Abstract
This study addresses the question of the origin of the long-latency responses evoked in flexors in the forearm by afferents from human hand muscles. The effects of electrical stimuli to the ulnar nerve at wrist level were assessed in healthy subjects using post-stimulus time histograms for flexor digitorum superficialis and flexor carpi radialis (FCR) single motor units (eight subjects) and the modulation of the ongoing rectified FCR EMG (19 subjects). Ulnar stimulation evoked four successive peaks of heteronymous excitation that were not produced by purely cutaneous stimuli: a monosynaptic Ia excitation, a second group I excitation attributable to a propriospinally mediated effect, and two late peaks. The first long-latency excitation occurred 8–13 ms after monosynaptic latency and had a high-threshold (1.2–1.5 × motor threshold). When the conditioning stimulation was applied at a more distal site and when the ulnar nerve was cooled, the latency of this late excitation increased more than the latency of monosynaptic Ia excitation. This late response was not evoked in the contralateral FCR of one patient with bilateral corticospinal projections to FCR motoneurones. Finally, oral tizanidine suppressed the long-latency high-threshold excitation but not the early low-threshold group I responses. These results suggest that the late high-threshold response is mediated through a spinal pathway fed by muscle spindle group II afferents. The second long-latency excitation, less frequently observed (but probably underestimated), occurred 16–18 ms after monosynaptic latency, had a low threshold indicating a group I effect, and was not suppressed by tizanidine. It is suggested that this latest excitation involves a transcortical pathway.
The role of secondary spindle afferents in human motor control was for long neglected, while that of primary spindle afferents was overestimated. One reason for this difference in the treatment of actions evoked by the two types of spindle afferent is methodological: Ia effects are easier to investigate because they appear first in motoneurones after peripheral stimulation and do so at lowest threshold, whereas it is impossible to stimulate group II afferents selectively, whether by electrical or mechanical stimuli (see Pierrot-Deseilligny & Burke, 2005). Only recently has it become possible to investigate group II pathways in the lower limb of human subjects. Most of the group II effects described so far are superimposed on group I effects, so that special tests are required to differentiate their contributions: cooling the nerve (Matthews, 1989; Schieppati & Nardone, 1997; Simonetta-Moreau et al. 1999) and depression of group II excitation by tizanidine, an α2 adrenergic receptor agonist (Corna et al. 1995; Marque et al. 2005). These investigations have shown that group II excitation of motoneurones, whether homonymous (Schieppati et al. 1995) or heteronymous (Simonetta-Moreau et al. 1999; Marque et al. 2005), is more potent than excitation by Ia afferents in corresponding pathways. These investigations have also led to the view that group II excitations play a major role in the normal control of bipedal stance (Nardone et al. 1990; Marchand-Pauvert et al. 2005) and gait (Berger et al. 1984; Sinkjær et al. 2000; Grey et al. 2001; Marchand-Pauvert & Nielsen, 2002).
In contrast, there is no unequivocal evidence for group II afferents contributing to the excitation of human upper limb motoneurones. The origin of the homonymous long-latency response to stretch in hand muscles has been vigorously debated. Stretch of the voluntarily activated flexor pollicis longus (FPL) produces large long-latency automatic responses (M2), following weak and inconstant responses at monosynaptic Ia spinal latency (M1). These long-latency responses were thought to be mediated through a transcortical Ia loop, because: (i) in the monkey, pyramidal tract neurones can respond very rapidly to a peripheral disturbance, (ii) there is ample time for afferent information carried by the fastest (Ia) afferents from the stretched muscles of the thumb to reach motor cortex and produce the corticomotoneuronal volleys responsible for the long-latency responses recorded in the FPL, and (iii) lesions of the dorsal columns cause the M2 response to disappear (for references see Marsden et al. 1983). However, alternative mechanisms have been proposed. (i) Cutaneous activation, which accompanies muscle stretch, was presumed to be entirely responsible for the M2 response (Darton et al. 1985), but the M2 responses that are elicited by moving a digit can survive after the digit has been locally anaesthetized (see Matthews, 1991). (ii) Because vibration – which excites rapidly conducting Ia afferents more than slowly conducting group II afferents – failed to elicit a long-latency response, it was suggested that the M2 response might be mediated by slower conducting group II afferents (Matthews, 1984). To confirm his slow-afferent hypothesis, Matthews (1989) used cooling of the arm (the rationale behind these experiments is given in Results), but the experiments rather supported the notion that the afferent limb of the long-latency stretch response of hand muscles does depend on Ia afferents. Further support for the existence of a stretch-induced transcortical Ia loop emerged from studies on patients with mirror movements because corticospinal axons branch abnormally to supply homologous motoneurones bilaterally. In these patients, stretch of an intrinsic hand muscle (first dorsal interosseus (FDI), and flexor pollicis brevis; Matthews et al. 1990) or of the FPL (Capaday et al. 1991) evoked typical long-latency stretch responses bilaterally, whereas the M1 response was restricted to the stretched muscle. ‘Nonetheless, the vindication of the transcortical hypothesis does not exclude all other possibilities, particularly for muscles less dominated by the cortex than those controlling the digits……The reflex action, if any, of the discharges elicited by stretch from muscle spindle secondary endings remains unknown’ (Matthews, 1991). Accordingly, the increased M2 response in wrist and other proximal muscles seen in patients with Parkinson's disease could result from overactivity in two parallel pathways (Limousin et al. 1999): one involving group I afferents and a transcortical pathway, the other group II afferents and a spinal pathway (Cody et al. 1986).
Taking advantage of our experience with lower-limb investigations (Simonetta-Moreau et al. 1999; Marque et al. 2005), we revisited the question, using electrical stimulation of the ulnar nerve at wrist level to investigate possible projections of group II afferents from hand muscles on motoneurones supplying flexors in the forearm. An advantage of this experimental protocol is the absence of recurrent projections from hand muscles, presumably because, as in the cat, the corresponding axons lack recurrent collaterals (see Katz et al. 1993). Thus, there is no risk that group II effects, which are elicited by volleys >1 × threshold of the motor response (MT) (Simonetta-Moreau et al. 1999), are masked by the recurrent inhibition evoked by antidromically conducted volleys in motor axons. A second advantage is the presence of heteronymous monosynaptic Ia projections from hand muscles to forearm motoneurones (Marchand-Pauvert et al. 2000), because they make it possible to compare the effects of either nerve cooling or tizanidine on the monosynaptic Ia excitation and on late high-threshold effects. A preliminary report of some of the results has been published in abstract form (Marchand-Pauvert, 2006).
Methods
The experiments were carried out on 26 healthy subjects (aged 22–70 years) and one patient with mirror movements (see below), all of whom had given informed written consent to the experimental procedures, which had been approved by the appropriate institutional ethics committees and conformed with the guidelines in the Declaration of Helsinki.
General experimental procedure
The subjects were comfortably seated in an armchair. The shoulder was in slight abduction (60 deg), the elbow semiflexed (110 deg) and the forearm and the hand pronated and supported by the arm of the chair, with a constant pressure throughout the experiment.
Recordings.
Electromyographic (EMG) activity was recorded by surface electrodes (silver plates, 0.8 cm diameter, 1.5 cm apart) secured to the skin over the corresponding muscle belly: flexor digitorum superficialis (FDS), flexor carpi radialis (FCR) and abductor digiti minimi (ADM) and, in some experiments (see below), also adductor pollicis (AP).
Conditioning stimuli.
Stimuli to mixed nerves were square electrical pulses of 1 ms duration delivered through bipolar surface electrodes (placed 1 cm apart, proximal cathode). They were applied to the median nerve in the ulnar groove (elbow level) and the ulnar nerve at wrist level (lateral aspect of the palmar side). In some experiments stimulation of hand muscle afferents in the ulnar nerve was also performed 5–7 cm more distal through a unipolar electrode (with the active cathode over the ADM and the anode on the dorsal side of the hand). The intensity of the stimulation was expressed as multiples of the motor threshold (×MT) evoked in the target muscle (ADM for ulnar stimulation at wrist level, AP for more distal stimulation of the ulnar nerve, FCR or FDS for median nerve stimulation at elbow level). The cutaneous sensation (local and radiating paraesthesiae) evoked by the ulnar nerve stimulation was mimicked by purely cutaneous stimuli applied through plate electrodes over the nerve projection to the fifth finger (allowance was made for the extra peripheral conduction time, see legend of Figs 2K and L, and 3E and F). The stimulus intensity was adjusted to mimic the sensation evoked by ulnar nerve stimulation; it was often impossible for the subject to make the distinction between the sensations elicited by the two stimulations.
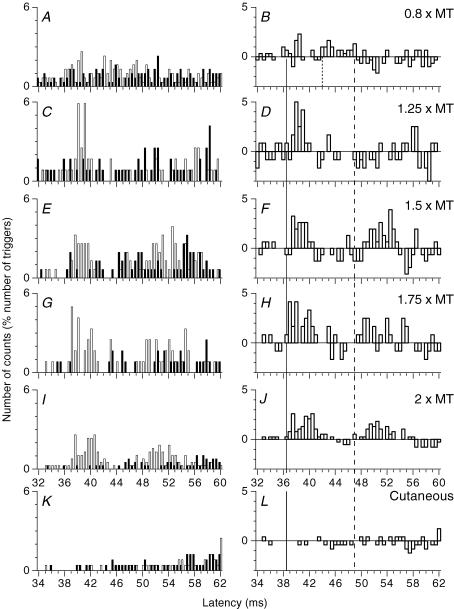
Figure 2. PSTHs for a FDS motor unit: effects of ulnar stimuli of various intensities and of cutaneous stimulation.
Same FDS unit as in Fig. 1A–D. A–J, histograms in A, C, E, G, I and K recorded with and without stimulation, as in Fig. 1A and C, and those in B, D, F, H, J and L representing the difference as in Fig. 1B and D. PSTHs are compared after electrical stimulation of the ulnar nerve at wrist level 0.8 (A and B), 1.25 (C and D), 1.5 (E and F), 1.75 (G and H) and 2 (I and J) × MT, and after electrical stimulation of the skin of the fifth finger, which reproduced the cutaneous sensation evoked by ulnar nerve stimulation at 2 × MT (K and L, the abscissa is shifted by 2 ms to make allowance for the supplementary afferent conduction time). Vertical lines indicate the latency of heteronymous Ia excitation (thick continuous line), non-monosynaptic propriospinally mediated group I excitation (dotted line, B) and late high-threshold excitation (dashed line).
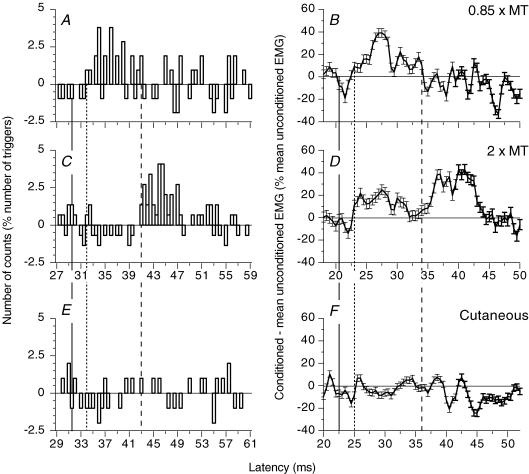
Figure 3. PSTHs for a FCR unit and modulation of the ongoing FCR EMG: effects of different stimuli.
PSTHs for a flexor carpi radialis (FCR) motor unit (A, C and E, the background firing has been subtracted) and modulation of the ongoing rectified FCR EMG (B, D and F, tonic voluntary contraction (∼10% of MVC), difference: conditioned EMG – mean unconditioned EMG as a percentage of the mean unconditioned EMG) recorded during the same experimental session after ulnar stimulation at wrist level (0.85 × MT, A and B; 2 × MT, C and D) and after electrical stimulation of the skin of the fifth finger, which reproduced the cutaneous sensation evoked by ulnar nerve stimulation at 2 × MT (E and F, the abscissa is shifted by 2 ms to make allowance for the supplementary afferent conduction time). (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 25.5 ms in the PSTH and 16.5 ms in the ongoing EMG (H reflex); distance wrist–elbow, 0.27 m; supplementary afferent conduction time for the ulnar Ia volley, 3.9 (0.27/69) ms; ulnar-induced heteronymous Ia excitation expected at 29.4 (25.5 + 3.9) ms in the PSTH and 20.4 (16.5 + 3.9) ms in the ongoing EMG). Vertical lines indicate the latency of the expected heteronymous Ia excitation (thick continuous vertical lines), non-monosynaptic propriospinally mediated group I excitation (dotted lines), and late high-threshold excitation (dashed lines).
Study of single motor units
Post-stimulus time histograms (PSTHs) for a voluntarily activated motor unit were constructed for the period following a conditioning stimulation in eight subjects. This process extracts from the naturally occurring spike train only those changes in firing probability that are time-locked to the stimulus (Stephens et al. 1976). Subjects were asked to perform weak tonic voluntary contractions – 1–2% of the maximal voluntary contraction (MVC) – to isolate a single motor unit. The corresponding motoneurones were therefore all in the low-threshold range. The same unit was kept long enough (several hours in some experiments) to investigate changes in the firing probability of the unit with various stimulus intensities and various experimental conditions (nerve cooling). Stimulation-induced effects were investigated in 36 FDS units (6 subjects) and 24 FCR units (7 subjects).
Method of PSTH.
EMG potentials were converted into standard pulses by a window discriminator with variable trigger levels. These pulses were fed to a computer, which subsequently triggered the stimulators about once every 0.5 s. PSTHs for single motor units were constructed for the 15–80 ms following the conditioning stimulation using a 0.5 ms bin width. Details of the particular PSTH technique used in this study are given elsewhere (see Pierrot-Deseilligny & Burke, 2005). Each stimulus was triggered at a fixed delay after the preceding motor unit action potential. This technique has advantages under two different conditions. (i) To favour the appearance of the peak of monosynaptic Ia excitation produced by ulnar and median nerve stimulations, stimulus delivery can be timed to avoid the period of afterhyperpolarization (AHP) following the previous motoneurone discharge. (ii) Conversely, the AHP can be used to attenuate the monosynaptic Ia-induced discharge of the motor unit. This discharge is followed by a depression due to the AHP, and this will obscure later EPSPs. Preventing the monosynaptic discharge of the motoneurone could allow late synaptic effects to become apparent. This can be done by delivering the stimulus at an appropriately short delay following the previous (spontaneous) discharge. The delay was chosen so that the AHP reduced the firing probability due to the monosynaptic Ia EPSP, but not the effects of the late synaptic events, which occurred later during the recovery from AHP. This explains why the monosynaptic Ia peaks are smaller in the present investigation than in a previous study (Marchand-Pauvert et al. 2000), in which the stimulation was triggered at a delay favouring early Ia effects. For example, the peak of heteronymous group Ia monosynaptic excitation was absent in the PSTH of the FCR unit illustrated in Fig. 3A. Histograms of the firing probability were constructed without stimulation (control situation, filled columns in the left panels of Figs 1A and C, and 2 and 4) and after the conditioning stimulus (open columns in the left panels of Figs 1A and C, and 2 and 4).
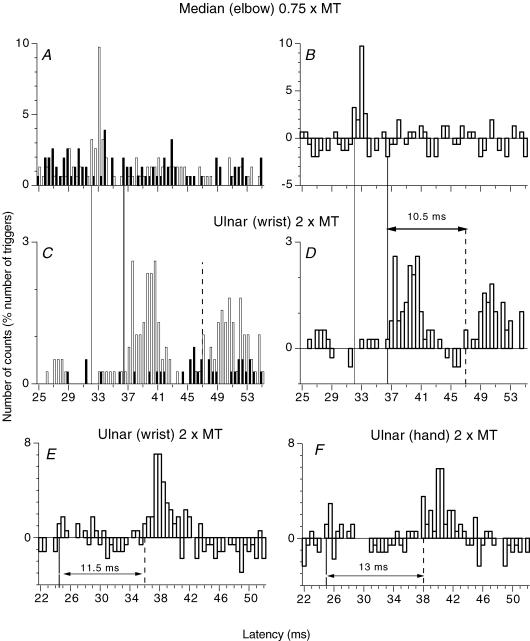
Figure 1. PSTHs for a FDS motor unit: monosynaptic Ia and late high-threshold excitations.
A and C, post-stimulus time histograms (PSTHs; 0.5 ms bin width) for a flexor digitorum superficialis (FDS; supplying the head for the fourth finger) motor unit without (filled bars) and with (open bars) stimulation of the homonymous median nerve at elbow level (A, 0.75 × motor threshold (MT)) and of the heteronymous ulnar nerve at wrist level (C, 2 × MT). B and D, the difference between the histograms with and without stimulation. In this and Figs 1E and F,2, 3A, C and E,4A, and 7A and B, the number of counts in each bin (expressed as a percentage of the number of triggers) is plotted against the latency after stimulation. (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 32 ms; distance between wrist and elbow stimulation sites, 0.305 m; conduction velocity in Ia afferents, 69 ms−1; supplementary afferent conduction time for the ulnar Ia volley, 4.4 (0.305/69) ms; ulnar-induced heteronymous Ia excitation expected at 36.4 (32 + 4.4) ms, corresponding to the latency (36.5 ms) of the early peak in C and D). E and F, effects of ulnar nerve stimulation (2 × MT) at wrist level (E) and 7 cm more distal (F, see Methods) are compared (another FDS unit than in A–D, the background firing has been subtracted) (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 21 ms; distance wrist–elbow, 0.23 m; supplementary afferent conduction time for the ulnar Ia volley, 3.3 (0.23/69) ms; ulnar-induced heteronymous Ia excitation expected at 24.3 (21 + 3.3) ms, corresponding to the latency (24.5 ms) of the early peak in E). Vertical lines indicate the latency of homonymous Ia excitation (thin continuous line), heteronymous Ia excitation (thick continuous line) and late high-threshold excitation (dashed line). Horizontal double-headed arrows in D–F indicate the time interval between heteronymous monosynaptic Ia and late high-threshold group II excitation.
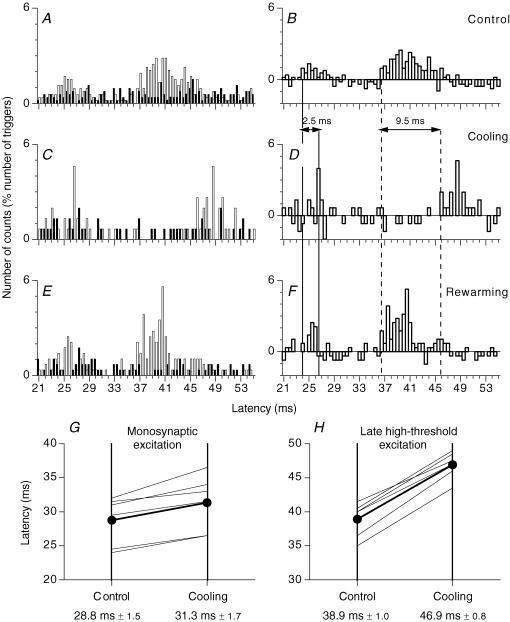
Figure 4. PSTHs for a FDS motor unit: effects of cooling the ulnar nerve.
A–F, PSTHs for the same FDS unit. Histograms in A, C and E, recorded with and without stimulation, as in Fig. 1A and C, and those in B, D and F representing the difference as in Fig. 1B and D. PSTHs are compared after electrical stimulation of the ulnar nerve at wrist level in the control situation (A and B), after cooling (13 min) of the ulnar nerve (C and D, the small excitation at 25–25.5 ms is not significant) and after rewarming (E and F). (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 20 ms; distance wrist–elbow, 0.27 m; supplementary afferent conduction time for the ulnar Ia volley, 3.9 (0.27/69) ms; ulnar-induced heteronymous Ia excitation expected at 23.9 (20 + 3.9) ms, corresponding to the latency (24 ms) of the early peak in B). Vertical lines indicate the latency before and after cooling of heteronymous Ia excitation (thick continuous vertical lines) and late high-threshold excitation (dashed lines). Horizontal double-headed arrows: delay caused by nerve cooling in the latencies of the two peaks of excitation. G and H, group data (6 FDS units). Each thin line represents one unit, and the thick lines (and •) represent the mean values for the group. The latency (ms) of the monosynaptic Ia (G) and late high-threshold peak (H) evoked in PSTHs are compared before (left vertical bar) and after cooling (right vertical bar). Mean (±1 s.e.m.) values in the group are shown beneath each corresponding vertical line.
Organization of the experiments.
A control histogram of firing probability without stimulation was constructed to assess the background firing probability of the unit. Ulnar stimulation at wrist level was randomly alternated with control trials in the same sequence with, in addition, either median stimulation (at elbow level) – to compare the latency of ulnar-induced effects to that of the homonymous monosynaptic Ia excitation (see Results) – or the purely cutaneous stimulation (see above). To clarify the differences between results obtained in control and conditioned situations, the control count was subtracted from the conditioned count for each bin in the PSTH. This accounts for the negative values in open columns on the right of Figs 1B and D, and 2 and 4, and in Figs 1E and F, 3A, C and E, and 7A and B. Changes in discharge probability were normalized as a percentage of the number of triggers (100–400 for each stimulus during a sequence). Sequences in which irregularities in the control sequence contributed significantly to the difference between the two situations were not retained for further analysis.
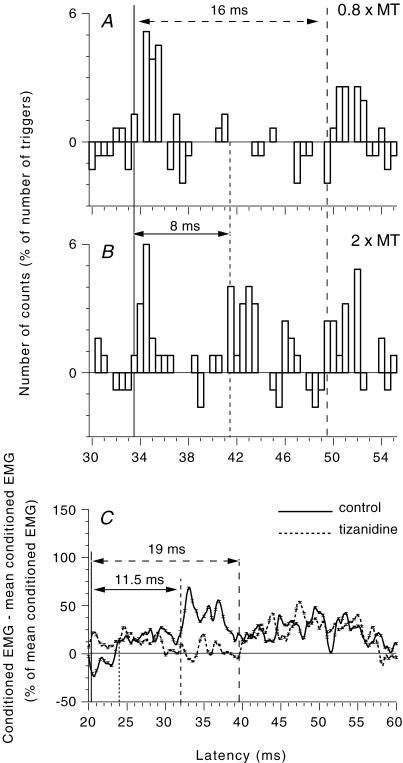
Figure 7. Evidence for a long-latency group I excitation in healthy subjects.
A and B, PSTHs for a FDS unit (the background firing has been subtracted). Effects of ulnar nerve stimulation at 0.8 (A) and 2 (B) × MT. (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 29 ms; distance wrist–elbow, 0.28 m; supplementary afferent conduction time for the ulnar Ia volley, 4 (0.28/69) ms; ulnar-induced heteronymous Ia excitation expected at 33 (29 + 4) ms, compatible with the latency (33.5 ms) of the early peak in A and B). C, modulation of the ongoing rectified FCR EMG (tonic voluntary contraction of ∼10–20% of MVC) after ulnar nerve stimulation at wrist level (2 × MT) in another healthy subject before (continuous line) and 90 min after oral intake (150 μg kg−1) of tizanidine (dashed line). (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 16.8 ms (H reflex); distance wrist–elbow, 0.26 m; supplementary afferent conduction time for the ulnar Ia volley, 3.7 (0.26/69) ms; ulnar-induced heteronymous Ia excitation expected at 20.5 (16.8 + 3.7) ms). Vertical lines indicate the latency of heteronymous Ia excitation (thick continuous line), non-monosynaptic propriospinally mediated group I excitation (dotted line), late high-threshold excitation (dashed line) and long-latency group I (probably transcortical) excitation (interrupted line with large gaps). Horizontal double-headed arrows indicate the time interval between monosynaptic latency and late group II excitation (continuous line) or transcortical group I excitation (interrupted line).
Statistical analysis.
The statistical analysis of changes in firing probability was confined to the window including visually located peaks of excitation. Consecutive bins with an increase in firing probability above the background discharge were grouped together and assessed with a χ2 test to determine the extent to which the distribution of firing probability after conditioning stimulation within this group differed from that in the control situation. For each intensity of ulnar nerve stimulation, a peak of excitation was accepted if the firing probability was significantly increased in a group of consecutive bins (at least P < 0.01). The conclusion on the ulnar nerve intensity threshold for evoking a late facilitation was therefore at least P < 0.05 (progressive Bonferroni correction). The onset of each peak (i.e. its latency) was taken as the initial bin of the first group of two or three consecutive bins which reached statistical significance (as a group) when lumped together (see Pierrot-Deseilligny & Burke, 2005). Indeed, excitation in the rising phase of the visually located peak was rarely significant in the very first bin. However, on average, the differences in the latencies of early and late responses (e.g. in cooling experiments) were very similar, whether latencies were measured on the first bin of the peak or the first bin to be significant by itself. Although the relation between the amplitude of a peak in the PSTH and that of the underlying EPSP is complex (Gustafsson & McCrea, 1984), the larger the EPSP, the higher the peak. Thus, the size of the peak was estimated as the sum of the difference (conditioned – unconditioned counts, expressed as a percentage of the number of triggers) in the consecutive bins with increased firing probability (e.g. the late peak in Fig. 1C and D reached 10.4% between 47 and 53 ms).
Multiple peaks.
The successive peaks described in the present investigation occurred at intervals of 8–13 ms at most. They cannot therefore be attributed to secondary and tertiary peaks in the PSTH reflecting the auto-correlation function of the motoneurone discharge (Türker & Powers, 1999, 2003), since these ‘synchronization-related errors’ occur with time lags of the same order as the spontaneous mean interspike interval (∼100 ms in this study).
Modulation of the ongoing EMG activity
In 19 subjects, ongoing FCR EMG activity was recorded during a tonic voluntary wrist flexion of 10–20% of MVC using a sampling rate of 2 kHz. EMG was filtered (100 Hz–1 kHz), amplified (×10 000), full-wave rectified for surface analysis and averaged for 60 ms against the conditioning stimulus. The rectified and integrated EMG activity was displayed on an oscilloscope so that, during a sequence, the subjects could maintain a constant contraction level, expressed as a percentage of the activity measured during a maximal tonic voluntary contraction lasting 5 s. Conditioned and unconditioned trials (i.e. trials in which the background EMG activity was measured) were randomly alternated (0.5 s) during short sequences of <100 s to avoid muscular fatigue. The data recorded during four sequences were averaged to produce a single run containing 200 conditioned and 200 unconditioned responses. The background EMG was measured in the corresponding unconditioned trials and then integrated over 60 ms to provide a fixed measure of baseline EMG within the sequence (mean unconditioned EMG). The difference between the grand average of conditioned values (in a single subject) and the baseline EMG was expressed as a percentage of this baseline (Figs 3B, D and F, and 5A, 6C and 7C). Statistical analysis was confined to the windows corresponding to the different peaks evoked by ulnar nerve stimulation (e.g. in Fig. 5A, the integration was performed between the dotted lines at 22.5 and 30 ms for the early non-monosynaptic group I facilitation and between the dashed lines at 31.5 and 40 ms for the late high-threshold facilitation). ANOVA and post hoc Scheffé's F test were used to determine whether the differences between conditioned and unconditioned EMG were significant for each subject.
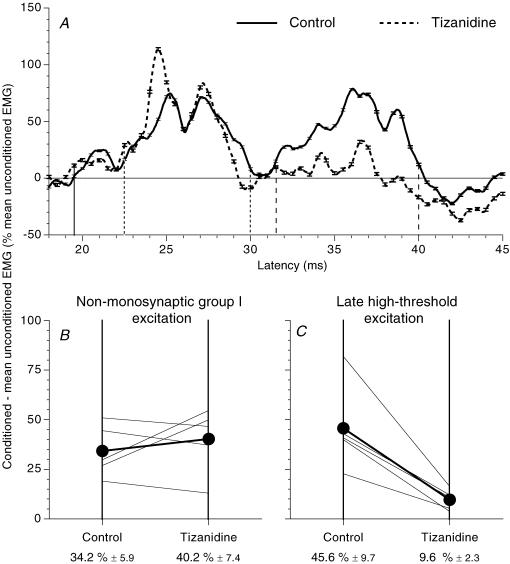
Figure 5. Effects of tizanidine on the ulnar-induced modulation of the ongoing rectified FCR EMG.
A, modulation of the ongoing rectified FCR EMG (tonic voluntary contraction of ∼10–20% of MVC, difference conditioned EMG – mean unconditioned EMG as a percentage of the mean unconditioned EMG) after ulnar nerve stimulation at wrist level (2 × MT) in one subject before (continuous line) and 90 min after oral intake (150 μg kg−1) of tizanidine (dashed line). (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 16 ms (H reflex); distance wrist–elbow, 0.23 m; supplementary afferent conduction time for the ulnar Ia volley, 3.3 (0.23/69) ms; ulnar-induced heteronymous Ia excitation expected at 19.3 (16 + 3.3) ms, corresponding to the latency (19.5 ms) of the early peak in (A). Vertical lines indicate the latency of heteronymous Ia excitation (thick continuous line), and the limits of the windows within which the non-monosynaptic propriospinally mediated group I excitation (dotted lines) and the late high-threshold excitation (dashed lines) were assessed. B and C, group data (5 subjects). Each thin line represents one subject, and the thick lines (and •) represent the mean values for the group. Non-monosynaptic group I (B) and late high-threshold (C) facilitations (difference: conditioned EMG – mean unconditioned EMG as a percentage of the mean unconditioned EMG) assessed within the windows corresponding to the limits between dotted and dashed lines, as in Fig. 5A are compared before (left vertical bar) and after tizanidine (right vertical bar). Mean (±1 s.e.m.) values in the group are shown beneath each corresponding vertical line.
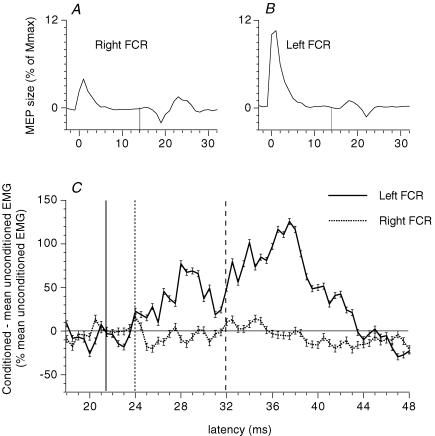
Figure 6. Results in a patient with mirror movements and bilateral corticospinal projections on FCRs of both sides.
A and B, motor-evoked potentials (MEPs) elicited in a patient (patient BS) with congenital mirror movements. Unilateral (right hemisphere) transcranial magnetic stimulation (TMS) over M1 through a figure-of-eight-shape coil, the centre of which was placed ∼1.5 cm anterior and ∼5.5 cm lateral to the vertex, produced a MEP of similar latency (14 ms) and amplitude (∼3% of Mmax) in the ipsilateral (A, right) and contralateral (B, left) voluntarily activated (5% of MVC) FCR. C, simultaneous modulation of the ipsilateral (continuous line) and contralateral (dotted line) FCR EMG by a unilateral (left) stimulation of the ulnar nerve at wrist level (2 × MT). (Calculations for heteronymous monosynaptic latency: median – induced monosynaptic latency, 17.5 ms (H reflex); distance wrist–elbow, 0.28 m; supplementary afferent conduction time for the ulnar Ia volley, 4 (0.28/69) ms; ulnar-induced heteronymous Ia excitation expected at 21.5 (17.5 + 4) ms. Vertical lines indicate the latency of the MEP (A and B, thin continuous lines), and in C they indicate the latency of heteronymous Ia excitation (thick continuous line), non-monosynaptic propriospinally mediated group I excitation (dotted line) and late high-threshold excitation (dashed line).
Nerve cooling
The effects of cooling the ulnar nerve on the latency of monosynaptic Ia and of late high-threshold excitations could be explored in the same FDS units during the same experimental session. An ice pack was placed against the palmar and ulnar aspect of the forearm, proximal to the stimulation site. Cooling was prolonged up to 30 min. This decreased the temperature of the skin of the forearm palmar side, measured with a digital thermometer, from 31–35 to 21–27°C. The latency of the ulnar-induced peaks of excitation in the PSTHs was compared before (control), during cooling and after rewarming (Fig. 4) in six FDS units (four subjects). Cooling-induced changes in the latency of the two peaks were tested in the group with the non-parametric Mann–Whitney U test.
Pharmacological experiments
The effects of ulnar nerve stimulation were compared before and after intake of a single oral dose of tizanidine (150 μg kg−1) in five subjects. It proved difficult to keep the same single unit discharging for a long time in the face of drug-induced sleepiness, requiring that ulnar-induced modulation of the ongoing FCR EMG activity be investigated (having first ensured that the modulation of the EMG faithfully reflected the group I and group II effects, see Fig. 3). The perceptible effects of the tizanidine began around 45–60 min after drug intake and the experiments lasted ∼120 min (when the subjects started to recover). Despite a tendency to sleepiness, the subjects were able to keep their tonic contraction at a constant level (10–20% of MVC). The difference between conditioned EMG and mean unconditioned EMG (expressed as a percentage of the mean unconditioned EMG) was compared before and 60–90 min after drug intake within windows confined to the peaks of the successive facilitations (e.g. 19.5–21.5, 22.5–30 and 31.5–40 ms in Fig. 5A). ANOVA and post hoc Scheffé's F test were performed to determine whether the changes induced by tizanidine in the ongoing EMG activity were significant for each subject. The non-parametric Wilcoxon matched-pairs signed-rank test and Mann–Whitney U test were used to compare tizanidine-induced changes in the amount of early and late facilitations in the group.
Patient with mirror movements
One patient (male, age 26) with congenital mirror movements associated with other congenital anomalies was investigated. Mirror movements of the wrist and the fingers were triggered from both sides. Unilateral transcranial magnetic stimulation (TMS) over M1 through a figure-of-eight-shape coil, the centre of which was placed ∼1.5 cm anterior and ∼5.5 cm lateral to the vertex, elicited nearly simultaneously a motor-evoked potential (MEP) in the FCR on both sides of the body at latencies similar to those of normal subjects (Fig. 6A and B). Thus, his mirror movements probably arose from abnormal branching of corticospinal axons to supply homologous motoneurones bilaterally (Matthews et al. 1990; Capaday et al. 1991). The effects of unilateral ulnar nerve stimulation at 2 × MT were investigated on the FCR ongoing EMG recorded simultaneously on both sides (Fig. 6C).
Results
Electrical stimulation of hand muscle afferents in the ulnar nerve at the wrist produced four peaks of excitation in both PSTHs and ongoing EMG recorded from the FDS and FCR muscles: (i) heteronymous monosynaptic Ia excitation, previously described (Marchand-Pauvert et al. 2000); (ii) a second low-threshold peak occurring ∼3–7 ms later, attributable to propriospinally mediated non-monosynaptic group I excitation (see Pierrot-Deseilligny & Burke, 2005); (iii) a long-latency high-threshold peak, which will be shown below to involve group II afferents and a spinal pathway; and (iv) an even later peak involving group I afferents and a probable transcortical pathway.
Excitation of FDS motoneurones by ulnar stimulation at wrist level
Monosynaptic Ia excitation.
Figure 1A and B shows that stimulation of the median nerve at elbow level (0.75 × MT, subthreshold for evoking an H reflex) produced in the PSTH for a FDS unit (supplying the head for the fourth finger) a highly significant (P < 0.001) peak of homonymous Ia excitation, beginning at 32 ms (thin continuous vertical line). After correction for the trigger delay of the unit, this latency corresponded to that of the FDS H reflex and can therefore be attributed to a monosynaptic Ia EPSP (Mao et al. 1984). Stimulation of the ulnar nerve (2 × MT) at the wrist (0.305 m more distal), elicited a first peak of excitation at 36.5 ms (Fig. 1C and D, thick continuous vertical line; P < 0.001), i.e. 4.5 ms later than the peak of homonymous Ia excitation. Given the conduction velocity of the fastest Ia afferents from hand muscles (69 ms−1; Marchand-Pauvert et al. 2000), the supplementary afferent peripheral conduction time from wrist to elbow level – 4.4 ms (0.305/69) – accounts for the 4.5 ms difference in the latencies of the two peaks of excitation. Thus, heteronymous and homonymous excitations had the same central delay. This is consistent with a heteronymous monosynaptic Ia connection, as demonstrated in a previous study (Marchand-Pauvert et al. 2000). Accordingly, the threshold of this early excitation was low (∼0.55 × MT, within the range found for group I afferents in the median and ulnar nerves, see Pierrot-Deseilligny & Burke, 2005). A similar heteronymous monosynaptic Ia excitation was found in 32/36 motor units (5/6 subjects).
Non-monosynaptic group I excitation.
Ulnar nerve stimulation at 0.8 × MT evoked a second peak at 42 ms (Fig. 2A and B). Its threshold was low (0.6 × MT) and it was not reproduced by purely cutaneous stimulation (Fig. 2K and L), indicating a group I effect. When the stimulus intensity was increased (1.25 × MT), this peak disappeared (Fig. 2C and D). This second peak, observed in 11/36 motor units (P < 0.01; 5/6 subjects), occurred 5.3 ± 0.4 ms later than monosynaptic Ia excitation, reflecting a longer central delay. For further arguments that this peak corresponds to propriospinally mediated group I excitation, see Discussion.
Late high-threshold excitation.
Ulnar nerve stimulation at wrist level (2 × MT) also evoked a late peak of excitation at the 47 ms latency, i.e. 10.5 ms later than the monosynaptic Ia excitation (Figs 1C and D, and 2I and J; dashed vertical line). The effects evoked by various intensities of ulnar stimulation in the same FDS unit are illustrated in Fig. 2. The late peak, which did not exist at 1.25 × MT (Fig. 2C and D), appeared at 1.5 × MT (Fig. 2E and F; P < 0.001) and its latency shortened somewhat at higher intensities (1.75–2 × MT; Fig. 2G–J). A purely cutaneous stimulus adjusted to evoke the same cutaneous sensation as ulnar nerve stimulation at 2 × MT (see Methods) produced no changes in the firing probability of the unit (Fig 2K and L). Ulnar nerve-induced late high-threshold excitation was observed in the PSTHs of 27/36 motor units (all six subjects). This facilitation occurred 10.6 ± 0.2 ms (range 8–13) later than the heteronymous monosynaptic Ia excitation. The lowest threshold observed for this late peak was 1.2 × MT. The mean size of the corresponding peak in the PSTHs was 15.6 ± 1.8% of the number of triggers, and its mean duration was 4.8 ± 0.4 ms. In 6/9 of the remaining units, the late excitation occurred with a low threshold (<1 × MT) and a longer latency (see Fig. 7A), possibly due to a transcortical group I pathway (see Discussion). In only one motor unit, was a late excitation produced by a pure cutaneous stimulation.
Effects produced by a more distal stimulation.
Because ulnar stimulation at wrist level evokes Ia excitation in FDS motoneurones, it would be conceivable that stimuli >1 × MT produce, in addition to the Ia volley elicited by electrical stimulation of afferent fibres, a later Ia discharge related to stimulation of efferent fibres: early discharge (Hunt & Kuffler, 1951) associated with the α volley and/or an activation of primary endings by the fusimotor volley. If this were the case, more distal stimulation should decrease the latency difference between early and late peaks, because the conduction distance along motor axons (from stimulation site to muscle spindles) would then be decreased. However, when comparing PSTHs obtained after stimulation (2 × MT) to the ulnar nerve at wrist level (Fig. 1E) and 7 cm more distal (Fig. 1F; see Methods), the difference between the latencies of the monosynaptic and late peaks was longer after distal stimulation (13 versus 11.5 ms), a finding that is not consistent with this possibility. Similar results were obtained for six units (three subjects). On average, distal stimulation increased the latency of the monosynaptic peak by 0.8 ± 0.1 ms and that of the late excitation by 2.7 ± 0.2 ms (P < 0.01; Mann–Whitney U test). This suggests that the late high-threshold excitation is mediated by afferents with a slower conduction velocity than Ia afferents, a conclusion confirmed by cooling experiments (see below). However, in two units in which the late peak had a low threshold (<1 × MT, as in Fig. 7A), distal stimulation increased the latency of the monosynaptic and the late peaks to the same extent, ∼1 ms (see transcortical group I excitation in Discussion).
Excitation of FCR motoneurones by ulnar stimulation at wrist level
Figure 3 compares the effects of ulnar stimulation on the PSTHs for a single FCR unit and on the ongoing FCR EMG recorded during the same experimental session. Thus, the PSTHs (Fig. 3A, C and E; difference between conditioned and control histograms) and the ongoing EMG (Fig. 3B, D and F; difference between conditioned and mean unconditioned EMG) were conditioned by the same conditioning stimuli: ulnar stimulation at wrist level (0.85 × MT in Fig. 3A and B; 2 × MT in Fig. 3C and D), purely cutaneous stimulation mimicking the sensation produced by ulnar stimulation at 2 × MT (Fig. 3E and F). Similar results – at the same latencies with respect to monosynaptic latency – were obtained with the two methods: (i) absence of excitation at the expected latency of heteronymous monosynaptic Ia excitation (thick continuous vertical lines; see legend of Fig. 3); (ii) low-threshold non-monosynaptic group I excitation at 0.85 × MT (Fig. 3A and B), 2.5 ms later than the expected monosynaptic Ia excitation (dotted vertical lines); (iii) significant late excitation at 2 × MT (Fig. 3C and D; P < 0.001), occurring 11.6 ms later than the expected monosynaptic Ia (dashed vertical lines); and (iv) absence of excitation produced by purely cutaneous stimuli (Fig. 3E and F). Similar results were obtained in the six subjects so tested with the two methods.
Monosynaptic Ia excitation.
Only in the PSTHs of 5/24 units was ulnar-induced heteronymous monosynaptic Ia excitation found. This relative rarity could have been due to the fact that the delay at which the stimulation was triggered was adjusted to favour late excitations rather than the monosynaptic Ia peak (see Methods).
Non-monosynaptic group I excitation.
Significant non-monosynaptic group I excitation following stimuli <1 × MT was observed in the PSTHs of 11/24 FCR units (P < 0.01; mean central delay 4.1 ± 0.4 ms; mean size 7.1 ± 1.4% of the number of triggers), and in the ongoing EMG of 17/19 subjects (mean central delay 3.3 ± 0.2 ms; mean amount of facilitation 27.2 ± 3.3% of the mean unconditioned EMG). When the stimulus intensity was increased (2 × MT), this peak – attributable to propriospinally mediated group I excitation (see Discussion) – disappeared from the PSTH of the units (Fig. 3C), but, although attenuated, was not completely suppressed in the ongoing EMG (Fig. 3D; see Discussion).
Late high-threshold excitation.
Ulnar-induced late high-threshold excitation was observed in the PSTHs of 19/24 motor units (6/7 subjects). It occurred 10.9 ± 0.3 ms (range 8–12.8 ms) after the expected latency of the monosynaptic Ia excitation, and had a duration of 8.2 ± 0.7 ms and size of 32.2 ± 3.9% of the number of triggers. The lowest threshold observed for this late peak was 1.2 × MT. Ulnar nerve stimulation at 2 × MT produced a late facilitation of the FCR ongoing EMG, not observed at intensities below 1 × MT, in 17/19 subjects. The amount of facilitation was 34.5 ± 4.5% of the mean unconditioned EMG, and the latency was 13.2 ± 0.7 ms longer than the expected latency of the monosynaptic Ia excitation. The persistence at 2 × MT of a non-monosynaptic group I excitation in the ongoing FCR EMG but not in the PSTHs of single units might account for the longer latency of the late high-threshold excitation in the former: recurrent inhibition produced by the discharge of FCR motoneurones in the non-monosynaptic group I excitation would delay the appearance of the following late excitation.
In four out of the five remaining units, the late excitation occurred with a low threshold (<1 × MT) and a longer latency, possibly involving a transcortical group I pathway (see Discussion). In only one motor unit, was late excitation produced by a purely cutaneous stimulus.
Further experiments to characterize the pathway(s) responsible for the late excitation
The long latency and high threshold of the excitation produced in most FDS and FCR units by ulnar stimuli at 2 × MT are compatible with an effect produced by afferents with a smaller diameter than Ia afferents (see Simonetta-Moreau et al. 1999; Marque et al. 2005). In addition, distal stimulation increased more the latency of the late excitation than the latency of monosynaptic Ia excitation (see Fig. 1E and F), and this finding also suggests that the late high-threshold excitation involves a spinal pathway fed by afferents with a slower conduction velocity than Ia fibres. This hypothesis was further confirmed by three kinds of experiments.
Effects of cooling the ulnar nerve.
The longer latency of the late response may result from either a slower conduction in the peripheral afferent pathway or a longer central pathway fed by group I afferents. To distinguish between these two possibilities, we used nerve cooling. The rationale was that this would decrease conduction velocity proportionally in large and small fibres (Paintal, 1965), thereby leading to a longer absolute delay in the transmission over a fixed distance of impulses travelling along group II fibres than for those travelling along group I afferents (see Matthews, 1989). Figure 4 illustrates the effects of cooling the ulnar nerve on the latencies of the ulnar-induced monosynaptic Ia and late high-threshold peaks produced in the PSTHs for a FDS MU. Before cooling (Fig. 4A and B), ulnar nerve stimulation at wrist level (2 × MT) produced a peak of monosynaptic Ia excitation at 24 ms (thick continuous vertical line, see legend of Fig. 4) and a late high-threshold peak at 36.5 ms (dashed vertical line). After cooling for 13 min (Fig. 4C and D), forearm skin temperature dropped from 32 to 22.7°C. The early peak was delayed by 2.5 ms (from 24 to 26.5 ms, see legend of Fig. 4), but the late high-threshold peak was delayed by 9.5 ms (from 36.5 to 46 ms), as indicated by double-headed horizontal arrows. After removal of the ice pack (28 min) and rewarming (45 min), the latency of the monosynaptic Ia and late high-threshold peaks returned to their control values (24 ms and 36.5 ms; Fig. 4E and F).
Group data.
Figure 4G and H shows the results obtained in six FDS units (four subjects) before (left vertical bar) and during (right vertical bar) cooling. In all units, cooling the ulnar nerve delayed more the late high-threshold excitation (Fig. 4H) than the monosynaptic Ia excitation (Fig. 4G). On average, cooling delayed the latency of the monosynaptic Ia peak by 2.5 ± 0.4 ms (from 28.8 ± 1.5 to 31.3 ± 1.3 ms), and that of the late peak by 8.0 ± 0.5 ms (from 38.9 ± 1.0 to 46.9 ± 0.8 ms). The mean lengthening of the latency during cooling was significantly greater for the late than for the monosynaptic Ia peak (P < 0.01; Mann–Whitney U test). This differential effect of cooling provides evidence that the longer latency of the late high-threshold responses is not due to a longer central pathway fed by group I afferents, but to the activation of peripheral afferents of slower conduction velocity, as has been demonstrated previously in the lower limb (see Pierrot-Deseilligny & Burke, 2005).
Pharmacological validation.
Tizanidine, an α2 adrenergic receptor agonist, selectively blocks transmission from group II afferents in the lumbar spinal cord both in the cat (Bras et al. 1990) and in human subjects (Corna et al. 1995; Marque et al. 2005). Assuming that it would have similar actions on circuits of the cervical cord, the effects of ulnar nerve stimulation on the ongoing FCR EMG activity were investigated before and after oral intake of tizanidine. In Fig. 5A, the modulation of the ongoing FCR EMG by ulnar stimulation (2 × MT) is compared before and after tizanidine in a single subject. In the control situation, ulnar stimulation produced three successive peaks of excitation: (i) a weak heteronymous monosynaptic Ia facilitation at 19.5 ms (continuous vertical line); (ii) a second facilitation at 22.5 ms (dotted vertical line), with a threshold <1 × MT, attributable to propriospinally mediated group I excitation; and (iii) a late facilitation at 31.5 ms (dashed vertical line, 12 ms after the monosynaptic Ia latency), lasting 9 ms, not recordable at intensities below 1 × MT. As in the lower limb (see Pierrot-Deseilligny & Burke, 2005), the late high-threshold facilitation was almost completely suppressed 90 min after oral intake of tizanidine, while the mono- and non-monosynaptic group I facilitations were not significantly modified.
Group data.
Figure 5B and C shows the amount of facilitation observed before (left vertical bar) and after (right vertical bar) tizanidine in the five subjects tested. The amount of propriospinally mediated group I excitation was not significantly changed (Fig. 5B): non-significant decrease in three subjects, non-significant increase in two subjects, and the mean group values were not significantly different (40.2 ± 7.4% after tizanidine versus 34.2 ± 5.9% before tizanidine; P = 0.68, Wilcoxon matched-pairs signed-rank test). Conversely, in all five subjects, the late high-threshold excitation was significantly depressed by tizanidine (Fig. 5C; mean values 9.6 ± 2.3% after tizanidine versus 45.6 ± 9.7% before tizanidine; P < 0.05, Wilcoxon matched-pairs signed-rank test). The differences in the effects of tizanidine on the non-monosynaptic group I and on late high-threshold excitations was statistically significant (P < 0.01, Mann–Whitney U test). Selective suppression of the late high-threshold excitation by tizanidine strongly supports the view that it is mediated through group II afferents.
Results obtained in the patient with mirror movements.
In previous studies, support for a transcortical Ia loop was derived from the existence of a bilateral M2 response in hand muscles after unilateral stretch in patients with mirror movements and abnormal bilateral corticospinal projections (see Introduction). Evidence against a transcortical Ia origin of the late high-threshold excitation was therefore sought in one patient with congenital mirror movements. Figure 6A and B shows the MEP evoked at a latency of ∼14 ms in the right (Fig. 6A) and left (Fig. 6B) FCR after unilateral stimulation of the right hemisphere. Figure 6C shows the effects of unilateral (left) ulnar stimulation (2 × MT) at wrist level on the modulation of the ongoing EMG of FCR of both sides (recorded simultaneously). On the ipsilateral side (continuous line), a potent late excitation occurred at 32 ms (dashed vertical line, 10.5 ms after the monosynaptic Ia latency), after the non-monosynaptic propriospinally mediated group I excitation, which appeared at 24 ms (dotted vertical line, 2.5 ms central delay; there was no heteronymous monosynaptic Ia excitation at 21.5 ms, see legend of Fig. 6). On the contralateral side (dotted line), there was no change in the ongoing EMG at any latency. The absence of a response in the contralateral FCR in a patient with bilateral corticospinal projections to FCR motoneurone pools is evidence against mediation of the late high-threshold response through a transcortical pathway, as addressed further in the Discussion.
Long-latency group I excitation.
The experiments illustrated in Fig. 7 show that ulnar nerve stimulation at wrist level can produce, not only the late group II response, but also group I excitation at an even longer latency. In the FDS unit illustrated in Fig. 7A and B, ulnar stimulation produced a heteronymous monosynaptic Ia excitation at 33.5 ms (continuous vertical line; see legend of Fig. 7A and B). At 0.8 × MT (Fig. 7A), a low-threshold long-latency excitation also occurred at 50 ms, i.e. 16.5 ms later. This excitation was considered muscle group I in origin, since it could not be reproduced by purely cutaneous stimulation. At 2 × MT (Fig. 7B), monosynaptic excitation was followed by two peaks of late excitation: the long-latency peak at 49.5 ms, which appeared 0.5 ms earlier than that at 0.8 × MT (interrupted vertical line with large gaps), and another peak of shorter latency but higher threshold at 41.5 ms (dashed vertical line, 8 ms later than the monosynaptic Ia excitation), due to group II excitation. A similar long-latency low-threshold group I excitation was found in 6/36 FDS (4/6 subjects) and 4/24 FCR units (2/7 subjects).
In addition, the modulation of the FCR ongoing EMG in one subject (Fig. 7C) before (continuous line) and after (dotted line) tizanidine suggested two components in the late excitation produced by ulnar nerve stimulation at 2 × MT (heteronymous Ia excitation latency estimated at 20.5 ms, see legend of Fig. 7C; non-monosynaptic group I excitation occurring at 24 ms not modified by tizanidine). Tizanidine had different effects on the initial and late parts of the long-latency excitation: the initial part of the late excitation occurring at 32 ms (dashed vertical line, 11.5 ms after monosynaptic latency) was almost completely suppressed by tizanidine, but the later excitation occurring at 39.5 ms (interrupted vertical line with large gaps, 19 ms after monosynaptic latency) was not modified. This part is probably attributable to the long-latency group I excitation (although it did not appear with stimuli <1 × MT; see Discussion).
Discussion
The present investigation shows that electrical stimulation of the ulnar nerve at wrist level can evoke four successive peaks of excitation in forearm flexor motoneurones: (i) monosynaptic Ia excitation, (ii) non-monosynaptic group I excitation probably mediated through cervical propriospinal neurones, (iii) late high-threshold excitation involving a spinal pathway fed by muscle spindle group II afferents, and (iv) a late low-threshold excitation probably involving group I afferents and a transcortical pathway. These four heteronymous excitatory pathways activated from hand muscle afferents are represented in the sketch in Fig. 8 and discussed in the following.
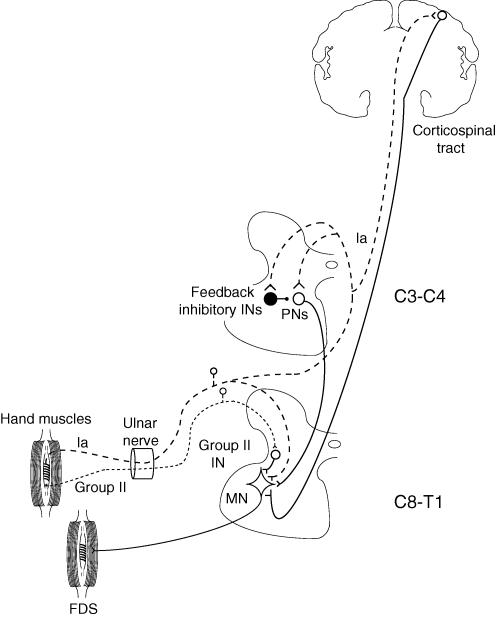
Figure 8. Sketch of the pathways mediating different excitations from hand muscles to FDS motoneurones.
Excitatory synapses are represented by Y-shaped bars and inhibitory synapses by small filled circles, excitatory interneurones by open circles, and inhibitory interneurones by large filled circles. Group II afferents from hand muscles in the ulnar nerve (dotted line) project to FDS motoneurones (MN) through an excitatory interneurone (Group II IN). Group Ia afferents from hand muscles in the ulnar nerve (dashed line) have three different excitatory projections on FDS MNs: (i) heteronymous monosynaptic segmental projection (Marchand-Pauvert et al. 2000), (ii) disynaptic projection through C3–C4 propriospinal neurones (PNs, which are inhibited from feedback inhibitory INs, also fed by the same group I afferents, Pierrot-Deseilligny & Burke, 2005), and (iii) transcortical projection through the corticospinal tract.
Monosynaptic Ia excitation
Significant ulnar-induced monosynaptic Ia excitation in the PSTHs of FDS units was more frequent in the present investigation (32/36 units) than in our previous paper (2/12 units, Marchand-Pauvert et al. 2000). This difference is most probably due to the different stimulus intensities used in the two studies: 2 × MT here versus stimuli below 1 × MT in the previous investigation. In human experiments, the nerve is stimulated through electrodes on the skin surface, and activation of all Ia afferents requires high stimulus intensities (3–4 × MT; Gracies et al. 1994). The duration of the peak of heteronymous monosynaptic Ia excitation observed with stimuli at 2 × MT (e.g. 5 ms in Fig. 2I and J) was also much longer than usual for a monosynaptic Ia peak. This long duration probably has a twofold origin: (i) a stimulus at 2 × MT will recruit slower Ia afferent fibres, and produce a longer composite Ia EPSP in the motoneurones (which is consistent with the finding that the duration of the monosynaptic peak in the same unit is much shorter (∼2 ms) with stimuli around 1 × MT; Fig. 2A–D); and (ii) axons of motoneurones supplying hand muscles lack recurrent collaterals (see Introduction), and the composite Ia EPSP is therefore not truncated by recurrent inhibition due to the antidromic motor volley at intensities above 1 × MT.
Propriospinally mediated non-monosynaptic group I excitation
Non-monosynaptic group I excitation, observed in ∼40% of FDS and FCR units, had all the characteristics of an effect mediated via C3–C4 propriospinal neurones: long central delay, low threshold and disappearance when the stimulation was increased (see Pierrot-Deseilligny & Burke, 2005). The finding that the more caudal the motoneurone pool in the spinal cord, the longer the mean central delay (FCR, C6–C8, 4.1 ms; FDS, C7–T1, 5.3 ms) also fits mediation of the excitation through interneurones located rostral to motoneurones, as are C3–C4 propriospinal neurones. The disappearance of the excitation in the PSTHs at 2 × MT reflects feedback inhibition of propriospinal neurones (see Pierrot-Deseilligny & Burke, 2005, and the sketch in Fig. 8). The absence of complete suppression in the EMG (Fig. 3D) could be explained by contractions of 10–20% of MVC recruiting motoneurones of higher threshold than in PSTH experiments (where all motoneurones are in the low-threshold range). Indeed, if data in the cat (Alstermark & Sasaki, 1986) apply to humans, propriospinal neurones transmitting the corticospinal command to these higher threshold motoneurones should receive a weaker inhibitory input than those projecting to low-threshold motoneurones.
Group II excitation
Evidence for group II excitation.
When the conditioning stimulus was applied at a more distal site (Fig 1E and F) and when the ulnar nerve was cooled (Fig. 4), the latency of the late excitation increased more than that of the monosynaptic Ia excitation. The former provides evidence against mediation of the late excitation by Ia afferents activated secondarily to stimulation of α and/or fusimotor fibres and the latter evidence against a transcortical Ia pathway. The data in Fig. 6 from the patient with bilateral corticospinal projections to FCR motoneurone pools also provides evidence against a transcortical pathway for the late high-threshold excitation. Finally, the suppression of the late high-threshold excitation by tizanidine (Fig. 5) provides further support for a group II origin of the late excitation (see Pierrot-Deseilligny & Burke, 2005).
Central and peripheral pathways.
Estimated central delay.
There are no data on group II pathways in the cervical enlargement, and it is therefore only possible to argue by analogy with what has been described for the lumbar enlargement both in cats (see Jankowska, 1992) and humans. Thus, it is assumed below that the shortest pathway has only one interneurone, which is probably not located at the same segmental level as motoneurones. The shortest central delay for group II pathways in the human lumbar spinal cord has been estimated at 3.8 ms (see Pierrot-Deseilligny & Burke, 2005), and the same value will be arbitrarily assumed in the following for the shortest central delay for group II pathways in the human cervical spinal cord.
Time taken by the group II volley to reach motoneurones and conduction velocity of group II afferents.
The distance between the ulnar nerve stimulation site and the spinal cord (C7 vertebra) was on average 0.70 m. Given a conduction velocity of 69 ms−1 for the fastest Ia afferents from hand muscles (Marchand-Pauvert et al. 2000), the afferent conduction time for the fastest Ia volley may be estimated at 10.1 ms (0.70/69). Since group II excitation occurred on average ∼11 ms after the monosynaptic Ia latency, the time for the fastest group II volley to reach motoneurones may be estimated at 21.1 ms (10.1 + 11). This time includes the afferent conduction time through slower afferents and the central delay required for the mediation through group II interneurones. Subtraction of 3.8 ms for the central delay (see above) from the time for the fastest group II volley to reach motoneurones gives a peripheral afferent conduction time of 17.3 (21.1–3.8) ms. Over the distance between the ulnar nerve stimulation site and the entrance of the afferent volleys to the spinal cord (0.70 m) this would give a conduction velocity of 40 ms−1 (0.70/0.0173) for the fastest group II afferents from hand muscles. Clearly many assumptions underlie this calculation (see above), but the value is consistent with that found for group II afferents from human foot muscles (39 ms−1; Marque et al. 2005).
Group II–Ia ratio.
Using these values, the ratio of the estimated conduction velocities of fastest group II to fastest group Ia afferents is ∼0.58 (40/69), which is in the same order of magnitude as the ratio between conduction velocities of group II and of Ia afferents in the cat (see Matthews, 1972). The ratio between the electrical thresholds of these two types of afferents (1.2/0.55 = 2.18) is also similar to that for the electrical thresholds of group II and group Ia afferents in the cat.
Origin of group II afferents.
Afferents within the group II range activated by the conditioning volleys may originate from spindle secondaries, but a contribution from cutaneous and/or joint afferents should also be considered. It is therefore important that stimuli to the skin of the fifth finger reproducing the cutaneous sensation produced by ulnar nerve stimuli at 2 × MT failed to produce the late excitation of either FDS (Fig 2K and L) or FCR (Fig. 3 and F) motoneurones. Stimulation of the skin may not equate precisely with stimulation of cutaneous afferents in a trunk nerve. However, the absence of effect from cutaneous afferents is consistent with animal data that tizanidine depresses responses of dorsal horn neurones to noxious skin stimuli, but does not modify responses to innocuous skin stimuli (Davies et al. 1984). It is therefore likely that the group II excitation suppressed by tizanidine was not related to an effect transmitted by cutaneous afferents mediating non-painful tactile sensations (see Marque et al. 2005). It appears also unlikely that joint afferents are sufficient by themselves to produce the late excitation. The threshold of joint afferents in the human lower limb is ∼1 × MT, i.e. between those for group I and group II afferents, much as has been described for joint afferents in the cat (MacLennan, 1972), and their effect is maximal with stimulus intensities at ∼1.4 × MT (Marchand-Pauvert et al. 2002). In contrast, the threshold for the late excitation investigated here is 1.2 × MT or above, and the maximal effect on the target motoneurones occurs at 1.8–2 × MT (Fig. 2C and J). It is also relevant that stimulation (2 × MT) at wrist level of the median nerve, which contains more cutaneous and joint afferents from the hand than the ulnar nerve, is less effective than ulnar volleys in evoking a late high-threshold excitation in FDS and FCR motoneurones (Marchand-Pauvert, 2006). It therefore seems likely that the group II afferents responsible for the late high-threshold excitation produced by ulnar nerve stimulation at wrist level are mainly muscle afferents. If cutaneous and joint volleys are insufficient by themselves to evoke the late excitation, they might, however, contribute to it, through their convergence with muscle group II afferents on the relevant interneurones (as described in the cat, Lundberg et al. 1987b).
Potency of heteronymous muscle spindle group II excitation.
The frequency of occurrence of group II excitation from hand muscles in FDS and FCR motoneurones was high (76% of the units). The amount of facilitation produced by group II excitation in individual units and in the ongoing FCR EMG was greater than that produced in corresponding spinal group I pathways (mono- and non-monosynaptic group I excitations). This holds true even when only the earlier part of the long-latency excitation (prior to 14 ms after the monosynaptic latency) is considered, i.e. when the high-threshold excitation is not contaminated by transcortical group I excitation (see below). This potent group II excitation from hand muscles contrasts with the failure to demonstrate a group II contribution to homonymous M2 responses to stretch in these muscles (see Introduction). This may be because more synchronized group II volleys are produced by electrical stimuli, whereas with stretch the low dynamic sensitivity of secondary endings would result in an even more dispersed afferent volley than occurs with group Ia afferents. Moreover electrical stimuli also produce synchronized cutaneous and joint volleys, which are insufficient by themselves to produce the late excitation but may converge on the relevant interneurones (see above). It is also possible that, as described in the hind limb of the low spinal cat (Lundberg et al. 1987a), muscle group II afferents have stronger projections on heteronymous than on homonymous motoneurones. This latter possibility appears plausible for human hand muscles, given that the spinal monosynaptic Ia projections from primary endings, the other spindle receptor, are weak on homonymous motoneurones and relatively strong on heteronymous motoneurones (see Pierrot-Deseilligny & Burke, 2005). The possible function of heteronymous group II muscle projections is discussed below.
Transcortical group I excitation
In some single units (Fig. 7A and B), stimulation of the ulnar nerve produced a twofold long-latency response with a high-threshold excitation occurring 8–13 ms after monosynaptic Ia latency, and a low-threshold excitation appearing later, at 16–18 ms. In two FDS motor units, the group I origin of the low-threshold long-latency excitation was confirmed: the increase in latency after distal stimulation (in experiments similar to that illustrated in Fig. 1E and F) was the same (1 ms) for the monosynaptic and the late peaks. Further support for a group I origin of the later part of the long-latency response was provided by its resistance to tizanidine in one subject (a property that contrasts with the suppression of the earlier late component classified as group II; Fig. 7C). The long latency of the responses transmitted by group I afferents implies a long central pathway. These group I responses occurred 16–18 ms later than monosynaptic latency, much as do transcortical M2 responses to stretch in intrinsic (17 ms, Matthews et al. 1990) and extrinsic (15 ms, Capaday et al. 1991) hand muscles. A transcortical pathway is therefore a good candidate. In comparison with homonymous stretch-induced transcortical group I responses, electrically induced transcortical group I excitation of heteronymous muscles was relatively rare in the present investigation (only 10/60 units), and was absent in the patient with bilateral corticospinal projections. Several factors may account for this discrepancy. First, projections of Ia afferents from hand muscles to target neurones might be less potent in heteronymous than in homonymous transcortical pathways. Secondly, the frequency of occurrence of the transcortical group I excitation was underestimated with the experimental protocol used. With the high-stimulus intensities used, a frequent and long-lasting group II excitation preceded and overlapped the transcortical response. Only when the late response had a low threshold (Fig. 7A) could it be attributed with certainty to long-latency group I excitation. However, few Ia afferents are activated with stimuli <1 × MT (Gracies et al. 1994). Thus, the absence of a late response with stimuli <1 × MT by no means excludes the possibility that transcortical group I pathways contribute to the late excitation at higher stimulus intensities. For example, in Fig. 7C, the late facilitation induced by ulnar stimulation at 2 × MT was biphasic with the late part (19 ms after monosynaptic latency) not modified by tizanidine. This later component was therefore attributable to transcortical group I excitation, even though not appearing with stimuli <1 × MT. Thirdly, recurrent inhibition and AHP of motoneurones following their discharge in the potent electrically induced group II response might prevent their activation by the later transcortical group I excitation. Finally, the conditioning volley is different, e.g. Ib afferents, which might be crucially involved in the genesis of the transcortical group I response (Matthews, 1989), are differentially activated by electrical stimulation and muscle stretch.
Significance of the findings
The long debate (see Introduction) concerning the origin of the long-latency responses to stretch in human arm muscles has been focused on homonymous M2 responses. The view has emerged that ‘in intrinsic hand muscles of primates, the segmental stretch reflex via primary and secondary spindle endings, may have been largely replaced by a transcortical loop for the more sophisticated functional demands during manipulative finger movements’ (Noth et al. 1991). Thus, in hand muscles, where homonymous spinal excitation from muscle spindles is weak, a transcortical group I loop is predominant (cf. Marsden et al. 1983; Matthews, 1991). In contrast, segmental reflexes involving most probably group II afferents are prevailing in more proximal muscles including wrist muscles (Cody et al. 1986, 1987), despite the presence of a transcortical component (Lee et al. 1983). Contrary to these previous studies, the present investigation explores heteronymous connections from distal to proximal muscles. It is shown that the long-latency excitation produced in FDS and FCR motoneurones by electrical stimulation of hand muscles afferents may have two components: one, dominant, transmitted via group II afferents and a spinal pathway, the other, weaker (but underestimated), mediated via group I afferents and a transcortical pathway.
Methodological considerations.
As seen above, it is not possible to differentiate these two components solely on threshold grounds. However, they may be distinguished on latency grounds: those classified as group II spinal responses occur at shorter latencies (8–13 ms later than monosynaptic latency) than presumed transcortical group I responses (at least 16 ms later than monosynaptic latency). Finally, suppression or not by tizanidine allows a confident distinction between the two responses.
Functional considerations.
During grasping and manipulative movements, γ (and β) drive to active muscles and stretch of the antagonists produce Ia and group II discharges from intrinsic hand muscles. This afferent inflow might be used to activate heteronymous spinal group II and transcortical Ia pathways projecting on motoneurones of proximal muscles. It will be shown in a forthcoming paper (see also Marchand-Pauvert, 2006) that spinal group II projections from hand muscles have a diffuse distribution on muscles operating at the wrist and the elbow. The distribution on antagonistic muscles suggests that heteronymous connections might be used to stabilize proximal joints and provide support to hand muscles during manipulative movements. Transcortical Ia responses also seem to spread to muscles other than the one being stretched, where they may contribute to maintain the stability of the arm. Thus, rapid and automatic transcortical group I reactions have been observed in proximal muscles after a perturbation localized to the thumb (Marsden et al. 1981). Moreover, transcortical Ia responses are highly flexible and they can even be routed to an antagonist if its contraction were mechanically advantageous (see Matthews, 1991).
The existence of two parallel pathways aiming at providing a support to hand muscles suggests that they have different functions. One can speculate that heteronymous group II spinal pathways contribute to maintain contractions of proximal muscles during grasping or manipulative movements. These proximal contractions, initiated by descending activation of α motoneurones, would be maintained by the potent excitation from hand muscle spindle secondaries activated by descending input to γ motoneurones (group II part of the ‘FRA hypothesis’, cf. Lundberg et al. 1987b). Moreover, γ motoneurones receive segmental excitation from group II afferents (Gladden et al. 1998), a positive feedback which might sustain the α motoneurone discharge. However, during manipulative movements, rapid and drastic adjustments, also concerning the supporting contractions of proximal muscles, are required for which group II pathways are not fitted. The low dynamic sensitivity of secondary endings does not allow for rapid adaptations, and their spinal connections are less rapidly flexible through descending control than are the direct transcortical ‘reflexes’. The ability of transcortical Ia pathways to elaborate quickly flexible responses (Marsden et al. 1981) might then be crucial. The dynamically sensitive, high conduction velocity Ia afferents could rapidly route the signal to the motor cortex to ‘make use of its machinery to establish complex and shifting patterns of connectivity’ (Matthews, 1991) and update proximal contractions.
Acknowledgments
The authors wish to express their gratitude to Professor David Burke and Dr Leonor Mazières for their very helpful comments and suggestions on the manuscript. This work was also supported by grants from Institut pour la Recherche Médicale (IRME). G. Lourenço and C. Iglesias were supported by grants from the University of Milan.
References
- Alstermark B, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 14. Differential projection to fast and slow motoneurones from excitatory C3–C4 propriospinal neurones. Exp Brain Res. 1986;63:530–542. doi: 10.1007/BF00237476. [DOI] [PubMed] [Google Scholar]
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal coordination of bilateral leg muscle activity during gait. J Physiol. 1984;405:1–37. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Jankowska E, Noga BR, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. Eur J Neurosci. 1990;2:1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol. 1991;440:243–355. doi: 10.1113/jphysiol.1991.sp018706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody FW, Goodwin CN, Richardson HC. Effects of ischaemia upon reflex electromyographic responses evoked by stretch and vibration in human wrist flexor muscles. J Physiol. 1987;391:589–609. doi: 10.1113/jphysiol.1987.sp016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody FW, MacDermott PB, Matthews PBC, Richardson HC. Observations on the genesis of the stretch reflex in Parkinson's disease. Brain. 1986;109:229–249. doi: 10.1093/brain/109.2.229. [DOI] [PubMed] [Google Scholar]
- Corna S, Grasso M, Nardone A, Schieppati M. Selective depression of medium-latency leg and foot muscle responses to stretch by an α2-agonist in humans. J Physiol. 1995;484:803–809. doi: 10.1113/jphysiol.1995.sp020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton K, Lippold OCJ, Shahani M, Shahani U. Long-latency spinal reflexes in humans. J Neurophysiol. 1985;53:1604–1618. doi: 10.1152/jn.1985.53.6.1604. [DOI] [PubMed] [Google Scholar]
- Davies J, Johnstone SE, Hill DR, Quillan JE. Tizanidine (Ds 103–282), a centrally acting muscle relaxant, selectively depresses excitation of feline dorsal horn neurons to noxious peripheral stimuli by an action at α2-adrenoreceptors. Neurosci Lett. 1984;48:197–202. doi: 10.1016/0304-3940(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Gladden MH, Jankowska E, Czarkowska-Bauch J. New observations on coupling between group II muscle afferents and feline γ-motoneurones. J Physiol. 1998;512:507–520. doi: 10.1111/j.1469-7793.1998.507be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies JM, Pierrot-Deseilligny E, Robain G. Evidence for further recruitment of group I fibres with high stimulus intensities when using surface electrodes in man. Electroencephalogr Clin Neurophysiol. 1994;93:353–357. doi: 10.1016/0168-5597(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534:925–933. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, McCrea D. Influence of stretch-evoked synaptic potentials on firing probability of cat spinal motoneurones. J Physiol. 1984;347:431–451. doi: 10.1113/jphysiol.1984.sp015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, Kuffler SW. Stretch receptor discharges during muscle contraction. J Physiol. 1951;113:298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Katz R, Mazzocchio R, Pénicaud A, Rossi A. Distribution of recurrent inhibition in the human upper limb. Acta Physiol Scand. 1993;149:189–198. doi: 10.1111/j.1748-1716.1993.tb09611.x. [DOI] [PubMed] [Google Scholar]
- Lee RG, Murphy JT, Tatton WG. Long-latency myotatic reflexes in man: mechanisms, functional significance, and changes in patients with Parkinson's disease or hemiplegia. Adv Neurol. 1983;39:489–508. [PubMed] [Google Scholar]
- Limousin P, Brown RG, Jahanshahi M, Asselman P, Quinn NP, Thomas D, Obeso JA, Rothwell JC. The effects of posteroventral pallidotomy on the preparation and execution of voluntary hand and arm movements in Parkinson's disease. Brain. 1999;122:315–327. doi: 10.1093/brain/122.2.315. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res. 1987a;65:271–281. doi: 10.1007/BF00236299. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res. 1987b;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- MacLennan CR. The behaviour of receptors of extramuscular and muscular origin with afferent fibres contributing to the group I and the groupII of the cat tibialis anterior muscle nerve. J Physiol. 1972;222.P:90–91P. [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurones of various leg muscles in man. Exp Brain Res. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V. Electrophysiological investigations of group II spinal reflexes in human upper and lower limbs. Proc Physiol Soc. 2006;1:SA4. [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Marque P, Iglesias C, Pierrot-Deseilligny E. Increase in group II excitation from ankle muscles to thigh motoneurones during human standing. J Physiol. 2005;566:257–271. doi: 10.1113/jphysiol.2005.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Pierrot-Deseilligny E. Monosynaptic Ia projections from intrinsic hand muscles to forearm motoneurones in humans. J Physiol. 2000;525:241–252. doi: 10.1111/j.1469-7793.2000.t01-1-00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Burke D, Pierrot-Deseilligny E. Suppression of the H reflex in humans by disynaptic autogenic inhibitory pathways activated by the test volley. J Physiol. 2002;542:963–976. doi: 10.1113/jphysiol.2002.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nielsen JB. Modulation of non-monosynaptic excitation from ankle dorsiflexor afferents to quadriceps motoneurones during human gait. J Physiol. 2002;538:647–657. doi: 10.1113/jphysiol.2001.012675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Nicolas G, Simonetta-Moreau M, Pierrot-Deseilligny E, Marchand-Pauvert V. Group II excitations from plantar foot muscles to human leg and thigh motoneurones. Exp Brain Res. 2005;161:486–501. doi: 10.1007/s00221-004-2096-6. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Human postural responses. Brain. 1981;104:513–534. doi: 10.1093/brain/104.3.513. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-latency automatic responses to muscle stretch in man: origin and function. Adv Neurol. 1983;39:509–539. [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Spindles and their Central Action. London: Arnold; 1972. p. 630p. [Google Scholar]
- Matthews PBC. Evidence from use of vibration that the human long-latency stretch reflex depends upon spindle secondary afferents. J Physiol. 1984;348:383–415. doi: 10.1113/jphysiol.1984.sp015116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Long-latency stretch reflexes of two intrinsic muscles of the human hand analysed by cooling the arm. J Physiol. 1989;419:519–538. doi: 10.1113/jphysiol.1989.sp017884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. The human stretch and the motor cortex. Trends Neurosci. 1991;14:87–91. doi: 10.1016/0166-2236(91)90064-2. [DOI] [PubMed] [Google Scholar]
- Matthews PBC, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. J Physiol. 1990;428:561–577. doi: 10.1113/jphysiol.1990.sp018228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone A, Giordano A, Corrà T, Schieppati M. Responses of leg muscles in humans displaced while standing. Effects of types of perturbation and of postural set. Brain. 1990;113:65–84. doi: 10.1093/brain/113.1.65. [DOI] [PubMed] [Google Scholar]
- Noth J, Schwarz M, Podoll K, Motamedi F. Evidence that low-threshold muscle afferents evoke long-latency stretch reflexes in human hand muscles. J Neurophysiol. 1991;65:1089–1097. doi: 10.1152/jn.1991.65.5.1089. [DOI] [PubMed] [Google Scholar]
- Paintal AS. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965;180:1–19. [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. Its Role in Motor Control and Movement Disorders. New York: Cambridge University Press; 2005. [Google Scholar]
- Schieppati M, Nardone A. Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J Physiol. 1997;503:691–698. doi: 10.1111/j.1469-7793.1997.691bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch responses of human foot muscles induced by perturbation of stance. Exp Brain Res. 1995;105:411–422. doi: 10.1007/BF00233041. [DOI] [PubMed] [Google Scholar]
- Simonetta-Moreau M, Marque P, Marchand-Pauvert V, Pierrot-Deseilligny E. The pattern of excitation of human lower limb motoneurones by probable group II muscle afferents. J Physiol. 1999;517:287–300. doi: 10.1111/j.1469-7793.1999.0287z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjær T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Usherwood TP, Garnett R. Technique for studying synaptic connections of single motoneurones in man. Nature. 1976;263:343–344. doi: 10.1038/263343a0. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Effects of large excitatory and inhibitory inputs on motoneurone discharge rate and probability. J Neurophysiol. 1999;82:829–840. doi: 10.1152/jn.1999.82.2.829. [DOI] [PubMed] [Google Scholar]
- Türker KS, Powers RK. Estimation of postsynaptic potentials in rat hypoglossal motoneurones: insights for human work. J Physiol. 2003;551:419–431. doi: 10.1113/jphysiol.2003.044982. [DOI] [PMC free article] [PubMed] [Google Scholar]