Abstract
Functional and structural studies demonstrate that Cl− channels of the ClC family have a dimeric double-barrelled structure, with each monomer contributing an identical pore. Studies with ClC-0, the prototype ClC channel, show the presence of independent mechanisms gating the individual pores or both pores simultaneously. A single-point mutation in the CBS-2 domain of ClC-0 has been shown to abolish slow gating. We have taken advantage of the high conservation of CBS domains in ClC channels to test for the presence of a slow gate in ClC-2 by reproducing this mutation (H811A). ClC-2-H811A showed faster opening kinetics and opened at more positive potentials than ClC-2. There was no difference in [Cl−]i dependence. Additional neutralization of a putative pore gate glutamate side chain (E207V) abolished all gating. Resolving slow and fast gating relaxations, however, revealed that the H811A mutation affected both fast and slow gating processes in ClC-2. This suggests that slow and fast gating in ClC-2 are coupled, perhaps with slow gating contributing to the operation of the pore E207 as a protopore gate.
ClC-2 is a broadly expressed Cl− channel member of the ClC family of membrane proteins (Jentsch et al. 2002). The function of ClC-2 is not well understood, but inactivation of the Clcn2 gene in mice leads to blindness and male infertility, prompting the suggestion that it could be involved in epithelial transport processes (Bösl et al. 2001; Nehrke et al. 2002). A possible role in epithelial transport is also supported by localization and functional studies in intestinal epithelia (Lipecka et al. 2002; Catalán et al. 2002, 2004; Zdebik et al. 2004). ClC-2 might be important in the control of intracellular Cl− ([Cl−]i) in neurones expressing inhibitory GABA receptors (Staley et al. 1996). Mutations in the human ClC-2 gene have been associated with idiopathic generalized epilepsy (Haug et al. 2003), but the pathophysiological mechanisms remain unclear (Niemeyer et al. 2004; Jentsch et al. 2005).
Much information about the gating of ClC channels has come from detailed studies of a Torpedo ClC-0 channel. ClC-0 was demonstrated to be a functional homodimer with the subunits forming parallel identical pores (Miller, 1982; Middleton et al. 1996; Ludewig et al. 1996). Structural data from the E. coli ClC homologue, EcClC, support the view that ClC channels are doubled-barrelled homodimers (Dutzler et al. 2002) and, despite the fact that it functions as an exchanger H+–Cl− (Accardi & Miller, 2004), it has provided important clues to the mechanism of ClC channel gating (Dutzler et al. 2003). In ClC-0 there is a fast gating process which controls independently the gating of each protopore (Miller, 1982). This is believed to involve the movement of a glutamate (E148 in EcClC and E166 in ClC-0) side-chain from an outermost Cl− binding site in the selectivity filter thus freeing the permeation pathway (Dutzler et al. 2003). In addition, a slow gating process can open or close both ClC-0 protopores simultaneously and has been termed the common gate. The two gating processes occur independently of each other and have opposite voltage dependencies, with the slow gate being favoured by hyperpolarization and fast gates opening with positive voltages (Miller, 1982). Functional and biochemical experiments also suggested a homodimeric structure for ClC-1, the main Cl− conductance of mammalian muscle (Fahlke et al. 1997; Saviane et al. 1999).
Gating of ClC-2 depends upon intra- but not extracellular Cl− and neutralization of E207 (which was erroneously referred to as E207 in Niemeyer et al. (2003)), homologous to E148 in EcClC, leads to loss of sensitivity to intracellular Cl− and, to a great extent, voltage. Experiments testing for transient activation by extracellular protons also demonstrated that E207 was not available for protonation in the closed channel state but became so after hyperpolarization. It was deduced that E207 is a hyperpolarization-dependent protopore gate in ClC-2 and that access of intracellular Cl− to a site normally occupied by its side-chain in the pore stabilizes the open state. A remaining hyperpolarization-dependent gate was speculated to correspond to the common gating of ClC-0 (Niemeyer et al. 2003).
The presence of a slow (common) gating mechanism in ClC-2 has not been proven. ClC-2 shows low activity under resting conditions but opens slowly upon hyperpolarization. A slow gating process in ClC-2, separate from the fast gating of protopores, has been surmised from temperature dependence, Cd2+ inhibition, and mutation of a cysteine residue known to alter common gating in ClC-0 (Zúñiga et al. 2004). The complex, multiexponential activation was ascribed to opening of a common gate acting on both protopores of a double-barrelled channel, with separate, [Cl−]i-dependent fast protopore gates that also respond to hyperpolarization present in parallel. Separation was, however, not clear and it was hypothesized that if present, the two processes must be rather strongly coupled in ClC-2. A similar conclusion has been reached from recent kinetic modelling of ClC-2 gating (de Santiago et al. 2005).
Recent work has investigated the role of C-terminus cystathionine β-synthase CBS domains in controlling gating of ClC-0, -1 and -2 (Estévez et al. 2004; Niemeyer et al. 2004; Hebeisen et al. 2004; Bennetts et al. 2005). Estévez et al. demonstrated that mutating H736 present in CBS2 of ClC-0 abolished common gating and inferred a similar effect in ClC-1. As CBS domains are highly conserved between ClC channels, we have explored for evidence of a separate common gate in ClC-2 that could be obtained by mutation of this conserved (H811) residue. We demonstrate that mutating H811 in ClC-2 has a profound effect on gating and that when combined with neutralization of E207 leads to the disappearance of all gating. Kinetic separation of slow and fast gating in H811-mutated ClC-2, however, reveals that these two processes cannot be affected separately and casts doubts on their presence as independent events in ClC-2.
Methods
The ClC-2 cDNA was ClC-2Δ77-86 from guinea-pig (Cid et al. 2000). Numbering corresponds to GenBank sequence no. AF113529. Mutants were generated using PCR and confirmed by sequencing. HEK-293 cells were grown and transiently transfected with expression plasmids for the various ClC-2 constructs and πH3-Cd8 to identify effectively transfected cells as previously described (Cid et al. 2000). Experiments were performed on cells in 35-mm cell culture plastic Petri dishes mounted directly on the microscope stage. The bath solution contained (mm): 140 NaCl, 2 CaCl2, 1 MgCl2, 22 sucrose and 10 Hepes, pH 7.4 adjusted with Tris. The pipette solution (35 mm Cl−) contained (mm): 100 sodium gluconate, 33 CsCl, 1 MgCl2, 2 EGTA, 1Na3ATP and 10 Hepes, pH 7.4 adjusted with Tris. In the 135 mm Cl−, 100 mm NaCl replaced the gluconate salt. Low Cl− solutions were prepared by equimolar replacement of 130 mm NaCl by sodium gluconate. Liquid junction potentials were calculated (Barry, 1994) and corrections applied when appropriate.
Standard whole cell patch-clamp recordings were performed as described elsewhere (Díaz & Sepúlveda, 1995) using an Axopatch 200B (Axon Instruments, USA) or an EPC-7 (List, Germany) amplifier. The bath was grounded via an agar–150 mm KCl bridge. Patch-clamp pipettes had resistances of 2–3 MΩ. The voltage pulse generator and analysis programs were from Axon Instruments. When giving trains of pulses, an interval of 60 s or 90 s between pulses was left at the holding potential to allow for complete current recovery. The currents generated by transfection were observed neither in untransfected cells nor in cells transfected with the πH3-Cd8 plasmid alone.
Time courses for current activation and deactivation were fitted by a double exponential plus a constant term equation as previously described (Cid et al. 2000). To estimate the apparent open probability, tail currents were obtained and their distribution as a function of voltage analysed by fitting a Boltzmann function of the form:
where Po and Pmin are open probability as a function of voltage and residual open probability independent of voltage, respectively. Po is assumed to reach a value of unity at full hyperpolarization. V0.5 is the voltage at which 50% activation occurs, and k is the slope factor.
The voltage dependence of fast and slow gating was resolved as follows: the open probability of the fast gate was calculated by comparing tail currents taken at 30 mV depolarizing pulses before (1Itail) and after (2Itail) a brief (10 ms) pulse to −200 mV. Assuming that the brief hyperpolarizing pulse opens the fast gate to a probability Pf = 1 (de Santiago et al. 2005), one can write Pf = 1Itail/2Itail. Ps can then be derived from overall Po values, estimated as described above, as Ps = Po/Pf.
Results
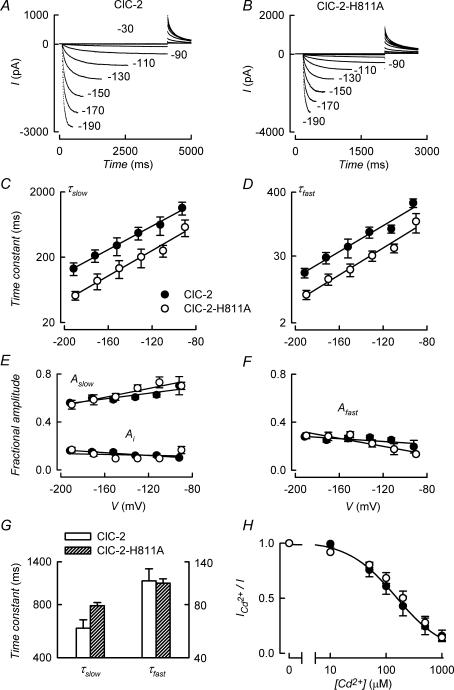
There is a high degree of sequence conservation between CBS domains in different ClC channels. Mutating a conserved histidine in CBS2 of ClC-0 has been shown to abolish the voltage dependence of the common gate leading to channels gated purely by the protopore gate (Estévez et al. 2004). This residue is conserved in ClC-2, number 811, and we have produced the equivalent mutation, ClC-2-H811A. In Fig. 1, currents evoked by voltage pulses in cells transfected with the H811A mutant of ClC-2 (Fig. 1B) are compared with those elicited by the WT channel (Fig. 1A). Currents appeared similar for WT and mutant, in that there was inward rectification with no current at depolarizing voltages. To quantify possible changes in activation and deactivation kinetics between the two channel types, their time dependence was analysed by fitting a double exponential plus a constant term equation to the current relaxations. Figure 1C and D shows the time constants for current activation whilst the deactivation time constants are in Fig. 1G. Activation of ClC-2 can be described by a bi-exponential kinetics plus an instantaneous term. Activation was faster for ClC-2-H811A at all voltages tested, but corresponding fractional amplitudes (Fig. 1E and F) did not differ significantly between mutant and WT channel. Closing of ClC-2 can be described by bi-exponential kinetics (Varela et al. 2002) and mutation H811A did not affect τf whilst it produced a small but significant increase in τs. The fractional contribution of each process to deactivation was about 0.5 and did not differ between channel types (data not shown).
Figure 1. Effect of H811A mutation on ClC-2-mediated chloride currents.
A and B, current traces mediated by ClC-2 (A) or ClC-2-H811A (B), elicited from a Vh of 0 mV in response to test pulses to the indicated potentials. These were followed by a pulse to 30 mV. The duration of the main pulses was increased at more positive voltages in order to approximate full activation of the conductance. For illustration purposes, the beginnings of the tail currents at 30 mV were set at the same time. C and D, effect of H811A mutation on activation. Slow (τs) and fast (τf) time constants were obtained from double-exponential plus a constant term fits to the time course and are plotted against voltage. E and F, fractional amplitudes (As and Af) of the slow and fast opening process as well as instantaneous component (Ai). G, time constants for the deactivation process at 30 mV after an activating pulse to −130 mV. The left- and right-hand axes apply to τs and τf, respectively. Means ± s.e.m. of 7 separate experiments for both WT and ClC-2-H811A. H, effect of Cd2+ on currents mediated by ClC-2 and ClC-2-H811A. Dose–response curves for the effect of extracellular Cd2+ on currents mediated by ClC-2 (•) and ClC-2-H811A (○) in response to a pulse to −130 mV (means ± s.e.m. of 3 experiments each). The line shows a fit of a hyperbola to the ClC-2 data, which gave an IC50 value of 151 μm.
Extracellular Zn2+ and Cd2+ inhibit ClC channels 0, 1 and 2 (Kurz et al. 1997; Rychkov et al. 1998; Chen, 1998; Clark et al. 1998; Varela et al. 2002) and in ClC-0 the extracellular divalent acts by a facilitation of slow gating leading to closure of the channel (Chen, 1998). We did not detect any difference in the potency of Cd2+ blockade between WT and ClC-2-H811A channels (Fig. 1H). This contrasts with the elimination of Zn2+ effect on the ClC-0 by H736A mutation (Estévez et al. 2004), equivalent to H811A in ClC-2.
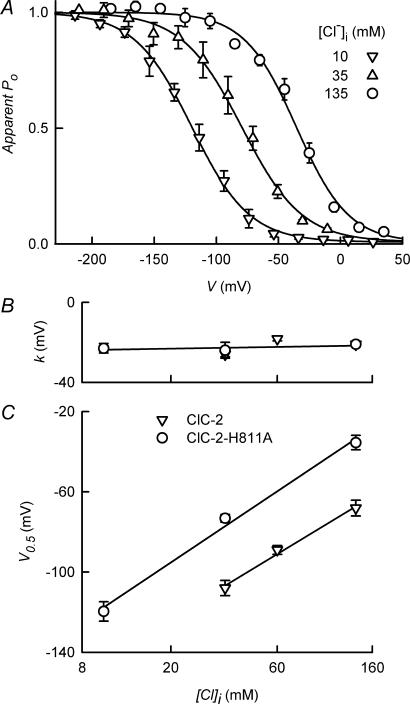
The fast and slow processes described by the time constants τf and τs have been associated with the fast (protopore) and slow (common) gating processes in ClC-2 (Zúñiga et al. 2004). This view has been supported by the recent development of a model for voltage-dependent ClC-2 gating in which protopore gating is associated with the fast and very fast (instantaneous component) opening processes and common gate with the slow relaxation (de Santiago et al. 2005). On the basis of this interpretation it might be speculated that the H811A mutation is affecting both protopore and common gates in ClC-2. If this were the case, the voltage dependence of the ClC-2-H811A channel, and perhaps its [Cl−]i dependence, should be altered. This was explored with the results in Fig. 2A, which shows steady-state activation curves for the H811A mutant of ClC-2 at [Cl−]i of 10, 35 and 135 mm. In WT ClC-2 at [Cl−]i 35 mm, significant activation takes place at potentials more negative than −50 mV, with half-maximal activation at −108 mV and slope factor of −26 mV (Niemeyer et al. 2003). In the H811A mutant there was a significant shift of the activation curve to more positive potentials (Fig. 2A, upright triangles) with respective V0.5 and slope factor values of −79 and −24 mV at [Cl−]i 35 mm. At 135 and 10 mm[Cl−]i activation curves were shifted in the depolarized direction and hyperpolarized direction, respectively (Fig. 2A). Slope factors were not altered by mutation compared with WT values (Fig. 2B). V0.5 values were displaced towards more positive voltage by about 35 mV in the ClC-2-H811A channels by comparison with the WT values (Fig. 2C). The slope describing the increase in V0.5 brought about by increasing [Cl−]i did not differ markedly between WT and mutant ClC-2. The H811A mutation in ClC-2 therefore decreases the energy required for channel opening but does not change its [Cl−]i dependence.
Figure 2. Steady-state activation for ClC-2-H811A.
A, apparent open probabilities, calculated as described in the text, are shown for ClC-2-H811A WT at three intracellular Cl− concentrations. The continuous lines are fits of a Boltzmann equation to the data (see Methods). B and C show the resulting slope factor k and V0.5 values, plotted against [Cl−]i. Values are means ± s.e.m. of 4–7 experiments. Data for wild-type ClC-2 (Catalán et al. 2004) are shown in B and C for comparison.
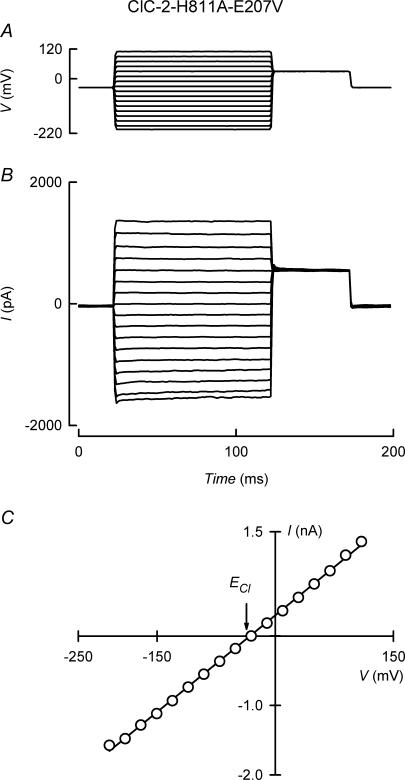
A mechanism has been proposed for protopore gating of ClC-2, where hyperpolarization is required to remove a pore carboxyl group provided by the side chain of E207, from an external Cl− binding site within the pore. Only then could Cl− move from the intracellular aspect of the selectivity filter to occupy the said external binding site (Niemeyer et al. 2003). If H811A mutation facilitated this hyperpolarization-dependent E207 side chain displacement, additional mutation neutralizing pore E207 might further open the channels. Figure 3B shows that, when studied at a range of voltages, the doubly mutated ClC-2-H811A–E207V channel was found open, and no time or voltage dependence was evident. Selectivity for Cl− was maintained, as seen from the reversal potential which was near ECl (Fig. 3C). This result suggests that additional mutation H811A might have indeed hit part of the mechanism facilitating pore gating in ClC-2.
Figure 3. Effect of double mutation H811A and E207V on ClC-2.
A and B, voltage protocol and currents in a cell transfected with ClC-2-H811A–E207V double mutant Cl− channels. C, the corresponding current–voltage relation. ECl indicates the position of the equilibrium potential for Cl− on the abscissa. Similar results were obtained in six separate experiments.
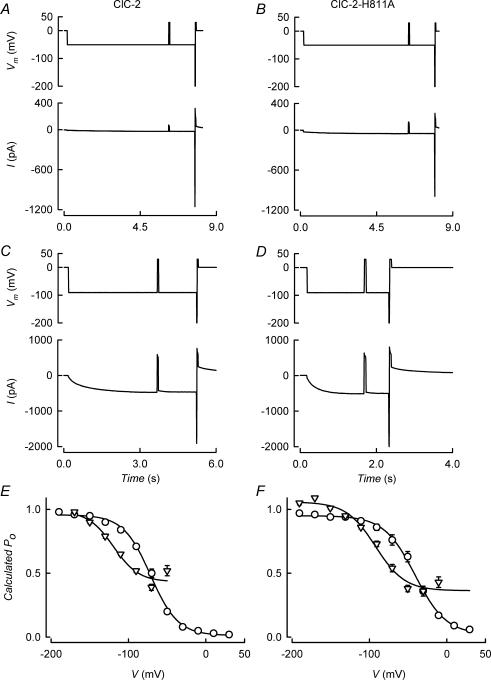
Channels carrying mutation E207V have been shown to lose intracellular Cl− dependence and become largely voltage-insensitive. Some voltage dependence remains in the E207V mutant, and if we accept that this pore-lining glutamate residue controls protopore fast gating, this residual voltage dependence might correspond to a slower component of gating. The picture given by ClC-2-E207V experiments is of shallow dependence on potential of the remaining gating, with a V0.5 of −134 to −120 mV and a minimal Po of 0.4–0.6 at positive potentials (Niemeyer et al. 2003). Separation between slow and fast components of gating in ClC-2 and their voltage dependence has been obtained by assuming that at potentials that saturate the conductance versus potential curve a Po of unity has been achieved and therefore that Pf and Ps are both equal to 1 (de Santiago et al. 2005). We tested this assumption by analysis of variance, which showed that apparent Po derived from relative conductance measurements coincides with the open probability of single channels (see supplemental Fig.1). Stimulation protocols for estimating Ps and Pf are seen in Fig. 4A–D. Pf was derived from comparing a tail current before and after giving a brief, 10 ms pulse to −200 mV, which is deemed not to affect markedly Ps but to evoke full opening of the fast gate (de Santiago et al. 2005). Ps is then inferred from Pf and total Po. Examples of the currents evoked by voltage protocols are shown in the lower panels of Fig. 4A–D for WT and H811A mutant channels. In Fig. 4E, the voltage dependence of the deduced Ps (triangles) and Pf (circles) values for wild-type channels are plotted. According to our calculations, the fast process opened the channels with a V0.5 of −72 ± 2 mV and a slope factor of −17.3 ± 1.0 mV (means ± s.e.m., n = 8) and there was no apparent offset at positive potentials. The slow gating process opened the channels with a V0.5 of −121 ± 2 mV and a slope factor of −18.0 ± 2.4 mV and had an offset minimal probability of 45 ± 6% (means ± s.e.m., n = 8) at positive potentials. Mutation H811A in ClC-2 affected both gating processes, as seen in Fig. 4F. Fast gating in ClC-2-H811A had a V0.5 of −43 ± 3 mV and a slope factor of −19.2 ± 1.5 mV (means ± s.e.m., n = 6), with Pf = 0 at positive potentials. Slow gating occurred with a V0.5 of −92 ± 5 mV and a slope of −19 ± 4 mV and had an offset of 36 ± 4% (means ± s.e.m., n = 6). If the voltage protocol used can in fact separate fast and slow gating processes, both are affected in a similar manner by H811A mutation.
Figure 4. Resolving slow and fast gating processes in the activation of ClC-2 and ClC-2-H811A.
A–D, upper panels, voltage protocols for estimating Ps and Pf values as described in the text. Brief −200 mV pulses are used to evoke full opening of the fast gate without affecting the slow gate and tail currents were obtained by pulsing to 30 mV. Lower panels, currents evoked by the voltage protocols for WT ClC-2 (A and C) and for H811A mutant (B and D) channels. E and F, voltage dependence of the deduced Ps (▿) and Pf (○) values for ClC-2 (E, means ± s.e.m., n = 9) and ClC-2-H811A (F, means ± s.e.m., n = 6) channels.
Discussion
ClC chloride channels show a conserved double-barrelled structure with two independent pores each formed by the folding of a single monomer which associates in a homodimeric structure (Miller, 1982; Dutzler et al. 2002). The model for channel gating in ClC-0, the prototype ClC channel, invokes two independent gates. A fast gate controls the individual pores of the single subunits, and a slow gate controls both of these protopores simultaneously. A gating mechanism has been proposed for protopore gating of ClC-2, where hyperpolarization is required to remove a pore carboxylate group from an external Cl− binding site within the pore (Niemeyer et al. 2003). In the present work we seek to determine whether a slow gating mechanism is present in ClC-2 equivalent to that described as a common gate in ClC-0.
Work with ClC-2–ClC-2 and ClC-2–ClC-0 concatamers has shown that ClC-2 forms functional homo- and heterodimers (Weinreich & Jentsch, 2001). It seems reasonable to assume that each ClC-2 monomer harbours a protopore. Evidence for a common (slow) gating process is less conclusive. Opening of ClC-2 channels is a function of voltage and can be described by a bi-exponential function with time constants that differ by around 10-fold, plus a third component too fast to resolve by exponential fit (Cid et al. 2000; Varela et al. 2002). It has been proposed that they might correspond to the equivalent fast and slow gating transitions in ClC-0 and, consequently, pertain to gating of protopores and a common gate, respectively (Zúñiga et al. 2004; de Santiago et al. 2005). It must be pointed out, however, that slow gating in ClC-0 differs in several respects from any aspect of gating in ClC-2. The common gate of ClC-0 is remarkably slow (time scale 10–100 s) and highly temperature dependent (Q10≈ 40) (Jentsch et al. 2002). The slow transition of ClC-2 is much faster (τs 0.2–2 s) and less temperature dependent (Q10 for τs≈ 5, similar to that of τf (Zúñiga et al. 2004)). In ClC-0, extracellular divalent cations block by closing the common gate of ClC-0 (Chen, 1998) and mutating an extracellular-facing cysteine residue abolishes common gating and the effect of Zn2+ (Lin et al. 1999). Extracellular Cd2+ blocks ClC-2 affecting fast and slow gating processes and mutation of the conserved cysteine decreases the effect of Cd2+ but affects both types of transitions (Zúñiga et al. 2004). Finally, our present results show that mutation of a conserved CBS residue (H811A) which abolishes common gating in ClC-0, affects slow and fast transitions in ClC-2 without altering its sensitivity to extracellular Cd2+. These divergences point to the idea that whilst slow and fast voltage-dependent transitions are present in ClC-2, the equivalent of an independent slow, common gate similar to that in ClC-0 is lacking.
Our present results show that the separate and independent gating processes seen in ClC-0 are absent from ClC-2. Fast, protopore gating in ClC-2 is a property of the outermost Cl− binding site in the pore involving removal of the E207 side-chain and entry of Cl− into the site from the intracellular aspect of the pore. This is supported by the disappearance of Cl− and most voltage dependence upon E207 neutralization, with the remaining Po dependence upon voltage resembling that of the slow gating described here and elsewhere (de Santiago et al. 2005). The disappearance of the slow-resembling gate remaining in the E207V mutant upon additional H811A CBS mutation might have led to the simple conclusion that protopore and slow gate had been hit separately. The picture is more complicated, however, as H811A mutation on its own affects slow and fast gating processes in a similar way. The position of α-helix R as deduced from the crystal structure of bacterial ClC shows that it participates in the selectivity filter and projects towards the cytoplasmatic side at its opposite end (Dutzler et al. 2002). This helix connects a central Cl− coordination site within the pore with the structures in the intracellular C-terminus such as the CBS domains. It has been speculated that this might be the way in which CBS domains contribute to gating regulation (Estévez et al. 2004). Interestingly, mutation K566Q in the R helix of rat ClC-2 produces a decrease in the apparent energy to open the channel (Jordt & Jentsch, 1997), similar to what is seen here with the H811A mutant. We have postulated that hyperpolarization is required to remove the E207 side-chain from an external binding site in the pore to allow Cl− occupancy (Niemeyer et al. 2003). This might require a pore conformational change as proposed for protopore gating in ClC-0 (Accardi & Pusch, 2003). It is conceivable that slow gating in ClC-2 contributes, or corresponds entirely, to this putative conformational change, perhaps exerting its effect at the central binding site of the pore through α-helix R. Whether slow gating in ClC-2 closes both pores simultaneously and the nature of the proposed coupling between its slow and fast gates (Zúñiga et al. 2004; de Santiago et al. 2005) are questions that will require further work probably involving single channel recording.
CBS domains have been proposed to regulate the activity of various proteins, including ClC channels, acting as sensors of the energy status of the cell through binding adenosine derivatives (Scott et al. 2004). It is possible that CBS domains form a continuous structure with the pore of ClC channels, allowing regulation of gating by intracellular factors. The effect of CBS domains on ClC-2 gating uncovered here by mutation might be part of a physiological mechanism.
Acknowledgments
This research was supported by Fondecyt 1030627. CECS is supported by a Millennium Science Initiative Institutional grant, and by grants from Fundación Andes, the Tinker Foundation and Empresas CMPC.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2005.102392
http://jp.physoc.org/cgi/content/full/jphysiol.2005.102392/DC1 and contains supplemental material consisting of a figure entitled: Variance–mean current relation analysis for ClC-2 channels.
This material can also be found as part of the full-text HTML version available from
References
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. doi: 10.1038/nature02314. [DOI] [PubMed] [Google Scholar]
- Accardi A, Pusch M. Conformational changes in the pore of CLC-0. J General Physiol. 2003;122:277–293. doi: 10.1085/jgp.200308834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Meth. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Bennetts B, Rychkov GY, Ng HL, Morton CJ, Stapleton D, Parker MW, Cromer BA. Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem. 2005;280:32452–32458. doi: 10.1074/jbc.M502890200. [DOI] [PubMed] [Google Scholar]
- Bösl MR, Stein V, Hubner C, Zdebik AA, Jordt SE, Mukhopadhyay AK, Davidoff MS, Holstein AF, Jentsch TJ. Male germ cells and photoreceptors, both dependent on close cell–cell interactions, degenerate upon ClC-2 Cl− channel disruption. EMBO J. 2001;20:1289–1299. doi: 10.1093/emboj/20.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán M, Cornejo I, Figueroa C, Niemeyer MI, Sepúlveda FV, Cid LP. Expression of ClC-2 chloride channels in surface epithelium of guinea pig colon: mRNA, protein and functional evidence. Am J Physiol. 2002;283:G1004–G1013. doi: 10.1152/ajpgi.00158.2002. [DOI] [PubMed] [Google Scholar]
- Catalán M, Niemeyer MI, Cid LP, Sepúlveda FV. Basolateral ClC-2 chloride channels in surface colon epithelium: regulation by a direct effect of intracellular chloride. Gastroenterology. 2004;126:1104–1114. doi: 10.1053/j.gastro.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chen TY. Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating. J General Physiol. 1998;112:715–726. doi: 10.1085/jgp.112.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid LP, Niemeyer MI, Ramírez A, Sepúlveda FV. Splice variants of a ClC-2 chloride channel with differing functional characteristics. Am J Physiol. 2000;279:C1198–C1210. doi: 10.1152/ajpcell.2000.279.4.C1198. [DOI] [PubMed] [Google Scholar]
- Clark S, Jordt SE, Jentsch TJ, Mathie A. Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J Physiol. 1998;506:665–678. doi: 10.1111/j.1469-7793.1998.665bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santiago JA, Nehrke K, Arreola J. Quantitattive analysis of the voltage-dependent gating of mouse parotid ClC-2 chloride channel. J General Physiol. 2005;126:591–603. doi: 10.1085/jgp.200509310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M, Sepúlveda FV. Characterisation of Ca2+-dependent inwardly rectifying K+ currents in HeLa cells. Pflugers Arch. 1995;430:168–180. doi: 10.1007/BF00374647. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol. 2004;557:363–378. doi: 10.1113/jphysiol.2003.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlke C, Knittle T, Gurnett CA, Campbell KP, George ALJ. Subunit stoichiometry of human muscle chloride channels. J General Physiol. 1997;109:93–104. doi: 10.1085/jgp.109.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet. 2003;33:527–532. doi: 10.1038/ng1121. [DOI] [PubMed] [Google Scholar]
- Hebeisen S, Biela A, Giese B, Muller-Newen G, Hidalgo P, Fahlke C. The role of the carboxyl terminus in ClC chloride channel function. J Biol Chem. 2004;279:13140–13147. doi: 10.1074/jbc.M312649200. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Poet M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl− channels gleaned from human genetic disease and mouse models. Annu Rev Physiol. 2005;67:779–807. doi: 10.1146/annurev.physiol.67.032003.153245. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz L, Wagner S, George AL, Jr, Rudel R. Probing the major skeletal muscle chloride channel with Zn2+ and other sulfhydryl-reactive compounds. Pflugers Arch. 1997;433:357–363. doi: 10.1007/s004240050288. [DOI] [PubMed] [Google Scholar]
- Lin YW, Lin CW, Chen TY. Elimination of the slow gating of ClC-0 chloride channel by a point mutation. J General Physiol. 1999;114:1–12. doi: 10.1085/jgp.114.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol. 2002;282:C805–C816. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- Ludewig U, Pusch M, Jentsch TJ. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Pheasant DJ, Miller C. Homodimeric architecture of a ClC-type chloride ion channel. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Phil Trans Roy Soc Lond B. 1982;299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Nehrke K, Arreola J, Nguyen HV, Pilato J, Richardson L, Okunade G, Baggs R, Shull GE, Melvin JE. Loss of hyperpolarization-activated Cl− current in salivary acinar cells from Clcn2 knockout mice. J Biol Chem. 2002;277:23604–23611. doi: 10.1074/jbc.M202900200. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Zúñiga L, Catalán M, Sepúlveda FV. A conserved pore-lining glutamate as a voltage- and chloride-dependent gate in the ClC-2 chloride channel. J Physiol. 2003;553:873–879. doi: 10.1113/jphysiol.2003.055988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, Yusef YR, Cornejo I, Flores CA, Sepúlveda FV, Cid LP. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol Genomics. 2004;19:74–83. doi: 10.1152/physiolgenomics.00070.2004. [DOI] [PubMed] [Google Scholar]
- Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, Bretag AH. Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J General Physiol. 1998;111:653–665. doi: 10.1085/jgp.111.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Conti F, Pusch M. The muscle chloride channel ClC-1 has a double-barrelled appearance that is differentially affected in dominant and recessive myotonia. J General Physiol. 1999;113:457–467. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the ClC-2 chloride channel. Neuron. 1996;17:543–551. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Varela D, Niemeyer MI, Cid LP, Sepúlveda FV. Effect of an N-terminus deletion on voltage-dependent gating of ClC-2 chloride channel. J Physiol. 2002;544:363–372. doi: 10.1113/jphysiol.2002.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich F, Jentsch TJ. Pores formed by single subunits in mixed dimers of different CLC chloride channels. J Biol Chem. 2001;276:2347–2353. doi: 10.1074/jbc.M005733200. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl− channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem. 2004;279:22276–22283. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
- Zúñiga L, Niemeyer MI, Varela D, Catalán M, Cid LP, Sepúlveda FV. The voltage-dependent ClC-2 chloride channel has a dual gating mechanism. J Physiol. 2004;555:671–682. doi: 10.1113/jphysiol.2003.060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://jp.physoc.org/cgi/content/full/jphysiol.2005.102392/DC1 and contains supplemental material consisting of a figure entitled: Variance–mean current relation analysis for ClC-2 channels.