Abstract
To date, no satisfactory explanation has been provided for the immediate increase in blood flow to skeletal muscles at the onset of exercise. We hypothesized that rapid vasodilatation is a consequence of release of a vasoactive substance from the endothelium owing to mechanical deformation of the vasculature during contraction. Rat soleus feed arteries were isolated, removed and mounted on micropipettes in a sealed chamber. Arteries were pressurized to 68 mmHg, and luminal diameter was measured using an inverted microscope. Pressure pulses of 600 mmHg were delivered for 1 s, 5 s, and as a series of five repeated 1 s pulses with 1 s between pulses. During application of external pressure the lumen of the artery was completely closed, but immediately following release of pressure the diameter was significantly increased. In intact arteries (series 1, n = 6) for the 1 s pulse, 5 s pulse and series of five 1 s pulses, the peak increases in diameter were, respectively, (mean ± s.e.m.) 16 ± 2, 14 ± 2 and 27 ± 3%, with respective times from release of pressure to peak diameter of 4.1 ± 0.3, 4.6 ± 0.7 and 2.8 ± 0.4 s. In series 2 (n = 9) the arteries increased diameter by 15 ± 2, 15 ± 2 and 30 ± 3% before and by 8 ± 1, 8 ± 1 and 21 ± 2% after removal of the endothelium with air. The important new finding in these experiments is that mechanical compression caused dilatation of skeletal muscle feed arteries with a time course similar to the change in blood flow after a brief muscle contraction. The magnitude of dilatation was not affected by increasing the duration of compression but was enhanced by increasing the number of compressions. Since removal of the endothelium reduced but did not abolish the dilatation in response to mechanical compression, it appears that the dilatation is mediated by both endothelium-dependent and -independent signalling pathways.
Our understanding of the mechanism of exercise hyperaemia remains unsatisfactory. Much of the research has focused on identifying or inhibiting potential vasodilator metabolites that might be responsible for the coupling of skeletal muscle blood flow and metabolic demand during steady-state exercise. Scant attention has been paid to the mechanism for the initial phase of increasing blood flow at the onset of exercise. Careful analysis of the blood flow response to a single contraction of the human forearm or canine hindlimb provided the initial clue that vasodilatation can occur rapidly (Tschakovsky et al. 1996, 2004; Naik et al. 1999). Additional support for the concept of rapid vasodilatation was garnered from the fact that contraction-induced increases in blood flow were prevented by clamping the smooth muscle membrane potential with intra-arterial infusion of K+ (Hamann et al. 2004a). Recent in situ studies of intramuscular arterioles in the cremaster and spinotrapezius provided definitive evidence of increased diameter within the first second following contraction (Mihok & Murrant, 2004; Murrant, 2005; VanTeeffelen & Segal, 2006). It is unlikely that a vasoactive substance from the skeletal muscle can account for this rapid vasodilatation because of the time required for diffusion of a vasoactive substance from the skeletal muscle myocyte to the vascular smooth muscle. Therefore, it appears that another explanation is needed.
Given that the vessel wall is known to respond to mechanical stimuli such as shear stress (Olesen et al. 1988) and cyclic stretch (Lamontagne et al. 1992), mechanical compression of the vasculature during contraction should be considered as a mechanism for exercise hyperaemia. Using ultrasound methods, it can be observed that the arteries of the human forearm are compressed and deformed during forceful contractions (unpublished observations). Moreover, it is known that intramuscular pressures can reach 570 mmHg during contraction (Sejersted et al. 1984), a level which far exceeds systolic arterial pressure. We reasoned that mechanical deformation of the vessel wall during contraction could cause release of soluble mediators that are responsible for the resultant vasodilatation. Both smooth muscle cells and endothelial cells of the vascular wall are known to be mechanosensitive. It has long been known that smooth muscle cells respond to changes in intravascular pressure, generally referred to as myogenic autoregulation. More recent attention has focused on mechanotransduction, particularly due to shear stress, by the vascular endothelium.
These experiments tested the hypothesis that rapid vasodilatation is a consequence of release of a vasoactive substance from the endothelium as a result of mechanical deformation of the vasculature during contraction. To test this hypothesis, we isolated feed arteries from skeletal muscle and studied their response to elevated external pressures in vitro in the presence and absence of endothelium.
Methods
All experiments were performed at Pepperdine University and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Pepperdine University.
Preparation of arteries
Male Sprague–Dawley rats (300–400 g body wt) were anaesthetized with pentobarbitone sodium (50 mg kg−1), and the calf muscle group was removed and transferred to a dissection chamber containing cold (4°C) Mops-buffered physiological saline solution (PSS; containing (mm): 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 sodium pyruvate, 0.02 EDTA and 3.0 Mops, pH 7.4). Rats were euthanized by oneumothorax. Soleus feed arteries were carefully isolated, removed and transferred to a vessel chamber (Living Systems, Burlington, VT, USA) containing warm PSS. Arteries were cannulated at one end with a glass micropipette filled with PSS–albumin solution (1 g bovine serum albumin (100 ml)−1) and tied to the pipette using a 0.1 nylon suture. The artery was flushed with PSS–albumin to remove red blood cells, and the other end was cannulated and tied.
After cannulation, the vessel chamber was transferred to the stage of an inverted microscope (UNICO, Dayton, NJ, USA) coupled with a video camera (Pulnix, San Jose, CA, USA), monitor (Sony), and video micrometer (Colorado Video). Luminal diameter was continuously monitored throughout the experiment and recorded on a computer using a data acquisition system (PowerLab, ADInstruments, Colorado Springs, CO, USA). The bath was maintained at 37°C by flowing warm PSS superfusate through the chamber throughout the experiment. Micropipettes were connected to independent reservoir systems, and arteries were pressurized by elevating both reservoirs to the same level. Luminal pressure was set at 60 cmH2O (46 mmHg) initially and raised to 90 cmH2O (68 mmHg) halfway through the 1 h equilibration period to mimic in vivo pressures, as previously described (Jasperse & Laughlin, 1997, 1999).
Experimental procedures
At the end of the 1 h equilibration period, the artery was exposed to acetylcholine (10−5m) to ensure that the endothelium was viable. After ACh was washed out and the artery reconstricted, the vessel chamber was filled with warm PSS, and the Plexiglass top was carefully installed to ensure there were no air bubbles in the chamber. Bath temperature was carefully monitored and maintained by continuing to perfuse the chamber with warm PSS. Extravascular pressure was monitored continuously with a pressure transducer connected to the chamber. Pressure pulses were delivered to the chamber via an electronically controlled solenoid valve system after momentarily closing the entrance and exit ports for the bath perfusate. Pressure pulses of 600 mmHg were chosen to mimic extravascular pressures experienced by arteries during maximal muscle contraction in vivo (Sejersted et al. 1984). Two series of experiments were performed.
Series 1.
The protocol for series 1 (n = 6) was designed to determine whether vascular compression elicits dilatation and whether the magnitude of dilatation is altered by the duration of compression or by repeated compressions. Pressure pulses were delivered in three different modes in randomized order: a single 1 s pulse; a single 5 s pulse; and five 1 s pulses with a 1 s period between pulses. The rationale behind this protocol was that contractions of 1 s duration produce a marked dilatation in vivo (Tschakovsky et al. 1996; Naik et al. 1999). The 5 s pulse was intended to establish whether a longer period of compression would yield a greater dilatation. Five 1 s pulses would have the same total duration of compression as the single 5 s pulse, but would have oscillations in pressure comparable to what would occur with repeated contractions. The pressure pulses were performed in duplicate and the order was randomized across vessels.
Series 2.
The protocol for series 2 (n = 9) was intended to ascertain whether the responses to vascular compression are dependent on an intact endothelium. First, the three different modes of pressure pulses were delivered as above with the endothelium intact. Then the chamber cover was removed and 5 ml of air was slowly and carefully pushed through the artery lumen. The lumen was refilled with PSS containing albumin and allowed to equilibrate for at least 15 min before acetylcholine (10−5m) was applied to the bath to confirm effective denudation of the endothelium. In two arteries where partial dilatation to acetylcholine remained, a second bolus of air was required to abolish the response to acetylcholine. Next, the chamber was filled with PSS, the cover replaced, and the pressure pulses repeated. Afterwards, the chamber cover was removed and acetylcholine (10−5m) and sodium nitroprusside (10−5m) were applied to the bath separately to ensure, respectively, the absence of endothelial responses and vascular smooth muscle viability.
Data analysis
Data were analysed off-line from the signals recorded via the video micrometer. Baseline diameters were measured immediately before pressure pulses, and peak diameter was identified as the highest value observed in the minute following pressure pulses. The time-to-peak dilatation was determined as the time between the release of compression and the peak diameter.
Only one artery from each rat was used, and data from duplicate measurements were averaged to yield a single value for that animal. To account for differences in baseline diameter among vessels, data were analysed as the percentage change from baseline to peak. Comparisons were performed by repeated measures analysis of variance with Tukey's post hoc tests where appropriate. The level of statistical significance was set at P ≤ 0.05. Data are presented as mean values ± s.e.m.
Results
All vessels exhibited spontaneous tone. From an initial diameter of 222 ± 9 μm, they constricted to 125 ± 8 μm during the 1 h equilibration period.
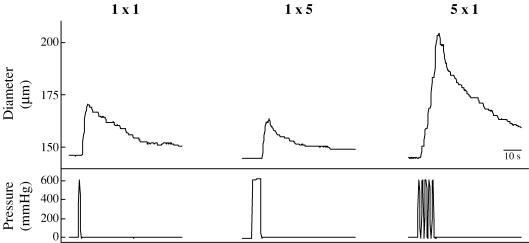
Figure 1 is a representative data record showing the response to the series of pressure pulses in a soleus feed artery with the endothelium intact. Compression of the artery invariably resulted in immediate dilatation, with the response to multiple pressure pulses being larger than that to single pressure pulses.
Figure 1. Response of a single soleus feed artery to external pressure.
1 × 1 signifies one pressure pulse of 1 s duration; 1 × 5 signifies one pressure pulse of 5 s duration; and 5 × 1 signifies five separate 1 s pulses with 1 s between each pulse. Diameters were tracked manually by moving a cursor on the video screen.
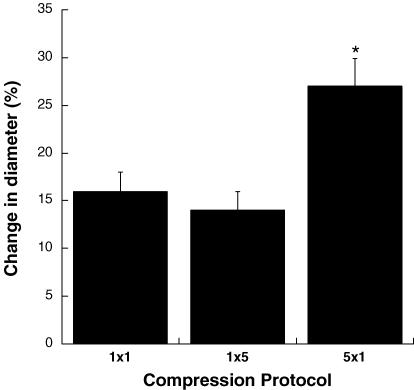
A summary of the responses in the six vessels comprising series 1 is shown in Fig. 2. All pressure pulses elicited significant increases in vessel diameter. There was no significant difference (P > 0.05) between the magnitude of vasodilatation produced by a single 1 s pressure pulse and a single 5 s pressure pulse. In contrast, a series of five 1 s pressure pulses produced a significantly greater dilatation (P < 0.01) than either of the single pressure pulses. The duration of the pressure pulse did not significantly affect (P > 0.05) the time-to-peak response for the single pressure pulses, with the delay being 4.1 ± 0.3 s for the 1 s pressure pulse and 4.6 ± 0.7 s for the 5 s pressure pulse. For the series of five 1 s pressure pulses, dilatation began in the interval between the first two pressure pulses and continued progressively until vessel diameter reached its maximum 2.8 ± 0.4 s following the end of the final pressure pulse, which was 10.8 s following the end of the initial pressure pulse. All vessels had a functional endothelium as indicated by a 60 ± 9% increase in diameter to acetylcholine.
Figure 2. Dilatation produced by compression of soleus feed arteries in series 1 (n = 6).
1 × 1 signifies one pressure pulse of 1 s duration; 1 × 5 signifies one pressure pulse of 5 s duration; and 5 × 1 signifies five separate 1 s pulses with 1 s between each pulse. *P < 0.01 compared to 1 × 1 and 1 × 5.
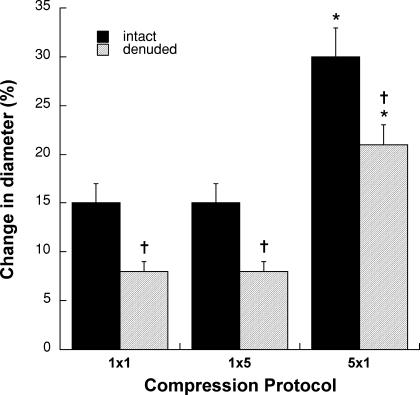
The major results from series 2 are depicted in Fig. 3. In intact vessels (as in series 1) there was no significant difference (P > 0.05) between the magnitude of dilatation produced by a single 1 s pressure pulse and a single 5 s pressure pulse. A series of five 1 s pressure pulses produced a significantly greater dilatation (P < 0.01) than either of the single pressure pulses. Removal of the endothelium significantly attenuated (P < 0.01), but did not abolish, the compression-induced dilatation. Approximately half of the response remained for the single pressure pulses, and three-quarters of the response remained for the multiple pressure pulses. Note that the absence of endothelium accounted for similar reductions in the magnitude of dilatation elicited by each compression protocol (∼9 μm). The time-to-peak dilatation was not altered by endothelial denudation (Table 1). Acetylcholine produced a 44 ± 8% increase in diameter in the intact vessels, but was virtually without effect (2 ± 1%) after denudation (P < 0.01). In contrast, sodium nitroprusside still produced a robust vasodilatation (44 ± 5%) after endothelial removal.
Figure 3. Dilatation produced by compression of intact and endothelium-denuded soleus feed arteries in series 2 (n = 9).
1 × 1 signifies one pressure pulse of 1 s duration; 1 × 5 signifies one pressure pulse of 5 s duration; and 5 × 1 signifies five separate 1 s pulses with 1 s between each pulse. *P < 0.01 compared to 1 × 1 and 1 × 5; †P < 0.01 compared to intact condition.
Table 1.
Time-to-peak response (mean ± s.e.m.) in series 2 experiments
| 1 s pulse | 5 s pulse | Five 1 s pulses | |
|---|---|---|---|
| Endothelium intact | 4.5 ± 0.2 s | 4.4 ± 0.3 s | 3.3 ± 0.2 s |
| Endothelium removed | 4.1 ± 0.3 s | 4.4 ± 0.2 s | 3.7 ± 0.5 s |
Discussion
The major findings of this study are: (1) mechanical compression caused immediate dilatation of soleus feed arteries; (2) the magnitude of dilatation was not affected by increasing the duration of compression; (3) the magnitude of dilatation was enhanced by increasing the number of compressions; (4) removal of the endothelium reduced but did not abolish the dilatation in response to mechanical compression; and (5) the time-to-peak response was 4–5 s and was not altered by removal of the endothelium. These results provide evidence to support a mechanism which could contribute to the rapid vasodilatation in contracting skeletal muscle. Vascular deformation as a result of elevated extravascular pressure within the contracting muscle may activate an intrinsic mechanosensitive mechanism that causes dilatation. Such a mechanism was originally posited by Mohrman & Sparks (1974) and recently put forward by Hamann et al. (2004b) and Tschakovsky et al. (2004) based on the in vivo blood flow response to contraction in humans.
In concordance with this hypothesis, brief periods of mechanical compression caused dilatation of skeletal muscle feed arteries. Although feed arteries, by definition, lie outside the muscle, soleus feed arteries are contained deep within the fascial sheath surrounding the gastrocnemius, soleus and plantaris muscle group. Thus, it is likely that soleus feed arteries in vivo are exposed to high extravascular pressures during contraction of the calf muscle group. Physical forces associated with contractile activity have previously been linked to the long-term growth of blood vessels (Brown & Hudlicka, 2003). The present work, along with the recent report of reactive hyperaemia-like changes in diameter of isolated gracilis arterioles by Koller & Bagi (2002), suggests a role for these physical forces in regulating acute changes in vessel diameter and blood flow.
Surprisingly, the duration of the pressure pulse had no effect on the resultant dilatation, as demonstrated by the fact that the magnitude of dilatation evoked by a single pressure pulse was ∼15% regardless of whether it was applied for 1 or 5 s. The same total time of compression (5 s) applied as five separate pressure pulses doubled the magnitude of dilatation to ∼30%. This suggests that the transducer responds to dynamic changes in pressure rather than to the duration of a static pressure.
The pressure applied in these experiments roughly mimics the extravascular pressure encountered during a maximal contraction (Sejersted et al. 1984). Since extravascular pressure in skeletal muscle is related to contraction force, vessels within the muscle are exposed to a wide range of pressures during dynamic exercise. It is clear that further studies need to be completed at lower pressures to allow a more complete understanding of the contribution of vascular compression to exercise hyperaemia. In addition, vascular responsiveness to diverse stimuli varies along the vascular tree (Jasperse & Laughlin, 2005). The response to mechanical compression in other portions of the skeletal muscle vasculature remains to be determined.
It is important to recognize that the magnitude of dilatation observed in these experiments (15–30%) was not trivial. According to Poiseulle's law, blood flow is proportional to the fourth power of the radius (r4). Thus, if there were uniform dilatation throughout the arterial resistance network, a 15% increase in diameter would translate to a 75% increase in blood flow and a 30% increase in diameter corresponds to a 185% increase in blood flow. Increases in blood flow of this magnitude would represent a substantial proportion of the increase in skeletal muscle blood flow in response to a brief maximal contraction.
Removal of the endothelium reduced but did not abolish the dilatation in response to mechanical compression. Thus, it would appear that the dilatation is mediated by both endothelium-dependent and -independent signalling pathways. Since endothelium removal accounted for similar reductions in the magnitude of dilatation for each mode, the mechanism responsible for greater dilatation to multiple pressure pulses evidently resides wholly within the vascular smooth muscle. Vascular smooth muscle relaxation would be expected under these conditions as part of the myogenic response. The vascular myogenic response is the reaction of a blood vessel to changes in transmural pressure: constriction to elevated pressure and dilatation to reduced pressure. The response is inherent to smooth muscle and is usually examined in the laboratory by altering intraluminal pressure. In this study, myogenic vasoconstriction established the baseline vascular tone upon which dilatation was observed in response to brief periods of elevated extravascular pressure. The mechanism for the interruption of myogenic tone cannot be discerned from these experiments. It has been established that changes in smooth muscle membrane potential and calcium mobilization are essential to the myogenic response (Schubert & Mulvany, 1999). The transduction of physical forces may involve mechanosensitive ion channels or the extracellular matrix and cytoskeleton of the vascular smooth muscle cell (Davis & Hill, 1999). Recently, integrins have been implicated in this mechanotransduction process (Martinez-Lemus et al. 2005). Although the specific mechanism responsible for the observed vasodilatation in response to vascular compression is unknown, it is likely to involve membrane hyperpolarization and calcium efflux.
Obvious candidate vasodilator substances for the endothelial component of the compression-induced vasodilatation include nitric oxide, prostaglandins or endothelium-derived hyperpolarizing factor. An increase in guanylate cyclase activity, indicating an increase in NO release, was demonstrated in the effluent from rhythmically compressed femoral artery rings (LaMontagne et al. 1992). In coronary arterioles, dilatation in response to longer periods of constant extravascular pressure (20–60 s) was NO dependent (Sun et al. 2004). To our knowledge, there have been no previous studies examining vascular deformation and the release of prostaglandins or endothelium-derived hyperpolarizing factor.
Identification of a vasodilator mechanism capable of responding rapidly enough to explain the prompt increase in blood flow at the onset of exercise has been a vexing problem for researchers for over a century. Metabolic processes seem too slow to account for the initial blood flow response, given the time required for diffusion of a vasoactive substance from the skeletal muscle myocyte to the vascular smooth muscle. The dilatation does not appear to be attributable to the autonomic nervous system (Buckwalter et al. 1997, 1998; Buckwalter & Clifford, 1999). Acetylcholine spillover from the motor nerve (Welch & Segal, 1997) does not seem to provide an adequate explanation for the initial vasodilatation (Dyke et al. 1998; Naik et al. 1999; Clifford et al. 2000). The data from this investigation support a relatively unexplored mechanism for initiating the increase in blood flow to contracting skeletal muscle, namely mechanical deformation of the vasculature. Evaluation of the time course of the response to compression showed an immediate increase in vessel diameter after release of pressure, with a peak diameter at 4–5 s. This time course is remarkably similar to what has been observed with muscle contractions of the human forearm or canine hindlimb. Studies using continous Doppler ultrasound measurements in humans show an immediate contraction-induced elevation in blood flow with a peak occurring 4–5 s following release of contraction (Leyk et al. 1994; Tschakovsky et al. 1996, 2004; Brock et al. 1998). When 1 s tetanic contractions were evoked by sciatic stimulation in anaesthetized dogs, blood flow was elevated within the first second following contraction and then increased progressively until reaching a peak at 4–7 s (Naik et al. 1999; Valic et al. 2005). The similarity in the time courses of the vascular response to compression and the blood flow response to muscle contraction provides credible evidence that mechanical deformation may be involved in initiating the blood flow response to contraction.
It should be made clear that we recognize that this is not the sole mechanism mediating exercise hyperaemia. In fact, arteriolar dilatation can occur in the absence of arteriolar compression, as elegantly shown by Berg et al. (1997). In addition, the tight coupling of blood flow to metabolism requires that other signals be involved during steady-state exercise. Thus, vascular deformation may be a key signalling event for the rapid vasodilatation at the onset of exercise, but it is just one part of the co-ordinated response of the skeletal muscle vascular network that brings about a normal blood flow response to exercise.
At least three attempts have been made to examine the in vivo response to compression of the vasculature. Mohrman & Sparks (1974) observed vasodilatation in response to elevated pressure in a cuff placed around the gastrocnemius muscle and concluded that extravascular compression may play a role in causing exercise hyperaemia. A later study (Bacchus et al. 1981) produced negative results with very brief pulsatile changes in extravascular pressure of 10–50 mmHg, but reported vasodilatation in response to 1 s pulses when the pressure exceeded 100 mmHg. Inflation of a blood pressure cuff around the human arm to 100 mmHg evoked a rapid increase in blood flow when the arm was in the dependant position (Tschakovsky et al. 1996). The hyperaemia was ascribed to the muscle pump but could have represented vasodilatation attributable to vascular deformation. The present data were acquired in an isolated vessel mounted in an airtight chamber, which provides more precise control of all variables and excludes any potential contribution of the muscle pump.
In summary, dilatation of skeletal muscle feed arteries was elicited by brief periods of mechanical compression which mimicked the extravascular pressure encountered during maximal skeletal muscle contractions. The magnitude of dilatation was not affected by increasing the duration of compression but was enhanced by increasing the number of compressions. The time course of dilatation was similar to the time course of the change in blood flow after a brief muscle contraction. Since removal of the endothelium reduced but did not abolish the dilatation in response to mechanical compression, it appears that the dilatation is mediated by both endothelium-dependent and -independent signalling pathways. These data support the concept that mechanical deformation of the vasculature may initiate rapid vasodilatation in contracting skeletal muscle.
Acknowledgments
The authors wish to thank Andrew Williams and Richard Rys for their design and fabrication of the solenoid-controlled pressurizing device used in these experiments. This project was supported by the National Heart, Lung, and Blood Institute and the Seaver Research Council of Pepperdine University.
References
- Bacchus A, Gamble G, Anderson D, Scott J. Role of the myogenic response in exercise hyperemia. Microvasc Res. 1981;21:92–102. doi: 10.1016/0026-2862(81)90007-8. [DOI] [PubMed] [Google Scholar]
- Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol. 1997;272:H2693–H2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol. 1998;85:2249–2254. doi: 10.1152/jappl.1998.85.6.2249. [DOI] [PubMed] [Google Scholar]
- Brown MD, Hudlicka O. Modulation of physiological angiogenesis in skeletal muscle by mechanical forces: involvement of VEGF and metalloproteinases. Angiogenesis. 2003;6:1–14. doi: 10.1023/a:1025809808697. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol. 1999;277:H1872–H1877. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. Autonomic control of skeletal muscle vasodilation during steady-state exercise. J Appl Physiol. 1997;83:2037–2042. doi: 10.1152/jappl.1997.83.6.2037. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Ruble SB, Mueller PJ, Clifford PS. Skeletal muscle vasodilation at the onset of exercise. J Appl Physiol. 1998;85:1649–1654. doi: 10.1152/jappl.1998.85.5.1649. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Valic Z, Naik JS, Buckwalter JB. Effect of vecuronium on the release of acetylcholine after nerve stimulation. J Appl Physiol. 2000;89:1249–1251. [PubMed] [Google Scholar]
- Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Dietz NM, Lennon RL, Warner DO, Joyner MJ. Forearm blood flow responses to handgripping after local neuromuscular blockade. J Appl Physiol. 1998;84:754–758. doi: 10.1152/jappl.1998.84.2.754. [DOI] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperaemia in canine skeletal muscle. J Physiol. 2004a;557:1013–1020. doi: 10.1113/jphysiol.2004.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann JJ, Buckwalter JB, Clifford PS, Shoemaker JK. Is the blood flow response to a single contraction determined by work performed? J Appl Physiol. 2004b;96:2146–2152. doi: 10.1152/japplphysiol.00779.2003. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Laughlin MH. Flow-induced dilation of rat soleus feed arteries. Am J Physiol. 1997;273:H2423–H2427. doi: 10.1152/ajpheart.1997.273.5.H2423. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol. 1999;86:6441–6449. doi: 10.1152/jappl.1999.86.2.441. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Laughlin MH. Skeletal muscle microcirculation and exercise. In: Shepro D, editor. Microvascular Research: Biology and Pathology. New York: Elsevier Academic Press; 2005. pp. 553–564. [Google Scholar]
- Koller A, Bagi Z. On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol Heart Circ Physiol. 2002;283:H2250–H2259. doi: 10.1152/ajpheart.00545.2002. [DOI] [PubMed] [Google Scholar]
- Lamontagne D, Pohl U, Busse R. Mechanical deformation of vessel wall and shear stress determine the basal EDRF release in the intact coronary vascular bed. Circ Res. 1992;70:123–130. doi: 10.1161/01.res.70.1.123. [DOI] [PubMed] [Google Scholar]
- Leyk D, Essfeld D, Baum K, Stegemann J. Early leg blood flow adjustment during dynamic foot plantarflexions in upright and supine body position. Int J Sports Med. 1994;15:447–452. doi: 10.1055/s-2007-1021086. [DOI] [PubMed] [Google Scholar]
- Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. Alphavbeta3- and alpha5beta1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol. 2005;289:H322–H329. doi: 10.1152/ajpheart.00923.2003. [DOI] [PubMed] [Google Scholar]
- Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol. 2004;82:282–287. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- Mohrman DE, Sparks HV. Myogenic hyperemia following brief tetanus of canine skeletal muscle. Am J Physiol. 1974;227:531–535. doi: 10.1152/ajplegacy.1974.227.3.531. [DOI] [PubMed] [Google Scholar]
- Murrant CL. Stimulation characteristics that determine arteriolar dilation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2005;289:R505–R513. doi: 10.1152/ajpregu.00571.2004. [DOI] [PubMed] [Google Scholar]
- Naik J, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–1746. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- Olesen SP, Clapham DE, Davies PR. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci. 1999;96:313–326. [PubMed] [Google Scholar]
- Sejersted OM, Hargens AR, Kardel KR, Blom P, Jensen O, Hermansen L. Intramuscular fluid pressure during isometric contraction of human skeletal muscle. J Appl Physiol. 1984;56:287–295. doi: 10.1152/jappl.1984.56.2.287. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Kaley G. Mechanical compression elicits NO-dependent increases in coronary flow. Am J Physiol Heart Circ Physiol. 2004;287:H2454–H2460. doi: 10.1152/ajpheart.00364.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol. 1996;271:H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol. 2005;98:72–76. doi: 10.1152/japplphysiol.00151.2004. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Segal SS. Coactivation of resistance vessels and muscle fibers with acetylcholine release from motor nerves. Am J Physiol. 1997;273:H156–H163. doi: 10.1152/ajpheart.1997.273.1.H156. [DOI] [PubMed] [Google Scholar]