Abstract
Clinical hyperglycaemia affects vascular endothelial function, but the effect on shear stress-induced arterial dilatation has not yet been established. We hypothesized that hyperglycaemia would inhibit this response via impaired glycocalyx mechanotransduction. Experiments were carried out in the anaesthetized pig in which pressure, blood flow and diameter of the left iliac artery were measured at two sites: proximal (d1) and distal (d2). Infusion of glucose, sufficient to raise blood glucose to 16–30 mm along the whole length of the artery, attenuated the shear stress-dependent dilatation in both sections of the artery with preservation of the responses to acetylcholine. The distal site was then isolated using snares and the lumen exposed to blood containing 25–35 mm glucose for 20 min. In the control situation, after exposure of both sections to normoglycaemia (5.7 mm glucose), both sections of artery showed increases in diameter in response to shear stress and acetylcholine. Hyperglycaemia attenuated the shear stress-dependent dilatation in the distal section only (P < 0.25), but not the response to acetylcholine. It is concluded from these results that the hyperglycaemia-impaired dilatation is consistent with loss of mechanotransducing properties of the endothelial glycocalyx by hyperglycaemia. These findings offer a possible explanation for the increased incidence of vascular disease in diabetic patients.
Diabetes mellitus (types 1 and 2) is associated with disease of arteries and the microcirculation, interpreted as resulting from dysfunction and/or destruction of the vascular endothelium. The fact that disease limitation is related to the tightness of blood glucose control would lead to the hypothesis that high blood glucose itself is the cause of the endothelial dysfunction (as reviewed by Bohlen, 2004; Triggle et al. 2005), but one could question whether the effect resulted from systemic hyperglycaemia or high glucose concentration in the vessel lumen.
If the latter, it is necessary to consider that all blood vessel linings are coated with the vascular endothelial glycocalyx, a highly negatively charged structure, rich in anionic sites mostly represented by the sialic acid moieties of glycoproteins and the sulphate and carboxyl groups of heparan sulphate proteoglycans, about 0.5 μm thick (Vink & Duling, 1996). The glycocalyx has been implicated as a protective factor against leucocyte adhesion to endothelial cells (Mullivor & Lipowsky, 2002) and myocardial oedema (Van den Berg et al. 2003) and as the sensor for vessel dilatation resulting from shear stress on the endothelial surface (Snow et al. 2001; proteoglycans interact via their transmembrane core protein with the cell cytoskeleton, see Weinbaum et al. 2003), i.e. the glycocalyx senses increased shear rate (Weinbaum et al. 2003) and signals the endothelial cell to release nitric oxide (Joannides et al. 1995). Impairment of flow-dependent dilatation has been shown in rabbit femoral and mesenteric arteries by neuroaminidase degradation of the sialic acid residues of endothelial glycocalyx (Hecker et al. 1993). In addition, nitric oxide production by cultured endothelial cells in response to fluid shear stress is inhibited after enzymatic removal of heparan sulphate proteoglycans (Florian et al. 2003).
In this study, the importance of the glycocalyx in mechanotransduction was studied in the intact animal by: (a) investigation of the pure shear-stress response, differentiated from the acetylcholine response; and (b) investigation of whether glucose produces a differential block of the former. The reasoning for (b) was that glucose interacts with the proteoglycans (Rosenberg et al. 1997) and glycosaminoglycans (Arisaka et al. 1995) of the glycocalyx, and this may take place under hyperglycaemic conditions. There is already evidence that glucose affects glycocalyx volume (Zuurbier et al. 2005). Unfortunately, systemic hyperglycaemia involves insulin release, and insulin has complex haemodynamic effects (Baron, 1994). Therefore, the eventual experimental approach undertaken in this study was the exploration of luminal hyperglycaemia.
Methods
This investigation was carried out under licenses issued by the Department of Health Ireland as directed by the Cruelty to Animals Act Ireland (1876) and EU Statutory Instructions (2002). Local ethical committee approval was obtained. Twenty-two female landrace pigs (18–31 kg) were sedated with ketamine (30 mg kg−1i.m.) and xylazine (2.7 mg kg−1i.m.) and then anaesthetized with a bolus, followed by a continuous infusion of sodium pentobarbitone (induction, 30 mg kg−1i.v.; maintenance 6 mg kg−1 h−1i.v.). Depth of anaesthesia was assessed by monitoring changes in heart rate and blood pressure in response to a nose pinch. At the end of the experimental procedures, animals were killed using a lethal intravenous injection of pentobarbitone.
Experimental procedures
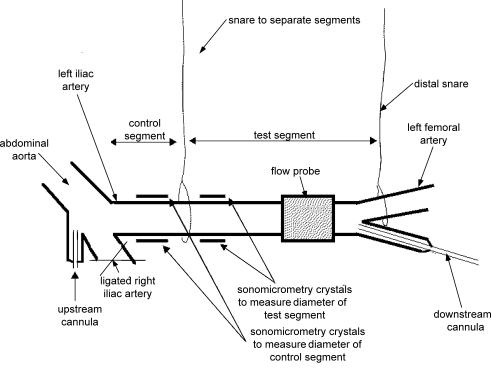
End-tidal partial pressure of CO2 was maintained between 34 and 40 mmHg by positive pressure ventilation using a Palmer pump via a tracheotomy; hypoxia was prevented by adding 40% oxygen to the inspired air. The left iliac artery was dissected from the aortic bifurcation to the deep femoral branch. A catheter-tip manometer, sometimes with lumen for infusion of acetylcholine (upstream cannula) was inserted via the right iliac artery or the mesenteric artery into the base of the aorta. The distal (test) segment was cannulated via the deep femoral artery. The right iliac artery, sacral artery, and all branches of the left iliac and mesenteric arteries were ligated. The details of the preparation are illustrated in Fig. 1.
Figure 1. Diagram of the preparation.
The upstream cannula (abdominal aorta) was inserted via the inferior mesenteric artery and was used for injection of acetylcholine or glucose in protocol 1. Both snares were occluded during protocol 2, so that glucose could be put into the test segment only. The distal snare was occluded and released (2 min) to produce reactive hyperaemia. The downstream cannula was used to introduce test solutions in protocol 2.
A snare was placed midway down the iliac artery, approximately 2 cm from where the artery leaves the abdominal aorta. Occlusion of this snare partitioned the artery into proximal (control) and distal (test) segments. A second snare was placed just above the femoral bifurcation. Temporary occlusion and release of this snare was used to study blood flow increases during reactive hyperaemia and to create a pouch at the test segment. Ultrasonic crystals were placed on diametrically opposite sides of the artery for continuous measurement of the diameter of both segments using sonomicrometry. The crystals measured the outer diameter of the artery; this was then corrected for the crystal lens effect and arterial wall thickness in the calculation of the internal diameters d1 (control) and d2 (test; Snow et al. 2001; Markos et al. 2002). An ultrasonic transit time flowmeter (Transonic Systems Inc., Ithaca, NY, USA) was placed proximal to the distal snare. Flow, arterial pressure, segment diameter, ECG and heart rate, were recorded using Power lab preamplifiers and software, and a Compaq PC. Shear stress was calculated continuously on-line by Power lab software. The whole animal was heparinised before commencement of the protocols.
Experimental protocol 1 (n = 7)
Through the upstream cannula, a 50% glucose solution (as used clinically for the treatment of hypoglycaemia) was infused at a rate calculated to achieve an approximate concentration of 40 mm in the blood flowing down the iliac artery and the effects of shear stress and ACh were observed before and after the infusion.
Experimental protocol 2 (n = 11)
In nine animals, a protocol was followed in which blood was withdrawn into a heparinised syringe from the animal and its glucose concentration was raised from normal (measured as 5.7 ± 0.9 mm, mean ± s.d., n = 9) to high (at least 20 mm). These samples were introduced into the distal arterial segment by closing both snares, stopping blood flow through the iliac artery, and irrigating the isolated distal segment for 20 min through the distal cannula (see Fig. 1); the fluid being changed every 2–3 min. In two animals, high glucose in saline (0.9%) was used.
We have previously established that snaring without irrigation had no effect on subsequent flow-dependent arterial dilatation. We started by introducing glucose at 20 mm and, if the effect on vasodilation was not reduced, increased the concentration, rather than the exposure time (the lower limb is ischaemic during the test). The concentration required to obtain a complete response was therefore variable (see Table 2). Each successful response was repeated at least twice in each preparation.
Table 2.
Matching of results of protocol 2 with criteria in each experiment
| Experiment number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Degree of block (%) | 100 | 100 | 100 | 100 | 96.9 | 66.4 | 87.8 | 100 | 80.1 | 35.6 | 100 |
| Shear response in control segment | ✓ | ✓ | ✓ | ✓ | 1† | 2† | ✓ | ✓ | ✓ | ✓ | ✓ |
| Peak diameter with ACh higher than with SS | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ |
| Response to ACh | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Recovery of SS response | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ |
| Range of SS similar, CvG | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Range of diameters similar, CvG | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Glucose in test segment (mm) | 20 | 40 | 40 | 44 | 66 | 34 | 47 | 40 | 34 | 70 | 82 |
| Glucose in B or S | S | S | B | B | B | B | B | B | B | B | B |
SS, shear stress; CvG, control versus glucose; B, blood; S, saline. †, recorded in segment 1 or 2 only because the other segment was not functional; ×.
Controls (n = 4)
In order to establish whether the results obtained were specific to d-glucose and not due to the protocol, two control procedures were carried out either in the test segment or in a separate preparation. Each intervention or administration of each substance was repeated at least twice.
First, to test that the administration of blood into the test segment did not affect responses, protocol 2 was repeated using normoglycaemic blood. Second, both snares were occluded with no fluids inserted to test that collapse of the artery below its normal diameter did not affect the response.
To ensure that the results were specific to the d-optical isomer of glucose, l-glucose (30 mm) in blood was infused into the test segment.
Two procedures were employed to test the mechanisms postulated to be involved in glucose-induced blockage of the shear stress response. First, hyaluronidase (0.28 μg ml−1) in blood was infused to show that disruption of the glycocalyx leads to attenuation of the shear stress response but preservation of the response to acetylcholine as predicted by the results reported by Mochizuki et al. (2003). Second, in order to exclude the participation of non-NO mediators in the responses (Pyke & Tschakovsky, 2005), l-NAME was administered systemically to reconfirm, in this preparation, that the responses to shear stress and acetylcholine are mediated by nitric oxide.
Calculation of shear stress (Nm−2)
Shear stress was calculated as:
where Q is the mean iliac blood flow (in ml min−1), D the mean external diameter (in mm), 1.5 is the assumed correction for lens and wall thickness, used to obtain internal from external diameter and 0.004 is the viscosity of blood (Nm−1 s).
The response was quantified as microns of increase of mean arterial diameter divided by the increase in mean shear rate.
Measurement of blood glucose
All glucose levels reported in results were measured using a Glucometer (Bayer) by withdrawing a 1 ml sample of blood and placing a drop of it on a Glucotide blood glucose test strip. If the blood glucose level was too high to be read by the glucometer, another 1 ml sample was taken and diluted, and the measurement repeated. The new reading was multiplied by the dilution factor to arrive at the blood glucose value. We started by introducing glucose at 20 mm and, if the effect on vasodilation was not reduced, increased the concentration rather than the exposure time (the lower limb is ischaemic during the test). The concentration required to obtain a complete response was therefore variable.
Statistics
Fisher's exact test, χ2 tests, and Wilcoxon matched-pairs signed-ranks test were carried out using InStat software (Graph Pad, San Diego, CA, USA). A value of 0.05 was regarded as significant.
Results
The upstream acetylcholine responses included a shear stress increase because acetylcholine causes downstream vasodilatation. In those instances in which a pure shear stress response was present, this was subtracted from the upstream acetylcholine response to assess the pure acetylcholine response. In those instances in which the shear stress response was completely blocked (protocol 2), the response observed with upstream acetylcholine was a pure acetylcholine response.
Protocol 1
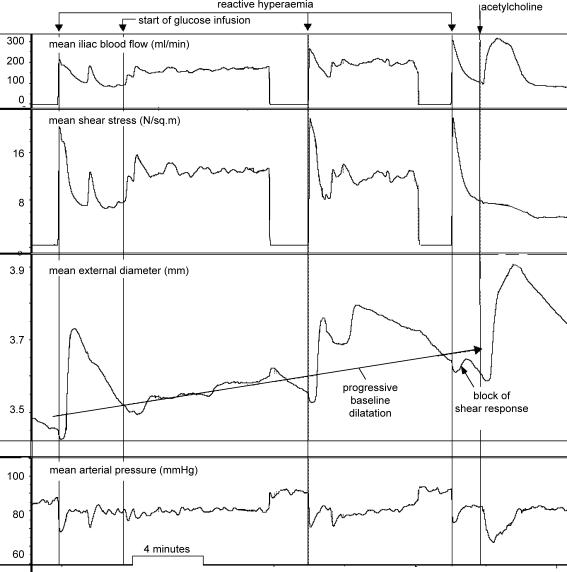
Upstream infusion of glucose caused attenuation of the shear stress-dependent response in both segments with preservation of the acetylcholine response (Fig. 2).
Figure 2. Record obtained with d-glucose infusion (protocol 1) showing (from top): mean blood flow, mean shear stress, mean arterial diameter and mean arterial pressure.
Reactive hyperaemia before the start of glucose infusion produces a large arterial diameter response. There is then progressive baseline dilatation. In the middle of the infusion, reactive hyperaemia produces an attenuated response, but after the end of the infusion, the shear stress response is almost abolished, but the artery still responds normally to acetylcholine.
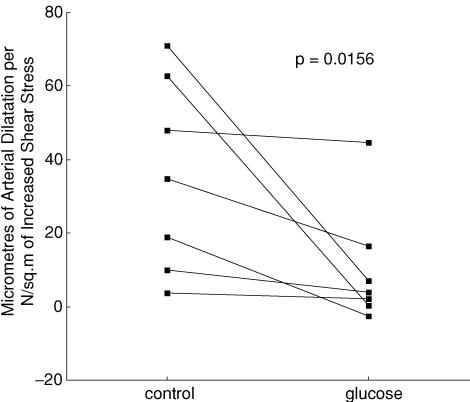
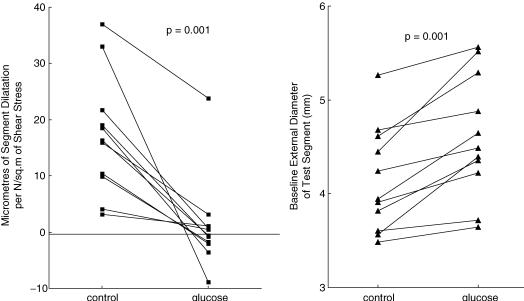
This protocol had the disadvantage of probably causing insulin release and insulin-dependent arterial dilatation (Fig. 2) (Toschi et al. 2002). Systemic infusion (n = 7) of d-glucose caused arterial dilatation and attenuated the shear stress-dependent dilatation from 35.6 ± 9.9 (mean ± s.e.m.) to 10.3 ± 6.2 μm N−1 m−2, P = 0.0156 (Wilcoxon matched-pairs signed-rank test). The results, in microns (μm) of arterial dilatation per unit of increased shear stress (newtons per meter squared), in the control situation and in the presence of hyperglycaemia, from the seven experiments, are shown in Fig. 3. The response was abolished or attenuated in all experiments, P = 0.0156. The results were subjected to criteria analysis (Table 1).
Figure 3. Plot of the ratio of the peak change of diameter per unit change of shear stress in the test segment (protocol 1), in control conditions and during d-glucose infusion.
Table 1.
Matching of results of protocol 1 with criteria in each experiment
| Experiment number | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Degree of block (%) | 52.7 | 60.5 | 41.8 | 90.2 | 100 | 99.3 | 7.2 |
| Peak diameter with ACh higher than with SS | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | n.m. |
| Response to ACh | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | n.m. |
| Range of SS similar | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Range of diameter response | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Glucose concentration (mm) | n.m. | 24 | 40 | 16 | 30 | 30 | 28 |
SS, shear stress; n.m., not measured.
One of these experiments failed the crucial test that diameter during the acetylcholine response should be greater than during the shear stress test. Counting this as a negative experiment, the probability that glucose infusion had no effect on shear stress response was < 0.0016 by Fisher's exact test.
Protocol 2
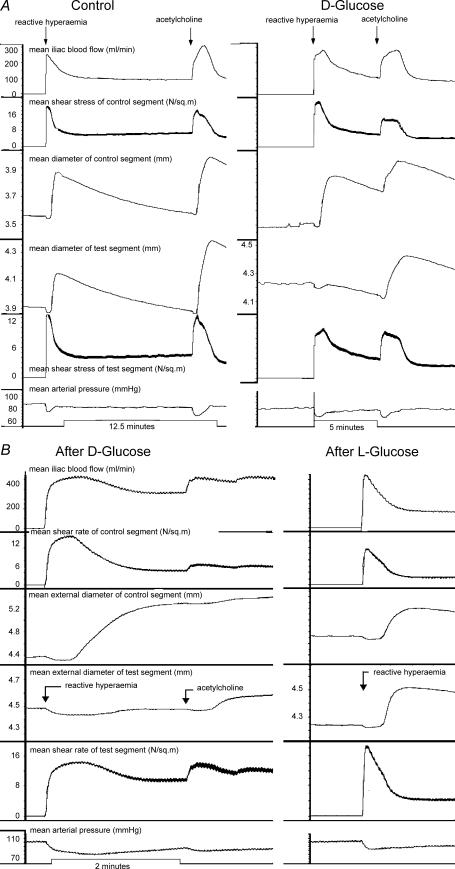
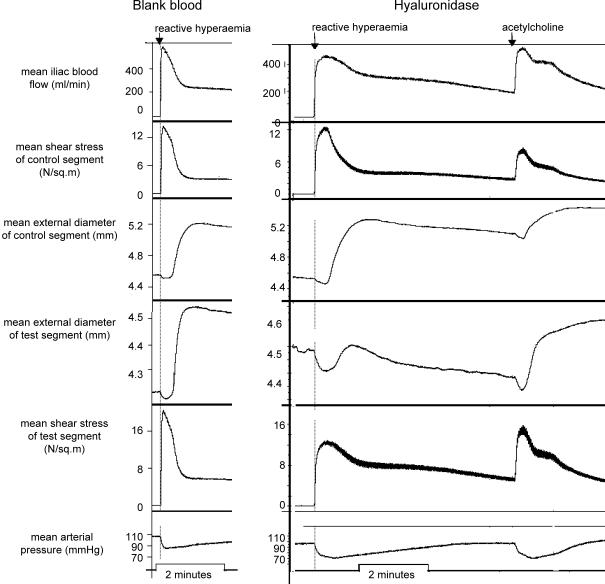
Exposure of the test segment lumen to glucose abolished or reduced the amplitude of the flow-dependent arterial dilatation in this segment, with preservation in the control segment (Fig. 4A and B); the acetylcholine response was preserved in both segments (Fig. 4) during repeated tests of both stimuli.
Figure 4. Records obtained in two experiments.
The records show (from top): mean iliac blood flow, mean shear stress in control segment, diameter of control segment (d1), diameter of test segment (d2), mean shear stress of test segment and mean arterial pressure, after exposure of d2 to d-glucose. A, left panel is a control record showing the presence of shear stress and ACh responses in both segments; right panel is a record after exposure of the lumen of the test segment to d-glucose, showing block of the shear stress response in the test segment and preservation of the acetylcholine response in both segments. The peak of the acetylcholine response in this test segment shows that nitric oxide was capable of dilating the artery to a much greater extent than during the blocked shear stress response. B, left panel shows effect of d-glucose in a similar experiment. The responses were similar to those shown in the right panel of A. Right panel of B shows that l-glucose in the same experiment had no effect. The concentration of glucose during these responses was 82 mm, the highest concentration used in all experiments in order to exclude the possibility of an osmotic effect.
The blockade of the shear stress-induced vasodilator response recovered in 20–40 min. Following this, reintroduction of high-glucose blood into the distal segment again resulted in differential blockade of the flow-dependent response. Luminal hyperglycaemia (n = 11) attenuated the shear stress-dependent dilatation in the test section only, from 17.2 ± 3.2 to 1.1 ± 2.5 μm N−1 m−2, P = 0.001, but not the response to acetylcholine.
The results in Fig. 4A and B are clear cut, but in the other experiments it may have been possible that a positive result was obtained artifactually. We therefore set criteria that should be met in the protocol (Table 2).
In all experiments, the attenuation of the flow response in d2 was accompanied by its preservation in d1. All experiments were associated with the presence of a static column of blood above the proximal snare, removal of which (after intraluminal glucose exposure), re-exposed d2 to arterial pressure, accompanied by a step up and a slow increase of the diameter of d2 before settling to a new steady value. Three of 11 experiments were unequivocal according to all the criteria in Table 2.
One experiment (no. 8) failed the vital test that diameter during an acetylcholine response should exceed diameter during the shear stress test. Counting this experiment as negative, the probability that the effect of glucose on the test segment occurred by chance was < 0.025 by χ2 test with correction for continuity, considered statistically significant.
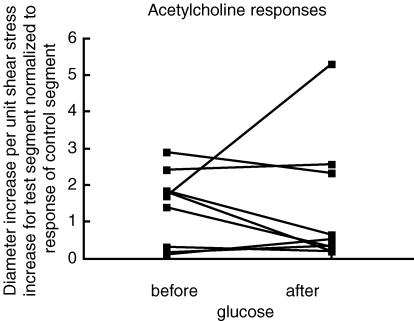
The quantified results for protocol 2, plotted in a similar manner to Fig. 3, are shown in Fig. 5. The response to shear stress diminished in all 11 experiments. It was also observed (right-hand panel) that there was always an increase in the baseline external diameter (P = 0.001). The responses to acetylcholine before and after glucose in the test segment (accompanied by block of the shear stress response) were measured in nine experiments. The increase in diameter was divided by the shear stress increase and normalized to the control segment response. The results are presented in Fig. 6. The P value was 0.6523 by Wilcoxon matched-pairs signed-ranks test (not significant).
Figure 5. Effects of intra-luminal d-glucose.
Left panel shows the change of diameter per unit change of shear stress on the test segment in protocol 2, in control conditions and after exposure of the lumen of the test segment to d-glucose (n = 11). Right panel shows change in baseline diameter (n = 11).
Figure 6. The change of diameter per unit change of shear stress on the test segment in protocol 2, normalized to the change of diameter per unit change of shear stress in the control segment.
This normalization was required because blood concentration of acetylcholine was unknown.
Controls
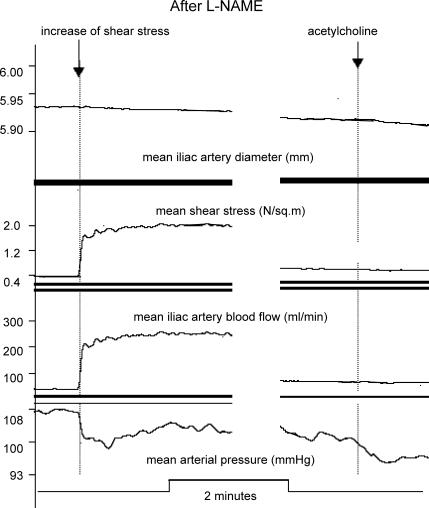
In the control experiments, occlusion of both snares, with or without insertion of normoglycaemic blood into the test segment, had no effect on the shear stress or acetylcholine responses (Fig. 4A). Likewise, l-glucose had no effect (Fig. 4B). Hyaluronidase caused attenuation of the shear stress response, with preservation of the acetylcholine response (Fig. 7), confirming the results of Mochizuki et al. (2003) by acting on the glycocalyx. l-NAME abolished both shear stress (Fig. 8) and acetylcholine responses, reconfirming, in this preparation, that these responses are mediated by nitric oxide.
Figure 7. Records obtained showing (from top): mean iliac blood flow, mean shear stress in control segment, diameter of control segment (d1), diameter of test segment (d2), mean shear stress of test segment and mean arterial pressure, after exposure of d2 to d-glucose.
Left panel shows control records illustrating the presence (with normoglycaemic blood in the test segment) of shear stress responses in both segments. Right panel is a record after exposure of the lumen of the test segment to hyaluronidase, showing block of shear stress response in test segment and preservation of acetylcholine response in both segments.
Figure 8. Records obtained after the systemic administration of l-NAME to abolish NO-mediated responses.
Left panel shows blocked response to increased shear rate; right panel shows blocked response to acetylcholine.
Discussion
It is postulated that impaired glycocalyx structure and function is a likely primary event in the causation of atherothrombotic vascular disease in diabetics and in patients presenting with acute coronary syndromes and occult insulin resistance (Stubbs et al. 1999b), known as the ‘metabolic syndrome’ (Park et al. 2005), and in their poor prognosis (Stubbs et al. 1999a). The immobilization of leucocytes at the endothelial layer after glycocalyx degradation is consistent with this view (Constantinescu et al. 2003). Such a primary event, located in the glycocalyx, has been shown to be initiated by tumour necrosis factor-α (TNF-α; Henry & Duling, 2000) and oxidized low-denisty lipoproteins (LDL; Vink et al. 2000; Constantinescu et al. 2003) and has also been postulated for vascular disease related to hyperlipidaemia (Vink et al. 2000; Constantinescu, 2002). Moreover, the fact that disturbed shear stress patterns initiate molecular changes leading to lipid accumulation in the arterial wall (Chien, 2003) indicates the primary role of the mechanotransducer in this process. This is also suggested by the finding that relatively high shear stress (protective) may suppress atherogenesis by altering the synthesis of glycosaminoglycans (Arisaka et al. 1995).
There is also evidence to suggest that glucose has an inflammatory effect, acting via generation of reactive oxygen species, and that this may be counteracted by an anti-inflammatory effect of insulin (Dandona et al. 2005). Contrary to this, it is claimed that, using euglycaemic clamp, insulin causes endothelium-dependent vasodilatation in large conduit arteries, probably by increasing oxidant stress (Arcaro et al. 2002; Singleton et al. 2003). Thus, oxygen radical production to inhibit glycocalyx function is another possible mechanism of action of glucose (Gross et al. 2003), although the failure to reverse glucose-induced impairment of relaxation in response to acetylcholine (Pieper et al. 1995) suggests otherwise.
Glucose infusion (protocol 1) had many disadvantages, including the fact that it took approximately an hour for shear stress responses to be inhibited; this is similar to the time taken for a change in glycocalyx permeability in mice (Zuurbier et al. 2005). By this time, arterial baseline dilatation had obscured the picture. It then took about 4 h for the blood glucose level to return to normal. Therefore, it was not possible to make repeated measurements within the lifetime of the preparation. Infusion of glucose results in an increase in iliac blood flow (Fig. 2), as in the coronary bed when insulin is held at normal levels with octreotide (Capaldo et al. 2005). Such an increase in flow will cause a baseline dilatation owing to shear stress-induced arterial dilatation. In protocol 1, as depicted by the data in Fig. 2, how does one explain the baseline dilatation when the shear stress response is blocked? In protocol 1, although the simulation of clinical conditions would certainly have caused insulin release, insulin rises within minutes after the start of an intravenous glucose infusion (Toschi et al. 2002). Insulin augments the effect of acetylcholine on peripheral arterioles and, to a lesser extent, that of nitroprusside (Rask-Madsen et al. 2003). Insulin also increases capillary blood volume (Dawson et al. 2002), endothelin-1 levels (Ferri et al. 1995; Cardillo et al. 1999), and serotonin (endothelium–NO)-dependent vasodilatation (Hermann et al. 2004). Remarkably, insulin infused intra-arterially does not increase blood flow (Cardillo et al. 1998, 1999), but only systemic hyperinsulinemia does so (Cardillo et al. 1998), i.e. there is an indirect mechanism by which hyperinsulinaemia causes generalized nitric oxide release (Cardillo et al. 1998). These complicated effects raise the possibility that the attenuation of the shear stress response seen with protocol 1 might not be only an effect of glucose on the glycocalyx. Also, this protocol does involve exposure of all tissue in the arterial wall to hyperglycaemia, not only the luminal surface.
As discussed above, results from protocol 1 are uncertain, so our conclusions have to be based on the results of protocol 2, which involved occlusion of the artery for 20 min. In protocol 2, after glucose, the positive response to acetylcholine was observed (in time) between two blocked shear stress responses. After glucose, the absence of a shear stress response was observed (in time) between two positive acetylcholine responses, i.e. ‘sandwich’ experiments, showing that the blockade of the shear stress response occurs at a time when the endothelial cells are capable of producing nitric oxide. The fact that glucose added to flowing blood (in protocol 1) attenuates the shear stress response in the presence of the acetylcholine response also shows that blockade of the shear stress response is caused by hyperglycaemia, and not by occlusion of the artery.
It is acknowledged that the glucose concentrations used in protocol 2 are higher than the clinical range of patients with glucose intolerance. The use of high blood glucose concentrations for 20 min (Table 2) was prompted by the fact that during the exposure time the hind leg of the animal becomes ischaemic. In pilot experiments, 20 min was found to be the minimum exposure time for blockade of the response. Since, in this protocol, the option of increasing the duration of exposure to 3 h used by Zuurbier et al. (2005) or 6 h used by Williams et al. (1998) was not possible, the concentration of glucose was increased instead. Experimentally, concentrations of 25 mm were used to demonstrate inhibition of the acetylcholine response after 3 h exposure in a rabbit model (Gomes et al. 2004) and in a rat model (Pieper et al. 1995). These concentrations are comparable to the higher levels that are seen in clinical practice (Gomes, 2004). Therefore, another advantage of our short exposure times is that the acetylcholine response would be preserved, enabling us to differentiate the mechanosensor defect from the ability of the endothelial cells to produce nitric oxide.
The data generated in protocol 2 may not reflect a direct action of glucose on the endothelial cells because it takes 5–10 days of direct exposure, not 20 min, to induce impairment of bradykinin-induced nitric oxide release (Tang & Li, 2004). Furthermore, such exposure of an artery ex vivo to glucose (Tang & Li, 2004) will expose all layers, not just the luminal surface, and will lead to inhibition of the acetylcholine response and the generation of free radicals (Yakubu et al. 2004).
A limitation of the present study was the difficulty in reproducing the blockade of the shear stress-dependent dilatation by glucose in all experiments according to the ideal experimental criteria (Tables 1 and 2). Thus it is possible to obtain results in which complete blockade is not evident. However, the overall results with intraluminal glucose (Fig. 5 and Table 2) suggest that the effect of glucose is genuine; the probability of this not being the case is estimated at < 0.05. There is no doubt that differential block of the shear stress response is possible with preservation of the acetylcholine response, demonstrated by the effects of d-glucose (Fig. 4) and hyaluronidase (Mochizuki et al. 2003). It is proposed, therefore, that the endothelial cells themselves do not sense the shear stress, but that this signal is transmitted to the endothelial cells by the glycocalyx.
The lack of effect of l-glucose would seem to exclude the possibility that the effect seen is an osmotic effect rather than one specific to d-glucose. The findings of a clear cut differential block of the shear stress response in protocol 2 (Fig. 4), together with the convincing examples of differential block with the first protocol (Fig. 2) lead us to conclude that d-glucose (probably luminal d-glucose) attenuates or abolishes endothelial mechanotransduction.
Normoglycaemic blood put into the test segment, or occlusion of the snares alone, had no effect on the shear stress response. The mere occlusion of the snares or the presence of stagnant blood is not responsible for the block following hyperglycaemia in this segment. This lack of effect of the snaring technique (which might affect oxygen and metabolite concentrations) on subsequent flow-dependent vasodilatation was demonstrated by the lack of effect of normoglycaemic blood (Fig. 7, left panel) and the lack of effect of l-glucose (Fig. 4B, right panel). Both these interventions involved double snaring.
Shear stress- and acetylcholine-induced arterial dilatation are mediated by nitric oxide release from endothelial cells. According to Hecker et al. (1993), shear stress also causes prostacyclin release. We do not think that prostacyclin is involved in our experiments, however, since shear stress responses were abolished by l-NAME, suggesting that all responses in our experiments are mediated by nitric oxide.
There are two main findings in this study. First, arterial dilatation in response to increased shear stress can be blocked while preserving the response of the endothelial cells to acetylcholine; this indicates that the shear stress signal is mediated by something between the blood and the cell, postulated to be the glycocalyx. Both of these responses are nitric oxide dependent, as shown by the l-NAME experiments. Second, such differential block can be obtained by exposing the lumen alone to higher than normal glucose concentrations. This block is specific to the d-optical isomer of glucose.
It is not clear to us whether the lack of shear stress stimulation of hyaluronan incorporation into the glycocalyx (Gouverneur et al. 2006) would happen if the arterial dilatation induced by shear stress were to be blocked by intraluminal dextrose. The doubt is caused by the fact that the shear stresses are still present and might induce hyaluronan incorporation independently. Dextrose and other atherogenic risk factors appear to cause thinning of the glycocalyx (Van den Berg et al. 2006), but whether this is related to block of shear stress-induced arterial dilatation is also uncertain. It seems to us important that future work should explore the effect or otherwise of other atherogenic risk factors than dextrose upon shear stress-induced arterial dilatation in order to determine whether there is an absolute correlation between positive effect and glycocalyx thinning effects.
Flow-mediated endothelial transduction (Davies, 1995) has been shown to involve extracellular matrix and cell proteins (Davies & Tripathi, 1993; Malek & Izomo, 1994; Girard & Nerem, 1995; Osol, 1995; Hutcheson & Griffith, 1996), such as F-actin, integrin and vimentin. Microtubules may also be involved (Sun et al. 2001). Moreover, it has been shown that mere defunctioning of glycocalyx hyaluronic acid and glycosoaminoglycans can abolish the shear stress dilatory response (Mochizuki et al. 2003). The findings of this study may indicate the following theoretical mode of action: the glycocalyx gel phase determines the mechanical response of the glycocalyx, while the proteinacious ‘hairs’ within the gel, which transmit the signal to the endothelial cellular cytoskeleton, depend for correct strain (in response to change in shear stress) upon the mechanical response of the gel phase. The strain transmitted to the cell cytoskeleton could then trigger the release of calcium ions (Muller et al. 1999), or increase intracellular calcium ion concentration from sodium–calcium exchange (Tang & Li, 2004), leading to activation of endothelial nitric oxide synthase (eNOS) and increased release of nitric oxide. Relaxation of vascular smooth muscle would then seem to be just a response to nitric oxide; mechanotransduction by vascular smooth muscle itself (Osol, 1995) appears to us unlikely.
Acknowledgments
The authors thank the Coronary Thrombosis Trust for supporting this work.
References
- Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, Bonadonna RC. Insulin causes endothelial dysfunction in humans. Circulation. 2002;105:576–589. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann NY Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:E187–E202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- Bohlen HG. Mechanisms for early microvascular injury in obesity and type II diabetes. Curr Hypertens Rep. 2004;6:60–65. doi: 10.1007/s11906-004-0013-9. [DOI] [PubMed] [Google Scholar]
- Capaldo B, Galderisi M, Turco AA, D'Erric A, Turco S, Rivellese AA, de Simone G, de Divitiis O, Riccardi G. Acute hyperglycemia does not affect the reactivity of coronary microcirculation in humans. J Clin Endocrinol Metab. 2005;90:3871–3876. doi: 10.1210/jc.2004-2207. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Nambi SS, Cannon RO III, Quon MJ, Panza JA. Vasodilator response to systemic but not to local hyperinsulinemia in the human forearm. Hypertension. 1998;32:740–745. doi: 10.1161/01.hyp.32.4.740. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83:131–151. doi: 10.1016/s0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- Constantinescu AA. Amsterdam: Academic Medical Center; 2002. Degradation of the endothelial glycocalyx by atherogenic factors. PhD Thesis, Medical Physics. [Google Scholar]
- Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Chaudhuri A, Garg R, Aljada A. Insulin infusion in acute illness. J Clin Invest. 2005;115:2069–2072. doi: 10.1172/JCI26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF. Flow-mediated endothelial transduction. Physiol Rev. 1995;75:519–559. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Tripathi SC. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ Res. 1993;72:239–245. doi: 10.1161/01.res.72.2.239. [DOI] [PubMed] [Google Scholar]
- Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282:E714–E720. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- Ferri C, Carlomagno A, Coassin S, Baldoncini R, Cassone Faldetta MR, Laurenti O, Properzi G, Santucci A, De Mattia G. Circulating endothlin-1 levels increase during euglycemic hyperinsulinemic clamp in lean NIDDM men. Diabetes Care. 1995;18:226–233. doi: 10.2337/diacare.18.2.226. [DOI] [PubMed] [Google Scholar]
- Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93:136–142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- Girard PR, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through the regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163:179–193. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- Gomes MB, Affonso FS, Cailleaux S, Almeida AL, Pinto LF, Tibirica E. Glucose levels observed in daily clinical practice induce endothelial dysfunction in the rabbit macro- and microcirculation. Fundam Clin Pharmacol. 2004;18:339–346. doi: 10.1111/j.1472-8206.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2006;290:H458–H462. doi: 10.1152/ajpheart.00592.2005. [DOI] [PubMed] [Google Scholar]
- Gross ER, LaDisa JF, Weihrauch D, Olson LE, Kress TT, Hettrick DA, Pagel PS, Warltier DC, Kersten JR. Reactive oxygen species modulate coronary wall shear stress and endothelial function during hyperglycemia. Am J Physiol Heart Circ Physiol. 2003;284:H1552–H1559. doi: 10.1152/ajpheart.01013.2002. [DOI] [PubMed] [Google Scholar]
- Hecker M, Mulsch A, Bassenge E, Busse R. Vasoconstriction and increased flow: two principal mechanisms of shear-dependent endothelial autocoid release. Am J Physiol. 1993;265:H828–H833. doi: 10.1152/ajpheart.1993.265.3.H828. [DOI] [PubMed] [Google Scholar]
- Henry CBS, Duling BR. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol. 2000;279:2815–2823. doi: 10.1152/ajpheart.2000.279.6.H2815. [DOI] [PubMed] [Google Scholar]
- Hermann TS, Ihlemann N, Dominguez H. Prolonged local forearm hyperinsulinemia induces sustained enhancement of nitric oxide-dependent vasodilation in healthy subjects. Endothelium. 2004;11:231–239. doi: 10.1080/10623320490904098. [DOI] [PubMed] [Google Scholar]
- Hutcheson LR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br J Pharmacol. 1996;118:720–726. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- Malek AM, Izomo S. Molecular aspect of signal transduction of shear stress in the endothelial cell. J Hypertens. 1994;12:989–999. [PubMed] [Google Scholar]
- Markos F, Hennessy BA, Fitzpatrick MO, Sullivan J, Snow HM. Reverse arterial wall stress causes nitric oxide-dependent vasodilatation in the anaesthetised dog. Pflugers Arch. 2002;445:51–54. doi: 10.1007/s00424-002-0915-9. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JAE, Kajiya F. Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol. 2003;285:H722–H726. doi: 10.1152/ajpheart.00691.2002. [DOI] [PubMed] [Google Scholar]
- Muller JM, Davis MJ, Kuo L, Chilian WM. Changes in coronary endothelial cell Ca2+ concentration during shear stress- and agonist-induced vasodilation. Am J Physiol Heart Circ Physiol. 1999;276:H1706–H1714. doi: 10.1152/ajpheart.1999.276.5.H1706. [DOI] [PubMed] [Google Scholar]
- Mullivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995;32:275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- Park SH, Lee WY, Rhee EJ, Jeon WK, Kim BI, Ryu SH, Kim SW. Relative risks of the metabolic syndrome according to the degree of insulin resistance in apparently healthy Korean adults. Clin Sci. 2005;108:553–559. doi: 10.1042/CS20040331. [DOI] [PubMed] [Google Scholar]
- Pieper GM, Meier DA, Hager SR. Endothelial dysfunction in a model of hyperglycemia and hyperinsulinemia. Am J Physiol Heart Circ Physiol. 1995;269:H845–H850. doi: 10.1152/ajpheart.1995.269.3.H845. [DOI] [PubMed] [Google Scholar]
- Pyke K, Tschakovsky M. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, Dominguez H, Ihlemamm N, Hermann T, Kober L, Torp-Pederson C. Tumor necrosis factor-alpha inhibits insulin's stimulating effect on glucose uptake and endothelium-dependent vasodilatation. Circulation. 2003;108:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Shworak NW, Liu J, Schwarz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. Specific structures emerge but how is synthesis regulated? J Clin Invest. 1997;100:S67–S75. [PubMed] [Google Scholar]
- Singleton JR, Smith AG, Russell JW, Feldman E. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52:2867–2873. doi: 10.2337/diabetes.52.12.2867. [DOI] [PubMed] [Google Scholar]
- Snow HM, Markos F, O'Regan D, Pollock K. Characteristics of arterial wall shear stress which cause endothelium-dependent vasodilatation in the anaesthetized dog. J Physiol. 2001;531:843–848. doi: 10.1111/j.1469-7793.2001.0843h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs PJ, Alaghband-Zadeh J, Laycock JF, Collinson PO, Carter GD, Noble MI. Significance of an index of insulin resistance on admission in non-diabetic patients with acute coronary syndromes. Heart. 1999a;82:443–447. doi: 10.1136/hrt.82.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs PJ, Laycock J, Alaghband-Zadeh J, Carter G, Noble MI. Circulating stress hormone and insulin concentration in acute coronary syndromes: identification of insulin resistance on admission. Clin Sci. 1999b;96:589–595. doi: 10.1042/cs0960589. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Sharma S, Koller A, Kaley G. Endothelial microtubule disruption blocks flow-dependent dilation of arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2087–H2093. doi: 10.1152/ajpheart.2001.280.5.H2087. [DOI] [PubMed] [Google Scholar]
- Tang Y, Li GD. Chronic exposure to high glucose impairs bradykinin-stimulated nitric oxide production by interfering with the phospholipase-C-implicated signalling pathway in endothelial cells: evidence for the involvement of protein kinase C. Diabetologia. 2004;47:2093–2104. doi: 10.1007/s00125-004-1589-y. [DOI] [PubMed] [Google Scholar]
- Toschi E, Camastra S, Sironi AM, Masoni A, Gastaldelli A, Mari A, Ferrannini E, Natali A. Effect of acute hyperglycemias on insulin secretion in humans. Diabetes Care. 2002;51:S130–S133. doi: 10.2337/diabetes.51.2007.s130. [DOI] [PubMed] [Google Scholar]
- Triggle CR, Howarth A, Cheng ZJ, Ding H. Twenty five years since the discovery of endothelium derived relaxing factor (EDRF): does a dysfunctional endothelium contribute to the development of type 2 diabetes? Can J Physiol. 2005;83:681–700. doi: 10.1139/y05-069. [DOI] [PubMed] [Google Scholar]
- Van den Berg BM, Spaan JA, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol. 2006;290:H915–H920. doi: 10.1152/ajpheart.00051.2005. [DOI] [PubMed] [Google Scholar]
- Van den Berg BM, Vink H, Spaan JAE. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- Vink H, Constantinescu AA, Spaan JA. Oxidized lipoproteins degrade the endothelial surface layer. Circulation. 2000;101:1500–1505. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci U S A. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SB, Goldfine AB, Timimi FK, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilatation in humans in vivo. Circulation. 1998;98:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- Yakubu MA, Sofola OA, Igbo I, Oyekan AO. Link between free radicals and protein kinase C in glucose-induced alteration of vascular dilation. Life Sci. 2004;75:2921–2932. doi: 10.1016/j.lfs.2004.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier CI, Demicri C, Koeman A, Vink H, Ince C. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol. 2005;99:1471–1476. doi: 10.1152/japplphysiol.00436.2005. [DOI] [PubMed] [Google Scholar]