Abstract
Fetal programming occurs when the normal pattern of fetal development is disrupted by an abnormal stimulus or ‘insult’ applied at a critical point in in utero development. This then leads to an effect, for example diabetes or hypertension, which manifests itself in adult life. As the placenta is the regulator of nutrient composition and supply from mother to fetus and the source of hormonal signals that affect maternal and fetal metabolism, appropriate development of the placenta is crucial to normal fetal development. Placental function evolves in a carefully orchestrated developmental cascade throughout gestation. Disruption of this cascade can lead to abnormal development of the placental vasculature or of the trophoblast. Timing of a developmental ‘insult’ will be critical in consequent placental function and hence programming of the fetus. The ‘insults’ that alter placental development include hypoxia and abnormal maternal nutrient status, to which the placenta may adapt by alterations in transporter expression and activity to maintain fetal growth or by epigenetic regulation of placental gene expression. Hypoxia is physiological for organogenesis and placental tissue normally exists in a relatively hypoxic environment, but intrauterine growth restriction (IUGR) and pre-eclampsia are associated with a greater degree of trophoblast hypoxia. The metabolic activity of placental mitochondria leads to oxidative stress even in normal pregnancy which is exacerbated further in IUGR, diabetic and pre-eclamptic pregnancies and may also give nitrative stress known to lead to covalent modification and hence altered activity of proteins. Hypoxia, oxidative and nitrative stress all alter placenta development and may be a general underlying mechanism that links altered placental function to fetal programming.
The placenta has several roles: providing an immune interface between the mother and the fetal allograft, serving to transport nutrients and waste products between mother and fetus and being a source of many peptide and steroid hormones that influence fetal, placental and maternal metabolism and development. By virtue of these roles the placenta is in a key position to play a direct role in fetal programming, i.e. by changing the pattern of developmental (hormonal) signals to the fetus or changing the pattern or amount of substrate transported to the fetus such that fetal development is altered, ultimately leading to cardiovascular or metabolic disease later in adult life. Epidemiological evidence has linked low birth weight and low placental weight to fetal programming. While these weights are an easily and accurately measured surrogate for an adverse intrauterine environment, manipulations that alter placental development in animal models and associative data on human placental development point to a more generalized role for the placenta in fetal programming. Fetal programming is not solely seen in IUGR pregnancies. Pregnancies complicated by diabetes and which lead to either small for gestational age (SGA) or large for gestational age (LGA) babies, by pre-eclampsia and by conditions where hypoxia, oxidative and nitrative stress are seen are associated with programming. Therefore is there a common mechanism linking these situations to fetal programming?
Critical periods in placental development
The placenta is in a constant state of growth and differentiation throughout gestation showing a 40-fold increase in fetal/placental weight ratio (a measure of placental efficiency) from 6 weeks to term (Benirschke & Kaufmann, 1990). This increased efficiency is achieved both by a 10-fold increase in the villous volume occupied by vasculature but also by an increase in trophoblast surface area from 0.08 to 12.5 m2 and a decrease in mean trophoblast thickness from 18.9 to 4.1 μm and hence the materno-fetal diffusion distance from 55.9 to 4.8 μm. These changes result from developmentally regulated periods of branching angiogenesis, non-branching angiogenesis, trophoblast differentiation and syncytium formation. Obviously disruption of the normal pattern of placental development will lead to a placenta with altered function. The timing of the disruption of this pattern will also be critical for placental function. Disruption during a period of angiogenesis will have different effects to disruption during a period of trophoblast growth and differentiation. Human data show that IUGR placentas are not simply smaller versions of a term placenta, they display alterations in placental vasculogenesis (Krebs et al. 1996), in trophoblast expression of transporters (Jansson et al. 2002) and trophoblast enzyme activity and hormone production (McMullen et al. 2004)
Placental vascular and trophoblast development and programming
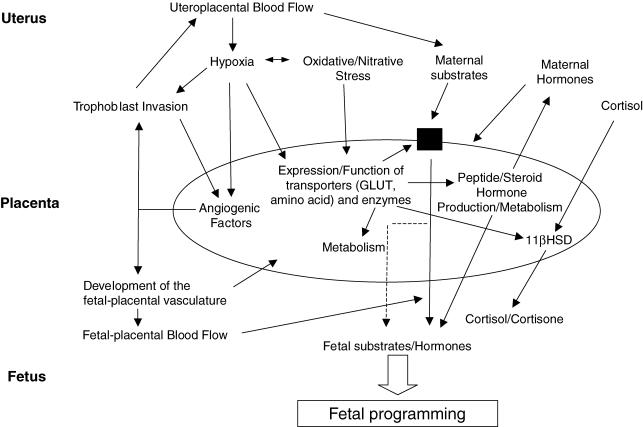
Placental nutrient transport has long been known to be dependent on vascular development which determines blood flow to both sides of the placenta and transport of flow-limited substrates (Fig. 1). Angiogenesis and vasculogenesis in both uteroplacental and fetal–placental circulations are important in this regard (Fig. 1). There is abundant evidence for alterations in these parameters in pregnancies complicated by IUGR, pre-eclampsia or diabetes. What has recently become clear, however, is the role of the trophoblast (both amount and function), in placental transporter activity, hormone production and substrate metabolism. Recent animal experiments have highlighted the involvement of both the vasculature and trophoblast in overall placental transport. In the Yorkshire pig, fetal growth from mid to late gestation is associated with an increase in area of endometrial attachment (placental exchange area) whereas in the Chinese Meishan pig fetus at this time, growth is associated with an increase in expression of angiogenic vascular endothelial growth factor (VEGF) mRNA, and density of placental blood vessels which increases placental efficiency (Vonnahme & Ford, 2004). In the over-nourished adolescent ewe, a model that results in IUGR, less proliferation of fetal trophectoderm and reduced expression of angiogenic factors in the placenta is seen. This leads to a reduction in placental mass, blood flow, fetal glucose, amino acids and oxygen concentration (Wallace et al. 2004). However, transporter activity was normal when adjusted to placental mass so in this model placental size limits fetal growth.
Figure 1. Placental adaptive responses and fetal programming.
The role of imprinted genes
At least 60 imprinted genes have been described in humans. Paternally derived imprinted genes enhance fetal growth while maternally imprinted genes suppress fetal growth (Reik et al. 2003). Knockout of paternally expressed Igf2 reduces placental growth while knockout of maternally expressed p57kip2 results in placental hyperplasia. Imprinted genes control both fetal and placental growth and may therefore control both the supply (placenta) and demand (fetus) of nutrients. In addition, several imprinted genes also encode for specific transporters in trophoblast. The paternally expressed Ata3 gene encodes a component of the system A amino acid transporter (Mizuno et al. 2002), while the maternally imprinted Impt/Slc22a11 gene encodes an organic cation transporter (Dao et al. 1998). Imprinting is also controlled by epigenetic mechanisms including DNA methylation and histone acetylation under the control of environmental factors and nutrients (Reik et al. 2003). This may provide a linkage between maternal nutrition and fetal placental growth.
Glucose and amino acid transporters
Alterations in expression of glucose and amino acid transporters in the placenta have been reported in pregnancies complicated by diabetes, IUGR and pre-eclampsia. In individuals with insulin-dependent diabetes mellitus plus a LGA baby, an increase in Glut 1 expression in the basement membrane is seen, accompanied by an increase in system A, a sodium-dependent transporter of neutral amino acids (Jansson et al. 1999). However, in pregnancies with gestational diabetes plus LGA no change in Glut 1 in the basement membrane, but an increase in system A, was seen (Jansson et al. 2001). The implication of this is that glucose transporters can be up-regulated (programmed) by hyperglycaemia in the first trimester leading to accelerated fetal growth in late gestation. In contrast, system A can be up-regulated in late gestation but not in the first trimester (Jansson et al. 2003).
There are at least 15 different amino acid transporters expressed in trophoblast (Jansson & Powell, 2000). There is evidence that the expression and activity of system A is reduced in IUGR placentas (where conversely there is no change in system y+ (a cationic amino acid transporter) (Mahendran et al. 1993; Jansson et al. 1998; Ayuk et al. 2002). However, system A is increased in diabetes (Jansson et al. 2002) and an inverse relationship has been reported between expression of system A in microvillous membranes and size at birth (Godfrey, 1998) perhaps due to up-regulation to maintain transport. Hypoxia, which has been associated with IUGR decreases expression of system A in trophoblast (Nelson et al. 2003) and may be a major regulator of expression. Inhibition of system A in rats causes growth restriction (Cramer et al. 2002) whereas in the growth-restricted placental-specific Igf2 knockout mouse, where there is a small placenta with abnormal barrier thickness and altered passive permeability, up-regulation of amino acid transport is seen (Sibley et al. 2004). All these data point towards an adaptive response of transporters in the placenta to altered growth but also that trophoblast membrane amino acid transport is a key regulator of fetal growth (Fig. 1).
Glucocorticoid action and metabolism
Glucocorticoids are key regulators of organ development and maturation. Exposure of the rat fetus to excess maternal or exogenous glucocorticoids causes growth restriction, hypertension, hyperglycaemia, increased activity of the hypothalamic pituitary adrenal axis (HPAA) and anxiety-like behaviour in aversive situations (Lindsay et al. 1996; Welberg et al. 2000). Glucocorticoids, which are produced by trophoblast and regulated by the activity of 11β hydroxysteroid dehydrogenase (11βHSD), impair the expression and function of Glut transporters (Hahn et al. 1999). The 11βHSD enzymes regulate exposure of the fetus to glucocorticoids. The trophoblast expresses 11βHSD2 that converts cortisol to inactive cortisone and this may protect the fetus against high levels of maternal cortisol (Krozowski et al. 1995) (Fig. 1). Expression and activity of placental 11βHSD2 is regulated in a cell- and gestational age-specific manner in both humans and baboons (Pepe et al. 2001). In humans, mutations in the 11βHSD2 gene have been reported in association with low birth weight and reduced 11βHSD2 activity, and increased fetal cortisol levels have been reported in association with IUGR (Seckl et al. 2000). 11βHSD2 expression increases with gestational age (Murphy & Clifton, 2003) and changes in oxygen tension alter 11βHSD2 expression and activity (Alfaidy et al. 2002). Thus, the reduced 11βHSD2 expression and activity in pre-eclampsia (Challis et al. 2002) may be due to a sustained placental hypoxia due to deficient trophoblast invasion in the first trimester. In a sheep model, maternal nutrient restriction for more than 45 days significantly decreased placental 11βHSD2 in addition to causing IUGR (McMullen et al. 2004). Overall, altered placental glucocorticoid metabolism is associated with nutrient restriction and hypoxia which both lead to IUGR and fetal programming. Again this points to a central role for the placenta.
Hypoxia, oxidative and nitrative stress
The placental pathologies that are associated with fetal programming including IUGR, pre-eclampsia and diabetes are also associated with hypoxia, oxidative and nitrative stress in the placenta. Low oxygen tension is physiological for organogenesis and is a key regulator of cellular events in early trophoblast differentiation (Genbacev et al. 1997). In early gestation the trophoblast develops by histiotrophic nutrition (Burton et al. 2001) but the establishment of intervillous blood flow at 10–12 weeks gestation subjects the trophoblast to oxidative stress (Jauniaux et al. 2000). This switch in oxygenation may also regulate trophoblast invasion as low oxygen tension prevents trophoblast differentiation to the invasive extravillous trophoblast pathway (Fig. 1).
The effect of hypoxia is mediated via the transcription hypoxia-inducible factor HIF-1α which activates gene transcription in response to varying oxygen concentrations. In mice with placentas null for Arnt, a HIF-1 subunit, there are reduced labyrinthine and spongiotrophoblast layers in the placenta, thus defining the role of hypoxia and HIF in determination of placental differentiation (Adelman et al. 2000).
Hypoxia has significant effects on placental development, causing hypercapillarization of the villous vasculature (Kingdom & Kaufmann, 1997), i.e. branching angiogenesis, an effect similar to that seen in vivo at high altitude and with maternal anaemia. This is due to hypoxic regulation of angiogenic mediators including VEGF and placental growth factor (PLGF), and angiopoietins (Charnock-Jones et al. 2004; Kaufmann et al. 2004) (Fig. 1). Hypoxia also increases the amount of villous trophoblast whereas strong oxygenation of villi reduces trophoblast proliferation (Kingdom & Kaufmann, 1997). Therefore oxygen regulates development of the villous vascular tree and villous trophoblast proliferation events that affect placental transport and thus may be a key event in programming.
The increasing metabolic activity of placental mitochondria throughout gestation results in increasing oxidative stress in a normal pregnancy. This oxidative stress is exacerbated in pregnancies complicated by pre-eclampsia or diabetes (Wang et al. 1992; Giugliano et al. 1996) and can be measured by production of reactive oxygen species or by decreased levels of antioxidant enzymes. The reactive oxygen species produced include superoxide and nitric oxide, both of which can be produced by trophoblast. We have recently shown that trophoblast and placental villous vascular endothelium express NADPH oxidase (NOX) 1 and 5 isoforms, which are probably the major enzymatic sources of superoxide in the placenta. In pregnancies complicated by pre-eclampsia, increased expression of both NOX 1 and 5 is seen (Cui et al. 2006). Trophoblast and placental vascular endothelium are also sites of production of nitric oxide. Upon interaction with locally produced superoxide this leads to production of the powerful pro-oxidant peroxynitrite. Peroxynitrite production and action can be localized by measurement of nitrotyrosine residues. A characteristic of peroxynitrite action is covalent modification of proteins by nitration leading to either gain or loss of function (Fig. 1). We have provided evidence that nitrotyrosine residues are increased in the placentas of pregnancies complicated by pre-eclampsia or diabetes (Myatt et al. 1996) and that treatment with peroxynitrite in vitro will alter placental vascular function (Kossenjans et al. 2000). Using proteomics, we have shown an increased abundance of nitrated proteins in the pre-eclamptic placenta and that phospho p38 MAP kinase, an enzyme that has a vital role in placental development, is nitrated in pre-eclampsia and has decreased catalytic activity (Webster RP, Brockman DE, Macha S & Myatt L, unpublished observations). Mice that are null for p38 MAP kinase are embryonic lethals and show decreased labyrinthine and spongiotrophoblast layers along with decreased vascularization in the labyrinthe (Mudgett et al. 2000). We also have preliminary evidence that poly ADP–ribose polymerase, acetyl coA transferase and the P2X4 receptor (Roberts VHJ, Webster RP, Brockman DE, Pitzer BA & Myatt L, unpublished observations) are nitrated in pre-eclampsia. Together these data suggest that hypoxia, oxidative and nitrative stress may all regulate placental development/function with consequent effects on fetal programming.
Summary
It is clear that the placenta is not simply a passive participant in pregnancy, supplying maternal substrates to the fetus. Rather it adapts to the maternal environment and changes both its structure and function with the net result being a change in both substrate supply to the fetus and the hormonal environment experienced by it. The placenta thus assumes an active role in programming the fetal experience in utero which leads to disease in adult life. While there is a surfeit of associative human and animal data that demonstrate this programming and the placental adaptation that occurs in response to an altered maternal environment, the mechanism(s) at the placental level remain elusive. As hypoxia mimics many of the effects that can be found in the placenta in association with fetal programming, we suggest that hypoxia and resultant oxidative and nitrative stress occurring at critical periods of placental development may be a common, although possibly not the only, underlying stimulus to the placental events associated with and leading to fetal programming.
References
- Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. Oxygen regulation of placental 11 beta-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metab. 2002;87:4797–4805. doi: 10.1210/jc.2002-020310. [DOI] [PubMed] [Google Scholar]
- Ayuk PT, Theophanous D, D'Souza SW, Sibley CP, Glazier JD. 1-arginine transport by the microvillous plasma membrane of the syncytiotrophoblast from human placenta in relation to nitric oxide production: effects of gestation, preeclampsia, and intrauterine growth restriction. J Clin Endocrinol Metab. 2002;87:747–751. doi: 10.1210/jcem.87.2.8204. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the Human Placenta. New York: Springer-Verlag; 1990. [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester – a review. Placenta. 2001;22(Suppl. A):S70–S77. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, Whittle WL, Newnham JP. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002;124:1–17. doi: 10.1530/rep.0.1240001. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25:103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Cramer S, Beveridge M, Kilberg M, Novak D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am J Physiol Cell Physiol. 2002;282:C153–C160. doi: 10.1152/ajpcell.2002.282.1.C153. [DOI] [PubMed] [Google Scholar]
- Cui XL, Brockman D, Campos B, Myatt L. Expression of NADPH oxidase isoform 1 (Nox1) in human placenta: involvement in preeclampsia. Placenta. 2006;27:422–431. doi: 10.1016/j.placenta.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D, Frank D, Qian N, O'Keefe D, Vosatka RJ, Walsh CP, Tycko B. IMPT1, an imprinted gene similar to polyspecific transporter and multi-drug resistance genes. Hum Mol Genet. 1998;7:597–608. doi: 10.1093/hmg/7.4.597. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Godfrey KM. Maternal regulation of fetal development and health in adult life. Eur J Obstet Gynecol Reprod Biol. 1998;78:141–150. doi: 10.1016/s0301-2115(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Graf R, Engelmann M, Beslagic D, Reul JM, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Wennergren M, Powell TL. Placental glucose transport in gestational diabetes mellitus. Am J Obstet Gynecol. 2001;184:111–116. doi: 10.1067/mob.2001.108075. [DOI] [PubMed] [Google Scholar]
- Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]
- Jansson T, Powell TL. Placental nutrient transfer and fetal growth. Nutrition. 2000;16:500–502. doi: 10.1016/s0899-9007(00)00323-3. [DOI] [PubMed] [Google Scholar]
- Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999;180:163–168. doi: 10.1016/s0002-9378(99)70169-9. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. discussion 623–616. [DOI] [PubMed] [Google Scholar]
- Kossenjans W, Eis A, Sahay R, Brockman D, Myatt L. Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am J Physiol Heart Circ Physiol. 2000;278:H1311–H1319. doi: 10.1152/ajpheart.2000.278.4.H1311. [DOI] [PubMed] [Google Scholar]
- Krebs C, Macara LM, Leiser R, Bowman AW, Greer IA, Kingdom JC. Intrauterine growth restriction with absent end-diastolic flow velocity in the umbilical artery is associated with maldevelopment of the placental terminal villous tree. Am J Obstet Gynecol. 1996;175:1534–1542. doi: 10.1016/s0002-9378(96)70103-5. [DOI] [PubMed] [Google Scholar]
- Krozowski Z, MaGuire JA, Stein-Oakley AN, Dowling J, Smith RE, Andrews RK. Immunohistochemical localization of the 11 beta-hydroxysteroid dehydrogenase type II enzyme in human kidney and placenta. J Clin Endocrinol Metab. 1995;80:2203–2209. doi: 10.1210/jcem.80.7.7608280. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- McMullen S, Osgerby JC, Thurston LM, Gadd TS, Wood PJ, Wathes DC, Michael AE. Alterations in placental 11 beta-hydroxysteroid dehydrogenase (11 betaHSD) activities and fetal cortisol: cortisone ratios induced by nutritional restriction prior to conception and at defined stages of gestation in ewes. Reproduction. 2004;127:717–725. doi: 10.1530/rep.1.00070. [DOI] [PubMed] [Google Scholar]
- Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Sotomaru Y, Katsuzawa Y, Kono T, Meguro M, Oshimura M, Kawai J, Tomaru Y, Kiyosawa H, Nikaido I, Amanuma H, Hayashizaki Y, Okazaki Y. Asb4, Ata3, and Dcn are novel imprinted genes identified by high-throughput screening using RIKEN cDNA microarray. Biochem Biophys Res Commun. 2002;290:1499–1505. doi: 10.1006/bbrc.2002.6370. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000;97:10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–744. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28:488–493. doi: 10.1161/01.hyp.28.3.488. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am J Physiol Cell Physiol. 2003;284:C310–C315. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- Pepe GJ, Burch MG, Albrecht ED. Localization and developmental regulation of 11beta-hydroxysteroid dehydrogenase-1 and -2 in the baboon syncytiotrophoblast. Endocrinology. 2001;142:68–80. doi: 10.1210/endo.142.1.7877. [DOI] [PubMed] [Google Scholar]
- Reik W, Constancia M, Fowden A, Anderson N, Dean W, Ferguson-Smith A, Tycko B, Sibley C. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl JR, Cleasby M, Nyirenda MJ. Glucocorticoids, 11beta-hydroxysteroid dehydrogenase, and fetal programming. Kidney Int. 2000;57:1412–1417. doi: 10.1046/j.1523-1755.2000.00984.x. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Coan PM, Ferguson-Smith AC, Dean W, Hughes J, Smith P, Reik W, Burton GJ, Fowden AL, Constancia M. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proc Natl Acad Sci U S A. 2004;101:8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme KA, Ford SP. Differential expression of the vascular endothelial growth factor-receptor system in the gravid uterus of yorkshire and Meishan pigs. Biol Reprod. 2004;71:163–169. doi: 10.1095/biolreprod.103.026344. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Aitken RP, Milne JS, Hay WW., Jr Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus. Biol Reprod. 2004;71:1055–1062. doi: 10.1095/biolreprod.104.030965. [DOI] [PubMed] [Google Scholar]
- Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynecol. 1992;167:946–949. doi: 10.1016/s0002-9378(12)80017-2. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–1054. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]