Abstract
The nature of muscle efferent fibre activation during whole body cooling was investigated in urethane-anaesthetized rats. Multiunit efferent activity to the gastrocnemius muscle was detected when the trunk skin was cooled by a water-perfused jacket to below 36.0 ± 0.7°C. That efferent activity was not blocked by hexamethonium (50 mg kg−1, i.v.) and was not associated with movement or electromyographic activity. Cold-induced efferent activity enhanced the discharge of afferent filaments from the isotonically stretched gastrocnemius muscle, demonstrating that it was fusimotor. Fusimotor neurons were activated by falls in trunk skin temperature, but that activity ceased when the skin was rewarmed, regardless of how low core temperature had fallen. While low core temperature alone was ineffective, a high core temperature could inhibit the fusimotor response to skin cooling. Fusimotor activation by skin cooling was often accompanied by desynchronization of the frontal electroencephalogram (EEG), but was not a simple consequence of cortical arousal, in that warming the scrotum desynchronized the EEG without activating fusimotor fibres. Inhibition of neurons in the rostral medullary raphé by microinjections of glycine (0.5 m, 120–180 nl) reduced the fusimotor response to skin cooling by 95 ± 3%, but did not prevent the EEG response. These results are interpreted as showing a novel thermoregulatory reflex that is triggered by cold exposure. It may underlie the increased muscle tone that precedes overt shivering, and could also serve to amplify shivering. Like several other cold-defence responses, this reflex depends upon neurons in the rostral medullary raphé.
In response to acute cold stress, a number of thermal defence responses are initiated by the brain. These include cutaneous vasoconstriction, shivering and non-shivering thermogenesis. When humans or animals are exposed to a cold environment, before the onset of overt shivering there is an increase in tonic motoneuron discharge (‘thermal muscle tone’), with a concomitant increase in metabolic heat production (Burton & Bronk, 1937; von Euler, 1961; Meigal et al. 2003). In lightly anaesthetized cats, thermal muscle tone is strongly modulated by stretch and postural reflexes (Burton & Bronk, 1937). It has been suggested that fusimotor neurons, acting via the stretch reflex, play important roles both in the generation of thermal muscle tone and in determining the pattern and amplitude of overt shivering (von Euler, 1961; Meigal et al. 2003).
It is not known whether fusimotor neurons are activated by the same central drive pathways that cause shivering. They may be studied independently of shivering in anaesthetized animals. The present study details the thermoregulatory control of fusimotor fibres in deeply anaesthetized rats. It then tests the hypothesis that, like several other cold-defence responses, thermoregulatory activation of fusimotor fibres depends on a synaptic relay in the medullary raphé.
Methods
Twenty adult male Sprague–Dawley rats (300–530 g) were used in this study. All experiments were carried out in accordance with guidelines of the National Health and Medical Research Council of Australia and were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute.
Animals were anaesthetized initially with pentobarbitone sodium (30 mg kg−1, i.p.), and the hair over the neck and trunk was shaved. The trachea was cannulated, and animals were then artificially ventilated with 2.0% isoflurane (Forthane; Abbott Australia Pty Ltd, Botany, NSW, Australia) in pure oxygen for the duration of the surgical preparation. Respiratory pressure was monitored via a pressure transducer attached to the respiratory line, and expired CO2 concentration was monitored by a CO2 analyser (ADC, Hoddesdon, Herts, UK). Ventilation was adjusted to keep expired CO2 between 3.5 and 4.5%. The right femoral artery and vein were cannulated for monitoring blood pressure and intravenous administration of drugs, respectively. A water-perfused Silastic® jacket (Dow Corning, Midland, MI, USA) was placed around the shaved trunk of the animal, and the temperature of the perfusion water was used to manipulate skin and core temperatures (Owens et al. 2002). Skin temperature was measured as the average from three thermocouples placed at sites across the trunk skin under the water-jacket. Core temperature was measured by a thermocouple inserted 5 cm into the rectum. Baseline core temperature during surgery was maintained around 37–38°C by perfusion of the jacket at 150–180 ml min−1 with water from a reservoir maintained at 43–45°C.
The left popliteal fossa was dissected to expose the nerves to the medial and lateral heads of the left gastrocnemius muscle, as detailed below. When surgery was complete, isofluorane was gradually withdrawn from the oxygen supply to the ventilator, and this anaesthetic agent was replaced by urethane (1.0–1.2 g kg−1, i.v.) (Sigma, St Louis, MO, USA). The depth of anaesthesia was frequently assessed throughout the experiment by testing withdrawal and corneal reflexes, and small additional doses of urethane (25–50 mg, i.v.) were administered if necessary to abolish those reflexes. This anaesthetic regime provides stable baseline conditions without abolishing thermoregulatory reflexes (Owens et al. 2002).
Muscle fibre recording
In 15 rats, efferent nerve activity was recorded from the central end of the cut lateral or medial gastrocnemius nerve in a pool filled with liquid paraffin. The nerve was dissected free, desheathed and placed over two silver-wire hooks. Its activity was recorded differentially, amplified (10 000- to 20 000-fold), filtered (bandpass 400–5000 Hz), and monitored continuously using an oscilloscope and audio amplifier. Spikes above a selected threshold voltage were detected with a time-window discriminator and counted in 5 s bins either on-line or off-line from a computer-based analysis system (CED POWER1401 and Spike 2 software; CED, Cambridge, UK).
In five rats, afferent activity was recorded from thin filaments of the otherwise intact medial or lateral gastrocnemius nerve. The gastrocnemius and soleus muscles, together with the Achilles tendon, were separated and mobilized, such that they could be freely extended with minimal disturbance to the surrounding tissues. The calcaneum was detached from the foot, and a long silk thread (metric 4) tied around the Achilles tendon. The thread was led over a pulley, where weights could be attached for isotonic stretch. Afferent nerve filaments split from the nerve were placed over a single silver-wire hook electrode, and their activity was recorded differentially with respect to a nearby thread of connective tissue placed over a second silver-wire hook. Filaments were selected to include few (2–5) stretch-sensitive afferent units. The activity was amplified (5000- to 10 000-fold), filtered (bandpass 100–3000 Hz), and counted in 5 s bins. In three of those five rats, a simultaneous recording was also made of efferent activity to the other gastrocnemius nerve, from its central cut end (as described above). Overall, muscle efferent activity was recorded from 18 rats and afferent activity from six nerves in five rats.
In six of the 18 rats in which muscle efferent recordings were made, the frontal electroencephalogram (EEG) was recorded from two stainless-steel screws implanted bilaterally through the frontal bone. Wires were attached, covered with dental cement (Reprosil®; Dentsply International Inc., Milford, DE, USA), and the signal between them was amplified (1000-fold) and filtered (1–100 Hz). Digital filters in Spike 2 were then used to reduce the bandpass to 1–4 Hz. In six of the 18 rats, a pair of silver wires was inserted into the gastrocnemius to record the electromyogram (EMG). This was done either into the muscle in the right leg, or into the medial head of the left gastrocnemius muscle, whose innervation was intact. The EMG signal was amplified (5000-fold) and filtered (bandpass 10–1000 Hz).
Experimental procedures
Skin cooling was performed by perfusion of cold water through the water-jacket for 30–165 s. This reversibly lowered skin temperature by 1.4–9.2°C. Repeated episodes of skin cooling could be used to lower core temperature (see Results).
Scrotal warming was used as an independent method to desynchronize the EEG (Kanosue et al. 1985) in four of the six rats in which the EEG was recorded together with muscle efferent activity. The scrotum was warmed by gently putting the finger of a rubber glove filled with hot water against it, and its surface temperature was measured by a thermocouple. In four of the 18 rats in which muscle efferent activity was recorded, hexamethonium chloride (50 mg kg−1 in saline; Sigma) was given intravenously to block postganglionic sympathetic activity.
In experiments where muscle afferent fibres were recorded, a weight was attached to the thread from the Achilles tendon sufficient to produce steady, tonic muscle afferent activity (20–50 g). This activity was allowed to adapt to a steady state before cooling commenced, and the muscle was kept isotonically stretched throughout the skin cooling series (40–60 min). After several cooling episodes, the remaining efferent pathway to the muscle was crushed and/or cut, in order to block fusimotor signals from reaching the muscle. The afferent response to skin cooling was then retested.
Glycine microinjection into the medullary raphé
In six of the 18 rats in which muscle efferent activity was recorded, including two rats in which the frontal EEG was also recorded, neuronal activity in the medullary raphé was inhibited by microinjection of glycine. The animal was mounted prone in a stereotaxic apparatus according to the co-ordinate system of Paxinos & Watson (1998), and a burr hole was made in the skull over the medial medulla. Glycine (Ajax Finechem, Seven Hills, NSW, Australia) was diluted with artificial cerebrospinal fluid and mixed with 1% red fluorescent microspheres (FluoSperes®; Molecular Probes) to identify the injection sites. A glass micropipette (tip o.d. ∼50 μm) filled with 0.5 m glycine was positioned stereotaxically into the rostral ventromedial medulla (3.0 mm posterior to lambda, 0.0–1.5 mm lateral to the mid-line and 9.5 mm deep to the dural surface), aimed at the ventral medullary raphé at the level of the caudal part of the facial nucleus (Morrison et al. 1999; Ootsuka et al. 2004).
Skin and core temperatures were carefully manipulated such that each repeated 40–90 s cooling episode was, as far as possible, of the same magnitude and made against the same background conditions in each animal. Glycine was injected through the micropipette in volumes of 120–180 nl over approximately 60 s. The cooling protocol was continued for several cycles afterwards. In three of the six rats the full protocol was repeated after intravenous hexamethonium (50 mg kg−1) injection. In two rats the same site was re-injected with glycine; in two other rats (including one of the above two rats) a second site at least 1 mm away was injected. Data from two further rats were excluded from this analysis because they received large raphé glycine injections (≥ 300 nl).
At the end of experiment, animals were deeply anaesthetized with pentobarbitone sodium (325 mg, i.v.) and perfused transcardially with saline followed by 4% paraformaldehyde. The brain was removed and placed in the same fixative at least overnight. After cryoprotection with 20% sucrose in phosphate-buffered saline, 40 μm frozen coronal sections were made of the medulla. The locations of microinjection sites were identified by detecting the fluorescent microspheres, using fluorescence microscopy (Leitz DMRBE, Leica, Germany). Relevant sections were photographed under both light and fluorescence optics using a digital camera (SPOT camera Real time; Diagnostic Instruments Inc., Sterling Heights, MI, USA). In each case the centre of the injection was identified and mapped onto standard sections of the atlas of Paxinos & Watson (1998).
Statistical analysis
Thresholds for muscle efferent fibre activation were estimated by plotting 5 s spike counts against either the absolute value of, or the decrease in skin temperature. Thresholds were measured from the intercept of the regression line (Owens et al. 2002).
In both muscle afferent recording experiments and raphé-glycine experiments, baseline muscle afferent or efferent fibre activity (spikes (5 s)−1) was measured as the average of the 5 s spike count over 1 min just before each cooling period. The peak values and peak increases of activity in response to cooling were averaged over 30 s periods. These responses were compared with baseline values using Student's paired t test.
To assess the response of muscle efferent fibres to skin cooling before and after glycine injections, comparisons were made between peak responses to the last two cooling episodes before glycine injection and the first episode after injection. The same procedure was used to compare the falls in skin and core temperature. To assess muscle afferent activity before and after ablating the efferent pathway (where there was no recovery process) comparisons were made between responses to the last two cooling episodes before, and the first two after cutting the nerve. In all these cases, baseline conditions and responses to cooling were compared using one-way ANOVA for repeated measures and Dunnett's test. In the text, changes in response are expressed as a percentage of the mean response to the two cooling episodes immediately preceding the intervention.
To assess the difference in response to glycine injections within versus outside the medullary raphé, changes in response (% of preinjection increase) for the first cooling episode after glycine injection were compared using Student's unpaired t test.
All values are shown as means ± s.e.m. and P < 0.05 was considered significant.
Results
Muscle efferent fibre response to skin cooling
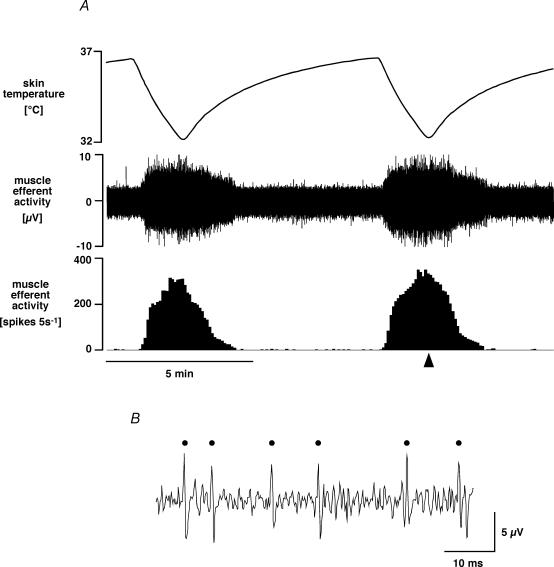
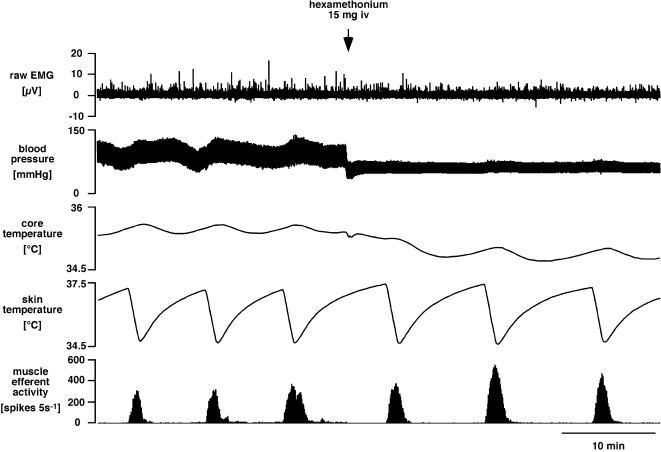
Figure 1 shows a representative example of efferent nerve activity recorded from the left medial gastrocnemius nerve. When cold water was perfused through the water-jacket for 1.5 min, skin temperature dropped by ∼4.5°C and there was a robust, reproducible increase in muscle efferent activity from low or absent levels at baseline. Muscle efferent activity stopped increasing and returned to baseline as the skin began to rewarm. This muscle efferent activity occurred in the absence of overt movement or electromyographic activation in innervated muscle (contralateral or other head of ipsilateral gastrocnemius; n = 6, Fig. 2) and was undiminished after hexamethonium (50 mg kg−1, n = 4). These observations indicate that the efferent activity was not due to α-motoneurons or sympathetic fibres, so by elimination, it was fusimotor.
Figure 1. Muscle efferent fibre response to skin cooling.
A, chart record from a representative experiment showing (from the top) skin temperature, raw efferent activity recorded from the central end of the medial gastrocnemius nerve, and 5 s counts of discriminated medial gastrocnemius efferent activity. B, expanded record of raw muscle efferent activity from the time indicated by the arrowhead. Counted spikes are marked by dots.
Figure 2. Comparison of muscle efferent activity with EMG activity during skin cooling, and the effect of ganglion blockade.
Traces (from the top) show raw EMG recorded from the left medial gastrocnemius muscle (whose innervation was intact), blood pressure, core (rectal) temperature, skin temperature and efferent activity (5 s counts) recorded from the left lateral gastrocnemius nerve. Note that muscle efferent activation by skin cooling occurred without EMG activation and was undiminished after ganglion blockade (hexamethonium, 15 mg i.v. at arrow).
Muscle afferent fibre response to skin cooling
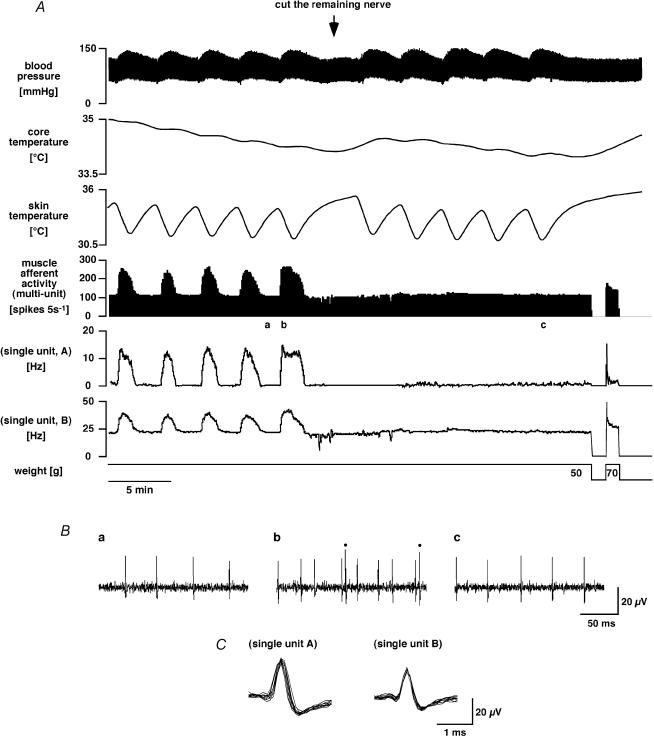
If muscle efferent activation by skin cooling was indeed due to fusimotor fibres, it should be able to potentiate muscle spindle afferent activity. Activity was therefore recorded from six fine afferent filaments peeled from the nerve to one head of the isotonically stretched gastrocnemius muscle in five rats, and responses to skin cooling were measured. A representative example is shown in Fig. 3. Muscle afferent fibres were initially silent, but were activated by isotonic stretch. As the stretch was maintained, some fibres fell silent while others adapted to a steady tonic discharge rate (within 10–390 s). At this point the animal was subjected to a sequence of skin cooling episodes. When skin was cooled for 30–135 s, skin temperature dropped by 3.6 ± 0.2°C (from 34.4 ± 0.1°C), and the (multiunit) muscle afferent response to isotonic stretch was reproducibly increased from 106 ± 16 to 177 ± 30 spikes (5 s)−1 (n = 6, P < 0.05). After the skin began to rewarm, muscle afferent activity returned to its baseline level. As may be seen in Fig. 3, the enhanced muscle afferent activity seen during cooling could be caused either by reactivation of completely adapted afferent fibres (Fig. 3A, unit A) or by increasing the discharge of tonically firing afferents (Fig. 3A, unit B). The activity of some afferent fibres was unaffected by skin cooling (not shown).
Figure 3. Muscle afferent fibre responses to skin cooling and their dependence on efferent innervation.
A, chart record showing (from the top) blood pressure, core (rectal) temperature, skin temperature, muscle afferent activity, multiunit 5 s counts and the firing rates of two single units (units A and B). The bottom trace indicates weights applied to the cut Achilles tendon. Muscle afferent activity was recorded from a small filament peeled from the lateral gastrocnemius nerve. Note how the afferent responses to skin cooling were abolished after the remaining efferent nerve connection to the muscle was severed (at arrow). B, expanded segments of raw muscle afferent activity taken from before (a) and during a skin cooling episode (b), and during a skin cooling episode after the nerve was severed (c), at times indicated by the corresponding letters in the above chart record (A). Dots in trace b indicate two spikes of single unit A; the remainder are from single unit B. Superimposed spike shapes of the two single units are shown below (C).
After several cooling episodes, the remaining efferent pathway to the muscle was crushed and/or cut during a cooling sequence. This had little or no effect on the tonic afferent activity while the skin was warm (Fig. 3). Thereafter, however, falls in skin temperature failed to increase muscle afferent activity during isotonic stretch (from 106 ± 30 to 110 ± 29 spikes (5 s)−1, n.s., n = 6), as shown in Fig. 3.
Effect of EEG changes on muscle efferent activity
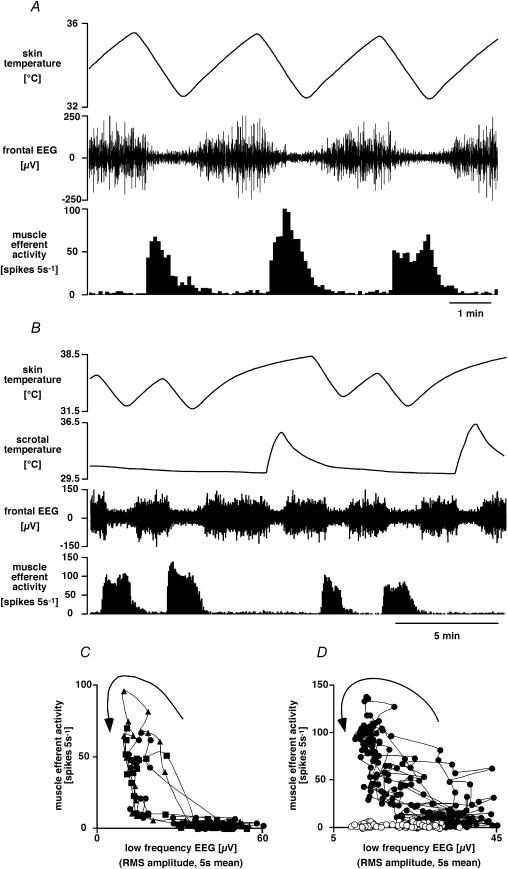
It has been noted that changing skin and hypothalamic temperatures may have effects on arousal and EEG state (Kanosue et al. 1985; Grahn & Heller, 1989; Grahn et al. 1989; Berner & Heller, 1998; Osaka, 2004), and that fusimotor activity may be closely linked with EEG state (von Euler & Söderberg, 1957). We therefore recorded the frontal EEG at the same time as muscle efferent activity and followed their responses to repeated skin cooling in six rats. As shown by the representative example in Fig. 4A, cooling episodes generally caused a decrease in the low frequency component (1–4 Hz) of the EEG (desynchronization) in parallel with muscle efferent activation. Closer inspection revealed that muscle efferent activity generally led the EEG desynchronization and ended before resynchronization, causing hysteresis in their relationship (Fig. 4C and D). In order to investigate whether the muscle efferent response to skin cooling was secondary to the EEG changes, warming the scrotum by 1.8–7.5°C from 30.9 ± 0.3°C was used as an alternative method to desynchronize the EEG (Kanosue et al. 1985). This was effective in three out of four rats. Scrotal warming, sufficient to desynchronize the EEG, had no effect on muscle efferent activity in two of the three rats; in the third rat the EEG desynchronization was accompanied by muscle efferent activation on some occasions, but not on others. Figure 4B and C illustrates a clear example where this procedure separated EEG desynchronization from muscle efferent activation.
Figure 4. Muscle efferent fibre response to skin cooling and its relation to cortical arousal.
A, chart record showing (from the top) skin temperature, filtered frontal EEG (bandpass 1–4 Hz) and muscle efferent activity (5 s counts). Note that skin cooling caused EEG desynchronization as well as muscle efferent activation. B, chart record from another experiment showing scrotal temperature in addition (second trace). Note that, like skin cooling, warming the scrotum desynchronized the EEG; but unlike skin cooling, it did not cause muscle efferent activation. C and D show plots of muscle efferent activity (5 s counts) versus filtered EEG amplitude (r.m.s. amplitude, 5 s mean) measured from the experiments shown in A and B, respectively. Note the hysteresis in these relations (time sequence indicated by arrows). In C, data from sequential cooling episodes are indicated by different symbols. In D, filled circles indicate episodes of skin cooling, open circles episodes of scrotal warming.
Effect of skin and core temperatures on muscle efferent activity
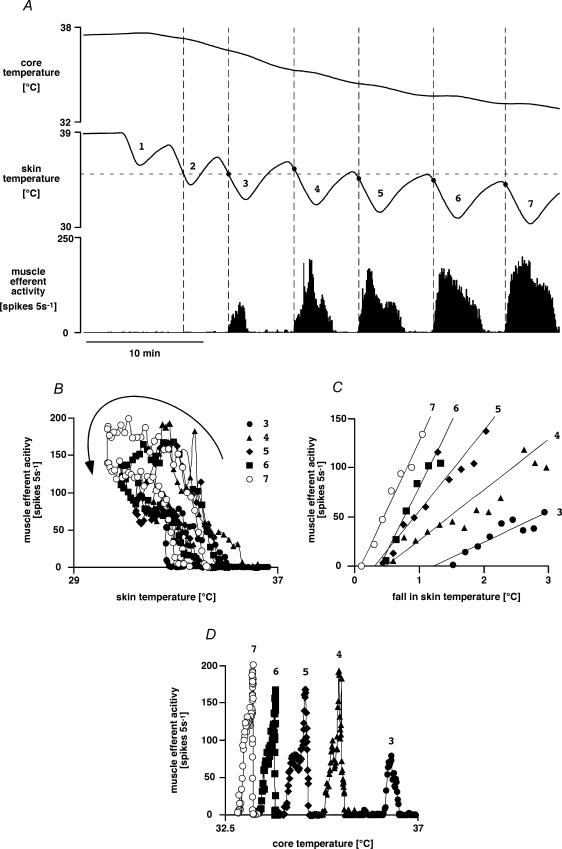
Seven rats were subjected to repeated skin cooling sequences similar to those shown in Fig. 5 while whole-nerve muscle efferent activity was recorded. Each skin cooling episode of 60–165 s lowered skin temperature by 4.7 ± 0.8°C (range 2.8–8.6°C, starting from 40.1–34.3°C) and reproducibly increased muscle efferent activity. The sequence of four to nine cooling episodes caused core temperature to fall from 37.6 ± 0.2 to 34.9 ± 0.6°C. There was hysteresis in the response to skin cooling (Fig. 5B), in that muscle efferents were activated only by falling skin temperature, and their activity fell to lower levels or ceased altogether during skin rewarming. Furthermore, muscle efferent activity always returned to baseline once the skin had rewarmed, regardless of how low core temperature had fallen.
Figure 5. Muscle efferent response to skin cooling: interaction with core temperature.
A, representative chart record showing responses to repeated skin cooling while core temperature was allowed to fall. Traces (from the top) show core (rectal) temperature, skin temperature and muscle efferent activity (5 s counts). Dots on the skin temperature trace (and dashed vertical lines drawn through them) indicate the onset of muscle efferent activation during each cooling episode. Note that when the expected threshold (horizontal dashed line) was crossed during the second cooling period, there was no muscle efferent activation. Note also that muscle efferent activity became stronger and more persistent during the later cooling periods when core temperature had fallen. B, C and D show plots of muscle efferent activity in the experiment illustrated in A plotted versus skin temperature (B, hysteresis indicated by arrow), versus the fall in skin temperature during the skin cooling phase of each cooling episode (C) and versus core temperature (D). Data from sequential cooling episodes are indicated by different symbols, and numbered as in A.
The skin temperature threshold for muscle efferent activation was estimated from the first cooling response in the sequence as 36.0 ± 0.7°C (range 34.1–38.3°C). It became evident, however, that the threshold followed the falling starting temperature during the sequence (Fig. 5A). We therefore calculated the threshold change in skin temperature to activate muscle efferents; this was −1.1 ± 0.3°C (range −0.5 ∼–2.3°C) over the sequence (41 cooling episodes in seven rats).
In every rat, however, there was evidence of interaction between the muscle efferent response to skin temperature and background conditions (putatively core temperature). For example, when baseline core (and skin) temperature was relatively high, skin cooling failed to activate muscle efferent fibres at the expected threshold temperature (shown by horizontal dashed line in Fig. 5A, see second cool; four out of four rats tested). Furthermore, when core temperature was low, the relationship between the increase in muscle efferent activity and the fall in skin temperature was steeper (Fig. 5C), and activity also persisted for a longer part of the skin rewarming phase (Fig. 5A). There was no direct relationship, however, between core temperature and muscle efferent activity (Fig. 5D).
Effect of neuronal inhibition in the medullary raphé on the muscle efferent response to skin cooling
Because inhibition of neurons in the rostal medullary raphé has been found to block sympathetic cutaneous vasoconstrictor responses to cold (Ootsuka et al. 2004), we tested whether it also affected the muscle efferent response to cooling. For this experiment, a regular protocol was used to establish a steady background against which to measure the effect of microinjecting glycine into the medullary raphé. Sequential cooling episodes lasting 40–90 s lowered skin temperature by 3.5 ± 0.2°C from 37.8 ± 0.2°C, and led to a delayed fall in core temperature of 0.4 ± 0.03°C from a baseline value of 36.2 ± 0.2°C (n = 6). In individual rats, baseline skin temperature and the fall in skin temperature with each cooling episode were kept as constant as possible (coefficients of variation 0.15 ± 0.04 and 3.5 ± 1.5%, respectively; n = 6). These skin cooling episodes increased muscle efferent activity from 0.5 ± 0.3 to 184 ± 56 spikes (5 s)−1 (40–357 spikes (5 s)−1, P < 0.05, n = 6)
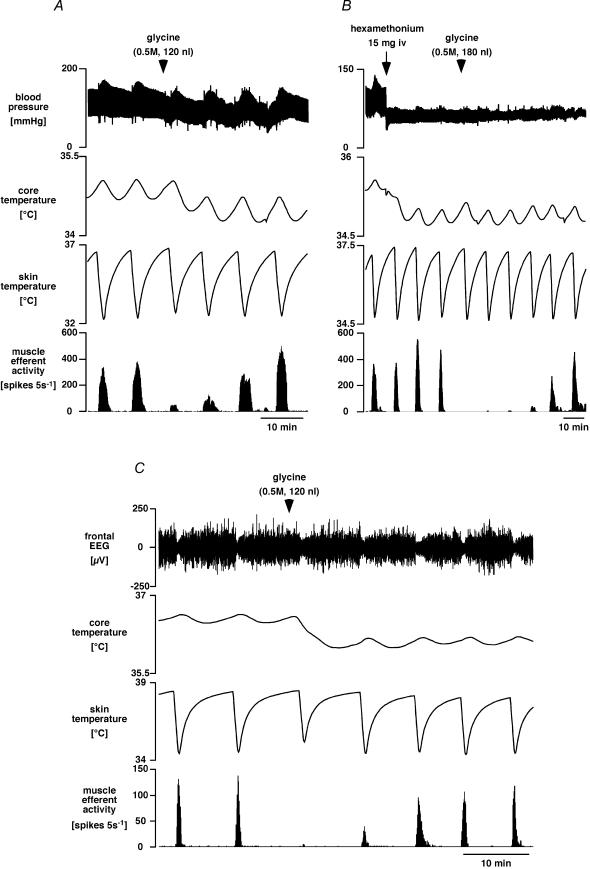
Figure 6 shows representative examples of the effect of glycine microinjection into the medullary raphé on muscle efferent responses. In five of the six rats, glycine (120–180 nl) was microinjected into sites verified histologically to be within the ventral medullary raphé; this did not affect the minimal baseline muscle efferent activity, but strongly inhibited its activation by skin cooling (to 5 ± 3% of preinjection levels; range 0–13%, P < 0.01, n = 5). In most (four out of five) cases the response recovered over the next 30–60 min (Fig. 6). Glycine injection usually also caused a small reduction in blood pressure, however, and always caused a reduction in core temperature (Fig. 6A and C). These effects were presumably a consequence of cutaneous vasodilatation (Ootsuka et al. 2004) and perhaps of reduced thermogenesis (Morrison, 2004). Because the lower core temperature and dilated cutaneous vessels after glycine might have had confounding effects on the experiment, we repeated the same protocol in two rats after giving hexamethonium to provide a steady background with a low blood pressure and a dilated cutaneous vascular bed. As can be seen in Fig. 6B, glycine microinjection into the medullary raphé was still able to abolish the muscle efferent response to skin cooling. The frontal EEG was also recorded in two of the rats receiving raphé glycine injections. In both cases, the desynchronization of the EEG by skin cooling persisted while the fusimotor response was inhibited by glycine (Fig. 6C). This was the case also in one further rat, which was excluded from the analysis (see Methods) because it received a large (300 nl) glycine injection into the medullary raphé.
Figure 6. Effect of neuronal inhibition in the medullary raphé on muscle efferent and EEG responses to skin cooling.
A, chart record showing sequential responses to skin cooling before and after microinjection of glycine (at arrowhead) into the medullary raphé. Traces show (from the top) blood pressure, core (rectal) temperature, skin temperature and muscle efferent activity (lateral gastrocnemius nerve, 5 s counts). B, chart record from another experiment showing the effect of glycine injection into the medullary raphé after ganglion blockade with hexamethonium (as indicated). C, chart record from a third experiment of this type, showing filtered (1–4 Hz) frontal EEG (top trace).
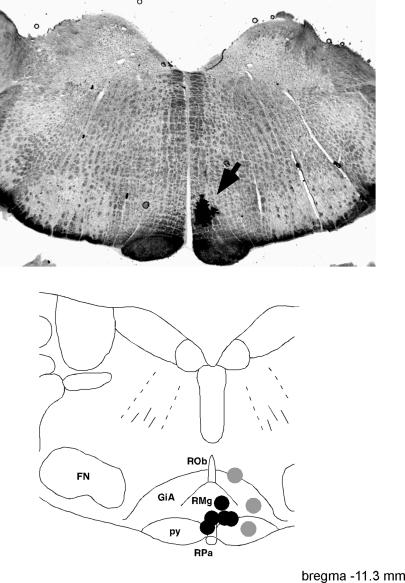
When the same dose of glycine was injected outside the medullary raphé (grey areas in the bottom panel of Fig. 7), it was less effective. There, two injections made after hexamethonium and one injection made before hexamethonium reduced the muscle efferent response to skin cooling to 58 ± 6% of preinjection values (range 48–60%, P < 0.05). When compared with injections made into the medullary raphé, their effect on cold-induced muscle efferent activity was significantly weaker (P < 0.01).
Figure 7. Glycine injection sites.
The top panel is a representative photomicrograph showing a dye mark (arrowed) where glycine was injected into the medullary raphé region. Bottom panel, grouped injection sites plotted onto a standard section, drawn with reference to the atlas of Paxinos & Watson (1998) at the level indicated. Sites where 120–180 nl glycine caused > 85% inhibition of the muscle efferent response to cooling (n = 5) are shown as black circles. Less effective injections (40–60% inhibition, n = 3) are indicated by grey circles. Abbreviations: GiA, gigantocellular reticular nucleus, alpha; FN, facial nucleus; py, pyramidal tract; RMg, raphé magnus nucleus; ROb, raphé obscurus nucleus; and RPa, raphé pallidus nucleus.
Discussion
New findings
The present study has characterized what we believe to be a thermoregulatory reflex, in which fusimotor fibres to the limb muscles are activated by cooling the skin. The relationship of this response to body core temperature and EEG state has been documented. Finally, evidence is presented that, like several other cold-defence responses, fusimotor activation depends upon neurons in the rostral medullary raphé.
Identity of muscle efferents activated by skin cooling
It is clear that the cold-activated efferent activity recorded in these experiments was of fusimotor fibres. This is supported by arguments against it being caused by any other efferent fibre type, in that it was not accompanied by evidence of α-motoneuron activity (movement, or electromyographic activity in innervated muscles), and it was unaffected by ganglion-blocking doses of hexamethonium. The positive argument for fusimotor fibre activation is that there was an accompanying sensitization of muscle spindle afferent activity. Although changes in muscle temperature secondary to skin cooling (via blood) could theoretically have influenced spindle afferent discharge, the disappearance of the sensitization once the efferent nerve supply was cut shows that any such direct effect was negligible.
The absence of EMG changes during cooling suggests that β-motoneurons, if present (Andrew et al. 1978), were not activated. The patterns of spindle afferent sensitization seen during cooling (steady increases in tonic firing with little or no adaptation to a maintained stretch, and de novo tonic activation) are characteristics of spindle sensitization by static but not dynamic fusimotor neurons (Andrew et al. 1978). Our protocol was not set up to detect dynamic fusimotor fibre actions, nor did we isolate single units and measure conduction velocities. We therefore conclude that while static fusimotor fibres were activated by skin cooling, we cannot say whether or not dynamic fusimotor fibres were activated as well.
Not all muscle afferent discharge was sensitized by skin cooling. There are at least three reasons why this may have happened in those cases: first, the fusimotor supply to the spindle under study may not have been activated, or activated sufficiently, by cooling; second, the process of dissecting afferent filaments could have damaged the efferent supply to that spindle; and third, the tonic afferent activity could have been caused by tendon organ rather than spindle discharge. We therefore cannot be sure about the proportion of fusimotor fibres activated by skin cooling; but the sensitization of afferents by cooling was common enough to be found in every experimen.
Previous work
In order to study the central control of fusimotor fibres, previous studies needed to use preparations in which fusimotor neurons were spontaneously active. This was achieved by using lightly anaesthetized or unanaesthetized preparations (von Euler & Söderberg, 1957; Schäfer & Schäfer, 1973). Under these conditions, fusimotor discharge is influenced strongly by local spinal reflexes (Hunt & Paintal, 1958; Alnaes et al. 1965) and by postural factors (Burton & Bronk, 1937). A change in fusimotor discharge may be detected by its effect on the muscle spindle afferent response to a standard stretch (von Euler & Söderberg, 1957; Sato, 1984). On this basis, von Euler & Söderberg (1957) stated that there was strong, tonic discharge from muscle spindles in the ‘lightly anaesthetised animal slightly below thermal balance’, but this was diminished by warming the preoptic/hypothalamic region with a thermode; Sato (1984) later confirmed this principle by showing increased muscle spindle responses when the preoptic region was cooled. The influence of cutaneous thermoreceptors was not investigated.
The present study, by contrast, used rats that were anaesthetized sufficiently deeply to prevent spontaneous fusimotor activity. We were then able to make the novel finding that fusimotor activity is generated as a reflex response to skin cooling, without any direct contribution from falls in core temperature. An inhibitory effect of a warm core temperature was confirmed, but only by its ability to block or attenuate responses to skin cooling.
von Euler & Söderberg (1957) considered that the fusimotor discharge was directly linked to cortical arousal, because they found parallel changes in fusimotor activity and EEG state, in that moderate hypothalamic warming caused EEG synchronization and decreased fusimotor activity, while strong heating desynchronized the EEG and increased fusimotor activity. Although our experiments confirmed that fusimotor discharge and EEG desynchronization commonly occurred together, our evidence does not support a direct, causal relationship. First, their timing did not accurately coincide, in that fusimotor activation during cooling episodes generally led EEG desynchronization, and finished earlier. Second, we were able to dissociate the two responses by using scrotal warming to desynchronize the EEG without activating fusimotor discharge. We were also able to block the fusimotor response to cooling, without blocking the EEG response, by inhibiting raphé neurons. This last point indicates that the brainstem pathways for the two effects are separate, and that the abolition of the fusimotor response by raphé glycine injections was not caused by interruption of an ascending ‘arousal’ pathway. We therefore conclude that, while skin cooling drives both responses, fusimotor activation is not a simple consequence of cortical arousal. Rather, fusimotor activation appears to be an independent reflex response to skin cooling. The inhibitory effect of core temperature on the fusimotor response to skin cooling further supports the concept that it is a thermoregulatory reflex.
Local cooling of the paw pad has been used in lightly anaesthetized cats to activate fusimotor fibres supplying muscles in the same limb (Lupandin, 1983; Lupandin & Kuz'mina, 1985). These effects are also prominent in spinal animals (Sato & Hasegawa, 1977; Sato, 1981, 1983), and may thus best be interpreted as segmental or local spinal reflexes. Cooling such a small body region is unlikely to provide much drive for thermoregulation. Under conditions where spinal reflexes provide the dominant drive, one may expect reflex responses to differ between the supplies to flexor and extensor muscles. Indeed, Lupandin reported such differences on cooling the paw pad (Lupandin, 1983; Lupandin & Kuz'mina, 1985). In line with that interpretation, we found, by contrast, that trunk skin cooling activated the fusimotor fibres to both extensor (the gastrocnemius) and flexor muscle (peroneal nerve. Tanaka M, Owens NC, Nagashima K, Kanosue K, McAllen RM, unpublished observations), suggesting that this was a generalized thermoregulatory response affecting at least the limbs, and perhaps the whole body.
The medullary raphé and fusimotor activity
The link between the medullary raphé and fusimotor activity was first addressed by Sato et al. (1990), who recorded from the ventral roots of anaesthetized rats whose preoptic regions had been destroyed by electrolytic lesions. In response to brief, tetanic electrical stimulation of the nucleus raphé magnus, these workers found that some fusimotor neurons were facilitated but most were inhibited, often for several minutes (Sato et al. 1990). Only the minority of facilitatory responses, however, could have perhaps involved the raphé–fusimotor pathway implicated in the present study.
It is believed that neurons in the ventral medullary raphé, level with the caudal part of the facial nucleus, are an essential synaptic relay mediating cold defence responses such as cutaneous vasoconstriction and non-shivering thermogenesis by interscapular brown adipose tissue (BAT; Blessing et al. 1999; Morrison, 1999; Morrison et al. 1999; Rathner & McAllen, 1999; Blessing & Nalivaiko, 2000; Tanaka et al. 2002), and it has been demonstrated that inhibition of neurons in the medullary raphé can block the sympathetic cutaneous vasoconstrictor (tail sympathetic nerve) responses to skin and core cooling (Ootsuka et al. 2004). Our results indicate that the fusimotor neuron activation by trunk skin cooling is also prevented when raphé neurons are inhibited. One hypothesis, then, might be that the same raphé neurons drive three responses to cold: cutaneous vasoconstriction, thermogenesis by BAT and fusimotor activation. We consider that this is unlikely, because the firing properties of fusimotor fibres (this study) differ significantly from those of cutaneous vasoconstrictor fibres in the rat's tail (Owens et al. 2002). Most strikingly, cutaneous vasocontrictor fibres are strongly activated by falls in core temperature as well as (less strongly) by falls in skin temperature (Owens et al. 2002); by contrast, under matching experimental conditions, fusimotor neurons did not respond at all to falls in core temperature. Interestingly, non-shivering thermogenesis, as measured by oxygen consumption in anaesthetized rats, is also activated by skin cooling rather than core cooling (Osaka, 2004), although a minor component of activity attributable to core cooling may also be observed in recordings from the nerve to interscapular BAT (Tanaka & McAllen, 2005). The different activation patterns of cutaneous vasoconstrictor, BAT and fusimotor fibres strongly suggest that the medullary raphé neurons controlling these cold-defence responses are separate.
Conclusion
The results of this study provide evidence for a thermoregulatory reflex which activates fusimotor fibres in response to skin cooling. This response is not secondary to cortical ‘arousal’ and, like several other cold-defence responses, it depends upon neurons in the medullary raphé.
We speculate that this reflex acts to increase the gain of the stretch reflex during cold exposure. Before shivering occurs, this increased gain would cause an increase in muscle tone, by enhancing tonic activity in (for example) postural muscles (Burton & Bronk, 1937; von Euler, 1961; Meigal et al. 2003). It would also help sustain or amplify shivering, the magnitude of which depends heavily upon the stretch reflex (Perkins, 1945; Lippold et al. 1959).
Acknowledgments
We are most grateful to David Trevaks for his expert help with technical and computing aspects of this study. We also thank the National Health and Medical Research Council of Australia for supporting this study.
References
- Alnaes E, Jansen JK, Rudjord T. Fusimotor activity in the spinal cat. Acta Physiol Scand. 1965;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- Andrew BL, Leslie GC, Part NJ. Some observations on the efferent innervation of rat soleus muscle spindles. Exp Brain Res. 1978;31:433–443. doi: 10.1007/BF00237300. [DOI] [PubMed] [Google Scholar]
- Berner NJ, Heller HC. Does the preoptic anterior hypothalamus receive thermoafferent information? Am J Physiol Regul Integr Comp Physiol. 1998;274:R9–R18. doi: 10.1152/ajpregu.1998.274.1.R9. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Yu Y-H, Nalivaiko E. Raphe pallidus and parapyramidal neurons regulate ear pinna vascular conductance in the rabbit. Neurosci Lett. 1999;270:33–36. doi: 10.1016/s0304-3940(99)00459-0. [DOI] [PubMed] [Google Scholar]
- Burton AC, Bronk DW. The motor mechanism of shivering and of thermal muscular tone. Am J Physiol. 1937;119:284. [Google Scholar]
- Grahn DA, Heller HC. Activity of most rostral ventromedial medulla neurons reflect EEG/EMG pattern changes. Am J Physiol Regul Integr Comp Physiol. 1989;257:R1496–R1505. doi: 10.1152/ajpregu.1989.257.6.R1496. [DOI] [PubMed] [Google Scholar]
- Grahn DA, Radeke CM, Heller HC. Arousal state vs. temperature effects on neuronal activity in subcoeruleus area. Am J Physiol Regul Integr Comp Physiol. 1989;256:R840–R849. doi: 10.1152/ajpregu.1989.256.4.R840. [DOI] [PubMed] [Google Scholar]
- Hunt CC, Paintal AS. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958;143:195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Nakayama T, Ishikawa Y, Hosono T, Kaminaga T, Shosaku A. Responses of thalamic and hypothalamic neurons to scrotal warming in rats: non-specific responses? Brain Res. 1985;328:207–213. doi: 10.1016/0006-8993(85)91031-5. [DOI] [PubMed] [Google Scholar]
- Lippold OCJ, Redfearn JWT, Vuco J. The influence of afferent and descending pathways on the rhythmical and arrhythmical components of muscular activity in man and the anaesthetized cat. J Physiol. 1959;146:1–9. doi: 10.1113/jphysiol.1959.sp006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupandin IuV. Regulation of the function of gamma- and alpha-motor neurons of antagonist muscles during cold tremor in the cat. [In Russian.] Neirofiziologiia. 1983;15:242–248. [PubMed] [Google Scholar]
- Lupandin IuV, Kuz'mina GI. Interaction of thermoreceptive and vestibular signalling in regulating the activity of flexor and extensor motor nuclei during cold tremor. [In Russian.] Fiziol Zh SSSR Im I M Sechenova. 1985;71:1433–1438. [PubMed] [Google Scholar]
- Meigal AY, Oksa J, Gerasimova LI, Hohtola E, Lupandin YV, Rintamaki H. Force control of isometric elbow flexion with visual feedback in cold with and without shivering. Aviat Space Environ Med. 2003;74:816–821. [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R290–R297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW, McAllen RM. Inhibition of rostral madullary raphé neurons prevents cold-induced activity in sympathetic nerves to rat tail and rabbit ear arteries. Neurosci Lett. 2004;357:58–62. doi: 10.1016/j.neulet.2003.11.067. [DOI] [PubMed] [Google Scholar]
- Osaka T. Thermogenesis elicited by skin cooling in anaesthetised rats: lack of contribution of the cerebral cortex. J Physiol. 2004;555:503–513. doi: 10.1113/jphysiol.2003.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat's tail. J Physiol. 2002;543:849–858. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Perkins JF. The role of the proprioceptors in shivering. Am J Physiol. 1945;145:264–271. doi: 10.1152/ajplegacy.1945.145.2.264. [DOI] [PubMed] [Google Scholar]
- Rathner JA, McAllen RM. Differential control of sympathetic drive to the rat tail artery and kidney by medullary premotor cell groups. Brain Res. 1999;834:196–199. doi: 10.1016/s0006-8993(99)01568-1. [DOI] [PubMed] [Google Scholar]
- Sato H. Fusimotor modulation by spinal and skin temperature changes and its significance in cold shivering. Exp Neurol. 1981;74:21–32. doi: 10.1016/0014-4886(81)90146-1. [DOI] [PubMed] [Google Scholar]
- Sato H. Effects of skin cooling and warming on stretch responses of the muscle spindle primary and secondary afferent fibers from the cat's tibialis anterior. Exp Neurol. 1983;81:446–458. doi: 10.1016/0014-4886(83)90274-1. [DOI] [PubMed] [Google Scholar]
- Sato H. Effects of changes in preoptic temperature on stretch response of muscle spindle endings in the cat's soleus muscle. Pflugers Arch. 1984;402:144–149. doi: 10.1007/BF00583326. [DOI] [PubMed] [Google Scholar]
- Sato H, Hasegawa Y. Reflex changes in discharge activities of gamma efferents to varying skin temperatures in cats. Pflugers Arch. 1977;372:195–201. doi: 10.1007/BF00585336. [DOI] [PubMed] [Google Scholar]
- Sato H, Hashitani T, Isobe Y, Furuyama F, Nishino H. Descending influences from nucleus raphe magnus on fusimotor neurone activity in rats. J Therm Biol. 1990;15:259–265. [Google Scholar]
- Schäfer SS, Schäfer S. The behavior of the proprioceptors of the muscle and the innervation of the fusimotor system during cold shivering. Exp Brain Res. 1973;17:364–380. doi: 10.1007/BF00234100. [DOI] [PubMed] [Google Scholar]
- Tanaka M, McAllen RM. A subsidiary fever center in the medullary raphé? Am J Physiol Regul Integr Comp Physiol. 2005;289:R1592–R1598. doi: 10.1152/ajpregu.00141.2005. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphé in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler C. Physiology and pharmacology of temperature regulation. Pharmacol Rev. 1961;13:361–398. [PubMed] [Google Scholar]
- von Euler C, Söderberg U. The influence of hypothalamic thermoceptive structures on the electroencephalogram and gamma motor activity. Electroencephalogr Clin Neurophysiol. 1957;9:391–408. doi: 10.1016/0013-4694(57)90029-9. [DOI] [PubMed] [Google Scholar]