Abstract
Recent studies demonstrate long-term programming of function of specific organ systems resulting from suboptimal environments during fetal life and development up to weaning. Nutrient restriction during pregnancy and lactation impairs overall fetal growth and development. We determined the effects of maternal protein restriction (MPR; 50% normal protein intake) during fetal development and/or lactation in rats on the function and ageing of the reproductive system of female progeny. Rats were fed either a control 20% casein diet (C) or a restricted diet (R) of 10% casein during pregnancy. After delivery mothers received either C or R diet until weaning to provide four groups, CC, RR, CR and RC. We report data on female offspring only. After weaning pups were fed the C diet. MPR increased maternal progesterone, corticosterone, oestradiol and testosterone concentrations at 19 days gestation. Reproductive and somatic phenotype was altered as pup birth weight was decreased, and ano-genital distance was increased by MPR. Pup corticosterone was decreased at 2 days postnatal (PN) life. Vaginal opening and timing of the first oestrus were delayed in RR and CR and these differences were not related to body weight. At 21 days PN oestradiol in RR and CR and progesterone in RR were reduced; at 70 days PN luteinizing hormone (LH) in all restricted groups was reduced in dioestrus while follicle stimulating hormone (FSH) was unchanged. Cycle length increased between 140 days and 1 year in RR and CR but remained unchanged in CC, providing evidence of premature ageing of reproductive function. Fertility rates declined over the same period in the three experimental groups but not CC. MPR in one of the two experimental periods, either pregnancy or lactation, resulted in decreased pup survival compared with CC and RR. These data show that MPR results in delayed sexual maturation and premature ageing of reproductive function.
Human epidemiological studies and controlled experimental investigations in several species, such as rodents and sheep, have demonstrated long-term programming of the function of specific organ systems as a result of exposure to a suboptimal environment during early fetal and postnatal development (Hoet & Hanson, 1999; Armitage et al. 2004). Nutrient restriction during pregnancy and lactation impairs overall fetal growth and development. A variety of nutrient restriction protocols have been studied ranging from mild 15% reduction in calories (Ozaki et al. 2001), through moderate 50% reduction (Ozaki et al. 2000) to severe 70% reduction (Woodall et al. 1996a,b; Vickers et al. 2001). In the several protein restriction models studied, birth weight is usually lowered (Galler & Tonkiss, 1991; Langley & Jackson, 1994; Holemans et al. 1999; Langley-Evans, 2000; Vickers et al. 2001). However, some studies report increased birth weight (Langley-Evans et al. 1996) or no effect (Langley et al. 1994). Birth weight is, however, a crude indicator of the degree of developmental compromise since developing mammals will recruit adaptive mechanisms to protect growth, especially of vital organs, during development.
In sheep reduced lifetime reproductive capacity has been demonstrated in ewes born to mothers undernourished during late pregnancy or the first months of life (Gunn, 1995; Rhind & McNeilly, 1998). Environmental challenges such as prenatal exposure to testosterone impair female reproductive capacity in sheep (Sharma et al. 2002; Savabieasfahani et al. 2005). In rodents prepubertal administration of oestradiol disrupts ovarian cyclicity in adult life (Rosa et al. 2003). Several studies have addressed the effects of fetal suboptimal nutrition on pubertal onset and development of female reproductive function (Engelbregt et al. 2000, 2002; Rae et al. 2002; Leonhardt et al. 2003). Increased nutritional demand on the mother by increasing litter size also alters sexual development of offspring (Engelbregt et al. 2001). However, the impact of maternal protein restriction on several important features of female reproduction such as alteration in cycle length and loss of fertility with ageing have not been studied.
We fed rats either a control 20% casein diet (C) or a restricted diet (R) of 10% casein during pregnancy. After delivery mothers received either the C or the R diet until weaning to provide four groups, CC, RR, CR and RC. After weaning pups were fed the C diet. In this report we present data on female offspring only. Maternal protein restriction altered key components of pregnant maternal steroid endocrinology as well as the endocrinolgy of female progeny. The onset of puberty was delayed and reproductive function aged more rapidly in groups that had been exposed to protein restriction during development. These data indicate that maternal protein restriction during development results in delayed sexual maturation and premature ageing of reproductive function.
Methods
Care and maintenance of animals
Details of protein restriction and generation of the pups have been published previously (Zambrano et al. 2005b). Briefly, 70 virgin female albino Wistar rats aged between 10 and 12 weeks and weighing 240 ± 20 g (mean ± s.e.m.) were obtained from the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (México City, México). Rats were maintained under controlled lighting (lights on from 07.00 h to 19.00 h at 22–23°C). All procedures were approved by the Animal Experimentation Ethics Committee of the Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán, México City.
Female rats were mated overnight with proven male breeders and the day on which spermatozoa were present in a vaginal smear was designated as the day of conception – day 0. Only rats who were pregnant within 5 days of introduction of the male were retained in the study. Pregnant rats were transferred to individual metabolic cages and allocated at random to one of two groups to be fed either a 20% casein (control diet – C) or 10% casein isocaloric diet (restricted diet – R) (Zambrano et al. 2005b). Food and water were available ad libitum for all animals.
Pregnant and lactating rats were weighed daily through pregnancy and until the pups were removed at weaning. Food was provided in the form of large flat biscuits which were retained behind a grill through which the rats nibbled the food. On day 20 post-conception, pregnant rats were transferred to normal rat cages to provide optimal conditions for delivery, which occurred in the early daylight hours between 09.00 h and 12.00 h on post-conceptual day 22. Day of delivery was considered as day 0 of postnatal life. We report data on female offspring only.
Experiment 1
A group of 30 pregnant rats (13 controls and 17 nutrient restricted, assigned at random) were studied for maternal and neonatal hormonal measurements. Pregnant rats were bled from the tail vein at 19 days gestation between 09.00 h and 10.00 h. Blood was collected into polyethylene tubes and allowed to clot at 4°C for 1 h. The blood samples were centrifuged at 3500 g for 15 min at 4°C. Serum samples were kept at −20°C until assayed. These rats were then allowed to deliver vaginally. Pup weight was recorded at birth. Ano-genital distance (AGD) was measured with calipers to enable determination of sex. Our published data indicate that female pups have an ano-genital distance of 1.67 ± 0.13 mm (n = 291 pups from 43 litters; mean ± s.e.m.) and males 3.26 ± 0.22 mm (n = 252 pups from 43 litters) (Zambrano et al. 2006). Thus a value of 2.5 mm is more than 2 s.d. from the mean of either group, and sex was judged according to whether the ano-genital distance was greater than (male) or less than (female) 2.5 mm. Mothers maintained their pups for 2 days postnatally on the diets they ate during pregnancy. On day 2 of postnatal life their pups were rapidly killed by decapitation between 09.00 h and 10.00 h. Trunk blood was collected and processed as described above.
Experiment 2
Sixteen control and 18 nutrient restricted pregnant rats underwent spontaneous vaginal delivery. Pup weight was recorded at birth. Ano-genital distance (AGD) was measured with calipers. To ensure homogeneity of subjects, litters of less than 12 or more than 14 pups were not included in the study. On day 1, litters of 12–14 pups were adjusted to 12 pups for each dam while maintaining as close to a 1: 1 sex ratio as possible. After removal of five control litters (2 with less than 12 pups and 3 with more than 14) and six protein-restricted litters (3 with less than 12 pups and 3 with more than 14) from the study, pups from 23 mothers were used. Four groups were established: CC (n = 5) in which dams who received the control diet during pregnancy continued to be fed the control diet during lactation; RR (n = 5) in which dams who had received the restricted diet during pregnancy continued to receive the restricted diet during lactation; CR (n = 6) in which dams who received the control diet during pregnancy received the restricted diet during lactation; and RC (n = 7) in which dams who received the restricted diet during pregnancy were provided with the control diet during lactation.
Postnatal maintenance
After weaning (postnatal day 21) males and females were separated and the females were maintained in groups of up to six until puberty and fed with control diet ad libitum. As the pups grew they were separated into groups of three to four. The pups continued to be weighed daily.
On the days on which blood sampling was performed, rats were rapidly killed by decapitation between 09.00 h and 10.00 h. Trunk blood was collected and processed as described above. Ovaries and uteri were dissected, cleaned of fat and weighed.
Evaluation of reproductive function and assessment of reproductive organs
Onset of puberty in the female pups was defined in two separate ways: as the time of vaginal opening and as the first oestrus. From the day at which vaginal opening occurred, a vaginal smear was obtained every 24 h to establish timing of the first oestrus. Vaginal smears were then continued to 70 days postnatal life and obtained daily again for 10 days at 140 days of age, at 1 year and at 22 months. Cyclicity was recorded following examination of the vaginal smears for the proportions of leucocytes, epithelial cells and cornified cells in the smear. The smears were assessed and cycles identified as described by Rosa et al. (2003). Cycles were counted as the interval between pro-oestrus and the next pro-oestrus (Rosa et al. 2003). When a cycle had completed less than 4 days at the beginning or the end of an observation period, this cycle was counted as 0.5 of a cycle. The average duration of the cycle was calculated for each animal for each period.
Fertility of the female offspring
Between 150 and 160 days postnatal life, two female rats from the same group were placed in a cage with a single fertile male for 5 days. At 1 year, to allow for longer cycles, the same procedure was conducted but males were left with females for 1 week. Results were expressed as the percentage of female rats per group that became pregnant. Pups from these pregnant rats were then entered in another study.
Radioimmunoassays
Serum FSH and LH concentrations were determined by double-antibody RIA using hormone standards and the specific antibodies provided by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK, Baltimore, MD, USA). The volume of samples assayed was l00 μl per tube. Rat FSH and LH were iodinated by the chloramine-T method, following separation of protein bound and free 125I by Sephadex G-100. Results were expressed in terms of NIDDK-rat-FSH-RP2 and NIDDK-rat-LH-RP3. Sensitivity of the RIA was 0.035 and 0.02 ng assay tube−1, respectively; FSH intra- and interassay coefficients of variation were < 9% and < 14%, respectively, and for LH < 7% and 12%, respectively. Serum rat prolactin was determined by RIA using commercial kits from Biocode-Hycel, Belgium (AH R011). The sensitivity was 1.07 ng ml−1. Only one assay was performed and the interassay coefficient of variation was < 6%.
Serum progesterone, oestradiol, testosterone and corticosterone concentrations were determined by RIA using commercial kits from DPC Coat-a-count (Diagnostic Product Corporation, Los Angeles, CA, USA) (TKPG2, TKE22, TKTT2 and TKRC1, respectively). Intra- and interassay coefficients of variation were 2% and 4% for progesterone, 3% and 2% for oestradiol, 3% and 5% for testosterone, and 2% and 4% for corticosterone, respectively. Sensitivity of the RIA was 0.02 ng ml−1 for progesterone, 0.8 pg ml−1 for oestradiol, 0.04 ng ml−1 for testosterone and 5.7 ng ml−1 for corticosterone.
Statistical analysis
Where more than one animal from any litter was studied, data from animals within the litter were averaged and analysis performed only on numbers of litters. All data are presented as the mean ± s.e.m. Statistical analysis was performed using multiple analysis of variance (ANOVA) followed by Tukey's test. Unpaired Student's t test and the χ2 test were also used when appropriate; P ≤ 0.05 was considered significant.
Results
Maternal body weight
Maternal body weights, litter size and percentage of pups of each sex did not differ between groups. Maternal weight gain was significantly lower in the nutrient restricted mothers (P ≤ 0.05; Table 1).
Table 1.
Maternal body weight (g) at onset of pregnancy and 20 days gestation (dG), and litter size of rats fed with control (20% casein) or restricted (10% casein) diet during pregnancy
| Control (n = 11) | Restricted (n = 12) | |
|---|---|---|
| Maternal body weight (g) at onset of pregnancy | 243.2 ± 2.93 | 246.3 ± 7.72 |
| Maternal body weight (g) at 20 dG | 384.3 ± 5.36 | 370.0 ± 6.82 |
| Maternal weight gain (g) at 20 dG | 140.9 ± 1.80 | 123.7 ± 2.56* |
| % weight gain at 20 dG | 57.9 ± 1.18 | 50.2 ± 1.75* |
| Litter size | 14.3 ± 0.37 | 13.7 ± 0.37 |
Unpaired Student's t test, mean ± s.e.m.; n = mothers or litters;.
P ≤ 0.05, different from control.
Maternal endocrinology
At 19 days of gestation, maternal progesterone was elevated in the protein restricted mothers (68.6 ± 6.5 ng ml−1) compared to mothers fed the control diet (50.9 ± 4.4 ng ml−1) (P ≤ 0.05). Prolactin was unchanged (control mothers: 24.9 ± 1.3 and restricted mothers: 20.3 ± 1.4 ng ml−1). We have previously reported that oestradiol, testosterone and corticosterone are elevated in maternal blood by nutrient restriction (P < 0.05) (Zambrano et al. 2005b).
Pup birth weight and morphometric measurements
Body weight of pups of protein restricted mothers had reduced birth weight compared to mothers fed the control diet, reduced abdominal diameter, and increased ratio of head: abdominal diameter, and ano-genital distance was increased by 29% as an absolute measure and 28% relative to body weight (Table 2).
Table 2.
Pup weight and morphometric measurments at birth of rats fed the control (20% casein) or restricted (10% casein) diet during pregnancy
| Control (n = 11) | Restricted (n = 12) | |
|---|---|---|
| Body weight (g) | 6.1 ± 0.08 | 5.7 ± 0.10** |
| Lenght (mm) | 48.3 ± 0.03 | 47.8 ± 0.02 |
| Ano-genital distance (mm) | 1.4 ± 0.04 | 1.8 ± 0.05** |
| Ano-genital distance (mm g−1) | 0.23 ± 0.01 | 0.32 ± 0.01** |
| Head diameter (mm) | 10.7 ± 0.07 | 10.6 ± 0.08 |
| Abdominal diameter (mm) | 13.3 ± 0.18 | 12.6 ± 0.13* |
| Head: abdominal ratio | 0.80 ± 0.01 | 0.84 ± 0.008* |
Unpaired Student's t test, mean ± s.e.m.; n refers to litters.
P ≤ 0.01
P ≤ 0.001 different from control.
Neonatal endocrinology
At 2 days postnatal age serum corticosterone in pups of control mothers was 132.4 ± 6.95 ng ml−1 and in pups of nutrient restricted mothers was 97.6 ± 5.29 ng ml−1 (P ≤ 0.001).
Postnatal growth
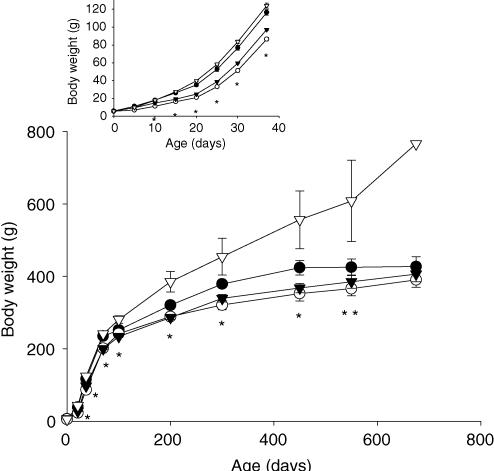
Figure 1 shows the body weights of the four groups of offspring from birth to 22 months. Offspring of both groups restricted during lactation were lighter than CC and RC throughout from day 10–450 (P ≤ 0.05). As they aged, pups from the RC group became heavier than all the other groups (P ≤ 0.05).
Figure 1. Offspring body weight (g) from birth to 22 months.
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). CC (•); RR (○); CR (▾); RC (▿). Insert represents 0–37 days enlarged. One way ANOVA followed by Tukey's test, mean ± s.e.m.; n = 4–7 litters. At 22 months, only 1 rat from RC group survived. *P ≤ 0.05 RR and CR different from CC and RC; **P ≤ 0.05 RC different from CC, RR and CR.
Onset of puberty indicated by vaginal opening and first oestrus
Vaginal opening occurred later in the RR and CR than the CC and RC groups (Tables 3, P ≤ 0.001). The timing of the first oestrus was similarly delayed (Table 3, P ≤ 0.001). Pup body weights at the time of vaginal opening and first oestrus were less in the RR and CR groups than CC and RC (Table 3, P ≤ 0.001).
Table 3.
Onset of puberty (d) defined as time of vaginal opening or first oestrus and body weight (g) at onset of puberty of offspring
| CC (n = 5) | RR (n = 5) | CR (n = 6) | RC (n = 7) | |
|---|---|---|---|---|
| Age at vaginal opening (d) | 37.0 ± 0.42a | 40.6 ± 0.39b | 38.8 ± 0.25b | 36.0 ± 0.37a |
| Body weight (g) at vaginal opening | 115.8 ± 2.53a | 101.3 ± 2.42b | 105.3 ± 1.31b | 118.8 ± 2.71a |
| Age at first oestrus (d) | 37.9 ± 0.47a | 40.9 ± 0.39b | 39.6 ± 0.33b | 36.9 ± 0.44a |
| Body weight (g) at first oestrus | 121.0 ± 3.08a | 104.8 ± 2.44b | 111.2 ± 1.11b | 124.0 ± 2.69a |
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). One way ANOVA and Tukey's test, mean ± s.e.m.; n refers to litters. P ≤ 0.001, data not sharing a letter are statistically different from other groups.
Ovarian and uterine weight
Ovarian and uterine weights were obtained at 21 days and around 70 days and 22 months of age in dioestrus (Table 4). There were no differences between right and left ovarian weight, and therefore combined ovarian weights are presented. At 21 days ovarian weight was greater in RC than RR and CR (P < 0.05). At 70 days ovarian weight was decreased in the RR group compared with CC (P < 0.05). There was a tendency to lower ovarian weight at 21 and 70 days in RR and CR groups. No differences were found in ovarian weigh at 22 months. Uterine weight was lower in RR group at 21 days compared with CC and RC.
Table 4.
Ovarian and uterine weight (mg) in the four groups of offspring at 21, 70 days and 22 months of postnatal life
| CC | RR | CR | RC | |
|---|---|---|---|---|
| 21 days | n = 5 | n = 5 | n = 6 | n = 7 |
| Body wt (g) | 46.7 ± 0.66a | 28.2 ± 0.56b | 27.6 ± 1.74b | 49.6 ± 1.90a |
| Ovarian wt (mg) | 9.2 ± 0.48a,b | 8.0 ± 0.52a | 7.0 ± 0.23a | 10.8 ± 0.32b |
| Uterine wt (mg) | 20.1 ± 1.15a | 12.3 ± 0.67b | 16.0 ± 0.58a,b | 19.4 ± 1.70a |
| 70 days | n = 5 | n = 5 | n = 5 | n = 5 |
| Body wt (g) | 242.0 ± 8.88a | 206.9 ± 3.51b | 205.9 ± 3.37b | 251.5 ± 7.98a |
| Ovarian wt (mg) | 62.1 ± 6.81a | 43.1 ± 3.90b | 50.0 ± 2.63a,b | 55.6 ± 2.92a |
| Uterine wt (mg) | 286.0 ± 8.70 | 320.1 ± 36.01 | 295.7 ± 14.03 | 346.0 ± 23.00 |
| 22 months | n = 4 | n = 5 | n = 5 | n = 1 |
| Body wt (g) | 420.4 ± 26.88 | 402.7 ± 20.70 | 400.2 ± 20.92 | 766.0 |
| Ovarian wt (mg) | 56.2 ± 2.95 | 59.8 ± 7.45 | 66.7 ± 7.43 | 69.0 |
| Uterine wt (mg) | 629.9 ± 45.63 | 614.2 ± 91.04 | 499.6 ± 30.33 | 495.0 |
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). Ovarian weight is expressed as the average of right and left ovaries, RC data at 22 months were not analysed because only one female survived to this age. One way ANOVA and Tukey's test, mean ± s.e.m.; n refers to litters. P ≤ 0.05, data not sharing a letter are statistically different from each other at the same age.
Hormone concentrations at 21 days, 70 days and 1 year
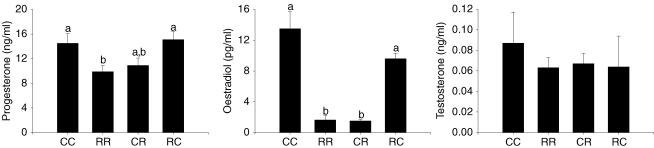
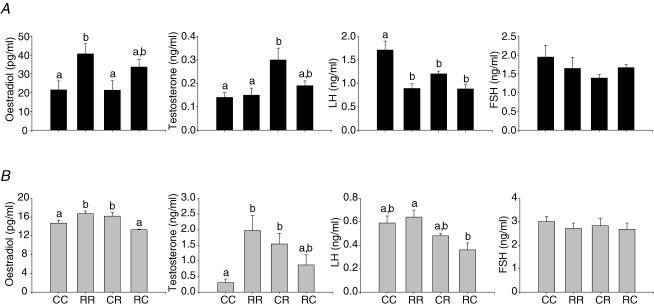
Hormonal data at 21 days are presented in Fig. 2. Oestradiol was lower in the RR and CR groups compared to CC and RC. Progesterone was only significantly different for RR and no differences were observed for testosterone at this age. At 70 days serum LH in dioestrus was lower in all three restricted groups compared with controls (Fig. 3). At one year testosterone and oestradiol were higher at oestrus in RR and CR than CC (Fig. 3). Values at older ages are not presented since animals had varying pregnancy histories.
Figure 2. Offspring serum hormone concentrations at 21 days of age.
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). One way ANOVA and Tukey's test, mean ± s.e.m.; n = 5–6 litters. P ≤ 0.05, data not sharing a letter are statistically different from each other at the same age.
Figure 3. Offspring serum oestradiol and testosterone in oestrus, and LH and FSH in dioestrus at 70 days (A, black bars) and one year of age (B, grey bars).
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). One way ANOVA and Tukey's test, mean ± s.e.m.; n = 4–5 litters. P ≤ 0.05. Note the change in scales to reflect hormone concentration differences at the two ages. Comparisons are only made between groups of the same age. Data not sharing a letter are statistically different from each other at the same age.
Analysis of oestrous cycle from vaginal opening to 70 days, 140 days, 1 year and 22 months
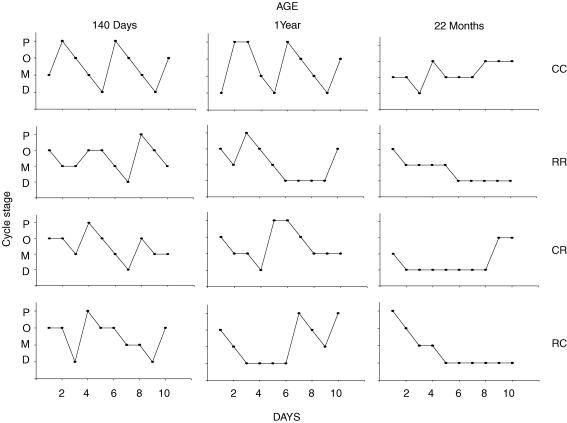
Figure 4 shows representative cycles from one animal in each group at 140 days, 1 year and 22 months of postnatal life. Immediately after puberty cycles tend to be short and frequent with no obvious difference between groups (data not shown). Table 5 presents the average cycle length and percentage of animals showing at least one cycle in a 10-day recording period at 140 days and 1 year postnatal age. At 140 days, a time at which fertility was maximal in all groups, cycles were clearly discernible in all animals in all groups and it is of interest that the only significant diference at this age is the longer cycle length in the RC group. By 1 year, cycle length was unchanged in CC but had increased in RR and CR compared with cycle length in the same group at 140 days (P ≤ 0.05). However, the cycle length did not differ between the three experimental groups and controls. At 22 months postnatal age only one animal in the CC and one animal in the CR group demonstrated any cycles.
Figure 4. Representative offspring oestrous cycle from one animal in each group at 140 days, 1 year and 22 months of postnatal life.
P, pro-oestrus; O, oestrus; M, metoestrus; D, dioestrus. Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter).
Table 5.
Cycle length at 140 days and one year of post natal age in offspring
| CC (n = 5) | RR (n = 5) | CR (n = 5) | RC (n = 4) | |
|---|---|---|---|---|
| Cycle length (d) at 140 days | 4.6 ± 0.16a | 4.9 ± 0.14a | 4.9 ± 0.33a | 6.3 ± 0.46b |
| % animals with at least one cycle at 140 days | 100.0 | 100.0 | 100.0 | 100.0 |
| Cycle length (d) at 1 year | 6.7 ± 0.91 | 9.7 ± 0.98* | 7.5 ± 0.69* | 7.4 ± 1.70 |
| % animals with at least one cycle at 1 year | 100.0 | 90.0 | 81.0 | 75.0 |
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). One way ANOVA and Tukey's test among groups same age, t test between same group different age, mean ± s.e.m.; n refers to litters. P ≤ 0.05, data not sharing a letter are statistically different from each other at the same age
P ≤ 0.01 different from same group at 140 d.
Fertility at 150 days and at 1 year
Fertility data are presented in Table 6. At 150 days fertility was maximal in all groups and in agreement with maximal fertility in our institutional records over the last 5 years. Fertility decreased between 150 days and 1 year; at 1 year fertility rate had fallen significantly by 40, 36 and 75% in RR, CR and RC offspring, respectively, compared with 150 days but not CC. At 1 year fertility was reduced in CR and RC compared with controls (Table 6). There were no differences in litter size or sex ratios in any of the pregnancies between groups at either age. Litter size was reduced with age in all groups but the sex ratio was unchanged (Table 7).
Table 6.
Fertility rate in the four groups of offspring at 150 days and 1 year of postnatal life
| CC (n = 5) | RR (n = 5) | CR (n = 5) | RC (n = 4) | |
|---|---|---|---|---|
| Fertility rate (%) at 150 days | 77.7 | 100.0 | 86.4 | 100.0 |
| Fertility rate (%) at 1 year | 66.6a | 60.0a* | 50.0b* | 25.0b* |
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). A χ2 test was performed; data not sharing a letter are statistically different from each other at the same age
different from same group at 150 d, P ≤ 0.05. n refers to litters.
Table 7.
Body weight (g) at onset of pregnancy and 20 days gestation (dG), litter size and sex ratio of pups at 150 days, and 1 year postnatal age
| CC | RR | CR | RC | |
|---|---|---|---|---|
| A. Pregnancy 150 days | n = 4 | n = 5 | n = 5 | n = 4 |
| Body weight (g) at onset of pregnancy | 293.1 ± 2.5a | 263.0 ± 2.7b | 258.0 ± 3.4b | 337.2 ± 16.1a |
| Body weight (g) at 20 dG | 383.9 ± 4.0a,c | 334.7 ± 4.3b | 367.1 ± 6.1a,b | 402.0 ± 18.2c |
| Weight gain (g) at 20 dG | 90.9 ± 1.3a | 71.7 ± 2.1b | 109.1 ± 2.5c | 65.0 ± 3.0b |
| % weight gain at 20 dG | 31.0 ± 1.8a | 27.3 ± 1.7a,c | 42.3 ± 2.3b | 19.3 ± 1.2c |
| Litter size | 14.1 ± 0.9 | 13.4 ± 1.1 | 12.2 ± 0.6 | 11.8 ± 1.9 |
| B. Pregnancy 1 year | n = 4 | n = 4 | n = 3 | n = 1 |
| Body weight (g) at onset of pregnancy | 394.2 ± 17.2 | 364.0 ± 23.1 | 333.8 ± 19.6 | 247.3 |
| Body weight (g) at 20 dG | 481.7 ± 13.5a | 439.1 ± 26.5a,b | 401.0 ± 15.6b | 361.3 |
| Weight gain (g) at 20 dG | 87.4 ± 20.9 | 75.1 ± 39.4 | 67.3 ± 16.7 | 114.0 |
| % weight gain at 20 dG | 23.9 ± 7.0 | 24.0 ± 14.0 | 24.1 ± 9.8 | 46.1 |
| Litter size | 5.5 ± 1.3* | 4.5 ± 2.1* | 4.5 ± 1.1* | 8.0 |
RC data at 1 year were not analysed because this group contained only one pregnancy. One way ANOVA followed by Tukey's test for same age different groups, t test for same group different age, mean ± s.e.m.; n refers to litters. P ≤ 0.05, data not sharing a letter are statistically different from each other at the same age
P ≤ 0.05 different from same group at 150 d.
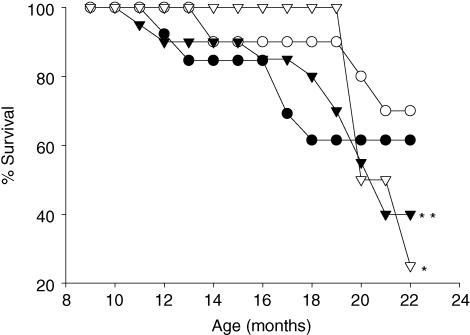
Survival rates at 22 months
Figure 5 shows the survival of pups in the four groups. At 21 months survival of RC pups was significantly lower than CC and RR. There was a tendency for RR to survive longer than CR (P = 0.07).
Figure 5. Percentage offspring survival at 22 months.
Maternal groups: mothers fed with control (C, 20% casein) or restricted (R, 10% casein) diet during pregnancy (first letter) and lactation (second letter). CC (•); RR (○); CR (▾); RC (▿). A χ2 test was performed, *P ≤ 0.05 different from CC and RR; **P = 0.07 CR versus RR. 100% represents all offspring alive in 5 litters in each group at 8 months of age.
Discussion
The goal of these studies was to observe reproductive outcomes of female pups delivered by mothers experiencing maternal protein restriction in two specific windows of their development – pregnancy and lactation. Each of the three experimental groups we have studied addresses the effects of a different challenge to the developing female rat compared with the CC group. The RR group is composed of offspring restricted for the whole period of pregnancy and lactation and thus there is no recuperation of prenatal restriction during lactation. The CR group provides information on the effects of protein restriction in lactation superimposed on a normal diet during the mother's pregnancy. Our study design addresses different questions from previous investigations of effects of maternal nutrient challenge during pregnancy and lactation on the reproductive capacity of female offspring. For example, Leonhardt et al. (2003) studied the effects of a global dietary reduction of 50% on postnatal function of the pituitary gonadal axis and leptin and the timing of puberty in males and females.
Birth weight and growth
Maternal nutrient restriction reduced the birth weight of female pups. Other studies of nutrient restriction have produced variable results. In some investigations birth weight was reduced (Galler & Tonkiss, 1991; Vickers et al. 2001), in others unchanged (Langley et al. 1994) and in some even increased (Langley-Evans et al. 1996). Factors that may alter response of the mother and fetus to nutrient restriction include the strain of rat, age of mothers and differences in the diets. It is of interest that the two groups whose mothers were restricted during lactation had lower body weights during the major part of the study but caught up with the body weight of the CC group in later life. We and others have shown that maternal nutrient restriction during lactation increases insulin sensitivity in early postnatal life (Petry et al. 1997; Shepherd et al. 1997; Zambrano et al. 2005a, 2006), but these offspring become insulin resistant later in life and put on weight to catch up with the controls.
One of the most interesting apsects of developmental programming is the passage of effects transgenerationally. We have shown that the second generation offspring of protein restricted rats have a lower birth weight (Zambrano et al. 2005a) and similar data are available for the children of the daughters of the Dutch Hunger Winter (Lumey et al. 1995). One simple explanation for the growth restriction in the second generation has been that there is increased maternal constraint on fetal growth as a result of altered uterine development in females nutritionally challenged when they themselves were developing. One study on growth of the fetal liver as a result of maternal protein restriction demonstrated an altered proportion of different cell types with an increase in gluconeogenic hepatic cells (Burns et al. 1997). It remains possible that although there is no overall difference in uterine weight, altered growth of specific tissues in the uterus could result from fetal and/or neonatal exposure to maternal protein restriction and play a role in transgenerational passage of epigenetic effects. The differences presented here in the maternal endocrine milieu – elevated maternal progesterone, testosterone, oestradiol and corticosterone – could all affect the growth of uterine, ovarian and neuroendocrine tissues in the female fetuses with consequences in their subsequent pregnancies.
Effects on sexual development and offspring endocrinology
The ano-genital distance in many species (including rats and mice) provides an external marker of sexual differentiation at birth. In the female sheep it has been shown that the ano-genital distance at birth is normally regulated by both oestradiol and testosterone (Kosut et al. 1997). Maternal steroids and/or their metabolites can cross the placenta, and thus exposure to endogenous fetal and transplacentally acquired maternal androgens in fetal life may play a role in the increased ano-genital distance we observed in female pups of protein restricted mothers (Graham & Gandelman, 1986). In our study maternal testosterone was elevated and thus may account for the increased ano-genital distance in the pups. We have previously shown an increase in the ano-genital distance at birth in male offspring of protein restricted mothers (Zambrano et al. 2005b). The elevation of maternal corticosterone in the dams subjected to protein restriction would be anticipated to depress the fetal pituitary-adrenal axis. This is a likely explanation for the lowered concentration of corticosterone observed in the 2-day-old offspring. Increased maternal glucocorticoid levels have been related to delay and decreased function in development of the sexual organs of male offspring (Page et al. 2001). Glucocorticoids could be implicated in later reproductive disturbances. It has been reported that dexamethasone inhibits ovarian function in immature female rats and inhibits the differentiation of granulose cells by FSH (Tohei & Kogo, 1999). Leonhardt et al. (2002) postulate that increased circulating glucocorticoid levels in female offspring could mediate the impaired development of Graafian follicles induced by maternal protein restriction.
Vaginal opening is considered a good marker of the onset of puberty. We observed significantly delayed vaginal opening in the two groups of rats whose mothers were restricted during lactation. Smith & Waddell (2000) demonstrated that administration of glucocorticoids to pregnant rats resulted in a subsequent delay in the onset of puberty in female pups. We demonstrated an increase in corticosterone levels in the mother restricted during pregnancy (Zambrano et al. 2005b), and these results may imply that fetal glucocorticoid exposure is an important determinant of the timing of puberty onset in female pups. In agreement with other authors (Engelbregt et al. 2000, 2002; Smith & Waddell, 2000) this delay was not related to body weight. Some authors have claimed that it is necessary to reach a particular body weight to initiate puberty (Frisch et al. 1977, 1996; Baker, 1985). Our data show that weight of the pup cannot be the only factor in determining the time of vaginal opening and the onset of puberty. Epigenetic factors during development clearly play a role that is independent of pup weight. Undernutrition before and during folliculogenesis delay fetal follicular development in sheep (Rae et al. 2001). The consequences of fetal androgen exposure are reflected in a progressive and dramatic impairment of fertility in the ovary-intact ewe, and the reasons for abnormal steroid feedback mechanisms may reside in sexually dimorphic inputs to the GnRH neurones. (Robinson et al. 2002). However, the precise mechanism by which maternal protein restriction programmes the delay in the onset of puberty in offspring remains unknown. Changes indicating delayed reproductive maturation – such as delayed testicular descent – occur in male offspring of mothers fed low protein diets (Zambrano et al. 2005b).
The decreased levels of LH at 70 days in all three groups experiencing maternal protein restriction at some stage of development (FSH was decreased as well but not significantly) and the increases of testosterone levels at 1 year presage potential reproductive problems including changes in the ovarian cycle. These changes could play a role in later fertility problems. This degree of maternal protein restriction leads to similar changes in reproductive hormones in male offspring (Zambrano et al. 2005b), indicating that a major effect of the challenge imposed on the developing offspring is to alter hypothalamo-pituitary endocrine function.
Cycle length and fertility rate
Our findings agree with the view that exposure to high concentrations of maternal steroids adversely affects both pituitary-gonadal and pituitary-adrenal function in the offspring. For example, it has been reported that maternal adrenocorticotropin injection diminishes reproductive capacity in adult life as shown by behavioural changes such as the frequency of copulation as well as ejaculation (Stylianopoulou, 1983). It was also found that neonatal administration of testosterone affect the sexual behaviour and cyclicity of female offspring (Stylianopoulou et al. 1983). Similarly, male offspring of prenatally stressed rats show decreased sexual activity (Anderson et al. 1986), delayed sexual maturation and reduced fertility rate (Zambrano et al. 2005b).
The irregular cycles in the female offspring in all groups from the onset of puberty until 70 days has important implications for studies in which females have been bred at the earliest possible age. The exciting studies of Wallace and colleagues in adolescent sheep indicate that outcomes in offspring in many rodent investigations in the literature may be influenced by maternal age (Wallace et al. 2000; Da Silva et al. 2001). One intriguing observation from our study is the significantly greater absolute weight gain in the original mothers who became pregnant at 80 days, 141 g in the controls (Table 1) compared with 91 g in the CC offspring in their pregnancies at 150 days (Table 7A) although both pregnancies delivered an average of 14 pups. These findings support the view that the younger pups are still growing during pregnancy. At 1 year maternal weight gain was still approximately 90 g although the total number of pups was only five. The decrease in litter size we observed with maternal age is very similar to that previously reported in Long-Evans rats in which litter size decreased from an average of 12 at 4 months of age to four at 1 year (Matt et al. 1986). All of these findings point to the need for control of maternal age, weight and parity in studies of developmental programming resulting from altered maternal nutrition.
Fertility rate was unaffected at 150 days postnatal life but was reduced at 1 year postnatal age in all three groups when compared to their fertility at 150 days. In keeping with this observation, the oestrous cycle length in the RR and CR groups increase between 150 days and 22 months. These findings indicate that some effects of nutrient restriction are not apparent at early stages of life but emerge in later life as do many of the consequences of developmental programming. Although anecdotal, it is of interest that the only female from the RC group who became pregnant was the only female that remained lean in the group. The remaining survivors who did not get pregnant weighed 489 ± 19.2 g (n = 3).
Longevity
Studies on the mechanisms of longevity clearly need to incorporate the concept that altered trajectories of ageing begin during fetal and neonatal development. The findings of Ozanne and Hales have introduced the important idea that differences in nutrition at different periods of development can interact to alter life span (Ozanne & Hales, 2004). Thus Gluckman & Hanson (2004) have introduced the idea of the predictive adaptive response in which the organism alters developing processes to enhance its chances of survival in a given environment. When the environment eventually experienced does not match the predicted environment, the organism's phenotype proves to be maladapted and life span is curtailed. The shortest lived group in our study was RC and the longest RR.
In conclusion, the finding that the major persistent effects on growth resulted from nutrient restriction of the mother during lactation is in agreement with the occurrence of the major part of cell division and organ and body growth in the postnatal period in the rat. The demonstration of significant effects on ovarian size, hormone levels and oestrous function and fertility resulting from nutrient restriction during development emphasizes the need for further studies of different exposures and outcomes related to developmental programming of the hypothalamo-pituitary-ovarian axis.
Acknowledgments
C.G. is a graduate student from Doctorado en Ciencias Biomédicas, UNAM supported by CONACyT (169728) and DGEP (UNAM), México. This work was partially funded by the NIH HD21350.
References
- Anderson RH, Fleming DE, Rhees RW, Kinghorn E. Relationships between sexual activity, plasma testosterone, and the volume of the sexually dimorphic nucleus of the preoptic area in prenatally stressed and non-stressed rats. Brain Res. 1986;370:1–10. doi: 10.1016/0006-8993(86)91098-x. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker ER. Body weight and the initiation of puberty. Clin Obstet Gynecol. 1985;28:573–579. doi: 10.1097/00003081-198528030-00013. [DOI] [PubMed] [Google Scholar]
- Burns SP, Desai M, Cohen RD, Hales CN, Iles RA, Germain JP, Going TC, Bailey RA. Gluconeogenesis, glucose handling, and structural changes in livers of the adult offspring of rats partially deprived of protein during pregnancy and lactation. J Clin Invest. 1997;100:1768–1774. doi: 10.1172/JCI119703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva P, Aitken RP, Rhind SM, Racey PA, Wallace JM. Influence of placentally mediated fetal growth restriction on the onset of puberty in male and female lambs. Reproduction. 2001;122:375–383. doi: 10.1530/rep.0.1220375. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, Houdijk ME, Popp-Snijders C, Delemarre-van de Waal HA. The effects of intra-uterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res. 2000;48:803–807. doi: 10.1203/00006450-200012000-00017. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, van Weissenbruch MM, Popp-Snijders C, Delemarre-van de Waal HA. Delayed first cycle in intrauterine growth-retarded and postnatally undernourished female rats: follicular growth and ovulation after stimulation with pregnant mare serum gonadotropin at first cycle. J Endocrinol. 2002;173:297–304. doi: 10.1677/joe.0.1730297. [DOI] [PubMed] [Google Scholar]
- Engelbregt MJ, van Weissenbruch MM, Popp-Snijders C, Lips P, Delemarre-van de Waal HA. Body mass index, body composition, and leptin at onset of puberty in male and female rats after intrauterine growth retardation and after early postnatal food restriction. Pediatr Res. 2001;50:474–478. doi: 10.1203/00006450-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Frisch RE. The right weight: body fat, menarche, and fertility. Nutrition. 1996;12:452–453. doi: 10.1016/s0899-9007(97)85084-8. [DOI] [PubMed] [Google Scholar]
- Frisch RE, Hegsted DM, Yoshinaga K. Carcass components at first estrus of rats on high-fat and low-fat diets: body water, protein, and fat. Proc Natl Acad Sci U S A. 1977;74:379–383. doi: 10.1073/pnas.74.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler JR, Tonkiss J. Prenatal protein malnutrition and maternal behavior in Sprague-Dawley rats. J Nutr. 1991;121:762–769. doi: 10.1093/jn/121.5.762. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Graham S, Gandelman R. The expression of ano-genital distance data in the mouse. Physiol Behav. 1986;36:103–104. doi: 10.1016/0031-9384(86)90081-8. [DOI] [PubMed] [Google Scholar]
- Gunn RG. Effects of nutrition in utero and in early life on the subsequent lifetime reproductive-performance of Scottish Blackface ewes in two management systems. Anim Sci. 1995;60:223–230. [Google Scholar]
- Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol. 1999;514:617–627. doi: 10.1111/j.1469-7793.1999.617ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans K, Gerber R, Meurrens K, De Clerck F, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81:73–79. [PubMed] [Google Scholar]
- Kosut SS, Wood RI, Herbosa-Encarnacion C, Foster DL. Prenatal androgens time neuroendocrine puberty in the sheep: effect of testosterone dose. Endocrinology. 1997;138:1072–1077. doi: 10.1210/endo.138.3.4993. [DOI] [PubMed] [Google Scholar]
- Langley SC, Browne RF, Jackson AA. Altered glucose tolerance in rats exposed to maternal low protein diets in utero. Comp Biochem Physiol Physiol. 1994;109:223–229. doi: 10.1016/0300-9629(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. discussion, 121. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr. 2000;51:11–17. doi: 10.1080/096374800100859. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Gardner DS, Jackson AA. Association of disproportionate growth of fetal rats in late gestation with raised systolic blood pressure in later life. J Reprod Fertil. 1996;106:307–312. doi: 10.1530/jrf.0.1060307. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Croix D, Dutriez-Casteloo I, Beauvillain JC, Dupouy JP. Effects of perinatal maternal food restriction on pituitary-gonadal axis and plasma leptin level in rat pup at birth and weaning and on timing of puberty. Biol Reprod. 2003;68:390–400. doi: 10.1095/biolreprod.102.003269. [DOI] [PubMed] [Google Scholar]
- Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Dupouy JP. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology. 2002;75:45–54. doi: 10.1159/000048220. [DOI] [PubMed] [Google Scholar]
- Lumey LH, Stein AD, Ravelli AC. Timing of prenatal starvation in women and birth weight in their first and second born offspring: the Dutch Famine Birth Cohort study. Eur J Obstet Gynecol Reprod Biol. 1995;61:23–30. doi: 10.1016/0028-2243(95)02149-m. [DOI] [PubMed] [Google Scholar]
- Matt DW, Lee J, Sarver PL, Judd HL, Lu JK. Chronological changes in fertility, fecundity and steroid hormone secretion during consecutive pregnancies in aging rats. Biol Reprod. 1986;34:478–487. doi: 10.1095/biolreprod34.3.478. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- Page KC, Sottas CM, Hardy MP. Prenatal exposure to dexamethasone alters Leydig cell steroidogenic capacity in immature and adult rats. J Androl. 2001;22:973–980. doi: 10.1002/j.1939-4640.2001.tb03438.x. [DOI] [PubMed] [Google Scholar]
- Petry CJ, Ozanne SE, Wang CL, Hales CN. Early protein restriction and obesity independently induce hypertension in 1-year-old rats. Clin Sci (Lond) 1997;93:147–152. doi: 10.1042/cs0930147. [DOI] [PubMed] [Google Scholar]
- Rae MT, Kyle CE, Miller DW, Hammond AJ, Brooks AN, Rhind SM. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim Reprod Sci. 2002;72:63–71. doi: 10.1016/s0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Rae MT, Palassio S, Kyle CE, Brooks AN, Lea RG, Miller DW, Rhind SM. Effect of maternal undernutrition during pregnancy on early ovarian development and subsequent follicular development in sheep fetuses. Reproduction. 2001;122:915–922. [PubMed] [Google Scholar]
- Rhind SM, McNeilly AS. Effects of level of food intake on ovarian follicle number, size and steroidogenic capacity in the ewe. Anim Reprod Sci. 1998;52:131–138. doi: 10.1016/s0378-4320(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Taylor JA, Foster DL, Padmanabhan V. In utero programming of sexually differentiated gonadotrophin releasing hormone (GnRH) secretion. Domest Anim Endocrinol. 2002;23:43–52. doi: 10.1016/s0739-7240(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Rosa ESA, Guimaraes MA, Padmanabhan V, Lara HE. Prepubertal administration of estradiol valerate disrupts cyclicity and leads to cystic ovarian morphology during adult life in the rat: role of sympathetic innervation. Endocrinology. 2003;144:4289–4297. doi: 10.1210/en.2003-0146. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Lee JS, Herkimer C, Sharma TP, Foster DL, Padmanabhan V. Fetal programming: testosterone exposure of the female sheep during midgestation disrupts the dynamics of its adult gonadotropin secretion during the periovulatory period. Biol Reprod. 2005;72:221–229. doi: 10.1095/biolreprod.104.031070. [DOI] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Crowther NJ, Desai M, Hales CN, Ozanne SE. Altered adipocyte properties in the offspring of protein malnourished rats. Br J Nutr. 1997;78:121–129. doi: 10.1079/bjn19970124. [DOI] [PubMed] [Google Scholar]
- Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141:2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- Stylianopoulou F. Effect of maternal adrenocorticotropin injections on the differentiation of sexual behavior of the offspring. Horm Behav. 1983;17:324–331. doi: 10.1016/0018-506x(83)90032-6. [DOI] [PubMed] [Google Scholar]
- Stylianopoulou F, Fameli M, Brountzos E, Contopoulos AN. Neonatal neural organizing effects of exogenous corticosteroids on sexual differentiation of the brain in the female rat. Horm Behav. 1983;17:332–341. doi: 10.1016/0018-506x(83)90033-8. [DOI] [PubMed] [Google Scholar]
- Tohei A, Kogo H. Dexamethasone increases follicle-stimulating hormone secretion via suppression of inhibin in rats. Eur J Pharmacol. 1999;386:69–74. doi: 10.1016/s0014-2999(99)00722-0. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Reddy S, Ikenasio BA, Breier BH. Dysregulation of the adipoinsular axis – a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J Endocrinol. 2001;170:323–332. doi: 10.1677/joe.0.1700323. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally-mediated placental growth restriction and fetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta. 2000;21:100–108. doi: 10.1053/plac.1999.0440. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996a;150:231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996b;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW. A low maternal protein diet during pregnancy and lactation has sex and window of exposure specific effects on offspring growth and food intake, glucose metabolism and serum leptin. J Physiol. 2006;571:221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz P. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005a;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano E, Rodriguez-Gonzalez GL, Guzman C, Garcia-Becerra R, Boeck L, Diaz L, Menjivar M, Larrea F, Nathanielsz PW. A maternal low protein diet during pregnancy and lactation in the rat impairs male reproductive development. J Physiol. 2005b;563:275–284. doi: 10.1113/jphysiol.2004.078543. [DOI] [PMC free article] [PubMed] [Google Scholar]