Abstract
Repeated motor activities like locomotion, mastication and respiration need rhythmic discharges of functionally connected neurons termed central pattern generators (CPGs) that cyclically activate motoneurons even in the absence of descending commands from higher centres. For motor pattern generation, CPGs require integration of multiple processes including activation of ion channels and transmitter receptors at strategic locations within motor networks. One emerging mechanism is activation of glutamate metabotropic receptors (mGluRs) belonging to group I, while group II and III mGluRs appear to play an inhibitory function on sensory inputs. Group I mGluRs generate neuronal membrane depolarization with input resistance increase and rapid fluctuations in intracellular Ca2+, leading to enhanced excitability and rhythmicity. While synchronicity is probably due to modulation of inhibitory synaptic transmission, these oscillations occurring in coincidence with strong afferent stimuli or application of excitatory agents can trigger locomotor-like patterns. Hence, mGluR-sensitive spinal oscillators play a role in accessory networks for locomotor CPG activation. In brainstem networks supplying tongue muscle motoneurons, group I receptors facilitate excitatory synaptic inputs and evoke synchronous oscillations which stabilize motoneuron firing at regular, low frequency necessary for rhythmic tongue contractions. In this case, synchronicity depends on the strong electrical coupling amongst motoneurons rather than inhibitory transmission, while cyclic activation of KATP conductances sets its periodicity. Activation of mGluRs is therefore a powerful strategy to trigger and recruit patterned discharges of motoneurons.
The making of a motor rhythm: concerted neuronal interactions produced by a central pattern generator network
Most motor activities (like locomotion, respiration, swallowing, suckling, etc.) require rapid, repeated contractions of selected groups of skeletal muscles. Rather than being simply driven by descending inputs or triggered by peripheral afferents, such motor rhythms are the expression of oscillatory discharges from an ensemble of neurons wired together to generate a coherent motor output. In analogy with networks amply studied in invertebrates, these circuits are collectively termed central pattern generators (CPG).
As far as the locomotor programme is concerned, the identification of the CPG neurons responsible for it and their precise connectivity remain unclear. Nonetheless, various models of CPG operation as well as available experimental evidence assume the existence of a class of interneurons (located ventrally to the spinal central canal) using commissural interneurons to distribute synaptic inputs to left and right motor pools of the leg muscles (Kiehn et al. 2000; Grillner & Wallen, 2002; Kiehn & Kullander, 2004). A recent model obtained from the feline spinal cord further distinguishes between rhythm-generating interneurons (the ‘clock’) which set the pace of locomotion, and the pattern-generating interneurons whose task is to distribute motor commands (of excitatory or inhibitory nature) to various motor pools (Lafreniere-Roula & McCrea, 2005). These motor signals would then be transmitted to motoneurons via premotoneurons. Within such a scheme, the role of motoneurons would not be the one of rhythm generation, but of pattern refinement obtained through the activation of certain intrinsic membrane conductances (Kiehn et al. 2000; Grillner & Wallen, 2002; Kiehn & Kullander, 2004). Although the topography of the spinal CPG remains elusive, there is broad interest in elucidating the cellular processes which can modulate CPG activity (Grillner et al. 2000; Fetz et al. 2000).
In the case of respiration, recent studies have highlighted the crucial role of distinct areas of the brainstem like the pre-Bötzinger complex and the parafacial respiratory nucleus as rhythm generators via a complex interaction between local neurons releasing glutamate, GABA or glycine (Greer et al. 2006). Such an activity would represent a potent inspiratory drive to accelerate the rapid maturation of motoneurons involved in diaphragm muscle contractions, though motoneurons themselves are not rhythmogenic. Recent investigations using fast Ca2+ imaging have shown that, at least in the case of the fetal mouse brain, the central pattern generator for respiration may be more distributed than hitherto supposed as firing of neurons in immature respiratory circuits is a stochastic process, suggesting that the rhythm does not depend on a single pacemaker (Eugenin et al. 2006).
The cyclic output of most motor circuits in the spinal cord or brainstem depends on the interplay between the excitation mediated by glutamate acting on ionotropic receptors, the GABA- and glycine-mediated inhibition, and the activity of voltage-sensitive channels (Grillner & Wallen, 2002; Alford et al. 2003; Kudo et al. 2004; Greer et al. 2006). Glutamate also activates metabotropic glutamate receptors (mGluRs) producing long lasting changes in neuronal excitability (Anwyl, 1999) and modulating rhythmic motor patterns generation (Krieger et al. 1998; El Manira et al. 2002). To illustrate the role of mGluRs as triggers and gain-setters of rhythmic oscillations, we shall discuss as examples the circuits within the lumbar spinal cord and the brainstem nucleus hypoglossus. Thus, spinal motoneurons send rhythmic signals to extensor and flexor muscles of the lower (hind) limbs (during locomotion) while hypoglossal motoneurons (HMs) cyclically contract tongue muscles during respiration, swallowing, chewing, etc. In the latter case rhythmic activity emerges from a single set of motoneurons supplying the tongue muscles, while in the case of the spinal cord alternating activity appears in two distinct sets of motoneuron pools. We shall examine whether mGluRs have a role in both networks to switch on rhythmogenesis that is usually silent.
Central pattern generators: localization
A combination of molecular, genetic and imaging studies has been employed to investigate the distribution of CPG neurons in lumbar spinal cord (Demir et al. 2002; Kiehn & Butt, 2003; Kiehn & Kullander, 2004; Hinckley et al. 2005; Kullander, 2005). Briefly, networks organizing locomotor activity are distributed throughout the lower thoracic and lumbar regions of the spinal cord. On each spinal side, the ventro-medial part of laminae VII, VIII and X contains the elements responsible for generation of rhythmicity.
Swallowing, sucking and respiration involve rhythmic movements of the tongue driven by synchronously active hypoglossal nuclei. Cunningham & Sawchenko (2000) showed that the main excitatory inputs to the hypoglossal nucleus come from the dorsal medullary reticular column (DMRC) and the nucleus of the tractus solitarius (NTS). The CPG for swallowing includes neurons localized in the NTS (responsible for timing, triggering and shaping the rhythm) and neurons localized in the ventrolateral medulla which sends swallowing commands to different orofaringeal motoneurons including hypoglossal ones (Jean, 2001). Lesion studies have shown that the neuronal networks responsible for NMDA-induced fictive sucking are located in the medulla oblongata (Nakamura & Katakura, 1995). For each inspiratory phase, the hypoglossus nucleus receives a rhythmic excitatory input from brainstem respiratory neurons within the pre-Bötzinger complex which appears to play an important role in the origin of respiration rhythmogenesis (Rekling & Feldman, 1998; Feldman et al. 2003).
Different groups of mGluRs
mGluRs are G-protein-coupled receptors that trigger intracellular signalling cascades to modulate neuronal signalling. mGluRs are divided into three groups, depending on sequence homology, transduction mechanisms and pharmacological profiles. The group I comprises mGluR1 and mGluR5, while group II consists of mGluR2 and mGluR3, and group III is made up by mGluR4, mGluR6, mGluR7 and mGluR8.
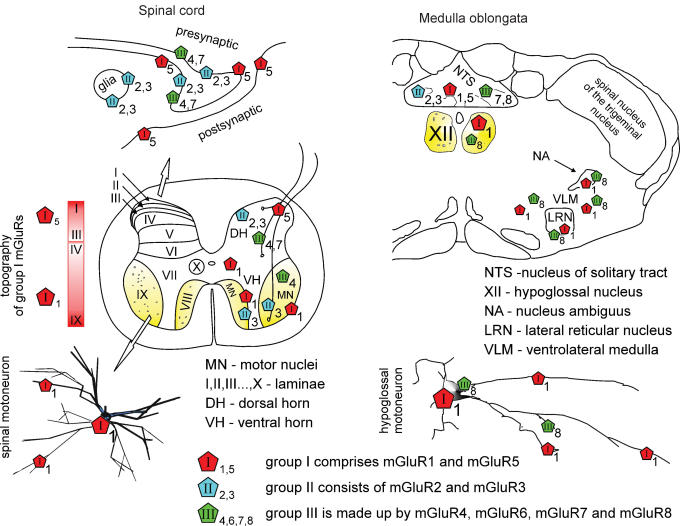
The group I mGluRs increase intracellular Ca2+ levels to activate protein kinase C. Both group II and group III mGluRs are coupled to inhibition of adenylyl cyclase. For reviews of mGluR electrophysiology, pharmacology, structure and second messengers mechanisms see Anwyl (1999), Pin et al. (1999), Schoepp et al. (1999) and Cartmell & Schoepp (2000). Figure 1 shows the distribution of group I mGluRs in the spinal cord and brainstem as these receptors are the largest group in terms of local expression.
Figure 1. Topography of mGluRs in the spinal cord and brainstem.
Left, top scheme shows that the majority of mGluR immunoreactivity is found on the perisynaptic and extrasynaptic plasma membrane of neurons, and partly on glia. Middle, schematic section of the adult rat spinal cord indicates that mGluR5s are densely expressed in laminae I–III of the dorsal horn (DH) with gradual decrease in deeper laminae. About half of vesicle-containing profiles stained for mGluR5 are also positively stained for GABA (Jia et al. 1999). mGluR1s are mostly distributed throughout laminae III–X (Berthele et al. 1999; Alvarez et al. 2000) with patchy immunoreactivity of varying intensity in somata and dendrites of spinal motor nuclei (MN) including motoneurons and interneurons (see inset marked by open arrow) of the ventral horn (VH), and in presynaptic axon terminals (Alvarez et al. 1997, 2000). Group II and III are scattered throughout the spinal cord with predominance in the DH (Berthele et al. 1999) and even some glial labelling (Ohishi et al. 1995; Jia et al. 1999; Azkue et al. 2000, 2001). In addition, group II mGluR3 mRNA is expressed in the small cells surrounding motoneurons, while group III mGluR4 mRNA is present in the spinal motoneurons (Berthele et al. 1999). Right, in the medulla oblongata group I (mGluR1a/5), group II (mGluR2/3) and group III (mGluR7/8) mGluRs (Hay et al. 1999; Pamidimukkala et al. 2002) show different distribution throughout subnuclei of NTS and of the ventrolateral medulla. Only mGluR1 and mGluR8 subtypes are expressed by hypoglossal motoneurons (XII; Hay et al. 1999; Pamidimukkala et al. 2002).
Possible scenarios of mGluR action on synaptic transmission
Approaches to understand the function of mGluRs during motor activity comprise studying either the effects of selective receptor agonists/antagonists on physiological responses or the phenotype of genetic models with receptor deletion (Aiba et al. 1994; Conquet et al. 1994; Li & Nattie, 1995; Fundytus et al. 2001; Mao et al. 2001; Shutoh et al. 2002). Another approach is to investigate in vitro spinal models which preserve CPG networks and generate rhythmic activities (for example El Manira et al. 2002; Whelan, 2003).
One important question is the origin of endogenous glutamate to activate mGluRs. In several brain areas glutamatergic neurons, firing at sufficiently high frequency, release an amount of glutamate that can temporarily overwhelm the normal amino acid uptake systems so that mGluRs are activated by overspill (Min et al. 1998; Gegelashvili et al. 2000; Reichelt & Knopfel, 2002). In general, even in relatively simple networks in vitro, the slow time course of the mGluR-mediated response (∼2 s) may reflect the prolonged rise in the concentration of intracellular signalling molecules, which in turn would promote pooling to activate common ion channel effectors (Mori & Gerber, 2002). It is therefore difficult to suppose that mGluR activity can induce a cycle-by-cycle modulation of relatively faster patterns in motor networks. It seems more likely that such a slow response due to mGluRs modulates neuronal excitability and thus constrains the network ability to generate patterned inputs at a certain frequency. The ability of endogenous glutamate to activate mGluRs may be enhanced by certain experimental in vitro conditions like the use of ambient temperature, which reduces the efficiency of glutamate uptake and slows down its diffusion to facilitate interaction with mGluRs (Asztely et al. 1997; Kullmann & Asztely, 1998). Nevertheless, since mGluR1 antagonists administered in vivo block hyperalgesia (Dolan & Nolan, 2002) or the stimulatory role of glutamate on respiration (Li & Nattie, 1995), it is clear that, even at physiological temperature, endogenous glutamate can activate mGluR1 receptors during intense network activity.
Group I mGluR antagonists do not block glutamatergic transmission evoked by single or low frequency afferent stimulation. Only when repeated network discharges evoke strong release of glutamate, blocking these receptors inhibits electrically induced synaptic transmission (Marchetti et al. 2003; Sharifullina et al. 2004). These data can suggest two possibilities not mutually exclusive: a fraction of the population of mGluRs is synaptically located (Alvarez et al. 2000; Hubert et al. 2001; Kuwajima et al. 2004), yet because of the intrinsically slow activation process, their contribution to synaptic events comes into action only with sustained neurotransmitter delivery occurs. A second possibility is that group I mGluRs are predominantly extrasynaptic and thus activated by glutamate spillover (Batchelor et al. 1994; Alford et al. 1995; Scanziani et al. 1997; Cochilla & Alford, 1998; Min et al. 1998; Huang & Bergles, 2004). While cumulative depolarization of spinal motoneurons evoked by trains of dorsal root stimuli is insensitive to selective blockers of group II or III mGluRs (Taccola et al. 2004a), the presynaptic location of group III receptors would make them suitable to control nociceptive inputs to the spinal cord dorsal horn (Azkue et al. 2001), in analogy with their role in glutamatergic transmission on brainstem auditory neurons (Billups et al. 2005). In the case of group II receptors (Azkue et al. 2000), their location appears to be mainly extrasynaptic.
Decreased spontaneous locomotor activity occurs in mGluR1−/− mice (Conquet et al. 1994), indicating an important role of such receptors in the control of motor patterns. Conversely, genetic models of mGluR2−/− (Yokoi et al. 1996), mGluR4−/− (Pekhletski et al. 1996), or mGluR6−/− (Takao et al. 2000) do not show changes in normal motor activity, indicating that these receptors are not activated by glutamate released during physiological activity in motor circuits. Nevertheless, such receptors may be a target for pharmacological modulation of network activity during pathological conditions (e.g. chronic inflammation and pain; Fisher et al. 2002) or after spinal lesion (Mills et al. 2002). On the basis of these considerations, the present review is focused on the role of mGluRs in motor networks.
Modulation of the lamprey respiratory and spinal locomotor networks by mGluRs
The lamprey spinal cord preparation is a useful model to study locomotor systems which, like in mammals, are activated by ionotropic glutamate receptors and modulated by mGluRs. Fictive swimming is typically induced by bath-applied NMDA because activation of NMDA receptors gives rise to plateau-like depolarizations suitable for CPG operation (see review by Grillner et al. 2000).
It was suggested by Cochilla & Alford (1998) that, in the larval lamprey, presynaptically localized group I mGluRs (activated by sustained release of glutamate) stimulate liberation of Ca2+ from ryanodine-sensitive intracellular stores. The enhanced presynaptic intracellular Ca2+ plus Ca2+ accumulation during repeated neuronal activity might facilitate further glutamate release essential for continuous fictive locomotion (Takahashi & Alford, 2002). In the adult lamprey, activation of group I mGluR1s speeds up locomotor frequency and increases NMDA-induced depolarization (Krieger et al. 1998, 2000) by increasing NMDA-induced influx of Ca2+ (Krieger et al. 2000). Since block of this subtype of mGluRs reduces locomotor frequency (Krieger et al. 1998; Kettunen et al. 2002), it seems that there is on-going activation of group I mGluR1 during fictive locomotion.
Unlike group I mGluR1s, mGluR5 activity decreases the frequency of NMDA-induced locomotor rhythm, suggesting mGluR5s as contributors to slowing down locomotor activity (Kettunen et al. 2002). The precise reason for the discrepancy between the effects of group I mGluR subtype activity remains unclear (El Manira et al. 2002). One possibility may reside in the fact that mGluR1 receptors depolarize spinal neurons by inhibiting a leak current to boost membrane depolarization and increase excitability of locomotor networks (Kettunen et al. 2003) including facilitation of NMDA receptors. mGluR5 receptors do not alter neuronal membrane potential or resistance, as their action is mainly linked to increasing intracellular Ca2+ and generating Ca2+ waves (Kettunen et al. 2002) which could reduce neuronal excitability via activation of Ca2+-dependent K+ conductances.
A physiological role for group II and III mGluRs in adult lamprey remains uncertain because such a receptor activity is limited to slight retardation of locomotor frequency and amplitude, possibly by direct depression of the release machinery for glutamate (Krieger et al. 1998).
On respiratory networks, block of group I mGluRs reversibly decreases respiratory frequency, while antagonism of group II mGluRs leads to frequency increase; both findings reveal endogenous activation of group I and II during respiration in the adult lamprey (Bongianni et al. 2002).
Role of mGluRs in rhythmic activity of the turtle
In vitro brainstem or spinal cord preparations of the turtle can generate rhythmic motor oscillations (Douse & Mitchell, 1990; Guertin & Hounsgaard, 1998a; Delgado-Lezama et al. 1999). To produce this pattern, it is possible to use either NMDA or cholinergic muscarinic agonists that induce intrinsic oscillations by independent cellular mechanisms (Guertin & Hounsgaard, 1998b, 1999). While oscillations ultimately need L-type calcium channel activation, only the oscillations evoked by NMDA/5-HT depend on voltage-sensitive NMDA-activated channels.
Since activation of mGluRs evokes plateau potentials of motoneurons and interneurons by facilitating voltage-activated Ca2+ channels (Delgado-Lezama et al. 1997; Svirskis & Hounsgaard, 1998; Perrier et al. 2002), it seems likely that mGluRs contribute to rhythm generation, though the precise identification of their subtypes remains a matter for future studies. Moreover, group I mGluR1 block suppresses the characteristic hyper-excitability induced by motor network activity (Alaburda & Hounsgaard, 2003).
Cellular mechanisms responsible for group I mGluR effects on excitability of rat spinal and brainstem motor networks
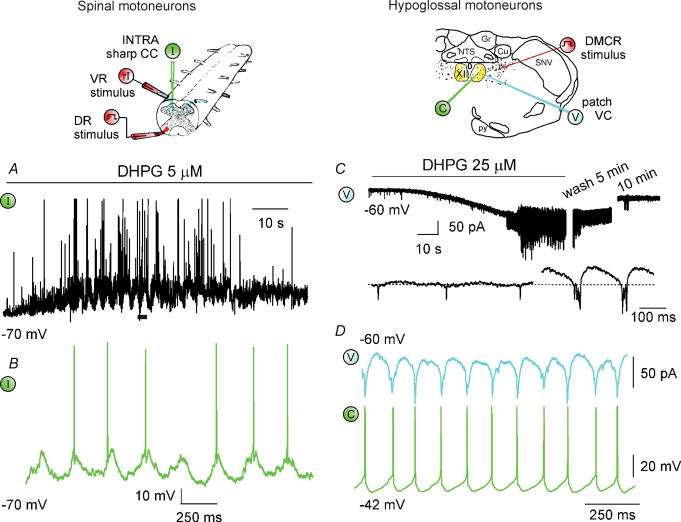
Figure 2 compares and contrasts the effects of group I mGluR activation by the selective agonist 3,5-dihydroxyphenyl-glycine (DHPG) on spinal (top left) or hypoglossal (top right) motoneurons and networks of in vitro rat preparations. Figure 2A and B shows intracellular (I, green) records from spinal motoneurons demonstrating onset of slow rhythmic oscillations (0.2–0.3 Hz) over a background of membrane depolarization, raised input resistance and increased synaptic activity (Marchetti et al. 2003). On top of slow oscillations, faster (4–11 Hz) oscillations with frequent spike firing are also apparent (Fig. 2B is a faster record of the trace indicated with a horizontal bar in A). This phenomenon is accompanied by a larger monosynaptic component of the DR-evoked response and by depression of the slower, polysynaptic phase probably due to facilitation of glycinergic inhibition (Marchetti et al. 2003). Recurrent inhibitory postsynaptic potentials (IPSP) due to Renshaw cell activity are, however, depressed (Marchetti et al. 2005). Oscillations have a network origin, are not intrinsic to motoneurons, and, because of their synchronicity, cannot evoke fictive standard locomotor patterns. Note that oscillations require mGluR5 activity, while membrane depolarization and resistance increase depend on mGluR1 activation (Marchetti et al. 2003).
Figure 2. Cellular actions of group I mGluR activation.
Left (top), schematic diagram showing the electrophysiological arrangements used for the rat isolated spinal cord preparation with sharp electrode intracellular recording (I; green) from lumbar motoneurons, DR electrical stimulation (step symbol; red) and ventral root (VR) antidromic stimuli to elicit recurrent IPSP (rI; red). A, application of DHPG induces membrane depolarization (with associated input resistance rise), membrane oscillations and enhancement in synaptic activity (large deflections are truncated spikes). B shows faster time base record of motoneuron oscillations with spikes taken from the trace marked by filled bar (voltage calibration in B applies also to A). Data are reproduced with permission from Marchetti et al. (2003, 2005). Right (top), schematic coronal section of the brainstem to show location of patch clamp electrode (under voltage clamp, VC, blue; current clamp, CC, green) for recording from HMs. The stimulating electrode is placed in the DMRC as shown (step symbol, red). C, under VC conditions application of DHPG induces inward current with emergence of fast oscillations and subsequent bursts; 10 min washout restores control conditions. Faster time base record taken from the trace depicts fast oscillations subsequently followed by bursts. D, paired recording from adjacent HMs shows strong phase coincidence of DHPG-evoked oscillatory patterns under voltage (top) and current (bottom) clamp. Data are reproduced, with permission, from Sharifullina et al. (2004, 2005).
Also on HMs (under voltage clamp; V, blue) DHPG induces rhythmic oscillations which initially comprise fast discharges (3–5 Hz) evolving into bursts (4–8 Hz) with superimposed fast oscillations (Fig. 2C, top trace; Sharifullina et al. 2005). The bottom record of Fig. 2C (faster timebase) shows the distinct features of fast oscillations and bursts. Bursts induce regular spontaneous firing with no change in input resistance. Approximately 50% of the recorded cells show rhythmic activity after DHPG application. Fast oscillations and bursts are mediated by gap junctions that functionally couple HMs (Sharifullina et al. 2005). Likewise, in the rat spinal cord, synchronous oscillations induced by DHPG are also inhibited by the gap junction blocker carbenoxolone, which does not affect fictive locomotor patterns (G. Taccola, unpublished observation).
In the nucleus hypoglossus, bursting depends on mGluR1 rather than mGluR5 receptors and it is paced by cyclic activation of ATP-sensitive K+ channels (KATP) (Sharifullina et al. 2005). Figure 2D shows tight burst coupling between two electrically connected motoneurons under voltage (V, blue, top) or current (C, green, bottom) clamp. Evoked excitatory responses are also enhanced by DHPG. Since on HMs bursting can take place with or without intact synaptic inhibition, it is clear that, in the brainstem, the rhythmogenic network does not involve changes in synaptic inhibition as observed in the spinal cord.
mGluRs tune motor networks in the brainstem and spinal cord
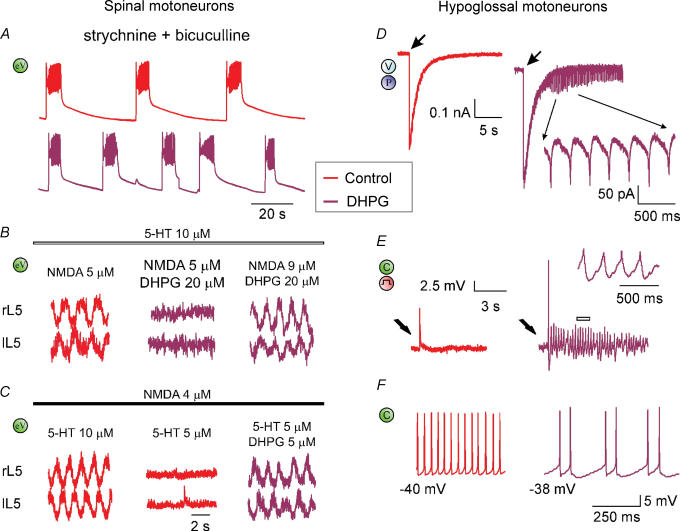
The functional consequences of mGluR1 activation on motor output are exemplified in Fig. 3.
Figure 3. Network actions of group I mGluR activation.
A, disinhibited bursting evoked by block of synaptic inhibition (red trace; 1 μ m strychnine plus 20 μ m bicuculline application) is accelerated by DHPG (5 μ m; crimson trace) which significantly reduces burst duration and periodicity. Because disinhibited bursting is believed to originate from the rhythmic discharges by CPG, this effect suggests activation of CPG interneurons by DHPG. eV (green), extracellular DC recording from VR. B, fictive locomotor patterns induced by co-application of NMDA and 5-HT is depressed by 20 μ m DHPG and restored by increasing the NMDA concentration to 9 μ m. C, conversely, fictive locomotor patterns brought below threshold by decreasing the 5-HT concentration are restored by adding a small (5 μ m) dose of DHPG. Data are reproduced, with permission, from Taccola et al. (2004b). D, fast inward current recorded (under voltage clamp, V) from single HM induced by puffer application (P) of AMPA is followed by rhythmic oscillations when occurring in coincidence with a concentration of DHPG (5 μ m) subthreshold for oscillations. E, electrically evoked EPSP (by stimulating the DMRC, see step symbol in red) recorded under current clamp (C, green) in control solution (red trace) generates spike and oscillations (crimson trace; see also inset at faster time scale for record corresponding to the open bar) when repeated in the presence of DHPG (5 μ m). F, comparison of two HMs (left and right) at similar membrane potential in the presence of DHPG (20 μ m). The one on the left (red) produces high-frequency firing without oscillations, while the one on the right (crimson) generates oscillations with lower firing rate. Data reproduced with permission from Sharifullina et al. (2005).
On spinal networks, the spontaneous rhythmic bursts which arise after block of synaptic inhibition (‘disinhibited bursting’) are shortened and accelerated by 5 μ m DHPG to demonstrate that CPG elements express group I mGluR receptors with excitatory function (Fig. 3A; Taccola et al. 2004b). Disinhibited bursting occurs spontaneously via activation of network glutamatergic ionotropic receptors predominantly of the AMPA type, while NMDA receptors simply modulate this pattern (Bracci et al. 1996). While disinhibited bursting cannot support locomotion in view of its slow frequency and lack of alternation, the current view is that it originates from the same CPG responsible for locomotion whenever the network brake on excitability is removed by application of glycine and GABA antagonists (Beato & Nistri, 1999).
Fictive locomotion can be induced by applying excitatory substances like NMDA and serotonin (5-HT) which generate sustained, rhythmic discharges alternating at various segmental levels, with locomotor-like frequency, to activate hindlimb flexors and extensor muscles (Kiehn et al. 2000; Kiehn & Butt, 2003; Kiehn & Kullander, 2004). As 5-HT operates via activation of multiple receptor classes with inhibitory and excitatory function on spinal networks (Bracci et al. 1998), it can per se trigger fictive locomotion in vivo (Kiehn & Butt, 2003) or in vitro (Beato et al. 1997) probably via coordinating left–right movements during this pattern (Zhong et al. 2006).
The complexity of simultaneous activation of distinct receptor subgroups and/or uneven distribution of analogous receptors within non-homogeneous populations of neurons, is demonstrated by the use of the group I mGluR agonist DHPG. In fact, high concentrations (20 μ m) of DHPG arrest the fictive locomotor pattern (Fig. 3B, middle) probably via over-activity of local inhibitory glycinergic pathways, an effect reversed by further network stimulation with a larger dose (9 μ m) of NMDA (Fig. 3B, right). Conversely, low concentrations (5 μ m) of DHPG actually trigger fictive locomotion when the concentration of excitatory agents is just below threshold (Fig. 3C) because CPG neurons contain DHPG-sensitive receptors as indicated by the action of this substance on disinhibited rhythmicity (see Fig. 3A). Furthermore, DHPG facilitates the alternating locomotor patterns associated with the network depolarization evoked by repeated DR stimuli (Taccola et al. 2004b). Activation of group I mGluRs by endogenous glutamate during fictive locomotion is shown by lengthening of the locomotor cycle period by group I antagonists (Taccola et al. 2004b).
On HMs (Fig. 3, right; Sharifullina et al. 2005), a low concentration of DHPG, subthreshold for oscillations (5 μ m), triggers them when applied in coincidence with a brief pulse application of AMPA (Fig. 3D). Likewise, the same concentration of DHPG unmasks rhythmic oscillations (Fig. 3E) during electrical stimulation of the DMRC input to motoneurons (see scheme at the top right of Fig. 2). The standard non-linear increment in the spike frequency with membrane depolarization is converted, in the presence of DHPG, into low frequency, regular firing (Fig. 3F) in which an oscillatory motoneuron (right) is compared with a non-oscillatory one (left) at similar membrane potential.
The similar oscillatory patterns produced by group I mGluR activity in rat spinal and brainstem circuits indicate that these mGluRs produce analogous electrical responses, though mediated by distinct receptor subtypes and with dissimilar functional consequence. In the rat brainstem, mGluR1-dependent oscillations stabilize HM firing at a steady level so as they might coordinate bilateral motor output for optimal recruitment of tongue muscles (Fig. 3F). Scant expression of mGluR5 receptors within this area (Hay et al. 1999) means that oscillatory activity is supported by mGluR1 receptors. The latter evoke membrane depolarization plus raised input resistance which are the two key factors to trigger motoneuron oscillations largely dependent on their gap junction connections and intrinsic conductances.
Conversely, while in the spinal cord, group I mGluRs are present within the locomotor CPG as shown by their acceleration of the disinhibited rhythm (Fig. 3A), they appear to be insufficiently expressed in this area to trigger locomotor activity. Furthermore, spinal motoneurons probably do not express electrical coupling as strong as the one found in the nucleus hypoglossus nor identical conductances. In view of this condition, the oscillatory activity of spinal motoneurons arises from distant sites within the dorsal horns where mGluR5s are strongly expressed. Such regions can therefore be seen as accessory spinal networks, the operation of which facilitates the onset of CPG activity and is then inhibited when alternating locomotor patterns emerge. Spinal topographic segregation of mGluR1 and mGluR5 receptors therefore ensures separation between neurons (including motoneurons) generating depolarization, and neurons inducing oscillations.
It is interesting that the role of mGluRs in the control of locomotor circuits appears to be evolution dependent. In fact, although mGluR1 receptors consistently accelerate the rhythmic oscillatory output of motor networks in the lamprey and rat spinal cord, mGluR5 receptors possess an inhibitory influence on fictive swimming as discussed earlier, while in the rat spinal cord they might contribute (via accessory motor circuits) to rhythmicity.
A scheme describing mGluR-dependent rhythmic operation of mammalian motor networks
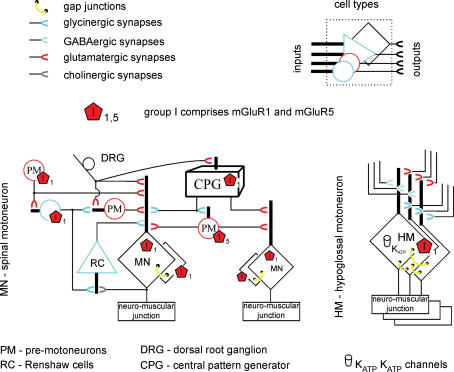
Figure 4 shows an idealized diagram to account for the action of mGluRs on synaptic pathways and oscillatory activity of spinal (left) and hypoglossal (right) motoneurons.
Figure 4. Idealized diagram of motor networks activated by group I mGluRs.
Different types of neuron are indicated by dissimilar cell body shapes: their inputs are compacted into a single dendritic shaft, while their output is indicated by a horseshoe symbol colour-coded according to the released transmitter. Group I mGluRs (mainly found at extrasynaptic sites) are assigned on the basis of cell type rather than specific cell compartments. Left, schematic representation of spinal networks (one side only of the segmental circuit is shown) with excitatory (glutamatergic, red; cholinergic, grey), inhibitory (GABAergic, light blue; glycinergic, dark blue) and electrical (yellow) synapses. CPG interneurons are collectively lumped into a black box with inputs from DRs, and output to premotoneurons and motoneurons. mGluR1 and mGluR5 receptors (red pentagons) are expressed by various cell types. While mGluR5 activity is believed to generate motoneuron oscillations, mGluR1 activity is thought responsible for network depolarization (including CPG elements and motoneurons). Gap junctions and depression of Renshaw cell activity probably concur to produce oscillation synchronicity. Note that facilitation of glycinergic interneuron (blue circle)-bearing mGluR1 receptors contributes to restrain network excitation. Right, network of HMs bearing mGluR1 receptors and coupled via gap junctions. HM expression of KATP channels enables pacing of bursting at low frequency. Network synaptic inputs important for oscillatory activity are also shown. Note apparent lack of mGluR5 receptors.
In the spinal cord (for the sake of simplicity one side only of the segmental network is shown here) the mGluR5 oscillators are suggested to be premotoneurons driving synchronous discharges to motoneurons. Rhythm synchronicity may arise because the activity of Renshaw cell interneurons is concomitantly depressed (Marchetti et al. 2005), and may be aided by a degree of motoneuron electrical coupling (Tresch & Kiehn, 2000). Such oscillations do not evolve into runaway excitation as there is concomitant facilitation (via mGluR1 receptor activation) of glycinergic transmission from premotoneurons to motoneurons. The duration of the oscillatory cycle might be controlled by periodic fluctuations in input resistance of premotoneurons and motoneurons due the strong increment of synaptic inputs and subsequent membrane shunting in analogy with the phenomenon recently reported for turtle motor networks (Alaburda et al. 2005).
In the brainstem, oscillations crucially depend on electrical coupling between motoneurons (Fig. 4, right) as mGluR1 activity functionally blends together clusters of motoneurons. Indeed, synchronous oscillations can be generated when synaptic inhibition is blocked (even though glycinergic transmission is facilitated by mGluRs; Donato & Nistri, 2000). However, for large neurons like HMs intense, rhythmic oscillations must be metabolically demanding because of the need to preserve the correct ionic homeostasis via the Na+–K+ and Ca2+-ATPase pumps. Since the latter are the main consumers of ATP (Ainscow et al. 2002; Watson et al. 2003), this phenomenon may cyclically deplete intracellular ATP to ensure activation of KATP channels that set burst frequency and duration. Normally KATP channels play a role in the rhythmic electrical discharges of brainstem respiratory neurons (Haller et al. 2001; Mironov & Richter, 2001) including HMs which fire in synchrony with inspiratory commands. Thus, activation of mGluR1 receptors amplifies a self-regulatory mechanism already operational under physiological conditions. Nevertheless, excessive concentrations of extracellular glutamate achieved, for example, via block of glutamate transporters can trigger rhythmic slow bursting which leads to motoneuron death due to excitotoxicity in which mGluR1 receptors appear to play an important role (Sharifullina & Nistri, 2006).
Conclusions
The hallmark of group I mGluR activation in spinal and brainstem networks is the onset of rhythmic oscillations. In vitro experiments cannot disclose the precise contribution of such oscillations to motor behaviour, but they do reveal a common strategy to synchronize and recruit motor circuits. The hypoglossal motor output triggered by mGluRs appears to be tightly controlled by the special properties (gap junctions and KATP channels) of its motoneurons as phase-locked motor commands are necessary for synchronous contraction of tongue bilateral muscles. The spinal locomotor output is a complex process of functional interaction amongst discrete motor modules, in which activation of widely distributed mGluRs can provide the synergy for generation of full motor patterns. Future studies with novel agents targeted at certain mGluR subtypes or isoforms specifically expressed by motor systems should further refine our understanding of the role of group I mGluRs on motor output and their interaction with group II and III mGluRs.
Acknowledgments
This work was supported by grants from MIUR (FIRB; PRIN), Fondazione Casali (Trieste), and FVG Region.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K+ channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci. 2003;23:8625–8629. doi: 10.1523/JNEUROSCI.23-25-08625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Russo R, MacAulay N, Hounsgaard J. Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci. 2005;25:6316–6321. doi: 10.1523/JNEUROSCI.0843-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford S, Schwartz E, Di Prisco GV. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. doi: 10.1177/1073858403009003014. [DOI] [PubMed] [Google Scholar]
- Alford S, Zompa I, Dubuc R. Long-term potentiation of glutamatergic pathways in the lamprey brainstem. J Neurosci. 1995;15:7528–7538. doi: 10.1523/JNEUROSCI.15-11-07528.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Dewey DE, Carr PA, Cope TC, Fyffe RE. Downregulation of metabotropic glutamate receptor 1a in motoneurons after axotomy. Neuroreport. 1997;8:1711–1716. doi: 10.1097/00001756-199705060-00029. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- Azkue JJ, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Bilbao A, Bidaurrazaga A, Grandes P. The metabotropic glutamate receptor subtype mGluR 2/3 is located at extrasynaptic loci in rat spinal dorsal horn synapses. Neurosci Lett. 2000;287:236–238. doi: 10.1016/s0304-3940(00)01189-7. [DOI] [PubMed] [Google Scholar]
- Azkue JJ, Murga M, Fernandez-Capetillo O, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Puente N, Bilbao A, Bidaurrazaga A, Kuhn R, Grandes P. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J Comp Neurol. 2001;430:448–457. doi: 10.1002/1096-9861(20010219)430:4<448::aid-cne1042>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Beato M, Bracci E, Nistri A. Contribution of NMDA and non-NMDA glutamate receptors to locomotor pattern generation in the neonatal rat spinal cord. Proc Roy Soc London B. 1997;264:877–884. doi: 10.1098/rspb.1997.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, Nistri A. Interaction between disinhibited bursting and fictive locomotor patterns in the rat isolated spinal cord. J Neurophysiol. 1999;82:2029–2038. doi: 10.1152/jn.1999.82.5.2029. [DOI] [PubMed] [Google Scholar]
- Berthele A, Boxall SJ, Urban A, Anneser JM, Zieglgansberger W, Urban L, Tolle TR. Distribution and developmental changes in metabotropic glutamate receptor messenger RNA expression in the rat lumbar spinal cord. Brain Res Dev Brain Res. 1999;112:39–53. doi: 10.1016/s0165-3806(98)00156-4. [DOI] [PubMed] [Google Scholar]
- Billups B, Graham BP, Wong AY, Forsythe ID. Unmasking group III metabotropic glutamate autoreceptor function at excitatory synapses in the rat CNS. J Physiol. 2005;565:885–896. doi: 10.1113/jphysiol.2005.086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Carfi M, Pantaleo T. Group I and II metabotropic glutamate receptors modulate respiratory activity in the lamprey. Eur J Neurosci. 2002;16:454–460. doi: 10.1046/j.1460-9568.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- Bracci E, Ballerini L, Nistri A. Spontaneous rhythmic bursts induced by pharmacological block of inhibition in lumbar motoneurons of the neonatal rat spinal cord. J Neurophysiol. 1996;75:640–647. doi: 10.1152/jn.1996.75.2.640. [DOI] [PubMed] [Google Scholar]
- Bracci E, Beato M, Nistri A. Extracellular K+ induces locomotor-like patterns in the rat spinal cord in vitro: comparison with NMDA or 5-HT induced activity. J Neurophysiol. 1998;79:2643–2652. doi: 10.1152/jn.1998.79.5.2643. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Alford S. Metabotropic glutamate receptor-mediated control of neurotransmitter release. Neuron. 1998;20:1007–1016. doi: 10.1016/s0896-6273(00)80481-x. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Matarese V, Conde F, Collingridge GL, Crépel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Hounsgaard J. Oscillatory interaction between dorsal root excitability and dorsal root potentials in the spinal cord of the turtle. Neuroscience. 1999;93:731–739. doi: 10.1016/s0306-4522(99)00187-6. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir R, Gao BX, Jackson MB, Ziskind-Conhaim L. Interactions between multiple rhythm generators produce complex patterns of oscillation in the developing rat spinal cord. J Neurophysiol. 2002;87:1094–1105. doi: 10.1152/jn.00276.2001. [DOI] [PubMed] [Google Scholar]
- Dolan S, Nolan AM. Behavioral evidence supporting a differential role for spinal group I and II metabotropic glutamate receptors in inflammatory hyperalgesia in sheep. Neuropharmacology. 2002;43:319–326. doi: 10.1016/s0028-3908(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Douse MA, Mitchell GS. Episodic respiratory related discharge in turtle cranial motoneurons: in vivo and in vitro studies. Brain Res. 1990;536:297–300. doi: 10.1016/0006-8993(90)90037-c. [DOI] [PubMed] [Google Scholar]
- El Manira A, Kettunen P, Hess D, Krieger P. Metabotropic glutamate receptors provide intrinsic modulation of the lamprey locomotor network. Brain Res Brain Res Rev. 2002;40:9–18. doi: 10.1016/s0165-0173(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Eugenin J, Nicholls JG, Cohen LB, Muller KJ. Optical recording from respiratory pattern generator of fetal mouse brainstem reveals a distributed network. Neuroscience. 2006;137:1221–1227. doi: 10.1016/j.neuroscience.2005.10.053. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y. Functions of mammalian spinal interneurons during movement. Curr Opin Neurobiol. 2000;10:699–707. doi: 10.1016/s0959-4388(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR1) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Dehnes Y, Danbolt NC, Schousboe A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem Int. 2000;37:163–170. doi: 10.1016/s0197-0186(00)00019-x. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Funk GD, Ballanyi K. Preparing for the first breath: prenatal maturation of respiratory neural control. J Physiol. 2006;570:437–444. doi: 10.1113/jphysiol.2005.097238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Cangiano L, Hu G, Thompson R, Hill R, Wallen P. The intrinsic function of a motor system – from ion channels to networks and behavior. Brain Res. 2000;886:224–236. doi: 10.1016/s0006-8993(00)03088-2. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Cellular bases of a vertebrate locomotor system-steering, intersegmental and segmental co-ordination and sensory control. Brain Res Rev. 2002;40:92–106. doi: 10.1016/s0165-0173(02)00193-5. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Chemical and electrical stimulation induce rhythmic motor activity in an in vitro preparation of the spinal cord from adult turtles. Neurosci Lett. 1998a;245:5–8. doi: 10.1016/s0304-3940(98)00164-5. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. NMDA-induced intrinsic voltage oscillations depend on L-type calcium channels in spinal motoneurons of adult turtles. J Neurophysiol. 1998b;80:3380–3382. doi: 10.1152/jn.1998.80.6.3380. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. L-type calcium channels but not N-methyl-D-aspartate receptor channels mediate rhythmic activity induced by cholinergic agonist in motoneurons from turtle spinal cord slices. Neurosci Lett. 1999;261:81–84. doi: 10.1016/s0304-3940(99)00013-0. [DOI] [PubMed] [Google Scholar]
- Haller M, Mironov SL, Karschin A, Richter DW. Dynamic activation of KATP channels in rhythmically active neurons. J Physiol. 2001;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay M, McKenzie H, Lindsley K, Dietz N, Bradley SR, Conn PJ, Hasser EM. Heterogeneity of metabotropic glutamate receptors in autonomic cell groups of the medulla oblongata of the rat. J Comp Neurol. 1999;403:486–501. [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jia H, Rustioni A, Valtschanoff JG. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J Comp Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- Kettunen P, Hess D, El Manira A. mGluR1, but not mGluR5, mediates depolarization of spinal cord neurons by blocking a leak current. J Neurophysiol. 2003;90:2341–2348. doi: 10.1152/jn.01132.2002. [DOI] [PubMed] [Google Scholar]
- Kettunen P, Krieger P, Hess D, El Manira A. Signaling mechanisms of metabotropic glutamate receptor 5 subtype and its endogenous role in a locomotor network. J Neurosci. 2002;22:1868–1873. doi: 10.1523/JNEUROSCI.22-05-01868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Butt SJ. Physiological, anatomical and genetic identification of CPG neurons in the developing mammalian spinal cord. Prog Neurobiol. 2003;70:347–361. doi: 10.1016/s0301-0082(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kullander K. Central pattern generators deciphered by molecular genetics. Neuron. 2004;41:317–321. doi: 10.1016/s0896-6273(04)00042-x. [DOI] [PubMed] [Google Scholar]
- Krieger P, Grillner S, El Manira A. Endogenous activation of metabotropic glutamate receptors contributes to burst frequency regulation in the lamprey locomotor network. Eur J Neurosci. 1998;10:3333–3342. doi: 10.1046/j.1460-9568.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- Krieger P, Hellgren-Kotaleski J, Kettunen P, El Manira AJ. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. J Neurosci. 2000;20:5382–5391. doi: 10.1523/JNEUROSCI.20-14-05382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Nishimaru H, Nakayama K. Developmental changes in rhythmic spinal neuronal activity in the rat fetus. Prog Brain Res. 2004;143:49–55. doi: 10.1016/s0079-6123(03)43005-7. [DOI] [PubMed] [Google Scholar]
- Kullander K. Genetics moving to neuronal networks. Trends Neurosci. 2005;28:239–247. doi: 10.1016/j.tins.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004;474:589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94:1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie EE. Prolonged stimulation of respiration by brain stem metabotropic glutamate receptors. J Appl Physiol. 1995;79:1650–1656. doi: 10.1152/jappl.1995.79.5.1650. [DOI] [PubMed] [Google Scholar]
- Mao L, Conquet F, Wang JQ. Augmented motor activity and reduced striatal preprodynorphin mRNA induction in response to acute amphetamine administration in metabotropic glutamate receptor 1 knockout mice. Neuroscience. 2001;106:303–312. doi: 10.1016/s0306-4522(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Taccola G, Nistri A. Distinct subtypes of group I metabotropic glutamate receptors on rat spinal neurons mediate complex facilitatory and inhibitory effects. Eur J Neurosci. 2003;18:1873–1883. doi: 10.1046/j.1460-9568.2003.02924.x. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Taccola G, Nistri A. Activation of group I metabotropic glutamate receptors depresses recurrent inhibition of motoneurons in the neonatal rat spinal cord in vitro. Exp Brain Res. 2005;164:406–410. doi: 10.1007/s00221-005-2368-9. [DOI] [PubMed] [Google Scholar]
- Mills CD, Johnson KM, Hulsebosch CE. Role of group II and group III metabotropic glutamate receptors in spinal cord injury. Exp Neurol. 2002;173:153–167. doi: 10.1006/exnr.2001.7828. [DOI] [PubMed] [Google Scholar]
- Min MY, Rusakov DA, Kullmann DM. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: role of glutamate diffusion. Neuron. 1998;21:561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol. 2001;533:227–236. doi: 10.1111/j.1469-7793.2001.0227b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Gerber U. Slow feedback inhibition in the CA3 area of the rat hippocampus by synergistic synaptic activation of mGluR1 and mGluR5. J Physiol. 2002;544:793–799. doi: 10.1113/jphysiol.2002.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res. 1995;23:1–19. [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, Li JL, Neki A, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neurosci Lett. 1995;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Hoang CJ, Hay M. Expression of metabotropic glutamate receptor 8 in autonomic cell groups of the medulla oblongata of the rat. Brain Res. 2002;957:162–173. doi: 10.1016/s0006-8993(02)03619-3. [DOI] [PubMed] [Google Scholar]
- Pekhletski R, Gerlai R, Overstreet LS, Huang XP, Agopyan N, Slater NT, Abramow-Newerly W, Roder JC, Hampson DR. Impaired cerebellar synaptic plasticity and motor performance in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. J Neurosci. 1996;16:6364–6373. doi: 10.1523/JNEUROSCI.16-20-06364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Pin JP, De Colle C, Bessis AS, Acher F. New perspectives for the development of selective metabotropic glutamate receptor ligands. Eur J Pharmacol. 1999;375:277–294. doi: 10.1016/s0014-2999(99)00258-7. [DOI] [PubMed] [Google Scholar]
- Reichelt W, Knopfel T. Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophysiol. 2002;87:1974–1980. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBotzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Sharifullina E, Nistri A. Glutamate uptake block triggers deadly rhythmic bursting of neonatal rat hypoglossal motoneurons. J Physiol. 2006 doi: 10.1113/jphysiol.2005.100412. DOI: 10.1113/jphysiol.2005.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifullina E, Ostroumov K, Nistri A. Activation of group I metabotropic glutamate receptors enhances efficacy of glutamatergic inputs to neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2004;20:1245–1254. doi: 10.1111/j.1460-9568.2004.03590.x. [DOI] [PubMed] [Google Scholar]
- Sharifullina E, Ostroumov K, Nistri A. Metabotropic glutamate receptor activity induces a novel oscillatory pattern in neonatal rat hypoglossal motoneurones. J Physiol. 2005;563:139–159. doi: 10.1113/jphysiol.2004.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh F, Katoh A, Kitazawa H, Aiba A, Itohara S, Nagao S. Loss of adaptability of horizontal optokinetic response eye movements in mGluR1 knockout mice. Neurosci Res. 2002;42:141–145. doi: 10.1016/s0168-0102(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Role of group II and III metabotropic glutamate receptors in rhythmic patterns of the neonatal rat spinal cord in vitro. Exp Brain Res. 2004a;156:495–504. doi: 10.1007/s00221-003-1798-5. [DOI] [PubMed] [Google Scholar]
- Taccola G, Marchetti C, Nistri A. Modulation of rhythmic patterns and cumulative depolarization by group I metabotropic glutamate receptors in the neonatal rat spinal cord in vitro. Eur J Neurosci. 2004b;19:533–541. doi: 10.1111/j.0953-816x.2003.03148.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Alford S. The requirement of presynaptic metabotropic glutamate receptors for the maintenance of locomotion. J Neurosci. 2002;22:3692–3699. doi: 10.1523/JNEUROSCI.22-09-03692.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao M, Morigiwa K, Sasaki H, Miyoshi T, Shima T, Nakanishi S, Nagai K, Fukuda Y. Impaired behavioral suppression by light in metabotropic glutamate receptor subtype 6-deficient mice. Neuroscience. 2000;97:779–787. doi: 10.1016/s0306-4522(00)00053-1. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neurosci. 2000;3:593–599. doi: 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Watson WD, Facchina SL, Grimaldi M, Verma A. Sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitors identify a novel calcium pool in the central nervous system. J Neurochem. 2003;87:30–43. doi: 10.1046/j.1471-4159.2003.01962.x. [DOI] [PubMed] [Google Scholar]
- Whelan PJ. Developmental aspects of spinal locomotor function: insights from using the in vitro mouse spinal cord preparation. J Physiol. 2003;553:695–706. doi: 10.1113/jphysiol.2003.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Zhong G, Diaz-Rios ME, Harris-Warrick RM. Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol. 2006;95:1545–1555. doi: 10.1152/jn.01103.2005. [DOI] [PubMed] [Google Scholar]