Abstract
Neural activity plays an important role in regulating synaptic strength and neuronal membrane properties. Attempts to establish guiding rules for activity-dependent neuronal changes have led to such concepts as homeostasis of cellular activity and Hebbian reinforcement of synaptic strength. However, it is clear that there are diverse effects resulting from activity changes, and that these changes depend on the experimental preparation, and the developmental stage of the neural circuits under study. In addition, most experimental evidence on activity-dependent regulation comes from reduced preparations such as neuronal cultures. This review highlights recent results from studies of the intact mammalian auditory system, where changes in activity have been shown to produce alterations in synaptic and membrane properties at the level of individual neurons, and changes in network properties, including the formation of tonotopic maps.
It is generally assumed that sensory input to the developing mammalian brain shapes the strength of synaptic connections and the membrane properties of neurons. However, although there is a wealth of experimental data showing that the gross aspects of neural pathways are dependent on sensory input, there is very little in vivo information on the effects of activity during development at the fundamental membrane and channel level, and much of our knowledge has come from neuronal culture systems. In addition to the familiar Hebbian plasticity, a prominent hypothesis to emerge from recent studies is the concept of homeostasis (Burrone & Murthy, 2003; Murphy, 2003; Turrigiano & Nelson, 2004; Thiagarajan et al. 2005). Homeostasis is commonly used to describe the attempt of a neuron to regulate its average firing rate to some ‘desired’ set point, in response to a change in the activity of the cell. The hypothetical homeostatic response may manifest itself as a change in the strength of synaptic inputs, and/or a change in the properties of the postsynaptic neuron. The basic premise of homeostasis is that, if the average firing rate of a neuron decreases, then the system will compensate by increasing the firing rate back to the ‘desired’ level by mechanisms that may include (1) increasing the strength of excitatory inputs, decreasing the strength of inhibitory inputs, or increasing the ratio of excitation to inhibition, and/or by (2) increasing the excitability of the postsynaptic neuron. (The opposite changes are proposed to occur as a consequence of an increase in the firing rate of a neuron.) In support of this hypothesis, a variety of experimental studies have shown that reduction in the firing rate of a neuron leads to an enhancement in the strength of excitatory inputs and a decrease in the strength of inhibitory inputs to that neuron. A change in synaptic strength may occur through a change in the total number of synaptic contacts, a change in presynaptic release, and/or a change in the postsynaptic response to neurotransmitter release. Distinct from homeostasis, Hebbian plasticity involves the strengthening of synaptic transmission through co-ordinated pre- and post-synaptic activity, and there is ample evidence for this process. Experimental manipulation of neuronal activity has led to a variety of effects, including changes in quantal size, attributed to changes in postsynaptic receptors (O'Brien et al. 1998; Turrigiano et al. 1998; Watt et al. 2000; Leslie et al. 2001; Kilman et al. 2002; Wierenga et al. 2005) or presynaptic changes in the amount of neurotransmitter packaged into vesicles (de Gois et al. 2005; Wang et al. 2005; Wilson et al. 2005), changes in quantal content without a change in quantal size (Paradis et al. 2001; Bacci et al. 2001), changes in synaptic size (Murthy et al. 2001) and changes in postsynaptic membrane properties (Daoudal & Debanne, 2003; Saar & Barkai, 2003; Zhang & Linden, 2003). Changes in neuronal excitability may occur through a variety of means, including a change in passive membrane properties (capacitance and resistance) or changes in voltage-activated currents (Daoudal & Debanne, 2003; Saar & Barkai, 2003; Zhang & Linden, 2003). In addition, morphological changes may alter the electrotonic architecture of the neuron. Experimental evidence has shown that changes in activity may alter the magnitude of voltage-activated sodium, potassium and calcium currents, and hyperpolarization-activated (Ih) currents (Daoudal & Debanne, 2003; Saar & Barkai, 2003; Zhang & Linden, 2003). For example, Desai et al. (1999) have demonstrated that, in visual cortex cultures, silencing of activity with TTX leads to a down-regulation of potassium currents and an up-regulation of sodium currents, with a resultant increase in cell excitability.
Thus, a variety of model systems have been used to demonstrate that activity can alter synaptic strength (both pre- and post-synaptically) and postsynaptic cell excitability. Some of these results are consistent with a so-called ‘homeostatic response’, and some are consistent with opposing mechanisms, such as ‘Hebbian’ strengthening of active synapses (Burrone & Murthy, 2003; Murphy, 2003). Furthermore, there is evidence that the response of a neuron to a change in activity may be different during development than in maturity (Burrone et al. 2002; Murphy, 2003). Despite these complications, recent studies, primarily using neuronal cultures, emphasize that the predominant response to a reduction in neuronal activity is a postsynaptic increase in quantal size (Turrigiano & Nelson, 2004). This raises the issue of what happens in the intact nervous system (Desai et al. 2002). This review highlights recent results from studies of the mammalian auditory system, in particular using deafness as a model of reduced or abolished sensory input during development. The results reveal that altered activity during development has multiple effects, including changes in excitatory and/or inhibitory synaptic transmission, and postsynaptic membrane properties. Furthermore, these changes may be different in different neuronal types, as previously described for long-term plasticity in the dorsal cochlear nucleus by Tzounopoulos et al. (2004). In addition, the results show that spontaneous activity during development is necessary for the proper formation of neural circuits (tonotopic maps) in central auditory nuclei.
Auditory pathways in the mammalian brainstem
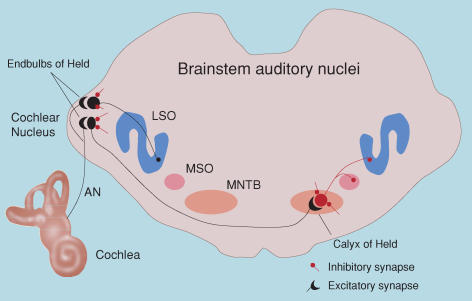
Figure 1 illustrates the pathways in the mammalian brainstem that contribute to the initial central processing of auditory input. Sound entering the ear stimulates hair cells in the cochlea. Hair cells make synaptic connection via special ribbon synapses with spiral ganglion cells, which in turn give rise to the primary auditory nerve fibres carrying auditory information to the brain. Under normal circumstances, the ribbon synapses continually release neurotransmitter, even in the absence of sound stimulation, and this results in the generation of spontaneous nerve impulses in the auditory nerves. The rate of spontaneous nerve impulses varies between auditory nerve fibres, but can reach high rates (100 Hz or more; Liberman, 1991). Spontaneous activity occurs before the opening of the ear canal and the onset of airborne sound-generated responses.
Figure 1. Major pathways in the mammalian auditory brainstem.
This schematic shows the auditory nerve (AN) arising from the cochlea and making monosynaptic connections with neurons in the cochlea nucleus of the brainstem (AVCN – anteroventral cochlear nucleus). Also shown are some of the major brainstem auditory nuclei: the medial nucleus of the trapezoid body (MNTB), the medial superior olive (MSO) and the lateral superior olive (LSO). Auditory nerve fibres make large excitatory synaptic contacts, the endbulbs of Held, with bushy cells in the AVCN. Globular bushy cells in the AVCN, in turn, make large calyceal contacts, the calyces of Held, with principal cells in the contralateral MNTB. Spherical bushy cells make ipsilateral contacts with the dendrites of neurons in the MSO. Excitatory neurons and synapses are shown in black; inhibitory neurons and synapses are shown in red.
An important aspect of the auditory system is the existence of tonotopic maps. The cochlea is arranged with the basal hair cells responding to highest sound frequencies and apical hair cells responding best to low frequencies. This spatial arrangement is maintained centrally, and most of the brainstem auditory nuclei are organized topographically according to their best response to acoustic frequencies (i.e. tonotopically). For example, the medial nucleus of the trapezoid body (MNTB) is organized with medial cells responding better to higher frequencies than lateral cells.
Figure 1 illustrates the major brainstem auditory nuclei and connections, highlighting several large synaptic connections which have received much attention for study; the endbulb of Held connection between auditory nerve fibres and bushy cells in the anteroventral cochlear nucleus (AVCN), and the calyx of Held connection arising from bushy cells and contacting principal cells in the MNTB (Yin, 2002). At these connections, both pre- and post-synaptic properties can be assessed directly (e.g. Forsythe, 1994; Isaacson & Walmsley, 1995; Borst & Sakmann, 1996; Sakaba et al. 2002; Schneggenburger et al. 2002; Taschenburger et al. 2002). In addition to large excitatory terminals, AVCN and MNTB neurons also receive many small inhibitory boutons (Leao et al. 2004b). MNTB principal neurons, in turn, send inhibitory inputs to the lateral superior olive (LSO) and medial superior olive (MSO). These pathways are involved primarily in sound localization, for which precise timing is required, involving both excitatory and inhibitory inputs (Friauf & Lohmann, 1999; FitzGerald et al. 2001; McAlpine et al. 2001; Paolini et al. 2001, 2004; Brand et al. 2002; Yin, 2002; Rubel & Fritzsch, 2002; Grothe, 2003; Kim & Kandler, 2003; McAlpine & Grothe, 2003; Mauk & Buonomano, 2004; Kandler & Gillespie, 2005).
Deafness-induced changes in central auditory pathways
Insight into the role of activity in regulating synaptic transmission and neuronal membrane properties has been gained by studying the effects of eliminating or reducing auditory nerve activity i.e. deafness. These studies have revealed a variety of changes in central auditory neurons following experimentally induced or naturally occurring deafness.
Cochlear ablation studies
Cochlear ablation, which destroys the hair cells and spiral ganglion cells (and hence the auditory nerve), has been reported to increase the excitability of brainstem auditory neurons (McAlpine et al. 1997; Kotak et al. 2005), reduce (Suneja et al. 1998; Mossop et al. 2000; Vale & Sanes, 2002; Vale et al. 2003) or increase inhibitory transmission (Suneja et al. 1998), increase (Vale & Sanes, 2002) or decrease (Kotak & Sanes, 1997) excitatory synaptic transmission, transiently reduce NMDA receptor expression (Nakagawa et al. 2000), reduce potassium-dependent chloride transport (Vale et al. 2003), depolarize neurons (Francis & Manis, 2000; Vale & Sanes, 2002), increase input resistance (Francis & Manis, 2000), elevate tyrosine kinase B levels (Suneja & Potashner, 2002), alter synaptic morphology (Russell & Moore, 2002), cause cell death (Hashisaki & Rubel, 1989; Mostafapour et al. 2000), alter GAP-43 expression (Illing et al. 1997), reduce protein synthesis (Sie & Rubel, 1992) and reduce calretinin expression (Zettel et al. 2003). These studies emphasize the wide range of possible effects resulting from the complete removal of the auditory nerve, as is the case for the recent in vitro models of altered activity. However, cochlear ablation is a severe method of silencing auditory nerve input, and recently, investigations have been extended to the study of deaf mutant animals. The results of these studies are the focus of the remainder of this review.
Naturally occurring deafness
There are a variety of naturally occurring animal models of deafness, some of which exhibit deficits from birth, and others which show age-related hearing loss (Keats & Berlin, 1999). DBA mice exhibit age-related hearing loss, beginning with a loss of high-frequency responses in spiral ganglion cells in the cochlea. Wang & Manis (2005) found that, in the AVCN of old DBA mice, synaptic transmission at the endbulb of Held–bushy cell connection is impaired; spontaneous mEPSC frequency is reduced, mEPSCs are slower and smaller, and release probability is lower in old DBA mice compared with young DBA mice. Wang & Manis (2005) suggested that auditory nerve activity regulates presynaptic release probability and postsynaptic receptor composition and kinetics at the endbulb of Held synapse. These results are opposite to those expected from a ‘homeostatic’ mechanism as commonly defined, and are more consistent with a ‘Hebbian’ reduction in synaptic strength due to a lack of correlated firing of pre- and post-synaptic cells.
Related structural analyses have examined synaptic features that may correlate with synaptic strength. Shaker-2 mice have dysfunctional cochlear hair cells from birth, and electron microscopy shows that the endbulbs of Held in adult deaf mice exhibit fewer vesicles and larger postsynaptic densities (Lee et al. 2003). This is similar to deaf white cats (Ryugo et al. 1997), which have endbulbs with a reduced synaptic vesicle density, structural abnormalities in endulb mitochondria, thickening of the pre- and post-synaptic densities, and enlargement of synaptic size. Ryugo et al. (1998) further demonstrated in the deaf white cats that there is a correlation between the amount of auditory nerve activity and the degree of abnormality in endbulb morphology. This is consistent with observations in normal cats in which endbulbs arising from auditory nerve fibres with a high spontaneous discharge rate are larger, with more synaptic specializations (Ryugo et al. 1996). These results support the proposal that activity during development induces the endbulbs to grow and generate more release sites, perhaps through the splitting of large specializations into multiple smaller specializations. Again, the functional consequences associated with these changes in synaptic structure appear to be opposite to the result expected from a homeostatic mechanism. Ryugo's group also studied small bouton terminals in the AVCN of deaf white cats (Redd et al. 2002). Compared with normal-hearing cats, bouton endings of congenitally deaf cats were smaller, but there was no difference in synaptic vesicle density or size of synaptic specializations. These anatomical observations indicate that, while synaptic structure may be different in congenitally deaf animals, the differences depend on the type of synaptic connection and/or the type of postsynaptic neuron.
Physiological aspects of synaptic transmission have been examined in the AVCN and MNTB of congenitally deaf (dn/dn) mice, which lack spontaneous and acoustically driven input due to cochlea hair cell dysfunction (Bock et al. 1982). The large excitatory calyceal synapse, the endbulb of Held, formed with AVCN bushy cells, has been studied by Oleskevich & Walmsley (2002) who found that, in congenitally deaf mice, the amplitude of evoked EPSCs is larger, due to an increased presynaptic release probability; a result that is consistent with homeostasis. There is also a much greater occurrence of delayed asynchronous mEPSCs following a train of stimuli (Fig. 2). These differences may be explained by impaired calcium buffering in the presynaptic terminal. In contrast to the results on the effects of reduced activity in neuronal culture systems, there appears to be no postsynaptic difference in quantal size, since the amplitude and time course of mEPSCs are the same in dn/dn and normal mice. Interestingly, no difference was found in synaptic transmission at the calyx of Held connection with MNTB principal cells, between normal and deaf mice (Oleskevich et al. 2004; Youssoufian et al. 2005). This is surprising as globular bushy cells give rise to the calyces of Held, and these cells have lost their major excitatory drive (the endbulbs of Held).
Figure 2. Excitatory synaptic transmission is greater in deaf mice.
A, reconstructions from electron micrographs of 4 different endbulbs of Held contacting a bushy cell in the AVCN. B shows the individual synaptic specializations contained within the boutons shown in A. C shows that both AMPA and NMDA components of the synaptic current arising from individual auditory nerve fibres are larger in deaf (dn/dn) mice. D illustrates that delayed asynchronous spontaneous release is much larger in deaf mice (insets). (A adapted from Nicol & Walmsley, 2002, with permission from Blackwell Publishing Ltd, B–D adapted from Oleskevich & Walmsley, 2002, with permission from Blackwell Publishing Ltd.)
In contrast to the lack of difference at the calyx of Held–MNTB cell connection, there are differences in the glycinergic inhibitory synaptic input to MNTB neurons between normal and deaf mice (Leao et al. 2004b). The amplitude of mIPSCs is smaller and the time course is slower in MNTB neurons from deaf mice, but their frequency is much higher than normal. The altered kinetics suggests a delay in the normal developmental glycine receptor subunit switch from alpha2 to alpha1, since heteromers with alpha1 subunits exhibit faster kinetics. However, anatomical data shows that there is also a much greater number of glycinergic synapses on MNTB neurons in deaf mice, which argues against a simple developmental delay due to a lack of activity (Leao et al. 2004b). A greater frequency of mIPSCs and a larger number of inhibitory synapses seems to be opposite to the result expected from a homeostatic response to a decreased excitatory drive to these cells.
Membrane properties of auditory brainstem neurons in deaf mice
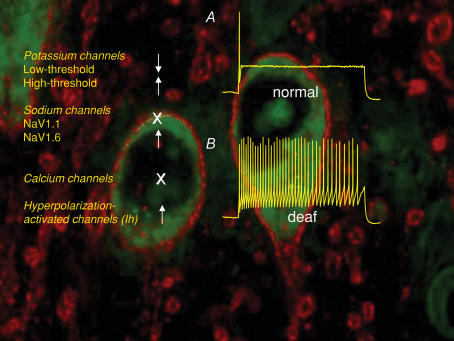
The postsynaptic membrane properties have also been examined in the AVCN and MNTB of normal and deaf (dn/dn) mice. In the AVCN, there appears to be no significant difference in the passive or active membrane properties of bushy cells, despite the loss of powerful excitatory auditory nerve input. However, MNTB principal cells in dn/dn mice exhibit a substantial increase in excitability, which is primarily due to smaller low-threshold potassium currents (Fig. 3; Dodson et al. 2002; Leao et al. 2004a). In addition, there is an increase in the high-threshold potassium currents and hyperpolarization-activated currents in the MNTB of dn/dn mice (Leao et al. 2004a, 2005b; see also Wang et al. 1998; Li et al. 2001; Lu et al. 2004). No differences were found between normal and deaf mice in voltage-activated calcium currents in the AVCN or MNTB (Leao et al. 2004a). These results show that a reduction in excitatory drive to auditory neurons can lead to no change (AVCN) or an increase (MNTB) in postsynaptic membrane excitability.
Figure 3. MNTB neurons are more excitable in MNTB neurons.
A illustrates that MNTB neurons usually respond to depolarizing currents with a single or a few, action potentials, whereas MNTB neurons in deaf (dn/dn) mice respond with multiple action potentials. Panels on the left indicate the up- (arrows up), down- (arrows down) or no change (X) in the magnitude of voltage-activated currents in MNTB neurons from deaf cf. normal mice. Background immunolabelling shows HCN1 immunoreactivity (red) on LSO neurons (green).
Tonotopic maps are disrupted in congenital deafness
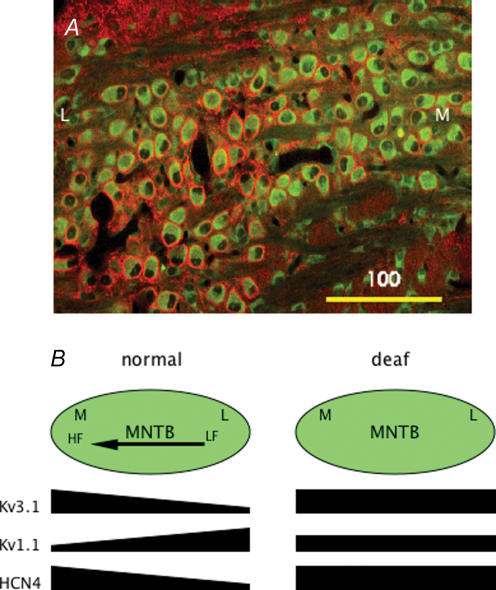
As indicated above, neurons in brainstem auditory nuclei such as the AVCN and MNTB are tonotopically organized. The membrane properties of neurons within a particular nucleus are systematically different, an arrangement which has previously been demonstrated in cochlea hair cells (Pantelias et al. 2001). von Hehn et al. (2004) used immunolabelling to show that the normal gradient of high-threshold potassium channel expression in MNTB neurons is disrupted in age-related hearing loss. Recently, Brew & Forsythe (2005) described a tonotopic gradient of low-threshold potassium currents in MNTB neurons (see also Barnes-Davies et al. 2004). Leao et al. (2005a) have recently reported that there are gradients of low- and high-threshold potassium currents, and hyperpolarization-activated currents in the MNTB of normal mice. These gradients do not all increase in the same direction. Importantly, all gradients appear to be absent in dn/dn mice (see Fig. 4). This demonstrates that spontaneous activity during development plays a critical role in the expression of gradients of voltage-activated currents and the formation of tonotopic maps.
Figure 4. Tonotopic gradients of channel expression are disrupted in deaf mice.
A illustrates an obvious medial (M) to lateral (L) gradient in pre- and post-synaptic immunolabelling of Kv3.4 channels (red) in MNTB neurons from a normal mouse. B illustrates a summary schematic (adapted from Leao et al. 2005a, with permission from Blackwell Publishing Ltd) of the MNTB (green shading) in normal and deaf (dn/dn) mice. Left panel shows the tonotopic (HF, high frequency; LF, low frequency) gradient of high-threshold potassium currents (Kv3.1), low-threshold potassium currents (Kv1.1) and hyperpolarization-activated currents (HCN4). Right panel shows that these gradients are not present in deaf (dn/dn) mice.
Conclusion
It is clear that neurons and circuits in the intact nervous system do not respond in a universal, stereotypical manner in response to a change in activity. Although the relevance of homeostasis and Hebbian processes to these responses both during development and in the mature nervous system is uncertain, the auditory system offers a valuable opportunity to investigate the mechanisms underlying activity-dependent changes in synaptic strength and membrane excitability. In this context, it is interesting to note that a recent study has demonstrated the restoration of synapses in congenitally deaf cats by stimulation of the auditory nerve by cochlear implants (Ryugo et al. 2005).
Acknowledgments
We would like to thank Mark Rich for invaluable discussions related to this review. This work was supported by NIH grant NS25547.
References
- Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. doi: 10.1523/JNEUROSCI.21-17-06588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Barker MC, Osmani F, Forsythe ID. Kv1 currents mediate a gradient of principal neuron excitability across the tonotopic axis in the rat lateral superior olive. Eur J Neurosci. 2004;19:325–333. doi: 10.1111/j.0953-816x.2003.03133.x. [DOI] [PubMed] [Google Scholar]
- Bock GR, Frank MP, Steel KP. Preservation of central auditory function in the deafness mouse. Brain Res. 1982;239:608–612. doi: 10.1016/0006-8993(82)90536-4. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Calcium influx and transmitter release at a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature. 2002;417:543–547. doi: 10.1038/417543a. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hear Res. 2005;206:116–132. doi: 10.1016/j.heares.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Burrone J, Murthy VN. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 2003;13:560–567. doi: 10.1016/j.conb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Burrone J, O'Byrne, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;240:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem. 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- de Gois S, Schafer MK-H, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nature Neuroscience. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald JV, Burkitt AN, Clark GM, Paolini AG. Delay analysis in the auditory brainstem of the rat: comparison with click latency. Hear Res. 2001;159:85–100. doi: 10.1016/s0378-5955(01)00325-2. [DOI] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HW, Manis PB. Effects of deafferentation on the electrophysiology of ventral cochlear nucleus neurons. Hear Res. 2000;149:91–105. doi: 10.1016/s0378-5955(00)00165-9. [DOI] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Grothe B. New roles for synaptic inhibition in sound localization. Nature Rev Neurosci. 2003;4:540–550. doi: 10.1038/nrn1136. [DOI] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol. 1989;283:5–73. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- Illing RB, Forster CR, Horvath M. Evaluating the plasticity potential of the auditory brain stem nucleus in the rat. Am J Otol. 1997;18(Suppl 6):S52–S53. [PubMed] [Google Scholar]
- Isaacson JS, Walmsley B. Counting quanta: direct measurements of transmitter release at a central synapse. Neuron. 1995;15:875–884. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. TINS. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats BJ, Berlin CI. Genomics and hearing impairment. Genome Res. 1999;9:7–16. [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAA receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Eliminaton and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Deafferentation weakens excitatory synapses in the developing central auditory system. Eur J Neurosci. 1997;9:2340–2347. doi: 10.1111/j.1460-9568.1997.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Leao RN, Berntson A, Forsythe ID, Walmsley B. Reduced low-voltage activated K+ conductances and enhanced central excitability in a congenitally deaf (dn/dn) mouse. J Physiol. 2004a;559:25–33. doi: 10.1113/jphysiol.2004.067421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Oleskevich S, Sun H, Bautista M, Fyffe RE, Walmsley B. Differences in glycinergic mIPSCs in the auditory brain stem of normal and congenitally deaf neonatal mice. J Neurophysiol. 2004b;91:1006–1012. doi: 10.1152/jn.00771.2003. [DOI] [PubMed] [Google Scholar]
- Leao RN, Sun H, Svahn K, Berntson A, Youssoufian M, Paolini AG, Fyffe REW, Walmsley B. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J Physiol. 2005a;571:563–578. doi: 10.1113/jphysiol.2005.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RN, Svahn K, Berntson A, Walmsley B. Hyperpolarization-activated (I) currents in auditory brainstem neurons of normal and congenitally deaf mice. Eur J Neurosci. 2005b;22:147–157. doi: 10.1111/j.1460-9568.2005.04185.x. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Cahill HB, Ryugo DK. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: an ultrastructural analysis of synapse morphology in the endbulbs of Held. J Neurocytol. 2003;32:229–243. doi: 10.1023/B:NEUR.0000010082.99874.14. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kaczmarek LK, Perney TM. Localization of two high-threshold potassium channel subunits in the rat central auditory system. J Comp Neurol. 2001;437:196–218. doi: 10.1002/cne.1279. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Central projections of auditory-nerve fibers of differing spontaneous rate. I. Anteroventral cochlear nucleus. J Comp Neurol. 1991;313:240–258. doi: 10.1002/cne.903130205. [DOI] [PubMed] [Google Scholar]
- Lu Y, Monsivais P, Tempel BL, Rubel EW. Activity-dependent regulation of the potassium channel subunits Kv1.1 and Kv3.1. J Comp Neurol. 2004;470:93–106. doi: 10.1002/cne.11037. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Grothe B. Sound localization and delay-lines – do the mammals fit the model? TINS. 2003;26:347–350. doi: 10.1016/S0166-2236(03)00140-1. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat Neurosci. 2001;4:396–401. doi: 10.1038/86049. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurons in the inferior colliculus of the monaurally deafened ferret to acoustic stimulation of the intact ear. J Neurophysiol. 1997;78:767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Buonomano DV. The neural basis of temporal processing. Ann Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Mostafapour SP, Cochran SL, Del Puerto NM, Rubel EW. Patterns of cell death in mouse anteroventral cochlear nucleus neurons after unilateral cochlea removal. J Comp Neurol. 2000;426:561–571. doi: 10.1002/1096-9861(20001030)426:4<561::aid-cne5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Murphy TH. Activity-dependent synapse development: changing the rules. Nature Neurosci. 2003;6:9–11. doi: 10.1038/nn0103-9. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorsky T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synaptic size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Sato K, Shiraishi Y, Kuriyama H, Altschuler RA. NMDAR1 isoforms in the rat superior olivary complex and changes after unilateral cochlear ablation. Brain Res Mol Brain Res. 2000;77:246–257. doi: 10.1016/s0169-328x(00)00059-0. [DOI] [PubMed] [Google Scholar]
- Nicol MJ, Walmsley B. Ultrastructural basis of synaptic transmission between endbulbs of Held and bushy cells in the rat cochlear nucleus. J Physiol. 2002;539:713–723. doi: 10.1113/jphysiol.2001.012972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol. 2002;540:447–455. doi: 10.1113/jphysiol.2001.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Youssoufian M, Walmsley B. Presynaptic plasticity at two giant auditory synapses in normal and deaf mice. J Physiol. 2004;560:709–719. doi: 10.1113/jphysiol.2004.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelias AA, Monsivais P, Rubel EW. Tonotopic map of potassium currents in chick auditory hair cells using an intact basilar papilla. Hear Res. 2001;156:81–94. doi: 10.1016/s0378-5955(01)00269-6. [DOI] [PubMed] [Google Scholar]
- Paolini AG, Clarey JC, Needham K, Clark GM. Fast inhibition alters first spike timing in auditory brainstem neurons. J Neurophysiol. 2004;92:2615–2621. doi: 10.1152/jn.00327.2004. [DOI] [PubMed] [Google Scholar]
- Paolini AG, FitzGerald JV, Burkitt AN, Clark GM. Temporal processing from the auditory nerve to the medial nucleus of the trapezoid body in the rat. Hearing Res. 2001;159:101–116. doi: 10.1016/s0378-5955(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarisation. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Redd EE, Cahill HB, Pongstaporn T, Ryugo DK. The effects of congenital deafness on auditory nerve synapses: Type I and Type II multipolar cells in the anteroventral cochlear nucleus of cats. J Assoc Res Otolaryngol. 2002;3:403–417. doi: 10.1007/s101620020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Russell FA, Moore DR. Ultrastructural transynaptic effects of unilateral cochlear ablation in the gerbil medial superior olive. Hear Res. 2002;173:43–61. doi: 10.1016/s0378-5955(02)00606-8. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Kretzmer EA, Niparko JK. Restoration of auditory nerve synapses in cats by cochlear implants. Science. 2005;310:1490–1492. doi: 10.1126/science.1119419. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Huchton DM, Niparko JK. Ultrastructural analysis of primary endings in deaf white cats: morphologic alterations in endbulbs of Held. J Comp Neurol. 1997;385:230–244. doi: 10.1002/(sici)1096-9861(19970825)385:2<230::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Rosenbaum BT, Kim PJ, Niparko JK, Saada AA. Single unit recordings in the auditory nerve of congenitally deaf white cats: morphological correlates in the cochlea and cochlear nucleus. J Comp Neurol. 1998;397:532–548. doi: 10.1002/(sici)1096-9861(19980810)397:4<532::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Wu M, Pongstaporn T. Activity-related features of synapse morphology: a study of endbulbs of held. J Comp Neurol. 1996;365:141–158. doi: 10.1002/(SICI)1096-9861(19960129)365:1<141::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Schneggenburger R, Neher E. Estimation of quantal parameters at the calyx of Held synapse. Neurosci Res. 2002;44:343–356. doi: 10.1016/s0168-0102(02)00174-8. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol. 1992;320:501–508. doi: 10.1002/cne.903200407. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998;154:473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ. TrkB levels in the cochlear nucleus after unilateral cochlear ablation: correlations with post-lesion plasticity. Brain Res. 2002;957:366–368. doi: 10.1016/s0006-8993(02)03679-x. [DOI] [PubMed] [Google Scholar]
- Taschenburger H, Leao RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:687–688. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hehn CA, Bhattacharjee A, Kaczmarek LK. Loss of Kv3.1 tonotopicity and alterations in cAMP response element-binding protein signaling in central auditory neurons of hearing impaired mice. J Neurosci. 2004;24:1936–1940. doi: 10.1523/JNEUROSCI.4554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. J Physiol. 1998;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li Y, Engisch KL, Nakanishi ST, Dodson SE, Miller GW, Cope TC, Pinter M, Rich MM. Activity-dependent presynaptic regulation of quantal size at the mammalian neuromuscular junction in vivo. J Neurosci. 2005;25:343–351. doi: 10.1523/JNEUROSCI.3252-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol. 2005;94:1814–1824. doi: 10.1152/jn.00374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MCW, MacLeod KM, Nelson S, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Vaoqui H, Murnick JG, Erickson JD, Lui G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TCT. Neural mechanisms of encoding binaural localization cues in the auditory brainstem. In: Oertel D, Popper AN, Fay RR, editors. Integrative Functions in the Mammalian Auditory Pathway. New York, U S A: Springer-Verlag; 2002. pp. 99–159. [Google Scholar]
- Youssoufian M, Oleskevich S, Walmsley B. Development of a robust central auditory synapse in congenital deafness. J Neurophysiol. 2005;94:3168–3180. doi: 10.1152/jn.00342.2005. [DOI] [PubMed] [Google Scholar]
- Zettel ML, O'Neill WE, Trang TT, Frisina RD. The effects of early bilateral deafening on calretinin expression in the dorsal cochlear nucleus of aged CBA/CaJ mice. Hear Res. 2003;183:57–66. doi: 10.1016/s0378-5955(03)00216-8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]