Abstract
Cholecystokinin (CCK) interacts with two types of G protein-coupled receptors in the brain: CCK-A and CCK-B receptors. Both CCK and CCK-B receptors are widely distributed in the hippocampal formation, but the functions of CCK there have been poorly understood. In the present study, we initially examined the effects of CCK on GABAA receptor-mediated synaptic transmission in the hippocampal formation and then explored the underlying cellular mechanisms by focusing on the dentate gyrus region, where the highest levels of CCK-binding sites have been detected. Our results indicate that activation of CCK-B receptors initially and transiently increased spontaneous IPSC (sIPSC) frequency, followed by a persistent reduction. The effects of CCK were more evident in juvenile rats, suggesting that they are developmentally regulated. Cholecystokinin failed to modulate the miniature IPSCs recorded in the presence of TTX and the amplitude of the evoked IPSCs, but produced a transient increase followed by a reduction in action potential firing frequency recorded from GABAergic interneurons, suggesting that CCK acts by modulating the excitability of the interneurons to regulate GABA release. Cholecystokinin reduced the amplitude of the after-hyperpolarization of the action potentials, and application of paxilline or charybdotoxin considerably reduced CCK-mediated modulation of sIPSC frequency, suggesting that the effects of CCK are related to the inhibition of Ca2+-activated K+ currents (IK(Ca)). The effects of CCK were independent of the functions of phospholipase C, intracellular Ca2+ release, protein kinase C or phospholipase A2, suggesting a direct coupling between the G proteins of CCK-B receptors and IK(Ca). Our results provide a novel mechanism underlying CCK-mediated modulation of GABA release.
While cholecystokinin (CCK) was originally discovered in the gastrointestinal tract (Mutt & Jorpes, 1968), it is one of the most abundant neuropeptides in the brain (Beinfeld et al. 1981). Cholecystokinin is present in unusually high concentrations in the cerebral cortex, hippocampus, amygdala, septum and olfactory tubercles (Rehfeld, 1978; Innis et al. 1979; Beinfeld et al. 1981; Beinfeld, 1983). In the brain, CCK exists in several biologically active molecular forms (Rehfeld et al. 1985), of which the sulphated C-terminal fragment (CCK-8S) is the most abundant entity, although the unsulphated octapeptide C-terminal fragment (CCK-8U) and the tetrapeptide C-terminal fragment (CCK-4) are also present in lower concentrations (Rehfeld, 1978). Cholecystokinin is involved in modulating numerous physiological functions including satiety, analgesia, learning and memory processes and in neuropsychiatric disorders such as anxiety and panic attack (Crawley & Corwin, 1994; Noble & Roques, 1999). However, the mechanisms of CCK in modulating those physiological functions and neuropsychiatric disorders are unknown, mainly because the cellular and molecular mechanisms of CCK in the brain are poorly understood.
The effects of CCK are mediated by two types of G protein-coupled receptors: CCK-A and CCK-B receptors (Jagerschmidt et al. 1995; Noble & Roques, 1999; Pommier et al. 1999, 2003). Whereas CCK-A receptors are present in peripheral tissues and a few discrete brain regions (Moran et al. 1986; Hill et al. 1987, 1990), CCK-B receptors are the predominant form found in the brain (Van Dijk et al. 1984). Both receptors are coupled to phospholipase C (PLC), leading to increases in intracellular Ca2+ release and activation of protein kinase C (PKC; Wank, 1995). In addition, CCK-A receptor activation increases adenylyl cyclase activity, which further enhances the generation of cyclic AMP and subsequent activation of protein kinase A (Wank, 1995), whereas CCK-B receptors elevate phospholipase A2 (PLA2) activity, resulting in the release of arachidonic acid (Pommier et al. 1999, 2003). These intracellular signals may be involved in the effects of CCK.
Cholecystokinin is extensively expressed in the hippocampal formation. Cholecystokinin immunoreactive fibres are located around the cell bodies in the entorhinal cortex, subiculum and stratum pyramidale of Ammon's horn, and among the granule cells and inner molecular layer of the dentate gyrus (Greenwood et al. 1981). The CCK immunoreactive neurons appear to be non-pyramidal cells and may represent a subpopulation of GABAergic interneurons (Somogyi et al. 1984; Hendry & Jones, 1985; Nunzi et al. 1985). In addition to containing CCK, the hippocampal formation also expresses CCK receptors. The dentate gyrus exhibits the highest levels of binding sites for CCK, and moderate to light labelling has been observed in the stratum pyramidale of CA3 and CA1 areas, respectively (Kritzer et al. 1988; Kohler & Chan-Palay, 1988). The type of CCK receptors in the hippocampus is CCK-B (Shigeyoshi et al. 1994).
The selective distribution of CCK in GABAergic interneurons and the wide expression of CCK-B receptors in the hippocampal formation suggest that CCK modulates GABAergic functions in the hippocampal formation. Indeed, CCK has been reported to increase GABA release from hippocampal slices (Perez de la Mora et al. 1993) and enhance GABAA receptor-mediated IPSCs in the CA1 region (Miller et al. 1997), although two studies suggest that CCK does not change GABA release from rat hippocampal synaptosomes (Breukel et al. 1997) and hippocampal slices (Migaud et al. 1994). To solve these controversies and to determine the involved cellular and molecular mechanisms, we initially examined the effects of CCK on GABAergic synaptic transmission in the hippocampal formation. We then explored the underlying mechanisms by focusing on the dentate gyrus of the hippocampus because the highest density of CCK receptors is detected in this region (Kritzer et al. 1988; Kohler & Chan-Palay, 1988). Our results demonstrate that CCK has a bidirectional regulation of GABAergic transmission: an initial transient increase followed by a persistent reduction in GABA release. The CCK-mediated transient increase in GABA release is related to the inhibition of Ca2+-activated K+ channel activity. The effects of CCK on GABA release are not related to PLC or PLA2 pathway, suggesting a direct coupling of G-proteins to Ca2+-activated K+ channels. Our results provide a novel cellular mechanism to explain the functions of CCK in the brain.
Methods
Hippocampal slice preparation
Horizontal hippocampal slices (400 μm) including the entorhinal cortex, subiculum and hippocampus were cut using a Vibratome (Leica VT1000S) usually from 10- to 20-day-old Sprague–Dawley rats as previously described (Lei & McBain, 2003). For those experiments examining the development of CCK-mediated modulation of GABA release, we expanded the age of the rats to 6- and 31-day-old-rats. Rats were deeply anaesthetized with isoflurane, rapidly decapitated, and the brain was dissected out in ice-cold saline solution that contained (mm): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2 and 10 glucose, saturated with 95% O2–5% CO2, pH 7.4. Slices were initially incubated in the above solution at 35°C for 40 min for recovery and then kept at room temperature until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of spontaneous, miniature and evoked GABAA receptor-mediated IPSCs
Whole-cell patch-clamp recordings using an Axopatch 200B or a Multiclamp 700B (Axon Instruments, Union City, CA, USA) in voltage-clamp mode were made usually from dentate gyrus granule cells visually identified with infrared video microscopy (Olympus BX51WI) and differential interference contrast optics unless stated otherwise. For the experiments examining the effects of CCK on GABAergic transmission in different regions of the hippocampal formation, we recorded from neurons in the entorhinal cortex and subiculum. The recording electrodes were filled with the following (mm): 100 caesium gluconate, 0.6 EGTA, 5 MgCl2, 8 NaCl, 2 ATP2Na, 0.3 GTPNa, 40 Hepes and 1 QX-314, pH 7.2–7.3 (adjusted with CsOH). The extracellular solution comprised the following (mm): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5 MgCl2, 2.5 CaCl2 and 10 glucose, saturated with 95% O2–5% CO2, pH 7.4. To record GABAA receptor-mediated spontaneous IPSCs (sIPSCs), the external solution was supplemented with dl-2-Amino-5-phosphono-pentanoic acid (dl-APV; 100 μ m) to block NMDA receptor-mediated responses and 6,7-dinitro-quinoxaline-2,3-dione (DNQX; 10 μ m) to block AMPA receptor-mediated responses. Under these conditions, the recorded inhibitory currents had a reversal potential of approximately −30 mV and were completely blocked by bicuculline methobromide (10 μ m), confirming that they were mediated by GABAA receptors. Usually sIPSCs were recorded at a holding potential of +30 mV (Lei & McBain, 2003). Miniature IPSCs (mIPSCs) were recorded by including TTX (0.5 μ m) in the above external solution to block action potential-dependent responses. Evoked IPSCs were recorded from dentate gyrus granule cells using the same internal and external solution by placing a stimulation electrode in the hilus. Data were filtered at 2 kHz, digitized at 10 kHz and acquired on-line using pClamp 9 (Clampex) software (Axon Instruments). The recorded sIPSCs and mIPSCs were subsequently analysed by Mini Analysis 6.0.1 (Synaptosoft Inc., Decatur, GA, USA). Each detected event was inspected visually to exclude obvious artifacts before analysis. Mean amplitude, frequency, cumulative amplitude and frequency histograms were calculated by this program. The recorded evoked IPSCs were analysed by pClamp 9 (Clampfit). Cholecystokinin was applied via the bath. To avoid desensitization induced by repeated applications of CCK, one slice was limited to only one application of CCK.
Recordings of action potentials
Spontaneous action potential firing was recorded from interneurons in the hilus with whole cell patch-clamp recordings in current-clamp mode. Caesium gluconate in the above intracellular solution was replaced with the same concentration of potassium gluconate, and QX-314 was omitted. Because dialysis of K+-containing internal solution into the cells can change the resting membrane potential and influence the spontaneous action potential firing, we waited for ∼15 min after the formation of whole-cell recordings to allow the resting membrane potential to stabilize. Data were obtained only from those cells displaying resting membrane potentials negative to −60 mV. Usually, for most of the cells a positive current injection was needed to elevate the resting membrane potential to approximately −45 mV to induce spontaneous action potential firing. Cholecystokinin was applied after the action potential firing had been stable for 5–10 min. The frequency of the action potentials was calculated by Mini Analysis 6.0.1.
Construction of voltage–current curves and recordings of Ca2+ channel currents
Voltage–current curves were constructed from the interneurons in the hilus. Potassium gluconate internal solution was used, and the external solution contained TTX (0.5 μ m) to block Na+ channels. Voltage–current relationships were obtained by using a ramp protocol from −100 to +40 mV at a speed of 0.08 mV ms−1. Calcium channel currents were recorded from the interneurons in the hilus. The external solution contained TTX (0.5 μ m) to block Na+ channels. The pipette solution contained (mm): 100 Cs-gluconate, 30 tetraethylammonium, 1 CaCl2, 1 MgCl2, 4 ATP, 0.3 GTP, 5 EGTA and 10 Hepes (pH adjusted to 7.2 with CsOH). Leak currents were subtracted using P/N leak subtraction in Clampex.
Outside-out nucleated patch recordings
Outside-out nucleated patch recordings from interneurons in the hilus were carried out as described by Lei et al. (2001). The pipettes contained potassium gluconate internal solution, and the external solution contained TTX (0.5 μ m). After formation of a whole-cell recording, a piece of membrane was excised by slowly pulling the electrode away from the patched interneuron with a slight negative pressure inside the pipette. Voltage–current relationship was then obtained from the excised patches before and during the application of CCK by using the ramp protocol from −100 to +40 mV at a speed of 0.08 mV ms−1.
Data analysis
Data are presented as the means ± s.e.m. Student's paired or unpaired t test or analysis of variance (ANOVA) was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P < 0.05.
Chemicals
CCK-8S, CCK-8U and CCK-4 were purchased from American Peptide Company. YM 022 was the product from Tocris. Lorglumide, SKF96365, U73122, thapsigargin, calphostin C and PACOCF3 were purchased from BIOMOL. The other chemicals were from Sigma.
Results
CCK bidirectionally modulates spontaneous IPSCs
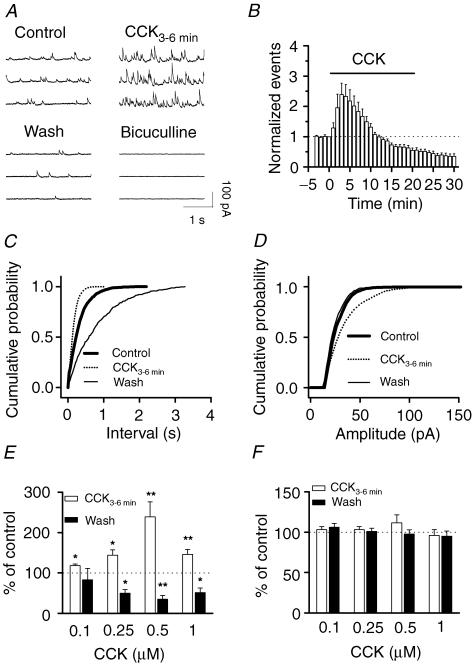
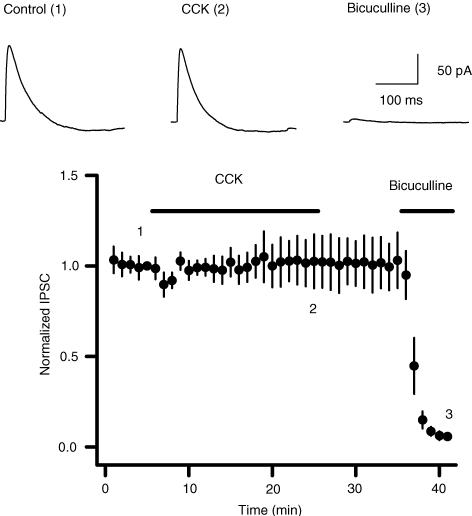
Although CCK has been reported to increase GABA release from rat cortical slices (Ferraro et al. 1999), rat nucleus accumbens (Lanza & Makovec, 2000) and the cerebral cortex of freely moving rats (Siniscalchi et al. 2003), there are inconsistent results as to whether CCK modulates GABA release in the hippocampus (Perez de la Mora et al. 1993; Migaud et al. 1994; Breukel et al. 1997; Miller et al. 1997). Since most of the studies were conducted by measuring GABA concentration in the perfusate of either hippocampal slices or hippocampal synaptosomes, which may represent a neutralized effect of CCK in all regions of the hippocampal formation, we studied the effects of CCK on GABAergic synaptic transmission by recording GABAA receptor-mediated spontaneous IPSCs from different regions of the hippocampal formation to solve these controversies. We initially examined the effects of CCK on sIPSCs recorded from dentate gyrus granule cells because the highest density of CCK receptors has been detected in the dentate gyrus (Kritzer et al. 1988; Kohler & Chan-Palay, 1988). Bath application of the sulphated octapeptide (CCK-8S, 0.1–1 μ m) produced a bidirectional modulation of sIPSC frequency recorded from dentate gyrus granule cells. Cholecystokinin transiently increased, and then gradually reduced sIPSC frequency (Fig. 1A, B, C and E). Spontaneous IPSC frequency usually increased to a maximum in 3–4 min after the beginning of CCK application (increased by 139 ± 37% at 0.5 μ m, n = 11, P = 0.004; Fig. 1A, B and E) and then began to decline (Fig. 1B). Cholecystokinin-induced gradual decline of sIPSC frequency is probably due to the agonist-induced desensitization of CCK receptors, because these receptors undergo considerable desensitization (Shinohara & Kawasaki, 1994). At the end of CCK application (0.5 μ m, 20 min), sIPSC frequency was reduced to 55 ± 8% of the initial value before CCK application (n = 11, P = 0.0002). The depressant effect of CCK was irreversible and became even more conspicuous after washing in CCK-free external solution. For 10 min after washing, sIPSC frequency was further reduced to 35 ± 9% of control values (n = 11, P = 0.00003, Fig. 1B and E). For five of these cells, sIPSC frequency did not recover even after 30 min washing (15 ± 6% of control, n = 5, P = 0.0001, data not shown), suggesting that CCK-induced persistent depression is irreversible. Cholecystokinin-mediated change in sIPSC amplitude was not consistent among cells, varying from an increase (Fig. 1D) through no change to slight reduction. It appeared that the effects of CCK on sIPSC amplitude were dependent on the initial frequency of sIPSCs. For the cells showing low initial sIPSC frequency, CCK usually increased sIPSC amplitude. However, for those cells displaying high initial sIPSC frequency, there was a temporal summation of sIPSCs (the increased sIPSCs overlapped each other) and the actual sIPSC amplitude was either unchanged or slightly reduced. The summarized data indicate that there is no significant difference in CCK-mediated change in sIPSC amplitude (Fig. 1F).
Figure 1. Bidirectional modulation of sIPSC frequency by CCK in dentate gyrus granule cells.
A, sIPSCs recorded from a granule cell before, during and after the application of CCK (0.5 μ m). Application of bicuculline (10 μ m) at the end of the experiment completely blocked sIPSCs, suggesting that the recorded events were mediated by GABAA receptors. B, summarized time course of sIPSC frequency recorded from 11 cells. Note that CCK increased sIPSC frequency to maximal by ∼3 min after the beginning of CCK application, but reduced it at the end of CCK application (20 min), and the depressant effects persisted after washing in CCK-free external solution for 10 min. C, cumulative frequency distribution of sIPSCs before, during and after the application of CCK. Note that within 3–6 min after application of CCK, sIPSC frequency was significantly increased (reduction in intervals), but after washing CCK away, sIPSC frequency was further reduced (increase in intervals). D, CCK slightly increased the amplitude of sIPSCs in this cell, but the averaged data demonstrate no significant difference for sIPSC amplitude during and after the application of CCK (F). E, summarized data for the changes in sIPSC frequency during and after application of CCK at different concentrations. Note that CCK increased sIPSC frequency at all the concentrations tested, but decreased sIPSC frequency after washing at concentrations equal to and higher than 0.25 μ m. F, the averaged amplitude of sIPSCs was not significantly changed during and after the application of CCK.
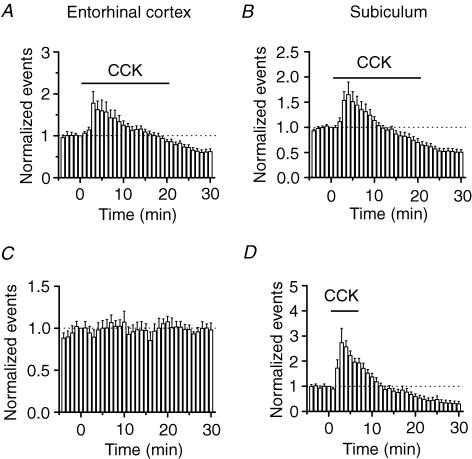
The hippocampal formation includes entorhinal cortex, dentate gyrus, CA3, CA1 and subiculum. Glutamatergic neurons in all these regions receive inhibition from GABAergic interneurons. Since the effects of CCK on GABAergic transmission onto the stratum pyramidale of the hippocampus have been elegantly studied (Miller et al. 1997), we next expanded our studies to other regions of the hippocampal formation. Dentate gyrus granule cells receive perforant pathway fibres from the neurons in layer II of the entorhinal cortex (Steward & Scoville, 1976; Ruth et al. 1982, 1988). We recorded sIPSCs from neurons in layer II of the entorhinal cortex and examined the effects of CCK on the sIPSCs of these neurons. Application of CCK (0.5 μ m) initially increased the frequency of sIPSCs to 178 ± 28% of control values (n = 14, P = 0.015), but subsequently depressed sIPSC frequency to 62 ± 6% of control values (n = 14, P < 0.0001, Fig. 2A). The extrinsic projections of the hippocampus are to the subiculum and then back to the entorhinal cortex. We recorded sIPSCs from neurons in the subiculum. Application of CCK (0.5 μ m) increased sIPSC frequency to 165 ± 25% of control values (n = 14, P = 0.02), followed by a reduction (51 ± 5% of control values, n = 14, P < 0.0001, Fig. 2B). All these data demonstrate that CCK has a biphasic effect on GABAergic transmission: an initial transient increase followed by a persistent reduction. We studied the mechanisms underlying CCK-mediated modulation of sIPSC frequency in the granule cells of the dentate gyrus further because the highest density of CCK receptors is expressed in the dentate gyrus region.
Figure 2. Cholecystokinin-mediated bidirectional modulation of sIPSCs in other regions of the hippocampal formation.
Application of CCK bidirectionally modulated sIPSC frequency recorded from neurons in the entorhinal cortex (A) and the subiculum (B). C, sIPSC frequency was stable in the absence of CCK, suggesting that the CCK-mediated late phase of reduction is not caused by a run-down of the recordings. D, application of CCK for a shorter time (6 min) induced bidirectional modulation of sIPSC frequency as well suggesting that CCK-mediated bidirectional modulation of sIPSC frequency is not caused by long-term application of CCK.
The following lines of evidence indicate that CCK-induced depression is a genuine effect. First, the depressant effect of CCK was not caused by instability of the recordings because sIPSC frequency recorded for 35 min without application of CCK was 98 ± 8% of the initial value (n = 5, P = 0.82, Fig. 2C). Furthermore, mIPSCs recorded using the same internal solution in the presence of TTX were stable after 20 min application of CCK (Fig. 4B). Second, CCK-mediated transient increase in sIPSC frequency was observed at each concentration tested (0.1–1 μ m), with an optimal response at 0.5 μ m (Fig. 1E). Cholecystokinin-induced late phase of depression was observed at concentrations equal to or higher than 0.25 μ m (Fig. 1E), suggesting that it is dose dependent and related to CCK-induced desensitization of CCK receptors. Third, the depressant effect was not caused by long-term application of CCK because application of CCK for a short time (6 min) still transiently increased sIPSC frequency to 273 ± 6% of control values (n = 6, P = 0.03) followed by a depression after 24 min washing in CCK-free external solution (32 ± 10% of control values, n = 6, P = 0.001, Fig. 2D). Because the half-disappearance time for CCK is more than 20 min in the brain (Deschodt-Lanckman et al. 1981), it is suggestive that the CCK-mediated late phase of depression is physiologically significant. To mimic the in vivo physiological condition, we applied CCK for 20 min for the rest of the experiments. We therefore conclude that CCK modulates sIPSCs in a bidirectional manner: an initial transient increase followed by a persistent reduction.
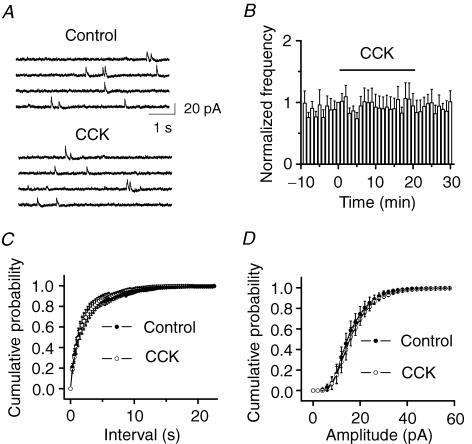
Figure 4. Cholecystokinin does not modulate the frequency and the amplitude of mIPSCs recorded in the presence of TTX.
A, mIPSC current traces recorded from a dentate gyrus granule cell before and during the application of CCK. B, time course of mIPSCs summarized from 6 granule cells. C, cumulative frequency distribution of mIPSCs before and during the application of CCK (n = 6, P = 0.45). D, cumulative amplitude distribution of mIPSCs before and during the application of CCK (n = 6, P = 0.97). Note that CCK changed neither the frequency nor the amplitude of mIPSCs.
CCK increases sIPSC frequency via the activation of CCK-B receptors
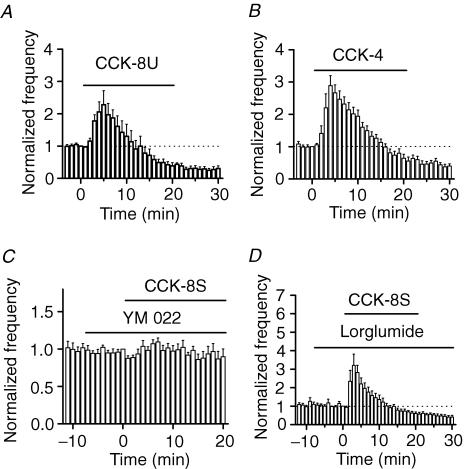
Although CCK-8S is the major form of CCK in the brain (Rehfeld et al. 1985; You et al. 1994), we also examined the effects of the unsulphated CCK octapeptide (CCK-8U) and the tetrapeptide (CCK-4). Application of CCK-8U (0.5 μ m) transiently increased sIPSC frequency to 228 ± 44% of control values (n = 7, P = 0.03) followed by a persistent reduction (30 ± 9% of control values, n = 7, P = 0.0002, Fig. 3A). Application of CCK-4 (0.5 μ m) also increased sIPSC frequency to 289 ± 31% of control values (n = 9, P = 0.0003) within 4 min of its application followed by a reduction (40 ± 7% of control values, n = 9, P < 0.0001, Fig. 3B). Because CCK-8U is a weak agonist for CCK-A receptors, but it is almost as potent as CCK-8S for CCK-B receptors (Wank, 1995), these results suggest that the effects of CCK on modulating sIPSC frequency are mediated by the activation of CCK-B receptors. To further identify the involved type of CCK receptors, we used the selective antagonists for CCK-A or CCK-B receptors. Application of the selective CCK-B receptor inhibitor, YM 022 (1 μ m, Nishida et al. 1994), alone did not significantly alter sIPSC frequency (100 ± 6% of control values, n = 7, P = 0.98, Fig. 3C). In the continuous presence of YM 022, application of CCK failed to significantly increase sIPSC frequency (89 ± 12% of control values, n = 7, P = 0.38, Fig. 3C), suggesting that CCK-B receptors are required for CCK-mediated modulation of sIPSCs. We also used a selective CCK-A receptor inhibitor, lorglumide (1 μ m, de Tullio et al. 1999) to examine the potential roles of CCK-A receptors. However, in the presence of lorglumide (1 μ m), CCK still transiently increased sIPSC frequency to 320 ± 61% of control values (n = 5, P = 0.02, Fig. 3D) followed by a reduction (40 ± 8% of control values, n = 5, P = 0.002, Fig. 3D), suggesting that CCK-A receptors are not involved. All these results unanimously demonstrate that the effects of CCK on sIPSCs are mediated by the activation of CCK-B receptors. This conclusion is also consistent with the previous observation that the CCK receptors in the hippocampus are CCK-B receptor subtype (Shigeyoshi et al. 1994). Because the sulphated CCK is the major form of CCK in the brain (Rehfeld et al. 1985; You et al. 1994), we used the sulphated CCK (CCK-8S) for the rest of the experiments.
Figure 3. Cholecystokinin increases sIPSC frequency via the activation of CCK-B receptors.
A, application of the unsulphated CCK octapeptide (CCK-8U, 0.5 μ m) transiently increased and then reduced sIPSC frequency. B, CCK-4 had the same effect as CCK-8S and CCK-8U. C, the effects of CCK were blocked by the CCK-B receptor antagonist YM 022, suggesting the involvement of CCK-B receptors. D, application of the CCK-A receptor antagonist lorglumide (1 μ m) failed to block the CCK-mediated bidirectional modulation of sIPSC frequency, suggesting that CCK-A receptors are not required.
CCK has no effects on miniature or evoked IPSCs
Several hypotheses could be proposed to explain CCK-mediated initial increases in sIPSCs, as follows: (1) CCK facilitates the generation of action potentials to increase the excitability of GABAergic interneurons to increase GABA release; (2) CCK increases Ca2+ channel activity to increase GABA release; (3) CCK modulates the exocytosis machinery downstream of Ca2+ influx; and (4) CCK increases the functions of postsynaptic GABAA receptors. We recorded the miniature IPSCs (mIPSCs) in the presence of TTX and the evoked IPSCs to test these hypotheses. Cholecystokinin (0.5 μ m) had no effects on either the frequency (control, 0.59 ± 0.18 Hz; CCK, 0.57 ± 0.15 Hz, n = 6, P = 0.69, Fig. 4A, B and C) or the amplitude (control, 21.8 ± 1.3 pA; CCK, 20.3 ± 1.3 pA, n = 6, P = 0.09, Fig. 3A, B and D) of mIPSCs recorded in the presence of TTX (0.5 μ m). Since mIPSCs recorded in the presence of TTX are generally considered to be action potential- and Ca2+-independent, this result suggests that CCK has no effects on either postsynaptic GABAA receptors or exocytosis downstream of Ca2+ influx. Moreover, if CCK acts by directly increasing Ca2+ channel activity, it should equally increase the evoked IPSCs because voltage-gated Ca2+ channels are functional for the evoked IPSCs. We recorded from dentate granule cells GABAA receptor-mediated IPSCs evoked by placing a stimulation electrode in the hilus. However, application of CCK (0.5 μ m) did not significantly change the amplitude of the evoked IPSCs (103 ± 15% of control values, n = 5, P = 0.87, Fig. 5), suggesting that CCK has no effects on presynaptic Ca2+ channels. Because action potentials underlying the evoked IPSCs are generated by exogenous stimulation-induced depolarization, whereas those responsible for spontaneous IPSCs are determined by the intrinsic excitability of neurons, the results that CCK modulates sIPSC frequency without altering the evoked IPSC amplitude suggest that CCK alters the excitability of GABAergic interneurons and regulates the generation of action potentials.
Figure 5. Cholecystokinin does not modulate the evoked IPSCs recorded from granule cells by placing a stimulation electrode in the hilus.
Upper panel shows the evoked IPSCs recorded at different time points in the figure. Lower panel shows the summarized data from 5 cells. Note that CCK failed to change the amplitude of the evoked IPSCs, and application of bicuculline (10 μ m) at the end of the experiments completely inhibited the evoked responses, suggesting that the evoked responses were mediated by GABAA receptors.
CCK modulates the excitability of the interneurons in the hilus
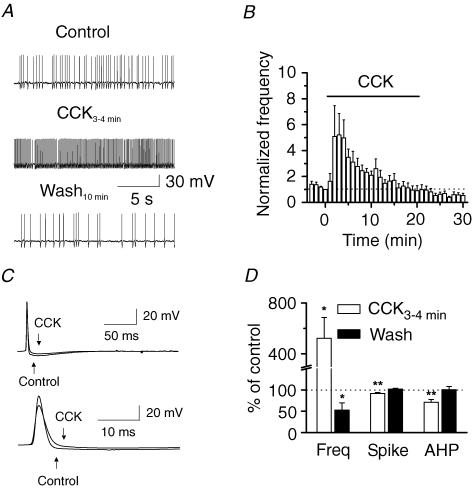
We then tested the hypothesis that CCK modulates GABA release via regulating the excitability (generation of action potentials) of GABAergic interneurons. We directly recorded spontaneous action potentials from the interneurons in the hilus. Caesium gluconate in the recording pipettes was replaced by the same concentration of potassium gluconate, and QX-314 was omitted. For most of the interneurons, application of CCK resulted in a transient increase, followed by a reduction in the frequency of action potential firing (Fig. 6A). However, there were still some interneurons in which the CCK-mediated late phase of reduction in action potential firing was not evident. The summarized data are shown in Fig. 6B and D. Cholecystokinin increased the spontaneous action potential firing frequency to 523 ± 164% of control values (n = 13, P = 0.02, Fig. 6A, B and D). While the action potential firing frequency at the end of CCK application was not significantly reduced to lower than the initial value (CCK after 20 min, 90 ± 39% of control, n = 13, P = 0.8, Fig. 6B), the action potential firing frequency was significantly lower than control values after 10 min washing in CCK-free external solution (53 ± 17% of control values, n = 13, P = 0.02, Fig. 6B and D). The reason underlying the CCK-mediated obvious late phase of depression for sIPSC frequency (Fig. 1B) versus the slight late phase of depression for action potential firing (Fig. 6B) may be that the former is the synchronized results from many interneurons whereas the later is the action of a single interneuron. Nonetheless, these results suggest that CCK regulates GABA release by modulating action potential firing of the interneurons. To detect the changes in action potential shape, we averaged the action potentials recorded before and during the application of CCK (Fig. 6C). Cholecystokinin significantly reduced the amplitudes of both the spike (92 ± 2% of control values, n = 9, P = 0.004, Fig. 6C and D) and the after-hyperpolarization (AHP; 71 ± 7% of control values, n = 9, P = 0.0004, Fig. 6C and D). The CCK-mediated slight reduction in spike amplitude may be due to the inactivation of Na+ channels, because a high frequency of firing is likely to depolarize the membranes and inactivate Na+ channels. The result that CCK reduces the amplitude of the AHP suggests that CCK increases action potential firing frequency by facilitating the achievement of the threshold to fire another action potential.
Figure 6. Cholecystokinin increases the action potential firing frequency of the interneurons and reduces the AHP of action potentials.
A, spontaneous action potentials recorded from an interneuron in the hilus before (top trace), during the application of CCK for 3–4 min (middle trace) and after washing for 10 min (bottome trace). Note that CCK initially increased the action potential firing frequency, but decreased it after washing in CCK-free external solution for 10 min. B, time course of the action potentials recorded from 13 neurons before, during and after the application of CCK. C, averaged action potentials before and during the application of CCK for 3–4 min in reduced (top traces) and enlarged scales (bottom traces). Note that CCK reduced the amplitudes of the spike and AHP. D, summarized data for the effects of CCK on action potential frequency, spike and AHP amplitudes.
CCK inhibits Ca2+-activated K+ channels
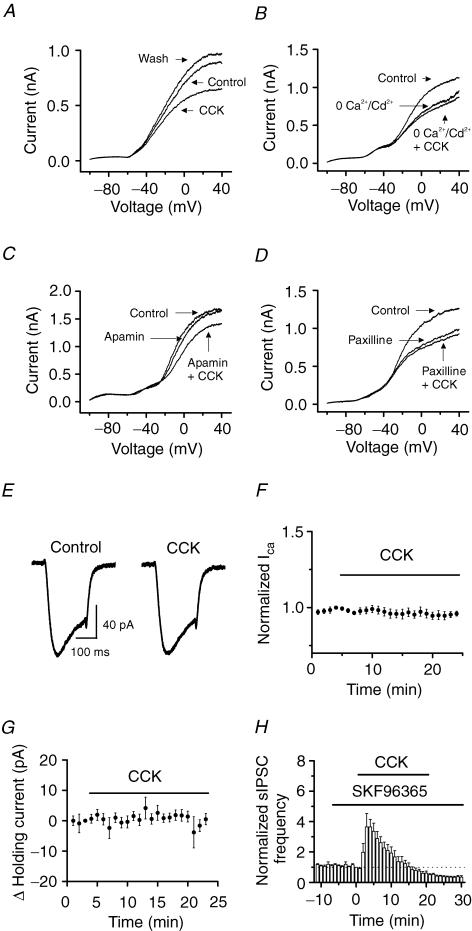
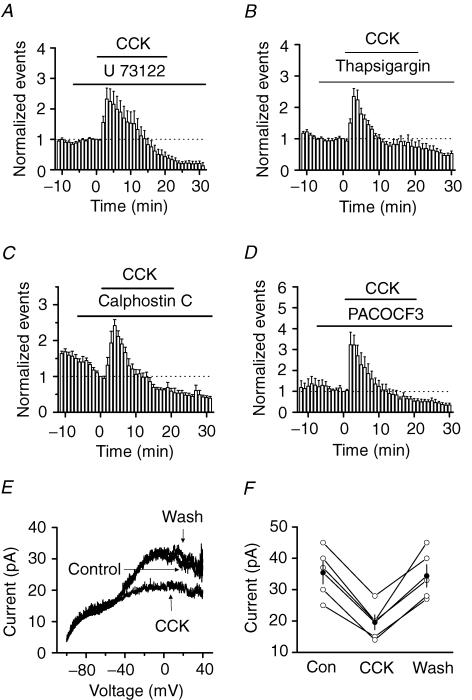
The amplitude of the AHP of the action potentials is determined by Ca2+-activated K+ channels (Storm, 1987). The result that CCK attenuated the amplitude of the AHP suggests that CCK depresses IK(Ca). We therefore next examined the effects of CCK on K+ channels of the interneurons in the hilus. We constructed the voltage–current relationship of the interneurons in the presence of TTX (0.5 μ m) by applying a ramp protocol (Fig. 7A). Application of CCK (0.5 μ m) had no conspicuous effects on the V–I curves at voltages close to or more negative than the resting membrane potential, but significantly reduced the outward current at depolarizing voltages (Fig. 7A). The amplitude of the current at +30 mV was significantly reduced from 1.09 ± 0.15 nA in control conditions to 0.72 ± 0.09 nA (n = 8, P = 0.006) by 3–5 min after the beginning of CCK application. After washing for 10 min in CCK-free external solution, the outward current was slightly but significantly higher than the initial control conditions (control, 1.09 ± 0.15 nA; wash, 1.18 ± 0.16 nA, n = 8, P = 0.02, Fig. 7A). This phenomenon (over-recovery) has also been observed for M-channels after inhibition by muscarine (Marrion et al. 1991; Chen et al. 1993; Tokimasa et al. 1996; Kurennyi et al. 1997), suggesting that some K+ channels share the same properties. We considered that the over-recovery of the outward K+ channels after CCK-mediated inhibition was at least part of the mechanism underlying the CCK-induced late phase of reduction (see Discussion). Cholecystokinin-mediated modulation of the outward current was due to the inhibition of IK(Ca) because when the extracellular solution was switched to a solution containing 0 Ca2+ but 100 μ m Cd2+ to block Ca2+ channels, CCK no longer inhibited the outward currents, although the outward current was reduced in this condition (0 Ca2++ 100 μ m Cd2+, 66 ± 2% of control values; 0 Ca2++ 100 μ m Cd2++ CCK, 67 ± 3% of control values, n = 6, P = 0.6, Fig. 7B). To further confirm the involvement of IK(Ca), we used IK(Ca) inhibitors. Application of the small-conductance IK(Ca) inhibitor, apamin (100 nm), did not apparently change the V–I curve (at +30 mV: control, 1.41 ± 0.13 nA; apamin, 1.40 ± 0.12 nA, n = 7, P = 0.53, Fig. 7C), nor did it block CCK-mediated inhibition of the outward currents (apamin, 1.40 ± 0.12 nA; apamin + CCK, 1.02 ± 0.13 nA, n = 7, P = 0.004, Fig. 7C), suggesting that the small-conductance IK(Ca) are not involved in the effects of CCK on the outward currents. We then used the large-conductance IK(Ca) inhibitors: paxilline and charybdotoxin. Application of paxilline (10 μ m) alone significantly reduced the outward current (control, 898 ± 71 pA; paxilline, 635 ± 89 pA, n = 7, P = 0.0003, Fig. 7D), followed by a slight reduction in the presence of CCK (paxilline, 635 ± 89 pA; paxilline + CCK, 587 ± 85 pA, n = 7, P = 0.04, Fig. 7D). Application of the large-conductance IK(Ca) inhibitor charybdotoxin (50 nm) alone significantly reduced the outward current (control, 876 ± 40 pA; charybdotoxin, 597 ± 36 pA, n = 5, P = 0.0001, data not shown). Subsequent application of CCK in the presence of charybdotoxin failed to significantly inhibit the outward currents further (charybdotoxin, 597 ± 36 pA; charybdotoxin + CCK, 588 ± 29 pA, n = 5, P = 0.38, data not shown). The slightly different effects of CCK in the presence of paxilline or charybdotoxin may be because of their distinct specificities for IK(Ca). Nevertheless, these results suggest that CCK modulates GABA release by regulating the large-conductance IK(Ca) in interneurons.
Figure 7. Cholecystokinin inhibits Ca2+-activated K+ channel currents recorded from hilar interneurons.
A, V–I curve recorded from an interneuron before, during and after the application of CCK. Note that CCK inhibited the amplitude of the outward current at potentials positive to −40 mV and that the outward current was over-recovered after washing in CCK-free extracellular solution. B, V–I curve recorded from an interneuron in the hilus when extracellular solution was switched from 2.5 mm Ca2+ (control) to 0 Ca2+ plus 100 μ m CdCl2 before and during the application of CCK. Note that removal of extracellular Ca2+ and addition of 100 μ m CdCl2 reduced the amplitude of the outward current and that application of CCK failed to change the outward current further in this condition. C, application of apamin (100 nm) failed to block CCK-induced depression of outward current, suggesting that CCK did not inhibit apamin-sensitive Ca2+-activated K+ channels. D, application of paxilline (10 μ m) inhibited the outward current, and subsequent application of CCK failed to change the outward current further, suggesting that CCK inhibited the large-conductance Ca2+-activated K+ channels. E, Ca2+ channel currents recorded from a hilar interneuron when the voltage was changed from −80 to −10 mV for 200 ms before and during the application of CCK. F, summarized data for the effects of CCK on Ca2+ channel currents from 6 cells. Note that CCK did not significantly modulate Ca2+ channel currents. G, CCK failed to change the holding current recorded at −55 mV. The average of the holding current recorded in the last minute before the application of CCK was subtracted to show the changes of the holding current. H, bath application of the receptor-operated cation channel blocker SKF 96365 (50 μ m) failed to block the effects of CCK on sIPSC frequency, suggesting that the effects of CCK on GABA release are unrelated to the functions of cation channels.
Cholecystokinin-mediated inhibition of IK(Ca) could be due to its inhibition of either Ca2+ channels to reduce Ca2+ influx or IK(Ca) channels per se. We next tested the effects of CCK on Ca2+ channels by recording Ca2+ channel currents from hilar interneurons. Cholecystokinin failed to significantly inhibit Ca2+ channels (96 ± 2% of control values, n = 6, P = 0.05, Fig. 7E and F), suggesting that CCK has no significant effects on Ca2+ channels of hilar interneurons. This result also suggests that the inhibitory effects of CCK on IK(Ca) are not mediated by the inhibition of voltage-gated Ca2+ channels, but by a direct modulation of IK(Ca).
Cholecystokinin has also been reported to inhibit K+ channels responsible for the control of resting membrane potentials (Branchereau et al. 1993; Cox et al. 1995; Miller et al. 1997) and to open cation channels to generate membrane depolarization (Wu & Wang, 1996a, b; Wang & Sims, 1998; Chakfe & Bourque, 2000, 2001). Either of these mechanisms would depolarize the membrane to change the holding current when the cells are held at voltages close to the resting membrane potentials. We next tested whether these mechanisms are involved by recording the holding currents at −55 mV, a potential close to the resting membrane potential. The extracellular solution contained (μ m): 0.5 TTX, 10 DNQX, 100 dl-APV and 10 bicuculline. However, CCK failed to change the holding currents (n = 8, P = 0.28, Fig. 7G), suggesting that it is unlikely that CCK-mediated increases in GABA release are caused by a change in the resting membrane potentials. We also tested the roles of receptor-operated cation channels by applying SKF 96365, a broadly used blocker of cation channels (Merritt et al. 1990). This compound blocks CCK-A receptor-mediated inward current (Tsujino et al. 2005). Application of SKF 96365 (50 μ m) alone did not significantly change sIPSC frequency (94 ± 6% of control values, n = 6, P = 0.3, Fig. 7H), nor did it block the effects of CCK on sIPSC frequency (CCK at 3 min, 365 ± 9% of control values, n = 6, P = 0.03; CCK at 20 min, 61 ± 13% of control values, n = 6, P = 0.03, Fig. 7H), suggesting that cation channels are unlikely to be involved. These results suggest that CCK-mediated increases in GABA release are unlikely to be caused by an inhibition of K+ channels responsible for the resting membrane potentials or opening of cation channels in the dentate gyrus region.
Inhibition of IK(Ca) inhibits the effects of CCK on GABA release
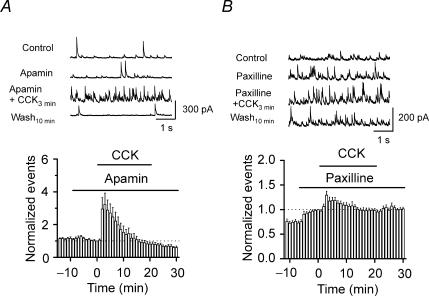
If IK(Ca) channels are responsible for CCK-mediated modulation of GABA release, application of IK(Ca) inhibitors should block or at least reduce the effects of CCK on sIPSC frequency. Therefore, we next tested the effects of IK(Ca) inhibitors on CCK-mediated changes in sIPSC frequency. Application of apamin (100 nm) alone did not significantly alter sIPSC frequency (90 ± 8% of control values, n = 7, P = 0.27, Fig. 8A) nor did it block the effects of CCK on sIPSCs (CCK at 3 min, 323 ± 69% of control values, n = 7, P = 0.02; wash at 10 min, 60 ± 9% of control values, n = 7, P = 0.004, Fig. 8A), suggesting that the small-conductance IK(Ca) is not involved in the effects of CCK on GABA release. However, application of paxilline (10 μ m) significantly increased sIPSC frequency to 142 ± 9% of control values (n = 10, P = 0.001, Fig. 8B), suggesting that the large-conductance IK(Ca) channels are involved in modulating GABA release. Furthermore, in the presence of paxilline, application of CCK only slightly increased sIPSC frequency to 129 ± 8% of control values (n = 10, P = 0.004, Fig. 8B) without significantly depressing the late phase of sIPSC frequency (95 ± 4% of control values, n = 10, P = 0.2, Fig. 8B), suggesting that the large-conductance IK(Ca) channels are required for the effects of CCK on GABA release. Similarly, application of charybdotoxin (50 nm), another large-conductance IK(Ca) channel inhibitor, significantly increased sIPSC frequency to 162 ± 20% of control values (n = 8, P = 0.02). However, coapplication of charybdotoxin and CCK only slightly increased sIPSC frequency to 127 ± 6% of control values (n = 8, P = 0.002, data not shown), but blocked the late phase of depression (80 ± 16% of control values, n = 8, P = 0.26, data not shown). Together, these data suggest that the large-conductance IK(Ca) channels are required for the effects of CCK on GABA release.
Figure 8. The large-conductance Ca2+-activated K+ channels are involved in CCK-mediated modulation of GABA release.
A, application of apamin (100 nm) failed to alter the CCK-induced change in GABA release. B, application of paxilline (10 μ m) alone increased sIPSC frequency and significantly inhibited the CCK-induced change in sIPSC frequency.
Phospholipase C, intracellular Ca2+ release, PKC and PLA2 are not required for CCK-mediated increases in GABA release
We next explored the signal transduction mechanisms underlying CCK-mediated modulation of GABA release. Activation of CCK-B receptors is coupled to PLC to generate two intracellular second messengers, IP3 to interact with IP3 receptors to increase intracellular Ca2+ release and diacylglycerol to activate PKC. We tested the roles of this pathway in CCK-mediated increases in GABA release. Application of the specific PLC inhibitor U 73122 (30 μ m) did not block the CCK-mediated initial increase (232 ± 36% of control values, n = 8, P = 0.008, Fig. 9A) or subsequent reduction in sIPSC frequency (43 ± 15% of control values, n = 8, P = 0.01, Fig. 9A), suggesting that the activity of PLC is not required for the effects of CCK. We also examined the roles of intracellular Ca2+ release and PKC in CCK-mediated modulation of GABA release. Bath application of thapsigargin (10 μ m) did not block the CCK-mediated initial increase in sIPSC frequency (212 ± 22% of control values, n = 7, P = 0.002, Fig. 9B), nor did it block the CCK-induced late phase of depression (wash for 10 min, 46 ± 7% of control values, n = 7, P = 0.0004, Fig. 9B), suggesting that intracellular Ca2+ release is not required for the effects of CCK.
Figure 9. Cholecystokinin-mediated modulation of GABA release is not dependent on PLC, intracellular Ca2+ release, PKC and PLA2.
A, application of the PLC inhibitor U 73122 (30 μ m) failed to block the effects of CCK on GABA release. B, application of thapsigargin (10 μ m) failed to block the effects of CCK on GABA release, suggesting that intracellular Ca2+ release is not required for the effects of CCK. C, application of the PKC inhibitor calphostin C (0.5 μ m) inhibited the basal level of sIPSC frequency, but failed to block the effects of CCK. D, application of the PLA2 inhibitor PACOCF3 did not block the effects of CCK on GABA release. E, CCK inhibited the outward current in an outside-out nucleated patch excised from an interneuron in the hilus, suggesting a direct coupling between G proteins and IK(Ca). F, summarized data from 5 outside-out nucleated patches.
We then applied calphostin C, a broad-spectrum protein kinase C inhibitor, to probe the roles of protein kinase C in CCK-mediated modulation of sIPSCs. Application of calphostin C (0.5 μ m) alone significantly reduced the frequency of sIPSCs (68 ± 4% of control values, n = 6, P = 0.001, Fig. 9C), suggesting that PKC is involved in the modulation of sIPSCs. However, in the presence of calphostin C, CCK still transiently increased (242 ± 17% of control values, n = 6, p = 0.0004, Fig. 9C) and subsequently reduced sIPSC frequency (59 ± 8% of control values at the end of CCK application, n = 6, P = 0.004, Fig. 9C). These results suggest that PKC activity is not involved in CCK-mediated modulation of GABA release. Because there are so many different isoforms of PKC and calphostin C is a broad-spectrum PKC inhibitor, the results that calphostin C inhibited the basal sIPSC frequency without blocking the effects of CCK suggest that the PKC isoform activated by CCK, if any, is not involved in modulating GABA release, although the activities of other PKC isoforms can increase GABA release.
While the PLC pathway is the major intracellular pathway activated by CCK, CCK-B receptor activation is also coupled to PLA2 (Pommier et al. 1999, 2003). We next examined the effects of PLA2 on CCK-mediated modulation of GABA release. Application of the specific PLA2 inhibitor PACOCF3 (Ackermann et al. 1995; 100 μ m) did not block the CCK-mediated initial increase in sIPSC frequency (CCK at 3 min, 322 ± 47% of control values, n = 5, P = 0.009, Fig. 9D) nor did it block the CCK-mediated late phase of reduction (CCK at 20 min, 53 ± 13% of control values, n = 5, P = 0.02; wash at 10 min, 35 ± 9% of control values, n = 5, P = 0.002, Fig. 9D), suggesting that the functions of PLA2 are not required for the effects of CCK on GABA release.
Since none of the inhibitors blocked the effects of CCK, we next tested a hypothesis that the effects of CCK are mediated by direct coupling of G proteins from CCK-B receptors to IK(Ca) without requiring intracellular second messengers. We used outside-out nucleated patches excised from the interneurons in the hilus to test this hypothesis. We reasoned that if intracellular second messengers are required, CCK should not change the V–I curve on outside-out patches when the integrity of intracellular signals is demolished after excision from interneurons. However, application of CCK still inhibited the outward current to 55 ± 3% of control values (at 0 mV, P < 0.001, n = 5, Fig. 9E and F), suggesting that no intracellular second messengers are required for the effects of CCK on GABA release. However, unlike the results of whole cell recordings (Fig. 7A), the outward current recorded from nucleated patches after washing in CCK-free solution was not significantly higher than the initial control values. The possible reason for the lack of over-recovery in nucleated patches is that the properties of IK(Ca) are changed after excision from the cell membrane. Nonetheless, this result further confirmed that direct coupling of G proteins from CCK-B receptors to IK(Ca) is responsible for CCK-mediated modulation of GABA release.
Developmental alteration of CCK-mediated modulation of GABA release
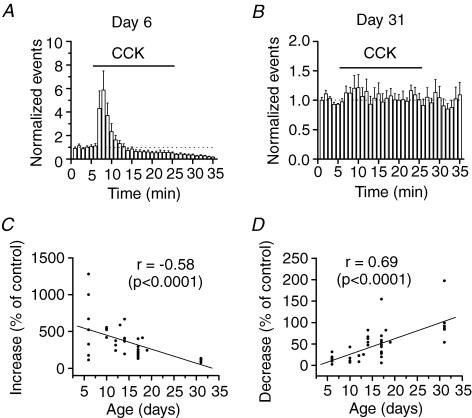
While most of the experiments were conducted on brain slices from 10- to 20-day-old rats because animals of this age range generate the best slices, we also expanded our experiments to include 6- and 31-day-old animals. While CCK still transiently increased, and then persistently reduced GABA release in 6-day-old animals (Fig. 10A), it had no significant effects on GABA release from 31-day-old animals (Fig. 10B), suggesting that its modulation of GABA release is developmentally regulated. We then retrospectively examined our data versus the animal ages recorded (Fig. 10C and D). The CCK-induced transient increase in sIPSC frequency was negatively correlated with the rat age (r = −0.58, P < 0.0001, Fig. 10C), but the CCK-induced late phase of reduction in GABA release was positively correlated with the animal age (r = 0.69, P < 0.0001, Fig. 10D). These results strongly indicate that CCK-mediated bidirectional modulation of GABA release is developmentally regulated and is more evident in juvenile animals.
Figure 10. Developmental alteration of CCK-mediated modulation of GABA release.
A, summarized time course of sIPSC frequency before, during and after the application of CCK from 7 dentate gyrus granule cells of 6-day-old rats. Note that CCK transiently increased sIPSC frequency, followed by a persistent reduction. B, summarized time course of sIPSC frequency before, during and after the application of CCK from 6 dentate gyrus granule cells of 31-day-old rats. Note that CCK did not significantly change sIPSC frequency. C, the CCK-mediated increase in sIPSC frequency was negatively correlated with the corresponding ages of the rats. D, the CCK-mediated late phase of depression in sIPSC frequency was positively correlated with the corresponding ages of the rats.
Discussion
In the present study, we have examined the effects of CCK on GABAergic synaptic transmission in the hippocampal formation. Our results demonstrate that CCK produces a bidirectional modulation of GABA release: an initial transient increase followed by a reduction. The CCK-mediated initial increase in GABA release is caused by an inhibition of IK(Ca) in GABAergic interneurons, leading to a reduction in the amplitude of after-hyperpolarization of action potentials that facilitates the achievement of action potential firing threshold. Cholecystokinin-mediated facilitation of action potential generation in GABAergic interneurons increases GABA release. The CCK-induced late phase of reduction may be caused by the over-recovery of IK(Ca) after activation of CCK-B receptors. The effects of CCK on GABA release are independent of intracellular second messengers (PLC, IP3, PKC and PLA2), suggesting a direct coupling of G-proteins and IK(Ca).
Ionic mechanisms underlying CCK-mediated modulation of GABA release
The magnitude and duration of the AHP are important factors in determining interspike interval and, thereby, neuronal firing rate. Our results indicate that CCK inhibits the amplitude of AHP of the interneurons in the hilus to increase action potential firing rate and GABA release. Consistent with our results, a variety of neurotransmitters (Madison & Nicoll, 1982; Pedarzani & Storm, 1995; Cloues & Sather, 2003) including neuropeptides (Jassar et al. 1999; Ogawa et al. 2005) increase action potential firing rate by depressing the AHP of action potentials. Since the AHP of the action potentials is determined by IK(Ca), we also explored the effects of CCK on IK(Ca) by using inhibitors for two types of IK(Ca) channels. Our results demonstrate that the apamin-sensitive small-conductance IK(Ca) channel does not participate in the modulation of GABA release because application of apamin alone did not change sIPSC frequency (Fig. 8A). Furthermore, the apamin-sensitive small-conductance IK(Ca) channel is unlikely to be involved in CCK-mediated modulation of GABA release because application of apamin failed to alter the effects of CCK on sIPSC frequency. However, our results demonstrate that the functions of the large-conductance IK(Ca) channel underlie CCK-mediated modulation of GABA release because the effects of CCK on sIPSC frequency were inhibited by application of the inhibitors for the large-conductance IK(Ca) channel. Consistent with our results, the large-conductance IK(Ca) channel has been reported to control the excitability of dentate gyrus (Brenner et al. 2005) and transmitter release at CA3–CA3 synapses (Raffaelli et al. 2004). More interestingly, IK(Ca) has been shown to be inhibited by a variety of neuropeptides including CCK in CA1 pyramidal neurons (Shinohara & Kawasaki, 1997), neurotensin in acutely dissociated neurons from the diagonal band of Broca (Jassar et al. 1999) and in neurons of the solitary tract nucleus (Ogawa et al. 2005) and tachykinins in NG 108-15 cells (Phenna et al. 1996). Added to this spectrum is our study showing that CCK inhibits IK(Ca) in interneurons where IK(Ca) controls spontaneous firing patterns (Goldberg & Wilson, 2005). There are two possible modes by which CCK could inhibit IK(Ca). First, CCK could inhibit Ca2+ channels to reduce Ca2+ influx, resulting in a reduction in IK(Ca). Second, CCK could directly inhibit IK(Ca) without affecting the functions of Ca2+ channels. The result that application of CCK failed to change the amplitude of Ca2+ channel currents recorded from the interneurons suggests that CCK acts by directly inhibiting IK(Ca) without modulating Ca2+ channels of the interneurons.
While our results demonstrate that the IK(Ca) channel is the major ion channel involved in CCK-mediated modulation of GABA release in the dentate gyrus region, CCK-mediated increases in GABA release in the CA1 region are related to inhibition of a resting K+ conductance (Miller et al. 1997). In fact, CCK suppresses both the resting K+ conductance and IK(Ca) in the CA1 region (Shinohara & Kawasaki, 1997). However, our results do not support a role for the inhibition of a resting K+ conductance on CCK-mediated modulation of GABA release in the dentate gyrus region because CCK had absolutely no effects on the holding current recorded from the hilar interneurons when the membrane was held at −55 mV. In fact, in both CA1 pyramidal neurons (Shinohara & Kawasaki, 1997) and hilar interneurons (Fig. 7), the major effect of CCK on the V–I curve is not within the voltages close to the resting membrane potential, but at more positive (> −40 mV) voltages, suggesting a predominantly inhibitory role on IK(Ca). Taken together, these results suggest that CCK controls GABA release by interacting with distinct ion channels in different brain regions.
In addition to modulating K+ channels, CCK has also been reported to activate cation channels in supraoptic nucleus neurons (Chakfe & Bourque, 2000, 2001) and acutely dissociated rat neostriatal neurons (Wu & Wang, 1996a, b). However, our results do not support a role for cation channels in CCK-mediated modulation of GABA release in the dentate gyrus region based on the following observations. First, CCK failed to change the holding current when the cell membrane was held at −55 mV, at which both cation channels and the K+ channels responsible for the resting membrane potentials are supposed to open. Second, application of the receptor-operated cation channel inhibitor SKF 96365 did not block the effects of CCK on sIPSCs. While our results do not support the involvement of cation channels and resting K+ channels, we cannot rule out the participation of other unidentified channels in CCK-mediated increases in GABA release because application of IK(Ca) inhibitors did not completely block CCK-mediated increases in sIPSC frequency. It is possible that CCK may have minor effects on other channels that participate in the modulation of GABA release. Further efforts are required to identify those channels.
While the mechanisms underlying the CCK-induced late phase of depression have not been completely elucidated, our results suggest that the over-recovery of IK(Ca) after CCK-induced depression is at least part of the mechanisms (Fig. 7A). The over-recovery of IK(Ca) can elevate the functions of IK(Ca) to increase the AHP, and to decrease action potential firing and GABA release. While we have observed a significant increase in IK(Ca) after washing out CCK (Fig. 7A), we failed to see statistically significant over-recovery of the AHP amplitude of action potentials (Fig. 6D), although we indeed observed that in some cells the AHP amplitudes after washing were larger than control values. The possible reason for this discrepancy is that the change in AHP amplitude is so subtle that it could not reliably be detected after averaging the action potentials. Interestingly, over-recovery of M-channel currents has also been observed after agonist-induced inhibition, and it is related to agonist-induced intracellular Ca2+ release (Marrion et al. 1991; Chen et al. 1993; Tokimasa et al. 1996; Kurennyi et al. 1997). Whether intracellular Ca2+ release underlies CCK-induced over-recovery of IK(Ca) remains to be determined.
Signalling mechanisms underlying CCK-induced modulation of GABA release
Our results demonstrate that CCK-B receptors are required for the effects of CCK on GABA release, consistent with the observations that CCK-B receptors are predominantly distributed in the hippocampus (Shigeyoshi et al. 1994) and that activation of CCK-B receptors increases GABA release from the anterior nucleus accumbens (Lanza & Makovec, 2000) and cerebral cortex (Ferraro et al. 1999; Siniscalchi et al. 2003). The next question is how activation of CCK-B receptors leads to the inhibition of IK(Ca) to increase GABA release. We first examined the roles of the second messengers coupled to CCK-B receptors, and our results do not support the involvement of any known second messengers in CCK-mediated modulation of GABA release because application of the inhibitors for PLC, intracellular Ca2+ release, PKC or PLA2 failed to block the effects of CCK on GABA release. Since G protein-coupled receptors modulate ion channels via either intracellular second messengers or direct G protein coupling, these results support a direct coupling of G proteins to CCK-B receptors and IK(Ca) channels. This notion is further supported by the result that application of CCK to the outside-out nucleated patches in which the intracellular second messengers are supposed to be disintegrated still led to an inhibition of the outward currents. Further evidence to support a direct coupling of G proteins and IK(Ca) is the time course of the effects of CCK on sIPSCs, because application of CCK increases sIPSCs frequency to the maximum in a short time (∼3 min), a time course too brief for second messengers to be involved. Consistent with our conclusion is the evidence showing that G proteins directly interact with IK(Ca) (Walsh et al. 1996). However, it is at present unclear whether G proteins modulate IK(Ca) via direct interaction with IK(Ca) channels or indirectly via other proteins associated with IK(Ca) channels. Further biochemical experiments are required to elucidate the interaction of G proteins and IK(Ca) channels in the interneurons.
Developmental modulation and physiological significance
We have also demonstrated that the effect of CCK on GABA release undergoes developmental modulation. The CCK-mediated initial increase and late phase of reduction are restricted to juvenile rats, but disappear when the animals are older (> 31 days). Presently, the mechanisms underlying the developmental modulation of the effects of CCK are unknown. Developmental changes in the properties of CCK-B receptors, G proteins and IK(Ca) may explain the distinct effects of CCK in animals of different ages.
There are inconsistent results regarding whether CCK modulates GABA release in different brain regions. Cholecystokinin has been reported to increase GABA release in cerebral cortex (You et al. 1997; Ferraro et al. 1999; Siniscalchi et al. 2003), neostriatum (You et al. 1996), anterior nucleus accumbens (Lanza & Makovec, 2000) and hippocampus (Perez de la Mora et al. 1993; Miller et al. 1997). By contrast, CCK has also been reported not to change GABA release in the frontal-parietal cortex (Hickling et al. 1997) and hippocampus (Migaud et al. 1994; Breukel et al. 1997). While the reasons underlying these controversies are unknown, CCK-mediated bidirectional modulation of GABA release may be relevant because most of those experiments were conducted by measuring the extracellular GABA concentration, which may reflect a neutralized effect of CCK on GABA release. Depending on the time when the samples were taken, it is likely that the CCK-mediated initial increase is attenuated by the late phase of reduction in GABA release, and the neutralized result would be no change in GABA release after application of CCK. Our results therefore provide a resolution to solve those controversies.
CCK has been implicated in modulating a variety of important brain functions including satiety, analgesia, learning and memory processes, and in neuropsychiatric disorders such as anxiety and panic attack (Sebret et al. 1999). GABAergic synaptic transmission underlies almost all of those physiological functions and neuropsychiatric disorders. For example, CCK–GABA interaction in the hippocampus has been demonstrated to elevate anxiety (Rezayat et al. 2005), which may be related to CCK-induced persistent reduction in GABA release. The present study is therefore likely to provide a novel cellular mechanism to explain the roles of CCK in these neuropsychiatric disorders and the physiological functions as well.
Acknowledgments
This work was supported by National Institutes of Health, National Center for Research Resources Grant 5P20RR017699-02.
References
- Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. Cholecystokinin in the central nervous system: a minireview. Neuropeptides. 1983;3:411–427. doi: 10.1016/0143-4179(83)90032-x. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ. The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res. 1981;212:51–57. doi: 10.1016/0006-8993(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Champagnat J, Denavit-Saubie M. Cholecystokinin-gated currents in neurons of the rat solitary complex in vitro. J Neurophysiol. 1993;70:2584–2595. doi: 10.1152/jn.1993.70.6.2584. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Breukel AI, Lopes da Silva FH, Ghijsen WE. Cholecystokinin (CCK-8) modulates vesicular release of excitatory amino acids in rat hippocampal nerve endings. Neurosci Lett. 1997;234:67–70. doi: 10.1016/s0304-3940(97)00678-2. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Bourque CW. Excitatory peptides and osmotic pressure modulate mechanosensitive cation channels in concert. Nat Neurosci. 2000;3:572–579. doi: 10.1038/75744. [DOI] [PubMed] [Google Scholar]
- Chakfe Y, Bourque CW. Peptidergic excitation of supraoptic nucleus neurons: involvement of stretch-inactivated cation channels. Exp Neurol. 2001;171:210–218. doi: 10.1006/exnr.2001.7780. [DOI] [PubMed] [Google Scholar]
- Chen H, Kurenny DE, Smith PA. Heparin prevents M-current over-recovery but not M-current suppression in bullfrog sympathetic ganglion neurones. Brain Res. 1993;625:323–327. doi: 10.1016/0006-8993(93)91075-4. [DOI] [PubMed] [Google Scholar]
- Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci. 2003;23:1593–1604. doi: 10.1523/JNEUROSCI.23-05-01593.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol. 1995;74:990–1000. doi: 10.1152/jn.1995.74.3.990. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Deschodt-Lanckman M, Bui ND, Noyer M, Christophe J. Degradation of cholecystokinin-like peptides by a crude rat brain synaptosomal fraction: a study by high pressure liquid chromatography. Regul Pept. 1981;2:15–30. doi: 10.1016/0167-0115(81)90062-8. [DOI] [PubMed] [Google Scholar]
- de Tullio P, Delarge J, Pirotte B. Recent advances in the chemistry of cholecystokinin receptor ligands (agonists and antagonists) Curr Med Chem. 1999;6:433–455. [PubMed] [Google Scholar]
- Ferraro L, Beani L, Trist D, Reggiani A, Bianchi C. Effects of cholecystokinin peptides and GV 150013, a selective cholecystokininB receptor antagonist, on electrically evoked endogenous GABA release from rat cortical slices. J Neurochem. 1999;73:1973–1981. [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci. 2005;25:10230–10238. doi: 10.1523/JNEUROSCI.2734-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood RS, Godar SE, Reaves TA, Jr, Hayward JN. Cholecystokinin in hippocampal pathways. J Comp Neurol. 1981;203:335–350. doi: 10.1002/cne.902030303. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Jones EG. Morphology of synapses formed by cholecystokinin-immunoreactive axon terminals in region superior of rat hippocampus. Neuroscience. 1985;16:57–68. doi: 10.1016/0306-4522(85)90047-8. [DOI] [PubMed] [Google Scholar]
- Hickling YM, Cheung NS, Larm JA, Cowen MS, Shulkes A, Beart PM. Cholecystokinin-GABA interactions in rodent cortex: analyses of cholecystokinin effects on K+- and l-glutamate-induced release of [3H]GABA from rat cortical slices and cultured mouse cortical neurones. Neurochem Int. 1997;30:171–179. doi: 10.1016/s0197-0186(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Hill DR, Campbell NJ, Shaw TM, Woodruff GN. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DR, Shaw TM, Graham W, Woodruff GN. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci. 1990;10:1070–1081. doi: 10.1523/JNEUROSCI.10-04-01070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Correa FM, Uhl GR, Schneider B, Snyder SH. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979;76:521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagerschmidt A, Guillaume N, Goudreau N, Maigret B, Roques BP. Mutation of Asp100 in the second transmembrane domain of the cholecystokinin B receptor increases antagonist binding and reduces signal transduction. Mol Pharmacol. 1995;48:783–789. [PubMed] [Google Scholar]
- Jassar BS, Harris KH, Ostashewski PM, Jhamandas JH. Ionic mechanisms of action of neurotensin in acutely dissociated neurons from the diagonal band of Broca of the rat. J Neurophysiol. 1999;81:234–246. doi: 10.1152/jn.1999.81.1.234. [DOI] [PubMed] [Google Scholar]
- Kohler C, Chan-Palay V. Cholecystokinin-octapeptide (CCK-8) receptors in the hippocampal region: a comparative in vitro autoradiographic study in the rat, monkey and the postmortem human brain. Neurosci Lett. 1988;90:51–56. doi: 10.1016/0304-3940(88)90785-9. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Innis RB, Goldman-Rakic PS. Regional distribution of cholecystokinin receptors in macaque medial temporal lobe determined by in vitro receptor autoradiography. J Comp Neurol. 1988;276:219–230. doi: 10.1002/cne.902760206. [DOI] [PubMed] [Google Scholar]
- Kurennyi DE, Chen H, Smith PA. Low concentrations of muscarine potentiate M-current in bullfrog sympathetic B-neurones. J Auton Nerv Syst. 1997;67:89–96. doi: 10.1016/s0165-1838(97)00103-3. [DOI] [PubMed] [Google Scholar]
- Lanza M, Makovec F. Cholecystokinin (CCK) increases GABA release in the rat anterior nucleus accumbens via CCKB receptors located on glutamatergic interneurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:33–38. doi: 10.1007/s002109900161. [DOI] [PubMed] [Google Scholar]
- Lei S, McBain CJ. GABAB receptor modulation of excitatory and inhibitory synaptic transmission onto rat CA3 hippocampal interneurons. J Physiol. 2003;546:439–453. doi: 10.1113/jphysiol.2002.034017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Orser BA, Thatcher GR, Reynolds JN, MacDonald JF. Positive allosteric modulators of AMPA receptors reduce proton-induced receptor desensitization in rat hippocampal neurons. J Neurophysiol. 2001;85:2030–2038. doi: 10.1152/jn.2001.85.5.2030. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982;299:636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Zucker RS, Marsh SJ, Adams PR. Modulation of M-current by intracellular Ca2+ Neuron. 1991;6:533–545. doi: 10.1016/0896-6273(91)90056-6. [DOI] [PubMed] [Google Scholar]
- Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, et al. SKF 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Roques BP, Durieux C. Effects of cholecystokinin octapeptide and BC 264, a potent and selective CCK-B agonist on aspartate and glutamate release from rat hippocampal slices. Neuropharmacology. 1994;33:737–743. doi: 10.1016/0028-3908(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Miller KK, Hoffer A, Svoboda KR, Lupica CR. Cholecystokinin increases GABA release by inhibiting a resting K+ conductance in hippocampal interneurons. J Neurosci. 1997;17:4994–5003. doi: 10.1523/JNEUROSCI.17-13-04994.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran TH, Robinson PH, Goldrich MS, McHugh PR. Two brain cholecystokinin receptors: implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- Mutt V, Jorpes JE. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968;6:156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Nishida A, Miyata K, Tsutsumi R, Yuki H, Akuzawa S, et al. Pharmacological profile of (R)-1-[2,3-dihydro-1-(2′-methylphenacyl)-2-oxo-5-phenyl-1H-1,4-benzodiazepin-3-yl]-3-(3-methylphenyl)urea (YM022), a new potent and selective gastrin/cholecystokinin-B receptor antagonist, in vitro and in vivo. J Pharmacol Exp Ther. 1994;269:725–731. [PubMed] [Google Scholar]
- Noble F, Roques BP. CCK-B receptor: chemistry, molecular biology, biochemistry and pharmacology. Prog Neurobiol. 1999;58:349–379. doi: 10.1016/s0301-0082(98)00090-2. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Gorio A, Milan F, Freund TF, Somogyi P, Smith AD. Cholecystokinin-immunoreactive cells form symmetrical synaptic contacts with pyramidal and nonpyramidal neurons in the hippocampus. J Comp Neurol. 1985;237:485–505. doi: 10.1002/cne.902370406. [DOI] [PubMed] [Google Scholar]
- Ogawa WN, Baptista V, Aguiar JF, Varanda WA. Neurotensin modulates synaptic transmission in the nucleus of the solitary tract of the rat. Neuroscience. 2005;130:309–315. doi: 10.1016/j.neuroscience.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Dopamine modulates the slow Ca2+-activated K+ current IAHP via cyclic AMP-dependent protein kinase in hippocampal neurons. J Neurophysiol. 1995;74:2749–2753. doi: 10.1152/jn.1995.74.6.2749. [DOI] [PubMed] [Google Scholar]
- Perez de la Mora M, Hernandez-Gomez AM, Mendez-Franco J, Fuxe K. Cholecystokinin-8 increases K+-evoked [3H] gamma-aminobutyric acid release in slices from various brain areas. Eur J Pharmacol. 1993;250:423–430. doi: 10.1016/0014-2999(93)90029-h. [DOI] [PubMed] [Google Scholar]
- Phenna S, Carpenter E, Peers C, Maudsley S, Gent JP. Inhibition of Ca2+-sensitive K+ currents in NG 108–15 cells by substance P and related tachykinins. Br J Pharmacol. 1996;119:315–320. doi: 10.1111/j.1476-5381.1996.tb15988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier B, Da Nascimento S, Dumont S, Bellier B, Million E, Garbay C, et al. The cholecystokininB receptor is coupled to two effector pathways through pertussis toxin-sensitive and -insensitive G proteins. J Neurochem. 1999;73:281–288. doi: 10.1046/j.1471-4159.1999.0730281.x. [DOI] [PubMed] [Google Scholar]
- Pommier B, Marie-Claire C, Da Nascimento S, Wang HL, Roques BP, Noble F. Further evidence that the CCK2 receptor is coupled to two transduction pathways using site-directed mutagenesis. J Neurochem. 2003;85:454–461. doi: 10.1046/j.1471-4159.2003.01690.x. [DOI] [PubMed] [Google Scholar]
- Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3–CA3 synapses in the rat hippocampus. J Physiol. 2004;557:147–157. doi: 10.1113/jphysiol.2004.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld JF. Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem. 1978;253:4022–4030. [PubMed] [Google Scholar]
- Rehfeld JF, Hansen HF, Marley PD, Stengaard-Pedersen K. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Ann N Y Acad Sci. 1985;448:11–23. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B. Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol Behav. 2005;84:775–782. doi: 10.1016/j.physbeh.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Ruth RE, Collier TJ, Routtenberg A. Topography between the entorhinal cortex and the dentate septotemporal axis in rats. I. Medial and intermediate entorhinal projecting cells. J Comp Neurol. 1982;209:69–78. doi: 10.1002/cne.902090107. [DOI] [PubMed] [Google Scholar]
- Ruth RE, Collier TJ, Routtenberg A. Topographical relationship between the entorhinal cortex and the septotemporal axis of the dentate gyrus in rats. II. Cells projecting from lateral entorhinal subdivisions. J Comp Neurol. 1988;270:506–516. doi: 10.1002/cne.902700404. [DOI] [PubMed] [Google Scholar]
- Sebret A, Lena I, Crete D, Matsui T, Roques BP, Dauge V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J Neurosci. 1999;19:7230–7237. doi: 10.1523/JNEUROSCI.19-16-07230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeyoshi Y, Okamura H, Inatomi T, Matsui T, Ito M, Kaji H, et al. Distribution of mRNA for CCK-B receptor in the brain of Mastomys natalensis: abundant expression in telencephalic neurons. Brain Res. 1994;640:81–92. doi: 10.1016/0006-8993(94)91859-7. [DOI] [PubMed] [Google Scholar]
- Shinohara S, Kawasaki K. Desensitization of cholecystokininB receptors in GH3 cells. J Neurochem. 1994;62:1352–1356. doi: 10.1046/j.1471-4159.1994.62041352.x. [DOI] [PubMed] [Google Scholar]
- Shinohara S, Kawasaki K. Electrophysiological changes in rat hippocampal pyramidal neurons produced by cholecystokinin octapeptide. Neuroscience. 1997;78:1005–1016. doi: 10.1016/s0306-4522(96)00653-7. [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Rodi D, Cavallini S, Marino S, Ferraro L, et al. Effects of cholecystokinin tetrapeptide (CCK4) and of anxiolytic drugs on GABA outflow from the cerebral cortex of freely moving rats. Neurochem Int. 2003;42:87–92. doi: 10.1016/s0197-0186(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Hodgson AJ, Smith AD, Nunzi MG, Gorio A, Wu JY. Different populations of GABAergic neurons in the visual cortex and hippocampus of cat contain somatostatin- or cholecystokinin-immunoreactive material. J Neurosci. 1984;4:2590–2603. doi: 10.1523/JNEUROSCI.04-10-02590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T, Simmons MA, Schneider CR, Akasu T. Hyperpolarizing shift of the M-current activation curve after washout of muscarine in bullfrog sympathetic neurons. Neurosci Lett. 1996;207:97–100. doi: 10.1016/0304-3940(96)12495-2. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Yamanaka A, Ichiki K, Muraki Y, Kilduff TS, Yagami K, et al. Cholecystokinin activates orexin/hypocretin neurons through the cholecystokinin A receptor. J Neurosci. 2005;25:7459–7469. doi: 10.1523/JNEUROSCI.1193-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk A, Richards JG, Trzeciak A, Gillessen D, Mohler H. Cholecystokinin receptors: biochemical demonstration and autoradiographical localization in rat brain and pancreas using [3H] cholecystokinin8 as radioligand. J Neurosci. 1984;4:1021–1033. doi: 10.1523/JNEUROSCI.04-04-01021.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Wilson SP, Long KJ, Lemon SC. Stimulatory regulation of the large-conductance, calcium-activated potassium channel by G proteins in bovine adrenal chromaffin cells. Mol Pharmacol. 1996;49:379–386. [PubMed] [Google Scholar]
- Wang B, Sims SM. CCK regulates nonselective cation channels in guinea pig gastric smooth muscle cells. Am J Physiol. 1998;274:G709–G717. doi: 10.1152/ajpgi.1998.274.4.G709. [DOI] [PubMed] [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang HL. The excitatory effect of cholecystokinin on rat neostriatal neurons: ionic and molecular mechanisms. Eur J Pharmacol. 1996a;307:125–132. doi: 10.1016/0014-2999(96)00213-0. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang HL. Gαq/11 mediates cholecystokinin activation of the cationic conductance in rat substantia nigra dopaminergic neurons. J Neurochem. 1996b;66:1060–1066. doi: 10.1046/j.1471-4159.1996.66031060.x. [DOI] [PubMed] [Google Scholar]
- You ZB, Godukhin O, Goiny M, Nylander I, Ungerstedt U, Terenius L, et al. Cholecystokinin-8S increases dynorphin B, aspartate and glutamate release in the fronto-parietal cortex of the rat via different receptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:576–581. doi: 10.1007/pl00004986. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Brodin E, Meana JJ, Morino P, Hokfelt T, et al. On the origin of striatal cholecystokinin release: studies with in vivo microdialysis. J Neurochem. 1994;62:76–85. doi: 10.1046/j.1471-4159.1994.62010076.x. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Pettersson E, Nylander I, Goiny M, Shou HZ, et al. Modulation of neurotransmitter release by cholecystokinin in the neostriatum and substantia nigra of the rat: regional and receptor specificity. Neuroscience. 1996;74:793–804. doi: 10.1016/0306-4522(96)00149-2. [DOI] [PubMed] [Google Scholar]