con-A on open-channel probability and/or unitary conductance (Bowie & Lange, 2002).
State-dependent modulation of KARs by Con-A
Although GluR6 subunits contain 10 N-glycosylated residues only nine are exposed to the extracellular surface and accessible to Con-A (Everts et al. 1999). The N-linked residue that does not bind Con-A is located in the pore region (Everts et al. 1999). All nine residues are positioned within or in close proximity to the agonist-binding domain of each GluR6 receptor subunit. Everts et al. (1999) have concluded that no single N-linked carbohydrate side chain is an absolute requirement for Con-A's effect, although the degree of modulation is significantly less with fewer residues present. Moreover, ectopic N-glycosylated sites introduced into the agonist-binding domain also impart sensitivity to Con-A and, as predicted, have a weaker effect compared to the greater number present on wild-type GluR6 receptors (Everts et al. 1999). This observation supports the hypothesis developed here that Con-A binds to different residues in agonist bound or unbound conformations determining the degree of modulation. We further qualify these observations by showing that removal of three amino acid residues (i.e. GluR6(Q)ΔNG5,6,7) is sufficient to abolish the modulation of Dom responses with only a partial effect on l-Glu and KA.
Although used extensively to study invertebrate and mammalian iGluRs, state-dependent binding and modulation by Con-A has been described in only a few studies (Evans & Usherwood, 1985; Everts et al. 1999). As discussed above, Con-A modulates GluR6 receptors by binding to residues in close proximity to the agonist-binding domain (Everts et al. 1997, 1999) and we show here that this property permits inferences to be made about conformations adopted by this structure. We propose that modulation of GluR6 KARs involves two distinct molecular events. Initially, Con-A binds to either agonist-bound, desensitized channels or GluR6 channels in the closed, unbound state. Due to architectural rearrangements that accompany agonist binding (Armstrong et al. 1998; Armstrong & Gouaux, 2000), the number of N-glycosylated residues accessible to Con-A (Everts et al. 1999; Fig. 2B) is different for desensitized and unbound channel conformations. At desensitized receptors, bound Con-A molecules do not affect receptor function with full agonists such as l-Glu (Fig. 2). However, subsequent agonist dissociation sets off changes in protein structure that promote cross-linking of bound Con-A molecules or adjacent amino acid residues to regulate gating behaviour. This process will be different if Con-A has initially bound to GluR6 receptors in the desensitized or closed, unbound state. We propose that this cross-linking event, in both cases, restricts allosteric movement(s) of the external surface of GluR6 receptors affecting gating behaviour.
Correlating Con-A modulation to conformational changes in GluR6 receptors
In principle, the state-dependence of Con-A modulation may reflect conformational changes to the dimer interface, the pore region or the agonist-binding domain. Although Con-A may affect the dimer–dimer interface, our previously published findings (Bowie et al. 2003) provide experimental evidence that does not support this possibility. Specifically, we have shown that Con-A binding to GluR6 KARs does not affect rates into and out of desensitization. Since Horning & Mayer (2004) have argued that the dimer interface of KARs (and AMPARs) determines desensitization kinetics, by implication, our data demonstrate that Con-A does not affect dimer–dimer interactions. Likewise, Con-A's action is unlikely to reflect binding to the pore since amino acid residues critical for lectin binding and modulation are distant from this region. Instead, our experiments on GluR6(Q)ΔNG5,6,7 receptors and work by Everts et al. (1999) demonstrate that amino acid residues critical for lectin modulation are located in and around the agonist-binding domain. Other mechanisms may emerge as our understanding of KARs progresses. However, given these limitations, the most straightforward explanation of our data is that Con-A modulation reports conformational changes in the agonist-binding domain. In support of this, recent X-ray analysis of the isolated ligand-binding core of GluR6 KARs (Mayer, 2005; Nanao et al. 2005) also reported that full and partial agonists elicit distinct conformational changes in this region of the protein.
A potential caveat amongst these studies is that our experiments have focused on desensitized receptors whereas crystal structures of iGluRs are thought to represent the agonist-binding domain in the activated state of the channel (Jin et al. 2003). Three possible explanations may account for this apparent discrepancy. The first possibility is that published structures of the KA (and AMPA) receptor ligand-binding core represents the conformation adopted during ion channel activation (i.e. channel openings) as already proposed (Armstrong et al. 1998; Hogner et al. 2002; Mayer, 2005) but does not represent the binding cleft during desensitization. However, an important issue is that unitary current measurements indicate that single AMPA or KA receptor activations are very short-lived, lasting only a few milliseconds (Swanson et al. 1996, 1997; Howe, 1996). Consequently, it is more likely that X-ray crystal structures of the ligand-binding core represent another conformational state that is more thermodynamically stable.
The second possibility, therefore, is that following agonist binding the ligand-binding core adopts a much more stable conformation such as the desensitized state. To date, the possibility that crystal structures of the iGluR ligand-binding core represent the desensitized state has not been examined experimentally though it has been suggested by some authors (Madden, 2002; Colquhoun & Sivilotti, 2004; Naur et al. 2005). In support of this, experimental protocols that require an extended incubation period with the ligand (e.g. radioligand-binding assays) are known to accumulate ligand-gated ion channels into high-affinity desensitized states (Colquhoun, 1998). By analogy, crystallization of the ligand-binding core may also promote formation of the desensitized state. Moreover, estimates of the apparent affinity of l-Glu for desensitized GluR6 receptors (IC50 = 0.44–0.5 μm (Paternain et al. 1998; A.Y.C. Wong, A.-M. L. Fay & D. Bowie, unpublished observations) and the isolated ligand-binding core (Ki = 1.4 μm) (Mayer, 2005) are almost identical whereas affinity for the activated state (EC50 = 694 μm) (Bowie et al. 2003) is more than 1000-fold lower.
The third and final possibility is that the conformation adopted by the ligand-binding core is identical whether the pore region is in the activated or desensitized state. This latter possibility would explain our observations on desensitized channels whilst agreeing with recent X-ray crystallographic data. However, this model, would have to reconcile with the fact that l-Glu evokes the largest peak response amongst all the agonists (see Fig. 3A) whereas the amplitude of its equilibrium response is the smallest (see Fig. 3C). In structural terms, the fact that partial agonists elicit responses of larger amplitude at equilibrium appears at odds with the proposed relationship between closure of the agonist-binding domain and agonist efficacy (Armstrong & Gouaux, 2000; Jin et al. 2003; Mayer, 2005). Clearly, further experimentation is required if these issues are to be resolved.
Acknowledgments
This work was supported by operating grants from the National Institutes of Health (RO1 MH62144) and Canadian Institutes of Health Research (MOP-62712). A.-M. L. Fay was funded by a predoctoral fellowship from the Fonds de Recherche en Santé du Québec. We thank Dr Adrian Wong and David MacLean for careful reading of the manuscript and interesting discussions. D. Bowie is a recipient of a Canada Research Chair award.
References
- Alt A, Weiss B, Ogden AM, Knauss JL, Oler J, Ho K, Large TH, Bleakman D. Pharmacological characterization of glutamatergic agonists and antagonists at recombinant human homomeric and heteromeric kainate receptors in vitro. Neuropharmacology. 2004;46:793–806. doi: 10.1016/j.neuropharm.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Ariens E. Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch Int Pharmacodyn Ther. 1954;99:32–49. [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Mayer M, Gouaux E. Tuning activation of the AMPA-sensitive GluR2 ion channel by genetic adjustment of agonist-induced conformational changes. Proc Natl Acad Sci U S A. 2003;100:5736–5741. doi: 10.1073/pnas.1037393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähring R, Mayer ML. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J Physiol. 1998;509:635–650. doi: 10.1111/j.1469-7793.1998.635bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M, Mayer ML. Trapping of glutamate and glycine during open channel block or rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol. 1995;483:367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol. 2002;539:725–733. doi: 10.1113/jphysiol.2001.013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Garcia EP, Marshall J, Traynelis SF, Lange GD. Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J Physiol. 2003;547:373–385. doi: 10.1113/jphysiol.2002.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci. 1998;18:8175–8185. doi: 10.1523/JNEUROSCI.18-20-08175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Binding, gating, affinity and efficacy: The interpretation of structure-activity relationships for agonists and of the effects of mutating receptors. Br J Pharmacol. 1998;125:923–948. doi: 10.1038/sj.bjp.0702164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D, Sivilotti LG. Function and structure in glycine receptors and some of their relatives. Trends Neurosci. 2004;27:337–344. doi: 10.1016/j.tins.2004.04.010. [DOI] [PubMed] [Google Scholar]
- del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Donevan SD, Beg A, Gunther JM, Twyman RE. The methylglutamate, SYM 2081, is a potent and highly selective agonist at kainate receptors. J Pharmacol Exp Ther. 1998;285:539–545. [PubMed] [Google Scholar]
- Evans ML, Usherwood PNR. The effect of lectins on desensitisation of locust muscle glutamate receptors. Brain Res. 1985;358:34–39. doi: 10.1016/0006-8993(85)90945-x. [DOI] [PubMed] [Google Scholar]
- Everts I, Petroski R, Kizelsztein P, Teichberg VI, Heinemann SF, Hollmann M. Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci. 1999;19:916–927. doi: 10.1523/JNEUROSCI.19-03-00916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P, Ogden DC, Colquhoun D. Conductances of single ion channels opened by nicotinic agonists are indistinguishable. Nature. 1984;309:160–162. doi: 10.1038/309160a0. [DOI] [PubMed] [Google Scholar]
- Gunther GR, Wang JL, Yahara I, Cunningham BA, Edelman GM. Concanavalin-A derivatives with altered biological activites. Proc Natl Acad Sci U S A. 1973;70:1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogner A, Kastrup JS, Jin R, Liljefors T, Mayer ML, Egebjerg J, Larsen IK, Gouaux E. Structural basis for AMPA receptor activation and ligand selectivity: crystal structures of five agonist complexes with the GluR2 ligand-binding core. J Mol Biol. 2002;322:93–109. doi: 10.1016/s0022-2836(02)00650-2. [DOI] [PubMed] [Google Scholar]
- Horning MS, Mayer ML. Regulation of AMPA receptor gating by ligand binding core dimers. Neuron. 2004;41:379–388. doi: 10.1016/s0896-6273(04)00018-2. [DOI] [PubMed] [Google Scholar]
- Howe JR. Homomeric and heteromeric ion channels formed from kainate-type subunits GluR6 and KA2 have very small, but different, unitary conductances. J Neurophysiol. 1996;76:510–519. doi: 10.1152/jn.1996.76.1.510. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat dorsal root ganglion neurons: activation by kainate and quisqualate, and blockade of desensitization by concanavalin A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- Jones KA, Wilding TJ, Huettner JE, Costa AC. Desensitization of kainate receptors by kainate, glutamate and diasteromers of 4-methylglutamate. Neuropharmacology. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Koshland DE, Jr, Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Koshland DE, Jr, Ray WJ, Erwin M. Protein structure and enzyme action. Fed Proc. 1958;17:1145–1150. [PubMed] [Google Scholar]
- Kuner T, Beck C, Sakmann B, Seeburg PH. Channel lining residues of the AMPA receptor M2 segment: structural environment of the Q/R site and identification of the selectivity filter. J Neurosci. 2001;21:4162–4172. doi: 10.1523/JNEUROSCI.21-12-04162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Karlin A, Seeburg PH, Sakmann B. Structure of the NMDA receptor channel M2 segment inferred from the accessibility of substituted cysteines. Neuron. 1996;17:343–352. doi: 10.1016/s0896-6273(00)80165-8. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, López-García JC. Molecular physiology of kainate receptors. Physiol Rev. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- Mathers DA, Usherwood PNR. Concanavalin A blocks desensitization of glutamate receptors on insect muscle fibres. Nature. 1976;259:409–411. doi: 10.1038/259409a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Crystal structures of the GluR5 and GluR6 ligand binding cores: molecular mechanisms underlying kainate receptor selectivity. Neuron. 2005;45:539–552. doi: 10.1016/j.neuron.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Vyklicky L. Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989;86:1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions; a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci U S A. 2005;102:1708–1713. doi: 10.1073/pnas.0409573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naur P, Vestergaard B, Skov LK, Egebjerg J, Gajhede M, Kastrup JS. Crystal structure of the kainate receptor GluR5 ligand-binding core in complex with (S)-glutamate. FEBS Lett. 2005;579:1154–1160. doi: 10.1016/j.febslet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Smith TC, Howe JR. Concentration-dependent substate behavior of native AMPA receptors. Nat Neurosci. 2000;3:992–997. doi: 10.1038/79931. [DOI] [PubMed] [Google Scholar]
- Stephenson R. A modification of receptor theory. Br J Pharmacol Chemother. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. J Physiol. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Green T, Sakai R, Contractor A, Che W, Kamiya H, Heinemann SF. Differential activation of individual subunits in heteromeric kainate receptors. Neuron. 2002;34:589–598. doi: 10.1016/s0896-6273(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tygesen CK, Rasmussen JS, Jones SV, Hansen A, Hansen K, Andersen PH. Stable expression of a functional GluR6 homomeric glutamate receptor channel in mammalian cells. Proc Natl Acad Sci U S A. 1994;91:13018–13022. doi: 10.1073/pnas.91.26.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]
- Yue K-T, MacDonald JF, Pekhletski R, Hampson DR. Differential effects of lectins on recombinant glutamate receptors. Eur J Pharmacol. 1995;291:229–235. doi: 10.1016/0922-4106(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]