Abstract
Vascular endothelial growth factor (VEGF) is the principal agent that increases microvascular permeability during physiological and pathological angiogenesis. VEGF is differentially spliced to form two families of isoforms: VEGFxxx, and VEGFxxxb. Whereas VEGF165 stimulates angiogenesis, VEGF165b is anti-angiogenic. To determine the effect of VEGF165b on permeability, hydraulic conductivity (Lp) was measured in individually perfused microvessels in the mesentery of frogs and rats. As with VEGF165, VEGF165b increased Lp in amphibian (2.4 ± 0.3-fold) and mammalian (1.9 ± 0.2-fold) mesenteric microvessels. A dose–response relationship showed that VEGF165b (EC50, 0.65 pm) was approximately 25 times more potent than VEGF165 (EC50, 16 pm) in amphibian microvessels. VEGF165 has been shown to increase permeability through VEGF receptor 2 (VEGF-R2) signalling. However, VEGF165b increased Lp of frog vessels to the same extent in the presence of the VEGF-R2 inhibitor ZM323881, indicating that it does not increase permeability via VEGF-R2 signalling, and was inhibited by the VEGF receptor inhibitor SU5416 at doses that are specific for VEGF receptor 1 (VEGF-R1). VEGF165b, in contrast to VEGF165, did not result in a sustained chronic increase in Lp. These results show that although VEGF165b is anti-angiogenic in the mesentery, it does signal in endothelial cells in vivo resulting in a transient, but not sustained, increase in microvascular Lp, probably through VEGF-R1.
The vascular endothelial growth factor (VEGF) isoform VEGF165 is a potent angiogenic factor that increases microvascular permeability both acutely (Bates & Curry, 1996; Wu et al. 1996; Fu & Shen, 2003, 2004) and chronically (Bates & Curry, 1996). VEGF is differentially spliced from eight exons to form multiple isoforms termed according to their amino acid number. Differential splicing of exons 6 and 7 results in isoforms of different heparin binding affinity (Houck et al. 1991), whereas differential splicing of exon 8 results in two families of isoforms which differ only in their terminal six amino acids (Bates et al. 2002). The pro-angiogenic, vasodilatory VEGFxxx (or conventional) isoforms are formed by proximal splice site selection in exon 8, resulting in an open reading frame of six amino acids being translated. The anti-angiogenic (or inhibitory) isoforms are formed by distal splice site selection 66 bases further into exon 8, and result in the translation of mRNA to protein from a different open reading frame, albeit of the same length (18 bases), resulting in proteins of the same size as the conventional isoforms. This family has been termed VEGFxxxb (Perrin et al. 2005). This family of isoforms was originally identified in human renal cortex (Bates et al. 2002), and has subsequently been shown to be widely expressed in human tissues at the mRNA (Bates et al. 2002) and protein levels (Woolard et al. 2004; Perrin et al. 2005; Y Qiu, unpublished data). The most studied isoform, VEGF165b, has been shown to bind to the VEGF receptor 2 (VEGF-R2), but not stimulate phosphorylation in heterologous expression systems, and inhibits VEGF165-mediated phosphorylation in human umbilical vein endothelial cells (HMVECs) (Woolard et al. 2004). VEGF165b inhibits VEGF165-induced endothelial cell proliferation and migration in vitro, arterial vasodilatation ex vivo and angiogenesis in vivo (Woolard et al. 2004). For these reasons, it has been termed an ‘inhibitory’ isoform. However, it was noted that in HMVECs, although VEGF-R2 was not phosphorylated, activation of downstream signalling pathways could be activated by VEGF165b, as Mitogen activated protein kinase (MAPK) and Akt were both phosphorylated after activation with VEGF165b.
The other major action of VEGF165 is its ability to increase dye extravasation in animal models. Macromolecular extravasation depends on many parameters, including measures of vascular permeability such as oncotic reflection coefficient (σ), hydraulic conductivity (Lp) and solute permeability (Pa), and hence VEGF is known as vascular permeability factor (Senger et al. 1983). VEGF165 has been widely implicated in the increased permeability of cancers (Senger et al. 1983), diabetic retinopathy (Tolentino et al. 1996), age-related macular degeneration (Schwesinger et al. 2001) and other angiogenic pathologies (Kunstfeld et al. 2004). Specifically, VEGF165 has been shown to increase vascular hydraulic conductivity and solute permeability in frog and rat microvessels (Bates & Curry, 1996; Wu et al. 1996; Fu & Shen, 2003, 2004). VEGF165b is also highly expressed in tissues that have a high hydraulic conductivity to water, such as the choroid plexus (HS Bevan, unpublished), and the renal glomerulus (Woolard et al. 2004), particularly by the podocytes surrounding the fenestrated glomerular endothelium (Cui et al. 2004). Therefore the aim of this study was to determine whether VEGF165b was able to increase permeability in a similar manner to VEGF165.
Methods
Materials
Male Wistar rats (350–600 g) were bred in-house. Male frogs (Rana temporaria, 6–8 cm) were supplied by Blades (UK). All regulated procedures were carried out under licence from the Home Office in accordance with local and national ethical guidelines and with the Animals (Scientific Procedures) Act 1986. Rat erythrocytes were collected by cardiac puncture from male Wistar rats anaesthetized with 5% halothane. All animals were humanely killed at the end of the procedure. Recombinant human VEGF165 and recombinant human VEGF165b were supplied by R & D Systems. ZM323881 and SU5416 were purchased from Calbiochem. All other chemicals were purchased from Sigma. Reagents were all water-soluble except ZM323881 and SU5416, which were soluble in dimethylformamide (DMF, final concentration in perfusate was 0.0001% for ZM323881 and 0.005% for SU5416). DMF does not change the basal permeability even at concentrations as high as 0.01%.
Rats were anaesthetized by intramuscular injection of ketamine (Vetalar, 25 mg ml−1), medetomidine (Domitor, 0.25 mg ml−1) and sterile water (1: 1: 2 v/v/v). The dose administered was 100 μl (100 g body weight)−1. A pre-operative dose was administered 5–10 min before the laparotomy. Anaesthesia was maintained by subcutaneous injection of ketamine–medetomidine into the nape of the animal's neck as required (approximately every hour). Throughout the experiment the upper surface of the rat mesentery was superfused with physiological mammalian Ringer solution containing (mm): NaCl 132, KCl 4.6, MgSO41.27, CaCl22, NaHCO325, d-glucose 5.5, Hepes acid 3.07 and 1.9 Hepes sodium salt, pH corrected to 7.45 ± 0.02 with 0.115 m NaOH at 37°C, if required. At the end of the experiment, the animals were killed by cervical dislocation while still under anaesthesia.
In vivo mesenteric microvascular preparation
Frogs were anaesthetized by immersion in 1.1 mg ml−1 3-aminobenzoic acid ethyl ester (MS222) in tap water. At the end of the experiment, they were killed by destruction of the brain and central nervous system Anaesthesia was maintained by superfusing the mesentery with 0.3 mg ml−1 MS222 in physiological frog Ringer solution containing (mm): NaCl 111, KCl 2.4, MgCl2 1, CaCl2 1.1, NaHCO3 0.2, d-glucose 5.5, Hepes acid 2.63 and Hepes sodium salt 2.37; pH corrected to 7.40 ± 0.02 with 0.115 m NaOH. A laparotomy was performed and the ileum was gently teased out with a moist cotton bud and draped over a transparent quartz pillar so that the mesentery could be visualized through a Leitz inverted microscope. Experiments were recorded using a video camera (Pulnix) connected through an electronic timer (FOR-A, Chesington, UK) to a video recorder (Panasonic). Experiments with frogs were performed at room temperature (20–22°C), and rat experiments were performed at 37°C.
Measurement of Lp
Lp was measured using the Landis–Michel technique (Michel et al. 1974) as previously described by us (Pocock & Bates, 2001). A straight true capillary or postcapillary venule (in frog: diameter, 15–40 μm; in rat: diameter, 15–30 μm) was selected that had flowing blood, was free of side branches for at least 800 μm and had no leucocytes adhering to the vessel wall. The vessel was cannulated with a bevelled glass micropipette connected to a manometer and perfused with 1% bovine serum albumin (BSA) in physiological Ringer solution containing rat erythrocytes as flow markers (baseline solution). Baseline Lp was measured for all vessels before the experiment was performed. Vessels with a baseline Lp > 10 × 10−7 cm s−1 cmH2O−1 were rejected. The pipette was refilled with test solutions as previously described (Hillman et al. 2001). In frogs, all experiments were performed at a pressure of 30 cmH2O, and pressures between 45 and 55cmH2O were used in rats. Any drugs used were made as stock solutions in water or vehicle before being diluted in the baseline solution. Downstream from the cannulation site, a pulled glass micropipette was used to occlude the vessel for approximately 5 s. The vessel was allowed to flow freely for at least 5 s between occlusions.
Administration of VEGF
Perfusion with VEGF was brought about by refilling the pipette perfusion system during perfusion with 1 ml VEGF (1 nm) as described in the Results. As soon as possible after the pipette had been refilled (and within 15 s) the vessel was occluded, and Lp recorded. Lp was measured every 15 s for up to 5 min. In some experiments, the pipette was refilled with control solution and the vessel perfused for at least 20 min to wash out any VEGF, and allow time for recovery (previously shown to require at least 15 min) (Bates & Curry, 1996). The pipette was then refilled with VEGF isoforms, and Lp measured again.
Calculation of Lp
The radius of the vessel (r), velocity of the marker cells (dL/dt) approximately 2 s after occlusion and the length (l) between the marker cell and the occlusion site were measured off-line from the video recording. Fluid flux per unit area (Jv/A) was calculated as:
| (1) |
Lp was calculated from the Starling equation (Eqn 2). Where ΔP was the hydrostatic pressure difference, Δπ the oncotic pressure difference between the capillary lumen and the interstitium, and σ the oncotic reflection coefficient. The hydrostatic pressure difference was calculated as the pressure in the manometer during occlusion (the capillary pressure) minus the interstitial pressure, which was previously shown to be zero (Kajimura et al. 2001). The effective oncotic pressure (σΔπ) of 1% BSA solution is 3.6 cmH2O.
| (2) |
Measurement of Lp, on day 2
Chronic Lp was measured as previously described (Bates, 1998). Briefly, after the final occlusion during measurement of Lp in response to application of VEGF165b or VEGF165, or perfusion with control solution (1% BSA), a map of the mesenteric microvascular architecture was made. The gut was then replaced in the body cavity of the frog and the skin and body wall sutured. The animal was then allowed to recover, the water changed and the frog kept at room temperature. After 24 h (day 2), the frog was anaesthetized as above and the mesentery exposed. The same vessel as cannulated before was found from the map. The Lp was measured as above for baseline solutions (1% BSA). The criteria for acceptable measurement of Lp on day 2 were essentially the same as those on day 1: the vessel must have freely moving red cells in it and no white cells adhering to and occluding the vessel. The differences between criteria for day 1 and day 2 were: there was no maximum Lp, there must have been at least 400 μm between the previous cannulation site and the last block site and microvessels with sluggish or intermittent flow on day 2 were accepted. Lp measurements were taken after perfusion for 15 min with 1% BSA. At the end of the experiment, frogs were killed by destruction of the brain and central nervous system (by crushing the cranium), while still unconsciousness.
Statistics
Baseline values are expressed as the mean Lp during the time perfused. Agonist perfusions are expressed as the peak (highest) Lp value during the first 60-s exposure and the mean Lp between 60 and 200-s exposure. The summary data for Lp are expressed as the medians ± semi-interquartile range unless stated otherwise, and normalized data (Lp divided by mean baseline Lp for that vessel) as mean ± s.e.m. The fold increase is the mean of the individual maximal increases, not the mean maximal increase divided by the mean baseline.
A Friedman test followed by a Dunn's post hoc test was used to compare Lp measurements for multiple comparisons of paired data, and a Kruskal–Wallis test was used for multiple comparisons of unpaired data. To compare Lp measurements for single comparisons, non-parametric paired (Wilcoxon) or unpaired (Mann Whitney U) tests were used. The Spearman rank correlation test was used to determine the significance of correlation between data groups.
Results
The anti-angiogenic VEGF165b isoform increases microvascular permeability of mesenteric microvessels
To determine the effects of VEGF165b on vessel permeability, frog vessels were perfused with 1% BSA until the baseline Lp was stable and then the pipette was refilled with 1 nm VEGF165b and the Lp measured approximately every 10 s for up to 5 min. Figure 1A shows a representative example from a single vessel of the changes in Lp over 200 s. An immediate transient increase in Lp was measured that rapidly returned to baseline within 1 min.
Figure 1. VEGF165b increases microvascular permeability in frog mesenteric microvessels.
A, a single vessel showing a representative increase in Lp in response to 1 nm VEGF165b. B, box plot showing Lp during basal conditions with 1% BSA, peak Lp during the first 60 s of exposure to 1 nm VEGF165b and mean Lp during 60–200 s with 1 nm VEGF165b. The box indicates the median and the 25% and 75% quartiles and the error bars denote the range. VEGF165b (1 nm) significantly increased the Lp during the first 60-s exposure (Friedman test, P < 0.0001, n = 20). C, paired Lp measurements in the same vessel under basal conditions (BSA) and the peak Lp during the first 60 s of exposure to VEGF165b. A total of 17 out of 20 of the vessels were classified as responders.
Figure 1B shows the summary data of the first 20 frog vessels that were exposed to VEGF165b. As the time course for the increase in Lp was reproducible, the data are expressed as the mean basal Lp with 1% BSA, the peak Lp during the first 60 s of exposure to VEGF165b and the mean Lp following the transient response (60–200 s after the initial exposure of VEGF165b). VEGF165b (1 nm) increased the Lp 2.4 ± 0.3-fold (mean ± s.e.m) from 0.9 ± 0.8 × 10−7 cm s−1 cmH2O−1 in the presence of 1% BSA to a peak Lp of 2.5 ± 1.2 × 10−7 cm s−1 cmH2O−1 during the first 60 s of perfusion with VEGF165b. The Lp after perfusion with VEGF165b for 60 s was 0.9 ± 0.7 × 10−7 cm s−1 cmH2O−1 (P < 0.0001, Friedman test, n = 20. BSA versus VEGF165b, P < 0.001; VEGF165b within 60 s versus VEGF165b after 60 s, P < 0.001, Dunn's post hoc multiple comparison test.) Figure 1C shows the paired data for these 20 vessels; 17 out of 20 of these vessels were classified as responders (i.e. the peak Lp in the presence of VEGF165b was greater than the mean basal Lp plus two standard deviations).
The experiments described above were performed in frog vessels; however, it is unclear as to whether or not frogs have the VEGF165b isoform therefore the experiments were repeated in rat mesenteric microvessels to verify the results in frog. The same protocol and analysis were applied to the experiments in rat vessels. Figure 2A is an example of a single vessel that was exposed to VEGF165b. A transient increase in Lp was measured that peaked within 60 s confirming the results shown in Fig. 1. The time course of the responses was slightly more variable in the rat but the peak Lp still occurred within the initial 60 s of exposure to VEGF165b. The summary data in rat vessels are shown in Fig. 2B. VEGF165b increased the Lp in rat vessels 1.9 ± 0.2-fold (mean ± s.e.m) from a baseline of 2.2 ± 1.1 × 10−7 cm s−1 cmH2O−1 with 1% BSA to a peak Lp of 3.4 ± 2.3 × 10−7 cm s−1 cmH2O−1 within the first 60 s of perfusion with 1 nm VEGF165b. The Lp then fell to 2.4 ± 0.6 × 10−7 cm s−1 cmH2O−1 after 60 s. There was a significant difference between the Lp values of these data sets (P < 0.01, Friedman test, n = 8). The Lp during the first 60 s of perfusion with VEGF165b was significantly increased above the basal Lp level with 1% BSA (P < 0.05, Dunn's post hoc test) and the Lp following the VEGF165b-induced transient response (P < 0.05, Dunn's post hoc test). The paired data for these eight rat vessels is shown in Fig. 2C. A total of 7 out of 8 of the rat vessels were classified as responders, showing an increased Lp compared with the baseline (BSA control).
Figure 2. VEGF165b increases microvascular permeability in rat mesenteric microvessels.
A, a single vessel showing a representative VEGF165b-induced increase in Lp in rat. B, box plot showing Lp during basal conditions with 1% BSA, peak Lp during the first 60 s of exposure to 1 nm VEGF165b and mean Lp during 60–200 s with 1 nm VEGF165b. The box indicates the median and the 25% and 75% quartiles and the error bars denote the range. VEGF165b (1 nm) significantly increased the Lp during the first 60 s of exposure (Friedman test, P < 0.01, n = 8). C, paired Lp measurements in the same vessels during basal conditions (BSA) and peak Lp during the first 60 s of exposure to VEGF165b. A total of 7 out of 8 of the vessels were classified as responders.
Is VEGF165b comparable to VEGF165?
Our next aim was to determine whether the response to VEGF165b was the same as to conventional VEGF165. As VEGF165 has been extensively studied in this laboratory in frog microvessels (Bates et al. 2001; Pocock & Bates, 2001; Whittles et al. 2002; Pocock et al. 2004; Glass et al. 2005) and VEGF165b caused a similar response in frog and rat vessels, the next set of experiments were performed in frog vessels. A total of 14 frog vessels were perfused with either 1 nm VEGF165 or 1 nm VEGF165b and the Lp was measured. Following no more than 5 min of perfusion with the agonist, the vessels were washed out with 1% BSA for at least 20 min (with at least two refillings of the pipette with 1% BSA). The basal Lp was measured once again before perfusion of the VEGF165b isoform and the Lp changes were measured.
Of the 14 vessels, half responded to both VEGF165 and VEGF165b, three vessels responded only to VEGF165b, one vessel responded only to VEGF165 and three vessels did not respond to either isoform. The results are summarized in Table 1. The discrepancies were not due to the order of the perfusions.
Table 1.
Not all vessels respond equally to VEGF165 and VEGF165b
| Number of vessels | |
|---|---|
| Total number of vessels to receive both treatments | 14 |
| Respond to VEGF165 and VEGF165b | 7 |
| Only respond to VEGF165b | 3 |
| Only respond to VEGF165 | 1 |
| Respond to neither VEGF165 nor VEGF165b | 3 |
An example of a single vessel that responded to both VEGF165 and VEGF165b is shown in Fig. 3A. VEGF165 (1 nm) was tested first and caused approximately a 3-fold increase in Lp (Fig. 3Aa). The vessel was washed for 20 min then perfused with 1 nm VEGF165b. Again, an approximate 3-fold increase in Lp was measured (Fig. 3Ab). The data were reproducible and so they could be expressed as time averaged data as shown in Fig. 3B. The data were averaged into 10-s time bins. Both VEGF165 (▴) and VEGF165b (□) caused a transient increase in Lp that peaked within the first 10 s of exposure.
Figure 3. Comparison of the effects of VEGF165b and VEGF165 in frog vessels.
A, representative Lp measurements from a single vessel that demonstrated an increase in Lp to both 1 nm VEGF165 (Aa) and 1 nm VEGF165b (Ab). B, frog vessels treated with either VEGF (▴) or VEGF165b (□). The data were averaged into 10-s time bins and expressed as mean ± s.e.m for each time point. The value y = 1 denotes no change from basal Lp. Note the second peak in Lp at 45 s when treated with VEGF165. C, there was no significant difference between the fold increase in Lp (mean ± s.e.m) with 1 nm VEGF165, 1 nm VEGF165b or 1 nm both isoforms (P > 0.05, Kruskal–Wallis test).
The normalized increases in Lp were examined for 1 nm VEGF, 1 nm VEGF165b and 1 nm both VEGF165 and VEGF165b. The summary data are shown in Fig. 3C. VEGF165 caused a 1.7 ± 0.2-fold increase (mean ± s.e.m, n = 16) in Lp, VEGF165b increased the Lp 3.1 ± 0.6-fold (n = 22) and the combination of VEGF165 and VEGF165b increased Lp 2.2 ± 0.5-fold (n = 9). There was no significant difference between these three groups (P > 0.05, Kruskal–Wallis test) indicating that there was no additive effect of VEGF165 and VEGF165b at these concentrations.
Dose dependency of the effect of VEGF165 and VEGF165b in frog microvessels
The percentage of vessels that responded to 1 nm VEGF165 (56%, 9 out of 16 vessels) was less than the percentage that responded to 1 nm VEGF165b (77%, 17 out of 22 vessels), although not significantly so (P > 0.1, Fisher's exact test). Therefore the potency of VEGF165b was compared to that of VEGF165 in frog vessels. Figure 4A shows an example of a vessel that was perfused with 1 pm VEGF165. Even at this low concentration, VEGF165 appeared to cause a small increase in the Lp in this example. Although on average this change was not greater than that measured when the pipette was refilled with 1% BSA, the timing of the peak response (immediately after refilling, which by itself does not cause an apparent increase in permeability) indicates that this was a response to VEGF165. At 1 pm, VEGF165b was still able to increase Lp above the control levels. The example shown in Fig. 4B demonstrated an increase in Lp that was comparable to that caused by 1 nm VEGF165b (see Fig. 1B).
Figure 4. The effect of VEGF165 and VEGF165b at lower concentrations in frog vessels.
A, a single vessel that gave a small increase in Lp when treated with 1 pm VEGF165. B, an example of a single vessel that responded to 1 pm VEGF165b. The peak Lp measured in this vessel was comparable to the peak Lp measured in vessels that were exposed to 1 nm VEGF165b.
Concentrations from 10 fm to 1 nm of VEGF165 or VEGF165b were perfused through frog vessels so that a dose–response curve could be plotted for both VEGF isoforms. A minimum of five vessels was used for each concentration. Figure 5A shows the dose–response curve for VEGF165 (▴) and VEGF165b (□) in frog vessels. There was a significant difference between the two curves (P < 0.005, F-test) even though there was no significant difference between the maximum responses at a concentration of 1 nm (Fig. 5A). The dose–response was normalized relative to the responses at the maximum and minimum concentrations as shown in Fig. 5B. There was a clear leftward shift in the dose–response curve for VEGF165b compared with VEGF165. The EC50 for VEGF165 was 1.6 × 10−11m (16 pm) whereas the EC50 for VEGF165b was significantly less at 6.5 × 10−13m (0.65 pm) (P < 0.05, F-test). This demonstrates that VEGF165b was approximately 25 times more potent than VEGF165 in frog in this system.
Figure 5. VEGF165b is a more potent vascular permeability factor than VEGF165.
A, VEGF165 (▴) and VEGF165b (□) increase Lp in frog microvessels in a concentration-dependent manner. B, the data from A normalized to the maximum and minimum responses. The dashed lines indicate the EC50 (VEGF165: EC50, 1.6 × 10−11; VEGF165b: EC50, 6.5 × 10−13, P < 0.05, F-test.) Data are expressed as means ± s.e.m.
The VEGF165b-induced increase in permeability is not mediated through VEGF-R2
VEGF165 has been shown to increase permeability (and specifically Lp) through VEGF-R2 in frogs (Whittles et al. 2002) and rats; therefore, our aim was to determine whether VEGF165b was also signalling through VEGF-R2 to increase permeability.
Frog vessels were perfused with 1% BSA until the basal Lp was stable, then the pipette was refilled with 1 nm VEGF165b and the Lp measured approximately every 10 s for up to 5 min. The vessels were washed out for 10 min with 1% BSA and then perfused with the specific VEGF-R2 inhibitor, ZM323881 (10 nm) for 10 min. Following pretreatment with ZM323881, the vessel was simultaneously perfused with ZM323881 and 1 nm VEGF165b for up to 5 min. This is the same protocol as used by Whittles et al. (2002) when determining whether VEGF165 was inhibited by ZM323881. In approximately half the vessels the inhibitor was given during the first VEGF165b perfusion instead of the second perfusion to rule out any differences that may occur over time. Figure 6A shows an example of a single vessel that was first exposed to VEGF165b alone (Fig. 6Aa). The vessel gave a representative increase in Lp to VEGF165b (approximately 2.5-fold increase). Following washout, the vessel was pretreated with 10 nm ZM323881 and then exposed to VEGF165b a second time in the presence of ZM323881 (Fig. 6Ab). Of all vessels tested, this vessel gave the greatest increase in Lp in the presence of ZM323881, providing a demonstration that VEGF165b does not signal through VEGF-R2. The data from 14 vessels was time averaged into 10-s time bins and is shown in Fig. 6B. VEGF165b and ZM323881 (▴) increased Lp with the same time course as VEGF165b alone (□).
Figure 6. VEGF165b increases Lp in the presence of VEGF-R2 inhibition.
A, a representative single frog vessel showing an increase in Lp with 1 nm VEGF165b in the absence (Aa) and presence (Ab) of VEGF-R2 inhibition with 10 nm ZM323881. This vessel gave the greatest response to VEGF165b in the presence of ZM323881. B, the time averaged data from 14 vessels that were treated with VEGF165b with and without ZM323881 (randomised order).
VEGF165b in the presence of ZM323881 increased the Lp 2.2 ± 0.3-fold (mean ± s.e.m) from a baseline Lp of 1.1 ± 0.8 × 10−7 cm s−1 cmH2O−1 with ZM323881 alone to a mean peak Lp of 1.9 ± 1.7 × 10−7 cm s−1 cmH2O−1 in the presence of ZM323881 and VEGF165b (Fig. 7A, P = 0.0001, n = 14, Wilcoxon test). The maximum increase (normalized to the baseline) is shown in Fig. 7B. In these 14 vessels, VEGF165b alone increased Lp 2.7 ± 0.4-fold (mean ± s.e.m). When the fold increases were compared to a refilling control, where the pipette was refilled with 1% BSA without agonist and analysed in the same way, both VEGF165b alone and VEGF165b with ZM323881 significantly increased the Lp above that of the refilling control (median of 1.37-fold increase, P < 0.005, Wilcoxon test). Figure 7C shows the paired data for the fold increases in Lp for VEGF165b alone and VEGF165b with ZM323881. There was no significant difference between the two data sets (P > 0.05, Wilcoxon test) showing that VEGF-R2 inhibition does not block the VEGF165b-induced increase in Lp.
Figure 7. VEGF165b increases Lp to the same magnitude even in the presence of VEGF-R2 inhibition.
A, box plot showing Lp during basal conditions in the presence of 10 nm ZM2323881 and peak Lp during the first 60 s of exposure to 1 nm VEGF165b with ZM323881. The box indicates the median and the 25% and 75% quartiles and the error bars denote the range (*P = 0.0001, Wilcoxon test, n = 14). B, mean ± s.e.m of the peak Lp during perfusion with 1 nm VEGF165b alone, 1 nm VEGF165b and 10 nm ZM323881 (n = 14) and the control refilling with 1% BSA (in a separate set of vessels, n = 6). *P < 0.005 versus a median of 1.37 (BSA control), Wilcoxon test. C, paired data from 14 vessels that were exposed to VEGF165b in the presence and absence of ZM323881. There was no significant difference between the magnitude of the Lp increase with either treatment (P > 0.05, Wilcoxon test).
The VEGF165b-induced increase in permeability is mediated through VEGF-R1
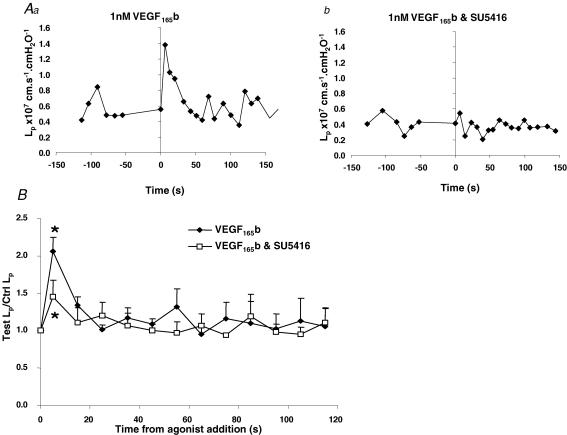
The VEGF receptor inhibitor SU5416, has been shown to inhibit VEGF-R1 phosphorylation with an IC50 of 90 nm, whereas the IC50 for VEGF-R2 was 1300 nm (Tille et al. 2003). Therefore we used this inhibitor to determine whether VEGF165b could increase Lp through VEGF-R1. Using the same protocol as described for ZM323881, Lp was measured in response to VEGF165b in the presence or absence of 200 nm SU5416. Figure 8Aa shows the effect of VEGF165b on a single vessel, and Fig. 8Ab shows that this response was abolished in the same vessel in the presence of SU5416. Figure 8B shows the mean results from seven vessels exposed to VEGF165b in the presence or absence of SU5416. Although VEGF165b increased Lp 2.3 ± 0.2-fold in these vessels in the absence of SU5416, this effect was significantly (P < 0.05, Wilcoxon paired test) attenuated by 200 nm SU5416. VEGF165b in the presence of SU5416 increased the Lp 1.45 ± 0.22-fold (mean ± s.e.m) to a mean peak of 2.3 ± 1.0 × 10−7 cm s−1 cmH2O−1 from a mean baseline of 1.2 ± 0.5 × 10−7 cm s−1 cmH2O−1 with SU5416 alone (Fig. 9A, P < 0.02, n = 7, Wilcoxon test). The results normalized to the baseline are shown in Fig. 9B. In these seven vessels, the mean ±s.e.m. peak increase induced by VEGF165b alone was 2.1 ± 0.2-fold (mean ± s.e.m) but in the presence of SU5416 1.78 ± 0.15 fold (mean ± s.e.m). Both VEGF165b alone and VEGF165b with SU5416 significantly increased the Lp above the refilling control with 1% BSA (median 1.37, P < 0.02, Wilcoxon test) indicating that 200 nm SU5416 attenuated but did not completely inhibit the Lp increase. Figure 9C shows the paired data for the fold increases in Lp for VEGF165b alone and VEGF165b with SU5416. There was a significantly reduced response in the presence of 200 nm SU5416 between the two data sets (P < 0.05, Wilcoxon test) showing that VEGF-R1 inhibition attenuates the VEGF165b-induced increase in Lp.
Figure 8. The VEGF165b-induced increase in Lp is inhibited by VEGF-R1 inhibition.
A, a representative single frog vessel showing an increase in Lp with 1 nm VEGF165b in the absence (Aa) and presence (Ab) of VEGF-R1 inhibition with 200 nm SU5416. B, the time averaged data from seven vessels that were treated with VEGF165b with and without SU5416 (randomised order).
Figure 9. The VEGF165b-induced increase in Lp is attenuated in the presence of VEGF-R1 inhibition.
A, box plot showing Lp during basal conditions in the presence of 200 nm SU5416 and peak Lp during the first 60 s of exposure to 1 nm VEGF165b with SU5416. The box indicates the median and the 25% and 75% quartiles and the error bars denote the range (*P < 0.05, Wilcoxon test, n = 7). B, mean ± s.e.m of the peak Lp during perfusion with 1 nm VEGF165b alone, VEGF165b and SU5416 (n = 7) and the control refilling with 1% BSA (in a separate set of vessels, n = 6). *P < 0.05 versus a median of 1.37 (BSA control), Wilcoxon test. C, paired data from seven vessels that were exposed to VEGF165b in the presence and absence of SU5416. There was a significant difference between the magnitude of the Lp increase with SU5416 treatment (P < 0.02, Wilcoxon test).
The VEGF165b-induced increase in permeability is not sustained chronically
We have previously described a biphasic permeability response to VEGF165 – a rapid transient increase that lasts a few seconds, and a sustained chronic response that lasts for 24 h, but resolves over 48 h. It is this sustained chronic response that results in the significant fluid flux during VEGF exposure. To determine whether VEGF165b could induce this chronic increase in permeability, mesenteric microvessels of frogs were perfused with VEGF165b, VEGF165 or BSA, and the permeability measured again. After 24 h, the permeability was measured in the same vessel (Fig. 10). In five vessels that increased permeability from mean ±s.e.m. of 3.2 ± 1.08 to 6.15 ± 1.68 × 10−7 cm s−1 cmH2O−1 VEGF165b did not significantly increase the permeability 24 hours later (mean 3.3 ± 0.5 × 10−7 cm s−1 cmH2O−1, P > 0.05, Friedman test followed by Dunnet's post hoc test), in contrast to VEGF165 (from 3.5 ± 0.7 to 20.9 ± 6.1 × 10−7 cm s−1 cmH2O−1, P < 0.001, Friedman test followed by Dunnet's post hoc test), whereas perfusion with control solution gave an increase from 2.9 ± 0.4 to 6.1 ± 1.7 × 10−7 cm s−1 cmH2O−1, n = 23 that did not reach significance (P > 0.1 Friedman test followed by Dunnet's post hoc test). These results show that VEGF165b, in contrast to VEGF165, does not increase Lp in a sustained and chronic manner.
Figure 10. : VEGF165b does not chronically increase Lp.
Bar chart showing Lp during basal conditions before perfusion with 1% BSA (control), VEGF165 or VEGF165b (□) and 24 h after a 10-min perfusion with test solution (BSA, ▪). The mean sustained Lp measured in the vessel, was not significantly different in control or VEGF165b-perfused vessels, but was significantly different in VEGF165-perfused vessels as previously described (P < 0.001, Dunnett's post hoc test).
Discussion
The novel VEGF isoform VEGF165b increases vascular permeability in vivo
This is the first demonstration that VEGF165b can stimulate a physiological change in intact vessels. VEGF165b acutely increased Lp in amphibian (frog, Fig. 1) and mammalian (rat, Fig. 2) mesenteric microvessels. Furthermore, the acute Lp response to VEGF165b seen in these vessels was similar in time course to that measured in response to conventional VEGF165 as previously demonstrated by us (Bates & Curry, 1996; Pocock et al. 2000; Pocock & Bates, 2001; Pocock et al. 2004). These results raise a number of questions concerning the physiological role of VEGF165b. The alternatively spliced anti-angiogenic family of isoforms are widely expressed throughout normal adult and fetal tissues, yet, unlike the VEGFxxx family, do not stimulate angiogenesis or increase vasodilatation. A number of roles for the VEGFxxxb family of isoforms can be postulated, including prevention of VEGF-induced angiogenesis, maturation of blood vessels or maintenance of normal permeability. The finding here that VEGF165b can transiently increase the Lp of intact microvessels was a surprising result. Care should be taken not to over-interpret these results, as a rapid transient increase in permeability to water in a vessel that has been acutely exposed to VEGF165b may not have the drastic consequences that administration of VEGF165 does. Lp is a very sensitive measure of fluid flux across a vessel wall, and does not measure permeability to solutes or protein flux, as has been the case for the majority of VEGF165 studies. It has yet to be seen whether VEGF165b can reduce the oncotic reflection coefficient in intact vessels, or alter the diffusive permeability to large molecules (Bates & Curry, 1996; Bates, 1998; Bates et al. 2001). VEGF165b does not cause arterial vasodilatation, which will automatically limit its permeabilizing effect as the increased delivery of fluid and solutes to tissues depend upon increased blood flow, which will not be forthcoming with VEGF165b. Moreover, VEGF-R2 activation by VEGF-C results in a chronic, sustained increase in permeability that occurs 20 min to 1 h after the initial transient increase. This event requires the initial signalling event, and is a result of downstream signalling of VEGF-R2 through NO production (Bates, 1998). VEGF165b does not appear to act through VEGF-R2, and therefore is unlikely to stimulate NO production, as the other VEGF receptor present in this tissue, VEGF-R1, through which VEGF165b works, does not activate endothelial NO synthase (eNOS) when stimulated.
VEGF165b is more potent than VEGF165
Experiments were performed to compare the dose–response curves of VEGF165- and VEGF165b-mediated increases in permeability in frog vessels as shown in Fig. 5. When the curves were normalized to the maximum and minimum concentrations, there was a clear leftward shift in the VEGF165b curve compared to the VEGF165 curve. The EC50 for VEGF165b was significantly less than that for VEGF165, demonstrating that VEGF165b is more potent in frog mesenteric vessels. The increased potency of VEGF165b may have fundamental importance for the regulation of Lp, particularly in vascular beds that produce both isoforms. For instance, the renal podocytes appear to have greater levels of VEGF165b than VEGF165 (Cui et al. 2004) and, therefore, the effect of VEGF165b on glomerular Lp requires further investigation. The very low EC50 for VEGF165b (0.65 pm) is significantly lower than the binding affinity of VEGFR2, the primary signalling receptor, for VEGF (75 pm Terman et al. 1992). It is closer to the binding affinity of the other primary VEGF receptor present in mesenteric microvessels, VEGF-R1 for VEGF165 (10 pm (Keyt et al. 1996)). The affinity of VEGF165b to VEGF-R2 is known to be equivalent to VEGF165, but its affinity with VEGF-R1 is not known.
VEGF165b does not signal an increase in permeability through VEGF-R2
We have previously demonstrated with the specific VEGF-R2 inhibitor, ZM323881, that VEGF165-induced increases in Lp are mediated through VEGF-R2 activation (Whittles et al. 2002), and this is in agreement with other studies showing that VEGF165 acts primarily through VEGF-R2 in endothelial cells in vivo (Brekken et al. 2000). In order to compare VEGF165b signalling to VEGF165 signalling we have used the same protocol as before. To our surprise, VEGF-R2 inhibition did not block the increase in permeability mediated by VEGF165b (Figs 7 and 8). Neither the magnitude nor the time course of the permeability increase was altered (Figs 6 and 7). The presence of VEGF-R2 and VEGF-R1 in frog tissues, including mesentery, has been confirmed by Western blot (Hillman et al. 2001). The mesentery has comparable levels of VEGF-R1 protein to lung; however, the levels of VEGF-R2 are greatly reduced compared to lung. Western blots are at best semiquantitative but there appears to be more VEGF-R1 than VEGF-R2 in frog mesentery. As a result, VEGF-R1 seems a likely target for VEGF165b signalling, and may explain why a greater proportion of the vessels responded to VEGF165b. The finding that a VEGF receptor inhibitor used at doses not thought to inhibit VEGF-R2 but to inhibit VEGF-R1 attenuates the response to VEGF165b is consistent with VEGF165b acting through VEGF-R1.
VEGF-R1 has been described as a decoy receptor for VEGF165 and negatively regulates vasculogenesis and angiogenesis during development (Yang et al. 2002). Mice that are deficient in VEGF-R1 die before birth due to severe cardiovascular defects (Fong et al. 1995). Activation of VEGF-R1 by VEGF165 promotes cell migration but not proliferation (Neufeld et al. 1999), therefore VEGF-R1 appears to have a role in organizing the blood vessels during development and is not simply a decoy receptor.
Placental growth factor (PlGF), a member of the VEGF superfamily, has been shown to signal through VEGF-R1 but not through VEGF-R2 (Sawano et al. 1996). PlGF has been shown to increase Lp in frog mesenteric microvessels (Hillman et al. 2001) demonstrating that permeability can be increased via receptors other than VEGF-R2 and probably VEGF-R1. Stacker et al. (1999) made a mutant form of VEGF, VEGF0, where the V3 domain of VEGF165 was replaced with the V3 domain of PlGF. VEGF0 was unable to bind to VEGF-R2, as assessed by BIAcore analysis, but retained its ability to bind to VEGF-R1. Using the Miles assay, they showed that VEGF0 was able to increase leakage of Evans Blue dye from dermal microvessels. They concluded that VEGF0 increased vascular permeability. The Miles assay does not, however, control for other haemodynamic factors such as increased blood flow due to vasodilatation, which is another VEGF165-induced effect (Bates et al. 1999), but increased Lp would increase Evans Blue solute flux in this assay. In another study, it was demonstrated that PlGF and a VEGF-R1-specific mutant of VEGF165 were unable to induce an increase in extravasation of Evans Blue dye suggesting that there was no increase in vascular permeability (Gille et al. 2001). However, upon closer examination of their methods it was found that the length of time that the microvessels were exposed to PlGF and the VEGF165 mutant were greater than our own of 60 min. The increase in permeability we measured occurred within 1 min of VEGF165b exposure. Furthermore, the permeability increase mediated by PlGF (Hillman et al. 2001) in this system occurred in seconds and had returned to basal levels by 20 min. PlGF did not increase permeability chronically but retained the ability to increase permeability acutely, whereas VEGF-R2 activation by VEGF-C resulted in a sustained chronic increase after 20 min. This could account for the discrepancy between our results and those by Gille and colleagues.
VEGF165b does not cause a sustained increase in permeability
The rapid transient increase in Lp that we measured in response to VEGF165b is unlikely to result in a large efflux of fluid, or tissue overhydration (oedema), as it is very transient. This would also be the case for the transient increase caused by VEGF165. VEGF165 has, however, been shown to cause large sustained increases in fluid (Strugar et al. 1994) and solute flux (Senger et al. 1983; Senger et al. 1986), probably due to the sustained increase in permeability that persists as long as 24 hours (Bates & Curry, 1996). This increase in permeability in single microvessels 24 h after a short bolus of VEGF165 (10 min) is a result of the VEGF165 signalling cascade that causes the initial transient (Bates, 1998). We show here that this chronic sustained increase in permeability does not occur in response to VEGF165b. This is in keeping with previous studies showing that activation of VEGF-R2 by VEGF-C, but not VEGF-R1 by PlGF, results in a sustained chronic increase in permeability (Hillman et al. 2001), suggesting that the two initial transients result in differing downstream permeability events. It also indicates that VEGF165b release is unlikely to result in oedema, increased vascular permeability or dye leakage in systems where VEGF165b is highly expressed, for example in the normal eye (Perrin et al. 2005).
VEGF165b has biological activity
Previous studies have shown that VEGF165b was predominantly inhibitory on VEGF-R2 signalling, endothelial migration, proliferation, angiogenesis and vasodilatation (Woolard et al. 2004). The only assay in which VEGF165b did stimulate any activity was measurement of MAPK and Akt phosphorylation in endothelial cells (which did contain VEGF-R1) (HS Beran, unpublished observations). Therefore it appears that VEGF165b is not simply an inhibitory isoform of VEGF, although it clearly is an anti-angiogenic isoform, but has signalling properties of its own, probably through VEGF-R1.
In summary we describe here that the anti-angiogenic, antivasodilatatory isoform of VEGF, VEGF165b, stimulates a transient increase in Lp, probably through VEGF-R1 activation. This demonstrates for the first time, a physiological effect of VEGF165b in intact microvessels in vivo and its mechanism of action, although its functional significance remains to be determined. This indicates that VEGF-R1 has an endogenous activating ligand – VEGF165b – in addition to PlGF.
Acknowledgments
This work was supported by the Wellcome Trust (69029), the British Heart Foundation (BB 2000003) and the Richard Bright VEGF Research Trust. The authors would like to acknowledge the technical assistance of Ms Leslie Sage.
References
- Bates DO. The chronic effect of vascular endothelial growth factor on individually perfused frog mesenteric microvessels. J Physiol. 1998;513:225–233. doi: 10.1111/j.1469-7793.1998.225by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- Bates DO, Curry FE. Vascular endothelial growth factor increases hydraulic conductivity of isolated perfused microvessels. Am J Physiol. 1996;271:H2520–H2528. doi: 10.1152/ajpheart.1996.271.6.H2520. [DOI] [PubMed] [Google Scholar]
- Bates DO, Heald RI, Curry FE, Williams B. Vascular endothelial growth factor increases Rana vascular permeability and compliance by different signalling pathways. J Physiol. 2001;533:263–272. doi: 10.1111/j.1469-7793.2001.0263b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6:83–96. [PubMed] [Google Scholar]
- Brekken RA, Overholser JP, Stastny VA, Waltenberger J, Minna JD, Thorpe PE. Selective inhibition of vascular endothelial growth factor (VEGF) receptor 2 (KDR/Flk-1) activity by a monoclonal anti-VEGF antibody blocks tumor growth in mice. Cancer Res. 2000;60:5117–5124. [PubMed] [Google Scholar]
- Cui TG, Foster RR, Saleem M, Mathieson PW, Gillatt DA, Bates DO, Harper SJ. Differentiated human podocytes endogenously express an inhibitory isoform of vascular endothelial growth factor (VEGF165b) mRNA and protein. Am J Physiol Renal Physiol. 2004;286:F767–F773. doi: 10.1152/ajprenal.00337.2003. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Fu BM, Shen S. Structural mechanisms of acute VEGF effect on microvessel permeability. Am J Physiol Heart Circ Physiol. 2003;284:H2124–H2135. doi: 10.1152/ajpheart.00894.2002. [DOI] [PubMed] [Google Scholar]
- Fu BM, Shen S. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc Res. 2004;68:51–62. doi: 10.1016/j.mvr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Gille H, Kowalski J, Li B, LeCouter J, Moffat B, Zioncheck TF, Pelletier N, Ferrara N. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276:3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- Glass CA, Pocock TM, Curry FE, Bates DO. Cytosolic Ca2+ concentration and rate of increase of the cytosolic Ca2+ concentration in the regulation of vascular permeability in Rana in vivo. J Physiol. 2005;564:817–827. doi: 10.1113/jphysiol.2005.083220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman NJ, Whittles CE, Pocock TM, Williams B, Bates DO. Differential effects of vascular endothelial growth factor-C and placental growth factor-1 on the hydraulic conductivity of frog mesenteric capillaries. J Vasc Res. 2001;38:176–186. doi: 10.1159/000051044. [DOI] [PubMed] [Google Scholar]
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Kajimura M, Wiig H, Reed RK, Michel CC. Interstitial fluid pressure surrounding rat mesenteric venules during changes in fluid filtration. Exp Physiol. 2001;86:33–38. doi: 10.1113/eph8602106. [DOI] [PubMed] [Google Scholar]
- Keyt BA, Berleau LT, Nguyen HV, Chen H, Heinsohn H, Vandlen R, Ferrara N. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271:7788–7795. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, et al. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Michel CC, Mason JC, Curry FE, Tooke JE, Hunter PJ. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974;59:283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- Pocock TM, Bates DO. In vivo mechanisms of vascular endothelial growth factor-mediated increased hydraulic conductivity of Rana capillaries. J Physiol. 2001;534:479–488. doi: 10.1111/j.1469-7793.2001.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock TM, Foster RR, Bates DO. Evidence of a role for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1015–H1026. doi: 10.1152/ajpheart.00826.2003. [DOI] [PubMed] [Google Scholar]
- Pocock TM, Williams B, Curry FE, Bates DO. VEGF and ATP act by different mechanisms to increase microvascular permeability and endothelial [Ca2+]i. Am J Physiol Heart Circ Physiol. 2000;279:H1625–H1634. doi: 10.1152/ajpheart.2000.279.4.H1625. [DOI] [PubMed] [Google Scholar]
- Sawano A, Takahashi T, Yamaguchi S, Aonuma M, Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7:213–221. [PubMed] [Google Scholar]
- Schwesinger C, Yee C, Rohan RM, Joussen AM, Fernandez A, Meyer TN, et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am J Pathol. 2001;158:1161–1172. doi: 10.1016/S0002-9440(10)64063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- Stacker SA, Vitali A, Caesar C, Domagala T, Groenen LC, Nice E, Achen MG, Wilks AF. A mutant form of vascular endothelial growth factor (VEGF) that lacks VEGF receptor-2 activation retains the ability to induce vascular permeability. J Biol Chem. 1999;274:34884–34892. doi: 10.1074/jbc.274.49.34884. [DOI] [PubMed] [Google Scholar]
- Strugar J, Rothbart D, Harrington W, Criscuolo GR. Vascular permeability factor in brain metastases: correlation with vasogenic brain edema and tumor angiogenesis. J Neurosurg. 1994;81:560–566. doi: 10.3171/jns.1994.81.4.0560. [DOI] [PubMed] [Google Scholar]
- Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tille JC, Wang X, Lipson KE, McMahon G, Ferrara N, Zhu Z, et al. Vascular endothelial growth factor (VEGF) receptor-2 signaling mediates VEGF-C(ΔNΔC)- and VEGF-A-induced angiogenesis in vitro. Exp Cell Res. 2003;285:286–298. doi: 10.1016/s0014-4827(03)00053-3. [DOI] [PubMed] [Google Scholar]
- Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, Ferrara N, Adamis AP. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–1828. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- Whittles CE, Pocock TM, Wedge SR, Kendrew J, Hennequin LF, Harper SJ, Bates DO. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002;9:513–522. doi: 10.1038/sj.mn.7800164. [DOI] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- Yang S, Toy K, Ingle G, Zlot C, Williams PM, Fuh G, Li B, de Vos A, Gerritsen ME. Vascular endothelial growth factor-induced genes in human umbilical vein endothelial cells: relative roles of KDR and Flt-1 receptors. Arterioscler Thromb Vasc Biol. 2002;22:1797–1803. doi: 10.1161/01.atv.0000038995.31179.24. [DOI] [PubMed] [Google Scholar]