Abstract
Secondary cerebral hypoperfusion is common following perinatal hypoxia–ischaemia. However, it remains unclear whether this represents a true failure to provide sufficient oxygen and nutrients to tissues, or whether it is simply a consequence of reduced cerebral metabolic demand. We therefore examined the hypothesis that cerebral oxygenation would be reduced during hypoperfusion after severe asphyxia, and further, that the greater neural injury associated with blockade of the adenosine A1 receptor during the insult would be associated with greater hypoperfusion and deoxygenation. Sixteen near-term fetal sheep received either vehicle or 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) for 1 h, followed by 10 min of severe asphyxia induced by complete occlusion of the umbilical cord. Infusions were discontinued at the end of the occlusion and data were analysed for the following 8 h. A transient, secondary fall in carotid artery blood flow and laser Doppler flow was seen from approximately 1–4 h after occlusion (P < 0.001), with no significant differences between vehicle and DPCPX. Changes in laser Doppler blood flow were highly correlated with carotid blood flow (r2 = 0.81, P < 0.001). Cortical metabolism was suppressed, reaching a nadir 1 h after occlusion and then resolving. Cortical tissue PO2 was significantly increased at 1, 2 and 3 h after occlusion compared to baseline, and inversely correlated with carotid blood flow (r2 = 0.69, P < 0.001). In conclusion, contrary to our initial hypothesis, delayed posthypoxic hypoperfusion was associated with suppression of cerebral metabolism and increased tissue PO2, and was not significantly affected by preceding adenosine A1 blockade. These data suggest that posthypoxic hypoperfusion is actively mediated and reflects suppressed cerebral metabolism.
Adverse neurological outcomes in newborn infants with hypoxic–ischaemic encephalopathy (HIE) are highly associated with poor cerebral perfusion in the first hours after birth (van Bel et al. 1993), raising the possibility that this poor perfusion may actually exacerbate injury (Osborn et al. 2003). Similarly, numerous experimental studies have shown that following initial restoration of cerebral blood flow after exposure to hypoxia–ischaemia, there is commonly a transient secondary fall in cerebral blood flow (Conger & Weil, 1995). The duration and speed of onset, and to a lesser extent the degree of the hypoperfusion, are broadly correlated with the severity of injury (Karlsson et al. 1994; Huang et al. 1999). Surprisingly, despite its near ubiquity, the significance of this delayed hypoperfusion remains highly controversial. Some data in adult species suggest that delayed hypoperfusion is due to impaired endothelial control of blood flow, and thus may extend the initial ischaemic injury (Hossmann, 1997; Ten & Pinsky, 2002). Other data suggest that it is actively mediated, and reflects reduced metabolic demand (Michenfelder & Milde, 1990; Gold & Lauritzen, 2002).
There are clinical and experimental data supporting the concept that this secondary hypoperfusion associated with HIE may represent a period of true, deleterious secondary ischaemia. For example, in newborn infants with moderate to severe hypoxic–ischaemic encephalopathy, van Bel and colleagues found a decrease in cerebral blood volume and oxygenated intracerebral haemoglobin using near-infrared spectroscopy in the first 12 h of life, consistent with decreased cerebral perfusion and oxygenation (van Bel et al. 1993). A similar reduction in cerebral oxygenation has been reported following severe asphyxia in preterm fetal sheep (Bennet et al. 1999). Further, there is evidence that this posthypoxic depression of blood flow and metabolism may be prevented by treatment with putative free radical antagonists (Rosenberg et al. 1989; Shadid et al. 1998), consistent with a toxic effect on the endothelium. However, although oxygen delivery was reduced during the posthypoxic secondary hypoperfusion period in neonatal lambs, cerebral metabolism was also reduced (Rosenberg, 1986, 1988), and others have found no significant change in arterio-venous oxygen content differences during postischaemic secondary hypoperfusion in near-term fetal sheep (Gunn et al. 1997), which would favour a primary reduction in cerebral metabolism.
We recently demonstrated that inhibition of the adenosine A1 receptor during brief but profound asphyxia in the near-term fetal sheep led to enhanced hippocampal and striatal injury (Hunter et al. 2003a). In the present study we used continuous measurements of local cortical tissue oxygenation, blood flow and metabolism in this protocol to investigate the hypothesis that secondary hypoperfusion following severe asphyxia in the immature brain would be associated with reduced tissue oxygenation, consistent with impaired coupling of cerebral blood flow and metabolism. We further examined whether the greater neural injury associated with preceding adenosine A1 receptor blockade (Hunter et al. 2003a) was associated with greater secondary hypoperfusion and deoxygenation.
Methods
Surgical procedures
Sixteen fetal sheep (gestation, 118–126 days) were operated on using sterile techniques under halothane anaesthesia (2%) (Mallard et al. 1992; Lan et al. 2000; Hunter et al. 2003a). All procedures were approved by the Animal Ethics Committee of the University of Auckland. Food but not water was withdrawn 18 h before surgery. Ewes were given 5 ml of Streptopen (procaine penicillin (250 000 IU) and dihydrostreptomycin (250 mg ml−1), Pitman-Moore, Wellington, New Zealand) intramuscularly for prophylaxis 30 min prior to the start of surgery. Anaesthesia was induced by intravenous (i.v.) injection of Saffan (alphaxalone and alphadolone; 3 mg kg−1, Schering-Plough Animal Health Ltd, Wellington, New Zealand) and general anaesthesia maintained using 2–3% halothane in O2. The depth of anaesthesia and maternal respiration were constantly monitored by trained anaesthetic staff. Under anaesthesia a 20-gauge catheter was placed in a maternal front leg vein, and the ewes were placed on a constant infusion saline drip to maintain maternal fluid balance.
Using sterile techniques, through a mid-line maternal abdominal incision the uterus was opened and the top half of the fetus exteriorised. Polyvinyl catheters were placed in each axillary artery, the right axillary vein and the amniotic sac. Two pairs of EEG electrodes (AS633–5SSF; Cooner Wire Co., Chatsworth, CA, USA) were placed on the dura over the parasagittal parietal cortex (5 mm and 10 mm anterior to bregma and 5 mm lateral) and secured with cyanoacrylate glue. A reference electrode was sewn over the occiput. A polyvinyl catheter was placed in the sagittal sinus approximately midway between the points where the coronal and lamboid sutures intersect the sagittal suture in order to measure oxygen content. This catheter was advanced 0.5–1.0 cm, such that the tip lay at or near the confluens sinum. An inflatable silicone occluder was placed around the umbilical cord of all fetuses (In Vivo Metric, Healdsburg, CA, USA).
A four-part composite probe (diameter ∼400 μm) containing emitting and receiving laser Doppler channels, a PO2 electrode and thermocouple was placed in the right parietal cortex approximately 5 mm lateral to the midline and 5 mm posterior to the coronal suture, to a depth of 5 mm below the dura, in the grey matter of the cortex (Oxford Optronix Inc., Oxford, UK) (Hunter et al. 2003a). An ultrasonic flow probe (3S, Transonic Systems Inc., Ithaca, NY, USA) was placed on the right carotid artery, near the angle of the jaw. A thermocouple (IT-18 thermometer, Physitemp, Clifton, NJ, USA) was inserted into the right lingual artery towards the carotid artery to measure arterial blood temperature, with a resolution of 0.01°C.
On completion of these procedures, all fetal leads were exteriorised through the maternal flank, the fetus returned to the uterus, the uterus and abdominal incisions repaired and a maternal long saphenous vein catheterised to provide access for postoperative care and killing. Antibiotics (80 mg gentamicin, Rousell, Auckland, New Zealand) were administered into the amniotic sac prior to closure of the uterus. Post-operatively, all sheep were housed together in separate metabolic cages with free access to water and food. They were kept in a temperature-controlled room (16 ± 1°C, humidity 50 ± 10%), in a 12-h day–night cycle. During postoperative recovery antibiotics were administered daily for 5 days i.v. to the ewe (600 mg benzylpencillin sodium (Crystapen) and 80 mg gentamicin). Fetal catheters were maintained patent by continuous infusion of heparinised isotonic saline (20 U ml−1 at 0.2 ml h−1), and the maternal catheter maintained by daily flushing with heparinised saline. Experiments were started after 3 days of postoperative recovery, and fetal arterial blood was taken daily from the brachial artery for pH, blood gas, glucose and lactate analysis to verify fetal health as previously reported (Hunter et al. 2003a), and microbial surveillance at post-mortem showed no evidence of fetal infection.
Recordings
Fetal mean arterial blood pressure (MAP) corrected for amniotic fluid pressure (Novatrans II, MX860; Medex Inc., Hilliard, OH, USA), carotid arterial blood flow (T208 Ultrasonic Flowmeter; Transonic Systems Inc.), cortical blood flow (laser Doppler flowmetry, Oxyflow; Oxford Optronics), cortical oxygen tension (Oxylite; Oxford Optronics), cortical temperature (Oxylite; Oxford Optronics), lingual arterial temperature and EEG were recorded continuously at 512 hertz (Hz) from 24 h before until 3 days after asphyxia. Data were stored to disk by custom software for off-line analysis (Labview for Windows, National Instruments Ltd, Austin, TX, USA).
The EEG signal was low-pass filtered with a 6th order low-pass Butterworth filter, with a cut-off frequency of 50 Hz. A power spectrum was then calculated from this 512 Hz sampled signal. For clarity of data display the EEG intensity was log transformed (dB, 20 × log(intensity)) (Williams et al. 1991). Carotid blood flow was measured as an index of changes in global cerebral blood flow (Gratton et al. 1996; Bishai et al. 2003; Gonzalez et al. 2005). Laser Doppler was used to measure local cortical blood flow (Bishai et al. 2003).
Brain heat production
Changes in the heat production of the brain were calculated as an index of local cerebral metabolism using the Fick principle (Hunter et al. 2003b). The difference between the temperature of arterial blood supplying the brain and the brain tissue itself gives the temperature increase resulting from brain metabolism. Multiplying this difference by local cortical blood flow (laser Doppler flow, expressed as percent change from baseline) provides the relative heat production in the local region of the brain in which the temperature sensor is placed.
Experimental protocol and drug treatment
As previously reported, fetuses were randomised to receive either vehicle or 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) infused into the right axillary vein at a rate of 3.6 mg min−1 for 10 min, and then at 0.75 mg min−1 for 60 min (Hunter et al. 2003a), a regimen known to block the bradycardia induced by cyclopentyl adenosine, a selective adenosine A1 agonist (Koos & Maeda, 2001). After infusion for 60 min, profound asphyxia was induced by complete occlusion of the umbilical cord for 10 min, confirmed by characteristic fetal cardiovascular and pH and blood gas changes (Hunter et al. 2003a). The infusion of DPCPX was discontinued at the end of the occlusion period. Fetal blood gases were measured at 8 min after the start of occlusion, then at 1, 2, 3, 4 and 6 h after umbilical cord occlusion. Three days after the hypoxic insult, the sheep were killed with sodium pentobarbitone (9 g i.v. to the ewe; Pentobarb 300, Chemstock International, Christchurch, New Zealand).
Data analysis
The effect of DPCPX on continuous variables such as cortical blood flow was evaluated by using a nested, random effect, 2-way repeated measures analysis of variance using JMP software version 5.1 (SAS Institute, Cary, NC, USA). Data were analysed using hourly averages up to 8 h after asphyxia. When an effect of Time or an interaction between Time and treatment Group was found, data were compared with baseline using the protected least significant difference test. Within subjects regression analysis by the method of Bland & Altman (1995) was used to examine the relationships between carotid blood flow and cortical blood flow, and between carotid blood flow and tPO2. This method removes the variation due to subjects and the experimental group, and expresses the variation due to the dependent variable of interest as a proportion of the sum of residual variance plus variance due the variable of interest. No effect of group was found within the regression model for either analysis. Data are shown as the mean ± s.e.m.
Results
Blood gases and tissue PO2
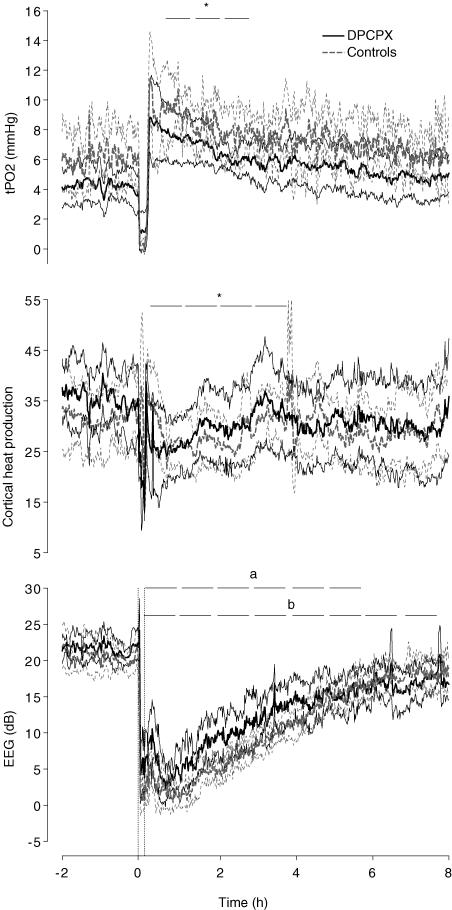
All animals had normal arterial pH and blood gases for our laboratory before occlusions, and there was no significant difference between groups in the baseline period. Fetal PO2 was significantly reduced 6 h after occlusion in both groups compared to baseline values (Table 1). There were no differences in fetal PCO2 within or between groups. Following asphyxia, there was a significant effect of Time (P < 0.001), Group (P < 0.05) and Time versus Group (P < 0.05) for fetal pH. Both control and DPCPX fetuses had a lower pH at 1 h compared to baseline values (P < 0.05). Overall, fetal pH was lower in the DPCPX group than controls after occlusion. Cerebral oxygen consumption (arterio-venous O2 content difference × percent baseline laser Doppler flow) was lower at 2, 3, 4 and 6 h after occlusion in controls and was lower at 2 and 3 h after occlusion in DPCPX infused fetuses (P < 0.05), with no significant difference between the groups (Table 1). There was a significant effect of Time on cortical tissue PO2 (tPO2) (P < 0.01), such that tPO2 was significantly increased at 1, 2 and 3 h after occlusion compared to baseline values (Fig. 1). There was no significant effect of treatment Group, and no significant interaction between Group and Time.
Table 1.
Time sequence of changes in arterial pH, blood gases and cerebral oxygen consumption after umbilical cord occlusion
| Baseline | + 8min Occ | 1 h | 2 h | 3 h | 4 h | 6 h | |
|---|---|---|---|---|---|---|---|
| Pa,O2 (mmHg) | |||||||
| Controls | 22.6 ± 1.4 | 8.8 ± 0.9* | 23.5 ± 1.1 | 21.9 ± 1.2 | 23.5 ± 2.0 | 21.0 ± 1.7 | 20.0 ± 1.6* |
| DPCPX | 19.1 ± 1.0 | 7.3 ± 1.3* | 20.4 ± 1.9 | 18.3 ± 1.4 | 18.1 ± 1.5 | 15.8 ± 1.0 | 15.5 ± 1.3* |
| Pa,CO2 (mmHg) | |||||||
| Controls | 45.6 ± 1.7 | 110.0 ± 2.7* | 46.1 ± 2.0 | 48.9 ± 2.2 | 46.6 ± 2.3 | 46.0 ± 2.0 | 49.4 ± 2.5 |
| DPCPX | 48.4 ± 2.0 | 115.5 ± 2.3* | 47.9 ± 1.5 | 47.0 ± 1.7 | 48.9 ± 1.4 | 50.1 ± 0.8 | 47.8 ± 1.2 |
| pH | |||||||
| Controls | 7.37 ± 0.01 | 6.99 ± 0.01* | 7.34 ± 0.02* | 7.37 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01 | 7.38 ± 0.01 |
| DPCPX | 7.38 ± 0.01 | 6.99 ± 0.01* | 7.30 ± 0.02* | 7.36 ± 0.02 | 7.37 ± 0.02 | 7.35 ± 0.02 | 7.36 ± 0.03 |
| CMRO2 | |||||||
| Controls | 125 ± 12 | — | 103 ± 7 | 79 ± 5* | 65 ± 14* | 83 ± 8* | 96 ± 8* |
| DPCPX | 110 ± 7 | — | 99 ± 9 | 72 ± 7* | 73 ± 12* | 88 ± 14 | 110 ± 7 |
+ 8 min Occ is time from start of occlusion, subsequent columns are time after occlusion. Values are mean ± s.e.m.; CMRO2: cerebral metabolic rate for oxygen.
P < 0.05 versus baseline.
Figure 1. Time sequence of changes in tPO2 (mmHg), cortical heat production and EEG intensity (dB) in DPCPX and vehicle control fetuses for 2 h before, during and 8 h after a 10-min umbilical cord occlusion.
The two vertical dashed lines depicted in the bottom graph indicate the start and finish of occlusion. The horizontal bars indicate significant hourly averages. Values are the mean ±s.e.m.*P < 0.05 versus baseline values for both groups, aP < 0.05 versus baseline values for controls, bP < 0.05 versus baseline values for DPCPX infused fetuses.
Haemodynamic changes
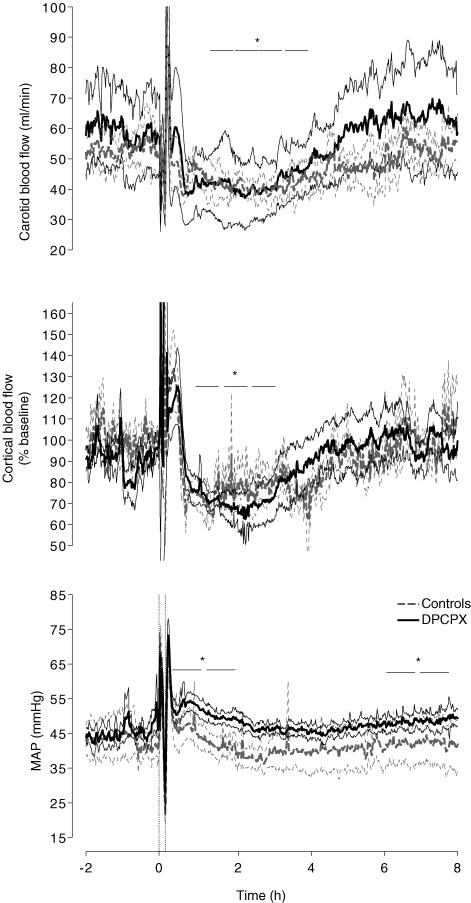
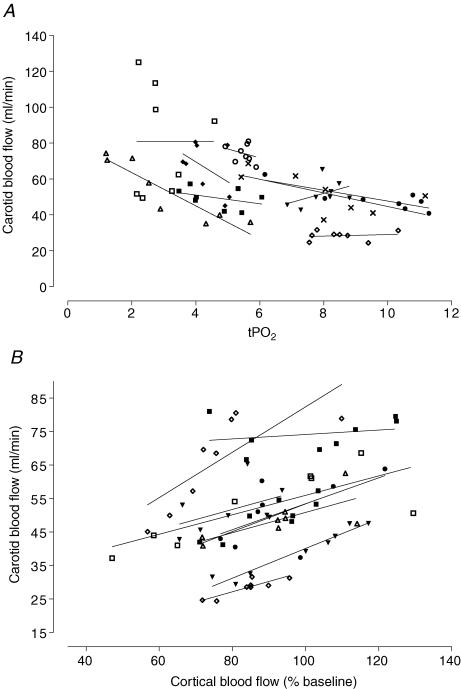
There was a significant effect of Time on changes in carotid blood flow (P < 0.001), such that carotid blood flow fell abruptly approximately 30 min after occlusion and transient hypoperfusion developed between 2 and 5 h after occlusion, with no significant difference between the groups (Fig. 2). Carotid blood flow was negatively correlated with tPO2 (Fig. 3, r2 = 0.69, P < 0.001) in this 8 h recovery period. Carotid blood flow was positively correlated with local cortical blood flow measured by laser Doppler (r2 = 0.81, P < 0.001). Cortical blood flow measured by laser Doppler showed a similar pattern of changes to carotid blood flow, with a significant fall at 2, 3 and 4 h compared to baseline values (Fig. 2, P < 0.05). There were no differences between controls and DPCPX infused fetuses up to 8 h after asphyxia.
Figure 2. Time sequence of changes in carotid blood flow (ml min−1), cortical blood flow (% baseline) and mean arterial blood pressure (mmHg) from 2 h before, during and up to 8 h after a 10-min umbilical cord occlusion.
The two vertical dashed lines depicted in the bottom graph indicate the start and finish of occlusion. The horizontal bars indicate significant hourly averages. Values are the mean ±s.e.m.*P < 0.05 versus baseline values for both groups.
Figure 3. Relationships between carotid blood flow and tPO2, and carotid blood flow and cortical blood flow (% baseline).
Individual animals from both groups are plotted at hourly time points after occlusion. Note that some animals had missing data for tPO2 or cortical flow. Carotid blood flow showed a negative within subjects linear correlation, calculated using the method of Bland & Altman (1995), with tPO2 (r2 = 0.69, P < 0.001, A) in this 8 h recovery period. Carotid blood flow was positively correlated with cortical blood flow (r2 = 0.81, P < 0.001, B).
There was a significant effect of Time for changes in fetal MAP (P < 0.001), such that there was a significant increase in MAP in both groups after asphyxia. There was a non-significant trend for MAP to be greater in the DPCPX treated fetuses. Overall, MAP was significantly increased at 1, 2, 7 and 8 h after occlusion compared with baseline (P < 0.05).
EEG intensity and cortical heat production
There was a significant effect of Time (P < 0.001), and a significant interaction between Time and Group (P < 0.05) on changes in EEG intensity, such that there was a significant decrease in EEG after asphyxia in controls up to 6 h, whereas EEG intensity tended to be greater in DPCPX animals between 1 and 4 h after asphyxia, but was still suppressed compared with baseline, 8 h after occlusion (Fig. 1). However, there was no significant difference between groups at any particular time point. Cerebral metabolism as measured by changes in cortical heat production showed a significant effect of Time, but no effect of Group, such that heat production was significantly suppressed compared to baseline from 1 to 4 h after occlusion (Fig. 1, P < 0.05).
Discussion
The present study demonstrates that, contrary to our initial hypothesis, transient delayed hypoperfusion following exposure to severe asphyxia was associated with suppressed cerebral metabolism and an increase, not decrease, in cortical tissue oxygenation. Although these results were derived from cortical measurements, together with the parallel changes in global blood flow and metabolism, these data strongly suggest that the fall in cerebral blood flow is a consequence of reduced cerebral metabolism, rather than being a cause of further injury. This finding is highly unlikely to be due to mild or trivial injury since this model is associated with marked neuronal loss particularly in the hippocampus (Mallard et al. 1992; Fujii et al. 2001; Hunter et al. 2003a) and with widespread lipid peroxidation, suggestive of severe oxidative stress (Miller et al. 2005). Supporting this interpretation, preceding treatment with the adenosine A1 receptor antagonist DPCPX, which we have previously shown to cause enhanced neural injury after 3 days recovery, was associated with a similar, evolving fall in cortical metabolism and reciprocal increase in tissue oxygenation.
Although this is the first description of the time course of secondary cerebral hypoperfusion in this particular model of profound asphyxia in near-term fetal sheep, the phenomenon has been consistently reported in different paradigms of reversible hypoxia–ischaemia in perinatal (Rosenberg, 1986, 1988; Gunn et al. 1997; Bennet et al. 1999) and adult animals (Rosenberg, 1988; Conger & Weil, 1995; Hossmann, 1997). Consistent with such previous reports, in the current study we observed an initial rapid recovery of carotid blood flow after release of the umbilical occluder, and there was an abrupt fall in flow from approximately 30 min, which gradually resolved by 6 h after occlusion.
This pattern of hypoperfusion was extremely similar in both carotid blood flow and local cortical flow measured by laser Doppler. Previous studies have suggested that carotid blood flow is a valid index of global cerebral blood flow. Changes in blood flow velocity in the carotid artery correlate well with microsphere measurements during physiological changes such as changes in arterial oxygen content (Rosenberg et al. 1985; van Bel et al. 1994; Gratton et al. 1996), carbon dioxide pressures (Gratton et al. 1996) and altered perfusion pressure except at exceptionally high levels (van Bel et al. 1994). Similarly, carotid blood flow correlated closely with relative change in laser Doppler measurements when perfusion pressure was altered using a range of interventions such as haemorrhage and increased intracranial pressure in the piglet (Kirkeby & Rise, 1999), and during seizures in the fetal sheep (Gonzalez et al. 2005). The present study supports these findings, and confirms a close relationship between carotid blood flow and local cortical flow even during pathological hypoperfusion.
The present finding that tissue oxygenation was increased is in contrast with previous studies that suggested that secondary hypoperfusion may be associated with impaired oxygenation. NIRS studies have shown that cerebral blood volume and oxygenation were decreased in the first 12 h of life in severely asphyxiated neonates (van Bel et al. 1993), and in preterm fetal lambs in the first few hours after the end of profound asphyxia (Bennet et al. 1999), suggesting that cerebral oxygen delivery is limited relative to requirements. The contrasting finding may be related to differences in maturational stage, severity of the insult, and species between studies. Further, the present study only examined oxygenation in the cortical grey matter, and thus it remains possible that there may be regional inhomogeneity, with reduced oxygenation elsewhere within the brain. However, it is important to note that NIRS measures changes in relative oxygenation of blood, not brain tissue partial pressures, and thus, these findings could reflect a relatively greater reduction in volume of the arterial compartment compared with the venous compartment during hypoperfusion, rather than the adequacy of tissue oxygenation. Similar to the present data, there is evidence that oxygen consumption is decreased during secondary hypoperfusion following asphyxia or ischaemia in the newborn and fetal lamb (Rosenberg, 1986; Gunn et al. 1997), with no changes in cerebral oxygen extraction (Gunn et al. 1997). Similarly, there is evidence of a significant increase in cytochrome oxidase after 30 min of hypoxia and hypercapnia in the newborn lamb, consistent with reduced oxygen utilisation by the brain during this phase (Shadid et al. 1999). These findings of reduced oxygen utilisation are consistent with the increased cortical tissue PO2 from the present study, which is the first direct evidence of tissue oxygenation during secondary hypoperfusion.
Consistent with the increase in cortical oxygenation in the present study, both global cerebral oxygen consumption and local cerebral metabolism, as measured by cortical heat production, were suppressed during secondary hypoperfusion. These data strongly indicate that postasphyxial hypoperfusion is mediated by intact coupling between cerebral blood flow and suppressed cerebral metabolism. Although local cerebral blood flow and metabolism are generally coupled under physiological conditions, the ratio appears to be dependent on the pattern of neural activity as well as its magnitude (Thompson et al. 2004). Our finding that cortical tPO2 was increased suggests that cerebral blood flow was not reduced as much as metabolism, which is consistent with these previous findings and suggests a non-linear relationship at this time. Further, it is possible that additional factors may also contribute to the hypoperfusion such as increased sympathetic nervous system activity (Quaedackers et al. 2004) or increased free radical release (Rosenberg et al. 1989; Shadid et al. 1998).
The mechanism of this suppression of cerebral metabolism after asphyxia is not known, but potentially, it could be a ‘passive’ function of cellular hypoxic injury, or an actively regulated inhibition which may help improve recovery; it is likely that both mechanisms are important. We have previously shown that adenosine A1 receptor blockade with DPCPX resulted in greater electrocortical activity during the first 5 min of asphyxia, with attenuated suppression of heat production and a marked increase in cerebral damage compared with controls (Hunter et al. 2003a). In contrast, in the present study DPCPX was associated with a minimal effect on the time course of recovery of EEG suppression, but no effect on cortical heat production or blood flow after asphyxia. This finding may in part reflect rapid clearance after the end of the infusion, but may also be due to the observation that the hypoxic increase in adenosine rapidly returns to normal after reoxygenation (Blood et al. 2002), reflecting the high activity of adenosine deaminase.
Other potential inhibitory neuromodulators, however, are known to be elevated after severe hypoxia, including the GABAA receptor agonist allopregnanolone (Nguyen et al. 2004), which is neuroprotective in adult models (He et al. 2004; Lapchak, 2004). Intriguingly this elevation is reported to resolve to preocclusion levels by 3–4 h after occlusion (Nguyen et al. 2004), consistent with the time course of suppressed metabolism in the present study. Further, the very rapid fall in carotid blood flow at the start of the phase of secondary hypoperfusion is highly suggestive of an active, neural mechanism, rather than a purely passive phenomenon. We have recently reported that an infusion of an α-adrenergic receptor antagonist shortly after the end of umbilical cord occlusion prevents secondary hypoperfusion of peripheral organs in the preterm fetal sheep; strikingly this treatment was associated with earlier onset of postasphyxial seizures (Quaedackers et al. 2004), raising the possibility that central inhibitory α2-adrenergic activity may be important after asphyxia. Although speculative at this time, this possibility is supported by data in adult models of ischaemia (Reis et al. 1997; Shuaib & Breker-Klassen, 1997; Golanov & Zhou, 2003).
In conclusion, we found that delayed secondary cerebral hypoperfusion following a severe asphyxial insult was associated with marked suppression of cerebral metabolism and a corresponding increase in cortical tPO2. These data strongly suggest that secondary cerebral hypoperfusion is a consequence of reduced cerebral metabolism. Hypoperfusion was not significantly affected by preceding adenosine A1 receptor blockade. We speculate that this transient suppression of cerebral metabolism may be actively regulated, and that it may help protect the brain from further injury.
Acknowledgments
This study was supported by the Health Research Council of New Zealand, Auckland Medical Research Foundation, Lottery Grants Board of New Zealand and USPHS NIH HL 654941.
References
- Bennet L, Rossenrode S, Gunning MI, Gluckman PD, Gunn AJ. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J Physiol. 1999;517:247–257. doi: 10.1111/j.1469-7793.1999.0247z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol. 2003;546:869–878. doi: 10.1113/jphysiol.2002.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: Part 1 – Correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AB, Hunter CJ, Power GG. The role of adenosine in regulation of cerebral blood flow during hypoxia in the near-term fetal sheep. J Physiol. 2002;543:1015–1023. doi: 10.1113/jphysiol.2002.023077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JD, Weil JV. Abnormal vascular function following ischemia-reperfusion injury. J Invest Med. 1995;43:431–442. [PubMed] [Google Scholar]
- Fujii E, Kodama Y, Takahashi N, Roman C, Ferriero D, Gregory G, et al. Fructose-1,6-bisphosphate did not affect hippocampal neuronal damage caused by 10 min of complete umbilical cord occlusion in fetal sheep. Neurosci Lett. 2001;309:49–52. doi: 10.1016/s0304-3940(01)02026-2. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Zhou P. Neurogenic neuroprotection. Cell Mol Neurobiol. 2003;23:651–663. doi: 10.1023/A:1025088516742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–7704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Hunter CJ, Bennet L, Power GG, Gunn AJ. Cerebral oxygenation during post-asphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab. 2005;25:911–918. doi: 10.1038/sj.jcbfm.9600087. [DOI] [PubMed] [Google Scholar]
- Gratton R, Carmichael L, Homan J, Richardson B. Carotid arterial blood flow in the ovine fetus as a continuous measure of cerebral blood flow. J Soc Gynecol Invest. 1996;3:60–65. doi: 10.1016/1071-5576(95)00047-X. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Gunn TR, de Haan HH, Williams CE, Gluckman PD. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest. 1997;99:248–256. doi: 10.1172/JCI119153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8:95–101. doi: 10.1097/00024382-199708000-00004. [DOI] [PubMed] [Google Scholar]
- Huang J, Kim LJ, Poisik A, Pinsky DJ, Connolly ES., Jr Titration of postischemic cerebral hypoperfusion by variation of ischemic severity in a murine model of stroke. Neurosurgery. 1999;45:328–333. doi: 10.1097/00006123-199908000-00027. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Bennet L, Power GG, Roelfsema V, Blood AB, Quaedackers JS, George S, Guan J, Gunn AJ. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke. 2003a;34:2240–2245. doi: 10.1161/01.STR.0000083623.77327.CE. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Blood AB, Power GG. Cerebral metabolism during cord occlusion and hypoxia in the fetal sheep: a novel method of continuous measurement based on heat production. J Physiol. 2003b;552:241–251. doi: 10.1113/jphysiol.2003.048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson BR, Grogaard B, Gerdin B, Steen PA. The severity of postischemic hypoperfusion increases with duration of cerebral ischemia in rats. Acta Anaesthesiol Scand. 1994;38:248–253. doi: 10.1111/j.1399-6576.1994.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Kirkeby OJ, Rise IR. Intracerebral laser Doppler blood flow measurements compared to blood flow in porcine internal carotid artery. J Clin Neurosci. 1999;6:389–394. doi: 10.1054/jocn.1999.0088. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Maeda T. Adenosine A2A receptors mediate cardiovascular responses to hypoxia in fetal sheep. Am J Physiol Heart Circ Physiol. 2001;280:H83–H89. doi: 10.1152/ajpheart.2001.280.1.H83. [DOI] [PubMed] [Google Scholar]
- Lan J, Hunter CJ, Murata T, Power GG. Adaptation of laser-Doppler flowmetry to measure cerebral blood flow in the fetal sheep. J Appl Physiol. 2000;89:1065–1071. doi: 10.1152/jappl.2000.89.3.1065. [DOI] [PubMed] [Google Scholar]
- Lapchak PA. The neuroactive steroid 3-α-ol-5-β-pregnan-20-one hemisuccinate, a selective NMDA receptor antagonist improves behavioral performance following spinal cord ischemia. Brain Res. 2004;997:152–158. doi: 10.1016/j.brainres.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Gunn AJ, Williams CE, Johnston BM, Gluckman PD. Transient umbilical cord occlusion causes hippocampal damage in the fetal sheep. Am J Obstet Gynecol. 1992;167:1423–1430. doi: 10.1016/s0002-9378(11)91728-1. [DOI] [PubMed] [Google Scholar]
- Michenfelder JD, Milde JH. Postischemic canine cerebral blood flow appears to be determined by cerebral metabolic needs. J Cereb Blood Flow Metab. 1990;10:71–76. doi: 10.1038/jcbfm.1990.9. [DOI] [PubMed] [Google Scholar]
- Miller SL, Yan EB, Castillo-Melendez M, Jenkin G, Walker DW. Melatonin provides neuroprotection in the late-gestation fetal sheep brain in response to umbilical cord occlusion. Dev Neurosci. 2005;27:200–210. doi: 10.1159/000085993. [DOI] [PubMed] [Google Scholar]
- Nguyen P, Yan EB, Castillo-Melendez M, Walker DW, Hirst JJ. Increased allopregnanolone levels in the fetal sheep brain following umbilical cord occlusion. J Physiol. 2004;560:593–602. doi: 10.1113/jphysiol.2004.069336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Heineman E, Gunn AJ, Bennet L. The role of the sympathetic nervous system in post-asphyxial intestinal hypoperfusion in the preterm sheep fetus. J Physiol. 2004;557:1033–1044. doi: 10.1113/jphysiol.2004.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Golanov EV, Galea E, Feinstein DL. Central neurogenic neuroprotection: central neural systems that protect the brain from hypoxia and ischemia. Ann N Y Acad Sci. 1997;835:168–186. doi: 10.1111/j.1749-6632.1997.tb48628.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg AA. Cerebral blood flow and O2 metabolism after asphyxia in neonatal lambs. Pediatr Res. 1986;20:778–782. doi: 10.1203/00006450-198608000-00016. [DOI] [PubMed] [Google Scholar]
- Rosenberg AA. Regulation of cerebral blood flow after asphyxia in neonatal lambs. Stroke. 1988;19:239–244. doi: 10.1161/01.str.19.2.239. [DOI] [PubMed] [Google Scholar]
- Rosenberg AA, Murdaugh E, White CW. The role of oxygen free radicals in postasphyxia cerebral hypoperfusion in newborn lambs. Pediatr Res. 1989;26:215–219. doi: 10.1203/00006450-198909000-00012. [DOI] [PubMed] [Google Scholar]
- Rosenberg AA, Narayanan V, Jones MD., Jr Comparison of anterior cerebral artery blood flow velocity and cerebral blood flow during hypoxia. Pediatr Res. 1985;19:67–70. doi: 10.1203/00006450-198501000-00018. [DOI] [PubMed] [Google Scholar]
- Shadid M, Hiltermann L, Monteiro L, Fontijn J, van Bel F. Near infrared spectroscopy-measured changes in cerebral blood volume and cytochrome aa3 in newborn lambs exposed to hypoxia and hypercapnia, and ischemia: a comparison with changes in brain perfusion and O2 metabolism. Early Hum Dev. 1999;55:169–182. doi: 10.1016/s0378-3782(99)00024-9. [DOI] [PubMed] [Google Scholar]
- Shadid M, Moison R, Steendijk P, Hiltermann L, Berger HM, van Bel F. The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr Res. 1998;44:119–124. doi: 10.1203/00006450-199807000-00019. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Breker-Klassen MM. Inhibitory mechanisms in cerebral ischemia: a brief review. Neurosci Biobehav Rev. 1997;21:219–226. doi: 10.1016/s0149-7634(96)00012-7. [DOI] [PubMed] [Google Scholar]
- Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8:242–250. doi: 10.1097/00075198-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Thompson JK, Peterson MR, Freeman RD. High-resolution neurometabolic coupling revealed by focal activation of visual neurons. Nat Neurosci. 2004;7:919–920. doi: 10.1038/nn1308. [DOI] [PubMed] [Google Scholar]
- van Bel F, Dorrepaal CA, Benders MJ, Zeeuwe PE, van de Bor M, Berger HM. Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics. 1993;92:365–372. [PubMed] [Google Scholar]
- van Bel F, Roman C, Klautz RJ, Teitel DF, Rudolph AM. Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr Res. 1994;35:329–333. doi: 10.1203/00006450-199403000-00011. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke. 1991;22:516–521. doi: 10.1161/01.str.22.4.516. [DOI] [PubMed] [Google Scholar]