Abstract
Cystic fibrosis transmembrane conductance regulator (CFTR) is an ion channel employing the ABC transporter structural motif. Deletion of a single residue (Phe508) in the first nucleotide-binding domain (NBD1), which occurs in most patients with cystic fibrosis, impairs both maturation and function of the protein. However, substitution of the Phe508 with small uncharged amino acids, including cysteine, is permissive for maturation. To explore the possible role of the phenylalanine aromatic side chain in channel gating we introduced a cysteine at this position in cysless CFTR, enabling its selective chemical modification by sulfhydryl reagents. Both cysless and wild-type CFTR ion channels have identical mean open times when activated by different nucleotide ligands. Moreover, both channels could be locked in an open state by introducing an ATPase inhibiting mutation (E1371S). However, the introduction of a single cysteine (F508C) prevented the cysless E1371S channel from maintaining the permanently open state, allowing closing to occur. Chemical modification of cysless E1371S/F508C by sulfhydryl reagents was used to probe the role of the side chain in ion channel function. Specifically, benzyl-methanethiosulphonate modification of this variant restored the gating behaviour to that of cysless E1371S containing the wild-type phenylalanine at position 508. This provides the first direct evidence that a specific interaction of the Phe508 aromatic side chain plays a role in determining the residency time in the closed state. Thus, despite the fact that this aromatic side chain is not essential for CFTR folding, it is important in the ion channel function.
The phenylalanine residue at position 508 in the first nucleotide-binding domain (NBD1) of cystic fibrosis transmembrane conductance regulator (CFTR) has been the focus of much attention because a mutation causing its deletion is the cause of most cystic fibrosis (Cheng et al. 1990). The mutation results in the biosynthetic arrest of the protein in the endoplasmic reticulum, where it is degraded rather than proceeding further through the secretory pathway to its functional site at the cell surface (Kopito, 1999). Recent structural studies (Lewis et al. 2005; Thibodeau et al. 2005) and experiments with intracellular CFTR folding (Du et al. 2005) suggest the deletion causes only a minor surface perturbation of NBD1 where it normally resides, and probably interferes with the normal domain assembly of the protein. When the protein is partially rescued by growth of cells at reduced temperature it has at least some chloride channel activity (Dalemans et al. 1991; Denning et al. 1992). The significance of the functional defects induced by Phe508 deletion is controversial (Schultz et al. 1999; Wang et al. 2000). When Phe508 is replaced by one of several other residues rather than being deleted, the protein is able to mature and be transported to the cell surface (Du et al. 2005). The fact that cysteine is one of the permissive residues at this position provides the opportunity to utilize a wide variety of sulfhydryl reactive reagents to investigate the role of this surface residue in CFTR ion channel function.
However, to specifically probe the 508 position with sulfhydryl reagents it is necessary to remove the 18 endogenous cysteines from CFTR. When this was done recently by Chen et al. (2004) they found that cysless CFTR did not mature and reach the surface of cells in which it was transiently expressed.
We now have generated BHK cell lines stably expressing cysless CFTR which does mature and function in cells cultured at reduced temperature. The cysless protein mediates cyclic AMP-stimulated 36Cl efflux from cells, binds and hydrolyses ATP and exhibits characteristic single-channel activity. Combining cysless CFTR single-channel recording with site-directed mutagenesis and cysteine chemical modification revealed that the side chain of Phe508 has a strong impact on CFTR function. This supports a direct role of the Phe508 side chain in CFTR channel gating and provides a tool with which to characterize the intramolecular interaction involved.
Methods
Stable expression of cysless CFTR in BHK cells
Mutagenesis of each of the cysteine codons in CFTR in the Bluescript cloning vector was performed by employing the Strategene Quick Exchange protocol such that alanine replaced all cysteines except Cys590 and Cys592, which were replaced by leucine. Following transfer to the pNUT expression plasmid, the mutagenized sequence was transfected into BHK-21 cells, and stably expressing lines were selected and maintained using methotrexate as previously described (Chang et al. 1993).
Isolation of membrane vesicles from BHK-21 cells
Membrane vesicles from BHK-21 cells stably expressing cysless CFTR variants were isolated as previously described (Aleksandrov & Riordan, 1998). To allow maturation of cysless CFTR variants, 10 dishes (150 mm) with 80% confluence were grown at 27°C for an additional 48 h in the presence of 2 mm butyrate. The cells were then scraped, pelleted by brief centrifugation, and resuspended in ice-cold hypotonic lysis buffer (10 mm Hepes, pH 7.2, 1 mm EDTA, 2 μg ml−1 leupeptin, 4 μg ml−1 aprotinin, 250 μg ml−1 benzamidine, 100 μg ml−1 Pefabloc, 7 μg ml−1 E64). Following 15 min incubation on ice, cells were lysed by 10 strokes in a tight-fitting Dounce homogenizer, followed by 15 strokes after the addition of an equal volume of sucrose buffer (500 mm sucrose, 10 mm Hepes, pH 7.2). The postnuclear supernatant (10 000 g, 10 min) was centrifuged at 100 000 g for 45 min to sediment microsomes. The pellet was resuspended in 1 ml of prephosphorylation buffer (250 mm sucrose, 10 mm Hepes, pH 7.2, 5 mm MgCl2) to yield a total protein concentration of about 1–3 mg ml−1. Vesicles with uniform diameter of ∼1 μm were obtained after brief sonication (3 × 20 s). The amount of CFTR protein in the vesicles was monitored by immunoblotting. The membrane vesicles were stored at −80°C until used.
SDS-PAGE and immunoblotting
Total cell lysates were separated on 4–20% gradient gels. For Western blotting, proteins were transferred to nitrocellulose (Bio-Rad) and probed with α-CFTR monoclonal antibody 596 recognizing an epitope between residues 1204 and 1211 in the second nucleotide-binding domain (NBD2) and detected by enhanced chemiluminescence (Pierce).
Immunofluorescence microscopy
Cells were grown on collagen-coated chamber slides (BD PharMingen), fixed in 4% paraformaldehyde, washed with PBS, permeabilized in 0.1% saponin and blocked with 5% normal goat serum and 1% bovine serum albumin. Incubation with primary antibody 596 ascites (1:1000) was followed by incubation with Alexa-488-labelled goat-antimouse IgG. Cells were observed in a Zeiss LSM510 confocal microscope.
36Cl efflux assay
Cells were grown to confluency in six-well culture plates and washed twice with efflux buffer containing 20 mm Hepes, pH 7.4, 11 mm glucose, 2 mm Ca(NO3)2, 2 mm Mg(NO3)2, 135 mm NaNO3, and loaded with 36Cl for 1 h at room temperature in 0.5 ml of the same buffer containing 1 μCi of the isotope. Prior to initiation of efflux, loaded cells were washed three times with 1 ml of efflux buffer. At time 0, efflux buffer was made to 10 μ m forskolin, 1 mm isobutylmethylxanthine and 100 μ m dibutyryl cAMP. Samples of 0.5 ml were collected and replaced with the same amount of fresh efflux buffer containing the agonists at 1 min intervals. Radioactivity in each collected sample was determined by scintillation counting.
Photoaffinity labelling with 8-azido 32P-labelled nucleotides
Photoaffinity labelling of wild-type and cysless CFTR in BHK membranes was carried out as previously described (Aleksandrov et al. 2001). To measure binding, membrane suspensions were incubated with 25 μ m 8N3[γ-32P]ATP or 8N3[α-32P]ADP in 40 mm Tris/HCl, pH 7.4, 5 mm MgCl2, 0.1 mm EGTA for 5 min on ice, and irradiated for 2 min in a Stratalinker UV cross-linker (254 nm). The labelled membranes were then incubated with tosyl L-I-tosylamide-2-phenylethye chloromethyl ketone (TPCK)-treated trypsin for 15 min on ice at an enzyme to membrane protein mass ratio of 1:200. Excess soybean trypsin inhibitor was added to terminate digestion and membranes were solubilized in RIPA buffer (50 mm Tris-HCl, pH 7.4, 1% deoxycholate, 1% Triton, 0.1% SDS and 150 mm NaCl) and immuno-isolated with antibodies L12B4 (recognizing NBD1) or 596 (recognizing NBD2) immobilized on Dynal beads. Sample buffer eluates from washed beads were separated by SDS-PAGE and transferred to nitrocellulose for autoradiography employing a Packard Instant Imager. To measure turnover of 8N3[γ-32P] ATP at the two ATP-binding sites, labelled membranes were incubated at 30°C for the time periods indicated in Fig. 2B.
Figure 2. Cysless CFTR retains nucleotide-binding activity at two sites.
A, tryptic fragments of the wild-type and cysless CFTR containing portions of nucleotide-binding sites I and II photolabelled with 8N3[γ-32P]ATP or 8N3[α-32P]ADP. The larger weak bands (site II at ∼105 kDa and site I at ∼75 kDa) are products of trypsin cleavage in the R domain near the middle of the CFTR sequence; the smaller more intensely labelled bands (site I at ∼40 kDa, and site II at ∼30 kDa) are products of more complete trypsin digestion (Aleksandrov et al. 2001). B, time courses of turnover of 8N3[γ-32P]ATP at sites I and II in wild-type and cysless CFTR. The incubation time is shown above the lines.
Single-channel measurements
CFTR single-channel measurements were made as previously described (Aleksandrov & Riordan, 1998). Microsomal membrane vesicles were prepared from BHK cells stably expressing cysless CFTR or other variants. Prior to use, membrane vesicles at a protein concentration of about 1 mg ml−1 in 10 mm Hepes, 5 mm MgCl2, 250 mm sucrose, pH 7.2, were phosphorylated by incubation for 15 min at room temperature with protein kinase A (100 units ml−1; Promega) and 2 mm Na2ATP. A planar lipid bilayer of 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-ethanolamine and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (Avanti Polar Lipids) at a 2:1 ratio was painted over a 0.2 mm aperture in a Teflon partition between cis and trans compartments of a chamber. All measurements were done at 30°C in symmetrical salt solution containing (mm): 300 Tris/HCl pH 7.2, 3 MgCl2 and 1 EGTA. The membrane voltage potential is the difference between cis and trans (ground) compartments. Single channel currents were measured at −75 mV under voltage-clamp conditions using an Axopatch 200B amplifier. Under this experimental condition the opening of any Cl− channel is monitored as a jump to the more negative current. Therefore in the all single-channel records shown in the paper the lower current level corresponds to the open state whereas the upper level corresponds to the closed state. Both chambers were magnetically stirred and controlled by a thermostat. Heating and temperature control were established using a Temperature Control System TC2BIP (Cell MicroControls). Ion channels were transferred from CFTR-containing membrane vesicles into the preformed lipid bilayer as a result of spontaneous fusion. To maintain uniform channel orientation and optimal functional state, 5 mm Na2ATP, 100 units ml−1 PKA and 10 μl membrane vesicles were added to the cis compartment only. Single-channel currents were measured under voltage-clamp conditions using an Axopatch 200B amplifier. The output signal was filtered with an 8-pole Bessel low-pass filter LPBF-48DG with cut off frequency of 50 Hz. For data analysis the signal was digitized (Digidata 1200; Axon Instruments) with sampling rate of 500 Hz and analysed using pCLAMP 6.0 (Axon Instruments) software. Dwell-time histograms for the open and closed states were plotted in the logarithmic binning mode and fitted by a single exponential function (Levenberg–Marquard method with value of 2.5 × 10−7 for convergence criterion). The mean dwell times for two different sets of experiments were considered as identical if the difference between them is in the limit of the sum of standard errors. Origin 4.1 (OriginLab Corp., Northampton, MA, USA) software was used to fit all-points histograms by multipeak Gaussians. Single-channel current was defined as the distance between peaks on the fitting curve and used for the calculation of the single-channel conductance. The probability of the single channel to be open (Po) was calculated as a ratio of the area under the peak for the open state to the total area under the both peaks on the fitting curve. The total number of active channels was determined as a maximum number of simultaneously open channels during the course of an experiment. Only records contained a single open level were used for the analysis. Records with Po values of less than 0.1 and number of openings not more than 2 × 102 were not considered as single-channel records and were used for the mean open time (τo) analysis only (Colquhoun & Hawkes, 1990). For these cases Po is the upper limit of the real value and the effective mean closed time is estimated as τc = τo(1 −Po)/Po.
Cysteine modification by methanethiosulphonate (MTS) reagents
MTS compounds were purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). Benzyl-methanethiosulphonate (MTSBn) and 2-trimethylammonioethylmethanethiosulphonate (MTSET) were dissolved in DMSO to prepare stock solutions of 10 mm concentration. Stock solutions were added into the cis compartment to a final concentration of 50 μ m in the course of single-channel recording to monitor any changes of the single-channel parameters in real time.
Results
Expression and activity of cysless CFTR
Not unexpectedly, mutagenesis of all 18 cysteine residues within CFTR impairs the ability of the protein to mature (Chen et al. 2004). However, we were able to promote the appearance of reasonable amounts of mature protein by stringent selection of stable transformants (Chang et al. 1993) and growth of cells at reduced temperature (Fig. 1). This was evidenced by appearance of the mature protein band C with complex oligosaccharide chains causing reduced mobility in SDS-PAGE (Fig. 1A). Immunofluorescence staining in Fig. 1B confirmed that some temperature-rescued cysless CFTR was associated with the plasma membrane. The amount of rescued band C was in the same range as for the wild type but the ratio of mature to immature bands was less than in the wild type (Fig. 1A and B). The surface cysless CFTR had cAMP stimulated Cl− channel activity as revealed in 36Cl efflux assays (Fig. 1C).
Figure 1. Cysless CFTR matures and functions at the surface of BHK cells.
A, Western blot showing the appearance of the mature 180 kDa band (C) in cells grown at 27°C, in addition to the immature 160 kDa band (B) visible at both temperatures. B, immunofluorescence staining shows wild-type and temperature-rescued cysless cystic fibrosis transmembrane conductance regulator (CFTR). Bars, 20 μm. C, 36Cl efflux from cells cultured at 37 and 27°C. Cells were loaded with 36Cl and incubated in efflux buffer. At time 0 (shown by arrow) 10 μ m forskolin, 1 mm isobutylmethylxanthine and 100 μ m dibutyryl cAMP were added. Sample (0.5 ml) were collected and replaced with the same amount of fresh efflux buffer containing the agonists at 1 min intervals. Each point is a mean value of three different experiments. For some time points the s.e.m. bar is hidden by symbol.
ATP binding and hydrolysis by cysless CFTR
As a hydrolysable-ligand gated channel, CFTR binds ATP at two sites, one of which also hydrolyses the nucleotide (Aleksandrov et al. 2002). As in other ABC proteins each of the two sites has contributions from both NBDs, with site I formed primarily by the Walker A P-loop of NBD1 and the signature sequence of NBD2, and site II by the P-loop of NBD2 and the signature of NBD1. To monitor these two sites in cysless CFTR we employed photoaffinity labelling with 8N3ATP and 8N3ADP. Figure 2A shows that there is binding of both nucleotides to both sites in the cysless as in the wild-type protein. The azido group at the 8 position of the adenine ring resulted in photolabelling of the NBD contributing the Walker A motif to the composite binding site, i.e. NBD1 of site I and NBD2 of site II. Thus, after trypsin digestion fragments, containing the labelled sites immunoprecipitated by antibodies recognizing epitopes in NBD1 (L12B4) and NBD2 (596) reflected nucleotide binding at sites I and II, respectively (bands of more rapid mobility). The less intense pair of more slowly migrating bands, representing ‘halves’ of CFTR produced by cleavage in the R domain, was coimmunoprecipitated by both antibodies as shown previously (Aleksandrov et al. 2001). Thus, this association between the front and back ‘halves’ was maintained in the cysless protein. Because in wild-type CFTR, site II is hydrolytic, 8N3[γ-32P]ATP is bound there and turns over rapidly whereas that bound at site I is stable (Aleksandrov et al. 2002). This was most evident with the smaller bands containing the photolabelled segments of site I (at ∼40 kDa) and site II (at ∼30 kDa), and occurred with cysless as well as wild-type CFTR (Fig. 2B). Thus, CFTR from which all cysteines had been removed not only matured and trafficked to the cell surface during culture at 27°C, but also produced a cAMP-stimulated Cl− permeability and bound and hydrolysed ATP.
Cysless CFTR single-channel properties
The presence of functional cysless CFTR ion channels in the lipid bilayer strongly correlated with the intensity of the mature band C in Western blots (Fig. 1A). No CFTR channels were recorded with membrane vesicles prepared from non-transfected BHK cells or cells transfected with CFTR mutants expressing only the immature form of CFTR (data not shown).
Cysless CFTR single-channel conductance was about 10% greater than that of wild-type CFTR under the same experimental conditions (compare Figs 3 and 4).
Figure 3. Single-channel activities of phosphorylated wild-type CFTR driven by different ATP analogues as ligands.
Ligand-type and numerical values of the single-channel parameters are shown above the traces. The top level of each trace is an ion channel closed state. All-points histograms used to calculate open channel conductance and probability of the single channel being open (Po) are shown on the left. Dwell-time histograms used to calculate mean open and mean closed times for the different ligands are shown on the right. Mean values and number of experiments are shown in Table 1.
Figure 4. Single-channel activities of phosphorylated cysless CFTR with different ATP analogues as ligands.
Ligand-type and numerical values of the single-channel parameters are shown above the traces. All-points histograms used to calculate open channel conductance and Po are shown on the left. Dwell-time histograms used to calculate mean open and mean closed times for the different ligands are shown on the right. Mean values and number of experiments are shown in Table 1.
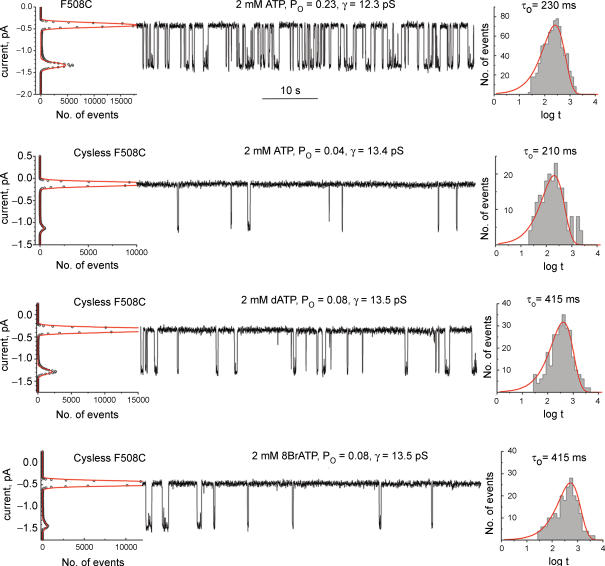
Wild-type CFTR ion channel mean open time (τo) was independent of the nucleotide concentration (Winter et al. 1994). However, different nucleotides (ATP, dATP, 8BrATP and 8N3ATP) supported CFTR ion channel gating that resulted in τo characteristic for each particular nucleotide (Fig. 3, Table 1). Thus τo may be considered as a signature of the ligand used to gate the CFTR channel. This behaviour was entirely maintained by the cysless channel (Fig. 4, Table 1), indicating that the channel open state is not influenced by the absence of all cysteines. In contrast, the mean closed times (τc) of cysless CFTR were substantially prolonged regardless of the ligand used. This was not due to impaired affinity of the nucleotide-binding sites in the cysless CFTR, as τc did not vary with increasing ligand concentration up to 10 mm (see Supplemental material). It is particularly remarkable that the difference in τc between cysless and wild-type CFTR was invariant for all ligands tested so that Δτc = τc,cysless−τc,wt = 1500 ± 100 ms. This ligand-independent increment in residence time of the closed state was the major functional difference between cysless and wild-type CFTR ion channels.
Table 1.
Basic parameters of wild-type and mutant CFTR channels
| Type | Ligand | Po | τo (ms) | τc (ms) | γ (pS) | n |
|---|---|---|---|---|---|---|
| Wild-type CFTR | ATP | 0.49 ± 0.03 | 220 ± 10 | 230 ± 10 | 12.3 ± 0.2 | 8 |
| dATP | 0.82 ± 0.03 | 420 ± 10 | 80 ± 10 | 12.3 ± 0.2 | 6 | |
| 8BrATP | 0.75 ± 0.02 | 510 ± 10 | 150 ± 10 | 12.3 ± 0.2 | 6 | |
| 8N3ATP | 0.58 ± 0.03 | 710 ± 10 | 560 ± 10 | 12.4 ± 0.2 | 5 | |
| F508C | ATP | 0.23 ± 0.03 | 230 ± 15 | 810 ± 10 | 12.3 ± 0.2 | 4 |
| Cysless CFTR | ATP | 0.12 ± 0.03 | 220 ± 15 | 1800 ± 120 | 13.5 ± 0.2 | 7 |
| dATP | 0.21 ± 0.03 | 410 ± 15 | 1600 ± 100 | 13.4 ± 0.2 | 4 | |
| 8BrATP | 0.24 ± 0.02 | 520 ± 15 | 1680 ± 120 | 13.2 ± 0.2 | 5 | |
| 8N3ATP | 0.26 ± 0.03 | 715 ± 15 | 2100 ± 160 | 13.4 ± 0.2 | 3 | |
| Cysless F508C | ATP | 0.04 ± 0.02 | 210 ± 20 | 5000 ± 850 | 13.4 ± 0.2 | 5 |
| dATP | 0.07 ± 0.02 | 415 ± 20 | 5200 ± 720 | 13.5 ± 0.2 | 3 | |
| 8BrATP | 0.09 ± 0.03 | 520 ± 20 | 5600 ± 900 | 13.5 ± 0.2 | 3 |
CFTR, cystic fibrosis transmembrane conductance regulator. Numerical values for the probability of the single channel being open (Po), the mean open time (τo), the mean closed time (τc) and γ are expressed as a mean values ± s.e.m. of n observations. All values are rounded to the nearest significant value. For cysless F508C, the effective values of τc were estimated as τo(1 − Po)/Po.
Influence of F508C substitution on channel gating
With a functional cysless CFTR, it became possible to focus on a single cysteine introduced at position 508 which is known to be permissive for maturation of the protein (Du et al. 2005). However, as a prelude to this, we first examined the single-channel properties of a F508C variant in the wild-type background (Fig. 5, upper trace). This revealed an approximate twofold reduction in Po relative to the wild-type CFTR and a marked increase in mean closed time. Mean open time was not different from the wild-type CFTR (see Table 1). These changes were qualitatively similar to those of cysless CFTR compared with wild type. With F508C in the cysless background there were even larger decreases in Po and increases in τc, with τo remaining unaltered (Fig. 5, second trace). Importantly, however, not only was τo unchanged with ATP as a ligand, but it also remained ligand specific as in the wild-type and cysless CFTR. This was indicated for cysless F508C in the lower two traces of Fig. 5 using the alternative ligands, dATP and 8BrATP, which resulted in the same increases in τo values relative to that with ATP as a ligand. Overall, the data in Fig. 5 revealed that the F508C substitution decelerated the gating of both wild-type and cysless CFTR primarily by prolonging the mean closed time. While informative, this large prolongation in cysless F508C limits detailed analysis of its gating kinetics.
Figure 5. Influence of F508C on CFTR channel gating.
Typical records of F508C and cysless F508C single-channel activity driven by different ligands at 30°C are shown in the middle of each panel. Numerical values of the single-channel parameters are shown above the traces. All-points histograms used to calculate open channel conductance and Po are shown on the left. Dwell-time histograms used to calculate mean open times for the different ligands are shown on the right. Mean values and number of experiments are shown in Table 1. Mean closed time for cysless F508C driven by different ligands was roughly estimated as τc = τo(1 −Po)/Po.
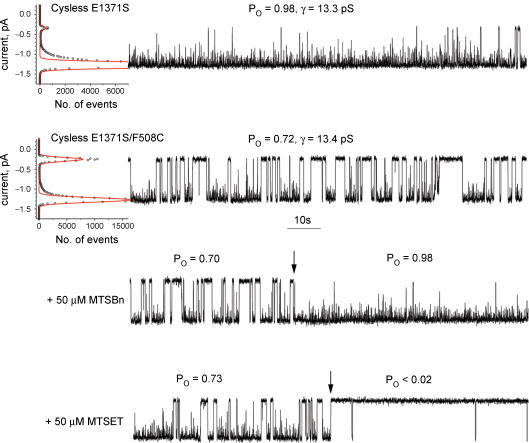
However, it was possible to circumvent this limitation by employing a variant in which the glutamate residue adjacent to the Walker B aspartate in NBD2 was mutated (E1371S). The effect of E1371S substitution on the wild-type CFTR ion channel function is shown in Fig. 6 (upper panel, Po = 0.97 ± 0.02, n = 5) and is in a good agreement with published data (Vergani et al. 2005). Importantly, this mutation had a similar but less complete effect on the cysless protein (Fig. 6, middle panel, Po = 0.82 ± 0.02, n = 6). While cysless E1371S was not completely locked open, its open probability was increased approximately sevenfold relative to the cysless wild-type protein (compare Fig. 4 upper panel, and Fig. 6 middle panel). The fact that the essentially non-hydrolytic cysless E1371S channel was able to open and close in a robust manner is of interest mechanistically. However, of practical importance for the utility of the cysless protein to study the role of the Phe508 residue in gating was the fact that the E1371S substitution also increased the activity of cysless F508C (Fig. 6, lower panel, Po = 0.25 ± 0.03, n = 4). Moreover, it was possible to differentiate better between cysless E1371S and cysless E1371S/F508C by using 8BrATP instead of ATP as a ligand (Fig. 7, first panel, Po = 0.97 ± 0.02, n = 4 and second panel (Fig. 7, second panel, Po = 0.71 ± 0.03, n = 4). This transformed the difference between them from the quantitative to the qualitative, where one was ‘locked open’ (single burst with Po≥ 0.95) while the other was obviously gated (multiple bursting with Po≤ 0.75).
Figure 6. Improvement of the cysless CFTR ion channel activity by introducing E1371S in NBD2.
The upper panel shows typical single-channel activity of the E1371S mutant at 30°C. The cysless E1371S single-channel record is shown in the middle panel. The influence of the further introduction of F508C in the cysless E1371S background on the ion channel gating is shown in the lower panel. The mean values of Po± s.e.m. and number of experiments are shown in the text. All records were done at 30°C and 2 mm ATP. Single-channel parameters are shown above each trace.
Figure 7. Aromatic side chain at position 508 is required to lock the channel open.
Cysless E1371S ion channel gated by 2 mm 8BrATP is shown in the upper panel. Effect of the introduction of F508C in the cysless E1371S background on the ion channel gating is shown in the second panel. The result of the chemical modification of the cysless E1371S/F508C channel by MTSBn at the cis side on the ion channel gating is shown by the arrow in the third panel. The effect of positively charged 2-trimethylammonioethylmethanethiosulphonate (MTSET) on the cysless E1371S/F508C ion channel function is shown by the arrow in the lower panel. The values of Po before and after chemical modification are shown above the traces. The mean values of Po± s.e.m. and number of experiments with chemical modifications are in the text. All records were done at 30°C and 2 mm 8BrATP.
Role of Phe508 aromatic side chain in gating
The fact that the presence of several different amino acids including cysteine in the 508 position enabled the protein to mature but with altered gating kinetics suggests that the aromatic side chain of phenylalanine is involved in the ion channel function. We reasoned that, if this were the case, modifications of C508 with a sulfhydryl reagent containing an aromatic ring might restore the original F508 gating properties. To test this assumption we used the cysless E1371S construct that is locked open with 8BrATP as a reference point (Fig. 7, upper panel). Under the same conditions this locked-open state could not be maintained when cysteine rather than phenylalanine was present at position 508. Instead, distinct opening and closing transitions occurred, although the overall open probability remained quite high (Fig. 7, second panel). The return of an aromatic ring to position 508 by the chemical modification with 50 μ m MTSBn restored the locked open state typical of cysless E1371S (Fig. 7, third panel, Po = 0.97 ± 0.02, n = 3). This strongly supports the notion that the aromatic side chain of Phe508 plays a role in channel gating. If this assumption is correct then modification of C508 with other MTS reagents not containing an aromatic ring should have a qualitatively different effect. This was found to be the case as the positively charged MTSET not only did not lock the cysless E1371S/F508C channel open but completely ablated gating (Fig. 7, lower trace, Po < 0.01, n = 3). This further strengthens the idea that specific interaction of Phe508 aromatic side chain is required for normal CFTR ion channel function. These qualitative differences in the gating kinetics provide the opportunity to monitor the process of chemical modification in real time at the single-molecule level.
Discussion
These experiments have two main outcomes. First they describe an initial characterization of cysless CFTR, indicating that it is functional, although with an extended single-channel closed time. Second, modification of a single cysteine (508C) in the cysless background with methanethiosulphonate reagents provide evidence of involvement of an aromatic moiety at this position in channel gating.
Neither ATP binding, nor the rate of ATP hydrolysis, is strongly affected by complete cysteine substitution in the mature CFTR molecule (Fig. 2). However the turnover of bound 8N3[γ-32P]ATP at the second ATP binding site provides only a qualitative measure of hydrolytic ability. Quantitative comparisons will require purification and conventional catalytic rate measurements, as has been done with wild-type CFTR (Li et al. 1996; Aleksandrov et al. 2002).
We employed single-channel recording to compare functional properties of the cysless and wild-type CFTR ion channels in more detail. It is important to distinguish between the requirements for this channel function and so-called conformational maturation. Conformational maturation, a term widely used in connection with the biosynthetic processing and ER export of CFTR, is not equivalent to functional competence. Some mutants can meet the requirements for maturation but are not sufficient for the normal protein function.
Our approach also illustrates the advantage of being able to introduce modifications of the cysteine after assembly has occurred rather than by mutagenesis. In the latter case, it is not possible to determine whether an effect on function has resulted indirectly from perturbed assembly. With the cysteine modification it is possible to monitor the impact of the induced structural perturbation at the single-molecule level in real time (see control in Supplemental material).
Complete cysteine substitution does not affect ion channel mean open time. Moreover, the open state has the same ligand specificity for both cysless and wild-type CFTR (see Table 1). Based on these experimental data we can conclude that complete cysteine substitution does not affect the open state structure. Therefore, we could expect the same structural organization for both cysless and wild-type CFTR under experimental conditions where the open state is predominant. The E1371 mutation that locks the channel open when 8BrATP is used as a ligand provides an identical structural background for both cysless and wild-type CFTR (Fig. 6 upper trace, Fig. 7 upper trace). That is why our experiments with the F508C mutation and its chemical modification were done on this particular background.
In contrast, there must be substantial differences between cysless and wild-type CFTR ion channels with respect to the structural organization within the closed state (see Supplemental material). These differences must account for the large increment in the mean closed time which persists even with ATP analogues that normally shorten the closed time of the wild-type CFTR ion channel (see Table 1). Mean closed time for wild-type CFTR over the range of nucleotide concentration close to saturation is ligand specific, as is the mean open time (see Table 1). However, cysless CFTR demonstrate the appearance of the additional ligand-insensitive component in the total mean closed time whereas mean open time for both mutants remains ligand specific. Within the open state configuration, cysless CFTR binding site(s) still recognize minor differences in the ligand chemical structure with the same efficiency as the wild-type CFTR indicating that the affinity of binding sites should be unchanged. At the same time, cysless CFTR cannot support the relevant difference in the closed times. This may be explained by less efficient coupling between channel gating and the hydrolytic cycle for the cysless protein compared with the wild-type CFTR. Mechanistically this means that not every hydrolytic cycle will induce ion channel gating, and nucleotide binding is not a rate-limiting step for cysless CFTR ion channel gating. In the framework of this speculation, the apparent contradiction between the strong difference in channel gating and hydrolytic activity for cysless and wild-type CFTR is resolved.
Thus, although its gating is not identical to wild-type, cysless CFTR provides a means of gaining further insight into the role of specific features of the molecule such as the Phe508 side chain. Previous studies have focused on the rescued ΔPhe508 channel from which Phe508 is simply absent (Kopito, 1999; Riordan, 1999). More recent studies examined the effects of replacement of Phe508 by all other amino acids on the structure of isolated NBD1 (Thibodeau et al. 2005) and on the maturation of the whole CFTR protein (Du et al. 2005). Although the F508C variant appeared to mature similarly as wild-type and mediated iodide efflux (Du et al. 2005), we show that at the single-channel level it greatly increased the channel mean closed time in both the wild-type and cysless background, suggesting that the phenylalanine side chain plays a role in channel gating. This could be confirmed using a site II ATPase-inhibited mutant (E1371S) which is locked open in both the wild-type and cysless backgrounds, while the F508C version of cysless E1371S was unable to maintain the locked open state. Neither total cysteine removal, nor F508C substitution, affects open state. On the contrary, both increase residency time in the closed state (Figs 3, 4 and 5). It should be remembered that brief closing events still occur in the locked-open state. In the restricted bandwidth of our single-channel recording they are visible as incomplete transitions and reflected in the asymmetric shape of the peak for the open state in the all-points histogram (Fig. 7, upper panel). We speculate that aromatic ring removal from position 508 could impede channel reopening and by doing so, transform some of these brief closings to long-lasting ones.
The ability of F508C version of cysless E1371S to maintain the locked open state was fully restored on modificaton of Cys508 with MTSBn. Thus either the aromatic ring of MTSBn or the native phenylalanine can support locking open, whereas the unmodified thiol cannot. In contrast, modification with the charged MTSET was functionally disruptive, completely ablating gating. This latter effect on channel function parallels the effects of mutagenic substitutions of Phe508 with large charged residues on CFTR maturation. Thus whereas the small cysteine side chain is compatible with maturation but not adequate for normal channel function, large charged groups are strongly detrimental to both.
Beyond the provision of a new tool for studies of CFTR structure and function, and demonstrating a role of the Phe508 aromatic side chain in the process responsible for the residency time in the closed state, mechanistic interpretations of the results remain largely speculative at this time.
Thus, just as removal of all endogenous cysteines apparently alters the gating response to ATP binding rather than binding itself, this also appears to be the case for the further increment in mean closed time caused by F508C. We postulate that a specific interaction of the wild-type aromatic phenylalanine side chain at this position is necessary for the normal more rapid exit from the closed state, and future experiments will attempt to identify the site of this interaction.
Acknowledgments
We wish to thank J. Stutts, S. Gabriel and T. Hegedus for critical reading and valuable comments. This work was supported by the NIDDK of the NIH.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2005.099457
http://jp.physoc.org/cgi/content/full/jphysiol.2005.099457/DC1 and contains supplemental material consisting of three figures.
Wild-type CFTR channel is insensitive to sulfhydryl group reduction but reversibly inhibited by DTNB to which the cysless CFTR channel is insensitive.
Single-channel activities of cysless and cysless F508C CFTR driven by 10 mM ATP.
Trypsin digestion patterns of cysless and wild-type CFTR.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Aleksandrov LA, Aleksandrov AA, Chang X-B, Riordan JR. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem. 2002;277:15419–15425. doi: 10.1074/jbc.M111713200. [DOI] [PubMed] [Google Scholar]
- Aleksandrov LA, Mengos A, Chang X-B, Aleksandrov AA, Riordan JR. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2001;276:12918–12923. doi: 10.1074/jbc.M100515200. [DOI] [PubMed] [Google Scholar]
- Aleksandrov AA, Riordan JR. Regulation of CFTR ion channel gating by MgATP. FEBS Lett. 1998;431:97–101. doi: 10.1016/s0014-5793(98)00713-3. [DOI] [PubMed] [Google Scholar]
- Chang X-B, Tabcharani JA, Hou Y-X, Jensen TJ, Kartner N, Alon N, Hanrahan JW, Riordan JR. Protein kinase A (PKA) still activates CFTR chloride channel after mutagenesis of all 10 PKA consensus phosphorylation sites. J Biol Chem. 1993;268:11304–11311. [PubMed] [Google Scholar]
- Chen EY, Bartlett MC, Loo TW, Clarke DM. The ΔF508 mutation disrupts packing of the transmembrane segments of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2004;279:39620–39627. doi: 10.1074/jbc.M407887200. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan C, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. Stochastic properties of ion channel openings and bursts in a membrane patch that contains two channels: Evidence concerning the number of channels present when a record containing only single openings is observed. Proc R Soc Lond B Biol Sci. 1990;240:453–477. doi: 10.1098/rspb.1990.0048. [DOI] [PubMed] [Google Scholar]
- Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Parvani A, Lecocq J-P, Lazdunski M. 'Altered chloride ion channel kinetics associated with the ΔF508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Du K, Sharma M, Lukacs GL. The ΔF508 cystic fibrosis mutation impairs domain–domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79(Suppl. 1):S167–S173. doi: 10.1152/physrev.1999.79.1.S167. [DOI] [PubMed] [Google Scholar]
- Lewis HA, Zhao X, Wang C, Sauder JM, Rooney I, Noland BW, Lorimer D, Kearins MC, Conners K, Condon B, Maloney PC, Guggino WB, Hunt JF, Emtage S. Impact of the ΔF508 mutation in first nucleotide-binding domain of human cystic fibrosis transmembrane conductance regulator on domain folding and structure. J Biol Chem. 2005;280:1346–1353. doi: 10.1074/jbc.M410968200. [DOI] [PubMed] [Google Scholar]
- Li C, Ramjeesingh M, Wang W, Garami E, Hewryk M, Lee D, Rommens JM, Galley K, Bear CE. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 1996;271:28463–28468. doi: 10.1074/jbc.271.45.28463. [DOI] [PubMed] [Google Scholar]
- Riordan JR. Cystic fibrosis as a disease of misprocessing of the cystic fibrosis transmembrane conductance regulator glycoprotein. Am J Hum Genet. 1999;64:1499–1504. doi: 10.1086/302429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz BD, Frizzell RA, Bridges RJ. Rescue of dysfunctional ΔF508-CFTR chloride channel activity by IBMX. J Membr Biol. 1999;170:51–66. doi: 10.1007/s002329900537. [DOI] [PubMed] [Google Scholar]
- Thibodeau PH, Brautigam CA, Machius M, Thomas PJ. Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol. 2005;12:10–16. doi: 10.1038/nsmb881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zeltwanger S, Hu S, Hwang T-C. Deletion of phenylalanine 508 causes attenuated phosphorylation-dependent activation of CFTR chloride channels. J Physiol 524. 2000;3:637–648. doi: 10.1111/j.1469-7793.2000.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MC, Sheppard DN, Carson MR, Welsh MJ. Effect of ATP concentration on CFTR Cl channels: a kinetic analysis of channel regulation. Biophys J. 1994;66:1398–1408. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type CFTR channel is insensitive to sulfhydryl group reduction but reversibly inhibited by DTNB to which the cysless CFTR channel is insensitive.
Single-channel activities of cysless and cysless F508C CFTR driven by 10 mM ATP.
Trypsin digestion patterns of cysless and wild-type CFTR.