Abstract
Early onset cerebral hypoperfusion after birth is highly correlated with neurological injury in premature infants, but the relationship with the evolution of injury remains unclear. We studied changes in cerebral oxygenation, and cytochrome oxidase (CytOx) using near-infrared spectroscopy in preterm fetal sheep (103–104 days of gestation, term is 147 days) during recovery from a profound asphyxial insult (n = 7) that we have shown produces severe subcortical injury, or sham asphyxia (n = 7). From 1 h after asphyxia there was a significant secondary fall in carotid blood flow (P < 0.001), and total cerebral blood volume, as reflected by total haemoglobin (P < 0.005), which only partially recovered after 72 h. Intracerebral oxygenation (difference between oxygenated and deoxygenated haemoglobin concentrations) fell transiently at 3 and 4 h after asphyxia (P < 0.01), followed by a substantial increase to well over sham control levels (P < 0.001). CytOx levels were normal in the first hour after occlusion, was greater than sham control values at 2–3 h (P < 0.05), but then progressively fell, and became significantly suppressed from 10 h onward (P < 0.01). In the early hours after reperfusion the fetal EEG was highly suppressed, with a superimposed mixture of fast and slow epileptiform transients; overt seizures developed from 8 ± 0.5 h. These data strongly indicate that severe asphyxia leads to delayed, evolving loss of mitochondrial oxidative metabolism, accompanied by late seizures and relative luxury perfusion. In contrast, the combination of relative cerebral deoxygenation with evolving epileptiform transients in the early recovery phase raises the possibility that these early events accelerate or worsen the subsequent mitochondrial failure.
Prematurely born infants continue to have a high rate of neurodevelopmental handicap, including overt cerebral palsy and cognitive and learning deficits (Marlow et al. 2005). Although the underlying pathogenesis of injury is complex and multifactorial (Volpe, 2001), exposure to hypoxia–ischaemia before, during or after birth is a major factor that contributes to adverse neurodevelopmental outcomes (Volpe, 2001). In the near-term brain there is now extensive clinical and experimental evidence that perinatal hypoxia–ischaemia can lead to a biphasic pattern of impaired oxidative energy metabolism, with initial recovery from the acute hypoxic depression, in a transient ‘latent’ phase, before secondarily falling as late as 6–15 h after reperfusion in the piglet (Lorek et al. 1994), the neonatal rat (Vannucci et al. 2004), and clinically (Azzopardi et al. 1989; Roth et al. 1997). There is little information available on the timing of these phases of injury in relevant preterm models. This is of considerable practical importance, because, as recently reviewed, the latent phase appears to correspond broadly with the practical window of opportunity for treatment (Gunn et al. 2005).
We and others have recently reported that exposure to severe hypoxia in the preterm fetal sheep is associated with diffuse white matter loss and severe subcortical neural injury (George et al. 2004; Welin et al. 2005; Dean et al. 2006), consistent with clinical observations (Barkovich & Sargent, 1995). Following this insult, the early recovery phase is marked by evolving abnormal neural activity, including frequent epileptiform transients (George et al. 2004; Dean et al. 2006), followed by delayed onset of large amplitude, stereotypical seizures, from approximately 10 h (Quaedackers et al. 2004a; Dean et al. 2006). The early EEG transients were maximal in the first 4 h after reperfusion, and were only seen in fetuses that later developed subcortical neuronal loss (George et al. 2004). Similar EEG changes have been observed in the preterm newborn; overall epileptiform transient activity is strongly associated with adverse outcome (Rowe et al. 1985; Hughes & Guerra, 1994; Vecchierini-Blineau et al. 1996; Marret et al. 1997; Biagioni et al. 2000; Okumura et al. 2003). In contrast, although seizures are associated with marked hypermetabolism with depletion of local metabolites, studies in rats suggest that the immature brain is relatively resistant to subsequent injury (Fernandes et al. 1999; Haas et al. 2001; Pereira de Vasconcelos et al. 2002). There is some evidence from near-term fetal sheep that delayed onset seizures after transient cerebral ischaemia are a marker or reflection of the onset of secondary failure of oxidative metabolism (Marks et al. 1996, 1999).
There is considerable evidence suggesting that mitochondrial dysfunction, with loss of cytochrome oxidase (CytOx) activity and depletion of high energy phosphates, is either causally related to, or at least tightly coupled with, the onset of delayed energy failure after hypoxic–ischaemic injury (Vannucci et al. 2004). There is a close correlation between histological loss of CytOx and neuronal loss (Dimlich et al. 1990; Wagner et al. 1990a; Nelson & Silverstein, 1994), and between the timing of loss of CytOx activity after severe anoxia in the cat and subsequent delayed neurological deterioration (Wagner et al. 1990b). These data are supported by in vitro evidence that the well described increase in intracellular calcium levels during hypoxia/reoxygenation triggers subsequent delayed functional impairment and morphological disintegration of mitochondria (Schild et al. 2003).
The time course of changes in CytOx can be monitored using near-infrared spectroscopy (NIRS). Studies using continuous NIRS monitoring in the newborn piglet have firstly confirmed evolving loss of CytOx after severe hypoxia–ischaemia (Chang et al. 1999), and secondly demonstrated a close correlation between CytOx and depletion of high energy metabolites on magnetic resonance spectroscopy (Tsuji et al. 1995; Chang et al. 1999; Peeters-Scholte et al. 2004). These data all relate to the term or post-term brain; there is no information on the evolution of mitochondrial dysfunction in the preterm brain, or when posthypoxic epileptiform transient activity and seizures occur in relation to the onset of failure of oxidative metabolism.
The goal of the present study was to examine the hypothesis that early EEG epileptiform transient activity after severe hypoxia in the preterm fetus occurs prior to the onset of secondary mitochondrial failure, whereas the onset of overt seizures would correspond with the onset of secondary energy failure as measured by changes in cerebral oxygenation and mitochondrial activity using NIRS. In terms of cerebral maturity the 0.7 gestation fetal sheep is comparable to the human brain at term at 28–32 weeks of gestation (McIntosh et al. 1979), prior to the onset of cortical myelination (Barlow, 1969).
Methods
All procedures were approved by the Animal Ethics Committee of The University of Auckland. Fourteen singleton Romney/Suffolk fetal sheep were operated on at 98–99 days of gestation (term = 147 days). Food, but not water was withdrawn 18 h before surgery. Ewes were given 5 ml of streptocin (procaine penicillin 250 000 IU and dihydrostreptomycin (250 mg ml−1); Stockguard Laboratories Ltd, Hamilton, New Zealand) intramuscularly for prophylaxis 30 min prior to the start of surgery. Anaesthesia was induced by i.v. injection of Alfaxan (3 mg kg−1; Alphaxalone, Jurox, Rutherford, NSW, Australia), and general anaesthesia maintained using 2–3% halothane in O2. Under anaesthesia a 20-g i.v. catheter was placed in a maternal front leg vein and the ewes were placed on a constant infusion saline drip to maintain maternal fluid balance. Ewes were not ventilated and the depth of anaesthesia, maternal heart rate and respiration were constantly monitored by trained anaesthetic staff.
All surgical procedures were performed using sterile techniques (Bennet et al. 1999). Following a maternal midline abdominal incision and exteriorization of the uterus and either the top or bottom half of the fetus, catheters were placed in the left fetal femoral artery and vein, right axillary artery and the amniotic sac. An ultrasonic blood flow probe (size 3S; Transonic Systems Inc., Ithaca, NY, USA) was placed around the left carotid artery to measure carotid blood flow (CaBF) as an index of global cerebral blood flow (van Bel et al. 1994; Bennet et al. 1999; Hunter et al. 2003; Gonzalez et al. 2005). Two small flexible fibre optic probes, used for the NIRS recordings, were placed biparietally on the skull 3.0 cm apart, 1.5 cm anterior to bregma, and secured using rapid setting dental cement (Rocket Red, Dental Adventures of America, Inc., Anaheim, CA, USA). Two pairs of EEG electrodes (AS633-5SSF, Cooner Wire Co., Chatsworth, CA, USA) were placed on the dura over the parasagittal parietal cortex (5 mm and 10 mm anterior to bregma and 5 mm lateral) and secured with cyanoacrylate glue. A reference electrode was sewn over the occiput. A pair of electrodes were sewn over the fetal chest to measure the fetal electrocardiogram (ECG). An inflatable silicone occluder was placed around the umbilical cord of all fetuses (In Vivo Metric, Healdsburg, CA, USA). All fetal leads were exteriorized through the maternal flank and a maternal long saphenous vein was catheterized to provide access for postoperative care and killing. Antibiotics (80 mg gentamicin, Rousell, Auckland, New Zealand) were administered into the amniotic sac prior to closure of the uterus.
Post-operatively all sheep were housed in separate metabolic cages with access to water and food ad libitum, together in a temperature-controlled room (16 ± 1°C, humidity 50 ± 10%) with a 12-h light–dark cycle. A period of 5 days postoperative recovery was allowed before experiments commenced, during which time antibiotics were administered to the ewe daily for four days i.v. (600 mg benzylpenicillin sodium; Novartis Ltd, Auckland, New Zealand, and 80 mg gentamicin). Fetal catheters were maintained patent by continuous infusion of heparinized saline (20 U ml−1 at 0.15 ml h−1) and the maternal catheter maintained by daily flushing.
Experimental procedures
Recordings
Fetal arterial blood pressure (Novatrans II, MX860; Medex Inc., Hilliard, OH, USA), corrected for maternal movement by subtraction of amniotic fluid pressure (Lawler & Brace, 1982), ECG, EEG and EMG were recorded continuously. The blood pressure signal was collected at 64 Hz and lowpass filtered at 30 Hz. The nuchal EMG signal was band-pass filtered between 100 Hz and 1 kHz, the signal was then integrated using a time constant of 1 s. The analog fetal EEG signal was lowpass filtered with the cut-off frequency set with the −3 dB point at 30 Hz, and digitized at 256 Hz (using analog to digital cards, National Instruments Corp., Austin, TX, USA). The intensity and frequency were derived from the intensity spectrum signal between 0.5 and 20 Hz (Williams et al. 1992). For data presentation, the total EEG intensity (power) was normalized by log transformation (dB, 20 × log intensity), and data from left and right EEG electrodes were averaged to give mean total EEG activity. The 90% spectral edge of the EEG, i.e. the frequency below which lay 90% of the EEG intensity, was calculated from the spectra. Data were collected by computer and stored to disk for off-line analysis.
Experimental protocol
Experiments were conducted at 103–104 days gestation. Fetal mean arterial blood pressure (MAP), corrected for maternal movement by subtraction of amniotic fluid pressure, CaBF, fetal heart rate (FHR) derived from the fetal ECG and NIRS signals were recorded continuously from the day before occlusion to 72 h after occlusion. Fetuses were randomly assigned to the sham control (n = 7) or the occlusion group (n = 7).
Fetal asphyxia was induced by rapid inflation of the umbilical cord occluder for 25 min with sterile saline of a defined volume known to completely inflate the occluder and totally compress the umbilical cord, as determined in pilot experiments with a Transonic flow probe placed around an umbilical vein (Bennet et al. 1999). Successful occlusion was confirmed by observation of a rapid onset of bradycardia with a rise in MAP, and by pH and blood gas measurements. The duration of occlusion was chosen as one that we have previously reported to represent an acute, severe, near-terminal insult but which could be survived without postasphyxial cardiac support (Bennet et al. 2000; Quaedackers et al. 2004a,b), and is associated with severe subcortical neuronal loss (Dean et al. 2006). All occlusions or sham occlusions were undertaken around 10.00 h.
Fetal arterial blood was taken at 15 min prior to asphyxia, 5 and 20 min during asphyxia and 2, 4, 6, 24, 48 and 72 h postasphyxia for pH and blood gas determination (Ciba-Corning Diagnostics 845 blood gas analyser and co-oximeter, MA, USA) and for glucose and lactate measurements (YSI model 2300, Yellow Springs, OH, USA). After the last blood sample ewes and fetuses were killed by an intravenous overdose of pentobarbitone sodium (9 g) to the ewe (Pentobarb 300; Chemstock International, Christchurch, New Zealand).
Near-infrared spectroscopy (NIRS) measurements
Concentration changes in fetal cerebral deoxy haemoglobin ([Hb]), oxyhaemoglobin ([HbO2]) and [CytOx] were measured using a NIR 500 spectrophotometer (Hamamatsu Photonics KK, Hamamatsu City, Japan) and data recorded by computer for off-line analysis. The principles of NIRS have been previously described (Reynolds et al. 1988). Briefly, near-infrared light, at four different wavelengths between 775 and 908 nm, was carried to the fetal head through a fibre optic bundle. Emerging light was collected by the second optode and transmitted to the spectrophotometer. Changes in the cerebral [HbO2], [Hb] and [CytOx] were calculated from the modified Lambert-Beer law using a previously established algorithm which describes optical absorption in a highly scattering medium (Reynolds et al. 1988; Wyatt et al. 1990). The NIRS measures obtained are relative changes from zero not absolute changes. Standardization of the distance between the optodes and fixation of the optodes to the surface of the skull by dental cement were used to reduce signal variability within and between subjects in this study.
Two key parameters were calculated: total haemoglobin ([THb]), the sum of [HbO2] and [Hb]; and [DHb], the difference between [HbO2] and [Hb]. THb is related to cerebral blood volume (CBV) by the cerebral haematocrit: CBV = [THb]/(HR) where H is the arterial haematocrit and R is the cerebral-to-large vessel haematocrit ratio, assumed to be 0.69 (Wyatt et al. 1990). THb is as an index of CBV given a stable blood haemoglobin and haematocrit (van Bel et al. 1993). DHb is a measure of total intravascular oxygenation in the brain (Brun et al. 1997).
Data analysis
Off-line analysis of the physiological data was performed using customised Labview programs (National Instruments). The raw EEG was assessed for epileptiform activity; specifically the presence of spikes, and sharp and slow waves (i.e. epileptiform transients). A spike was defined as having a sharp outline and duration of less than 70 ms. Sharp waves were assessed as transient high frequency events of moderate to high amplitude (50–200 μV), single or repeated mono- or diphasic transients lasting 100–250 ms, typically superimposed on a flat EEG background (Scher, 2003; George et al. 2004; Dean et al. 2006). Transients were counted up to 7 h after occlusion from the raw EEG record per minute and the total number per hour was summed. Seizures were identified visually and defined as the concurrent appearance of sudden, repetitive, evolving stereotyped waveforms in the EEG signal lasting more than 10 s and of an amplitude greater than 20 μV (Scher et al. 1993).
Data were analysed using SPSS for windows (SPSS, Chicago, IL, USA). For the analysis of the recovery data after occlusion (from 1 to 72 h post-occlusion), the baseline period was taken as the mean of the 12 h before occlusion. For between group comparisons two way analysis of variance for repeated measures was performed. When statistical significance was found between groups or between group and time analysis of covariance (ANCOVA) was used to compare selected time points, using the baseline control periods prior to occlusion as a covariate. Statistical significance was accepted when P < 0.05. Data are means ± s.e.m.
Results
Baseline
All fetuses had normal FHR, MAP, blood gases, acid–base, glucose and lactate status, and EEG signals before each experiment, according to the standards of our laboratory. Values and statistical comparisons for arterial pH, blood gases, and glucose and lactate levels for the sham control and occlusion groups are presented in Table 1. Umbilical cord occlusion was associated with marked fetal hypoxia and acidosis (Table 1), bradycardia, hypotension, cerebral hypoperfusion and EEG suppression (P < 0.001 data not shown), similar to our previous reports in this model (Bennet et al. 1999, 2000; George et al. 2004; Quaedackers et al. 2004a,b).
Table 1.
Fetal arterial pH, blood gases, glucose and lactate values for control (C) and umbilical cord occlusion (O) groups 15 min before (control) and at the end of asphyxia (20 min), and after either sham or 25 min umbilical cord occlusion
| Control | 20 min | 2 h post | 4 h post | 6 h post | 24 h post | 48 h post | 72 h post | ||
|---|---|---|---|---|---|---|---|---|---|
| pHa | C | 7.36 ± 0.01 | 7.38 ± 0.01 | 7.39 ± 0.01 | 7.38 ± 0.00 | 7.39 ± 0.01 | 7.37 ± 0.01 | 7.36 ± 0.01 | 7.34 ± 0.02 |
| O | 7.37 ± 0.01 | 6.85 ± 0.05§ | 7.36 ± 0.01 | 7.39 ± 0.01 | 7.4.0 ± 0.01 | 7.36 ± 0.01 | 7.37 ± 0.01 | 7.38 ± 0.01 | |
| Pa,CO2 (mmHg) | C | 49.1 ± 1.8 | 48.1 ± 1.5 | 46.0 ± 1.2 | 48.0 ± 1.5 | 48.6 ± 1.6 | 47.6 ± 2.1 | 48.6 ± 1.9 | 46.6 ± 2.0 |
| O | 49.1 ± 1.3 | 138.9 ± 11.8§ | 44.6 ± 1.1 | 46.4 ± 1.3 | 45.8 ± 1.3 | 46.6 ± 1.5 | 47.1 ± 1.6 | 44.1 ± 1.0 | |
| Pa,O2 (mmHg) | C | 24.2 ± 0.9 | 23.8 ± 1.1 | 23.4 ± 0.9 | 23.8 ± 0.9 | 23.4 ± 0.8 | 22.7 ± 0.7 | 22.6 ± 0.6 | 24.0 ± 0.8 |
| O | 22.4 ± 0.8 | 10.8 ± 2.2§ | 25.9 ± 1.3 | 22.7 ± 1.2 | 24.2 ± 1.0 | 26.5 ± 1.2§ | 27.0 ± 1.4§ | 28.7 ± 0.9§ | |
| Hb (g dl−1) | C | 8.7 ± 0.2 | 8.9 ± 0.1 | 9.0 ± 0.3 | 9.0 ± 0.2 | 8.9 ± 0.2 | 8.9 ± 0.2 | 9.2 ± 0.2 | 10.0 ± 0.4 |
| O | 8.5 ± 0.3 | 8.9 ± 0.4 | 9.3 ± 0.5 | 9.0 ± 0.4 | 9.1 ± 0.5 | 9.2 ± 0.4 | 8.9 ± 0.3 | 9.2 ± 0.4 | |
| Hct (%) | C | 25.8 ± 0.8 | 26.0 ± 0.5 | 26.4 ± 0.7 | 26.5 ± 0.7 | 26.1 ± 0.5 | 26.1 ± 0.8 | 27.3 ± 0.7 | 29.4 ± 1.2 |
| O | 24.9 ± 0.9 | 26.1 ± 1.0 | 27.4 ± 1.3 | 26.6 ± 1.2 | 26.6 ± 1.4 | 27.0 ± 1.2 | 26.1 ± 0.9 | 27.3 ± 1.2 | |
| Lactate (mmol l−1) | C | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.1 |
| O | 0.7 ± 0.1 | 6.9 ± 0.2§ | 2.6 ± 0.3§ | 2.2 ± 0.4* | 1.8 ± 0.2* | 1.2 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | |
| Glucose (mmol l−1) | C | 0.9 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| O | 1.0 ± 0.1 | 0.7 ± 0.1* | 1.2 ± 0.1* | 1.2 ± 0.1* | 1.6 ± 0.2† | 1.5 ± 0.1† | 1.2 ± 0.1 | 1.1 ± 0.1 |
Data are mean ± s.e.m.
P < 0.05
P < 0.01
‡P < 0.005
P < 0.001 (between group comparisons, data compared by ANOVA).
Blood pressure, carotid blood flow, EEG amplitude and frequency
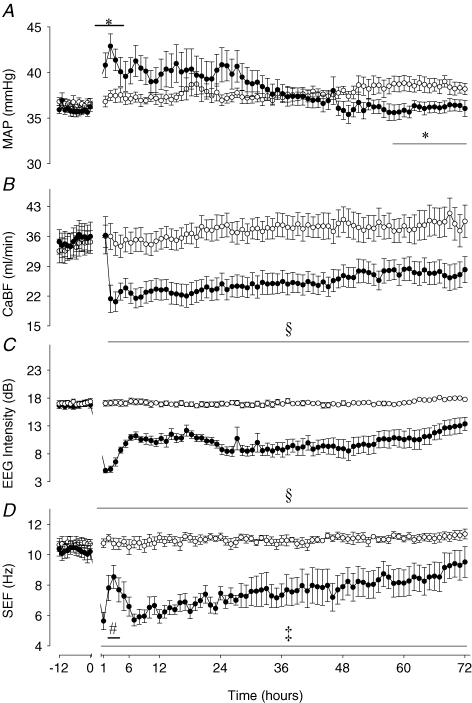
Data for the control and occlusion group fetuses for MAP, CaBF and EEG during recovery versus the 12 h baseline before occlusion are shown in Fig. 1. In the occlusion group MAP remained elevated above baseline for the first 3 h (P < 0.05); the peak in pressure occurred at 2 h post-occlusion (42.9 ± 1.4 mmHg versus 37.4 ± 0.5 mmHg). MAP tended to be higher than controls during the subsequent 28 h post-occlusion, but this was not significant. Between 57 and 72 h after occlusion MAP was significantly lower than control group values (P < 0.05). Carotid blood flow returned to control group values in the first hour, but thereafter was significantly reduced compared to the control group for the entire recovery period (P < 0.001). The nadir in the fall in CaBF was at 3 h post-occlusion (20.6 ± 2.3 ml min−1versus 34.3 ± 2.9 ml min−1, P < 0.001).
Figure 1. Time sequence of changes in fetal mean arterial blood pressure, carotid blood flow, electroencephalographic intensity and spectral edge frequency before and after asphyxia in preterm fetal sheep.
MAP, fetal mean arterial blood pressure (mmHg); CaBF, carotid blood flow (ml min−1); EEG, electroencephalographic intensity (dB); SEF, spectral edge frequency (Hz). •, asphyxia induced by 25 min of umbilical cord occlusion (n = 7); ○, sham occlusion (n = 7). Occlusion data not shown. Data are mean ± s.e.m. hourly averages. *P < 0.05, ‡P < 0.005, §P < 0.001, occlusion group versus sham control group, ANOVA; #P < 0.05 versus 1st hour after occlusion.
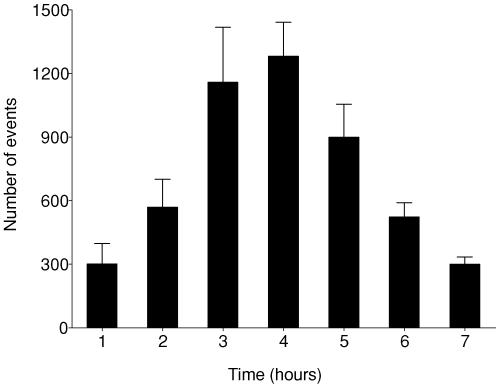
Mean EEG intensity was significantly suppressed during the entire post-asphyxial recovery period (P < 0.001 versus sham controls, Fig. 1). Seizures started at 8.0 ± 0.5 h post-occlusion and continued for a mean of 18.5 ± 5.8 h, although some fetuses exhibited seizures as late as 48–72 h (Figs 2 and 3). A mean total of 72.5 ± 11.3 seizure events occurred in the occlusion group over the 3 day recovery period. Examples of these seizures are given in Figs 2D and 3. No seizures were seen in the sham control group. EEG spectral edge frequency was significantly suppressed throughout the recovery period after occlusion (P < 0.005 versus sham controls, Fig. 1). There was a transient, relative increase in spectral edge frequency 2–4 h after occlusion (P < 0.05 versus the first hour after occlusion), which paralleled the time course of epileptiform transient activity (Figs 2 and 4). Epileptiform transients peaked at around 4 h (1282 ± 161 events per hour, mean amplitude 66.2 ± 6.0 μV, P < 0.0001 versus sham controls, Fig. 4). Examples of this activity are shown in Fig. 2B and C (compared to normal EEG activity, Fig. 2A). Epileptiform transients were not seen in sham controls.
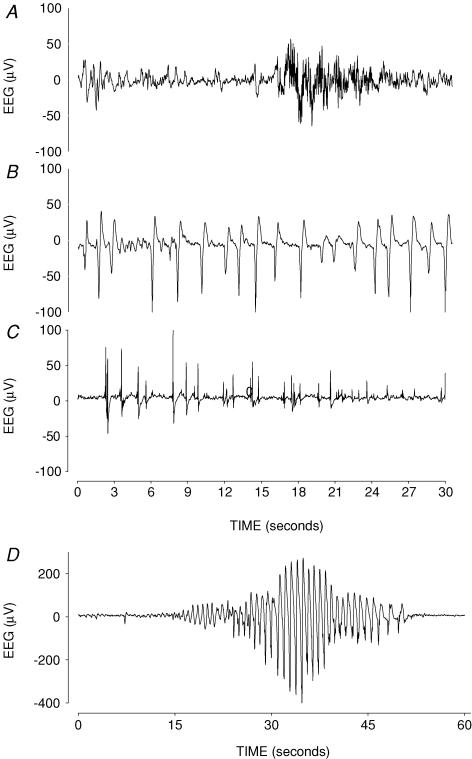
Figure 2. Examples of raw electroencephalographic (EEG) data from fetuses in the occlusion group.
Examples of raw electroencephalographic (EEG) data from fetuses in the occlusion group, showing normal discontinuous mixed frequency EEG activity before occlusion (A), examples of epileptiform transients superimposed on a suppressed EEG background at 1 and 4 h after occlusion, respectively (B and C). D, an example of a seizure approximately 12 h after occlusion, displaying the typical stereotypic, evolving pattern.
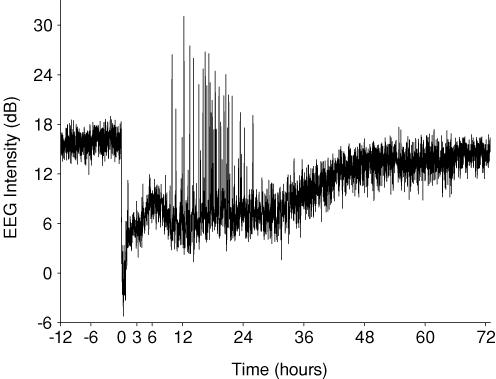
Figure 3. An example of changes in EEG intensity from a fetus in the occlusion group.
The occlusion occurs at time zero. Note the profound suppression of EEG activity during and immediately after occlusion. From 8 h this fetus developed electroclinical seizures shown by high amplitude, short duration events (for an example of a single seizure see Fig. 2D). Data are continuous 1 min averages.
Figure 4. Time sequence of changes in electroencephalographic transient activity (events per hour) in the occlusion group during the first 7 h recovery from umbilical cord occlusion.
NIRS
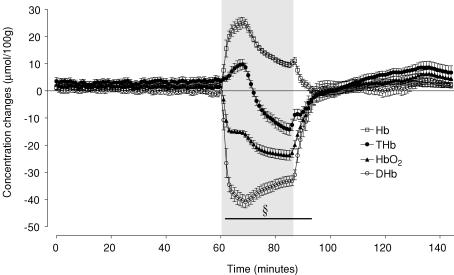
The changes in NIRS parameters during umbilical cord occlusion are shown in Fig. 5. Occlusion associated with a marked, sustained fall in [HbO2] (P < 0.001) and corresponding rise in [Hb] (P < 0.001) throughout the period of occlusion, leading to a considerable fall in [DHb] which was maximal after 8 min of occlusion (−40.4 ± 2.4 μmol (100 g)−1versus 0.4 ± 0.7 in sham controls, P < 0.001). In contrast, [THb] showed an initial rise for the first 8 min (P < 0.01) then progressively fell and was significantly reduced for the last 13 min of the occlusion (−14.2 ± 2.2 versus 5.5 ± 2.0 μmol (100 g)−1, at the end of the occlusion, P < 0.001). There were no changes in the sham control group during this interval (data not shown).
Figure 5. Time sequence of acute concentration changes in fetal deoxygenated haemoglobin, total haemoglobin, oxygenated haemoglobin and delta haemoglobin measured by near infrared spectroscopy before and after asphyxia.
Hb, deoxygenated haemoglobin; THb, total haemoglobin; HbO2, oxygenated haemoglobin; DHb, delta haemoglobin ( = HbO2– Hb). Asphyxia induced by 25 min of umbilical cord occlusion shown by the grey box. For clarity only the significant changes in DHb are shown; §P < 0.001 versus sham controls. There was no change in sham control values during this interval (not shown). Data are mean ± s.e.m. 1 min averages.
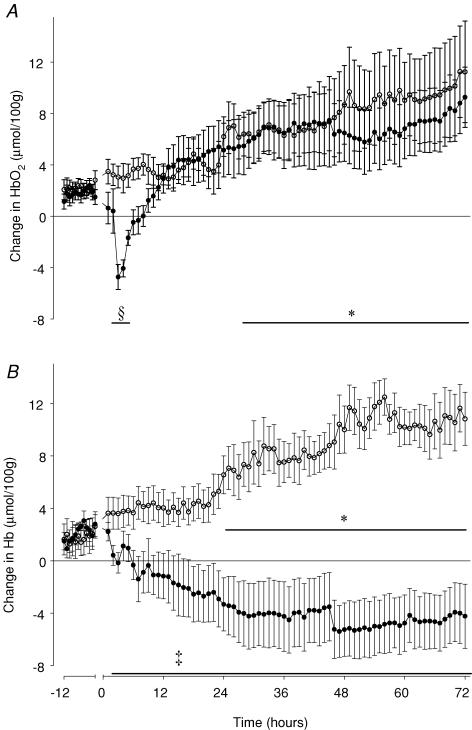
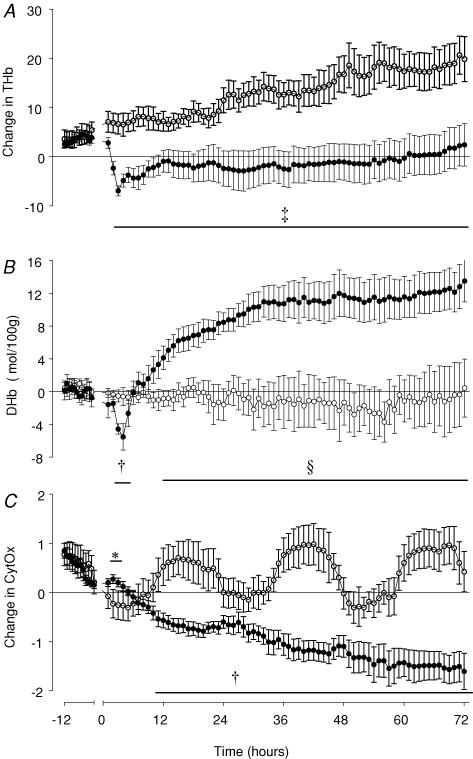
All parameters showed a rapid initial recovery to control values over the first 7 min after release of occlusion. Sham controls showed a progressive, parallel rise in [Hb] and [HbO2], and thus in [THb] over the 72 h recovery period (P < 0.05, from 23 h and 29 h after occlusion, respectively, until 72 h versus baseline recordings), but no significant change in [DHb]. In the occlusion group there was a significant fall in [HbO2] for the first 8 h after reperfusion, with a nadir in [HbO2] seen at 3 h (P < 0.001, Fig. 6A). Thereafter there was no significant difference between groups. There was a significant and sustained fall in [Hb] for the duration of recovery, particularly marked after 12 h (P < 0.005, Fig. 6B). There was a rapid initial fall in [THb] which reached a nadir at 3 h after occlusion (Fig. 7A). Thereafter values stabilized, and remained below control group values for the remainder of the study (P < 0.005). [DHb] initially returned to control values after the end of occlusion, then exhibited a brief fall at 3 and 4 h (P < 0.01, Fig. 7B). From 5 h onward [DHb] exhibited a progressive rise, and was significantly higher than control group values from 12 h after occlusion until the end of the study (P < 0.001).
Figure 6. Time sequence of changes in fetal oxygenated haemoglobin (A) and deoxygenated haemoglobin (B) measured by near infrared spectroscopy before and after asphyxia.
HbO2, oxygenated haemoglobin (A); Hb, deoxygenated haemoglobin (B). •, asphyxia induced by 25 min of umbilical cord occlusion (n = 7); ○, sham occlusion (n = 7). Occlusion data not shown. Data are mean ± s.e.m. hourly averages. ‡P < 0.005, §P < 0.001, occlusion group versus sham control group, ANOVA. *P < 0.05 sham control group versus baseline.
Figure 7. Time sequence of changes in fetal total haemoglobin, delta haemoglobin and cytochrome oxidase measured by near infrared spectroscopy before and after asphyxia in preterm fetal sheep.
THb, total haemoglobin ( = HbO2+ Hb); DHb, delta haemoglobin ( = HbO2-Hb), a measure of relative intracerebral oxygenation; CytOx, cytochrome oxidase. •, asphyxia induced by 25 min of umbilical cord occlusion (n = 7); O, sham occlusion (n = 7). Occlusion data not shown. Data are mean ± s.e.m. hourly averages. *P < 0.05, †P < 0.01, ‡P < 0.005, §P < 0.001, occlusion group versus sham control group, ANOVA.
The sham control group showed a diurnal pattern of [CytOx] changes throughout the study, with peak levels occurring at night. In the occlusion group, [CytOx] was transiently increased between 2 and 3 h after the end of occlusion (P < 0.05, Fig. 7C), and thereafter progressively fell throughout the recovery period. [CytOx] fell to significantly lower than the first 3 h of recovery by 5 h after occlusion (P < 0.05), and was significantly lower than sham control group values from 10 h (P < 0.01) for the remainder of the experiment. Occlusion was associated with a loss of the diurnal pattern in [CytOx] seen in the control group.
Discussion
The present study demonstrates that severe asphyxia in the preterm fetal sheep leads to a consistent pattern of evolving mitochondrial dysfunction, with initial recovery of CytOx values after the end of the asphyxic insult in a ‘latent’ phase, followed by a progressive fall, starting from approximately 5 h, and still continuing as late as 48 h after asphyxia. These changes were associated with distinctive changes in EEG recordings and cerebral oxygenation. The latent phase was marked by epileptiform EEG transient activity despite profound suppression of background EEG activity, whereas overt seizures were not seen until after the onset of the secondary fall in CytOx levels. These observations support previous suggestions that delayed posthypoxic seizures are generally a marker for the onset of the terminal secondary deterioration, effectively reflecting progression of the execution phase of cell death (Marks et al. 1996; Gunn et al. 1999; Marks et al. 1999).
Cytochrome oxidase is the terminal electron acceptor of the mitochondrial electron transport chain and is responsible for over 90% of cellular oxygen consumption. Energy generated in this process is used for ATP synthesis (Wong-Riley, 1989; Cooper et al. 1997). Although measurement of CytOx by NIRS is complex (Cooper et al. 1997; Cooper & Springett, 1997), accurate measurement of CytOx changes, using the algorithm developed by Wray et al. (1988), has been demonstrated in the adult rat brain (Cooper et al. 1997). The finding in the present study that the pattern of changes in CytOx was completely different from that of any of the components of haemoglobin (Fig. 7) strongly supports the conclusion that the measurement algorithm eliminates significant ‘cross-talk’ of signals from haemoglobin in the system (Wray et al. 1988).
In the early latent phase of recovery from severe hypoxia in the present study, when CytOx was normal, carotid blood flow, and cerebral blood volume, as measured by total Hb, initially recovered, but then fell reaching a nadir around 3 h after asphyxia. This fall in cerebral perfusion was not due to changes in blood pressure, which was normal or mildly elevated for most of the recovery period, but rather was mediated by increased vascular resistance (Bennet et al. 1999). This is consistent with findings in the preterm infant where systemic hypoperfusion (as estimated from flow in the superior vena cava) is only associated with hypotension in a subset of infants (Kluckow, 2005). This raises the question of what is mediating this vasoconstriction and whether it contributes to injury. A delayed fall in cerebral blood flow has been widely observed in many models (Conger & Weil, 1995), and yet its significance remains unclear. Some data in adult species suggest that delayed hypoperfusion is due to impaired endothelial control of blood flow, and thus may extend the initial ischaemic injury (Hossmann, 1997; Ten & Pinsky, 2002). Other data suggest that it is actively mediated, and simply reflects reduced metabolic demand (Michenfelder & Milde, 1990; Gold & Lauritzen, 2002).
In the current study, despite the presence of epileptiform transients, mean EEG intensity and spectral edge frequency were markedly suppressed during the latent phase, which may be taken to suggest that, overall, reduced cerebral blood flow reflected reduced cerebral activity. During this early recovery period, similarly to previous findings in younger fetuses (Bennet et al. 1999), there was a marked transient fall in oxygenated haemoglobin that was maximal at 3 h after occlusion, which in turn would suggest a relative impairment of cerebral oxygenation that could compromise cerebral recovery. Nevertheless, such a fall in HbO2 could merely reflect the fall in total blood flow. Thus in the present study we evaluated an alternative measure of the relative adequacy of perfusion, DHb, the difference between the content of oxygenated and deoxygenated haemoglobin, which is a reliable measure of changes in intracerebral oxygenation (Brun et al. 1997). If arterial oxygen saturation is stable this parameter correlates closely with perfusion changes due to severe hypotension or to changes in intracranial pressure (Tsuji et al. 1998; Soul et al. 2000). A further advantage of this measure, for the present study, is that there was a significant increase in total Hb levels in sham controls over time, reflecting a rise in both oxygenated and deoxygenated haemoglobin, similar to a previous report (Bennet et al. 1999). In part this apparent increase in cerebral haemoglobin concentration is likely to be a reflection of the rapid increase in brain size at this age (approximately a doubling in weight every fortnight), leading to a real maturational increase in cerebral blood volume, although we cannot rule out other causes of a baseline shift. Critically, there was no significant relative change in HbO2 and Hb in the controls, such that delta Hb recordings in the sham control group remained wholly stable, demonstrating that regardless of the specific mechanisms of the apparent increase in total Hb over time, there was no relative change between oxygenated and deoxygenated haemoglobin during the recording period.
In the present study delta Hb measurements suggested that there was a true reduction in cerebral oxygenation at 3 and 4 h after occlusion, although the decrease was of short duration, and relatively modest compared to that seen during asphyxia (Fig. 5). Thus, overall, it is likely that the rapid onset, secondary hypoperfusion in the first hours after severe hypoxia mainly reflects a reduction in cerebral metabolism. It is unknown whether this reduction is purely a ‘passive’ function of cellular hypoxic injury and loss of cellular homeostasis, or whether it may in part be actively mediated. Nevertheless, the period of transient deoxygenation suggests that an imbalance between neuronal activity and perfusion did occur during the latent phase, albeit briefly. It is unclear whether this deoxygenation promotes or accelerates further tissue injury and thus contributes to the subsequent loss of mitochondrial function. It is of interest that the period of cerebral deoxygenation was associated with a mild but significant increase in CytOx compared to baseline. Since acute hypoxia is reported to increase CytOx in fetal sheep (Newman et al. 2000), this supports the concept that there was some degree of cerebral hypoxic stress. The mechanisms of the brief fall in oxygenation are unknown. However, it is striking that epileptiform transient activity and the relative rise in EEG frequency were maximal at the time of the nadir in DHb. Thus, potentially during these early hours in the recovery process there may be a transient imbalance between activity and suppression.
From approximately 5 h after occlusion, CytOx levels gradually began to fall, and were significantly reduced compared with sham controls from 10 h after occlusion. In parallel with this fall, although carotid blood flow and cerebral blood volume remained depressed, DHb progressively rose to well above control values. This dramatic increase in intracerebral oxygenation is strongly indicative of a relative luxury perfusion. Although there are few data in the preterm infant, a delayed increase in perfusion has been shown at term both after severe transient cerebral ischaemia in fetal sheep (Abi Raad et al. 1999) and in infants with severe encephalopathy (Ilves et al. 2004). This temporal relationship between the fall in CytOx and increase in cerebral oxygenation at a time when total cerebral blood flow was reduced is wholly consistent with delayed loss of mitochondrial oxidative capacity, which would lead to reduced tissue oxygen consumption. A similar secondary fall in CytOx has been documented using NIRS in some newborn infants with severe hypoxic–ischaemic encephalopathy (van Bel et al. 1993), as well as in experimental preparations (Marks et al. 1996). Recent data in the neonatal rat suggest that loss of high energy metabolites occurs in parallel with loss of neuronal cell markers, and thus, that markers of impaired mitochondrial activity such as loss of CytOx are primarily markers of cell death (Vannucci et al. 2004). Whether this is the case, or whether mitochondrial failure precedes cell death but represents a pivotal, execution step, the present data strongly indicate that cell loss was occurring up to approximately 48 h after release of occlusion.
The pattern of epileptiform activity in the latent phase, and after the onset of the secondary deterioration in mitochondrial activity, were strikingly different. In the latent phase, we observed frequent brief fast, sharp and slow wave epileptiform transients with very little background activity. In contrast, overt seizures occurred in the secondary phase, from approximately 8 h, and continuing for a mean of 18 h. The relationship between posthypoxic epileptiform transients and seizures and evolving neural injury is an area of considerable controversy. Although longer seizures are associated with a fall in cortical tissue oxygenation in the fetal sheep (Gonzalez et al. 2005), and very prolonged, intense induced seizures can unquestionably cause injury in their own right (Wasterlain et al. 2002), there is little experimental evidence that spontaneous seizures after hypoxia–ischaemia substantially further increase neural injury. Indeed, we have previously shown that complete suppression of postischaemic seizures in the near-term fetal sheep with a potent NMDA receptor antagonist did not reduce parasagittal cortical infarction, and was associated with only a modest reduction in neuronal loss in ‘penumbral’ regions (Tan et al. 1996). Similarly, Yager et al. (2004) have shown that induced seizures did not increase hypoxic–ischaemic injury in the neonatal rat if secondary hyperthermia was prevented. It is likely that this reflects the consistent finding that although the immature brain does seem to be more prone to posthypoxic hyperactivity (Jensen et al. 1991), it is much more resistant to seizure induced damage than in adulthood (Fernandes et al. 1999; Lado et al. 2000; Haas et al. 2001).
We observed in the present study that seizures began at a time when CytOx was secondarily falling from its initial postocclusion values and intracerebral oxygenation was increasing, but when these parameters had yet to become significantly different from sham controls. Given this timing, it is possible that the intermittent depletion of local metabolites by these seizures (Miller et al. 2002) may have exacerbated the secondary deterioration in mitochondrial function. Overall, however, given that CytOx had already begun to fall, and that the slope of the fall did not seem to become steeper after the onset of seizures, these data favour the concept that seizures are primarily a manifestation of developing cell loss, rather a major cause of injury. In contrast, the earlier epileptiform transients in the latent phase may, paradoxically, be a more important therapeutic target. We have previously shown that these events are strongly associated with adverse outcome after severe hypoxia in the preterm fetal sheep (George et al. 2004), consistent with some clinical reports (Hughes & Guerra, 1994; Okumura et al. 2003). We propose that these events may be a manifestation of the posthypoxic hyperexcitability of the glutamate receptor that has been demonstrated in infant rats (Jensen et al. 1998). Thus these events may favour progression of intracellular cell death pathways by increasing posthypoxic intracellular influx of cytotoxic factors, such as calcium (Mies et al. 1994; Hossmann, 1996).
A potential limitation of the present study is that the epileptiform activity was measured from the cortex, whereas the NIRS measurements represent a global estimate of cerebral oxygenation, incorporating subcortical as well as cortical areas. Nevertheless it is likely that the abnormal EEG activity arose from the subcortical regions, which are severely injured in the present model (Dean et al. 2006), and propagate to the cortex. Supporting the hypothesis that the early onset EEG transients are closely associated with pathogenic cellular events, we have recently reported that a brief infusion of a glutamate antagonist in the same model as the present study both markedly suppressed the postocclusion transients and reduced neuronal loss (Dean et al. 2006). These data are also consistent with evidence from studies of therapeutic hypothermia in the near-term fetal sheep that postischaemic cooling is no longer neuroprotective when it is initiated after the onset of delayed seizures (Gunn et al. 2005).
The results of the present study clearly relate to a subset of cases of preterm brain injury. Overt perinatal insults in preterm infants are actually more common than at term (de Vries et al. 1998; Low et al. 2003), and modern imaging has indicated that profound hypoxia in premature infants leads to subcortical neuronal injury with cortical sparing (Barkovich & Sargent, 1995; de Vries et al. 1998), consistent with that seen in the present model (Dean et al. 2006). Thus, although no one experimental model can possibly represent the full spectrum of preterm neural injury, the present findings suggest that at least some cases of preterm neural injury will demonstrate a biphasic pattern of secondary energy failure.
Finally, intriguingly, in the present study we observed a pronounced diurnal rhythm in CytOx in sham controls, with maximal levels at night. There is evidence that that there is a diurnal rhythm in CytOx activity in the suprachiasmatic nucleus in adults (Ximenes da Silva et al. 2000) with CytOx activity being the highest at the middle of the day, decreasing at the end of the light period. Speculatively, this could be mediated by melatonin which regulates diurnal changes in enzyme activity in the fetus during light cycle changes (Breen et al. 1996). A different cycle between the adult and fetus is consistent with the greater activity of the fetus at night, in contrast to adults, who are more active during the day (Schmidt et al. 2000), and evidence that, at least near-term, fetuses spend more time in the higher-energy demanding (REM equivalent) sleep state at night (Richardson, 1992). Mean levels of CytOx and cerebral blood volume during REM sleep have been shown to significantly exceed those during waking and slow-wave sleep even in the adult cat (Vern et al. 1988). Although the preterm fetus shows dysynchronous EEG activity, the suprachiasmatic nucleus is active in the fetal sheep from as early as 75 days of gestation (Breen et al. 1996), and we have previously demonstrated a diurnal rhythm in fetal heart rate and heart rate variability at 0.7 gestation (Quaedackers et al. 2005).
In conclusion, the current data taken together indicate that following profound hypoxia in preterm fetal sheep there is delayed onset of progressive mitochondrial failure, associated with evidence of relative luxury perfusion, suggesting an evolving impairment of cerebral oxygen metabolism. Frequent but brief epileptiform transients peaked in the latent phase before these changes, and were associated with a transient relative fall in intracerebral oxygenation, whereas overt seizures were not seen until after the start of the fall in mitochondrial function, at a time when intracerebral oxygenation was normal. These data are consistent with evidence in older animals that the latent phase, before the onset of mitochondrial failure, provides the key ‘window of opportunity’ for intervention (Gunn et al. 2005). Practical implications of these findings are that EEG monitoring which permits continuous assessment of the amplitude and frequency components of the raw EEG may be particularly important in selecting infants at risk of evolving injury, and that NIRS monitoring may help to refine assessment.
Acknowledgments
These studies were supported by The Health Research Council of New Zealand, the Auckland Medical Research Foundation, and the Lottery Grants Board of New Zealand.
References
- Abi Raad R, Tan WK, Bennet L, Gunn AJ, Davis SL, Gluckman PD, et al. Role of the cerebrovascular and metabolic responses in the delayed phases of injury after transient cerebral ischemia in fetal sheep. Stroke. 1999;30:2735–2742. doi: 10.1161/01.str.30.12.2735. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Wyatt JS, Cady EB, Delpy DT, Baudin J, Stewart AL, et al. Prognosis of newborn infants with hypoxic-ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–451. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol. 1995;16:1837–1846. [PMC free article] [PubMed] [Google Scholar]
- Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol. 1969;135:249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- Bennet L, Quaedackers JS, Gunn AJ, Rossenrode S, Heineman E. The effect of asphyxia on superior mesenteric artery blood flow in the premature sheep fetus. J Pediatr Surg. 2000;35:34–40. doi: 10.1016/s0022-3468(00)80009-3. [DOI] [PubMed] [Google Scholar]
- Bennet L, Rossenrode S, Gunning MI, Gluckman PD, Gunn AJ. The cardiovascular and cerebrovascular responses of the immature fetal sheep to acute umbilical cord occlusion. J Physiol. 1999;517:247–257. doi: 10.1111/j.1469-7793.1999.0247z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagioni E, Bartalena L, Boldrini A, Pieri R, Cioni G. Electroencephalography in infants with periventricular leukomalacia: prognostic features at preterm and term age. J Child Neurol. 2000;15:1–6. doi: 10.1177/088307380001500101. [DOI] [PubMed] [Google Scholar]
- Breen S, Rees S, Walker D. The development of diurnal rhythmicity in fetal suprachiasmatic neurons as demonstrated by fos immunohistochemistry. Neuroscience. 1996;74:917–926. doi: 10.1016/0306-4522(96)00128-5. [DOI] [PubMed] [Google Scholar]
- Brun NC, Moen A, Borch K, Saugstad OD, Greisen G. Near-infrared monitoring of cerebral tissue oxygen saturation and blood volume in newborn piglets. Am J Physiol. 1997;273:H682–H686. doi: 10.1152/ajpheart.1997.273.2.H682. [DOI] [PubMed] [Google Scholar]
- Chang YS, Park WS, Lee M, Kim KS, Shin SM, Choi JH. Near infrared spectroscopic monitoring of secondary cerebral energy failure after transient global hypoxia–ischemia in the newborn piglet. Neurol Res. 1999;21:216–224. doi: 10.1080/01616412.1999.11740921. [DOI] [PubMed] [Google Scholar]
- Conger JD, Weil JV. Abnormal vascular function following ischemia-reperfusion injury. J Invest Med. 1995;43:431–442. [PubMed] [Google Scholar]
- Cooper CE, Cope M, Quaresima V, Ferrari M, Nemoto E, Springett R, et al. Measurement of cytochrome oxidase redox state by near infrared spectroscopy. Adv Exp Med Biol. 1997;413:63–73. doi: 10.1007/978-1-4899-0056-2_7. [DOI] [PubMed] [Google Scholar]
- Cooper CE, Springett R. Measurement of cytochrome oxidase and mitochondrial energetics by near infrared spectroscopy. Philos Trans R Soc Lond B Biol Sci. 1997;352:669–676. doi: 10.1098/rstb.1997.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean JM, George SA, Wassink G, Gunn AJ, Bennet L. Suppression of post hypoxic-ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology. 2006;50:491–503. doi: 10.1016/j.neuropharm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Dimlich RV, Showers MJ, Shipley MT. Densitometric analysis of cytochrome oxidase in ischemic rat brain. Brain Res. 1990;516:181–191. doi: 10.1016/0006-8993(90)90917-z. [DOI] [PubMed] [Google Scholar]
- Fernandes MJ, Dube C, Boyet S, Marescaux C, Nehlig A. Correlation between hypermetabolism and neuronal damage during status epilepticus induced by lithium and pilocarpine in immature and adult rats. J Cereb Blood Flow Metab. 1999;19:195–209. doi: 10.1097/00004647-199902000-00011. [DOI] [PubMed] [Google Scholar]
- George S, Gunn AJ, Westgate JA, Brabyn C, Guan J, Bennet L. Fetal heart rate variability and brainstem injury after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R925–R933. doi: 10.1152/ajpregu.00263.2004. [DOI] [PubMed] [Google Scholar]
- Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci U S A. 2002;99:7699–7704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Hunter CJ, Bennet L, Power GG, Gunn AJ. Cerebral oxygenation during post-asphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab. 2005;25:911–918. doi: 10.1038/sj.jcbfm.9600087. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Battin M, Gluckman PD, Gunn TR, Bennet L. Therapeutic hypothermia: from lab to NICU. J Perinat Med. 2005;33:340–346. doi: 10.1515/JPM.2005.061. [DOI] [PubMed] [Google Scholar]
- Gunn AJ, Bennet L, Gunning MI, Gluckman PD, Gunn TR. Cerebral hypothermia is not neuroprotective when started after postischemic seizures in fetal sheep. Pediatr Res. 1999;46:274–280. doi: 10.1203/00006450-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8:195–208. [PubMed] [Google Scholar]
- Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8:95–101. doi: 10.1097/00024382-199708000-00004. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Guerra R. The use of the EEG to predict outcome in premature infants with positive sharp waves. Clin Electroencephalogr. 1994;25:127–135. doi: 10.1177/155005949402500404. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Bennet L, Power GG, Roelfsema V, Blood AB, Quaedackers JS, et al. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke. 2003;34:2240–2245. doi: 10.1161/01.STR.0000083623.77327.CE. [DOI] [PubMed] [Google Scholar]
- Ilves P, Lintrop M, Metsvaht T, Vaher U, Talvik T. Cerebral blood-flow velocities in predicting outcome of asphyxiated newborn infants. Acta Paediatr. 2004;93:523–528. doi: 10.1080/08035250410024745. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Applegate CD, Holtzman D, Belin TR, Burchfiel JL. Epileptogenic effect of hypoxia in the immature rodent brain. Ann Neurol. 1991;29:629–637. doi: 10.1002/ana.410290610. [DOI] [PubMed] [Google Scholar]
- Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC. Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J Neurophysiol. 1998;79:73–81. doi: 10.1152/jn.1998.79.1.73. [DOI] [PubMed] [Google Scholar]
- Kluckow M. Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum Dev. 2005;81:429–437. doi: 10.1016/j.earlhumdev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lado FA, Sankar R, Lowenstein D, Moshe SL. Age-dependent consequences of seizures: relationship to seizure frequency, brain damage, and circuitry reorganization. Ment Retard Dev Disabil Res Rev. 2000;6:242–252. doi: 10.1002/1098-2779(2000)6:4<242::AID-MRDD3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lawler FH, Brace RA. Fetal and maternal arterial pressures and heart rates: histograms, correlations, and rhythms. Am J Physiol. 1982;243:R433–R444. doi: 10.1152/ajpregu.1982.243.3.R433. [DOI] [PubMed] [Google Scholar]
- Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, et al. Delayed (‘secondary’) cerebral energy failure after acute hypoxia- ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Low JA, Killen H, Derrick EJ. Antepartum fetal asphyxia in the preterm pregnancy. Am J Obstet Gynecol. 2003;188:461–465. doi: 10.1067/mob.2003.37. [DOI] [PubMed] [Google Scholar]
- Marks KA, Mallard CE, Roberts I, Williams CE, Gluckman PD, Edwards AD. Nitric oxide synthase inhibition and delayed cerebral injury after severe cerebral ischemia in fetal sheep. Pediatr Res. 1999;46:8–13. doi: 10.1203/00006450-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Marks KA, Mallard EC, Roberts I, Williams CE, Sirimanne ES, Johnston B, et al. Delayed vasodilation and altered oxygenation after cerebral ischemia in fetal sheep. Pediatr Res. 1996;39:48–54. doi: 10.1203/00006450-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- Marret S, Parain D, Menard JF, Blanc T, Devaux AM, Ensel P, et al. Prognostic value of neonatal electroencephalography in premature newborns less than 33 weeks of gestational age. Electroencephalogr Clin Neurophysiol. 1997;102:178–185. doi: 10.1016/s0013-4694(96)95655-6. [DOI] [PubMed] [Google Scholar]
- McIntosh GH, Baghurst KI, Potter BJ, Hetzel BS. Foetal brain development in the sheep. Neuropathol Appl Neurobiol. 1979;5:103–114. doi: 10.1111/j.1365-2990.1979.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Michenfelder JD, Milde JH. Postischemic canine cerebral blood flow appears to be determined by cerebral metabolic needs. J Cereb Blood Flow Metab. 1990;10:71–76. doi: 10.1038/jcbfm.1990.9. [DOI] [PubMed] [Google Scholar]
- Mies G, Kohno K, Hossmann KA. Prevention of periinfarct direct current shifts with glutamate antagonist NBQX following occlusion of the middle cerebral artery in the rat. J Cereb Blood Flow Metab. 1994;14:802–807. doi: 10.1038/jcbfm.1994.100. [DOI] [PubMed] [Google Scholar]
- Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- Nelson C, Silverstein FS. Acute disruption of cytochrome oxidase activity in brain in a perinatal rat stroke model. Pediatr Res. 1994;36:12–19. doi: 10.1203/00006450-199407001-00003. [DOI] [PubMed] [Google Scholar]
- Newman JP, Peebles DM, Harding SR, Springett R, Hanson MA. Hemodynamic and metabolic responses to moderate asphyxia in brain and skeletal muscle of late-gestation fetal sheep. J Appl Physiol. 2000;88:82–90. doi: 10.1152/jappl.2000.88.1.82. [DOI] [PubMed] [Google Scholar]
- Okumura A, Hayakawa F, Kato T, Maruyama K, Kubota T, Suzuki M, et al. Abnormal sharp transients on electroencephalograms in preterm infants with periventricular leukomalacia. J Pediatr. 2003;143:26–30. doi: 10.1016/S0022-3476(03)00182-3. [DOI] [PubMed] [Google Scholar]
- Peeters-Scholte C, van den Tweel E, Groenendaal F, van Bel F. Redox state of near infrared spectroscopy-measured cytochrome aa3 correlates with delayed cerebral energy failure following perinatal hypoxia-ischaemia in the newborn pig. Exp Brain Res. 2004;156:20–26. doi: 10.1007/s00221-003-1761-5. [DOI] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Ferrandon A, Nehlig A. Local cerebral blood flow during lithium-pilocarpine seizures in the developing and adult rat: role of coupling between blood flow and metabolism in the genesis of neuronal damage. J Cereb Blood Flow Metab. 2002;22:196–205. doi: 10.1097/00004647-200202000-00007. [DOI] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Fraser M, Gunn AJ, Bennet L. Cardiovascular and endocrine effects of maternal dexamethasone treatment in preterm fetal sheep. Br J Obstet Gynaecol. 2005;112:182–191. doi: 10.1111/j.1471-0528.2004.00344.x. [DOI] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Heineman E, Gunn AJ, Bennet L. The role of the sympathetic nervous system in post-asphyxial intestinal hypoperfusion in the preterm sheep fetus. J Physiol. 2004a;557:1033–1044. doi: 10.1113/jphysiol.2004.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedackers JS, Roelfsema V, Hunter CJ, Heineman E, Gunn AJ, Bennet L. Polyuria and impaired renal blood flow after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2004b;286:R576–R583. doi: 10.1152/ajpregu.00592.2003. [DOI] [PubMed] [Google Scholar]
- Reynolds EO, Wyatt JS, Azzopardi D, Delpy DT, Cady EB, Cope M, et al. New non-invasive methods for assessing brain oxygenation and haemodynamics. Br Med Bull. 1988;44:1052–1075. doi: 10.1093/oxfordjournals.bmb.a072289. [DOI] [PubMed] [Google Scholar]
- Richardson BS. The effect of behavioral state on fetal metabolism and blood flow circulation. Semin Perinatol. 1992;16:227–233. [PubMed] [Google Scholar]
- Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, et al. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev Med Child Neurol. 1997;39:718–725. doi: 10.1111/j.1469-8749.1997.tb07372.x. [DOI] [PubMed] [Google Scholar]
- Rowe JC, Holmes GL, Hafford J, Baboval D, Robinson S, Philipps A, et al. Prognostic value of the electroencephalogram in term and preterm infants following neonatal seizures. Electroencephalogr Clin Neurophysiol. 1985;60:183–196. doi: 10.1016/0013-4694(85)90030-6. [DOI] [PubMed] [Google Scholar]
- Scher M. Neonatal seizures: an expression of fetal or neonatal brain disorders. In: Stevenson DK, Benitz WE, Sunshine P, editors. Fetal and Neonatal Brain Injury. Mechanisms, Management and the Risks of Practice. Cambridge, UK: Cambridge University Press; 2003. pp. 735–784. [Google Scholar]
- Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia. 1993;34:284–288. doi: 10.1111/j.1528-1157.1993.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Schild L, Huppelsberg J, Kahlert S, Keilhoff G, Reiser G. Brain mitochondria are primed by moderate Ca2+ rise upon hypoxia/reoxygenation for functional breakdown and morphological disintegration. J Biol Chem. 2003;278:25454–25460. doi: 10.1074/jbc.M302743200. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Kott M, Muller T, Schubert H, Schwab M. Developmental changes in the complexity of the electrocortical activity in foetal sheep. J Physiol Paris. 2000;94:435–443. doi: 10.1016/s0928-4257(00)01087-1. [DOI] [PubMed] [Google Scholar]
- Soul JS, Taylor GA, Wypij D, Duplessis AJ, Volpe JJ. Noninvasive detection of changes in cerebral blood flow by near-infrared spectroscopy in a piglet model of hydrocephalus. Pediatr Res. 2000;48:445–449. doi: 10.1203/00006450-200010000-00005. [DOI] [PubMed] [Google Scholar]
- Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, et al. Accumulation of cytotoxins during the development of seizures and edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res. 1996;39:791–797. doi: 10.1203/00006450-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Ten VS, Pinsky DJ. Endothelial response to hypoxia: physiologic adaptation and pathologic dysfunction. Curr Opin Crit Care. 2002;8:242–250. doi: 10.1097/00075198-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44:591–595. doi: 10.1203/00006450-199810000-00020. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Naruse H, Volpe J, Holtzman D. Reduction of cytochrome aa3 measured by near-infrared spectroscopy predicts cerebral energy loss in hypoxic piglets. Pediatr Res. 1995;37:253–259. doi: 10.1203/00006450-199503000-00001. [DOI] [PubMed] [Google Scholar]
- van Bel F, Dorrepaal CA, Benders MJ, Zeeuwe PE, van de Bor M, Berger HM. Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics. 1993;92:365–372. [PubMed] [Google Scholar]
- van Bel F, Roman C, Klautz RJ, Teitel DF, Rudolph AM. Relationship between brain blood flow and carotid arterial flow in the sheep fetus. Pediatr Res. 1994;35:329–333. doi: 10.1203/00006450-199403000-00011. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. J Cereb Blood Flow Metab. 2004;24:1090–1097. doi: 10.1097/01.WCB.0000133250.03953.63. [DOI] [PubMed] [Google Scholar]
- Vecchierini-Blineau MF, Nogues B, Louvet S, Desfontaines O. Positive temporal sharp waves in electroencephalograms of the premature newborn. Neurophysiol Clin. 1996;26:350–362. doi: 10.1016/s0987-7053(97)89149-6. [DOI] [PubMed] [Google Scholar]
- Vern BA, Schuette WH, Leheta B, Juel VC, Radulovacki M. Low-frequency oscillations of cortical oxidative metabolism in waking and sleep. J Cereb Blood Flow Metab. 1988;8:215–226. doi: 10.1038/jcbfm.1988.52. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- de Vries LS, Eken P, Groenendaal F, Rademaker KJ, Hoogervorst B, Bruinse HW. Antenatal onset of haemorrhagic and/or ischaemic lesions in preterm infants: prevalence and associated obstetric variables. Arch Dis Child Fetal Neonatal Ed. 1998;78:F51–F56. doi: 10.1136/fn.78.1.f51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KR, Kleinholz M, Myers RE. Delayed decreases in specific brain mitochondrial electron transfer complex activities and cytochrome concentrations following anoxia/ischemia. J Neurol Sci. 1990a;100:142–151. doi: 10.1016/0022-510x(90)90025-i. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Kleinholz M, Myers RE. Delayed onset of neurologic deterioration following anoxia/ischemia coincides with appearance of impaired brain mitochondrial respiration and decreased cytochrome oxidase activity. J Cereb Blood Flow Metab. 1990b;10:417–423. doi: 10.1038/jcbfm.1990.72. [DOI] [PubMed] [Google Scholar]
- Wasterlain CG, Niquet J, Thompson KW, Baldwin R, Liu H, Sankar R, et al. Seizure-induced neuronal death in the immature brain. Prog Brain Res. 2002;135:335–353. doi: 10.1016/S0079-6123(02)35031-3. [DOI] [PubMed] [Google Scholar]
- Welin AK, Sandberg M, Lindblom A, Arvidsson P, Nilsson UA, Kjellmer I, et al. White matter injury following prolonged free radical formation in the 0.65 gestation fetal sheep brain. Pediatr Res. 2005;58:100–105. doi: 10.1203/01.PDR.0000163388.04017.26. [DOI] [PubMed] [Google Scholar]
- Williams CE, Gunn AJ, Mallard C, Gluckman PD. Outcome after ischemia in the developing sheep brain: an electroencephalographic and histological study. Ann Neurol. 1992;31:14–21. doi: 10.1002/ana.410310104. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosciences. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wray S, Cope M, Delpy DT, Wyatt JS, Reynolds EO. Characterization of the near infrared absorption spectra of cytochrome aa3 and haemoglobin for the non-invasive monitoring of cerebral oxygenation. Biochim Biophys Acta. 1988;933:184–192. doi: 10.1016/0005-2728(88)90069-2. [DOI] [PubMed] [Google Scholar]
- Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, et al. Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol. 1990;68:1086–1091. doi: 10.1152/jappl.1990.68.3.1086. [DOI] [PubMed] [Google Scholar]
- Ximenes da Silva A, Gendrot G, Serviere J, Lavialle M. Daily changes of cytochrome oxidase activity within the suprachiasmatic nucleus of the Syrian hamster. Neurosci Lett. 2000;286:139–143. doi: 10.1016/s0304-3940(00)01096-x. [DOI] [PubMed] [Google Scholar]
- Yager JY, Armstrong EA, Jaharus C, Saucier DM, Wirrell EC. Preventing hyperthermia decreases brain damage following neonatal hypoxic-ischemic seizures. Brain Res. 2004;1011:48–57. doi: 10.1016/j.brainres.2004.02.070. [DOI] [PubMed] [Google Scholar]