Abstract
Electrophysiological recordings were used to investigate the degree of pelvic/visceral convergent inputs onto single medullary reticular formation (MRF) neurons. A total of 94 MRF neurons responsive to bilateral electrical stimulation of the pelvic nerve (PN) in 12 urethane-anaesthetized male rats were tested for responses to mechanical stimulation of the urinary bladder, urethra, colon and penis, and electrical stimulation of the dorsal nerve of the penis (DNP) and abdominal branches of the vagus. Responses to distension of the bladder were found for 51% (n = 48) of the MRF neurons tested. Of these 48, 71% responded to urethral infusion, 81% responded to colon distension, 100% responded to penile stimulation (and DNP), and 85% responded to vagal stimulation, with 62% responding to stimulation of all four of these territories. This high degree of visceral convergence (i.e. 62%) in a subset of PN-responsive MRF neurons is significantly greater than for the subset of PN-responsive MRF neurons that did not respond to urinary bladder distension (i.e. out of the 46 remaining neurons, none responded to all four of the other pelvic/visceral stimuli combined). These results suggest that the neurons processing information from the urinary bladder at this level of the neural axis are likely to be important for mediating interactions between different visceral organs for the coordination of multiple pelvic/visceral functions.
Normal functions related to the male pelvic/visceral organs, such as urination, defaecation, and ejaculation, involve coordination between the different organ systems. One example of a process in which coordination is important is ejaculation, which consists of emission of the seminal fluid from the ejaculatory ducts to the proximal urethra, bladder neck closure and anterograde ejaculation out of the proximal urethra (Seftel et al. 1991; Truitt & Coolen, 2002). Disruption of this coordination, such as following spinal cord injury, results in the failure of bladder neck closure and thus a subsequent retrograde ejaculation leading to infertility (Heruti et al. 2001).
Another example in which coordination between the pelvic organs is important is during voiding of the urinary bladder, which requires contraction of the external anal sphincter, thereby preventing defaecation. In humans, distension of the urinary bladder produces contractions of the anal sphincter (Basinski et al. 2003). Experimental studies using cats have shown that distension of the urinary bladder both inhibits colonic contractions (Bouvier et al. 1990) and produces simultaneous contraction of the anal sphincter (Bouvier & Grimaud, 1984). The reverse also occurs, i.e. the urinary system is inhibited during defaecation. In humans, functional stimulation of the anal sphincter inhibits detrusor muscle contraction (Cheng et al. 2002). In animal studies, distension of the rectum has been shown to inhibit urinary bladder activity in female rats (Sugaya et al. 1998), and stimulation of colonic branches of the pelvic nerve in cats has been shown to have an inhibitory effect on micturition (Floyd et al. 1978).
Interactions and functional coordination between various organ systems creates a situation where a pathological condition in one pelvic/visceral organ can affect the normal function of another organ. For example, some patients who suffer from irritable bowel syndrome have micturition problems, such as voiding incontinence (Whorwell et al. 1986a). In addition, 46% of female patients who suffer from urinary tract problems have sexual dysfunction, such as decreased sexual activity, disorders in sexual arousal, and dyspareunia (pain with intercourse) (Salonia et al. 2004). In an experimental study using female rats, urinary bladder inflammation was shown to decrease uterine contractions (Dmitrieva et al. 2001), thereby demonstrating that the pathology of one organ, such as the urinary bladder, can affect the function of another.
The neural mechanisms underlying the interactions between the various pelvic/visceral organs are likely to be mediated by both the peripheral and central nervous systems. For the reflex circuitries to be efficient in coordinating pelvic/visceral functions there must be some sort of viscero-visceral convergence within the CNS, both in the spinal cord itself, as well as supraspinally. In the spinal cord, various neuroanatomical studies have shown innervation of different visceral organs by the same set of neurons (Russo & Conte, 1996; Nadelhaft & Vera, 2001). Also, various electrophysiological studies have shown that individual neurons in the spinal cord of rats (Berkley et al. 1993; Qin & Foreman, 2004), cats (Foreman et al. 1984) and primates (Milne et al. 1981) respond to different pelvic/visceral and somatic stimuli. At the brainstem level, numerous regions, including Barrington's nucleus, the solitary nucleus and the nucleus gracilis, have been shown to receive viscero-visceral convergent inputs (Hubscher & Berkley, 1994; Berkley & Hubscher, 1995; Rouzade-Dominguez et al. 2003b).
The medullary reticular formation (MRF) is another brainstem region that receives multiple pelvic/visceral organ inputs. The extensive connections of MRF neurons with the forebrain, limbic system, brainstem nuclei, cerebellum and spinal cord, are an indication of the potential importance of these neurons in the functional coordination between different systems (Brodal et al. 1980; Van Bockstaele et al. 1993; Tavares & Lima, 1994; Cobos et al. 2003; Sun & Panneton, 2005). MRF neurons, for example, that are responsive to electrical stimulation of dorsal nerve of penis (DNP) and mechanical stimulation of penis (low- and high-threshold stimuli) also respond to electrical stimulation of the pelvic nerve (PN), as well as to mechanical stimulation of the perineum and limbs (Hubscher & Johnson, 1996).
Previous neuroanatomical and electrophysiological studies have, however, focused primarily on either inputs to MRF from territories involved with one pelvic organ system (such as from either the bladder and urethra or from the colon and rectum or from the genitalia) or on somato-visceral convergence (Roy et al. 1992; Hubscher & 1996,Johnson, 2004; Almeida & Lima, 1997; Nadelhaft & Vera, 2001; Hubscher et al. 2004). Given the vast array of inputs that have been shown to go to the MRF from the pelvic viscera, as well as convergent inputs from widespread somatic territories, it is hypothesized that there is a significant degree of overlap from different pelvic territories onto individual MRF neurons. Using electrophysiological studies, a defined region of the MRF was searched for neurons responsive to PN stimulation. Each PN-responsive neuron was then tested for responsiveness to urinary bladder (UB) distension and urethral stimulation, electrical stimulation of the DNP and abdominal branches of the vagus, mechanical stimulation of the genitalia, and distension of the distal colon.
Methods
Animal preparation
A total of 12 male Wister rats (120 days of age) were used. Each animal was anaesthetized with 50% urethane (1.2 g kg−1). The jugular vein, carotid artery and trachea were exposed and intubated for anaesthetic supplement (5% urethane, as needed, based on assessing withdrawal reflexes), blood pressure monitoring, and respiratory rate/end-expired PCO2 level monitoring, respectively. Using an oesophageal heat sensor probe connected to a thermometer, the animal temperature was monitored throughout the experiment, and maintained at around 37°C using a circulating water-heating pad.
Each animal was mounted onto a stereotaxic device. A dorsal incision was made to gain access to the brainstem. The dorsal surface of rostral medulla was exposed by removing some occipital bone and suctioning the caudal midline portion of the cerebellum (Hubscher & Johnson, 1996, 1999).
Electrical nerve stimulation
A dorsal incision, through the gluteus superficialis and biceps femoris muscles, was made in each animal to expose the PN and DNP bilaterally. The somato-motor branch of the PN was cut and a portion removed to avoid undesirable contractions; only the viscerocutaneous branch was prepared for stimulation. The nerves were separated from the connective tissues and placed on specially fabricated bipolar electrodes (Hubscher & Johnson, 1996). The stimulus intensity, for both nerves, was set at 30–50 μA, 0.1 ms duration, with trains of 14 pulses at 70 pulses s−1, 100 ms train duration, 1 train s−1 (Hubscher & Johnson, 1996).
A bipolar electrode was introduced alongside the oesophageal thermometer probe, just caudal to the oesophageal hiatus, in order to stimulate the abdominal branches of the vagus nerve that are situated on the external wall of the oesophagus (Hubscher et al. 2004). The stimulus intensity was set at 8 mA for 2 ms duration. This stimulus intensity produces compound action potentials in the vagus nerve, as recorded with bipolar hook electrodes in the neck where the vagus runs along the common carotid artery (Hubscher et al. 2004).
Mechanical stimulation of pelvic/visceral organs
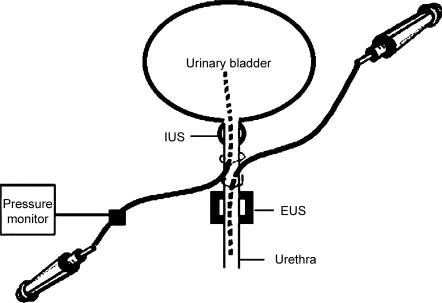
A midline abdominal incision was made to expose the UB and proximal urethra. UB and urethral catheters, comprising PE 60 tubing attached to a syringe, were implanted through an incision in the proximal urethra, with the UB catheter directed rostrally toward the UB, and the urethral catheter directed caudally toward the distal urethra (see Fig. 1). The ureters were tied close to the UB and were cut proximal to the suture and drained externally using PE 10 tubing (Kakizaki & de Groat, 1997). The abdominal incision was then closed.
Figure 1. Diagram illustrating the insertion location of urinary bladder (UB) and urethral catheters into the proximal urethra.
A 10-mm-long balloon, made from condom latex material and attached to a 25 g × 3/4 in catheter (Terumo Corporation, Tokyo, Japan), was inserted intra-anally for distal colon distension (Berkley et al. 1993). The UB and colon catheters were connected to a pressure monitor (World Precision Instruments). Pressures were recorded on videotape and analysed offline using Data Wave software (http://www.dwavetech.com). Once fully distended (2–3 s), the UB and colon remained distended until a response was obtained up to a maximum of 10 s before deflation.
Electrophysiological recordings
An epoxylite-insulated tungsten microelectrode with standard tip (Frederick Haer and Co., Bowdoinham, ME, USA) was lowered from the dorsal surface with a motorized drive (Frederick Haer and Co.) into the MRF. Stereotaxic coordinates were 3400 μm rostral to obex and 400 μm and 800 μm lateral to midline on both sides of the brainstem (four tracks per animal, in total). The search area for each dorso-ventral track covered a length of 2800–3000 μm, which penetrated the rostral part of the nucleus reticularis gigantocellularis (Gi), the Gi pars alpha (GiA) and the medial part of lateral paragigantocellular nucleus (LPGi) (Hubscher & Johnson, 1999).
Bilateral electrical stimulation of the PN (bPN) was used as the search stimulus. Once a neuron was found to be responsive to bPN (twice- or half-background activity for excitatory and inhibitory responses, respectively, and at least three spikes for non-spontaneous units; Hubscher & Johnson, 2003, 2004), responses of that neuron to distension of the UB and colon were tested, as well as infusion of the urethra. Responses to electrical stimulation of the DNP and the abdominal branches of the vagus nerve were then tested. In addition, the responses to mechanical (stroke and pinch) stimulation of different somatic regions, such as the penis, scrotum, trunk, upper and lower limbs, ears, eyelids and face, were tested. All of the spike recordings made while testing the PN-responsive MRF neurons were recorded to videotape and analysed offline using Data Wave software.
Histology
At the end of the experiment, each animal was perfused using 0.9% normal saline followed by 4% paraformaldehyde introduced through the left ventricle. The brainstem was extracted from each animal and sectioned at 100 μm thickness on a vibratome, and then processed histologically and stained with cresyl violet. The electrophysiological tracks were visualized in these sections under the light microscope, thereby confirming the location of each electrode track (Hubscher & Johnson, 1999). Stereotaxic depth measurements obtained relative to the dorsal surface were used to plot the location of each PN-responsive neuron that was recorded along each of the electrode tracks.
Data analysis
Data was analysed for significance using Student's t test and the χ2 test. Results were considered significant when P < 0.05. Spike histograms were generated using the Data Wave program. The study was performed in accordance with guidelines of the Animal Care and Use Committee (IACUC) of the University of Louisville School of Medicine, and the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, publication no. 0-309-05377-3).
Results
A total of 94 MRF neurons in 24 tracks responded to bPN. About half of these neurons responded to distention of the UB. A comparison between the characteristics of PN-responsive MRF neurons that did and did not respond to UB distension is presented in Table 1. There were no significant differences (P > 0.05) in background activity, neuronal responses and nerve response latencies between these two groups of MRF neurons. Although the majority of responses were excitatory, inhibitory and complex (mixed excitatory and inhibitory) neuronal responses were also observed. The percentage of inhibitory PN-responsive MRF neurons was significantly higher for those responding to UB distension (77% of the total number of inhibitory responses).
Table 1.
Characteristics of medullary reticular formation (MRF) neurons
| Neurons responsive to PN and UB distension (n = 48) | Neurons responsive to PN but NOT UB distension (n = 46) | |
|---|---|---|
| Background activity | ||
| Neurons with spontaneous background activity | 37.5% | 26.1% |
| Mean spontaneous background activity (spikes s−1) | 14.7 ± 1.9 | 18.2 ± 3.7 |
| Neuronal responses | ||
| Percentage of neurons excitatory | 77.1% | 87.0% |
| Percentage of neurons inhibitory | 20.8%* | 6.5% |
| Percentage of neurons complex | 2.1% | 6.5% |
| Response latencies | ||
| PN | 141.1 ± 11.6 ms | 140.3 ± 12.5 ms |
| DNP | 152.7 ± 13.1 ms | 160.9 ± 18.1 ms |
| Vagus | 196.8 ± 25.8 ms | 195.2 ± 19.6 ms |
| UB distension | 3.8 ± 0.6 s | NA |
| Colon distension | 3.7 ± 0.6 s | 2.5 ± 0.5 s |
Significantly different (χ2, P < 0.05) from those MRF neurons not responding to (UB) distension. PN, pelvic nerve; DNP, dorsal nerve of the penis; NA, not applicable.
The distribution of the excitatory, inhibitory and complex neurons differed from one MRF subregion to another (Table 2). There were, however, no significant differences in the pattern of distribution of PN-responsive neurons that did and did not respond to UB distension. The location of a sample of neurons from four animals (selected at random) is illustrated in Fig. 2. Note the ventral location of neurons that were inhibited by PN and bladder stimulation. Examples from electrophysiological recordings are provided for individual neurons with excitatory (Fig. 3) and inhibitory (Fig. 4) responses to both PN and UB distension. An example of a PN-responsive MRF neuron that did not respond to UB distension is provided in Fig. 5.
Table 2.
Distribution of PN-responsive neurons within the MRF
| Neurons responsive to PN and UB distension (n = 48) | Neurons responsive to PN but NOT UB distension (n = 46) | |
|---|---|---|
| Gi neuronal responses | ||
| No. excitatory | 27 | 28 |
| No. inhibitory | 3 | 1 |
| No. complex | 0 | 1 |
| DPGi neuronal responses | ||
| No. excitatory | 4 | 4 |
| No. inhibitory | 0 | 0 |
| No. complex | 1 | 0 |
| GiA + RMg neuronal responses | ||
| No. excitatory | 6 | 8 |
| No. inhibitory | 7* | 2 |
| No. complex | 0 | 2 |
Significantly different (χ2, P < 0.05) from those MRF neurons not responding to urinary bladder (UB) distension. DPGi, dorsal paragigantocellular nucleus; Gi, gigantocellular reticular nucleus; GiA, Gi pars alpha; RMg, raphe magnus nucleus.
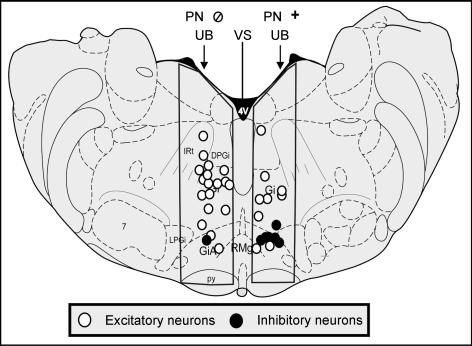
Figure 2. Distribution of PN-responsive MRF neurons that did and did not respond to UB distension.
Example showing the distribution of pelvic nerve (PN)-responsive medullary reticular formation (MRF) neurons (recorded from 4 animals) that did and did not respond to UB distension (right and left side, respectively). Note the distribution of excitatory versus inhibitory responses of neurons in the outlined area under investigation. Also note that the left/right distribution is for illustrative purposes only. Neurons were found in the indicated regions on both sides of the MRF for both groups. The cross section is adapted from atlas Fig. 65 (Paxinos & Watson, 1998). 4V, 4th ventricle; 7, facial nucleus; DPGi, dorsal paragigantocellular nucleus; Gi, gigantocellular reticular nucleus; GiA, Gi pars alpha; IRt, intermediate reticular nucleus; LPGi, lateral paragigantocellular nucleus; py, pyramidal tract; RMg, raphe magnus nucleus.
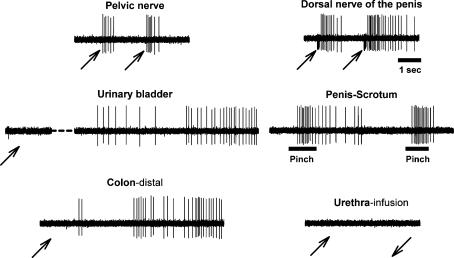
Figure 3. Sample raw record of convergent excitatory inputs to MRF.
Example of a single neuron recording in the left nucleus reticularis pars alpha obtained during testing of electrical stimulation of PN and dorsal nerve of the penis (DNP), UB and distal colon distension, urethral infusion and pinching of the glans penis and scrotum. As shown, this neuron responded to UB and distal colon distension, but not to urethral infusion. This neuron also responded to pinching the ear, eyelid, face, forepaw and hindpaw (not shown). There was no response to electrical stimulation of the abdominal branches of the vagus (not shown). Note the stimulus artifact in the DNP record and that the urinary bladder had a 14 s response delay (partial record shown). The upward arrows indicate the beginning of the stimulus, the downward arrow indicates the end of the stimulus, and bars indicate the onset and duration of the mechanical stimulus. The response discharge to UB and colon distension lasted for 46 and 20 s, respectively. The time scale for the DNP record applies to all traces.
Figure 4. Sample histogram of convergent inhibitory inputs to MRF.
Histogram showing an example of inhibitory responses of a single neuron, located in the left nucleus reticularis gigantocellularis pars alpha, to bilateral electrical stimulation of the DNP, pinching of the glans penis and scrotum, and distension of the UB and distal colon. The neuron also responded to pinching the dorsal trunk, eyelid, forepaw, hindpaw, and electrical stimulation of PN and abdominal branches of vagus (not shown). The response latency for UB and distal colon distension was about 2 s, and it had a response discharge of about 10 and 7 s, respectively. The bin width is 0.25 sec. Symbols as in Fig. 3.
Figure 5. Example of an MRF neuron not responsive to bladder distention.
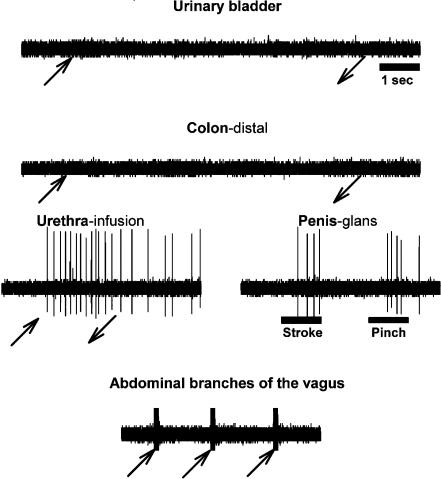
Record showing an example of the responses of a single neuron located in the left nucleus reticularis gigantocellularis to UB and distal colon distension, urethral infusion, low- and high-threshold stimulation of the glans penis, and electrical stimulation of the abdominal branches of the vagus. As shown, this neuron responded to stimulation of one pelvic/visceral organ (urethra), but didn't respond to the other pelvic/visceral organs (UB and distal colon) or to electrical stimulation of abdominal branches of the vagus (stimulus artifact can be seen in record). Symbols as in Fig. 3.
Viscero-visceral convergence
Many of the PN-responsive MRF neurons studied that were excited or inhibited by UB distension also responded to stimulation of other organs/nerves (urethral infusion, colon distension, penis stimulation, DNP and abdominal branches of the vagus stimulation – 62% responded to all of these stimuli) as well as numerous cutaneous territories (face, eyelid and trunk). A summary comparing the convergent responses of PN-responsive MRF neurons that did and did not respond to UB distension is presented in Table 3. Note that the degree of convergence from multiple pelvic/visceral territories was significantly greater for those neurons responding to UB distension. In fact, the amount of convergence for those responding to all the other pelvic/visceral territories tested was quite different for the subsets of neurons responding and not responding to UB distension (62 versus 0%, respectively, responded to urethra as well as colon and vagal stimulation).
Table 3.
Summary of MRF viscero-visceral and somato-visceral convergence in PN-responsive MRF neurons that did and did not respond to UB distension
| Region stimulated | Neurons responsive to PN and UB distension | Neurons responsive to PN but NOT UB distension |
|---|---|---|
| DNP | 100% | 100% |
| Vagus (abdominal branches) | 85.4%* | 52.2% |
| Urethra (infusion) | 70.8%* | 15.2% |
| Distal colon (distension) | 81.3%* | 13.0% |
| Glans penis | ||
| LT + HT | 27.8% | 12.1% |
| HT only | 72.2% | 87.9% |
| Face | ||
| LT + HT | 6.3% | 0.0% |
| HT only | 45.8% | 28.3% |
| Eyelid | ||
| LT + HT | 35.4%* | 10.9% |
| HT only | 56.3% | 67.4% |
| Dorsal trunk | ||
| LT + HT | 20.8%* | 2.2% |
| HT only | 68.8%* | 43.5% |
Significantly different (χ2, P < 0.05) from those not responding to UB distension. LT, low threshold, HT, high threshold.
Of the total number of PN-responsive MRF neurons, 66 (70.2%) had convergent inputs from one or more of the pelvic/visceral stimuli examined. All the neuronal responses of the MRF neurons to UB and colon distension were in the noxious range (70 mmHg pressure). The average response latency (from the beginning of distension to the beginning of the response) for the MRF neurons to UB and colon distension is provided in Table 1. Note, though, that the time until the balloon was fully distended was approximately 2–3 s. For a few MRF neurons, the response delay exceeded the 10 s stimulus duration (i.e. responded after deflation – as for the example shown in Fig. 3). In addition, all the responses to UB, colon and urethra had after discharges that lasted anywhere from several seconds, to on occasion several minutes (see examples in Figs 3 and 4). The mean MRF neuronal responses to UB and colon distension lasted 10.4 ± 1.3 and 9.7 ± 1.4 s, respectively (i.e. total response duration – see response criteria in Methods).
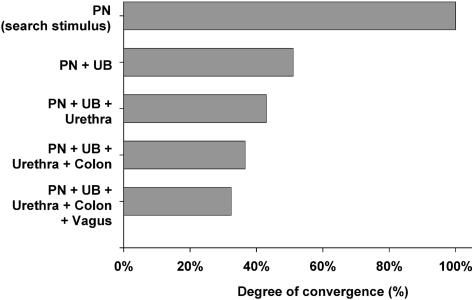
Most MRF neurons that responded to the stimulation of one visceral organ responded to stimulation of other visceral organs (i.e. viscero-visceral convergence). The degree of viscero-visceral convergence in the MRF is illustrated graphically in Fig. 6. Note that many responses were found for electrical stimulation of the abdominal branches of the vagus nerve, which is known to innervate a number of pelvic/visceral organs, including the stomach, liver, pancreas, colon and the female reproductive organs (Sauter et al. 1983; Prechtl & Powley, 1985; Schoenen et al. 1992; Altschuler et al. 1993; Hubscher & Berkley, 1995). Examples illustrating varying degrees of viscero-visceral convergence onto individual PN-responsive MRF neurons can be seen by comparing responses shown in Figs 3, 4, 5 and 7.
Figure 6. Viscero-visceral convergence in MRF.
Illustration of the degree of viscero-visceral convergence to MRF neurons responsive to bilateral electrical stimulation of the PN (the search stimulus). Most of the neurons that are responsive to one pelvic/visceral organ also respond to others.
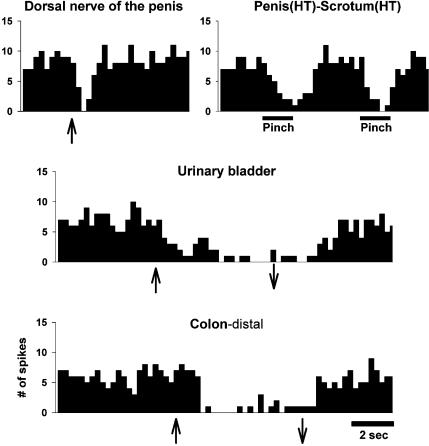
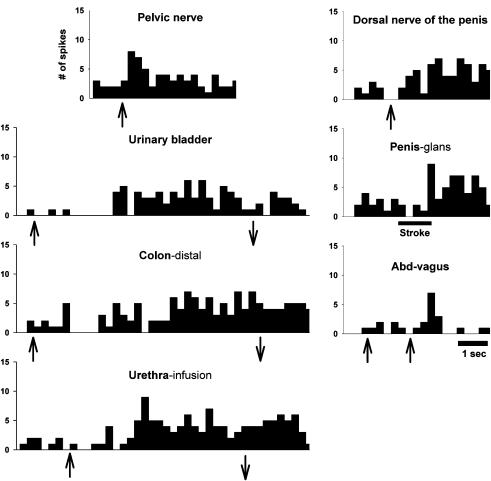
Figure 7. Example showing extent of visceral convergence in MRF.
Histogram showing an example of excitatory responses for a single neuron located in the left nucleus reticularis gigantocellularis to bilateral stimulation of PN and dorsal nerve of the penis, UB and distal colon distension, urethral infusion, glans penis stroke (low-threshold (LT) stimulus) and electrical stimulation of the abdominal branches of the vagus. The same neuron responded also to stroking (LT) the dorsal trunk, stretching (LT) the eyelid, and pinching the face, forepaw and hindpaw (not shown). This neuron had convergent inputs from all the pelvic/visceral organs tested, as well as responses to stimulation of the abdominal branches of the vagus. The response discharge to UB and colon distension lasted for 15 and 17 s, respectively. The bin width is 0.25 sec. Symbols as in Fig. 3.
Note that about 8.5% of the neurons responding to UB and colon distension did not respond to urethral infusion (see example in Fig. 3). Also, only 5.6% of the neurons responding to UB distension and urethral infusion did not respond to colon distension. In addition some of the neurons not responding to UB distension responded to stimulation of one of the other pelvic viscera (urethra only (8.5%), see example in Fig. 5; colon only (2.8%)). All neurons responding to both urethra and colon responded to UB distension.
Somato-visceral convergence into MRF
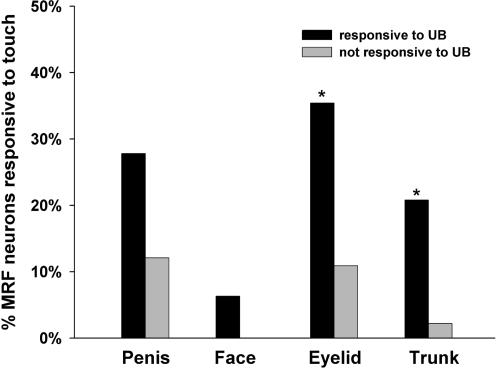
All of the PN-responsive MRF neurons had convergent input from somatic territories (bilateral). Of the 94 PN-responsive MRF neurons, 27.7% responded to both low- and high-threshold stimulation of somatic receptive fields, whereas 72.3% responded to high-threshold levels of stimulation only. The subregion distribution of PN-responsive MRF neurons with responses to both low- and high-threshold levels of stimulation was as follows: 73.0% located in Gi, 11.5% located in DPGi, and 15.4% located in GiA and raphe magnus nucleus; these levels were not significantly different from the distribution of the PN-responsive MRF neurons that only had responses to high-threshold levels of stimulation. The PN-responsive MRF neurons that responded to UB distension were more responsive to low-threshold stimulation of several different somatic territories (see Fig. 8) compared with the neurons that did not respond to UB distension (43.8 versus 10.9%, respectively).
Figure 8. Summary of MRF responses to low threshold levels of stimulation.
Diagram comparing the percentage of responses to LT stimulation of different somatic receptive fields for those PN-responsive MRF neurons that did, versus those that did not, respond to UB distension. As shown, PN-responsive MRF neurons that respond to UB distension have more LT responses. *Significant difference (χ2, P < 0.05).
Discussion
The results of the present study indicate that there is a high degree of convergence from the pelvic viscera (UB, colon and urethra) to single MRF neurons that are responsive to electrical stimulation of the PN. All of these neurons were responsive to electrical stimulation of the DNP and mechanical stimulation of the glans penis (low-threshold and/or high-threshold), and many responded to stimulation of the abdominal branches of the vagus, consistent with previous results (Hubscher & Johnson, 1996; Hubscher et al. 2004). The same region also has somatic convergent inputs from different parts of the body (limbs, perineum, face and trunk), a finding also consistent with previous findings (Hubscher & Johnson, 1996). A significant amount of viscero-visceral convergence, in addition to a high degree of somato-visceral convergence, indicate that these areas of the MRF (e.g. Gi, GiA) are likely to play a role in coordinating the eliminative functions of different pelvic viscera (micturition, defaecation and ejaculation). It is possible, though, that at least some of the visceral inputs to MRF result from ascending inputs that have already converged at lower spinal cord levels, as seen for spinal neurons that have been shown to receive either both UB and colon inputs (Qin & Foreman, 2004), or both UB and urethral inputs (Russo & Conte, 1996).
UB/urethral input to MRF
More than half of the PN-responsive MRF neurons responded to distension of the UB. Most of these neurons were also responsive to urethral stimulation. The high degree of convergence from UB and urethra to single MRF neurons indicates that these neurons may play a role in coordinating or modulating the micturition process. Neuroanatomical tracing studies using transsynaptic tracers are consistent with these electrophysiological findings. For example, pseudorabies virus transfection studies (using two histologically distinguished modified types of pseudorabies virus) show double labelling of many MRF neurons following injection of the UB and external urethral sphincter (Nadelhaft & Vera, 2001). Only a very small number of double-labelled neurons are found, however, in the pontine micturition centre (Nadelhaft & Vera, 2001), which is known to be involved in the control of urine voiding and storage through its facilitatory and inhibitory projections, respectively, to lumbosacral spinal neurons that contribute to the functioning of the UB and the external urethral sphincter (Holstege et al. 1986; Noto et al. 1989; Kruse et al. 1990; Yoshimura, 1999; Griffiths, 2002).
A few studies have demonstrated the ability of MRF neurons to modulate the function of the pelvic organs involved in micturition. In one study using cats, MRF neurons were shown to be activated during voiding (Sugaya et al. 2003). In the same study, MRF neurons were shown to have reciprocal connections with the pontine micturition centre and to project axons to the spinal cord, suggesting that two descending spinal pathways from the pontine micturition centre play important roles in micturition, one of which projects by way of the MRF (Sugaya et al. 2003). In another study using cats, electrical stimulation of the MRF inhibited UB contractions (McMahon & Spillane, 1982).
In the present study, most of the inhibitory neurons in the MRF region examined were located in ventral areas (GiA and raphe magnus), which is consistent with previous results (Hubscher & Johnson, 1996). This segregation of the inhibitory and excitatory neurons coincides with segregation between the areas that have inhibitory versus excitatory descending influences on spinal reflexes, as shown in a study examining the effect of electrical stimulation of MRF subregions on the nociceptive tail-flick reflex (Zhuo & Gebhart, 1990).
Convergence of UB and colon inputs in the MRF
Most of the UB-responsive neurons responded to colon distension. Other electrophysiological studies also demonstrate the convergence of UB and colon inputs to single neurons in the brainstem, including studies of neurons within Barrington's nucleus (Rouzade-Dominguez et al. 2003b), the lateral medulla (Robbins et al. 2005) and rostral medulla (Baez et al. 2005), although the latter study tested UB voiding and not distension. Neurons in Barrington's nucleus that have been implicated to play a functional role in the micturition process are double labelled following pseudorabies virus transfection of the UB and colon (Rouzade-Dominguez et al. 2003a),
MRF neurons are likely to be part of the neural circuitry involved in coordinating eliminative processes, including micturition and defaecation. The underlying mechanisms for this control are not known. Lumbosacral spinal neurons have recently been shown in the rat to receive viscero-visceral convergent inputs from the bladder and colon (Qin & Foreman, 2004). These spinal neurons are obvious targets for brainstem modulation of the cross talk that is likely to be occurring between the various visceral organs.
The responses of the MRF neurons to UB and colon distension were in the noxious range (70 mmHg pressure). These results may indicate a role of MRF neurons in visceral nociception as well. It has been shown that the MRF can modulate nociceptive processing by its descending (excitatory and inhibitory) inputs (McCreery et al. 1979; Haber et al. 1980; Gebhart et al. 1983; Zhuo & Gebhart, 1990, 2002a,b), and work as relay nuclei to higher centres for nociceptive processing and ultimately pain perception (Foreman et al. 1984).
Convergence of UB and colon with genital inputs in the MRF
All the PN-responsive MRF neurons (thus all UB and colon-responsive MRF neurons) were responsive to electrical stimulation of dorsal nerve of the penis and mechanical stimulation of penis. These results indicate a role of the MRF neurons in the coordination of urogenital functions. For example, a subpopulation of MRF neurons may inhibit both voiding and defaecation circuitries during ejaculation. The brainstem neurons may also facilitate other aspects that are necessary for normal ejaculation, such as excitation of neurons important for closure of the internal urethral sphincter, which is important in order to avoid retrograde ejaculation.
The MRF has been shown to have a modulatory effect over sexual functions through its descending projections (Marson & McKenna, 1990; Johnson & 1998, Hubscher, 2000). For example, recordings from the motor branch of the pudendal nerve to MRF stimulation demonstrate that MRF neurons (mainly within the LPGi) have an inhibitory effect on the pudendal reflex (Johnson & Hubscher, 1998) and an excitatory effect on sympathetic fibres in the pudendal nerve (Johnson & Hubscher, 2000). Also, injection of an anterograde neuronal tracer (Fluoro-Ruby) into Gi and LPGi (Hermann et al. 2003) and raphe obscurus nucleus (Hermann et al. 1998) shows direct projections from these nuclei to motor neurons labelled with a retrograde neuronal tracer (Fluoro-Gold) injected into the external urethral sphincter, ischiocavernosus and bulbospongiosus muscles. On the other hand, other studies indicate that there are descending inputs from MRF to the spinal cord motor neurons that innervate different pelvic organs. For example, transneuronal-tracing studies with pseudorabies virus injected into UB (Marson, 1997; Sugaya et al. 1997; Zermann, 2002), prostate (Zermann, 2002), penis (Marson et al. 1993) and ischiocavernosus and bulbospongiosus muscles (Marson & McKenna, 1996) labels neurons in different brainstem nuclei (including the MRF nuclei under investigation in this study).
Convergence of vagal inputs into MRF
The PN-responsive MRF neurons that responded to UB distension have significantly higher convergent inputs from abdominal branches of vagus than neurons that did not respond to UB distension. The high convergence of pelvic/visceral inputs with the input resulting from vagus nerve stimulation could indicate a dual innervation of the pelvic/visceral organs. Other studies have demonstrated vagal innervation to pelvic/visceral organs. For example, bilateral vagotomy, in female rats, eliminates the responses of solitary nucleus neurons to uterine horn distension, and affects the response to cervical and vaginal stimuli (Hubscher & Berkley, 1995). Neurons in the dorsal motor nucleus of the vagus and the solitary nucleus have been labelled with neuroanatomical tracers from all areas of the colon, with the exception of rectum (Altschuler et al. 1993). In addition, tracers injected into the urinary bladder labels some nodose ganglion neurons. Capsaicin treatment doesn't affect this pattern of labelling in the nodose ganglion (Jancso & Maggi, 1987), indicating that the UB may be innervated by myelinated fibres in the vagus nerve.
Somato-visceral convergence in the MRF
All the PN-responsive MRF neurons have convergent inputs from cutaneous regions all over the body. The convergence of somatic inputs from all over the body to the same neurons that are responsive to the pelvic/visceral organs may indicate a role of MRF in controlling the body posture during micturition and/or preventing unwanted reflexes that may interrupt micturition (Baez et al. 2005). The significance for an increase in the amount of low-threshold sensory inputs to the MRF for neurons responding to urinary bladder distension (as indicated in Fig. 8) is not known, but perhaps relates to the maintenance of body posture during micturition.
The high degree of pelvic/visceral and somatic convergence onto single MRF neurons could also explain the diffuse and overlapping perception of pelvic visceral pain in some pelvic/visceral pathologies (Whorwell et al. 1986b; Longstreth, 1994; Cervero & Laird, 1999). In the present study, some of the PN-responsive MRF neurons had complex response properties, having opposite responses from stimulation of different areas (usually stimulation of the face evoked an inhibitory response with the remaining convergent responsive territories evoking excitatory responses). This may indicate the ability of MRF neurons to modulate cutaneous and/or visceral pain through somatic stimulation of other areas (see Fig. 3 in Hubscher & Johnson, 2004).
Viscero-visceral convergence in the MRF
The high degree of convergence of afferent inputs from multiple visceral organs into various regions of the CNS (including the MRF) provides a way for the CNS to control and coordinate functions between various pelvic/visceral organs, and may also explain how a pathology associated with one organ can affect the physiology/functioning of another organ (Qin et al. 2005; Berkley, 2005). The importance of regions located supraspinally in viscero-visceral interactions is apparent from the interruption of the physiological functions of the pelvic/visceral organs after spinal cord injury (Heruti et al. 2001). The MRF is one of the supraspinal regions that can play a key role in modulating these pelvic/visceral functions.
The ability of MRF neurons to modulate and/or coordinate multiple pelvic/visceral functions, as discussed in the present paper, necessitates the convergence of sensory inputs from different internal organs onto the same neurons. The results of this study indicate that the PN-responsive MRF neurons that respond to UB distension show a high degree of viscero-visceral convergence, which may indicate a role for a subset of neurons at this level of the neural axis in the modulation of spinal reflexes for the coordination of functions associated with these organs, such as elimination (urination, defaecation, ejaculation).
Acknowledgments
We wish to thank James Armstrong for excellent technical assistance. This study was supported by a grant from the NIH (NS40919).
References
- Almeida A, Lima D. Activation by cutaneous or visceral noxious stimulation of spinal neurons projecting to the medullary dorsal reticular nucleus in the rat: a c-fos study. Eur J Neurosci. 1997;9:686–695. doi: 10.1111/j.1460-9568.1997.tb01417.x. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Escardo J, Lynn RB, Miselis RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology. 1993;104:502–509. doi: 10.1016/0016-5085(93)90419-d. [DOI] [PubMed] [Google Scholar]
- Baez MA, Brink TS, Mason P. Roles for pain modulatory cells during micturition and continence. J Neurosci. 2005;25:384–394. doi: 10.1523/JNEUROSCI.3536-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basinski C, Fuller E, Brizendine EJ, Benson JT. Bladder–anal reflex. Neurourol Urodyn. 2003;22:683–686. doi: 10.1002/nau.10101. [DOI] [PubMed] [Google Scholar]
- Berkley KJ. A life of pelvic pain. Physiol Behav. 2005;86:272–280. doi: 10.1016/j.physbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nat Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH, Wall PD. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993;69:545–556. doi: 10.1152/jn.1993.69.2.545. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Grimaud JC. Neuronally mediated interactions between urinary bladder and internal anal sphincter motility in the cat. J Physiol. 1984;346:461–469. doi: 10.1113/jphysiol.1984.sp015035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Grimaud JC, Abysique A. Effects of stimulation of vesical afferents on colonic motility in cats. Gastroenterology. 1990;98:1148–1154. doi: 10.1016/0016-5085(90)90327-w. [DOI] [PubMed] [Google Scholar]
- Brodal A, Walberg F, Berkley KJ, Pelt A. Anatomical demonstration of branching olivocerebellar fibres by means of a double retrograde labelling technique. Neuroscience. 1980;5:2193–2202. doi: 10.1016/0306-4522(80)90136-0. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- Cobos A, Lima D, Almeida A, Tavares I. Brain afferents to the lateral caudal ventrolateral medulla: a retrograde and anterograde tracing study in the rat. Neuroscience. 2003;120:485–498. doi: 10.1016/s0306-4522(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Dmitrieva N, Johnson OL, Berkley KJ. Bladder inflammation and hypogastric neurectomy influence uterine motility in the rat. Neurosci Lett. 2001;313:49–52. doi: 10.1016/s0304-3940(01)02247-9. [DOI] [PubMed] [Google Scholar]
- Floyd K, McMahon SB, Morrison JF. Inhibition of the micturition reflex by stimulation of pelvic nerve afferents from the colon. J Physiol. 1978;284.P:39–40P. [PubMed] [Google Scholar]
- Foreman RD, Blair RW, Weber RN. Viscerosomatic convergence onto T2–T4 spinoreticular, spinoreticular–spinothalamic, and spinothalamic tract neurons in the cat. Exp Neurol. 1984;85:597–619. doi: 10.1016/0014-4886(84)90034-7. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Sandkuhler J, Thalhammer JG, Zimmermann M. Inhibition of spinal nociceptive information by stimulation in midbrain of the cat is blocked by lidocaine microinjected in nucleus raphe magnus and medullary reticular formation. J Neurophysiol. 1983;50:1446–1459. doi: 10.1152/jn.1983.50.6.1446. [DOI] [PubMed] [Google Scholar]
- Griffiths DJ. The pontine micturition centres. Scand J Urol Nephrol. 2002;Suppl.:21–26. doi: 10.1080/003655902320765926. [DOI] [PubMed] [Google Scholar]
- Haber LH, Martin RF, Chung JM, Willis WD. Inhibition and excitation of primate spinothalamic tract neurons by stimulation in region of nucleus reticularis gigantocellularis. J Neurophysiol. 1980;43:1578–1593. doi: 10.1152/jn.1980.43.6.1578. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Bresnahan JC, Holmes GM, Rogers RC, Beattie MS. Descending projections from the nucleus raphe obscurus to pudendal motoneurons in the male rat. J Comp Neurol. 1998;397:458–474. [PubMed] [Google Scholar]
- Hermann GE, Holmes GM, Rogers RC, Beattie MS, Bresnahan JC. Descending spinal projections from the rostral gigantocellular reticular nuclei complex. J Comp Neurol. 2003;455:210–221. doi: 10.1002/cne.10455. [DOI] [PubMed] [Google Scholar]
- Heruti RJ, Katz H, Menashe Y, Weissenberg R, Raviv G, Madjar I, Ohry A. Treatment of male infertility due to spinal cord injury using rectal probe electroejaculation: the Israeli experience. Spinal Cord. 2001;39:168–175. doi: 10.1038/sj.sc.3101120. [DOI] [PubMed] [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Berkley KJ. Responses of neurons in caudal solitary nucleus of female rats to stimulation of vagina, cervix, uterine horn and colon. Brain Res. 1994;664:1–8. doi: 10.1016/0006-8993(94)91946-1. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Berkley KJ. Spinal and vagal influences on the responses of rat solitary nucleus neurons to stimulation of uterus, cervix and vagina. Brain Res. 1995;702:251–254. doi: 10.1016/0006-8993(95)01121-8. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Responses of medullary reticular formation neurons to input from the male genitalia. J Neurophysiol. 1996;76:2474–2482. doi: 10.1152/jn.1996.76.4.2474. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Effects of acute and chronic midthoracic spinal cord injury on neural circuits for male sexual function. I. Ascending pathways. J Neurophysiol. 1999;82:1381–1389. doi: 10.1152/jn.1999.82.3.1381. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Responses of thalamic neurons to input from the male genitalia. J Neurophysiol. 2003;89:2–11. doi: 10.1152/jn.00294.2002. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Johnson RD. Effects of chronic dorsal column lesions on pelvic viscerosomatic convergent medullary reticular formation neurons. J Neurophysiol. 2004;92:3596–3600. doi: 10.1152/jn.00310.2004. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Kaddumi EG, Johnson RD. Brain stem convergence of pelvic viscerosomatic inputs via spinal and vagal afferents. Neuroreport. 2004;15:1299–1302. doi: 10.1097/01.wnr.0000128428.74337.ef. [DOI] [PubMed] [Google Scholar]
- Jancso G, Maggi CA. Distribution of capsaicin-sensitive urinary bladder afferents in the rat spinal cord. Brain Res. 1987;418:371–376. doi: 10.1016/0006-8993(87)90106-5. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Hubscher CH. Brainstem microstimulation differentially inhibits pudendal motoneuron reflex inputs. Neuroreport. 1998;9:341–345. doi: 10.1097/00001756-199801260-00030. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Hubscher CH. Brainstem microstimulation activates sympathetic fibers in pudendal nerve motor branch. Neuroreport. 2000;11:379–382. doi: 10.1097/00001756-200002070-00031. [DOI] [PubMed] [Google Scholar]
- Kakizaki H, de Groat WC. Reorganization of somato-urethral reflexes following spinal cord injury in the rat. J Urol. 1997;158:1562–1567. [PubMed] [Google Scholar]
- Kruse MN, Noto H, Roppolo JR, de Groat WC. Pontine control of the urinary bladder and external urethral sphincter in the rat. Brain Res. 1990;532:182–190. doi: 10.1016/0006-8993(90)91758-9. [DOI] [PubMed] [Google Scholar]
- Longstreth GF. Irritable bowel syndrome and chronic pelvic pain. Obstet Gynecol Surv. 1994;49:505–507. doi: 10.1097/00006254-199407000-00027. [DOI] [PubMed] [Google Scholar]
- Marson L. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. [PubMed] [Google Scholar]
- Marson L, McKenna KE. The identification of a brainstem site controlling spinal sexual reflexes in male rats. Brain Res. 1990;515:303–308. doi: 10.1016/0006-8993(90)90611-e. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1996;374:161–179. doi: 10.1002/(SICI)1096-9861(19961014)374:2<161::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marson L, Platt KB, McKenna KE. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience. 1993;55:263–280. doi: 10.1016/0306-4522(93)90471-q. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Bloedel JR, Hames EG. Effects of stimulating in raphe nuclei and in reticular formation on response of spinothalamic neurons to mechanical stimuli. J Neurophysiol. 1979;42:166–182. doi: 10.1152/jn.1979.42.1.166. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Spillane K. Brain stem influences on the parasympathetic supply to the urinary bladder of the cat. Brain Res. 1982;234:237–249. doi: 10.1016/0006-8993(82)90865-4. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Foreman RD, Giesler GJ, Jr, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain. 1981;11:163–183. doi: 10.1016/0304-3959(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Separate urinary bladder and external urethral sphincter neurons in the central nervous system of the rat: simultaneous labeling with two immunohistochemically distinguishable pseudorabies viruses. Brain Res. 2001;903:33–44. doi: 10.1016/s0006-8993(01)02349-6. [DOI] [PubMed] [Google Scholar]
- Noto H, Roppolo JR, Steers WD, de Groat WC. Excitatory and inhibitory influences on bladder activity elicited by electrical stimulation in the pontine micturition center in the rat. Brain Res. 1989;492:99–115. doi: 10.1016/0006-8993(89)90893-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Prechtl JC, Powley TL. Organization and distribution of the rat subdiaphragmatic vagus and associated paraganglia. J Comp Neurol. 1985;235:182–195. doi: 10.1002/cne.902350204. [DOI] [PubMed] [Google Scholar]
- Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport. 2004;15:467–471. doi: 10.1097/00001756-200403010-00017. [DOI] [PubMed] [Google Scholar]
- Qin C, Malykhina AP, Akbarali HI, Foreman RD. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology. 2005;129:1967–1978. doi: 10.1053/j.gastro.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Robbins MT, Uzzell TW, Aly S, Ness TJ. Visceral nociceptive input to the area of the medullary lateral reticular nucleus ascends in the lateral spinal cord. Neurosci Lett. 2005;381:329–333. doi: 10.1016/j.neulet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez ML, Miselis R, Valentino RJ. Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: substrates for pelvic visceral coordination. Eur J Neurosci. 2003a;18:3311–3324. doi: 10.1111/j.1460-9568.2003.03071.x. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez ML, Pernar L, Beck S, Valentino RJ. Convergent responses of Barrington's nucleus neurons to pelvic visceral stimuli in the rat: a juxtacellular labelling study. Eur J Neurosci. 2003b;18:3325–3334. doi: 10.1111/j.1460-9568.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- Roy JC, Bing Z, Villanueva L, Le Bars D. Convergence of visceral and somatic inputs onto subnucleus reticularis dorsalis neurones in the rat medulla. J Physiol. 1992;458:235–246. doi: 10.1113/jphysiol.1992.sp019415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A, Conte B. Afferent and efferent branching axons from the rat lumbo-sacral spinal cord project both to the urinary bladder and the urethra as demonstrated by double retrograde neuronal labeling. Neurosci Lett. 1996;219:155–158. doi: 10.1016/s0304-3940(96)13204-3. [DOI] [PubMed] [Google Scholar]
- Salonia A, Zanni G, Nappi RE, Briganti A, Deho F, Fabbri F, Colombo R, Guazzoni G, Di Girolamo V, Rigatti P, Montorsi F. Sexual dysfunction is common in women with lower urinary tract symptoms and urinary incontinence: results of a cross-sectional study. Eur Urol. 2004;45:642–648. doi: 10.1016/j.eururo.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Sauter JF, Niijima A, Berthoud HR, Jeanrenaud B. Vagal neurons and pathways to the rat's lower viscera: an electrophysiological study. Brain Res Bull. 1983;11:487–491. doi: 10.1016/0361-9230(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Schoenen J, Delwaide PJ, Legros JJ, Franchimont P. Bulbospinal neuronopathy (letter; comment) Neurology. 1992;42:1252–1253. doi: 10.1212/wnl.42.6.1252-b. [DOI] [PubMed] [Google Scholar]
- Seftel AD, Oates RD, Krane RJ. Disturbed sexual function in patients with spinal cord disease. Neurol Clin. 1991;9:757–778. [PubMed] [Google Scholar]
- Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Oda M. Evidence for involvement of the subcoeruleus nucleus and nucleus raphe magnus in urine storage and penile erection in decerebrate rats. J Urol. 1998;159:2172–2176. doi: 10.1016/S0022-5347(01)63300-7. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Ogawa Y, Hatano T, Nishijima S, Matsuyama K, Mori S. Ascending and descending brainstem neuronal activity during cystometry in decerebrate cats. Neurourol Urodyn. 2003;22:343–350. doi: 10.1002/nau.10115. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett. 1997;223:197–200. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]
- Sun W, Panneton WM. Defining projections from the caudal pressor area of the caudal ventrolateral medulla. J Comp Neurol. 2005;482:273–293. doi: 10.1002/cne.20434. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D. Descending projections from the caudal medulla oblongata to the superficial or deep dorsal horn of the rat spinal cord. Exp Brain Res. 1994;99:455–463. doi: 10.1007/BF00228982. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002;297:1566–1569. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Akaoka H, Aston-Jones G. Brainstem afferents to the rostral (juxtafacial) nucleus paragigantocellularis: integration of exteroceptive and interoceptive sensory inputs in the ventral tegmentum. Brain Res. 1993;603:1–18. doi: 10.1016/0006-8993(93)91293-2. [DOI] [PubMed] [Google Scholar]
- Whorwell PJ, Lupton EW, Erduran D, Wilson K. Bladder smooth muscle dysfunction in patients with irritable bowel syndrome. Gut. 1986a;27:1014–1017. doi: 10.1136/gut.27.9.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwell PJ, McCallum M, Creed FH, Roberts CT. Non-colonic features of irritable bowel syndrome. Gut. 1986b;27:37–40. doi: 10.1136/gut.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N. Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol. 1999;57:583–606. doi: 10.1016/s0301-0082(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Zermann DH. Efferent control of different visceral pelvic organs by spinal and supraspinal centres. Scand J Urol Nephrol. 2002;Suppl.:27–33. doi: 10.1080/003655902320765935. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Pain. 1990;42:337–350. doi: 10.1016/0304-3959(90)91147-B. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Facilitation and attenuation of a visceral nociceptive reflex from the rostroventral medulla in the rat. Gastroenterology. 2002a;122:1007–1019. doi: 10.1053/gast.2002.32389. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Modulation of noxious and non-noxious spinal mechanical transmission from the rostral medial medulla in the rat. J Neurophysiol. 2002b;88:2928–2941. doi: 10.1152/jn.00005.2002. [DOI] [PubMed] [Google Scholar]