Abstract
Many animal studies and human epidemiological findings have shown that impaired growth in utero is associated with physiological abnormalities in later life and have linked this to tissue programming during suboptimal intrauterine conditions at critical periods of development. However, few of these studies have considered the contribution of the placenta to the ensuing adult phenotype. In mammals, the major determinant of intrauterine growth is the placental nutrient supply, which, in turn, depends on the size, morphology, blood supply and transporter abundance of the placenta and on synthesis and metabolism of nutrients and hormones by the uteroplacental tissues. This review examines the regulation of placental nutrient transfer capacity and the potential programming effects of nutrition and glucocorticoid over-exposure on placental phenotype with particular emphasis on the role of the Igf2 gene in these processes.
Introduction
The pattern of intrauterine development and size at birth, in particular, are critical in determining life expectancy. The smaller the neonate the less likely it is to survive at birth and the more prone it is to adult-onset, degenerative diseases such as hypertension and type 2 diabetes (Barker, 1994). In mammals, the major determinant of intrauterine growth is the placental supply of nutrients to the fetus, which occurs primarily by diffusion and transporter mediated transport (Sibley et al. 1997). In turn, these processes depend upon the size, morphology, blood supply and transporter abundance of the placenta and on the synthesis and metabolism of nutrients and hormones by the placenta itself. Few of the experimental or epidemiological studies of the developmental origins of adult disease have considered the placental contribution to the ensuing phenotype. Even fewer have examined programming of the placenta per se. This review examines the regulation of placental nutrient transfer capacity with particular emphasis on the potential programming effects of nutrition and glucocorticoid over-exposure during pregnancy.
Gross placental structure and the morphology of the materno-fetal interface involved in nutrient transfer vary widely between genera (Steven, 1975; Wooding & Flint, 1994). Primates have a discoid, haemochorial placenta with the fetal trophoblast in direct contact with maternal blood. There are therefore four membranes plus a variable thickness of cytoplasm between the maternal and fetal circulations in these animals. Rodents also have a discoid, haemochorial placenta but with additional trophoblast layers. This produces a materno-fetal exchange barrier with six membranes, located in the labyrinthine zone of the rodent placenta. In contrast, the placenta of ruminants, equids and pigs is non-invasive and epitheliochorial, retaining both the uterine epithelium and maternal endothelium. This results in a significantly thicker placental barrier of eight membranes and accompanying cytoplasm than found in haemochorial placentas. In ruminants the areas of materno-fetal interface are confined to discrete circular or oval structures, known as placentomes, whereas in equids and pigs the placental exchange surface is diffuse and covers the entire uterus. These species differences in morphological organization of the placental exchange barrier have implications for transplacental nutrient transfer and the programming of placental phenotype by environmental factors (Wooding & Fowden, 2006).
Factors affecting placental nutrient transfer capacity
Size
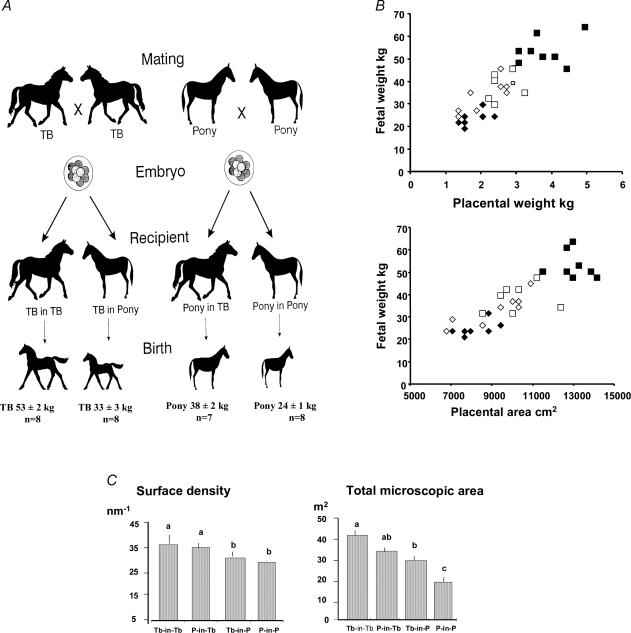
Placental size directly affects the capacity for nutrient transfer via changes in the surface area for transport and, when measured as placental weight, is positively correlated to bodyweight at term in a wide range of species (Baur, 1977; Mellor, 1983). In some animals (e.g. human, horse), placental weight increases progressively throughout pregnancy while, in others (e.g. rodents, sheep), it rises to a plateaux at mid to late gestation and then declines towards term (Stegeman, 1975; Baur, 1977; Coan et al. 2004). In sheep, experimental restriction of placental size from early in gestation by removal of implantation sites, multiple pregnancy or by adverse environmental conditions, such as heat stress, retards fetal growth but often increases the fetal to placental weight ratio in late gestation (Owens et al. 1989; Vatnick et al. 1991; Thureen et al. 1992). In species like horses, cows and pigs, with wide breed differences in size, placental growth can be manipulated by transferring embryos from large breeds into recipient mothers of smaller breeds and vice versa (Fig. 1; Ferrell, 1991; Wilson et al. 1998; Allen et al. 2002). Birth weight is either enhanced above or constrained below the genetic potential for the breed by these techniques but is still directly related to placental weight and surface area (Fig. 1; Ferrell, 1991; Wilson et al. 1998). Most of these methods of restricting placental growth are associated with postnatal abnormalities in cardiovascular, metabolic and endocrine function (McMillen & Robinson, 2005; Fowden et al. 2005).
Figure 1. Effects of embryo transfer between breeds on placental and fetal weight in equids.
The effects of transferring embryos between Thoroughbred (TB) and pony mares on body weight of the foals at birth (A) the relationship between foal birth weight and placental weight and gross surface area (B) and stereological measurements of the surface density of the microcotyledons and total microscopic area of feto-maternal contact in the placenta (C) of the four groups of foals (Thoroughbred in Thoroughbred, TB-in-TB, ▪; Pony in Thoroughbred, P-in-TB, □; Pony in Pony, P-in-P, ♦; Pony in Thoroughbred, P-in-TB, ⋄). Data from Allen et al. (2002).
Both under- and over-nutrition during pregnancy affect placental size, although the specific effects depend on the severity, duration and gestational age at the onset of nutritional perturbation (Heasman et al. 1999). In sheep, moderate undernutrition during the peri-conceptual period alone has no effect on placental and fetal weights in late gestation (Oliver et al. 2005) but, when the period of undernutrition is extended to cover the period of rapid placental growth, placental weight is frequently increased near term (Fig. 2). This overgrowth appears to compensate for the reduced nutrient availability early in gestation as fetal weight is normal, or even enhanced, in late gestation after restoration of normal nutrition (Kelly, 1992). Similar compensatory increases in placental weight have been observed in response to undernutrition in pregnant pigs, rats and humans (Pond et al. 1991; Woodall et al. 1996; Lumey, 1998). By contrast, moderate undernutrition during mid to late gestation when the placenta has formed tends to reduce placental weight near term (Fig. 2). When nutrient deprivation occurs throughout pregnancy in sheep and rats, fetal and placental weights both decrease but, generally, more fetus is produced per gram of placenta than in normally nourished animals (Woodall et al. 1996: Osgerby et al. 2002). Similar increases in placental efficiency are observed when placental and fetal growth are retarded by glucocorticoid administration during late gestation (Johnson et al. 1979; Jensen et al. 2002; Ain et al. 2005). Exposure to poor nutrition or glucocorticoids at critical stages of placental development therefore appears to increase the efficiency with which the small placenta transfers nutrients to the fetus.
Figure 2. The effects of maternal undernutrition on ovine placental weight.
The profile of ovine placental weight in normally nourished animals and the percentage change in placental weight observed in late gestation (between vertical lines) after periods of moderate maternal undernutrition either during implantation and the phase of rapid placental growth (striped bars) or during the plateaux in placental weight later in gestation (stippled bars). Data from Davis et al. 1981; Mellor, 1983; Faichney & White, 1987; Holst et al. 1992; McGrabb et al. 1992; Heasman et al. 1998; Dandrea et al. 2001; Steyn et al. 2001; Edwards & McMillen, 2001.
The actual placental capacity for glucose transfer increases between mid and late gestation and in some, but not all, models of placental size restriction (Owens et al. 1989; Molina et al. 1991; Thureen et al. 1992; Carver & Hay, 1995; Wallace et al. 2002). In the small placenta of carunclectomised ewes, clearance of 3-O-methyl glucose, a non-metabolisable glucose analogue, is increased per kg placenta in late gestation (Owens et al. 1987). Similarly, placental glucose transfer measured as umbilical uptake per kg placenta is increased in the small placenta of ewes with prolonged hypoglycaemia, when the reduced transplacental glucose concentration gradient is taken into account (Carver & Hay, 1995). However, in heat stressed ewes, less glucose is transfered across the small placenta per unit weight at the mean transplacental glucose concentration gradient observed in controls (Thureen et al. 1992). In addition, weight specific rates of placental glucose transport are unaffected by placental size in nutritionally manipulated adolescent ewes, despite an increased fetal to placental weight ratio when placental growth is restricted by overnutrition (Wallace et al. 2002). The phenotype of the small placenta therefore differs with the mode of growth retardation (Sibley et al. 2005). In some instances, the increased efficiency of the small placenta is due to a genuine increase in the efficiency of placental nutrient transport. In others, the increased fetal to placental weight ratio must reflect fetal adaptations to the small placenta that maximize transplacental concentration gradients and partitioning of placentally derived nutrients into fetal growth.
Morphology
Changes in both the gross morphology and ultrastructure of the placenta occur with increasing gestational age and in response to nutritional and endocrine manipulations (Stegeman, 1975; Baur, 1977; Sibley et al. 2005). Many of these changes are interrelated and lead to alterations in surface area, vascularity, barrier thickness and cell composition of the placenta, all of which influence its transport characteristics (Sibley et al. 1997). For instance, the reduction in binucleate cell population induced in the ovine placenta by early cortisol exposure may influence expansion of the feto-maternal syncytium, barrier thickness and placental hormone secretion (Ward et al. 2002).
The gross morphology of ovine placentomes changes progressively throughout gestation with peak numbers of the more everted placentome types at 125–135 days of gestation (Fig. 3). The proportion of these C/D type placentomes is reduced by early glucocorticoid exposure (Ward et al. 2006), which is consistent with their decreased frequency just before term when fetal cortisol concentrations are rising naturally (Fig. 3). In contrast, the proportion of C/D type placentomes in late gestation is increased at high altitude (Penninga & Longo, 1998) and by undernutrition earlier in gestation (Fig. 3). Previous studies have speculated that placentome eversion is an adaptation to increase nutrient delivery to the fetus (Alexander, 1964; Penninga & Longo, 1998; Heasman et al. 1999). However, placental glucose delivery appears to be unrelated to placentome type in normal conditions and is reduced to a greater extent, rather than enhanced, in fetuses with larger proportions of everted placentomes when cortisol levels are elevated during late gestation (Ward et al. 2006).
Figure 3. Regulation of gross ovine placental morphology.
A meta-analysis of the percentage of the total placentome number classified as everted C/D type placentomes in single fetuses with respect to gestational age (term 145–150 days) in normal nutritional conditions (control, ▵) and after either undernutrition earlier in gestation (▴) or cortisol exposure for 5–10 days before delivery ( ). Dashed lines connect control and experimental data from the individual studies. Data from Alexander (1964); Vatnick et al. (1991); Tangalakis et al. (1995); Heasman et al. 1998; Penninga & Longo (1998); Steyn et al. 2001; Gardner et al. 2002; Osgerby et al. 2004; Ward et al. (2006).
). Dashed lines connect control and experimental data from the individual studies. Data from Alexander (1964); Vatnick et al. (1991); Tangalakis et al. (1995); Heasman et al. 1998; Penninga & Longo (1998); Steyn et al. 2001; Gardner et al. 2002; Osgerby et al. 2004; Ward et al. (2006).
In several species, placental surface area for nutrient exchange increases 5- to 15-fold between mid and late gestation, even when there is no change in placental weight (Baur, 1977; Roberts et al. 2001; Coan et al. 2004). In sheep and horses, this occurs by elongation and increased branching of the fetal villi (Stegeman, 1975; Macdonald & Fowden, 1997). In addition, barrier thickness decreases between mid and late gestation in these and other species, which reduces the diffusion distance between the maternal and fetal capillaries (Steven, 1982; Roberts et al. 2001; Coan et al. 2004). Together, these morphological changes increase the theoretical diffusion capacity of the mouse placenta by 15- to 20-fold between mid and late gestation (Coan et al. 2004). When placental growth is impaired by undernutrition throughout pregnancy in guinea pigs, total placental weight and surface area are reduced by 60–70% and barrier thickness increased by 40% in late gestation (Roberts et al. 2001). The proportion of the placenta occupied by the labyrinthe zone involved in nutrient exchange is also reduced in these circumstances. Nutrient restriction may therefore impair the functional capacity of the guinea pig placenta to a greater extent than expected from the reduction in placental weight alone.
Reciprocal embryo transfer between large and small breeds has shown that growth of the placental exchange surface is controlled by both the maternal and fetal genome (Ferrell, 1991; Wilson et al. 1998; Allen et al. 2002). When the genetic potential for placental growth is constrained in the Thoroughbred foal by the smaller surface density of microcotyledons in the pony uterus, the chorionic villi appear to elongate, which increases the total microscopic area of feto-maternal contact in absolute terms (Fig. 2) and as a proportion of gross surface area (Allen et al. 2002). This leads to a larger fetus than would be produced by a pony genotype within the pony uterus (Fig. 2, Allen et al. 2002). However, in pigs, the increase in fetal weight produced by transfering embryos from small breeds into the uteri of larger breeds is due to increased placental vascularity rather than weight (Biensen et al. 1999).
Placental vascularity rises between early and late gestation in sheep due to increases in both the number and surface density of the capillaries, particularly on the fetal side of the placentomes (Stegeman, 1975; Reynolds et al. 2005). These changes are accompanied by exponential increases in blood flow in the umbilical and uterine circulations and by enhanced placental expression of the angiogenic factor vascular endothelial growth factor (VEGF) and its receptor (Silver et al. 1982: Reynolds et al. 2005). Increased blood flow is therefore an important contributory factor to the ontogenic increment in placental transport capacity, at least for substances, such as oxygen, that cross the placenta by flow-limited diffusion. When placental growth is compromised by heat stress of adult ewes or overnutrition of adolescent ewes, placental vascularity decreases in association with reduced VEGF and VEGF receptor expression in the placentomes (Reynolds et al. 2005). Similar decreases in angiogenic factor expression are observed in term placentomes from adult ewes undernourished from early in gestation (Redmer et al. 2004). However, weight specific rates of umbilical and uterine blood flow in late gestation are unaffected by heat stress, glucocorticoid administration, hypoglycaemia, acute fasting, and more prolonged nutritional manipulations earlier in pregnancy (Thureen et al. 1992; Carver & Hay, 1995; Fowden et al. 1998b; Wallace et al. 2002; Jellyman et al. 2004).
Transporter abundance
Tranporter mediated transfer depends on the abundance, localization and affinity of the specific protein carriers. In human and rat placenta, at least nine different amino acid transport systems have been identified, each with distinct functional characteristics yet overlapping substrate specificity (Regnault et al. 2005). For instance, the System A family of amino acid transporters, which transfer small neutral amino acids, has three known carrier proteins in the placenta. Each is encoded by a different gene, which can be regulated independently (Constancia et al. 2005). In contrast, only two of the 12 different isoforms of glucose transporters have been identified in placental membranes directly involved in transplacental glucose flux (Zhou & Bondy, 1993; Currie et al. 1997; Bell et al. 1999). These two transporters, GLUT 1 and 3, have different affinities and Km values and are found in different placental membranes depending on species and placental type (Wooding et al. 2005). In the multilayered epitheliochorial placenta of ruminants and equids, they are localized so that glucose uses both isoforms sequentially in crossing from the maternal to fetal circulations (Wooding et al. 2005).
Activity of the placental glucose and amino acid transport systems is influenced by gestational age and a range of environmental factors including heat stress, hypoxia, under- and over-nutrition, as well as exposure to hormones, such as glucocorticoids, growth hormone and leptin (Carver & Hay, 1995; Ross et al. 1996; Harding et al. 1997; Hanguel-de Mouzon & Shafrir, 2001; Wallace et al. 2002; Jansson et al. 2003; Ward et al. 2004). Less is known about the regulation of the abundance of the actual transporter proteins. In rodents and sheep, placental GLUT 3 mRNA and protein levels increase as gestation advances whereas GLUT 1 abundance is unaffected or decreases towards term (Zhou & Bondy, 1993; Bell et al. 1999). During the second half of gestation, placental GLUT abundance is also altered in an isoform-specific manner by glucocorticoid administration and by variations in nutrient availability induced by fasting, restriction of dietary intake, diabetes and direct maternal infusions of glucose and insulin (Hahn et al. 1999; Das et al. 1998, 2000; Bell et al. 1999; Hanguel-de Mouzon & Shafrir, 2001; Langdown & Sugden, 2001; Dandrea et al. 2001; Lesage et al. 2002). Both increases and decreases in GLUT protein abundance are observed in response to these manipulations depending on their timing and duration (Hahn et al. 1999; Bell et al. 1999; Langdown & Sugden, 2001; Dandrea et al. 2001). Taken together, these observations indicate that placental GLUT protein abundance is responsive to environmental conditions and/or the concomitant changes in fetal growth that these conditions induce.
Nutrient synthesis and metabolism
Placental nutrient delivery to the fetus is also determined by the utilization and production of nutrients by the placenta itself. In ruminants and equids, the uteroplacental tissues use 50–70% of the glucose and oxygen taken up by the uterus (Fowden, 1997). They also produce lactate for fetal use and metabolize amino acids by deamination and transamination as part of a multiorgan system supplying essential and gluconeogenic amino acids to the fetus (Hay, 1995; Regnault et al. 2005). The fluxes of carbohydrates and amino acids into and across the ovine placenta are responsive to a range of environmental factors including nutritional state, placental size, temperature and hormone concentrations (Owens et al. 1989; Liu et al. 1994; Harding et al. 1997; Ross et al. 1996; Timmerman et al. 2000; Fowden et al. 2001). Early glucocorticoid exposure in sheep increases uteroplacental glucose consumption and reduces the absolute amount and proportion of glucose taken up by the uterus that is delivered to the fetus (Barbera et al. 1997; Ward et al. 2004). Fasting and moderate maternal undernutrition for short periods also reduces placental glucose consumption but has no effect on the partitioning of glucose between the uteroplacental and fetal tissues during the hypoglycaemic period (Hay et al. 1983; Fowden et al. 1998b). However, when maternal hypoglycaemia is prolonged for 3 weeks or more by maternal insulin administration, the uteroplacental tissues conserve glucose for their own use and transfer proportionately less glucose to the fetus, although absolute rates of glucose utilization are reduced in both the fetus and placenta (Carver & Hay, 1995). Placental lactate production and delivery to the fetus also decrease in response to undernutrition and glucocorticoid treatment but increase above normal values during maternal insulin-like growth factor (IGF)-I treatment and re-feeding after a short period of undernutrition (Liu et al. 1994; Harding & Johnston, 1995; Ward et al. 2004). Similarly, there are changes in the placental handling of specific amino acids in response to undernutrition and glucocorticoid treatment, which probably reflect alterations in individual amino acid concentrations and/or placental aminotransferase activities (Liechty et al. 1987; Ross et al. 1995; Thureen et al. 2000).
Hormone synthesis and metabolism
The placenta produces a number of hormones including steroids, peptides, glycoproteins and eicosanoids, which are released into both the maternal and fetal circulations (Fowden & Forhead, 2004). Some of these hormones, such as progesterone and placental lactogen, have metabolic effects in the mother that favour glucose delivery to the fetus. Others like the prostaglandins (PG) F2α and E2 affect fetal endocrine function, regional blood flow and myometrial contractility, which affect placental nutrient and oxygen transfer indirectly (Whittle et al. 2001). In sheep, horses and monkeys, undernutrition during late gestation increases uteroplacental production of PGF2α and PGE2 (Binienda et al. 1989; Fowden et al. 1994). These increments in PG synthesis are directly related to the fall in uteroplacental glucose consumption and can be reversed by restoring the normal circulating glucose concentration either by re-feeding or glucose infusion into the fasted mares and ewes (Fowden et al. 1994). In part, these nutritionally induced increases in uteroplacental PG production may be due to the concomitant rise in glucocorticoid concentrations as cortisol has been shown to increase PGH synthase activity and production of PGF2α and PGE2 in human and ovine placentae (Whittle et al. 2001). Cortisol also alters placental steroidogenesis and placental lactogen secretion with potential consequences for mammary gland development and nutrition after birth (Whittle et al. 2001; Ward et al. 2002).
The placenta also inactivates hormones like the PGs, catecholamines, glucocorticoids and thyroxine (Fowden & Forhead, 2004). For instance, the placenta of sheep, horses, humans and non-human primates contains PG dehydrogenase (PGDH), which converts biologically active PGs to their inactive keto forms (Binienda et al. 1989; Fowden et al. 1994; Whittle et al. 2001). Undernutrition during late gestation increases production of these PG metabolites while glucocorticoid administration decreases PGDH activity and, thereby, enhances uteroplacental production of the primary PGs (Fowden et al. 1994). Similarly, in many species, placental 11β-hydoxysteroid dehydrogenase type 2 (11βHSD2) inactivates glucocorticoids and limits fetoplacental exposure to the higher maternal glucocorticoid concentrations (Seckl & Meaney, 2004). Placental activity of this enzyme is down-regulated by undernutrition, hypoxaemia, alcohol and glucocorticoid administration (Whorwood et al. 2001; Clarke et al. 2002; Moritz et al. 2005). This increases glucocorticoid exposure and compromises fetoplacental development (Fowden & Forhead, 2004; Moritz et al. 2005). Certainly, in humans and rats, placental 11βHSD2 activity is directly related to birth weight while, in rats, inhibition of this enzyme during pregnancy leads to fetoplacental growth retardation and abnormalities in cardiovascular and metabolic function in the adult offspring (Seckl & Meaney, 2004).
Mechanisms of placental programming
The molecular mechanisms by which environmental signals alter placental nutrient transport capacity remain unknown but may involve the imprinted Igf2 gene. This gene controls placental growth and its placental expression is down-regulated in rodents by conditions known to reduce placental size, such as nutrient restriction and glucocorticoid administration (Fowden, 2003; Ain et al. 2005). Disruption or deletion of Igf2 gene expression causes placental growth retardation while, conversely, Igf2 over-expression by imprint relaxation or disruption of the IGF-II clearance receptor leads to placentomegaly (Table 1). Both up- and down-regulation of Igf2 gene expression alter placental phenotype and efficiency (Table 1; Fowden et al. 2006).
Table 1.
The effects of deletion or disruption of genes relating IGF-II bioavailability on growth of the placenta and fetus and on the fetal to placental weight ratio in mice in late gestation
| Gene | Placental weight | Fetal weight | F:P weight ratio |
|---|---|---|---|
| Igf2 | 75% | 60% | 85% |
| Placental specific Igf2P0 | 65% | 75% | 115–130% |
| Igf-2R | 140% | 140% | 100% |
| H19 | 140% | 130% | 90% |
| Igf-2R and H19 | 230% | 200% | 80% |
The data show knockout percentage wild-type. Data from Efstratiadis (1998); Chiao et al. (2002); Constancia et al. (2005).
Over-expression of the Igf2 gene by deletion of the H19 gene, which normally silences the maternal Igf2 allele, leads to a disproportionate increase in the area of the labyrinthine zone, the region of the placenta responsible for nutrient exchange in rodents during the second half of gestation (Chiao et al. 2002; Coan et al. 2005). Conversely, Igf2 deletion from all fetoplacental tissues produces a disproportionate decrease in labyrinthine area, which is accompanied by general hypoplasia and a specific loss of glycogen cells in the junctional zone (Lopez et al. 1996; Coan et al. 2005). However, when Igf2 deficiency is induced solely in the nutrient exchange area by deletion of the labyrinth-specific Igf2P0 promoter, all placental layers are proportionately smaller, although placental and fetal growth retardation are less severe in these mutants than in the complete Igf2 null near term (Coan et al. 2005; Constancia et al. 2005). Nevertheless, surface area is reduced by 50% and barrier thickness increased by 28% in the Igf2P0 null placenta by term (Sibley et al. 2004). These observations suggest that expression of the Igf2P0 transcript in the labyrinth is essential for normal growth of all placental zones but that, nearer term, local expression of Igf2 derived from all four Igf2 promoters or circulating IGF-II produced by fetal tissues may also influence placental growth.
Despite the small size and reduced diffusion capacity of the Igf2P0 null placenta (Constancia et al. 2002), the weight of fetus produced per gram of placenta is greater in these mutants than in their wild-type littermates (Table 1). At day 16 of gestation, the Igf2P0 null placenta transfers 30–60% more glucose and methyl-aminoisobutyric acid (MeAIB) per gram than the wild-type placenta (Fig. 4). These changes are accompanied by placental up-regulation of the Slc2a3 and Slc38a4 genes, which encode GLUT3 and an isoform of the System A family of amino acid transporter, respectively (Constancia et al. 2005). The small Igf2P0 null placenta therefore responds to the nutrient demand signals of the fetal tissues still expressing Igf2 by increasing nutrient transporter abundance to help maintain the fetal growth rate. In contrast, up-regulation of placental glucose and System A amino acid transport is not seen in the small placenta of the complete Igf2 null, which lacks Igf2 in all fetoplacental tissues (Constancia et al. 2005). Indeed, these placentae are less efficient and transfer less MeAIB per gram than their wild-type counterparts (Fig. 4). They also have reduced expression of the Slc38a2 isoform of the System A amino acid transporter and altered expression of the System XAG− and System Y+ amino acid transporters in the labyrinthine and junctional zones (Matthews et al. 1999; Constancia et al. 2005). Placental nutrient transfer capacity in mice is therefore responsive to fetal nutrient demands and regulated by the interplay between placental and fetal Igf2 (Constancia et al. 2005). However, whether fetal nutrient demands and other environmental signals act on the placental Igf2 gene to change its imprint status, promoter usage or expression from the active allele remains unknown.
Figure 4. Effects of deleting the Igf2 gene on placental nutrient transfer.
Ratios of mutant to wild-type placental transfer of [14C]glucose (A) and [14C]methyl-AIB (B) expressed per gram placenta in Igf2P0 and complete Igf2 null mice at day 16 and day 19 of gestation (Term 21 days). Bar 95% confidence limits. Ratio significantly different from 1: ***P < 0.001 **P < 0.01 *P < 0.05. n = 11 litters for each marker at each age. Data from Constancia et al. (2002, 2005).
Conclusions
Placental nutrient transfer capacity is responsive to environmental stimuli and can adapt to help maintain the fetal nutrient supply, particularly when the placenta is small. Changes in nutrient and glucocorticoid availability alter placental development and the ensuing phenotype, in part, via Igf2 and other growth factors (Fig. 5). Nutritional and endocrine signals also alter the synthesis and metabolism of nutrients and hormones by the placenta per se which, directly and indirectly, influence the balance and absolute amounts of specific nutrients supplied to the fetus (Fig. 5). Environmentally induced reductions in placental enzymes, such as 11βHSD2, will exacerbate the placental actions of the glucocorticoids (Fig. 5) and increase fetal glucocorticoid exposure with consequences for growth and differentiation of fetal tissues more generally (Fowden et al. 1998a). Placental phenotype is also regulated by the genetic drive for fetal growth, although the nature of the fetal nutrient demand signals that regulate placental growth and nutrient transfer capacity remains unknown (Constancia et al. 2005). Indeed, little is known about epigenetic mechanisms that control the placental capacity for nutrient transfer at the molecular level or the extent to which changes in placental phenotype induced early in gestation persist to programme nutrient transfer closer to term.
Figure 5. Regulation of placental nutrient transfer processes.
Schematic diagram showing the effects of environmental factors in regulating the placental capacity for nutrient transfer. Composite data from sheep and mice. BNC, binucleate cells; GLUT, glucose transporters; 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2; oPL, ovine placental lactogen.
Acknowledgments
We would like to thank our many colleagues who have helped with the work and preparing this review. We would also like to thank the BBSRC for their financial support.
References
- Ain R, Canhan LN, Soares MJ. Dexamethasane-induced intrauterine growth retardation impacts the placental prolactin family, insulin-like growth factor-II and Akt signaling pathway. J Endo. 2005;185:253–263. doi: 10.1677/joe.1.06039. [DOI] [PubMed] [Google Scholar]
- Alexander G. Studies on the placenta of the sheep (Ovis avis L.): placental size. J Reprod Fert. 1964;7:289–305. doi: 10.1530/jrf.0.0070289. [DOI] [PubMed] [Google Scholar]
- Allen WR, Wilsher S, Turnbull C, Stewart F, Ousey J, Rossdale PD, Fowden AL. Influence of maternal size on placental, fetal and postnatal growth in the horse. I. Development in utero. Reproduction. 2002;123:454–465. [PubMed] [Google Scholar]
- Barbera A, Wilkening RB, Teng C, Battaglia FC, Meschia G. Metabolic alterations in the fetal hepatic and umbilical circulations during glucocorticoid-induced parturition in sheep. Ped Res. 1997;41:242–248. doi: 10.1203/00006450-199702000-00015. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies and Disease. London: BMJ Publishing Group; 1994. [Google Scholar]
- Baur R. Morphometry of the placental exchange area. Adv Anat Emb Cell Bio. 1977;53:1–63. doi: 10.1007/978-3-642-66603-2. [DOI] [PubMed] [Google Scholar]
- Bell AW, Hay WW, Ehrhardt RA. Placental transport of nutrients and its implications for fetal growth. J Reprod Fert Suppl. 1999;54:401–410. [PubMed] [Google Scholar]
- Biensen NJ, Wilson ME, Ford SP. The impacts of uterine environment and fetal genotype on conceptus size and placental vascularity during late gestation in pigs. J Anim Sci. 1999;77:954–959. doi: 10.2527/1999.774954x. [DOI] [PubMed] [Google Scholar]
- Binienda A, Marsmann A, Mitchell MD, Gleed RD, Figueroa JP, Nathanielsz PW. Effect of food withdrawal on arterial blood glucose and plasma 13,14-dihydro-15-keto-prostaglandin F2α concentrations and nocturnal myometrial electromyographic activity in the pregnant rhesus monkey in the last third of gestation: a mode of preterm labor? Am J Obstet Gynecol. 1989;160:746–750. doi: 10.1016/s0002-9378(89)80073-0. [DOI] [PubMed] [Google Scholar]
- Carver TD, Hay WW. Uteroplacental carbon substrate metabolism and O2 consumption after long term hypoglycaemia in pregnant sheep. Am J Physiol. 1995;269:E299–E308. doi: 10.1152/ajpendo.1995.269.2.E299. [DOI] [PubMed] [Google Scholar]
- Chiao E, Fisher P, Crisponi L, Deiana M, Dragatsis I, Schlersinger D, Pilia G, Efstratiadis A. Overgrowth of a mouse model of the Simpson–Golabi–Behmel syndrome is independent of IGF signalling. Devel Biol. 2002;243:185–206. doi: 10.1006/dbio.2001.0554. [DOI] [PubMed] [Google Scholar]
- Clarke K, Ward JW, Forhead AJ, Giussani DA, Fowden AL. Regulation of 11β-hydroxysteroid dehydrogenase type 2 activity in ovine placenta by fetal cortisol. J Endo. 2002;172:527–534. doi: 10.1677/joe.0.1720527. [DOI] [PubMed] [Google Scholar]
- Coan PM, Ferguson-Smith AC, Burton GJ. Developmental dynamics of the definitive mouse placenta assessed by stereology. Biol Reprod. 2004;70:1806–1813. doi: 10.1095/biolreprod.103.024166. [DOI] [PubMed] [Google Scholar]
- Coan P, Ferguson-Smith AC, Burton GJ. Imprinted genes in the placenta – a review. Placenta. 2005;26(Suppl. A):S10–S20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Constancia M, Angiolini E, Sandovic I, Smith P, Smith R, Kelsey G, Dean W, Ferguson-Smith A, Sibley C, Reik W, Fowden AL. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc Natl Acad Sci U S A. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constancia M, Hemburger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF2 is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Currie MJ, Bassett NS, Gluckman PD. Ovine glucose transporter-1 and -3: partial sequence and development expression in the placenta. Placenta. 1997;18:393–401. doi: 10.1016/s0143-4004(97)80039-2. [DOI] [PubMed] [Google Scholar]
- Dandrea J, Wilson ME, Gopalakrishna G, Heasman L, Budpa H, Stephenson T, Symonds ME. Maternal nutritional manipulation of placental growth and glucose transporter 1 (GLUT1) abundance in sheep. Reproduction. 2001;122:793–800. [PubMed] [Google Scholar]
- Das UG, Farouk Sadiq H, Soares MJ, Hay WW, Devaskar SU. Time dependent physiological regulation of rodent and ovine glucose transporter (GLUT-1) protein. Am J Physiol. 1998;274:R339–R347. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- Das UG, He J, Ehrhardt RA, Hay WW, Devaskar SW. Time dependent physiological regulation of ovine placental Glut-3 transporter protein. Am J Physiol. 2000;279:R2252–R2261. doi: 10.1152/ajpregu.2000.279.6.R2252. [DOI] [PubMed] [Google Scholar]
- Davis SR, Rattray RV, Petch ME, Duganzich DM. Interrelationships of placental development with nutrition in pregnancy and lamb birth weight. Proc NZ Soc Prod. 1981;41:218–223. [Google Scholar]
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A. Genetics of mouse growth. Int J Dev Biol. 1998;42:955–976. [PubMed] [Google Scholar]
- Faichney GJ, White GA. Effects of maternal nutritional status on fetal and placental growth and on fetal urea synthesis in sheep. Aust J Biol Sci. 1987;40:365–377. doi: 10.1071/bi9870365. [DOI] [PubMed] [Google Scholar]
- Ferrell CL. Maternal and fetal influences on uterine and conceptus in the cow. I. Growth of tissues of the gravid uterus. J Anim Sci. 1991;69:1945–1953. doi: 10.2527/1991.6951945x. [DOI] [PubMed] [Google Scholar]
- Fowden AL. Comparative aspects of fetal carbohydrate metabolism. Equin Vet J. 1997;24(Suppl.):19–25. doi: 10.1111/j.2042-3306.1997.tb05074.x. [DOI] [PubMed] [Google Scholar]
- Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–526. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Giussanni DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–734. doi: 10.1016/j.earlhumdev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long term consequences of the life insurance? Proc Nutr Soc. 1998a;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Mundy L, Silver M. Developmental regulation of glucogenesis in the sheep fetus during late gestation. J Physiol. 1998b;508:937–947. doi: 10.1111/j.1469-7793.1998.937bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Ouey JC, Forhead AJ. Comparative aspects of prepartum maturation; provision of nutrients. Pferdeheilkunde. 2001;17:653–658. [Google Scholar]
- Fowden AL, Ralph M, Silver M. Nutritional regulation of uteroplacental prostraglandin production and metabolism in pregnant ewes and mares during late gestation. Exp Clin Endo. 1994;102:212–221. doi: 10.1055/s-0029-1211285. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Hormone Res. 2006 doi: 10.1159/000091506. in press. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Ward JW, Giussani DA, Fowden AL. The effects of a reversible period of adverse intrauterine conditions during late gestation on fetal and placental weight and placetome distribution in sheep. Placenta. 2002;23:459–466. doi: 10.1053/plac.2002.0830. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Graf R, Engleman M, Beslapic D, Reul JM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endo Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- Hanguel-de Mouzon S, Shafrir E. Carbohydrate and fat metabolism and related hormonal regulation in normal and diabetic placenta. Placenta. 2001;22:619–627. doi: 10.1053/plac.2001.0698. [DOI] [PubMed] [Google Scholar]
- Harding JE, Evans PC, Gluckman PD. Maternal growth hormone treatment increases placental diffusion capacity but not fetal or placental growth in sheep. Endo. 1997;138:5352–5358. doi: 10.1210/endo.138.12.5584. [DOI] [PubMed] [Google Scholar]
- Harding JE, Johnston BM. Nutrition and fetal growth. Reprod Fert Dev. 1995;7:539–547. doi: 10.1071/rd9950539. [DOI] [PubMed] [Google Scholar]
- Hay WW. Regulation of placental metabolism by glucose supply. Reprod Fert Dev. 1995;7:365–375. doi: 10.1071/rd9950365. [DOI] [PubMed] [Google Scholar]
- Hay WW, Sparks JW, Wilkening RB, Battaglia FC, Meschia G. Partition of maternal glucose production between conceptis and maternal tissues in sheep. Am J Physiol. 1983;245:E347–E350. doi: 10.1152/ajpendo.1983.245.4.E347. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Firth K, Stephenson T, Symonds ME. Influence of restricted maternal nutrition in early to mid gestation on placental and fetal development at term in sheep. Ped Res. 1998;44:546–551. doi: 10.1203/00006450-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Stephenson TJ, Symonds ME. The influence of maternal nutrient restriction in early to mid-gestation on placental and fetal development in sheep. Proc Nutr Soc. 1999;58:283–288. doi: 10.1017/s0029665199000397. [DOI] [PubMed] [Google Scholar]
- Holst PJ, Allan CJ, Gilmour AR. Effects of restricted diet during mid pregnancy of ewes on uterine and fetal growth and lamb birth weight. Aust J Agric Res. 1992;43:315–324. [Google Scholar]
- Jansson T, Yives K, Wengren M, Powell TL. Glucose transport and system A activity in the syncytiotrophoblast microvillous and basal membranes in intrauterine growth retardation. Placenta. 2003;23:386–391. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- Jellyman JK, Gardner DS, Fowden AL, Guissani DA. Effects of dexamethasone on the uterine and umbilical vascular beds during basal and hypoxemic conditions in sheep. Am J Obstet Gynec. 2004;190:825–835. doi: 10.1016/j.ajog.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Jensen EG, Gallaher BW, Breier BH, Harding JE. The effect of chronic maternal cortisol infusion on the late-gestation fetal sheep. J Endo. 2002;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- Johnson JWC, Mitzner W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. Am J Obstet Gynec. 1979;133:677–684. doi: 10.1016/0002-9378(79)90018-8. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Nutrition and placental development. Proc Nutr Soc Aust. 1992;17:203–211. [Google Scholar]
- Langdown ML, Sugden MC. Enhanced placental GLUT1 and GLUT3 expression in dexamethasone-induced fetal growth retardation. Mol Cell Endo. 2001;185:109–117. doi: 10.1016/s0303-7207(01)00629-3. [DOI] [PubMed] [Google Scholar]
- Lesage J, Hahn D, Leonhardt M, Bondeau B, Breant B, Duprey JP. Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression but does not correlate with endogenous corticosterone levels. J Endo. 2002;174:37–43. doi: 10.1677/joe.0.1740037. [DOI] [PubMed] [Google Scholar]
- Liechty EA, Barone S, Nutt M. Effect of maternal fasting on ovine fetal and maternal branched-chain amino acid transamitrase activities. Biol Neonate. 1987;52:166–173. doi: 10.1159/000242706. [DOI] [PubMed] [Google Scholar]
- Liu L, Harding JE, Evans PC, Gluckman PD. Maternal insulin-like growth factor-I infusion alters feto-placental carbohydrate and protein metabolism in pregnant sheep. Endo. 1994;135:895–900. doi: 10.1210/endo.135.3.8070384. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Dikkes P, Zurakkowski D, Villa-Komarott L. Insulin-like growth factor II affects the appearance and glycogen content of glycogen cells in the murine placenta. Endo. 1996;137:2100–2108. doi: 10.1210/endo.137.5.8612553. [DOI] [PubMed] [Google Scholar]
- Lumey LH. Compensatory placental growth after restriction of maternal nutrition in early pregnancy. Placenta. 1998;19:105–111. doi: 10.1016/s0143-4004(98)90105-9. [DOI] [PubMed] [Google Scholar]
- Macdonald AA, Fowden AL. Microscopic anatomy of the ungulate placenta. Equine Vet J. 1997;24(Suppl.):7–13. doi: 10.1111/j.2042-3306.1997.tb05072.x. [DOI] [PubMed] [Google Scholar]
- McGrabb GH, Hosking BJ, Egan AR. Changes in the maternal body and feto-placental growth following various lengths of feed restriction during mid-pregnancy in sheep. Aust J Agri Res. 1992;43:1429–1440. [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of metabolic syndrome: prediction, plasticity and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- Matthews JC, Beveridge MJ, Dialynas E, Bartke A, Kilberg MS, Novak DA. Placental anionic and cationic amino acid transporter expression in growth hormone over expressing and null IGF-II or null IGF-I receptor mice. Placenta. 1999;20:639–650. doi: 10.1053/plac.1999.0421. [DOI] [PubMed] [Google Scholar]
- Mellor DJ. Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br Vet J. 1983;139:307–324. doi: 10.1016/s0007-1935(17)30436-0. [DOI] [PubMed] [Google Scholar]
- Molina RD, Meschia G, Battaglia FC, Hay WW. Gestational maturation of placental glucose transfer capacity in sheep. Am J Physiol. 1991;261:R697–R704. doi: 10.1152/ajpregu.1991.261.3.R697. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Boon WM, Wintour EM. Glucocortioid programming and adult disease. Cell Tissue Res. 2005;322:81–88. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Hawkins P, Harding JE. Periconceptual undernutrition alters growth trajectory and metabolic and endocrine response to fasting in late gestation fetal sheep. Ped Res. 2005;57:591–598. doi: 10.1203/01.PDR.0000155942.18096.9C. [DOI] [PubMed] [Google Scholar]
- Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on ovine fetal growth. J Endo. 2002;173:131–141. doi: 10.1677/joe.0.1730131. [DOI] [PubMed] [Google Scholar]
- Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on the placental growth trajectory and the uterine insulin-like growth factor axis in the pregnant ewe. J Endo. 2004;182:89–103. doi: 10.1677/joe.0.1820089. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Restriction of placental size in the sheep enhances the efficiency of placental transfer of antipyrine, 3-O-methy-D-glucose but not of urea. J Dev Physiol. 1987;9:457–464. [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Glucose metabolism in pregnant sheep when placental growth is restricted. Am J Physiol. 1989;257:R350–R357. doi: 10.1152/ajpregu.1989.257.2.R350. [DOI] [PubMed] [Google Scholar]
- Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]
- Pond WG, Maurer RR, Klindt J. Fetal organ response to maternal protein deprivation during pregnancy in swine. J Nutr. 1991;121:504–509. doi: 10.1093/jn/121.4.504. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Dom Anim Endo. 2004;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Regnault TRH, Friedman JE, Wilkening RB, Anthony RV, Hay WW. Fetoplacental transport and utilization of amino acids in IUGR. A review. Placenta. 2005;26(Suppl. 1):S52–S62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borawicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta. 2005;26:689–708. doi: 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Sohlstrom A, Kind KL, Karl RA, Khong TY, Robinson JS, Owens PC, Owens JA. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta. 2001;22:177–185. doi: 10.1053/plac.2000.0602. [DOI] [PubMed] [Google Scholar]
- Ross JC, Fennessey PV, Wilkening RP, Battaglia FC, Meschia G. Placental transport and fetal utilization of leucine in a model of fetal growth retardation. Am J Physiol. 1996;270:E491–E503. doi: 10.1152/ajpendo.1996.270.3.E491. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. Glucocorticoid programming. Ann NY Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GB, Fowden AL, Constancia M. Placenta-specific Igf2 regulates the diffusional exchange characteristics of the mouse placenta. Pro Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C, Glazier J, D'Souza A. Placental transporter activity and expression in relation to fetal growth. Exp Physiol. 1997;82:389–402. doi: 10.1113/expphysiol.1997.sp004034. [DOI] [PubMed] [Google Scholar]
- Sibley C, Turner MA, Cetin I, Ayuk P, Boyd R, D'Souza SW, Glazier JD, Greenwood SL, Jansson T, Powell T. Placental phenotypes of intrauterine growth. Ped Res. 2005;58:827–832. doi: 10.1203/01.PDR.0000181381.82856.23. [DOI] [PubMed] [Google Scholar]
- Silver M, Barnes RJ, Comline RS, Burton GJ. Placental blood flow: some fetal and maternal cardiovascular adjustments during gestation. J Reprod Fert Suppl. 1982;31:139–160. [PubMed] [Google Scholar]
- Stegeman HJ. Placental development in the sheep and its relation to fetal development. BiJdragen V Dierkunde. 1975;44:3–73. [Google Scholar]
- Steven DH. Comparative Placentation. London: Academic Press; 1975. [Google Scholar]
- Steven DH. Placentation in the mare. J Reprod Fert. 1982;31:41–55. [PubMed] [Google Scholar]
- Steyn C, Hawkins P, Saito T, Noakes DE, Kingdon JCP, Hanson MA. Undernutrition during the first half of gestation increases the predominance of fetal tissue in late gestation ovine placentomes. Eur J Obstet Gynec Reprod Biol. 2001;98:165–170. doi: 10.1016/s0301-2115(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Moritz KM, Shandley Y, Wintour EM. Effect of maternal glucocorticoid treatment on ovine fetal fluids at 0.6 gestation. Reprod Fert Dev. 1995;7:1595–1598. doi: 10.1071/rd9951595. [DOI] [PubMed] [Google Scholar]
- Thureen PJ, Anderson SM, Hay WW. Regulation of uterine and amino acid uptakes by maternal amino acid concentrations. Am J Physiol. 2000;279:R849–R859. doi: 10.1152/ajpregu.2000.279.3.R849. [DOI] [PubMed] [Google Scholar]
- Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am J Physiol. 1992;263:R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- Timmerman M, Teng C, Wilkening RP, Fennessey P, Battaglia FC, Meschia G. Effect of dexamethasone on fetal hepatic glutamine-glutanate exchange. Am J Physiol. 2000;278:E839–E845. doi: 10.1152/ajpendo.2000.278.5.E839. [DOI] [PubMed] [Google Scholar]
- Vatnick I, Schoknect IPA, Danigrad R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol. 1991;15:351–356. [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Milne S, Hay WW. Placental glucose transport in growth restricted pregnancies induced by overnourishing adolescent sheep. J Physiol. 2002;547:85–94. doi: 10.1113/jphysiol.2002.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JW, Forhead AJ, Wooding FBP, Fowden AL. Functional significance and cortisol dependence of the gross morphology of ovine placetome during late gestation. Biol Reprod. 2006 doi: 10.1095/biolreprod.105.046342. in press. [DOI] [PubMed] [Google Scholar]
- Ward JW, Wooding FBP, Fowden AL. The effect of cortisol on the binucleate cell population in the ovine placenta during late gestation. Placenta. 2002;23:451–458. doi: 10.1053/plac.2002.0834. [DOI] [PubMed] [Google Scholar]
- Ward JW, Wooding FPB, Fowden AL. Ovine feto-placental metabolism. J Physiol. 2004;554:529–541. doi: 10.1113/jphysiol.2003.054577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle WL, Patel FA, Alfaidy N, Holloway AC, Fraser M, Gyomovey S, Lye SJ, Gibb W, Challis JRG. Glucocorticoid regulation of human and ovine parturition: the relationship between fetal hypothalamic-pituitary-adrenal axis activation and intrauterine prostaglad production. Biol Reprod. 2001;64:1019–1032. doi: 10.1095/biolreprod64.4.1019. [DOI] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to mid gestatic programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11β hydroxsterond dehydrogenase isoforms, and type I angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Biensen NJ, Young CR, Ford SP. Development of Meishan and Yorkshire litter mate conceptures in either a Meishan or Yorkshire uterine environment to Day 90 of gestation or to term. Biol Reprod. 1998;58:905–910. doi: 10.1095/biolreprod58.4.905. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the sonatrotrophic axis and postnatal growth. J Endocrinol. 1996;150:231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- Wooding FBP, Flint APF. Placentation. In: Lamming GE, editor. Marshall's Physiology of Reproduction. 4. London: Chapman & Hall; 1994. pp. 233–460. [Google Scholar]
- Wooding FBP, Fowden AL. Nutrient transfer in the equine placenta: correlation of structure and function. Equine Vet J. 2006 doi: 10.2746/042516406776563341. in press. [DOI] [PubMed] [Google Scholar]
- Wooding FBP, Fowden AL, Bell AW, Ehrhardt RA, Limesand SW, Hay WW. Localisation of glucose transport in the ruminant placenta: implications for sequential use of transporter isoforms. Placenta. 2005;26:626–640. doi: 10.1016/j.placenta.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Zhou A, Bondy CA. Placental transporter gene expression and metabolism in the rat. J Clin Invest. 1993;91:845–852. doi: 10.1172/JCI116305. [DOI] [PMC free article] [PubMed] [Google Scholar]