Abstract
Prenatal stress (PS) and maternal exposure to exogenous glucocorticoids can lead to permanent modification of hypothalamo-pituitary-adrenal (HPA) function and stress-related behaviour. Both of these manipulations lead to increased fetal exposure to glucocorticoids. Glucocorticoids are essential for many aspects of normal brain development, but exposure of the fetal brain to an excess of glucocorticoids can have life-long effects on neuroendocrine function. Both endogenous glucocorticoid and synthetic glucocorticoid exposure have a number of rapid effects in the fetal brain, including modification of neurotransmitter systems and transcriptional machinery. Such fetal exposure permanently alters HPA function in prepubertal, postpubertal and ageing offspring, in a sex-dependent manner. Prenatal stress and exogenous glucocorticoid manipulation also lead to the modification of behaviour, brain and organ morphology, as well as altered regulation of other endocrine systems. It is also becoming increasingly apparent that the timing of exposure to PS or synthetic glucocorticoids has tremendous effects on the nature of the phenotypic outcome. Permanent changes in endocrine function will ultimately impact on health in both human and animal populations.
The ability of the early environment to modify hypothalamo-pituitary-adrenal (HPA) function in adulthood was described nearly 50 years ago (Levine, 1957). However, more recent human epidemiological evidence indicating that the fetal and early postnatal environment can influence susceptibility to later disease (Barker, 2002) has rejuvenated the field. Such observational studies have revealed that plasma cortisol levels in adulthood are correlated with birth weight and risk for glucose intolerance, hypertension, and dyslipidaemia (metabolic syndrome) (Levitt et al. 2000; Barker, 2002; Ward et al. 2004b). As a result of these associations, it has been proposed that programming of the HPA axis in utero is linked to the development of cardiovascular disease, insulin resistance and diabetes in later life (Ward et al. 2004a; Phillips et al. 2005).
A considerable array of manipulations in early development have been shown to permanently modify the development and subsequent function of HPA function in newborn, juvenile and adult offspring of many species. These include primates, guinea pigs, sheep, cattle, goats, pigs, rats and mice. Broadly, these can be split into prenatal and postnatal manipulations. Examples of prenatal manipulation are prenatal stress (PS), exposure to synthetic glucocorticoids and nutrient restriction. Postnatal manipulations that modify HPA function in adult offspring include neonatal handling, maternal deprivation, modified maternal behaviour, exposure to synthetic glucocorticoids and infection. In this review, focus will be placed on the impact of PS and prenatal exposure to synthetic glucocorticoids on HPA function after birth. These manipulations have considerable clinical implication given the prevalence of maternal anxiety/stress during pregnancy and the incidence of preterm labour which necessitates the use of antenatal glucocorticoid therapy to promote fetal lung maturation.
Why do mammals have the ability to programme HPA function and behaviour in their offspring? This is best conceptualized in animal populations. A pregnant animal exposed to a hostile environment requires increased vigilance (e.g. high predation) for survival. It is therefore logical for a ‘stress’ signal to be transmitted to the fetus which by programming development of the fetal HPA axis and stress-related behaviour leads to an enhanced ability to survive after birth. As humans, perhaps we have inherited a sophisticated mechanism to adapt our offspring to the environment into which they are to be born. However, if this process is set in motion by a compromised or modified pregnancy (e.g. placental insufficiency, stress, nutrient restriction or glucocorticoid treatment), whether or not this is related to the environment into which the fetus will be born, the outcome will be modification of endocrine, behavioural, cardiovascular and metabolic regulation.
HPA axis development
The timing of maturation of the HPA axis relative to birth is highly species specific and is linked to landmarks of brain development (Dobbing & Sands, 1979). In animals that give birth to mature young (primates, sheep and guinea pigs) maximal brain growth and a large proportion of neuroendocrine maturation takes place in utero (Dobbing & Sands, 1979; Matthews, 1998; Challis et al. 2000). In contrast, in species that give birth to immature young (rats, rabbits and mice), much neuroendocrine development occurs in the postnatal period. As a result, fetal/neonatal manipulations will impact on different stages of neuroendocrine development depending on the species studied.
Rapid maturation of HPA function occurs during fetal life in many mammalian species (for review, see Challis et al. 2000; Matthews, 2002). There are exponential increases in fetal plasma cortisol concentrations in the last 10 days of gestation in humans, horses, pigs, sheep and guinea pigs (Fowden et al. 1998). Increases in fetal ACTH precede those of cortisol, consistent with the role of adrenocorticotrophin (ACTH) in adrenal development (Challis et al. 2000; Owen & Matthews, 2003). Fetal plasma ACTH and corticosterone concentrations also increase significantly in species that give birth to immature young (rats and mice), but changes are of a lesser magnitude. In these rodents, a critical phase of HPA development occurs after birth: the stress–hyporesponsive period (Sapolsky & Meaney, 1986). It is likely that this phase occurs prenatally or perinatally in precocious species.
The near-term elevation of fetal plasma ACTH concentrations in the presence of high circulating glucocorticoid is somewhat paradoxical, as glucocorticoids are known to negatively feedback on HPA axis function in the fetal sheep and guinea pig (Fig. 1) (Matthews & Challis, 1995; Unno et al. 1998; McCabe et al. 2001). Detailed analysis has identified increased central drive at the level of the fetal paraventricular nucleus (corticotrophin-releasing hormone and arginine vasopressin) in late gestation (Myers et al. 1993; Matthews & Challis, 1995; Butler et al. 1999), and this coincides with an alteration in glucocorticoid feedback sensitivity at a number of levels within the HPA axis (Andrews & Matthews, 2000; Owen & Matthews, 2003; Setiawan et al. 2004). The prepartum surge of circulating fetal glucocorticoid is vital for the development of many organ systems including the lung, liver and kidney (Liggins, 2000). This surge is also critical for normal brain and neuroendocrine development. Indeed, we have recently identified that there is a very dramatic elevation in transcriptional activity (inducible nerve growth factor A (NGFI-A) expression) in the hippocampus in the approach to term and that this significantly correlates with fetal plasma cortisol concentrations (Fig. 2). Further, premature exposure of the fetal brain to glucocorticoids increases hippocampal NGFI-A expression, suggesting that glucocorticoids are involved in pre-birth hippocampal activation (Andrews et al. 2004). The functional significance of this process remains to be determined.
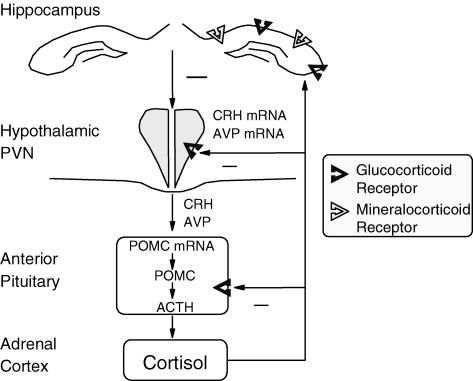
Figure 1. The hypothalamo-pituitary-adrenal (HPA) axis.
Parvocellular neurons in the PVN produce corticotrophin-releasing hormone (CRH) and vasopressin (AVP), which in turn stimulate adrenocorticotrophin (ACTH) synthesis and release from the anterior pituitary corticotroph cells. ACTH then initiates the production and release of cortisol from the adrenal cortex. Glucocorticoids act at multiple loci within the body to maintain homeostasis, but also act in the brain to modify behaviour and learning. Due to the damaging effects of extended glucocorticoid exposure the HPA axis is tightly regulated. Glucocorticoids feedback, via glucocorticoid and mineralocorticoid receptors in the limbic system and glucocorticoid receptors in the PVN and anterior pituitary, to decrease HPA activity. POMC, pro-opimelanocortin.
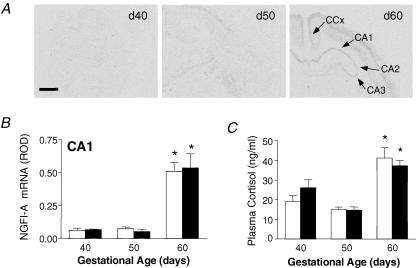
Figure 2.
A, representative expression of inducible nerve growth factor A (NGFI-A) mRNA in coronal sections of fetal guinea pig hippocampus at gestational days (d) 40, 50 and 60. NGFI-A mRNA expression is shown in hippocampal subfields (CA1, CA2 and CA3), and cingulate cortex (CCx). Scale bar: 1.5 mm. B, relative levels of NGFI-A mRNA expression in the hippocampus (CA1) in female (open bars) and male (filled bars) fetuses in the second half of gestation. *Significant (P < 0.05) differences compared to previous gestational age. C, plasma cortisol concentrations in female (open bars) and male (filled bars) fetuses in late gestation. *Significant (P < 0.05) differences compared to previous gestational age. Adapted from Andrews et al. (2004) with permission from Blackwell Publishing Ltd.
Development of glucocorticoid receptors and mineralocorticoid receptors
In guinea pigs, glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) mRNAs are present in the fetal cortex and all regions of the hippocampus and dentate gyrus by gestational day 40 (term ∼70 days). There is a dramatic increase in hippocampal GR mRNA between gestational days 40 and 50, but a decrease in MR mRNA levels indicating differential developmental regulation (Matthews, 1998; Owen & Matthews, 2003). After 50 days of gestation, hippocampal GR mRNA and protein levels increase to a peak at term, with little further change in MR. Given that there is a reduction in glucocorticoid feedback sensitivity in late gestation, an increase in hippocampal GR expression is counterintuitive. However, recently we have identified that other aspects of steroid receptor signalling may be altered at this time. There is a significant decrease in the expression of steroid receptor coactivator-1 (SRC-1) mRNA and protein over the second half of gestation in the fetal guinea pig hippocampus (Setiawan et al. 2004). Steroid receptor coactivators enhance the transcriptional activity of a number of nuclear receptors through histone acetyltransferase activity and the recruitment of other basal transcription machinery (Spencer et al. 1997; Meijer et al. 2000; Meijer, 2002). A decrease in SRC-1 levels may contribute to increased fetal HPA activity in late gestation by decreasing glucocorticoid signalling in the hippocampus even in the presence of rising GR levels. In the paraventricular nucleus (PVN), GR mRNA levels are higher at gestational day 40 than at any other stage of fetal or postnatal life, directly contrasting the situation in the hippocampus. Near term, there is a dramatic reduction (50%) in GR mRNA in the guinea pig (Matthews, 1998; Owen & Matthews, 2003). We have shown a similar decrease in GR mRNA in the fetal sheep PVN near term (Andrews & Matthews, 2000). Such a decrease in GR expression in the PVN would also act to reduce glucocorticoid feedback sensitivity, facilitating the exponential increase in fetal plasma ACTH and cortisol concentrations that occurs near term.
The profile of central MR and GR expression is different in species that give birth to immature young. In the rat, GR and MR in the brain are low throughout gestation, but increase rapidly after birth, consistent with the postnatal nature of brain and HPA development in this species (Diaz et al. 1998). However, there are distinct developmental profiles for GR and MR in the fetal rat brain. GR mRNA is present in the hippocampus, hypothalamus and pituitary by gestational day 13 (term ∼21 days), and levels increase around term. In contrast, hippocampal MR is not detectable until gestational days 16–17 (Diaz et al. 1998; Kretz et al. 2001).
GR mRNA is present in the metanephros, gut, muscle, spinal cord and dorsal root ganglia, periderm, sex chords of the testis, and adrenal of the human embryo by 8–10 weeks of life (Costa et al. 1996; Condon et al. 1998). High levels of GR mRNA are present in the human fetal lung by 12 weeks of gestation. Unfortunately, there is no information regarding developmental changes in GR expression in the human fetus later in gestation or at any stage in the developing fetal brain. In summary, it is clear that normal development of GR and MR expression follows an ordered temporal and spatial profile, and that this is highly species-specific.
Programming of the HPA axis: prenatal stress
Guinea pigs
Early studies demonstrated that a single maternal exposure (3 h) to a strobe light stressor on day 60 of gestation (term ∼70 days) in the guinea pig resulted in adult offspring with reduced basal and activated HPA function (Cadet et al. 1986). We have shown that an acute period (48 h) of maternal nutrient restriction during the period of maximal fetal brain growth (day 50 of gestation) in the guinea pig, results in young adult offspring with modified HPA function in adult life and that this effect is highly sex-specific (Lingas & Matthews, 2001). Restriction of maternal nutrient intake (48 h) resulted in a 50% decrease in fetal plasma glucose concentrations and intrauterine growth restriction (IUGR). This treatment significantly increases maternal cortisol secretion (Lingas et al. 1999), which in turn reaches the fetus and modifies fetal HPA activity (Lingas et al. 1999; Go et al. 2001). Adult male guinea pigs born to nutrient-restricted mothers exhibit reduced basal and stress-induced HPA activity. In contrast, young adult female offspring from the same litters exhibited elevated basal and activated HPA function (Lingas & Matthews, 2001). In a more recent study, we have demonstrated that short focused exposure of maternal psychological stress (high frequency strobe light; 3 × 2 h), at critical times of fetal brain development, result in adult offspring that exhibit altered HPA regulation; however, more importantly the effects are highly specific to the stage of gestation at which pregnant mothers were exposed to stress (Kapoor & Matthews, 2005). Twenty-four hour plasma sampling revealed that adult male offspring born to mothers exposed to PS (3 × 2 h) on gestational days 50–52 (maximal brain growth) exhibited elevated basal adrenocortical activity, while those born to mothers exposed to PS on gestational days 60–62 exhibited normal basal adrenocortical activity but heightened adrenocortical responsiveness to challenge (Fig. 3). Interestingly, the adult males born to mothers exposed to stress at gestational day 50 also exhibited a 70% reduction in plasma testosterone concentrations (Kapoor & Matthews, 2005). Testosterone has an inhibitory effect on HPA axis function by decreasing arginine vasopressin (AVP) levels in the median eminence (Viau & Meaney, 2004). It is possible that the elevation in basal adrenocortical activity in offspring whose mothers were exposed to PS on gestational day 50 is in part mediated by the decrease in plasma testosterone in these animals. Studies are currently underway to determine the influences of PS at various stages of gestation on HPA function in female offspring.
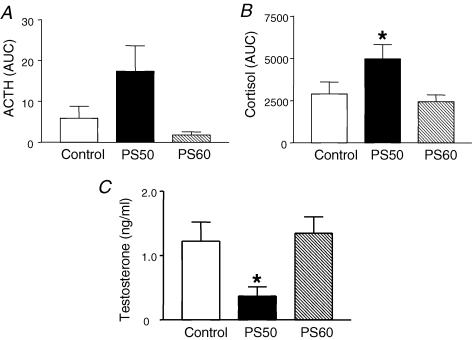
Figure 3.
Twenty-four hour plasma ACTH (A) and cortisol (B) concentrations (area under the curve, AUC; mean ± s.e.m.) in male offspring born to mothers that were undisturbed throughout pregnancy (Control), exposed to a strobe light on gestational days 50–52 (PS50) or gestational days 60–62 (PS60) *P < 0.05 PS50 versus control. C, plasma testosterone concentrations (mean ± s.e.m.) in the same male offspring *P < 0.05 PS50 versus control. Adapted from Kapoor & Matthews (2005) with permission from Blackwell Publishing Ltd.
Rats
In rat offspring, PS is generally associated with increased peak and/or extended pituitary–adrenal response duration, though there are many variations to this general pattern (Weinstock, 2005; Koenig et al. 2005). The majority of studies have been undertaken in male offspring and this has represented a limitation. It is now emerging that very significant sex differences in endocrine outcome exist following PS and other early manipulations of the fetal environment. Basal plasma ACTH and corticosterone concentrations are elevated in female rats born to PS dams (Peters, 1982; McCormick et al. 1995; Weinstock et al. 1998; Ward et al. 2000), and this is associated with adrenal hypertrophy (Ward et al. 2000). However, other studies have failed to report increased basal HPA activity following PS (Kay et al. 1998). These differences between studies may result from differences between the PS protocols adopted as well as variation in the time of day when blood samples were taken. In this regard, PS has been shown to cause a phase shift in the circadian corticosterone rhythm in adult offspring (Koehl et al. 1997, 1999).
Few studies have compared the impact of PS on HPA activity in male and female offspring using identical prenatal protocols and results have been variable (Weinstock et al. 1992; McCormick et al. 1995; Koehl et al. 1999; Szuran et al. 2000). Basal ACTH, but not corticosterone, is elevated in adult female offspring born to prenatally stressed dams and pituitary–adrenocortical responsiveness to stress is elevated in these animals (McCormick et al. 1995). In contrast, basal HPA activity is not different and responses to stress are reduced in male offspring born to PS mothers (McCormick et al. 1995). Another study, utilizing a similar PS protocol has reported increased basal and stress-stimulated corticosterone concentrations in adult female but not male offspring (Szuran et al. 2000). A recent study has extended these observations, showing that corticosterone responses to stress are increased in female but not male PS offspring, and that habituation to repeated stress is prevented in male offspring whose mothers were exposed to PS (Bhatnagar et al. 2005). Interestingly, no habituation was identified in females in either control or PS offspring. Studies in the rat indicate that the female HPA axis may be more susceptible to PS-induced programming. It is also clear that there is tremendous variability in HPA outcome following PS in male offspring. This is not surprising given the wide spectrum of experimental approaches used to induce stress in the pregnant dams and the variability in methods used to activate HPA function in the offspring. With respect to the latter, activation of distinct central neuronal pathways are known to be stress-specific (Herman et al. 1996; Pacak & Palkovits, 2001). The development of each of these pathways is probably affected in a unique fashion by PS. Interestingly, a recent study has demonstrated that PS over the last 4 days of gestation results in young adult male offspring that exhibit reduced plasma testosterone concentrations (Gerardin et al. 2005). While adrenal weight was shown to be decreased in these animals HPA function was not assessed. The reduction in testosterone following PS is similar to that described in the guinea pig above (Kapoor & Matthews, 2005), though the relationship to altered HPA function is less clear.
Primates and other species
Prenatal stress in pregnancy has been shown to modify HPA function in offspring of other species, including primates, cows, goats and pigs. In the monkey, juvenile offspring whose mothers were repeatedly exposed to unpredictable noise during mid- to late gestation (days 90–145) in a novel cage exhibited higher plasma ACTH levels under both basal and stressed conditions and significantly elevated basal plasma cortisol concentrations (Clarke et al. 1994). A more recent study has determined that juvenile monkeys born to mothers exposed to 6 weeks of PS (daily acoustic startle) in early (gestational days 50–92) or late (gestational days 105–147) exhibit increased basal cortisol concentrations and reduced inhibition following overnight dexamethasone suppression (Coe et al. 2003). Interestingly, there did not appear to be an effect of the timing of PS exposure in this study. In calves whose mothers were subjected to transport stress during pregnancy, clearance of cortisol was slower than in controls and pituitary weights significantly increased (Lay et al. 1997a,b). In goats, repeated transport in the last third of pregnancy had no effect on basal cortisol concentrations in goat offspring but did significantly influence the sympatho-adrenomedullary system (Roussel et al. 2005). In pigs, stimulation of maternal cortisol release by weekly maternal ACTH injections in pregnant sows for 6 weeks resulted in offspring (60 days of age) with a higher adrenal cortex: medulla ratio. Under stressful conditions, the pigs in the maternal ACTH-treated group also showed significantly higher plasma cortisol responses (Haussmann et al. 2000). A more recent study has shown that the HPA outcome in pigs following prenatal manipulation of maternal glucocorticoid concentrations is dependent on the timing of exposure (Kranendonk et al. 2005). Offspring (6 weeks old) whose mothers were treated with hydrocortisone in mid-gestation exhibited elevated basal salivary cortisol levels, while those exposed in early and late gestation exhibited normal basal cortisol but attenuated responses to an ACTH challenge. These studies again indicate that the timing of manipulation is critical. Maternal social stress during pregnancy in pigs results in female offspring (67 days of age) that exhibit elevated salivary cortisol responses to social mixing (Jarvis et al. 2006). The effects of PS on domestic species are becoming increasingly recognized as there are clearly implications for animal production efficiency.
Humans
While a number of studies have linked maternal stress/anxiety during pregnancy to behavioural deficits in children (for recent reviews, see Weinstock, 2005; Austin et al. 2005; Van den Bergh et al. 2005), very few studies have considered the function of the HPA axis. A recent study undertaken in a sample of 10-year-old children from the Avon Longitudinal Study of Parents and Children (ALSPAC) has demonstrated for the first time a significant link between prenatal anxiety, particularly in late pregnancy, and individual differences in salivary cortisol (O'Connor et al. 2005). A relatively small study has also identified a link between maternal anxiety and salivary cortisol in children at 5 years of age. Children whose mothers exhibited higher levels of morning cortisol during pregnancy, and more fear of bearing a handicapped child, showed higher levels of salivary cortisol (Gutteling et al. 2005). The same group showed similar associations in another group of children at 4–6 years of age (Gutteling et al. 2004). Clearly further studies are required to fully understand the relationship between PS/anxiety during pregnancy and HPA function in human children and adults.
Altered central HPA regulation
Alterations of HPA activity following PS probably involves modification of regulation of central drive to the HPA axis. This may include altered expression of central GR and MR systems. Juvenile and adult rats born to PS mothers exhibit reduced hippocampal GR and MR expression which is associated with a marked increase in stress-level plasma corticosterone and a longer duration in corticosterone response (Henry et al. 1994; Maccari et al. 1995; Barbazanges et al. 1996; Koehl et al. 1997). Piglets whose mothers were subjected to PS (daily restraint, last 5 weeks of gestation) exhibit reduced hypothalamic GR binding sites but increased hippocampal GR sites with no effect on the hippocampal MR possibly indicating decreased negative feedback at the PVN and enhanced facilitation of the HPA response (Kanitz et al. 2003). Some studies also indicate the possibility that programming of GR and MR, like the HPA axis, is sex-specific with the effect of prenatal glucocorticoids being greater in female offspring. In a study in which rats were subjected to stress throughout pregnancy, only female offspring born to PS mothers showed significantly fewer hippocampal GR binding sites indicating reduced GR density (Weinstock et al. 1992). Lower hippocampal GR binding has implications for hippocampal negative feedback and termination of the pituitary–adrenal response following activation. When pregnant rats were stressed daily during the last week of pregnancy (days 15–19) by restraint, female offspring once again showed higher basal corticosterone levels and a lower density of hippocampal GR (Szuran et al. 2000). Interestingly, in our studies where 48 h maternal nutrient restriction (gestation day 50) was shown to modify HPA function in adult guinea pig offspring, there were no significant differences in MR and GR expression in the hippocampus or PVN, but a significant reduction of GR expression in the anterior pituitary (Lingas & Matthews, 2001). Together these data suggest that multiple mechanisms are involved in the programming of HPA function following maternal exposure to stress in pregnancy. Clearly further work is required to unravel these mechanisms.
How are PS effects transduced to the fetus
Maternal stress leads to numerous cardiovascular and endocrine changes in the mother, including increases in plasma ACTH, β-endorphin, glucocorticoid and catecholamine concentrations. The placenta forms a structural and biochemical barrier to many of these maternal factors, though a number will still enter the fetus. There may also be indirect effects on the fetus via modification of placental function. For example, maternal glucocorticoids will probably stimulate corticotrophin-releasing hormone (CRH) production by the placenta (in species where the placenta produces CRH) which will in turn also activate the fetal HPA axis (Challis et al. 2000). In addition, increased maternal catecholamine concentrations will lead to constriction of placental blood vessels and may lead to fetal hypoxia (Ohkawa et al. 1991), which will in turn activate the fetal HPA axis (Challis et al. 2000). It is also possible that fetal hypoxia will lead to activation of the fetal sympathetic nervous system, a system that has also been shown to be programmed by the early environment (for review see Young, 2002). Programming of the sympathetic nervous system and neurotransmitter systems in the brain will ultimately lead to altered physiological responses to stress in offspring.
While other factors are doubtless important, glucocorticoids have become a popular candidate for mediating the effects of PS on HPA function and behaviour after birth. Maternal and fetal plasma corticosterone are significantly elevated after maternal stress in rats (Takahashi et al. 1998; Koehl et al. 1999). In the guinea pig, cortisol concentrations in the maternal plasma are 10-fold those in the fetus; PS results in significant increases in plasma glucocorticoid concentrations in both mother and fetus (Dauprat et al. 1984; Cadet et al. 1986). Interestingly, an earlier study demonstrated that greater glucocorticoid transfer occurs across the placenta of female compared to male fetuses (Montano et al. 1993). This may account, in part, for the increased effects of PS in female offspring. It is also known that there are increases in basal maternal HPA activity and plasma glucocorticoid concentrations in many species in late gestation (Challis et al. 2000; Dunn et al. 2005). While changes in maternal plasma corticosteroid-binding globulin (CBG) will, in part, compensate by binding the increased circulating cortisol, maternal free cortisol concentrations do increase significantly in the second half of gestation (Dunn et al. 2005). In addition, elegant studies have shown a reduced maternal responsiveness to stress in late gestation (Brunton et al. 2005; Ma et al. 2005). Together, increased basal maternal HPA activity and reduced responsiveness to stress may account for the timing-specific impact of PS on HPA function in offspring later in life.
Under normal circumstances, access of maternal endogenous glucocorticoid (corticosterone or cortisol) to the fetus is low. This results from the placental expression of 11β-hydroxysteroid dehydrogenase (11β-HSD) (Burton & Waddell, 1999). 11β-HSD interconverts cortisol and corticosterone to inactive products (cortisone, 11-dehydrocorticosterone). There are two isoforms, 11β-HSD type 1 which is bi-directional and, type 2 which is uni-directional (cortisol to cortisone). The efficiency of placental 11β HSD2 varies amongst species; however, it is generally accepted that placental 11β-HSD2 is of primary importance in excluding maternal endogenous glucocorticoid from the fetus. Indeed, it has been shown in the guinea pig that there is a decrease in placental 11β-HSD2 in the final approach to birth and this is associated with increased transfer of maternal cortisol to the fetus (Sampath-Kumar et al. 1998). The impact of PS on placental 11β-HSD2 expression has not been well characterized; however, a recent study has indicated that PS can influence placental 11β-HSD2 activity (Welberg et al. 2005). Also, placental 11β-HSD2 activity is reduced in human intrauterine growth-restricted (IUGR) pregnancies (McTernan et al. 2001).
A number of approaches have been used to determine the role of maternal glucocorticoids in the programming of HPA function during PS. In one study, pregnant rats were adrenalectomized and basal levels of corticosterone replaced. In this group, PS had no effect on HPA function in the adult offspring (Barbazanges et al. 1996), suggesting that maternal glucocorticoid, or a factor stimulated by glucocorticoids, passes to the fetus to mediate PS-induced changes in HPA function. Further support for the ‘glucocorticoid hypothesis’ has been provided by an elegant series of studies in which 11β-HSD activity was pharmacologically manipulated. Blockade of 11β-HSD during pregnancy, and therefore an increased transfer of glucocorticoid from mother to fetus, results in offspring that exhibit elevated basal and stress-stimulated HPA activity (for review see Seckl et al. 2000).
The fact that glucocorticoids play a central role in the programming of HPA function logically leads one to question the impact of synthetic glucocorticoid administration during pregnancy. Approximately 7% of pregnant women are at risk of preterm labour in the western world. These women are routinely treated with synthetic glucocorticoids at between 24 and 34 weeks gestation to reduce respiratory distress syndrome in newborns. The two most commonly administered synthetic glucocorticoids are betamethasone and dexamethasone. Although there have been no reports of adverse effects of a single dose of antenatal glucocorticoid treatment in children, evidence is emerging that multiple courses may have negative neurological outcomes later in postnatal life (for review see Andrews & Matthews, 2003). Surveys of obstetrical practice in Australia and the UK indicate that until recently repeated dose regimens have been common (Quinlivan et al. 1998; Brocklehurst et al. 1999). In North America, a National Institutes of Health consensus update conference (Gilstrap, 2001) recommended limiting the use of multiple doses to ongoing clinical trials and similar recommendations have been made in Europe.
Unlike the situation for endogenous glucocorticoids, synthetic glucocorticoids are not metabolized by placental 11β-HSD2. However, emerging evidence suggests that the multidrug resistance protein P-glycoprotein (P-gp) may play a role in limiting entry of maternally administered synthetic glucocorticoids to the fetus (Sun et al. 2005; Kalabis et al. 2005). Notwithstanding, synthetic glucocorticoids do enter the fetal circulation (Moss et al. 2003). It is important to identify the significant physiological differences between fetal exposure to increased endogenous glucocorticoid and synthetic glucocorticoids. Endogenous glucocorticoids bind to both GRs and MRs as described above, and effects on the fetal brain are probably mediated by both of these receptors. In contrast, synthetic glucocorticoids bind predominantly to the GR as the MR has low affinity for synthetic glucocorticoids. In addition to binding to classic GR, synthetic glucocorticoids may also bind to neurosteroid receptors in the brain (Kliewer et al. 1998).
Programming of the HPA axis: synthetic glucocorticoids
Guinea pigs
Administration of dexamethasone to pregnant guinea pigs during the period of maximal brain growth (gestational day 50; term ∼70 days) alters corticosteroid receptor expression in the developing limbic system in both a region- and a sex-specific manner (Dean & Matthews, 1999). We have shown that repeated antenatal dexamethasone exposure decreases fetal cortisol concentrations and hypothalamic CRH mRNA expression (McCabe et al. 2001). These studies indicate that synthetic glucocorticoids enter the fetal brain. Similar repeated maternal treatment with synthetic glucocorticoids programmes HPA function in young postpubertal adult guinea pig offspring in a sex-specific manner. Young adult male offspring (∼75 days of age) display reduced basal and activated plasma cortisol levels, associated with an increased hippocampal expression of MR mRNA (Liu et al. 2001). In contrast, adult female offspring exposed to the same prenatal treatment exhibit increased basal and activated plasma cortisol levels in the follicular and early luteal phases, while the effect is reversed in the late luteal phase (Liu et al. 2001). Plasma testosterone concentrations were almost doubled in young adult male offspring whose mothers were treated with glucocorticoids in late pregnancy (Liu et al. 2001). Testosterone inhibits HPA function (Viau, 2002), thus the reduction in HPA function that we have reported in adult male guinea pigs following synthetic glucocorticoid exposure is probably modulated, at least partially, by increased testosterone concentrations. Further studies are required to identify the impact of prenatal synthetic glucocorticoid treatment on development and subsequent function of the hypothalamo-pituitary-gonadal (HPG) axis, particularly with respect to the interaction between the HPG and HPA axes in both males and females. In more recent studies, we have shown that the effects of prenatal synthetic glucocorticoid exposure appear to change as a function of age. When male guinea pig offspring whose mothers had been treated in a similar fashion to that described above (Liu et al. 2001) were assessed at 150 days rather than in young adulthood (∼75 days), the effects on HPA function and feedback regulation were quite different. Basal plasma cortisol concentrations were not different between prenatal treatment groups and plasma testosterone concentrations were normalized. Interestingly, hippocampal MR mRNA remained elevated in animals that had been exposed to synthetic glucocorticoids in utero. Together these observations would suggest that the increased testosterone was an important factor in the inhibition of HPA activity in the males that had been prenatally exposed to dexamethasone (Banjanin et al. 2004). Also, the older animals became hypertensive, exhibiting increased mean arterial pressure. Hippocampal MR has been implicated in cardiovascular regulation, and we suggest that the increased MR mRNA observed in these animals may be, in part, responsible for the increase in blood pressure. Further studies are required to explore this possibility.
Rats
In the rat, daily treatment with synthetic glucocorticoids in the third week or throughout gestation (∼22 days) results in elevated basal plasma corticosterone levels in adult male offspring (Levitt et al. 1996; Welberg & Seckl, 2001). This is associated with increased blood pressure in adulthood. In another study, maternal synthetic glucocorticoid treatment on gestational days 17, 18 and 19 resulted in adult male offspring that show mount increased corticosterone responses to stress (Muneoka et al. 1997). More recently, a comprehensive study has determined in adult male offspring that prenatal synthetic glucocorticoid exposure leads to significant increases in basal morning (08.00 h) plasma ACTH and corticosterone concentrations whereas levels at the evening (20.00 h) diurnal peak were similar to controls. No effect of prenatal dexamethasone exposure on HPA function was observed in adult female offspring, indicating striking sex differences in the rat (O'Regan et al. 2004). Interestingly, synthetic glucocorticoid treatment on gestational days 17 and 19 did not alter basal HPA function in prepubertal offspring, though there were differences in the ratio of AVP and CRH in the median eminence of these young rats (Bakker et al. 1995), suggesting that programmed changes in HPA function may develop as animals mature postnatally.
The mechanisms that underlie the programming of adult HPA function are dependent on dose and timing of exposure (Welberg & Seckl, 2001). Adult male rat offspring whose mothers were exposed to synthetic glucocorticoid in the last week of gestation exhibit reduced hippocampal GR and MR expression and no change in GR expression in the PVN. The reduced hippocampal glucocorticoid feedback sensitivity in these animals is associated with increased levels of CRH mRNA in the PVN, and an overall elevation of HPA activity. In contrast, in offspring born to mothers treated daily with synthetic glucocorticoid throughout pregnancy there are no changes in hippocampal corticosteroid receptors, but increased MR and GR mRNA in the basolateral nucleus of the amygdala. From previous descriptions of the excitatory influence that central amygdaloid GR occupation may exert over HPA activity (Herman & Cullinan, 1997) these data would suggest that long-term exposure to synthetic glucocorticoids in utero may increase forward drive to the HPA axis (Welberg & Seckl, 2001). Glucocorticoid feedback may also be altered as a result of local free intrahippocampal cortisol concentrations. It has recently been shown that daily maternal treatment with dexamethasone over the last week of gestation results in an increase in intrahippocampal 11β-HSD1 concentrations in the first week of neonatal life (Wan et al. 2005). This enzyme amplifies the glucocorticoid effect by converting biologically inactive 11-ketone metabolites into active glucocorticoid. Whether the effect identified in newborn rats persists into adulthood remains to be determined. Another recent study has elegantly demonstrated that perinatal exposure to dexamethasone has profound effects on pituitary morphology in adult offspring. Fetal exposure to glucocorticoids resulted in a significant reduction in corticotroph number and granule migration, while effects in the early neonatal period were less profound (Theogaraj et al. 2005).
Primates and other species
A longitudinal analysis in sheep has also identified that prenatal synthetic glucocorticoid exposure is associated with alterations in HPA function that vary as a function of age (Sloboda et al. 2002). A single maternal injection of betamethasone on gestational day 104 had little effect on HPA function in offspring at 6 months of age but led to offspring with significantly elevated basal and stimulated plasma cortisol concentrations at 1 year of age. The fact that the effects of prenatal synthetic glucocorticoid change over time indicate the importance of studying outcome at several different times during the life course.
While a number of sophisticated studies undertaken in rats, sheep and guinea pigs have shown long-term profound effects on HPA function, care must be applied in extrapolation to the human due to variability in neurodevelopmental profiles. Limited studies have been undertaken to establish the long-term effects of fetal exposure to synthetic glucocorticoids on HPA function in the primate (Uno et al. 1994). Pregnant rhesus monkeys were treated daily with dexamethasone (4 × 1.25 mg kg−1) commencing on 132 days of gestation. Basal and stress-stimulated cortisol levels were elevated in the offspring (10 months of age) born to dexamethasone-treated mothers (Uno et al. 1994). Collectively, these studies demonstrate how short-term fetal exposure to synthetic glucocorticoids can effectively programme HPA function.
Humans
Virtually nothing is currently known about the impact of prenatal synthetic glucocorticoid exposure on HPA function in human infants, children or adults. A very recent report of follow-up in the original antenatal betamethasone trial indicates that there was no clinical effect on cardiovascular risk factors at 30 years of age, though there were indications of insulin resistance, particularly in women (Dalziel et al. 2005).
Transgenerational effects of prenatal glucocorticoid exposure
Recently, we have determined that repeated exposure to antenatal glucocorticoids leads to transgenerational effects on HPA function and behaviour (Kostaki et al. 2005). Female and male offspring (F2) born to mothers (F1) that had been exposed to synthetic glucocorticoid during the F0 pregnancy, but had themselves gone through an undisturbed pregnancy, exhibited significant reductions in basal and stress-stimulated salivary cortisol levels. Interestingly, reproductive capacity was significantly affected in the F1 females that had been exposed to synthetic glucocorticoid during fetal life (F0 pregnancy) (Dunn et al. 2005). Though, to our knowledge, no other studies have investigated transgenerational influences of prenatal synthetic glucocorticoid exposure on HPA function, another study has identified transgenerational influences on liver enzyme function (Drake et al. 2005). The mechanisms by which transgenerational programming takes place remain to be elucidated though they probably involve epigenetic effects along with altered maternal endocrine and cardiovascular adaptation to pregnancy. Further studies are clearly warranted.
Mechanisms of HPA programming
From the above discussion, it would appear that the programming of HPA function involves modification of glucocorticoid negative feedback at the level of the limbic system, hypothalamus and pituitary, but that the effect at each of these sites is dependent on the duration and timing of the manipulation. An elegant series of studies in the rat has provided further mechanistic insight on HPA programming. Handling of neonatal rats in the first 2 weeks of life results in elevated hippocampal GR, increased glucocorticoid negative feedback and a reduction in HPA activity when animals reach adulthood. This up-regulation of hippocampal GR appears to involve an elevation of serotonin (Meaney et al. 2000). Indeed, serotonin has been shown to up-regulate GR mRNA and binding in primary cultures of embryonic hippocampal cells from mice, rats and guinea pigs (Meaney et al. 2000; Erdeljan et al. 2001, 2005). This effect is mediated via serotonin (5-HT7) receptors and appears to be permanent involving altered methylation of the GR promoter (Meaney et al. 2000; Weaver et al. 2004). Interestingly, this effect can be pharmacologically reversed in adulthood (Weaver et al. 2005).
Neonatal handling also increases thyroid activity in the neonate, and thyroid hormone has been shown to increase serotonin turnover in the hippocampus. Therefore, it has been suggested that the ascending serotonin neurons are stimulated by thyroid hormones to increase hippocampal serotonin and thence GR expression (Meaney et al. 2000). Interestingly, exposure of the fetal guinea pig to synthetic glucocorticoid causes a significant increase in fetal plasma thyroid hormone concentrations and up-regulates GR mRNA (Dean & Matthews, 1999). Further, prenatal synthetic glucocorticoid exposure advances maturation of the serotonin transporter system in the developing rat brain (Slotkin et al. 1996, 2005). Together these data indicate that the ascending serotonergic system is probably involved in glucocorticoid-induced HPA programming during prenatal life. However, this is clearly not the only route by which prenatal glucocorticoid exposure influences HPA function. Many studies have identified influences of PS and synthetic glucocorticoid on other central neurotransmitter systems that are involved in the regulation of HPA function (Weinstock, 2005). Further, both PS and synthetic glucocorticoids have been shown to have a long-term impact on brain structures, particularly in the hippocampus, which may in turn influence HPA function (for review see Owen et al. 2005). As has been illustrated above, both PS and synthetic glucocorticoids can also influence the development and subsequent function of other neuroendocrine systems including the hypothalamo-pituitary-gonadal and the hypothalamo-pituitary-thyroid systems. Such modification will indirectly influence HPA function.
Clinical significance of programming the HPA axis
Alterations in the regulation of HPA activity throughout life will impact on adult health due to altered tissue exposure to endogenous glucocorticoids. Chronically elevated plasma cortisol has been associated with atherosclerosis, immunosuppression, depression and cognitive impairment, as well as elevated cholesterol levels and increased incidence of diabetes (Sapolsky et al. 2000; Lupien & Lepage, 2001). At the level of the CNS, it is possible that a compromise in the developing hippocampus (i.e. reduction of pyramidal neurons or alterations in axonal/dendritic processes and synaptogenesis), associated with synthetic glucocorticoid exposure, may decrease the age at which hippocampal deficits (associated with normal ageing) are first noted. Prenatally programmed increases in HPA function will exacerbate this hippocampal deficit, and in turn lead to further increases in HPA function (due to reduced glucocorticoid negative feedback; see Fig. 1). In this regard, there is a reduction in the efficiency of glucocorticoid feedback as humans age, resulting in extended HPA responses to stress and elevated exposure to cortisol. This has been linked to a reduction in hippocampal volume and impaired cognitive ability (Lupien & Lepage, 2001). For these reasons, follow-up studies in humans and animal models, to investigate the impact of PS and synthetic glucocorticoid exposure, may fail to identify significant functional deficits in the hippocampus until adulthood or early old age.
Conclusions
The prenatal environment has profound influences on endocrine function throughout life. These effects appear to be highly dependant on the timing of exposure. In this regard, we provide evidence that specific phases of brain development are probably more susceptible to the programming effects of an adverse intrauterine environment and/or prenatal exposure to glucocorticoids. Emerging evidence indicates that effects are highly sex-dependent reinforcing the importance of follow-up studies in males and females. Prolonged elevation of HPA function is detrimental and can lead to long-term pathologies including cardiovascular, metabolic and cognitive/behavioural dysfunction. The mechanisms of endocrine programming are complex involving multiple pathways and complex hormonal and neuronal interactions. Finally, it is clear that a number of dramatic events occur within the normal brain in the final approach to term, and further detailed studies are required to better understand the role of this final maturation in the function of the brain after birth.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP-49511) and the Natural Sciences and Engineering Council of Canada.
References
- Andrews MH, Kostaki A, Setiawan E, McCabe L, Owen D, Banjanin S, Matthews SG. Developmental regulation of the 5-HT7 serotonin receptor and transcription factor NGFI-A in the fetal guinea-pig limbic system: influence of GCs. J Physiol. 2004;555:659–670. doi: 10.1113/jphysiol.2003.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MH, Matthews SG. Regulation of glucocorticoid receptor mRNA and heat shock protein 70 mRNA in the developing sheep brain. Brain Res. 2000;878:174–182. doi: 10.1016/s0006-8993(00)02735-9. [DOI] [PubMed] [Google Scholar]
- Andrews MH, Matthews SG. Antenatal glucocorticoids: Is there cause for concern. Fetal Matern Med Rev. 2003;14:329–354. [Google Scholar]
- Austin MP, Leader LR, Reilly N. Prenatal stress, the hypothalamic-pituitary-adrenal axis, and fetal and infant neurobehaviour. Early Hum Dev. 2005;81:917–926. doi: 10.1016/j.earlhumdev.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Bakker JM, Schmidt ED, Kroes H, Kavelaars A, Heijnen CJ, Tilders FJH, Van Rees EP. Effects of short-term dexamethasone treatment during pregnancy on the development of the immune system and the hypothalamo-pituitary adrenal axis in the rat. J Neuroimmunol. 1995;63:183–191. doi: 10.1016/0165-5728(95)00152-2. [DOI] [PubMed] [Google Scholar]
- Banjanin S, Kapoor A, Matthews S. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–318. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13:364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Lee TM, Vining C. Prenatal stress differentially affects habituation of corticosterone responses to repeated stress in adult male and female rats. Horm Behav. 2005;47:430–438. doi: 10.1016/j.yhbeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we prescribing multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol. 1999;106:977–979. doi: 10.1111/j.1471-0528.1999.tb08440.x. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Meddle SL, Ma S, Ochedalski T, Douglas AJ, Russell JA. Endogenous opioids and attenuated hypothalamic-pituitary-adrenal axis responses to immune challenge in pregnant rats. J Neurosci. 2005;25:5117–5126. doi: 10.1523/JNEUROSCI.0866-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton PJ, Waddell BJ. Dual function of 11β-hydroxysteroid dehydrogenase in placenta: Modulating placental glucocorticoid passage and local steroid action. Biol Reprod. 1999;60:234–240. doi: 10.1095/biolreprod60.2.234. [DOI] [PubMed] [Google Scholar]
- Butler TG, Schwartz J, McMillen IC. Functional heterogeneity of corticotrophs in the anterior pituitary of the sheep fetus. J Physiol. 1999;516:907–913. doi: 10.1111/j.1469-7793.1999.0907u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet R, Pradier P, Dalle M, Delost P. Effects of prenatal maternal stress on the pituitary adrenocortical reactivity in guinea-pig pups. J Dev Physiol. 1986;8:467–475. [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Condon J, Gosden C, Gardener D, Nickson P, Hewison M, Howie AJ, Stewart PM. Expression of type 2, 11beta-hydroxysteroid dehydrogenase and corticosteroid hormone receptors in early human fetal life. J Clin Endocrinol Metab. 1998;83:4490–4497. doi: 10.1210/jcem.83.12.5302. [DOI] [PubMed] [Google Scholar]
- Costa A, Rocci MP, Arisio R, Benedetto C, Fabris C, Bertino E, Botta G, Marozio L, Mostert M, Urbano D, Emanuel A. Glucocorticoid receptors immunoreactivity in tissue of human embryos. J Endocrinol Invest. 1996;19:92–98. doi: 10.1007/BF03349843. [DOI] [PubMed] [Google Scholar]
- Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- Dauprat P, Monin G, Dalle M, Delost P. The effects of psychosomatic stress at the end of pregnancy on maternal and fetal plasma cortisol levels and liver glycogen in guinea-pigs. Reprod Nutr Dev. 1984;24:45–51. doi: 10.1051/rnd:19840105. [DOI] [PubMed] [Google Scholar]
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253–259. doi: 10.1016/s0006-8993(99)02064-8. [DOI] [PubMed] [Google Scholar]
- Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11β-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci. 1998;18:2570–2580. doi: 10.1523/JNEUROSCI.18-07-02570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Dunn E, Owen D, Kostaki A, Matthews SG. Prenatal glucocorticoid exposure affects reproductive capacity and adrenocortical function in mature female guinea pig offspring. Pediatr Res. 2005;58:O-008. Abstract. [Google Scholar]
- Erdeljan P, Andrews MH, MacDonald JF, Matthews SG. Glucocorticoids and serotonin alter glucocorticoid receptor mRNA levels in fetal guinea-pig hippocampal neurons, in vitro. Reprod Fertil Dev. 2005;17:743–749. doi: 10.1071/rd05043. [DOI] [PubMed] [Google Scholar]
- Erdeljan P, MacDonald JF, Matthews SG. Glucocorticoids and serotonin alter glucocorticoid receptor (GR) but not mineralocorticoid receptor (MR) mRNA levels in fetal mouse hippocampal neurons, in vitro. Brain Res. 2001;896:130–136. doi: 10.1016/s0006-8993(01)02075-3. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- Gerardin DC, Pereira OC, Kempinas WG, Florio JC, Moreira EG, Bernardi MM. Sexual behavior, neuroendocrine, and neurochemical aspects in male rats exposed prenatally to stress. Physiol Behav. 2005;84:97–104. doi: 10.1016/j.physbeh.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Gilstrap LC. Antenatal corticosteroids revisited: repeat courses. National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol. 2001;98:144–150. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- Go KS, Lingas R, Wheeler MB, Irwin DM, Matthews SG. Decreased CRH mRNA expression in the fetal guinea pig hypothalamus following maternal nutrient restriction. Brain Res. 2001;896:179–182. doi: 10.1016/s0006-8993(01)02089-3. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Maternal prenatal stress and 4–6 year old children's salivary cortisol concentrations pre- and post-vaccination. Stress. 2004;7:257–260. doi: 10.1080/10253890500044521. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children's cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Carroll JA, Weesner GD, Daniels MJ, Matteri RL, Lay DC., Jr Administration of ACTH to restrained, pregnant sows alters their pigs' hypothalamic-pituitary-adrenal (HPA) axis. J Anim Sci. 2000;78:2399–2411. doi: 10.2527/2000.7892399x. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Jarvis S, Moinard C, Robson SK, Baxter E, Ormandy E, Douglas AJ, Seckl JR, Russell JA, Lawrence AB. Programming the offspring of the pig by prenatal social stress: Neuroendocrine activity and behaviour. Horm Behav. 2006;49:68–80. doi: 10.1016/j.yhbeh.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod. 2005;73:591–597. doi: 10.1095/biolreprod.105.042242. [DOI] [PubMed] [Google Scholar]
- Kanitz E, Otten W, Tuchscherer M, Manteuffel G. Effects of prenatal stress on corticosteroid receptors and monoamine concentrations in limbic areas of suckling piglets (Sus scrofa) at different ages. J Vet Med A Physiol Pathol Clin Med. 2003;50:132–139. doi: 10.1046/j.1439-0442.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo-pituitary-adrenal axis activity in male guinea pig offspring. J Physiol. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63:397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JF, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signalling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Koehl M, Barbazanges A, Le Moal M, Maccari S. Prenatal stress induces a phase advance of circadian corticosterone rhythm in adult rats which is prevented by postnatal stress. Brain Res. 1997;759:317–320. doi: 10.1016/s0006-8993(97)00394-6. [DOI] [PubMed] [Google Scholar]
- Koehl M, Darnaudéry M, Dulluc J, Van Reeth O, Le Moal M, Maccari S. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Kostaki A, Owen D, Li D, Matthews SG. Transgenerational effects of prenatal glucocorticoid exposure on growth, endocrine function and behaviour in the guinea pig. Pediatr Res. 2005;58:P1–P052. Abstract. [Google Scholar]
- Kranendonk G, Hopster H, Fillerup M, Ekkel ED, Mulder EJ, Wiegant VM, Taverne MA. Lower birth weight and attenuated adrenocortical response to ACTH in offspring from sows that orally received cortisol during gestation. Domest Anim Endocrinol. 2005 doi: 10.1016/j.domaniend.2005.07.001. DOI 10.1016/j.domaniend.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kretz O, Schmid W, Berger S, Gass P. The mineralocorticoid receptor expression in the mouse CNS is conserved during development. Neuroreport. 2001;12:1133–1137. doi: 10.1097/00001756-200105080-00017. [DOI] [PubMed] [Google Scholar]
- Lay DCJ, Randel RD, Friend TH, Carroll JA, Welsh THJ, Jenkins OC, Neuendorff DA, Bushong DM, Kapp GM. Effects of prenatal stress on the fetal calf. Domest Anim Endocrinol. 1997a;14:73–80. doi: 10.1016/s0739-7240(96)00115-4. [DOI] [PubMed] [Google Scholar]
- Lay DCJ, Randel RD, Friend TH, Jenkins OC, Neuendorff DA, Bushong DM, Lanier EK, Bjorge MK. Effects of prenatal stress on suckling calves. J Anim Sci. 1997b;75:3143–3151. doi: 10.2527/1997.75123143x. [DOI] [PubMed] [Google Scholar]
- Levine S. Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science. 1957;156:258–260. doi: 10.1126/science.156.3772.258. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young South African adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611–4618. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Liggins GC. The role of the hypothalamic-pituitary-adrenal axis in preparing the fetus for birth. Am J Obstet Gynecol. 2000;182:475–477. doi: 10.1016/s0002-9378(00)70241-9. [DOI] [PubMed] [Google Scholar]
- Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 hours) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 1999;846:236–242. doi: 10.1016/s0006-8993(99)02058-2. [DOI] [PubMed] [Google Scholar]
- Lingas R, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–E739. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Lepage M. Stress, memory, and the hippocampus: can't live with it, can't live without it. Behav Brain Res. 2001;127:137–158. doi: 10.1016/s0166-4328(01)00361-8. [DOI] [PubMed] [Google Scholar]
- Ma S, Shipston MJ, Morilak D, Russell JA. Reduced hypothalamic vasopressin secretion underlies attenuated adrenocorticotropin stress responses in pregnant rats. Endocrinology. 2005;146:1626–1637. doi: 10.1210/en.2004-1368. [DOI] [PubMed] [Google Scholar]
- McCabe L, Marash D, Li A, Matthews SG. Repeated antenatal glucocorticoid treatment decreases hypothalamic corticotropin releasing hormone mRNA but not corticosteroid receptor mRNA expression in the fetal guinea-pig brain. J Neuroendocrinol. 2001;13:425–431. doi: 10.1046/j.1365-2826.2001.00649.x. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86:4979–4983. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Dev Brain Res. 1998;107:123–132. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Challis JR. Regulation of CRH and AVP mRNA in the developing ovine hypothalamus: effects of stress and glucocorticoids. Am J Physiol. 1995;268:E1096–E1107. doi: 10.1152/ajpendo.1995.268.6.E1096. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: The effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC. Coregulator proteins and corticosteroid action in the brain. J Neuroendocrinol. 2002;14:499–505. doi: 10.1046/j.1365-2826.2002.00795.x. [DOI] [PubMed] [Google Scholar]
- Meijer OC, Steenbergen PJ, de Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- Montano MM, Wang M-H, Vom Saal FS. Sex differences in plasma corticosterone in mouse fetuses are mediated by differential placental transport from the mother and eliminated by maternal adrenalectomy or stress. J Reprod Fertil. 1993;99:283–290. doi: 10.1530/jrf.0.0990283. [DOI] [PubMed] [Google Scholar]
- Moss TJ, Doherty DA, Nitsos I, Harding R, Newnham JP. Pharmacokinetics of betamethasone after maternal or fetal intramuscular administration. Am J Obstet Gynecol. 2003;189:1751–1757. doi: 10.1016/s0002-9378(03)00825-1. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Mikuni M, Ogawa T, Kitera K, Kamei K, Takigawa M, Takahashi K. Prenatal dexamethasone exposure alters brain monoamine metabolism and adrenocortical response in rat offspring. Am J Physiol. 1997;273:R1669–R1675. doi: 10.1152/ajpregu.1997.273.5.R1669. [DOI] [PubMed] [Google Scholar]
- Myers DA, Myers TR, Grober MS, Nathanielsz PW. Levels of corticotropin-releasing hormone messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus and proopiomelanocortin mRNA in the anterior pituitary during late gestation in fetal sheep. Endocrinology. 1993;132:2109–2116. doi: 10.1210/endo.132.5.8386607. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Ohkawa T, Takeshita S, Murase T, Okinaga S, Arai K. The effect of an acute stress in late pregnancy on hypothalamic catecholamines of the rat fetus. Nippon Sanka Fujinka Gakkai Zasshi. 1991;43:783–787. [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programmes gender specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Owen D, Andrews MH, Matthews SG. Maternal adversity, glucocorticoids and programming of neuroendocrine function and behaviour. Neurosci Biobehav Rev. 2005;29:209–226. doi: 10.1016/j.neubiorev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144:2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Peters DAV. Prenatal stress: Effects on brain biogenic amine and plasma corticosterone levels. Pharmacol Biochem Behav. 1982;17:721–725. doi: 10.1016/0091-3057(82)90353-7. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Bennett FI, Wilks R, Thame M, Boyne M, Osmond C, Forrester TE. Maternal body composition, offspring blood pressure and the hypothalamic-pituitary-adrenal axis. Paediatr Perinat Epidemiol. 2005;19:294–302. doi: 10.1111/j.1365-3016.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians – a survey of clinical practice. Aust N Z J Obstet Gynaecol. 1998;38:1–7. doi: 10.1111/j.1479-828x.1998.tb02947.x. [DOI] [PubMed] [Google Scholar]
- Roussel S, Boissy A, Montigny D, Hemsworth PH, Duvaux-Ponter C. Gender-specific effects of prenatal stress on emotional reactivity and stress physiology of goat kids. Horm Behav. 2005;47:256–266. doi: 10.1016/j.yhbeh.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sampath-Kumar R, Matthews SG, Yang K. 11beta-hydroxysteroid dehydrogenase type 2 is the predominant isozyme in the guinea pig placenta: decreases in messenger ribonucleic acid and activity at term. Biol Reprod. 1998;59:1378–1384. doi: 10.1095/biolreprod59.6.1378. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11β-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000;57:1412–1417. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Owen D, McCabe L, Kostaki A, Andrews MH, Matthews SG. Glucocorticoids do not alter developmental expression of hippocampal or pituitary steroid receptor coactivator-1 and -2 in the late gestation fetal guinea pig. Endocrinology. 2004;145:3796–3803. doi: 10.1210/en.2003-1723. [DOI] [PubMed] [Google Scholar]
- Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Barnes GA, McCook EC, Seidler FJ. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Dev Brain Res. 1996;93:155–161. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300892. DOI 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2005 doi: 10.1016/j.placenta.2005.05.007. DOI 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–581. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Theogaraj E, John CD, Christian HC, Morris JF, Smith SF, Buckingham JC. Perinatal glucocorticoid treatment produces molecular, functional, and morphological changes in the anterior pituitary gland of the adult male rat. Endocrinology. 2005;146:4804–4813. doi: 10.1210/en.2005-0500. [DOI] [PubMed] [Google Scholar]
- Unno N, Wu WX, Ding XY, Li C, Hing WK, Nathanielsz PW. The effects of fetal adrenalectomy at 110 days gestational age on AVP and CRH mRNA expression in the hypothalamic paraventricular nucleus of the ovine fetus. Dev Brain Res. 1998;106:119–128. doi: 10.1016/s0165-3806(97)00203-4. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol. 2004;181:223–231. doi: 10.1677/joe.0.1810223. [DOI] [PubMed] [Google Scholar]
- Wan S, Hao R, Sun K. Repeated maternal dexamethasone treatments in late gestation increases 11beta-hydroxysteroid dehydrogenase type 1 expression in the hippocampus of the newborn rat. Neurosci Lett. 2005;382:96–101. doi: 10.1016/j.neulet.2005.02.066. [DOI] [PubMed] [Google Scholar]
- Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 2000;70:359–366. doi: 10.1016/s0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- Ward AM, Moore V, Steptoe A, Cockington R, Robinson JS, Phillips DIW. Size at birth and cortisol responses to psychological stress: evidence for fetal programming. J Hypertens. 2004a;I22:2295–2301. doi: 10.1097/00004872-200412000-00011. [DOI] [PubMed] [Google Scholar]
- Ward AM, Syddall HE, Wood PJ, Chrousos GP, Phillips DI. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: low birth weight and central HPA regulation. J Clin Endocrinol Metab. 2004b;89:1227–1233. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998;16:289–295. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11beta-hydroxysteroid dehydrogenase type 2 activity. J Endocrinol. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- Young JB. Programming of sympathoadrenal function. Trends Endocrinol Metab. 2002;13:381–385. doi: 10.1016/s1043-2760(02)00661-6. [DOI] [PubMed] [Google Scholar]