Abstract
Control mechanisms for potassium (K+) excretion in humans developed in Palaeolithic times when diets were sodium poor and episodically K+ rich. Nevertheless, our understanding of the regulation of K+ excretion comes from experiments in rats with large sodium and K+ intakes. Our objective was to identify how K+ excretion was regulated when rats consumed a low NaCl diet to reflect Palaeolithic conditions. Rats that were given mineralocorticoids plus either NaCl, mannitol, or NaHCO3 had a small kaliuresis. In contrast, KCl load induced a large kaliuresis and a near-maximal luminal [K+] in the terminal cortical collecting duct ([K+]CCD). The time course of events was important. The rise in the [K+]CCD was prompt, but the initial kaliuresis was only modest. Over the next 4 h, kaliuresis increased markedly due solely to a higher calculated distal flow rate, which appeared to be due to diminished reabsorption of NaCl in the loop of Henle; of note, the measured papillary [K+] rose. In summary, the increase in the [K+]CCD in rats given KCl is likely to be due to an increase in the number of luminal K+ channels rather than to mechanisms that are known to induce a lumen-negative voltage in cortical distal nephron segments. The higher distal flow rate might be due to a higher interstitial [K+], which inhibited NaCl reabsorption in the loop of Henle. Thus, to understand which of the potential control mechanisms are operating, one must look very closely at the conditions imposed by the experimental setting.
Our present understanding of the regulation of the renal excretion of potassium (K+) is derived mainly from experiments in fed rats. These data have provided valuable information because of the use of invasive procedures (e.g. micropuncture) and electrophysiology techniques (Giebisch, 1998). Nevertheless, it is extremely important to recognize that rats fed their regular chow have an extremely high intake of K+ and NaCl, not because their diet is particularly rich in these electrolytes, but rather because smaller animals must eat more to have a higher heat production rate to maintain body temperature (Schmidt-Nielsen, 1991). For comparison with humans, if the intakes in the rat are expressed per kilogram body weight to adjust for the size of organs where K+ is stored prior to its excretion, the daily rate of excretion of K+ is 10-fold higher in fed rats (10–15 mmol (kg body weight)−1; Cheema-Dhadli et al. 2002) than in humans (1–1.5 mmol of K+ (kg body weight)−1). In addition, fed rats excreted 3–5 times more NaCl per kilogram body weight as compared to human subjects that consumed a typical western diet. Therefore, conclusions drawn about control mechanisms of K+ excretion in the fed rat describe the need to achieve very high rates of K+ excretion under conditions where the intake of NaCl is high and hence distal delivery of Na+ to and the flow rate in the late cortical distal nephron may not limit this excretion. These regulatory events might differ significantly when the goal is to increase the excretion of K+ rather quickly from very small to modestly high rates in an episodic fashion when there is a low intake of Na+, conditions that reflect the Palaeolithic diet.
To gain further insights into the mechanisms that regulate the excretion of K+ in humans, our experiments were designed to reflect conditions in humans in Palaeolithic times because this is when important control mechanisms should have developed. The requirements for the control of K+ excretion are first to limit the excretion of K+ when K+ intake is low and second to cause a large episodic rise in the rate of excretion of K+ when K+ intake is high. Because there was very little intake of NaCl in the Palaeolithic setting, mechanisms would be needed to ensure that there would be a periodic and high delivery of sodium (Na+) to the late cortical distal nephron to permit episodic K+ excretion. On the other hand, it is critical to limit the distal delivery of Na+ to avoid excreting appreciable quantities of Na+ because of the low intake of NaCl in this setting.
Results to be reported indicate that administering mineralocorticoids, increasing the distal delivery of Na+ with Cl− or HCO3−, or raising the flow rate in the terminal cortical collecting duct (CCD) by administering mannitol was not a sufficient stimulus to induce high rates of excretion of K+. In contrast, when rats were given a supplement of K+, there was a very high rate of excretion of K+ that had two distinct phases. In the initial 2 h, there was a near-maximal concentration of K+ in the luminal fluid in the late cortical distal nephron ([K+]CCD), perhaps due to the presence of more K+ ion channels in the luminal membranes. Nevertheless, the kaliuresis was only modestly high. In contrast there was a large increase in the rate of excretion of K+ due entirely to a rise in the flow rate in the terminal CCD in the next 4 h. This second delayed form of regulation was accompanied by signs of inhibition of the reabsorption of Na+ and Cl− in the loop of Henle and an increase in the [K+] in the renal papilla. These results provide new insights into the regulation of the excretion of K+. They also point out the relative importance of factors that led to a high [K+]CCD and those that led to a high flow rate in this nephron segment were different in both their mechanism and timing. Hence it is necessary to examine regulatory mechanisms in the context of the stimuli that are present at the time of study.
Methods
Rats
Rats were cared for in accordance with the principles and guidelines of the Canadian Council on Animal Care. The Animal Care Committee of St Michael's Hospital approved the study protocol.
Protocol
Adult male Wister rats (weight 300–400 g) were housed in individual metabolic cages so that complete collections of urine could be obtained by spontaneous voiding. At the end of each collection period, rats were placed on a plastic sheet, their tails were lifted and gentle suprapubic pressure was applied to improve bladder emptying. In all the reported results, the rate of excretion of creatinine was in a similar range (60–90 nmol min−1).
All rats had free access to water, and they consumed a low electrolyte diet for 5 days (Na+ 1 mmol kg−1, K+ 0 mmol kg−1, Cl− 0 mmol kg−1, ICN Pharmaceuticals, Montreal, Canada). They were given 2 μg of desmopressin acetate (dDAVP, Ferring Co., Ontario, Canada) at 10.00 h and 17.00 h on days 3–5 to ensure that V2 actions of vasopressin levels were not a variable because this hormone can augment the rate of excretion of K+ in certain experimental settings (Alfaidy et al. 1997; Ecelbarger et al. 2000).
Studies to examine control of K+ excretion
Effect of mineralocorticoids
The objective was to determine if mineralocorticoids would increase the rate of excretion of K+ and/or the [K+]CCD appreciably in rats on the low electrolyte diet. Rats (n = 7) were given 5 μg of deoxycorticosterone (DOC) per kilogram body weight at 17.00 h on day 4 and again at 09.00 h on day 5 by the intraperitoneal route. The control group of rats (n = 7) was treated in an identical fashion, but they did not receive DOC. As soon as the 24-h urine collection was complete on the morning of day 5, rats were anaesthetized (nitrous oxide, oxygen and isoflurane (2.5%) administered at 2 l min−1). An arterial blood sample was drawn by needle puncture from the aorta, following which they were killed by decapitation under anaesthesia according to the guidelines of the Canadian Council on Animal Care.
Effect of NaHCO3 or NaCl on K+ excretion parameters in rats pretreated with DOC.
The objective was to determine whether a supplement of NaCl or NaHCO3 to augment the distal delivery of Na+, with or without Cl−, would increase the rate of excretion of K+ and/or the calculated parameters of K+ excretion in the terminal cortical distal nephron in rats that consumed the low electrolyte diet for 5 days. Because the organic anions that accompany K+ in a Palaeolithic diet (e.g. citrate or malate) are converted to HCO3−in vivo, the NaHCO3 group provided an opportunity to examine whether HCO3− had a unique role to stimulate K+ excretion in this setting. In the NaCl group (n = 10), rats were given 1500 μmol of NaCl as an isotonic solution by the intraperitoneal route on the morning of day 5. This dose represents ∼1/2 of the intake of NaCl in rats consuming their usual chow. In the NaHCO3 group, the following dosing pattern was established in preliminary experiments as one that induced bicarbonaturia, a urine pH > 7.4, and a large increment in the Na+ excretion rate. On the evening of day 4, rats (n = 7) received 1500 μmol of NaHCO3 in their drinking water that, in this instance, also contained sucrose. On the morning of day 5, each rat was given 1500 μmol of isotonic NaHCO3 by the intraperitoneal route. Urines were collected for 2 h. A blood sample was taken at the end of the 2 h urine collection period.
Effect of KCl on K+ excretion parameters in rats pretreated with DOC
In preliminary studies, the rate of excretion of K+ rose modestly in the initial 2 h, but then increased markedly thereafter. Hence there were two protocols carried out in the KCl group. First, to examine which component of the K+ excretion process in the cortical distal nephron was responsible for the initial increment in K+ excretion, the first group of rats (n = 10) was given KCl (1500 μmol) on the morning of day 5 via the intraperitoneal route. Urine was collected for 2 h and a blood sample was taken at the 2 h time. In the second protocol, an additional group of rats (n = 7) were treated in an identical fashion and they were given a second 1500 μmol supplement of isotonic KCl via the intraperitoneal route at the 2 h time for the time course experiments because this dose of K+ was able to sustain a positive balance of K+ and a plasma K+ concentration that was similar at the 2 h and 6 h times. Urine was collected every 2 h for a 6-h period, and a blood sample was taken at the 6 h time.
Effect of mannitol on K+ excretion in rats pretreated with DOC
The objective was to determine whether an increase in the flow rate in the late cortical distal nephron would augment the rate of K+ excretion in rats that consumed the low electrolyte diet for 5 days. These flow rates were designed to be similar to those in rats given the KCl supplement. On the morning of day 5, one group of rats (n = 8) was given 3 ml of a solution containing 300 μmol of mannitol per ml by the intraperitoneal route and urine was collected for 120 min. A second group of rats (n = 8) was treated in an identical fashion except that they received 6 ml of a solution containing 300 μmol of mannitol per ml by the intraperitoneal route and urine was also collected for 120 min.
Concentration of K+ in the renal papilla
The purpose of these experiments was to measure the K+ concentration in the renal papilla at the 2 h time in separate groups of rats that were treated as above with NaCl or KCl. The procedure was previously described (Gowrishankar et al. 1998). Rats (n = 6 in each group) were decapitated at the set time point for the study and their kidneys were removed as rapidly as possible. Each kidney was sliced along its longitudinal axis with a sharp knife to expose intact papilla. The papillary tips were rapidly excised from both kidneys, blotted, and transferred immediately to a preweighed plastic vial and sealed. The vial was weighed and 1 ml of the solution used for flame photometer measurement of Na+ and K+ was added. The tissue was homogenized and Na+ and K+ were measured by flame photometry. In another six rats in each group, the renal papilla was excised in an identical fashion and desiccated to measure its water content by difference in the wet and dry weights.
Non-invasive tools to reflect the parameters of K+ excretion in the terminal CCD in vivo
[K+]CCDin vivo
An estimate of the [K+]CCD was obtained by dividing the urine K+ concentration (UK) by the urine/plasma osmolality ratio ((U/P)osm) when vasopressin acted (eqn (1)). This calculation adjusts for water reabsorption in the medullary collecting duct (West et al. 1986a).
| (1) |
Flow rate in the terminal CCD in vivo
Since some osmoles are absorbed in the medullary collecting duct, this calculation provides a minimum estimate of the flow rate in the terminal cortical collecting duct when vasopressin acts (Steele et al. 1994) (eqn (2)). The rationale is that the osmolality in this luminal fluid will be equal to the plasma osmolality (Posm) in this setting. Therefore this flow rate is directly related to delivery of osmoles to the terminal CCD, which is reflected the rate of excretion of osmoles.
| (2) |
Analytical techniques
The concentrations of Na+ (PNa) and K+ (PK) in plasma and urine (UNa and UK) were determined by flame photometry (Radiometer, FLM-3, London, ON, Canada), the concentrations of Cl− in plasma (PCl) and urine (UCl) were determined by electromimetic titration (Chloride meter, CMT 10, London Scientific Ltd, London, Ontario), osmolality was measured by freezing point depression (Advanced Instruments Inc., Needham Heights, MA, USA), and blood gas analysis was performed at 37°C with a digital pH/blood gas analyser (Corning 178 blood pH analyser, Corning, NY, USA). The concentrations of urea and creatinine in plasma and urine were measured as previously described (Halperin et al. 1985; Cheema-Dhadli & Halperin, 1993).
Statistical analysis
Results are reported as means ± s.e.m. Statistical analysis was performed on the group mean values using Student's unpaired t test. A P-value that was less than 0.05 was considered to be statistically significant.
Results
Characteristics of the model
The concentrations of K+ in plasma in rats fed regular chow or the low electrolyte diet were not significantly different (4.0 ± 0.1 and 3.9 ± 0.1 mmol l−1); the same applied to the concentrations of Na+ (142 ± 1 and 139 ± 1 mmol l−1, respectively) and Cl− (99 ± 1 and 101 ± 1 mmol l−1, respectively) in plasma. Because of the high alkali content in the form of potassium citrate in rat chow and its absence in the low electrolyte diet (Cheema-Dhadli et al. 2002), the pH and the concentration of bicarbonate in plasma were significantly higher in rats consuming regular chow. The concentration of creatinine in plasma was also somewhat higher in these rats (36 ± 1 and 31 ± 1 μmol l−1, respectively, Table 1). Rats consuming the regular diet excreted 3.3 mmol of Na+ day−1 and 5.2 mmol of K+ day−1 whereas rats on the low electrolyte diet for 5 days had a 10- to 20-fold lower daily excretion of Na+ and Cl+, while the daily excretion of K+ was 60-fold lower (Table 1).
Table 1.
Effect of diet on 24-h urine values
| Usual chow | Low electrolyte | |
|---|---|---|
| Plasma | ||
| pH | 7.45 ± 0.01 | 7.40 ± 0.02* |
| Na+ (mmol l−1) | 142 ± 1 | 139 ± 1 |
| K+ (mmol l−1) | 4.0 ± 0.1 | 3.9 ± 0.1 |
| Cl− (mmol l−1) | 99 ± 1 | 101 ± 1 |
| HCO3− (mmol l−1) | 30 ± 1 | 26 ± 1* |
| Creatinine (μmol l−1) | 36 ± 1 | 31 ± 1* |
| Urine | ||
| Volume (ml day−1) | 25 ± 2 | 2.1 ± 0.7* |
| Na+ (mmol day−1) | 3.3 ± 0.12 | 0.16 ± 0.04* |
| K+ (mmol day−1) | 5.2 ± 0.19 | 0.08 ± 0.02* |
| Cl− (mmol day−1) | 4.1 ± 0.11 | 0.25 ± 0.07* |
A 24-h urine was obtained on day 5 in rats (n = 16) that ate their usual chow and in another group of rats (n = 7) that consumed the low electrolyte diet for 5 days. Results are reported as the mean ± s.e.m.
P < 0.05 for the effect of the diet.
Effect of DOC on the rate of excretion of K+
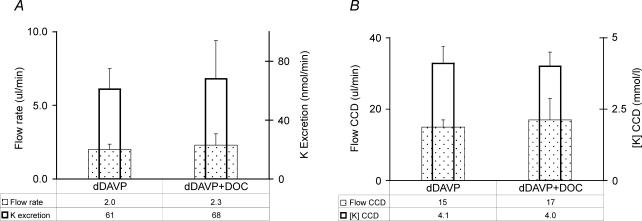
To evaluate the relative importance of factors that might regulate the secretion of K+ in the late cortical distal nephron, we first tested whether this rate would rise appreciably when DOC was administered for 2 days to rats consuming the low electrolyte diet. As shown in Fig. 1A, there were no significant changes in the urine flow rate or in the rate of excretion of K+ when DOC was administered. Similarly, the estimated values for [K+]CCD and the flow rate in the terminal CCD were not significant different in the rats that received DOC (Fig. 1B). There was no decline in the rate of excretion of Na+ (Na+ 116 ± 26 versus 161 ± 44) in the DOC-treated rats.
Figure 1. Effect of DOC on the parameters for K+ excretion in rats consuming the low electrolyte diet.
The results are given as the mean ± s.e.m. for 11 rats in each group. A, urinary flow rate (depicted by the stippled, wider rectangles) and rate of excretion of K+ (depicted by the open, narrower rectangles). B, flow rate in the terminal CCD (depicted by the stippled, wider rectangles) and [K+]CCD (depicted by the open, narrower rectangles). There were no significant differences in these results. In A and B, dDAVP alone is shown on the left, and dDAVP plus DOC on the right.
Effect of NaHCO3 or KCl on the rate of excretion of K+ in rats pretreated with DOC
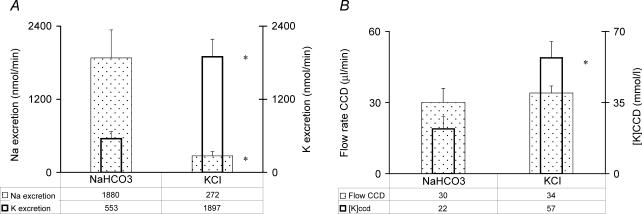
In the NaCl group, there was no significant increase in the rate of excretion of Na+ when urine was collected for only 2 h. Therefore a second group of rats were given NaHCO3 to increase the rate of Na+ excretion in this time period and also if there is a special role for bicarbonate to modulate K+ excretion. Data on K+ excretion from these rats for this 2-h period were compared to the rats that received KCl. In the NaHCO3 group, there was a high urine pH as compared to the KCl group (7.8 ± 0.23 versus 6.0 ± 0.04, respectively, P < 0.05). Similarly, the plasma HCO3− concentration was higher in rats given NaHCO3 (33 ± 1.0 versus 20 ± 0.5 mmol l−1, respectively, P < 0.05). There was no significant difference in the urine flow rate in the NaHCO3versus the KCl groups (5.2 ± 1.0 and 5.9 ± 0.5 μl min−1, respectively) or in the estimated flow rate in the terminal CCD (30 ± 1 and 34 ± 3 μl min−1, respectively). Nevertheless, rats treated with NaHCO3 had a much higher rate of excretion of Na+ as compared to the KCl group (1880 ± 469 and 272 ± 72 nmol min−1, respectively, P < 0.05, Fig. 2A). In contrast, the rate of excretion of K+ was 3-fold higher in the KCl group (1897 ± 290 and 553 ± 118 nmol min−1, respectively, P < 0.05). In addition, the calculated [K+]CCD was also close to 3-fold higher in the KCl group (57 ± 8 mmol l−1 and 22 ± 6 mmol l−1, respectively, P < 0.05, Fig. 2B).
Figure 2. Effect of NaHCO3 or KCl on the parameters for K+ excretion in rats consuming the low electrolyte diet.
The results are the mean ± s.e.m. for 7 rats that received NaHCO3 and the 10 rats that received KCl. A, rate of excretion of Na+ (depicted by the stippled, wider rectangles) and rate of excretion of K+ (depicted by the open, narrower rectangles). B, flow rate in the terminal CCD (depicted by the stippled, wider rectangles) and [K+]CCD (depicted by the open, narrower rectangles). In A and B, the NaHCO3 group is shown on the left and the KCl group on the right. *P < 0.05 for the effect of an infusion of KCl.
Effect of distal flow rate on the rate of excretion of K+ in rats pretreated with DOC
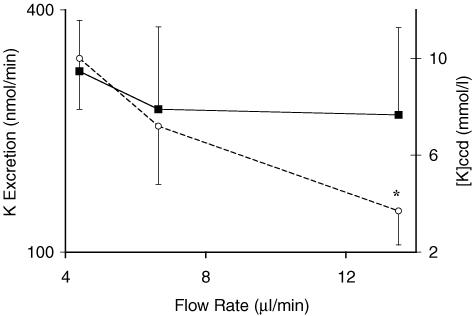
Two groups of rats were given mannitol to increase the urine flow rate. The urine flow rate in the control group was 4.4 ± 0.2 μl min−1, whereas it rose to 6.7 ± 0.8 μl min−1 in the low dose mannitol group and to 13.5 ± 2.2 μl min−1 in the high dose mannitol group. Over this range of flow rates, there was no significant change in the rate of excretion of K+ (Fig. 3). Accordingly, [K+]CCD was 2–3 fold lower at the highest flow rate (10 ± 2.1 mmol l−1 in control group and 4 ± 1.4 mmol l−1 in the high dose mannitol group, P < 0.05). Hence raising the flow rate did not cause a rise in the rate of excretion of K+ or in the [K+]CCD.
Figure 3. Effect of an infusion of mannitol on the rate of excretion of K+ and [K+]CCD in rats pretreated with DOC.
The results are the mean ± s.e.m. for 7 rats in each group. The urine flow rate is shown on the x-axis; the two higher flow rates were caused by an infusion of mannitol (see Methods for details). ▪, rate of excretion of K+; this rate was not significantly different at the different urine flow rates. ○, [K+]CCD; this latter concentration was significantly lower at the highest urine flow rate. *P < 0.05 for the effect of a change in the urine flow rate.
Time course and components of the kaliuretic response in the rats given KCl and pretreated with DOC
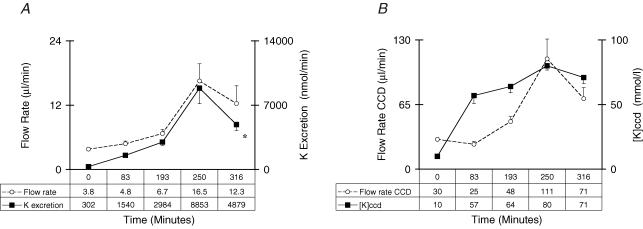
In preliminary experiments, we noted that there was a marked, but delayed rise in the rate of K+ excretion 2 h after KCl was administered. Accordingly, a more detailed study was carried out to determine which component of K+ excretion (the estimated [K+]CCD and flow rate in the terminal cortical distal nephron) would be responsible for the large rise in K+ excretion in the next 4 h. The concentration of K+ in plasma was 4.8 ± 0.4 at the 2 h time and 4.7 ± 0.1 at the 6 h time in these rats. Of great interest, while the [K+]CCD did not rise further in the last 4 h period, there was a large and significant rise in the rate of K+ excretion (peak K+ excretion rate was 8853 ± 1695 nmol min−1), and a parallel rise in the urine flow rate (peak urine flow rate was 16.5 ± 3.2 μl min−1) at the 4 h time point. In addition, the estimated flow rate in the terminal CCD was 3- to 4-fold higher when comparing the 2 h and the 4 h time points (30 ± 1 and 111 ± 20 μl min−1, respectively, P < 0.05, Fig. 4).
Figure 4. Time course for K+ excretion parameters in rats given a KCl supplement.
For details, see Methods. The time after administering KCl is shown on the x-axis. A, ○, the flow rate in the terminal CCD μl min−1; ▪, rate of excretion of K+ in μmol min−1. B, ○, the urine flow rate in μl min−1; ▪, [K+]CCD. Notice how closely the rate of excretion of K+ mirrors the urine flow rate and that the [K+]CCD rises initially and does not change appreciably thereafter. *P < 0.05 for the effect of an infusion of KCl.
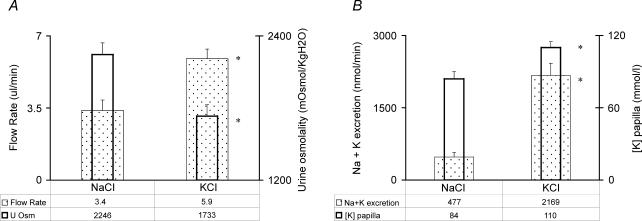
To study the effect of KCl on loop of Henle function, two additional groups of rats were employed. The control group was given NaCl and the experimental group received an equivalent dose of KCl. Measurements were made at the 2 h time. The urine flow rate was significantly higher in the KCl group (5.9 ± 0.5 and 3.4 ± 0.5 μl min−1, respectively, P < 0.05) as was the rate of excretion of Na++ K+ (2169 ± 257 and 477 ± 92 nmol min−1, respectively, P < 0.05, Fig. 5). In addition, Uosm was significantly lower in the KCl group (1733 ± 94 and 2246 ± 94 mosmol (kg H2O)−1, respectively, P < 0.05).
Figure 5. Effect of KCl administration on loop of Henle function.
The results are the mean ± s.e.m. for 7 rats in the NaCl group and 10 rats in the KCl group. A, urinary flow rate (depicted by the stippled, wider rectangles) and urine osmolality (depicted by the open, narrower rectangles). B, Na+ plus K+ excretion rate (depicted by the stippled, wider rectangles) and papillary [K+] (depicted by the open, narrower rectangles). In A and B, the NaCl supplement group is shown on the left and the KCl supplement group on the right. *P < 0.05 for the effect of an infusion of KCl.
Papillary measurements
The concentration of K+ in the papillary interstitial compartment was significantly higher in the KCl as compared to the NaCl group (110 ± 6 mmol l−1 and 84 ± 5 mmol l−1, respectively, P < 0.05).
Discussion
Control mechanisms for the excretion of K+ in mammalian species including rats and humans developed in Palaeolithic times. Nevertheless, our present understanding of control of K+ excretion is derived mainly from studies in rats, a species with a large intake of K+ and Na+ salts. Our objective was to determine which factor(s) that might regulate the excretion of K+ actually have a high degree of control strength under dietary conditions that mimic those in Palaeolithic times (Eaton & Konner, 1985). The principal new results were that in the absence of a K+ load, mineralocorticoids, distal delivery of Na+ and/or HCO3−, and a high distal flow rate were not sufficient stimuli, alone or in combination, to induce a large kaliuresis. There was a large increase in K+ excretion following a KCl load, but two different mechanisms were involved. In the first 2 h after KCl was administered, there was a modest rise in K+ excretion due only to a large rise in the [K+]CCD. In the next 4 h, the rise in the [K+]CCD was sustained, but now there was a large kaliuresis that was due primarily to a large increase in the flow rate to the cortical distal nephron. Its cause appeared to be the consequence of inhibition of the reabsorption of Na+ and Cl− in the medullary thick ascending limb of the loop of Henle (mTAL).
Because the late cortical distal nephron is not accessible to invasive testing in an intact animal or human, indirect tests must be used to gain insights about the control strength of individual factors that have been suggested to influence the K+ secretory process in vivo. Interpretations can be more robust if separate analyses of the components of the K+ excretion formula (concentration and flow rate) in the urine, and more importantly, in the luminal fluid exiting the terminal cortical collecting duct. As illustrated in Fig. 4, much greater insights can be provided if these parameters could be analysed at several time points in non-anaesthetized animals.
The rationale and the experimental support for tests that were relied on to provide information about the components of the K+ secretory process in the cortical distal nephron were previously described (West et al. 1986b; Ethier et al. 1990b; Steele et al. 1994). Because we must use the osmolality ratio in the urine versus plasma, it is essential that data be gathered when vasopressin acts; hence all rats were treated with dDAVP and had urine osmolalities that were > 4-fold higher than in plasma.
One can estimate the [K+]CCD by dividing the K+ concentration in the urine by the ratio of the urine osmolality to that of plasma. In addition, this osmolality ratio and the osmole excretion rate provide a minimum estimate of flow rate in the terminal cortical distal nephron (eqns (1) and (2)) (Ethier et al. 1990a). One weakness when using these tests is that some osmoles, including K+, are reabsorbed in the medullary collecting duct. When this occurs, the absolute value for the [K+]CCD will be over-estimated and flow rate in the terminal cortical collecting duct will be under-estimated. Because the calculations were done after giving a large electrolyte supplement, the magnitude of these types of error were minimized because only a small proportion of Na++ K+ and Cl− delivered to the MCD is reabsorbed in this nephron segment when rats are given a supplement of electrolytes (Sonnenberg, 1974). Hence, while the values for the [K+] and the flow rate in the terminal cortical collecting duct should only be viewed as estimates, the fact that their magnitude was so high in the rats treated with KCl adds confidence to their ability to reflect values in vivo, at least in a semiquantitative fashion.
The discussion to follow will be divided into two sections, comments about the factors controlling the secretion of K+ in the cortical distal nephron and an analysis of the control of the reabsorption of Na+ and Cl− in the mTAL in a setting that was designed to reflect the episodic kaliuresis of Palaeolithic times.
Factors controlling K+ secretion in the cortical distal nephron
There are two ways to increase the rate of secretion of K+ in the late cortical distal nephron, increase the flow rate and/or the [K+]CCD in these nephron segments (Halperin & Kamel, 1998).
Raise the flow rate
A higher flow rate can increase the rate of K+ secretion in rats consuming their regular diet that contains large amounts of NaCl and K+ (Good & Wright, 1979). Nevertheless, a large increase in the flow rate in the terminal cortical collecting duct did not increase the rate of excretion of K+ when the flow rate was increased 3- to 4-fold in rats on the low electrolyte diet that were given mannitol (Fig. 3).
Raise the [K+]CCD
Because all rats on the low electrolyte diet were pretreated with DOC and did not have an appreciable increase in K+ excretion, this hormone was not important to initiate a large kaliuresis (Table 1). The driving force for K+ secretion in the late cortical distal nephron is a lumen-negative voltage, which is generated when Na+ is absorbed faster than accompanying anions (usually Cl−) (Velazquez et al. 1987). This is most evident when there is little distal delivery of Cl− or if the reabsorption of Cl− is diminished, e.g. by the presence of bicarbonaturia and/or a high luminal fluid pH (Carlisle et al. 1991). Nevertheless, even when Na+ was delivered to the cortical distal nephron with HCO3− rather than Cl− ions, there was insufficient K+ secreted to raise the rate of K+ excretion to levels seen with KCl (Fig. 2).
Role of the KCl load
To interpret the results in rats given KCl, we shall first consider how high the [K+]CCD can rise in vivo. The maximum luminal osmolality in the cortical nephron segments is equal to the interstitial osmolality or Posm (∼300 mosmol (kg H2O)−1); in addition, close to half of the urine osmoles are usually urea. Moreover, K+ ions are usually accompanied by an equivalent number of monovalent anions (Cl− and HCO3−). Hence, the maximum [K+]CCD would be ∼75 mmol l−1. To have this maximum [K+]CCD, there must also be very little Na+ in the fluid exiting the terminal CCD. Interestingly, the [K+]CCD was close to this maximum value and there was little Na+ in the urine in rats on a low electrolyte diet that received the KCl load (Fig. 4). Hence to excrete more K+, there must be a higher distal flow rate.
Number of K+ channels in the luminal membrane of the cortical distal nephron.
The rate of excretion of K+ and the [K+]CCD were an order of magnitude higher in rats given KCl as compared to all other groups. Therefore either there was a more negative lumen voltage and/or more luminal K+ channels in the cortical distal nephron in rats given KCl. Because the delivery of Na+ with HCO3− in rats that were pretreated with DOC did not augment the [K+]CCD appreciably, it is possible that the higher rate of net K+ secretion in the cortical distal nephron in the KCl group was due to more luminal ROMK or maxi-K+ channels because a low K+ intake decreases the number of luminal K+ channels (Palmer et al. 1994; Palmer & Frindt, 1999). In this regard, dietary K+ intake appears to be an important regulator of the ROMK-like 35 pS K+ channel (Wang, 2004).
Factors controlling the flow rate in the terminal cortical distal nephron
The time course of the events that led to a kaliuresis with the administration of KCl reveals new insights about control of K+ excretion in this setting. While the initial phase was modest and due to a near-maximum increase in [K+]CCD, the subsequent large increase in K+ secretion required an increased flow rate in the CCD. It appears that Na+ and Cl− reabsorption in the mTAL was inhibited in this setting because there was an initial significant fall in the Uosm along with a rise in the rate of excretion of electrolytes (Fig. 5). Therefore another objective of this study was to improve our understanding of the mechanism whereby a high intake of K+ might inhibit the reabsorption of Na+ and Cl− in the mTAL in rats consuming a low electrolyte diet. Stokes (1982) suggested that an elevated K+ concentration in the medullary interstitial compartment could be the signal to inhibit Na+ and Cl− reabsorption in the mTAL. Its first step is a diminished negative voltage across the basolateral membrane of the mTAL due to this higher interstitial [K+]. As a result, the exit of Cl− ions should decrease until the intracellular [Cl−] rises. This higher [Cl−] in mTAL cells might slow the reabsorption of Na+ and Cl− from its lumen. The net result is an increased delivery of Na+ and Cl− to the cortical distal nephron. If true, the degree of inhibition of Na+ and Cl− reabsorption in the mTAL should be very sensitive to the medullary interstitial [K+]. In support of this view, the papillary [K+] was higher as was the flow rate exiting the terminal CCD in rats given KCl as compared to NaCl (Fig. 5). While the papillary [K+] does not directly reflect the [K+] in the outer medullary interstitial compartment, it is reasonable to presume that a larger papillary [K+] will lead to a higher [K+] in the interstitial compartment in the medulla. Nevertheless, this comment must remain a speculation because of the need to carry out in vivo studies, but it is included because of its important potential implications.
It is also important to consider the nephron segment that added K+ to the medullary interstitial compartment. Jamison (1987) suggested that the nephron site where K+ was reabsorbed was the outer MCD and that this process was a passive one. Because the permeability of the outer MCD for K+ is very low (Stokes, 1982), this is an unlikely mechanism. Stokes, on the other hand, suggested that K+ could enter the medullary interstitial compartment after reabsorption in the mTAL. Nevertheless, this source of K+ entry should not be stimulated appreciably by the amount of K+ ingested. We postulate that it is possible that more K+ was absorbed by the H+,K+-ATPase in the MCD (Wingo & Armitage, 1993; Nakamura et al. 1998). The H+,K+-ATPase in the MCD has an increased activity during K+ depletion whereas our proposed mechanism requires an increased flux in this transporter in response to a K+ load. We offer the following speculation to clarify this issue. When the K+ load is first absorbed, the [K+] in plasma rises at a time when the H+,K+-ATPase might still be active. Therefore the increased delivery of K+ to the inner MCD can raise the medullary interstitial [K+], which leads to a greater inhibition of mTAL function as observed in vitro by Stokes and supported by the significant early fall in Uosm in our time course experiment (Fig. 5). With time, when the rise in the [K+] in plasma is sustained, there will be fewer luminal H+,K+-ATPase units in the inner MCD, so the rate of K+ reabsorption should decline. As a result, a new steady state might occur where distal delivery of Na+ and Cl− is small enough to maintain high rates of K+ secretion, but not large enough to produce a natriuresis.
Concluding remarks
To understand the regulation of the excretion of an important dietary constituent like K+ in humans, it is important that the experimental setting mimic as closely as possible conditions in Palaeolithic times when control mechanisms likely developed. Accordingly, we studied rats with a low intake of K+ and NaCl. When this was done, factors such as mineralocorticoid levels, distal delivery of Na+, HCO3−, and volume – alone or in combination did not augment the net secretion of K+. With a K+ load, the initial small kaliuretic response was due to a higher [K+]CCD. We speculate that a possible control site was to increase the number of K+ channels in the luminal membrane of principal cells in the CCD. The next regulatory step was an increased flow rate in the late cortical distal nephron due to inhibition of mTAL function. Now a large kaliuresis could occur because there were a sufficient number of luminal K+ channels. Hence there are times when controls influence the [K+]CCD and others when they influence the flow rate in the terminal cortical distal nephron. These data provide new insights into the control of K+ excretion that may have implications for our understanding of the disorder in patients with a dyskalemia.
Acknowledgments
We are extremely grateful to Dr Man Oh for very helpful discussions and suggestions during the preparation of this manuscript. We are also indebted to Stella Tang for expert technical assistance and Siu Yee Lee for secretarial assistance. This work was supported by grant MT-15485 from the Canadian Institutes for Health Research.
References
- Alfaidy N, Blot-Chabaud M, Bonvalet J-P, Farman N. Vasopressin potentiates mineralocorticoid selectivity by stimulating 11β hydroxysteroid dehydrogenase in rat collecting duct. J Clin Invest. 1997;100:2437–2442. doi: 10.1172/JCI119785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle EJF, Donnelly SM, Ethier J, Quaggin SE, Kaiser U, Vasuvattakul S, Kamel KS, Halperin ML. Modulation of the secretion of potassium by accompanying anions in humans. Kidney Int. 1991;39:1206–1212. doi: 10.1038/ki.1991.152. [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S, Halperin ML. Diurnal excretion of nitrogen and sulphur after meals containing protein: indications for postprandial synthesis of proteins. Can J Physiol Pharm. 1993;71:120–127. doi: 10.1139/y93-017. [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S, Lin S-H, Halperin ML. Mechanisms used to dispose of a progressively increased alkali load in the rat. Am J Physiol. 2002;282:F1049–F1055. doi: 10.1152/ajprenal.00006.2001. [DOI] [PubMed] [Google Scholar]
- Eaton SB, Konner M. Paleolithic nutrition. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- Ecelbarger CA, Kim GH, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol. 2000;279:F46–F53. doi: 10.1152/ajprenal.2000.279.1.F46. [DOI] [PubMed] [Google Scholar]
- Ethier JH, Honrath U, Veress A, Sonnenberg H, Halperin ML. Nephron site responsible for the reduced kaliuretic response to mineralocorticoids during hypokalemia in rats. Kidney Int. 1990a;38:812–817. doi: 10.1038/ki.1990.275. [DOI] [PubMed] [Google Scholar]
- Ethier JH, Kamel KS, Magner PO, Lemann JJ, Halperin ML. The transtubular potassium concentration in patients with hypokalemia and hyperkalemia. Am J Kidney Dis. 1990b;15:309–315. doi: 10.1016/s0272-6386(12)80076-x. [DOI] [PubMed] [Google Scholar]
- Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- Good DW, Wright FS. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979;236:F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- Gowrishankar M, Lenga I, Cheung RY, Cheema-Dhadli S, Halperin ML. Minimum urine flow rate during water deprivation: Importance of the permeability of urea in the inner medulla. Kidney Int. 1998;53:159–166. doi: 10.1046/j.1523-1755.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- Halperin M, Goldstein M, Vinay P, Gougoux A, Pichette C, Jungas R. Regulation of the maximum rate of renal ammoniagenesis in the dog with chronic metabolic acidosis: a quantitative analysis. Am J Physiol. 1985;248:F607–F615. doi: 10.1152/ajprenal.1985.248.4.F607. [DOI] [PubMed] [Google Scholar]
- Halperin ML, Kamel KS. Potassium. Lancet. 1998;352:135–142. doi: 10.1016/S0140-6736(98)85044-7. [DOI] [PubMed] [Google Scholar]
- Jamison RL. Potassium recycling. Kidney Int. 1987;31:695–703. doi: 10.1038/ki.1987.54. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Wang S, Galla JH, Soleimani M. Potassium depletion increases HCO3− reabsorption in outer medullary collecting duct by activation of colonic H-K-ATPase. Am J Physiol. 1998;274:F687–F692. doi: 10.1152/ajprenal.1998.274.4.F687. [DOI] [PubMed] [Google Scholar]
- Palmer L, Antonian J, Frindt G. Regulation of the apical K and Na channels and Na/K pumps in the rat cortical collecting tubule by dietary K. J General Physiol. 1994;105:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L, Frindt G. Regulation of the apical K channels in the rat cortical collecting tubule during changes in K intake. Am J Physiol. 1999;277:F805–F812. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K. Scaling: Why Is Animal Size So Important? Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- Sonnenberg H. Medullary collecting duct function in antidiuretic and in salt or water diurectic rats. Am J Physiol. 1974;226:501–506. doi: 10.1152/ajplegacy.1974.226.3.501. [DOI] [PubMed] [Google Scholar]
- Steele A, DeVeber H, Quaggin SE, Scheich A, Ethier J, Halperin ML. What is responsible for the diurnal variation in potassium excretion? Am J Physiol. 1994;36:R554–R560. doi: 10.1152/ajpregu.1994.267.2.R554. [DOI] [PubMed] [Google Scholar]
- Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb. J Clin Invest. 1982;70:219–229. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez H, Ellison DH, Wright FS. Chloride-dependent potassium secretion in early and late renal distal tubules. Am J Physiol. 1987;253:F555–F562. doi: 10.1152/ajprenal.1987.253.3.F555. [DOI] [PubMed] [Google Scholar]
- Wang WH. Regulation of renal K transport by dietary K intake. Annu Rev Physiol. 2004;66:547–569. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- West ML, Bendz O, Chen CB, Singer G, Richardson RMA, Sonnenberg H, Halperin ML. Development of a test to evaluate the transtubular potassium concentration gradient in the cortical collecting duct in vivo. Min Electrolyte Metab. 1986a;12:226–233. [PubMed] [Google Scholar]
- West ML, Marsden PA, Richardson RMA, Zettle RM, Halperin ML. New clinical approach to evaluate disorders of potassium excretion. Min Electrolyte Metab. 1986b;12:234–238. [PubMed] [Google Scholar]
- Wingo CS, Armitage FE. Potassium transport in the kidney: regulation and physiologic relevance of H+,K+-ATPase. Sem Nephrol. 1993;13:213–224. [PubMed] [Google Scholar]