Abstract
Isolated whole skeletal muscles fatigue more rapidly than isolated single muscle fibres. We have now employed this difference to study mechanisms of skeletal muscle fatigue. Isolated whole soleus and extensor digitorum longus (EDL) muscles were fatigued by repeated tetanic stimulation while measuring force production. Neither application of 10 mm lactic acid nor increasing the [K+] of the bath solution from 5 to 10 mm had any significant effect on the rate of force decline during fatigue induced by repeated brief tetani. Soleus muscles fatigued slightly faster during continuous tetanic stimulation in 10 mm[K+]. Inhibition of mitochondrial respiration with cyanide resulted in a faster fatigue development in both soleus and EDL muscles. Single soleus muscle fibres were fatigued by repeated tetani while measuring force and myoplasmic free [Ca2+] ([Ca2+]i). Under control conditions, the single fibres were substantially more fatigue resistant than the whole soleus muscles; tetanic force at the end of a series of 100 tetani was reduced by about 10% and 50%, respectively. However, in the presence of cyanide, fatigue developed at a similar rate in whole muscles and single fibres, and tetanic force at the end of fatiguing stimulation was reduced by ∼80%. The force decrease in the presence of cyanide was associated with a ∼50% decrease in tetanic [Ca2+]i, compared with an increase of ∼20% without cyanide. In conclusion, lactic acid or [K+] has little impact on fatigue induced by repeated tetani, whereas hypoxia speeds up fatigue development and this is mainly due to an impaired Ca2+ release from the sarcoplasmic reticulum.
Extensive activation of skeletal muscle leads to a decline in contractile function known as fatigue. Fatigue in vivo can be either central or peripheral, i.e. due to a decreased ability of the nervous system to activate the muscle cells, or due to impaired function of the muscle cells themselves (Gandevia, 2001). Mechanisms of peripheral fatigue can be studied in isolated muscle preparations that are activated without the involvement of the nervous system. Such studies have revealed two fundamentally different mechanisms underlying the decreased force production in fatigued muscle cells: (i) impaired excitability of the sarcolemma due to a depolarization caused by altered Na+–K+ gradients; (ii) metabolic changes associated with anaerobic ATP metabolism that cause impaired intracellular Ca2+ handling and/or defective function of the contractile proteins (Fitts, 1994; Allen et al. 1995).
Lactate and hydrogen ions accumulate within muscle cells during most types of intense activity. The resulting acidification has been considered as a major cause of peripheral fatigue (Fitts, 1994). However, later studies showed that acidosis has only a minor depressive effect on force production in mammalian muscle cells studied at physiological temperatures (Pate et al. 1995; Westerblad et al. 1997). Intriguingly, recent results indicate that acidosis may actually counteract fatigue development by depressing the negative effects of increased extracellular [K+] (Nielsen et al. 2001). Subsequent studies showed that acidosis decreases the sarcolemmal Cl− permeability, thereby reducing the Na+ current required to generate action potentials that can propagate into the t-tubular system and activate the t-tubular voltage sensors (Pedersen et al. 2004, 2005).
Apart from glycogen breakdown and production of lactate and hydrogen ions, anaerobic metabolism also involves breakdown of creatine phosphate (CrP) and accumulation of creatine and inorganic phosphate ions (Pi). Pi has been suggested to have a central role in fatigue by inhibiting cross-bridge force production as well as sarcoplasmic reticulum (SR) Ca2+ release (Westerblad et al. 2002).
We have noted a markedly faster decline of force during fatiguing stimulation of isolated whole slow-twitch soleus muscles as compared to single soleus fibres. For instance, tetanic force is decreased to about 40% of the control after 100 repeated tetani in whole soleus muscles (Dahlstedt et al. 2000), whereas a similar stimulation scheme minimally affects force in single soleus fibres (Bruton et al. 2003). To gain further insights into mechanisms of peripheral fatigue, we now investigated possible mechanisms of the faster fatigue development in whole muscles. In an initial series of experiments, isolated whole muscles were fatigued by repeated short tetani while exposed to lactic acid (10 mm) or an increased bath [K+] (10 mm). We hypothesized that application of lactic acid would increase fatigue resistance by preventing action potential propagation failure, whereas exposure to increased [K+] would have the opposite effect. However, neither of these interventions affected the rate of fatigue development. In a subsequent series of experiments, isolated whole muscles and single soleus fibres were fatigued in the presence of cyanide, which inhibits mitochondrial respiration. This resulted in equally fast fatigue development in whole muscles and single fibres, suggesting that limited O2 diffusion plays a key role in fatigue.
Methods
General
Adult, male mice (NMRI strain) were killed by rapid neck disarticulation. All procedures were approved by the Stockholm North local ethical committee. Intact slow-twitch soleus and fast-twitch extensor digitorum longus (EDL) muscles were isolated and mounted at optimum length (i.e. where maximum tetanic force was obtained) in a stimulation chamber (Myobath, World Precision Instruments), which had a volume of 10 ml and was filled with Tyrode solution (see below). The isolated muscles were stimulated with supramaximal, 0.5 ms current pulses delivered via two plate electrodes lying parallel to the long axis of the muscle. Single muscle fibres were dissected from soleus muscles, mounted at optimum length in a stimulation chamber and superfused with Tyrode solution (Bruton et al. 2003). To measure myoplasmic free [Ca2+] ([Ca2+]i), the isolated fibre was injected with the fluorescent indicator indo-1 (Molecular Probes/Invitrogen); the methods for measuring indo-1 fluorescence and translating it to [Ca2+]i are described elsewhere (Bruton et al. 2003).
Solutions
The following Tyrode solution was used under control conditions (mm): NaCl 121; KCl 5.0; CaCl2 1.8; MgCl2 0.5; NaH2PO4 0.4; NaHCO3 24.0; EDTA 0.1; glucose 5.5; 0.2% fetal calf serum. The solution was continuously bubbled with 5% CO2–95% O2, which gives a bath pH of 7.4. Test solutions were prepared by: (i) adding 10 mm lactic acid (bath pH 7.1); (ii) increasing [K+] to 10 mm while decreasing [Na+] to 116 mm; (iii) adding 2 mm sodium cyanide (NaCN).
All reagents and enzymes used in the study were from either Sigma-Aldrich or Boehringer Ingelheim, unless stated otherwise.
Stimulation protocol for whole muscles
Experiments were performed at both 25°C and 35°C, since the effects of high K+ and acidosis on force exhibit large temperature differences (Westerblad et al. 1997; Pedersen et al. 2003). Muscles from both legs were mounted in separate stimulation chambers and allowed to rest for 30 min in standard Tyrode solution. Contractions were induced by stimulating at 50 Hz and 70 Hz for soleus muscles and 70 Hz and 100 Hz for EDL muscles at 25°C and 35°C, respectively; with these frequencies tetanic force was about 80% of the maximum that can be achieved. The tetanus duration was 600 ms for soleus and 300 ms for EDL muscles. After producing a control tetanus, the solution was changed to a test solution with lactic acid or increased [K+] in one chamber, whereas the other muscle remained in standard Tyrode solution. Tetanic contractions were produced at ∼2 min intervals for 20 min, and fatiguing stimulation then started. In experiments where mitochondrial respiration was inhibited with cyanide (Albaum et al. 1946; Sahlin & Katz, 1986; Adler et al. 1999), one muscle was exposed to cyanide for 5 min before fatiguing stimulation started. During fatigue induction, muscles were stimulated at 2 s intervals, and a total of 100 and 50 tetani were given to soleus and EDL muscles, respectively. The more demanding fatiguing stimulation for soleus was used to obtain a similar force decrease in the two muscles.
In a separate set of experiments, fatigue was induced by continuous tetanic stimulation under control conditions and with 10 mm[K+] (25°C). Soleus muscles were then stimulated at 50 Hz for 60 s while EDL muscles were stimulated at 70 Hz for 15 s.
Measurements of metabolites
Soleus and EDL muscles were stimulated, respectively, by repeated 50 Hz or 70 Hz tetani (as described above) in the presence or absence of cyanide (30°C). Muscles were frozen in liquid nitrogen immediately after the cessation of fatiguing stimulation. Muscles were freeze-dried, cleaned from connective tissue, extracted with ice-cold 0.5 m perchloric acid, and centrifuged. The supernatant was neutralized with 2.2 m KHCO3, centrifuged and the latter supernatant was stored at −80°C. Metabolites were analysed with enzymatic techniques adapted for fluorometry, measuring changes in NADH or NADPH as described elsewhere (Bruton et al. 1997). The concentration of metabolites was adjusted to the mean total Cr content (sum of CrP and Cr).
Stimulation protocol for single soleus muscle fibres
The temperature of the solution in the single fibre stimulation chamber was 25–27°C. After being injected with indo-1 (see above), the isolated fibre was allowed to rest for ∼60 min. Cells that, after this rest period, produced less than 90% of the pre-injection tetanic force were discarded. Each fibre was exposed to two series of fatiguing tetani, one in standard Tyrode solution and one after 5 min exposure to cyanide. The order was randomized so that half of the fibres were first fatigued under control conditions and the other half in the presence of cyanide. Fibres were allowed to rest for at least 60 min between the two fatigue runs. Fatigue was induced by giving 100 tetani (70 Hz, 500 ms) at 2 s intervals, except in two fatigue runs in the presence of cyanide where stimulation was stopped when force had decreased to 40% of the initial.
Measurements and statistical analyses
In whole-muscle experiments, we measured the maximum force developed in test contractions and throughout fatiguing stimulation. In single-fibre experiments, force and [Ca2+]i were measured as the mean during the last 100 ms of the tetanus. Data are expressed as mean ± s.e.m. Student's paired t test was used to establish significant differences between treated and control preparations. The significance level was set at P < 0.05.
Results
Application of lactic acid or increasing K+ has no marked effect on fatigue induced by repeated tetanic stimulation
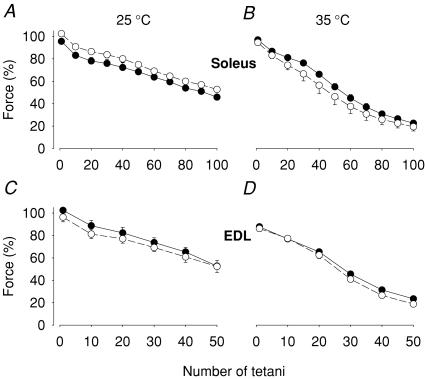
Exposure to lactic acid (10 mm) for 20 min had no significant effect on the tetanic force produced before or during fatiguing stimulation in either soleus or EDL muscles (Fig. 1).
Figure 1. Exposure to lactic acid had no significant impact on fatigue development.
Soleus (A and B) and EDL (C and D) muscles were fatigued by repeated tetani in the presence of 10 mm lactic acid (•) or under control conditions (○). Experiments were performed at 25°C (A and C) or 35°C (B and D). Data represent mean ± s.e.m.; n = 4 at 25°C and 6 at 35°C. The force in each muscle was normalized to that prior to the 20 min ± lactic acid incubation period.
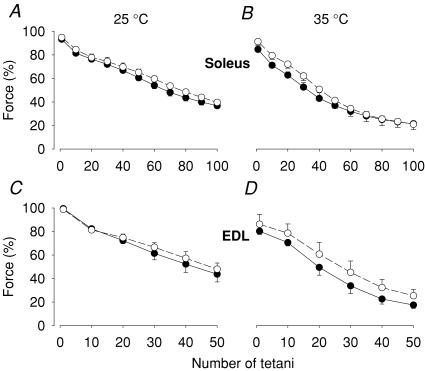
In an early series of experiments, the bath [K+] was increased from 5 to 12 mm (25°C), and after 20 min exposure, tetanic force was decreased by 22.3 ± 6.7% in soleus and 19.7 ± 5.2% in EDL muscles (n = 3). Due to the marked decrease in pre-fatigue force, we did not perform any further experiments with 12 mm K+. Exposure for 20 min to 10 mm K+ at 35°C resulted in some, although not significant, decrease in pre-fatigue tetanic force in both soleus and EDL muscles. Fatigue development was not faster in 10 mm compared to 5 mm K+ (Fig. 2).
Figure 2. Increasing extracellular [K+] had no significant effect on fatigue induced by repeated tetanic contractions.
Soleus (A and B) and EDL (C and D) muscles were fatigued in 10 mm (•) or 5 mm (○) [K+]. Experiments were performed at 25°C (A and C) or 35°C (B and D). Data represent mean ± s.e.m.; n = 6 at 25°C and 5 at 35°C. The force in each muscle was normalized to that prior to the 20 min incubation period in 10 mm or 5 mm[K+].
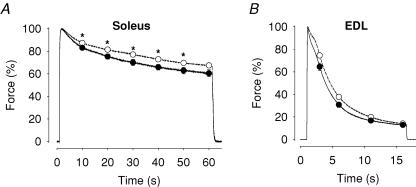
The lack of effect of increased [K+] on fatigue induced by repeated brief tetani was unexpected. We therefore performed an additional set of experiments where fatigue was induced by a continuous tetanic contraction, which, compared to repeated short tetani, induces an increased stress on action potential propagation, especially in the t-tubular system (Lännergren & Westerblad, 1987; Westerblad et al. 1990; Duty & Allen, 1994). With continuous tetanic stimulation, mean force was generally lower in 10 mm than in 5 mm K+ in both soleus and EDL muscles (Fig. 3), but the difference was small and only significant (P < 0.05) in soleus muscles between 10 s and 50 s of stimulation.
Figure 3. The force decrease during continuous tetanic stimulation was slightly larger when the bath [K+] increased.
Soleus (A) and EDL (B) muscles were fatigued by continuous tetanic stimulation in 10 mm (•) or 5 mm (○) [K+]. Experiments were performed at 25°C. Data represent mean ± s.e.m. (n = 4). *Significant difference between 10 mm and 5 mm[K+] (P < 0.05). For each muscle, the maximum force during the continuous tetanus was set to 100%.
Fatigue development in whole muscles is faster in the presence of cyanide
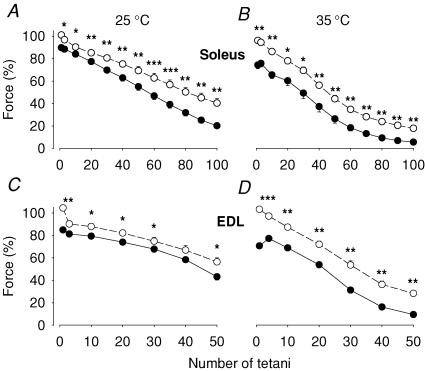
Since neither application of lactic acid nor increasing [K+] had any significant impact on fatigue induced by repeated tetani, it appears that these factors cannot explain the faster fatigue development in isolated whole muscles as compared to single fibres. We therefore investigated the role of hypoxia, by producing fatigue in muscles where mitochondrial respiration was blocked by cyanide. Tetanic force was significantly decreased after 5 min exposure to cyanide in both muscles and at both temperatures (Fig. 4). The forces during fatigue were also markedly smaller in the presence of cyanide. Under control conditions, tetanic force showed a rapid decrease during the first few tetani. This pattern was altered in cyanide-exposed muscles where force initially showed little change or actually increased. To illustrate this difference, measurements of force in the third fatiguing tetanus are included in Fig. 4.
Figure 4. The force during repeated tetanic stimulation was markedly decreased when mitochondrial respiration was blocked by cyanide.
Soleus (A and B) and EDL (C and D) muscles were fatigued in the presence of 2 mm cyanide (•) or under control conditions (○). Experiments were performed at 25°C (A and C) or 35°C (B and D). Data represent mean ± s.e.m. (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 versus control. The force in each muscle was normalized to that prior to the 5 min ± cyanide incubation period.
To confirm that anaerobic metabolism was accelerated in cyanide-exposed muscles, we measured metabolites in soleus and EDL muscles (n = 6). In the fatigued state, CrP was significantly lower in muscles exposed to cyanide than in controls (soleus 2.4 ± 0.5 versus 13.4 ± 1.9 μmol (g dry muscle)−1, P < 0.01; EDL 1.9 ± 0.3 versus 19.6 ± 2.3 μmol (g dry muscle)−1, P < 0.001) and there was a corresponding increase in Pi (soleus 58.7 ± 3.3 versus 40.1 ± 3.0 μmol (g dry muscle)−1, P < 0.01; EDL 88.1 ± 3.9 versus 61.2 ± 2.8 μmol (g dry muscle)−1, P < 0.001). Lactate was also higher in cyanide-exposed than control muscles (soleus 58.1 ± 4.3 versus 37.3 ± 5.2 μmol (g dry muscle)−1, P < 0.05; EDL 102.2 ± 5.5 versus 64.2 ± 1.0 μmol (g dry muscle)−1, P < 0.001). Thus, cyanide-exposed muscles exhibited increased non-oxidative ATP production.
Force declines rapidly during fatiguing stimulation of single soleus fibres in the presence of cyanide
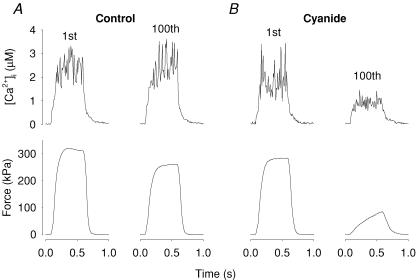
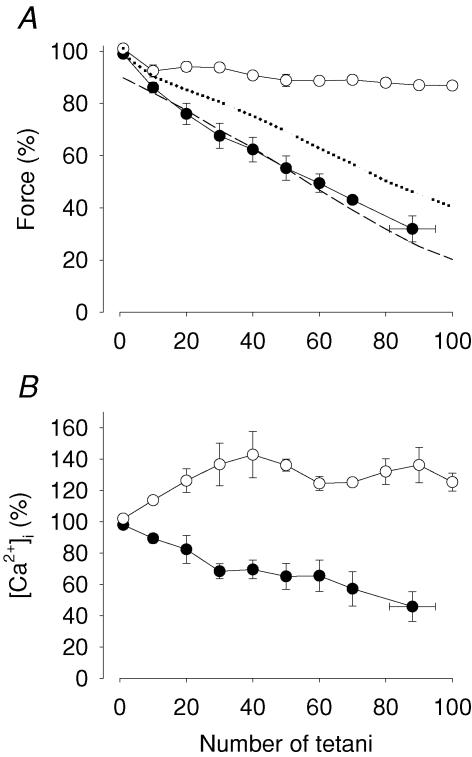
Figure 5 shows representative original records of tetanic [Ca2+]i and force obtained in a single soleus fibre that was first fatigued under control conditions and subsequently, after a 60 min recovery period, in the presence of cyanide. There was a striking difference between the two fatigue runs in that little change in [Ca2+]i and force was observed during the series of 100 tetani in control, whereas both [Ca2+]i and force were substantially decreased in the fatigued state in the presence of cyanide. Mean data (n = 4) showed a decrease in tetanic force of only ∼10% at the end of fatiguing stimulation under control conditions, whereas force was markedly decreased in the presence of cyanide (Fig. 6A). Tetanic [Ca2+]i increased during the initial part of fatiguing stimulation in control, whereas it showed a monotonic decrease in the presence of cyanide (Fig. 6B). Thus, the reduction in tetanic force during fatigue in cyanide is associated with a decreased SR Ca2+ release.
Figure 5. Representative [Ca2+]i and force records obtained from fatigue runs produced in a single soleus fibre under control conditions and subsequently in the presence of cyanide.
Traces obtained from the first and last (100th) tetani of fatiguing stimulation in normal Tyrode solution (A) and in the presence of 2 mm cyanide (B). The fibre was allowed to rest for 60 min between the two fatigue runs.
Figure 6. Force and [Ca2+]i of single soleus fibres decreased rapidly during repeated tetanic stimulation in the presence of cyanide.
Relative tetanic force (A) and [Ca2+]i (B) during fatigue in the presence of 2 mm cyanide (•) or under control conditions (○). Force and [Ca2+]i of the first fatiguing tetanus was set to 100% and data represent mean ± s.e.m. (n = 4). Force and [Ca2+]i of the two groups were significantly different (P at least < 0.01) at all time points except for force at 10 tetani. Dotted and dashed lines in A show mean forces from whole soleus muscles fatigued by repeated tetani at 25°C in control and in the presence of cyanide, respectively (data taken from Fig. 4).
For comparison Fig. 6A also includes mean data from whole soleus muscles fatigued with (dashed line) and without (dotted line) cyanide. It is clear that while whole muscles fatigued much faster than single fibres under control conditions, the rate of fatigue was virtually identical during cyanide exposure.
Discussion
To obtain a better understanding of mechanisms underlying peripheral fatigue, we studied possible causes of the faster fatigue development during repeated tetanic stimulation in isolated whole muscles as compared to single muscle fibres. Our results indicate that the faster fatigue development in whole muscles is not the result of impaired action potential propagation due to increased extracellular [K+]. Instead, the results suggest that fatigue is a consequence of hypoxia, because whole muscles and single muscle fibres fatigued at the same rate when mitochondrial respiration was inhibited by cyanide.
In the first series of experiments, muscles were exposed to 10 mm lactic acid, which is similar to the venous lactate concentration measured in humans during exhaustive exercise (Juel et al. 2004). During the pre-fatigue incubation period, this is expected to result in an influx of lactate and hydrogen ions mainly via the lactate–H+ cotransporter (Juel, 1997), resulting in a decrease in intracellular pH of ∼0.2 pH units (Nielsen et al. 2001). During the subsequent fatiguing stimulation period, the added lactic acid would hinder the export of lactate and hydrogen ions produced by glycogenolysis and in this way exaggerate the fatigue-induced acidification. Nevertheless, the addition of lactic acid did not have any marked effect on fatigue development in either soleus or EDL muscles, which agrees with recent results on rat soleus muscles (Kristensen et al. 2005). Thus, these results support the conclusion that accumulation of lactate and hydrogen ions is not a major cause of fatigue (Pate et al. 1995; Bangsbo et al. 1996; Bruton et al. 1998; Posterino et al. 2001; Lamb, 2002; Westerblad et al. 2002). Furthermore, the present results do not support the hypothesis that acidosis may delay fatigue development by improving action potential propagation (Nielsen et al. 2001; Pedersen et al. 2004, 2005). One likely reason for this is that action potential propagation failure is not a limiting factor during fatigue induced by repeated, brief tetani, and this will be discussed below.
In the next series of experiments, muscles were exposed to an increased [K+] of 10 mm, which is similar to the maximum venous plasma [K+] during intense exercise (Sejersted & Sjøgaard, 2000). During exercise each action potential results in efflux of K+ (and influx of Na+), and hence the interstitial [K+] in muscles will increase and be higher than that in the venous plasma (Sejersted & Sjøgaard, 2000). In fact, interstitial [K+] > 10 mm has been measured during intense exercise with microdialysis probes (Juel et al. 2000; Nielsen et al. 2004). Thus, it is clear that during repeated contractions, extracellular [K+] can reach levels that markedly depress force production when applied to isolated muscles stimulated at long intervals (Clausen et al. 1993). Having this in mind, we expected that force would decrease more rapidly during fatigue when [K+] of the bath solution was increased from 5 mm to 10 mm. However, the force decrease during repeated tetanic stimulation was not affected by the increased bath [K+] (Fig. 2). One possible mechanism behind this unexpected result involves the Na+–K+ pumps. These are activated by increases in extracellular [K+] and intracellular [Na+] (Sejersted & Sjøgaard, 2000; Clausen, 2003). When rested fibres are exposed to increased [K+], there is no immediate increase in intracellular [Na+], and the Na+–K+ pumps are therefore not fully activated. During repeated contractions, on the other hand, both extracellular [K+] and intracellular [Na+] increases, which effectively activates the pumps (Clausen et al. 1998). Furthermore, repeated contractions appear to increase the sensitivity of the pumps to intracellular [Na+] (Clausen, 2003). Since Na+–K+ pumping is electrogenic and causes a hyperpolarization, effective activation of the pumps would counteract the depolarization induced by increasing [K+] during fatiguing stimulation (Sejersted & Sjøgaard, 2000; Clausen, 2003).
In an additional set of experiments, muscles were fatigued by a continuous tetanic contraction which, compared to repeated short tetani, induces an increased stress on action potential propagation, especially in the t-tubular system (Westerblad et al. 1990; Duty & Allen, 1994). In these experiments, mean force was more reduced at increased bath [K+], but the difference compared to normal [K+] was limited (Fig. 3). Thus, the present results indicate that action potential failure due to increased extracellular or t-tubular [K+] is not a key factor in skeletal muscle fatigue.
The O2 delivery to muscle cells of isolated whole muscles depends on diffusion from the surface of the muscle. Therefore muscle cells in deeper parts of isolated muscles will experience a hypoxic/anoxic milieu, especially during fatiguing stimulation when O2 demand is increased (Barclay, 2005). Isolated whole muscles would then depend more on anaerobic metabolism during fatiguing stimulation, and this could lead to faster fatigue development. Bearing this in mind, we compared fatigue development in the absence and presence of cyanide, which reversibly blocks mitochondrial respiration (Adler et al. 1999). Both soleus and EDL muscles displayed a larger force decrease during fatigue in the presence of cyanide (Fig. 4), which indicates that aerobic metabolism occurred in both muscles, and that this reduced the force decline during fatigue. Accordingly, our measurement of metabolites in fatigued soleus and EDL muscles showed a larger decrease of CrP and increase of Pi and lactate ions in the presence than in the absence of cyanide. We also studied fatigue in single soleus fibres, where problems with O2 diffusion do not exist. Under control conditions, isolated soleus fibres exhibited only limited force decrease during fatiguing stimulation (Figs 5 and 6), which is consistent with the idea that the faster fatigue development in whole muscles is due to hypoxia. This idea was further supported by the fact that single soleus fibres fatigued markedly faster when mitochondrial respiration was inhibited by cyanide. In fact, they then fatigued at a rate similar to that of cyanide-exposed whole soleus muscles (see Fig. 6A). Moreover, a markedly accelerated fatigue development in the presence of cyanide has previously been observed in single fast-twitch fibres of mouse flexor digitorum brevis muscles (Lännergren & Westerblad, 1991; Westerblad & Allen, 1991). Thus, these results indicate that hypoxia, and the resultant increased dependency on anaerobic metabolism, plays a central role in fatigue. Since lactate and hydrogen ions had no significant impact on fatigue development in the present experiments (see above), the link between anaerobic metabolism and faster fatigue development most likely involves a net breakdown of ATP or increased CrP hydrolysis leading to accumulation of Pi.
Numerous studies on skinned muscle fibres have shown that increased [Pi] reduces both the cross-bridge force production and the myofibrillar Ca2+ sensitivity (Cooke & Pate, 1985; Millar & Homsher, 1990). Thus, direct effects of increased [Pi] on the contractile machinery may contribute to the more pronounced decrease in force during fatigue in the presence of cyanide.
A faster fatigue development accompanied by decreased tetanic [Ca2+]i has been observed in cyanide-exposed single fast-twitch fibres of mouse flexor digitorum brevis muscles (Westerblad & Allen, 1991). In line with this, the present results show a marked decrease of tetanic [Ca2+]i during fatigue of single soleus fibres exposed to cyanide that did not occur under control conditions (Figs 5 and 6). Thus, one reason for the accelerated force decrease during fatigue in cyanide is impaired SR Ca2+ release. There are several mechanisms by which decreases in [ATP] or [CrP] or increased [Pi] can reduce SR Ca2+ release; for instance (i) a net breakdown of ATP and an associated increase in [Mg2+] and ATP breakdown products may inhibit the SR Ca2+ release channels (Blazev & Lamb, 1999; Laver et al. 2001), and (ii) increased [Pi] may result in Ca2+–Pi precipitation in the SR lumen and hence a decrease in the [Ca2+] available for release (Fryer et al. 1995; Dahlstedt & Westerblad, 2001; Dahlstedt et al. 2003; Dutka et al. 2005). Alternatively, decreased [CrP] and a net ATP breakdown may impair action potential propagation, and hence decrease SR Ca2+ release, via mechanisms that are not directly dependent on an increased extracellular [K+]. For example, a decreased energy buffering may decrease the action potential amplitude by limiting the hyperpolarizing action of the ATP-driven Na+–K+ pumps or by opening of ATP-sensitive K+ channels (KATP channels); both of these mechanisms have been associated with fatigue (Duty & Allen, 1995; Gong et al. 2003; Petersen et al. 2005). Further experiments are required to distinguish between these possible causes of decreased SR Ca2+ release in hypoxic muscles.
One important question that arises from the present results is: which type of fatiguing pattern resembles that in vivo, the relatively fast decline in force seen in whole muscles or the fatigue-resistant pattern observed in single fibres? Mouse soleus muscles consist of slow-twitch type 1 and fast-twitch type 2A fibres (Marechal & Beckers-Bleukx, 1993). Motor units with these fibre types show little force decrease when they are fatigued by repeated tetanic contractions in situ (Burke et al. 1973; Kugelberg & Lindegren, 1979). Furthermore, during fatigue induced by repeated tetani, force was only reduced by 20% after 120 tetani in rat soleus muscles stimulated in situ (Roy et al. 2002), which compares to a 60% decrease after ∼80 tetani in isolated rat soleus muscles stimulated with a similar protocol in vitro (Lunde et al. 2001). Thus, the limited force decline during fatigue in isolated single fibres more closely resembles that in vivo, whereas isolated whole muscles fatigue prematurely due predominantly to limitations in the O2 diffusion to deeper parts of the muscle.
Acknowledgments
The present study was supported by the Swedish Research Council (proj 3642 & 10842), the Swedish National Center for Sports Research, and Funds at the Karolinska Institutet.
References
- Adler M, Lebeda FJ, Kauffman FC, Deshpande SS. Mechanism of action of sodium cyanide on rat diaphragm muscle. J Appl Toxicol. 1999;19:411–419. doi: 10.1002/(sici)1099-1263(199911/12)19:6<411::aid-jat597>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Albaum HG, Tepperman J, Bodansky O. The in vivo inactivation by cyanide of brain cytochrome oxidase and its effect on glycolysis and on the high energy phosphorus compounds in brain. J Biol Chem. 1946;164:45–51. [PubMed] [Google Scholar]
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Madsen K, Kiens B, Richter EA. Effect of muscle acidity on muscle metabolism and fatigue during intense exercise in man. J Physiol. 1996;495:587–596. doi: 10.1113/jphysiol.1996.sp021618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay CJ. Modelling diffusive O2 supply to isolated preparations of mammalian skeletal and cardiac muscle. J Muscle Res Cell Motil. 2005;26:225–235. doi: 10.1007/s10974-005-9013-x. [DOI] [PubMed] [Google Scholar]
- Blazev R, Lamb GD. Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres. J Physiol. 1999;520:203–215. doi: 10.1111/j.1469-7793.1999.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J, Westerblad H. Effects of CO2-induced acidification on the fatigue resistance of single mouse muscle fibers at 28°C. J Appl Physiol. 1998;85:478–483. doi: 10.1152/jappl.1998.85.2.478. [DOI] [PubMed] [Google Scholar]
- Bruton JD, Tavi P, Aydin J, Westerblad H, Lännergren J. Mitochondrial and myoplasmic Ca2+ in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Wretman C, Katz A, Westerblad H. Increased tetanic force and reduced myoplasmic [Pi] following a brief series of tetani in mouse soleus muscle. Am J Physiol. 1997;272:C870–C874. doi: 10.1152/ajpcell.1997.272.3.C870. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., III Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T. Na+–K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+–K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Nielsen OB, Harrison AP, Flatman JA, Overgaard K. The Na+,K+ pump and muscle excitability. Acta Physiol Scand. 1998;162:183–190. doi: 10.1046/j.1365-201X.1998.0295e.x. [DOI] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Tavi P, Westerblad H. Creatine kinase injection restores contractile function in creatine-kinase-deficient mouse skeletal muscle fibres. J Physiol. 2003;547:395–403. doi: 10.1113/jphysiol.2002.034793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of skeletal muscle deficient of creatine kinase. FASEB J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Westerblad H. Inhibition of creatine kinase reduces the rate of fatigue-induced decrease in tetanic [Ca2+]i in mouse skeletal muscle. J Physiol. 2001;533:639–649. doi: 10.1111/j.1469-7793.2001.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium-phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–C1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Duty S, Allen DG. The distribution of intracellular calcium concentration in isolated single fibres of mouse skeletal muscle during fatiguing stimulation. Pflugers Arch. 1994;427:102–109. doi: 10.1007/BF00585948. [DOI] [PubMed] [Google Scholar]
- Duty S, Allen DG. The effects of glibenclamide on tetanic force and intracellular calcium in normal and fatigued mouse skeletal muscle. Exp Physiol. 1995;80:529–541. doi: 10.1113/expphysiol.1995.sp003865. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gong B, Legault D, Miki T, Seino S, Renaud JM. KATP channels depress force by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol Cell Physiol. 2003;285:C1464–C1474. doi: 10.1152/ajpcell.00278.2003. [DOI] [PubMed] [Google Scholar]
- Juel C. Lactate–proton cotransport in skeletal muscle. Physiol Rev. 1997;77:321–358. doi: 10.1152/physrev.1997.77.2.321. [DOI] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–E251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kristensen M, Albertsen J, Rentsch M, Juel C. Lactate and force production in skeletal muscle. J Physiol. 2005;562:521–526. doi: 10.1113/jphysiol.2004.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E, Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Lamb GD. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil. 2002;23:81–91. doi: 10.1023/a:1019932730457. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. Action potential fatigue in single skeletal muscle fibres of Xenopus. Acta Physiol Scand. 1987;129:311–318. doi: 10.1111/j.1748-1716.1987.tb08074.x. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Lenz GKE, Lamb GD. Regulation of the calcium release channel from rabbit skeletal muscle by the nucleotides ATP, AMP, IMP and adenosine. J Physiol. 2001;537:763–778. doi: 10.1111/j.1469-7793.2001.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde PK, Dahlstedt AJ, Bruton JD, Lännergren J, Thorén P, Sejersted OM, Westerblad H. Contraction and intracellular Ca2+ handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res. 2001;88:1299–1305. doi: 10.1161/hh1201.092041. [DOI] [PubMed] [Google Scholar]
- Marechal G, Beckers-Bleukx G. Force-velocity relation and isomyosins in soleus muscles from two strains of mice (C57 and NMRI) Pflugers Arch. 1993;424:478–487. doi: 10.1007/BF00374911. [DOI] [PubMed] [Google Scholar]
- Millar NC, Homsher E. The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Biol Chem. 1990;265:20234–20240. [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol. 2004;554:857–870. doi: 10.1113/jphysiol.2003.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol. 1995;486:689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Clausen T, Nielsen OB. Loss of force induced by high extracellular [K+] in rat muscle: effect of temperature, lactic acid and β2-agonist. J Physiol. 2003;551:277–286. doi: 10.1113/jphysiol.2003.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, de Paoli F, Nielsen OB. Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol. 2005;125:237–246. doi: 10.1085/jgp.200409173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Murphy KT, Snow RJ, Leppik JA, Aughey RJ, Garnham AP, Cameron-Smith D, McKenna MJ. Depressed Na+–K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+–K+-ATPase mRNA expression following intense exercise. Am J Physiol Regul Integr Comp Physiol. 2005;289:R266–R274. doi: 10.1152/ajpregu.00378.2004. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Dutka TL, Lamb GD. L(+)-lactate does not affect twitch and tetanic responses in mechanically skinned mammalian muscle fibres. Pflugers Arch. 2001;442:197–203. doi: 10.1007/s004240100528. [DOI] [PubMed] [Google Scholar]
- Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR. Mechanical properties of the electronically silent adult rat soleus muscle. Muscle Nerve. 2002;26:404–412. doi: 10.1002/mus.10219. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Katz A. The content of NADH in rat skeletal muscle at rest and after cyanide poisoning. Biochem J. 1986;239:245–248. doi: 10.1042/bj2390245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Lännergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause? News Physiol Sci. 2002;17:17–21. doi: 10.1152/physiologyonline.2002.17.1.17. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lee JA, Lamb AG, Bolsover SR, Allen DG. Spatial gradients of intracellular calcium in skeletal muscle during fatigue. Pflugers Arch. 1990;415:734–740. doi: 10.1007/BF02584013. [DOI] [PubMed] [Google Scholar]