Abstract
We have examined over the course of a 1-week period the independent and combined effects of chronically increased muscle contraction and the peroxisome proliferator-activated receptor (PPAR)α and PPARγ activators, Wy 14,643 and rosiglitazone, on the expression and plasmalemmal content of the fatty acid transporters, FAT/CD36 and FABPpm, as well as on the rate of fatty acid transport. In resting muscle, the activation of either PPARα or PPARγ failed to induce the protein expression of FAT/CD36. PPARα activation also failed to induce the protein expression of FABPpm. In contrast, PPARγ activation induced the expression of FABPpm protein (40%; P < 0.05). Chronic muscle contraction increased the protein expression of FAT/CD36 (∼50%; P < 0.05), whereas FABPpm was slightly increased (12%; P < 0.05). Neither PPARα nor PPARγ activation altered the contraction-induced expression of FAT/CD36 or FABPpm. Changes in protein expression of FAT/CD36 or FABPpm, induced by either contractions or by administration of rosiglitazone, were largely attributable to increased transcription. The contraction-induced increments in FAT/CD36 were accompanied by parallel increments in plasmalemmal FAT/CD36 and in rates of fatty acid transport (P < 0.05). Up-regulation of FABPpm expression was, however, accompanied by a reduction in plasmalemmal FABPpm, which did not affect the rates of long chain fatty acid (LCFA) transport. These studies have shown that in skeletal muscle (i) neither PPARα nor PPARγ activation alters FAT/CD36 expression, (ii) PPARγ activation selectively up-regulates FABPpm expression and (iii) contraction-induced up-regulation of LCFA transport does not appear to occur via activation of either PPARα or PPARγ.
Long chain fatty acids (LCFAs) regulate gene expression (Abumrad et al. 1999) and provide the components for cellular membranes and cellular metabolism (Abumrad et al. 1998). An important locus of control for these processes may reside at the level of the plasma membrane, the barrier to the intracellular environment. For many years, it had been thought that fatty acids enter the cell via unregulated diffusion (Hamilton & Kamp, 1999); however, there is strong support for the concept that fatty acid entry into cells occurs, in part, via a regulated protein-mediated mechanism (Abumrad et al. 1981, 1984). Several fatty acid transport proteins are thought to be involved in this process, including the 40-kDa plasma membrane fatty acid-binding protein (FABPpm) and the 88-kDa fatty acid translocase (FAT/CD36) (Abumrad et al. 1999). Altering the plasmalemmal content of FAT/CD36 and/or FABPpm results in concomitant changes in the rates of LCFA transport (Luiken et al. 2001, 2002b; Steinberg et al. 2002; Clarke et al. 2004; Koonen et al. 2004).
The peroxisome proliferator-activated receptors (PPARs) are ligand-inducible transcription factors of the nuclear hormone receptor superfamily that activate gene expression, particularly those genes involved in the regulation of fatty acid metabolism. Currently, three isoforms of PPARs (α, γ and δ) are known to exist. Generally, PPARα and PPARγ appear to govern the expression of genes regulating β-oxidation and adipogenic processes, respectively, whereas more recently, it has been shown that PPARδ is also implicated in processes regulating β-oxidation (Escher & Wahli, 2000; Chen et al. 2001; Dressel et al. 2003; Gilde et al. 2003; Hevener et al. 2003; Tanaka et al. 2003). As changes in FAT/CD36 and FABPpm result in concomitant changes in the rates of LCFA transport, which affects rates of LCFA metabolism (Steinberg et al. 2002; Bonen et al. 2004; Campbell et al. 2004; Chabowski et al. 2005; Coort et al. 2005), it is possible that these LCFA transporters are also regulated by PPARs. It is known that the FAT/CD36 gene contains a peroxisome-proliferator response element (PPRE) in its 5′ non-coding regions (Schoonjans et al. 2002), but the FABPpm gene has not been assessed yet for the presence of a PPRE. While it is not known whether FABPpm is regulated by PPARs, PPARα appears to regulate FAT/CD36 mRNA expression in liver (Motojima et al. 1998), whereas in adipose tissue (Motojima et al. 1998) and the aorta (Chen et al. 2001) this same transporter is regulated by PPARγ. Thus, it appears that PPARα and PPARγ regulate the LCFA transporter FAT/CD36 in a tissue-specific manner.
Skeletal muscle is a key tissue involved in fatty acid metabolism. Based on the positive relationship between PPARα mRNA and FAT/CD36 mRNA in human muscle, it has been suggested that PPAR mediates regulation of LCFA transporters (Zhang et al. 2004). It is interesting that chronically increased muscle activity is a stimulus for increasing the expression of FAT/CD36 and FABPpm (Bonen et al. 1999; Koonen et al. 2004), and that PPARα (Horowitz et al. 2000) and PPARγ (Kawamura et al. 2004) is also a stimulus for their increased expression. This may indicate that the contraction-induced expression of FAT/CD36 and FABPpm in muscle involves the activation of PPARα and/or PPARγ. Therefore, in the present study we examined whether muscle activity-induced up-regulation of FAT/CD36 and FABPpm occurred via induction of either PPARα or PPARγ. For these purposes, we examined the independent effects of muscle contraction, Wy 14,643, a PPARα activator, and rosiglitazone, a PPARγ activator, on the expression of FAT/CD36 and FABPpm mRNA abundance and protein levels in rat skeletal muscle. We also examined the combined effects of muscle contraction and Wy 14,643 and muscle contraction and rosiglitazone on FAT/CD36 and FABPpm mRNA and protein expression. Finally, in all these experiments we also examined the plasmalemmal content of FAT/CD36 and FABPpm, and the rates of LCFA transport.
Methods
Animals
Sprague-Dawley rats weighing 357 ± 4.8 g were used. The rats were housed in an air-conditioned room on a reverse 12 h light–12 h dark cycle. Animals were provided with rat chow and water ad libitum. Ethical approval was obtained for this work from the Animal Care Committees at the University of Waterloo and at the University of Guelph.
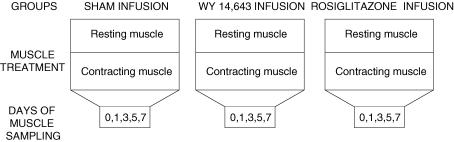
Three experimental groups were used to examine the independent and combined effects of PPAR activators and muscle contraction (Fig. 1): (i) in the first group, the PPARα activator Wy 14,643 had been infused for up to 1 week; (ii) in the second group, the PPARγ activator rosiglitazone had been infused for up to 1 week; and (iii) a sham control group was not treated with either Wy 14,643 or rosiglitazone. Within each of these three groups, the extensor digitorum longus (EDL) muscle in one leg was chronically stimulated to contract. The contralateral EDL muscle in the same animals served as a resting control for the respective treatments. These experimental treatments (contraction and/or drug) were maintained for 0, 1, 3, 5 or 7 days (Fig. 1).
Figure 1. Schematic representation of the experimental design.
There were three experimental groups in which saline (sham), WY 14,643 or Rosiglitazone were infused continuously for 7 days, using an implanted mini-osmotic pump. In addition, in each animal, EDL muscles in one leg were induced to contract for up to 7 days while the contralateral EDL was not stimulated (rest). The animals were killed after 1,3,5 and 7 days of treatment.
Chronic electrical stimulation of rat muscles
Muscles were stimulated using chronic low-frequency stimulation, as we have previously described (Johannsson et al. 1996; McCullagh et al. 1997; Bonen et al. 1999). Briefly, rats were anaesthetized with Somnotol (6 mg (100 g body weight)−1, i.p.). Induction of anaesthesia was confirmed by the absence of responses to both foot pinching and touching the eyelids. Once full anaesthesia had been induced, two stainless steel electrodes were implanted onto either side of the peroneal nerve of one of the hindlimbs. These electrodes were passed subcutaneously and exteriorized at the back of the neck, where they were attached to a miniature electronic stimulator. Sham operation was performed on the contralateral hindlimb and served as control. The animals were allowed to recover for 7 days prior to the initiation of the stimulus pulses (10 Hz; duration, 50 μs). The peroneal nerve, which innervates the EDL muscle, was stimulated for 24 h for 0, 1, 3, 5 or 7 days. The stimulators were turned off for 6 h prior to removal of the muscles. To remove the muscles, the rats were anaesthetized with an injection of Somnotol (6 mg · 100 g body weight−1, i.p.) after which the EDL muscles from both legs were excised. Immediately after removal, muscle samples were frozen in liquid nitrogen and stored at −80°C until required for further analyses, unless otherwise noted. These treatments yielded muscles that had been chronically stimulated (contraction) or that had not been electrically activated (resting).
Wy 14,643 and rosiglitazone
Fifteen minutes before the start of chronic electrical stimulation, an osmotic minipump (Alzet, Durect Corporation, Cupertino, CA, USA), which released Wy 14,643 (Cedarlane, Laboratories, Hornby, ON, Canada) or rosiglitazone (a gift from GlaxoSmithKline) at a constant rate, was inserted subcutaneously into the dorsal thoracic region of the anaesthetized rats. Implantation took approximately 10 min. The osmotic pump released 1 mg per day of either Wy 14,643 (vehicle, DMSO) or rosiglitazone (vehicle, 15% ethanol) at a constant rate of 41.7 μg h−1 for up to 7 days. Animals were killed with an overdose of somnotol (100–150 mg (100 g body weight)−1, i.p.) on days 1, 3, 5 and 7 and the EDL muscles from both legs were removed. The muscles were frozen in liquid nitrogen and stored at −80°C until required for further analyses.
Blood sampling
On the days that the animals were killed, blood samples were obtained via cardiac puncture. Serum was extracted and stored at −20°C. These samples were analysed for glucose (Sigma-Aldrich, St Louis, MO, USA), insulin (Linco, St Louis, MO, USA) and fatty acid (WAKO) concentrations using commercially available kits.
Fatty acid transporters
RNA isolation
Total RNA was isolated from the skeletal muscle samples (days 5 and 7) through the use of a modified guanidine isothiocyanate/caesium chloride centrifugation method (Chirgwin et al. 1979). Frozen muscle tissues were homogenized in 4 m guanidine isothiocyanate (8 ml) and then layered slowly onto 5.7 m caesium chloride solution (3.3 ml). The samples were centrifuged for 23 h at 20°C in an SW-41 Ti rotor (Beckman Canada, Mississauga, ON, Canada) at 150 000 g. The RNA pellets were then recovered and purified by two precipitations in cold, filtered ethanol at −80°C.
Northern blot analysis
For electrophoresis, 2 μg (FABPpm) and 3 μg (FAT) of total RNA were used on a 1.2% (w/v) formaldehyde agarose gel (Sambrook & Russel, 2001). The RNA was then transferred to a positively charged nylon membrane (Roche Diagnostics, Laval, Quebec, Canada) and cross-linked using ultraviolet light (GS-Gene Linker, Bio-Rad, Richmond, CA, USA). To ensure that the RNA was intact and equally loaded, the cross-linked membrane was stained with Northern Blot Staining Solution (Sigma-Aldrich) and scanned into the computer for later densitometry and normalization of blots using the 28S ribosomal RNA signal. The cDNA for FAT (Abumrad et al. 1993) had been previously subcloned into the EcoRI site of pBluescript(KS) and the orientation checked by digestion with Acc I. To produce the digoxigenin (DIG)-labelled antisense riboprobe, the template DNA was linearized with Ase I, and T3 RNA polymerase was used. The size of the resulting transcript was approximately 1.6 kb. The cDNA for mitochondrial AspAT (Mattingly et al. 1987), which is identical to FABPpm (Bradbury & Berk, 2000), had been previously subcloned into pBluescript(KS) and contained a 2.3-kb EcoR1 fragment. The orientation was checked by digestion of template DNA with Hind III restriction enzyme. To produce the DIG-labelled antisense RNA riboprobe, the template DNA was linearized with Xho I and T7 RNA polymerase was used.
The RNA transcription ingredients included 1–2 μg DNA template and the nucleotide triphosphate mixture (2.5 mm cytosine triphosphate, 2.5 mm GTP, 2.5 mm ATP, 1.625 mm uridine triphosphate (UTP) (Promega, Madison, WI, USA) and 0.875 mm DIG-11 UTP (Roche Diagnostics)), 20 mm dithiothreitol (Promega) and 1X RNA polymerase buffer maintained at room temperature. The final two ingredients, 0.5 μg RNase inhibitor and the appropriate RNA polymerases were added cold, and the whole probe mixture was incubated for 2 h at 37°C. The DNA template was then digested by adding 1 μl RNase-free DNase I (Promega) and incubated for 10 min at 37°C. The probe mixture was precipitated by storing in ethanol at −80°C for 30 min, centrifuged at 14 000 g for 15 min and resuspended in 20 ml hybridization buffer.
The cross-linked membranes were prehybridized in the above buffer, preheated to 68°C, for approximately 8 h on a shaker set at 68°C. The prehybridization buffer was then replaced with buffer containing the prepared DIG-labelled antisense RNA probes, specific for either FABPpm or FAT. The membranes were then incubated with the probe overnight at 68°C. A chemiluminescense detection system (Roche Diagnostics) was used to visualize the mRNA blots. Quantification of blots was performed with Gene Tool (Syngene, Perkin-Elmer, Woodbridge, ON, Canada).
Western blotting
FAT/CD36 and FABPpm protein concentrations were determined in homogenized EDL muscles and separated using SDS/PAGE. FAT/CD36 and FABPpm were detected as we have described in detail previously (Bonen et al. 2000; Luiken et al. 2001, 2002a). Proteins were visualized by chemiluminescence detection, according to the manufacturer's instructions (Hyperfilm-ECL; Amersham). Quantification of blots was performed with Gene Tool.
Preparation of giant sarcolemmal vesicles
Giant sarcolemmal vesicles were prepared as described previously (Bonen et al. 2000; Koonen et al. 2002, 2004). Briefly, by using a scalpel blade, 1- to 3-mm thick slices of muscle were obtained and these were incubated for 1h in 140 mm KCl-10 mm MOPS (pH 7.4), aprotinin (30 μg ml−1), collagenase type VII (13 U ml−1), and PMSF (0.14 mg ml−1) in a shaking 34°C water bath. Supernatant fractions were then collected and filtered through cheesecloth. The remaining tissue was rinsed with KCl-MOPS and 10 mm EDTA, and the resulting sceond supernatant fraction was filtered through the cheesecloth and pooled with the first to a volume of 7.5 ml. To this supernant fraction a Percoll solution (1.75 ml: 16% Percoll, 26 mm KCL, 10 μg ml−1 aprotinin) was added and the resulting suspension was placed at the bottom of a density gradient consisting of a 1-ml KCl-MOPS (containg 0.14 mg ml−1 PMSF) upper layer and a 3 ml middle layer of 4% Nycodenz (w/v). Following a 60 g centrifugation for 45 minutes at room temperature, the vesicles were harvested from the interface separating the upper and middle layers, diluted with KCl-MOPS and recentrifuged for 5 minutes at 12000 g. The resulting pellets were resuspended with KCl-MOPS to protein concentrations of 2–3 mg ml−1.
Palmitate uptake by giant vesicles
Palmitate uptake into giant sarcolemmal vesicles was performed as previously described (Bonen et al. 1998, 1999, 2000; Luiken et al. 1999). Briefly, 40 μl KCl–Mops with 0.1% bovine serum albumin (BSA), containing both 3H-radiolabelled (0.3 μCi) and unlabelled palmitate (14 μm) and 0.06 μCi [14C]mannitol, were added to 40 μl vesicle suspension. Following a 15-s incubation at room temperature, the palmitate uptake was terminated by the addition of a stop solution consisting of 1.4 ml ice-cold KCl–MOPS, 2.5 mm HgCl2 and 0.1% BSA. Following centrifugation at maximum speed (8050 kg) for 1 min in a tabletop microfuge (Beckman Canada), the supernatant fraction was discarded and the resulting radioactivity in the tip of the tube was determined, from which rates of palmitate transport were calculated. To measure non-specific palmitate uptake, the stop solution was added before the addition of the radiolabelled palmitate.
Statistical analysis
We pooled the data for the mRNA analyses from days 5 and 7, because there was no difference between mRNA abundance on these days. Subsequently, the mRNA data where analysed using a paired t test for each experimental treatment. Analyses of variance were used to examine the changes over time (days 0, 1, 3, 5 and 7) in LCFA transport proteins in control and experimental muscles. Post hoc analyses were performed using Fisher's least-squares difference (LSD) test. t tests were used to compare the changes in rates of LCFA transport. All data are reported as means ± s.e.m.
Results
Treatment with Wy 14,643 or rosiglitazone did not alter the circulating insulin or glucose concentrations in healthy animals (Table 1). This finding is similar to previous studies in which rosiglitazone or Wy 14,643 did not lower glucose and/or insulin concentration in lean mice, although reductions did occur in Wy 14,643- or rosiglitazone-treated ob/ob mice (Muurling et al. 2003; Ide et al. 2004; Wilson-Fritch et al. 2004). Circulating fatty acid concentrations were reduced by 30% in the animals treated with Wy 14,643 (P < 0.05, Table 1) and there was a tendency for rosiglitazone to lower circulating fatty acid concentrations (−21%; P = 0.075, Table 1). Previously, rosiglitazone has been found to reduce circulating fatty acid concentrations in obese Zucker rats (Coort et al. 2005), but not in ob/ob or lean mice (Muurling et al. 2003; Dhindsa et al. 2005). In another study, Wy 14,643 was found to reduce circulating fatty acids in ob/ob mice, wheras there was a tendency to do so in lean mice (Ide et al. 2004), as in the healthy animals in the present study.
Table 1.
Glucose, insulin and fatty acid concentrations in control and experimental animals
| Treatment Group | Glucose (mm) | Insulin (ng ml−1) | Fatty acids (mm) |
|---|---|---|---|
| Control (n = 8) | 11.1 ± 0.9 | 5.1 ± 1.0 | 0.34 ± 0.05 |
| WY 14,643 (n = 8) | 11.9 ± 0.8 | 5.4 ± 1.1 | 0.24 ± 0.02* |
| Rosiglitazone (n = 8) | 10.3 ± 0.4 | 5.0 ± 0.3 | 0.27 ± 0.02** |
Data are means ± s.e.m. Data were obtained from animals treated for 7 days
P < 0.05 versus control group
P = 0.075 versus control group.
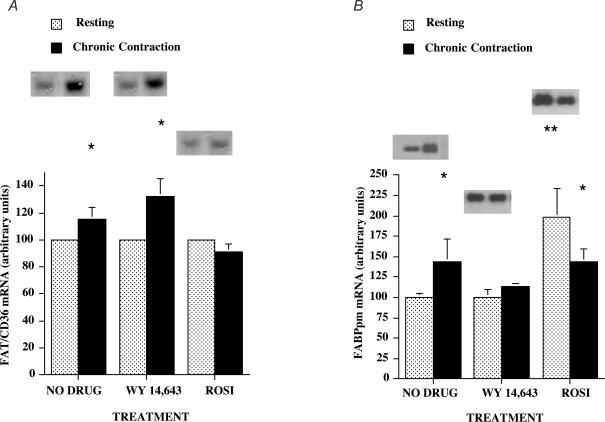
Effects of muscle contraction, Wy 14,643 and rosiglitazone on fatty acid transporter mRNAs: FAT/CD36 mRNA
Resting muscle
There were no differences among the groups in FAT/CD36 mRNA (P > 0.05, Fig. 2A). Therefore, within each group these data were normalized to 100 (arbitrary units).
Figure 2. FAT/CD36 mRNA (A) and FABPpm mRNA (B) in resting and chronically contracting muscles treated with the PPARα activator Wy 14,643 and the PPARγ activator rosiglitazone.
Data are means ± s.e.m. In each treatment group, data from day 5 and day 7 were pooled and mRNA data were normalized to 285 ribosomal RNA. n = 6–8 muscles for each group. *P < 0.05, resting muscle versus chronically contracting muscle. **P < 0.05, resting muscle + rosiglitazone versus resting muscle no drug, and resting muscle + Wy 14,643.
Chronic muscle contraction
There was a small, but consistent, increase in FAT/CD36 mRNA (+15%; P < 0.05) in the chronically contracting muscles and in contracting muscles treated with Wy 14,643 (+32%; P < 0.05), although these increments in the two groups did not differ (P > 0.05, Fig. 2A). In contrast, treatment with rosiglitazone inhibited the contraction-induced increase in FAT/CD36 mRNA (Fig. 2A).
FABPpm mRNA
Resting muscle
Compared to the untreated muscles, there was no change in FABPpm mRNA in the muscles of animals treated with Wy 14,643 (P > 0.05). In contrast, in the rosiglitazone-treated animals, FABPpm mRNA was markedly increased in the resting muscles (+98%; P < 0.05, Fig. 2B).
Chronic muscle contraction
In the chronically contracting muscles, compared to control muscles, FABPpm mRNA was increased by 44% (P < 0.05, Fig. 2B). A small, consistent increase (+13%; P < 0.05) was observed in chronically contracting muscles of the Wy 14,643-treated animals (Fig. 2B). In the rosiglitazone-treated animals, FABPpm mRNA was increased (+44%; P < 0.05, Fig. 2B). This increase was less (P < 0.05) than the rosiglitazone-induced increase in FABPpm mRNA (+98%) in the resting muscles of the same animals (Fig. 2B).
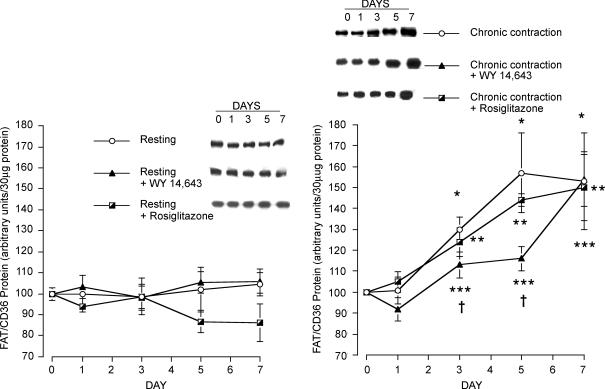
Effects of muscle contraction, Wy 14,643 and rosiglitazone on fatty acid transporter proteins: FAT/CD36 protein
Resting muscle
A 7-day infusion of either Wy 14,643 or rosiglitazone failed to alter expression of FAT/CD36 protein in muscle (Fig. 3A). However, in these same animals, the PPARα agonist, Wy 14,643, increased FAT/CD36 protein in the liver by 80% (P < 0.05, data not shown).
Figure 3. FAT/CD36 protein in resting (A) and chronically contracting muscles (B) treated with the PPARα activator Wy 14,643 and the PPARγ activator rosiglitazone.
Data are means ± s.e.m. n = 4 – 5 muscles for each group at each data point. *P < 0.05, chronic contraction versus day 0. **P < 0.05, chronic contraction + rosiglitazone versus day 0. ***P < 0.05, chronic contraction + Wy 14,643 versus day 0. †P < 0.05, chronic contraction + Wy 14,643 versus chronic contraction, and chronic contraction + Wy 14,643 versus chronic contraction + rosiglitazone.
Chronic muscle contraction
Chronic muscle stimulation for 7 days progressively increased FAT/CD36 protein expression. After 3 days, FAT/CD36 expression was increased by 30% (P < 0.05), and was further increased in the next few days, attaining a plateau at day 5 (+57%; and +53% at day 7) of chronic stimulation (P < 0.05, Fig. 3B).
The combined effects of muscle contraction and administration of rosiglitazone also lead to increments in FAT/CD36 protein expression (day 3, +24%; day 5, +44%; day 7, +50%; P < 0.05). However, these increments did not differ from those observed in the chronic contraction group that had not been treated with rosiglitazone (P > 0.05, Fig. 3B).
Infusion with Wy 14,643 initially inhibited the chronic contraction-induced increase in FAT/CD36, as the FAT/CD36 increments observed on day 3 (+13%) and day 5 (+16%) were lower than those observed in the chronically contracting muscles that had not been treated with Wy 14,643 (day 3, +30%; day 5, +57%; P < 0.05). However, by day 7 these increases in FAT/CD36 (+50 to +57%) were similar among all the groups (P > 0.05, Fig. 3B).
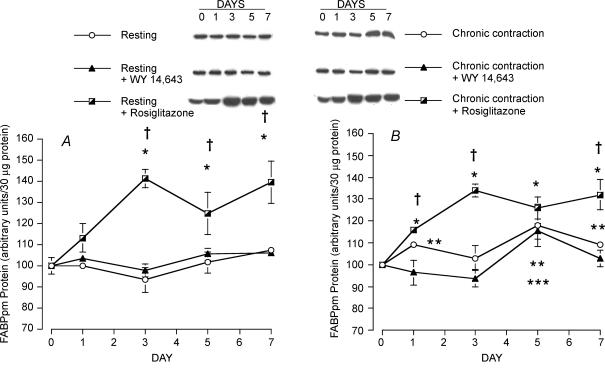
FABPpm protein
Resting muscle
During 7 days of infusion with Wy 14,643, FABPpm expression was not altered (P > 0.05, Fig. 4A). In contrast, infusion of rosiglitazone induced a marked increase in FABPpm expression in resting muscle (P < 0.05, Fig. 4A). Specifically, after 1 day of infusion, FABPpm was increased by 13% (P < 0.05), and there was a 41% increase in FABPpm (P < 0.05) by day 3. This up-regulation by FABPpm was maintained at days 5 (+25%) and 7 (+39%) of rosiglitazone infusion (Fig. 4A).
Figure 4. FABPpm protein in resting (A) and chronically contracting muscles (B) treated with the PPARα activator Wy 14,643 and the PPARγ activator rosiglitazone.
Data are means ± s.e.m. n = 4 – 5 muscles for each group at each data point. *P < 0.05, resting muscle + rosiglitazone versus day 0, and chronic contraction + rosiglitazone versus day 0. **P < 0.05, chronic contraction versus day 0. ***P < 0.05, chronic contraction + Wy 14,643 versus day 0. †P < 0.05, chronic contraction + rosiglitazone versus resting muscle and chronic contraction, and chronic contraction + rosiglitazone versus resting muscle + Wy 14,643 and chronic contraction + Wy 14,643.
Chronic muscle contraction
There was only a small but significant increase in FABPpm (+9 to +18%; P < 0.05) after 5–7 days in chronically stimulated muscles and in the chronically stimulated Wy 14,643-treated animals (+15% after 5 days, P < 0.05, Fig. 4B). The combined chronic muscle contraction and rosiglitazone treatment increased FABPpm expression by 16%, 34%, 26% and 32% on days 1, 3, 5 and 7, respectively (P < 0.05, Fig. 4B). These increases were almost identical to the changes in FABPpm observed in the non-contracting muscles in the same animals (i.e. +13%, +41%, +25% and +32% on days 1, 3, 5 and 7, respectively, P > 0.05, Fig. 4A).
Effects of muscle contraction, Wy 14,643 and rosiglitazone on plasmalemmal fatty acid transporters
Plasma membrane FAT/CD36
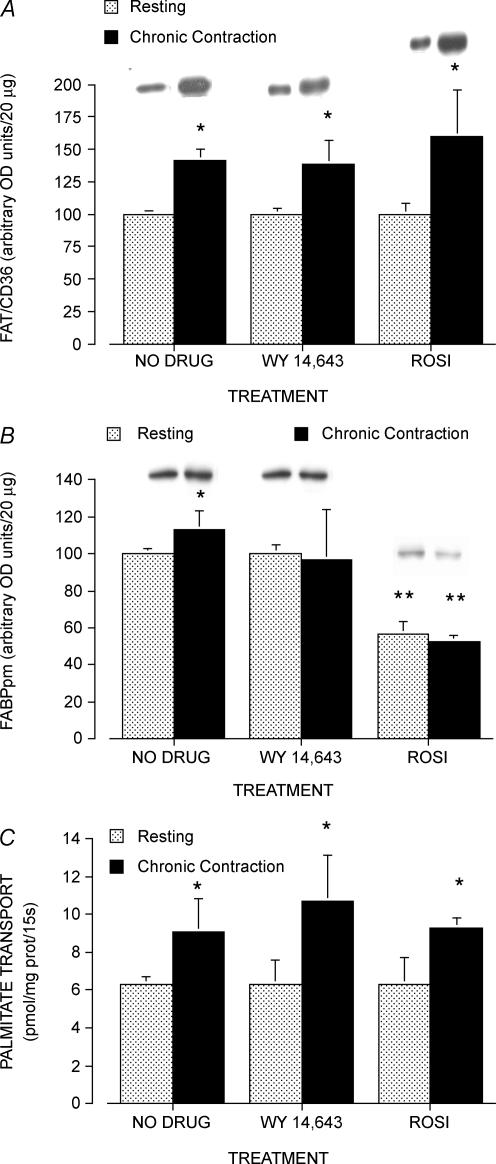
There were no differences in the plasmalemmal content of FAT/CD36 in the resting muscles in the three groups of rats (P > 0.05) and, therefore, these data in each group were set to 100 (arbitrary units). In the chronically contracting muscles, plasma membrane FAT/CD36 level was increased by 41% (P < 0.05, Fig. 5A), whereas in the chronically stimulated muscles treated with Wy 14,643 and rosiglitazone, plasmalemmal FAT/CD36 level was increased by 39% (P < 0.05) and 60% (P < 0.05, Fig. 5A), respectively. These increments in plasma membrane FAT/CD36 level did not differ among the groups (P > 0.05).
Figure 5. Plasmalemmal FAT/CD36 (A) and FABPpm protein levels (B) and rates of palmitate transport into giant sarcolemmal vesicles (C) and in resting and chronically contracting muscles treated with the PPARα activator Wy 14,643 and the PPARγ activator rosiglitazone.
Data are means ± s.e.m. n = 4 – 5 muscles for each group. *P < 0.05, resting muscle versus chronically contracting muscle (with and without Wy 14,643 and/or rosiglitazone). **P < 0.05, rosiglitazone-treated muscles versus all other treatments.
Plasma membrane FABPpm
There was no change in the plasmalemmal content of FABPpm among the resting muscles, either in the presence or absence of Wy 14,643 (P < 0.05, Fig. 5B). In chronically contracting muscle there was a small, consistent increase in plasmalemmal FABPpm (+13%; P < 0.05, Fig. 5B), whereas no changes in plasmalemmal FABPpm were observed in contracting muscles treated with Wy 14,643 (P > 0.05, Fig. 5B). In contrast, with the 7-day administration of rosiglitazone, the plasmalemmal content of FABPpm was decreased by ∼40% in both the control and chronically stimulated muscles (P < 0.05, Fig. 5B).
Effects of muscle contraction, Wy 14,643 and rosiglitazone on rates of fatty acid transport
In the resting muscles, rates of palmitate transport into giant sarcolemmal vesicles did not differ among the three treatment groups (P > 0.05, Fig. 5C). In the chronically contracting muscles, the rates of palmitate transport were increased in each of the three groups (P < 0.05, Fig. 5C). These increases ranged from +44% in the chronically stimulated muscles to +60% and +48% in the chronically stimulated muscles treated with Wy 14,643 and rosiglitazone, respectively. These increments did not differ significantly among the three groups (P > 0.05, Fig. 5C).
Discussion
In this study we examined the independent and combined effects of chronically increased muscle contraction and the PPARα and PPARγ activators (Wy 14,643 and rosiglitazone, respectively) on the expression and plasmalemmal content of the fatty acid transporters, FAT/CD36 and FABPpm, as well as on the rate of fatty acid transport. The present study confirms previous reports (Bonen et al. 1999; Koonen et al. 2004) that chronic muscle contraction increases the expression of FAT/CD36 and FABPpm. In addition, a number of novel observations were made. (i) Despite the known Wy 14,643-mediated induction of FAT/CD36 in liver (Motojima et al. 1998 and data not shown), no such induction was observed in resting skeletal muscle in either FAT/CD36 or FABPpm protein expression. (ii) Rosiglitazone administration increased FABPpm protein expression, but not FAT/CD36 protein expression, in resting skeletal muscle. (iii) These rosiglitazone-induced increments in FABPpm protein expression were accompanied by a decrease in plasmalemmal FABPpm, which failed to alter the rate of palmitate transport. (iv) In the contracting muscles, the increase in FAT/CD36 protein expression was not attenuated by rosiglitazone and only temporarily by Wy 14,643 (days 1–5, not day 7). (v) Increments in FABPpm were not altered by Wy 14,643, and the rosiglitazone-induced increments in FABPpm were unaltered by muscle contraction. (vi) The rates of LCFA transport were not influenced by either rosiglitazone or Wy 14,643 in either resting or contracting muscles. (vii) Finally, the contraction-induced up-regulation of LCFA transport is mainly associated with an increase in plasmalemmal FAT/CD36, not plasmalemmal FABPpm.
FAT/CD36 responses to low-frequency chronic stimulation, Wy 14,643 and rosiglitazone
The response of FAT/CD36 to PPAR activators is controversial. Motojima et al. (1998) observed that PPARα activation via Wy 14,643 induced FAT/CD36 mRNA in liver, but not in adipose tissue, of mice. Others have shown that activation of PPARα by Wy 14,643 does occur in skeletal muscle (Brun et al. 1999; Wu et al. 1999; Clapham et al. 2001). Thus, in agreement with Motojima et al. (1998), the results from our study also indicate that a tissue-specific response to PPARα activation occurred; with Wy 14,643 infusion there was a large increase in hepatic FAT/CD36 protein (+80%, data not shown) whereas no increase in skeletal muscle FAT/CD36 protein or mRNA was found in the same animals. Similarly, activation of PPARγ by rosiglitazone in skeletal muscle also failed to induce FAT/CD36. This result parallels observations in diabetic mice in which rosiglitazone administration failed to alter the abundance of the FAT/CD36 transcript in muscle (Albrektsen et al. 2002). In C2C12 muscle cells, FAT/CD36 mRNA abundance was not altered by activation of either PPARα or PPARγ (Dressel et al. 2003), although PPARγ activation by rosiglitazone induced FAT/CD36 protein expression in cultured muscle cells obtained from type 2 diabetic patients (Wilmsen et al. 2003). Collectively, these studies suggest that induction of FAT/CD36 expression by PPARα and/or PPARγ activators is tissue specific (liver versus muscle), while species differences (rodent versus human) may also be occurring.
In contrast to the results with PPARα or PPARγ activation, FAT/CD36 protein expression was shown to increase in response to chronic contraction. This has been observed previously in our laboratory (Bonen et al. 1999). The present study shows that this contraction-induced increase in FAT/CD36 was not influenced by the concomitant activation of either PPARα or PPARγ. In the chronically contracting muscles, there was an increase in both FAT/CD36 mRNA and protein expression. However, it is unclear why rosiglitazone treatment during chronic stimulation prevented the contraction-induced increase in FAT/CD36 mRNA. Collectively, these results suggest that rosiglitazone exerts its effects on FAT/CD36 expression at the level of transcription rate and/or mRNA stability.
FABPpm responses to Wy 14,643, rosiglitazone and chronic stimulation
The lack of FABPpm induction in the resting muscles treated with Wy 14,643, indicates that, just as in liver (Motojima et al. 1998), Wy 14,643 does not induce FABPpm expression in skeletal muscle. This suggests that this protein is not regulated by the PPARα pathway in either liver (Motojima et al. 1998) or skeletal muscle (present study). Similarly, the changes in FABPpm protein expression in muscle during chronic stimulation were minor, although there was some up-regulation at the mRNA level.
The finding that rosiglitazone administration increased both FABPpm mRNA accumulation and protein demonstrates that FAT/CD36 and FABPpm can be independently regulated, although the candidate pathways involved remain to be determined. This rosiglitazone-induced up-regulation of FABPpm is only one of a few reports to demonstrate that selected proteins can be up-regulated by rosiglitazone in skeletal muscle. It is widely believed that rosiglitazone affects primarily adipose tissue gene expression. Because plasmalemmal FABPpm was reduced, it appears that the increase in FABPpm expression was confined to other subcellular sites. While FABPpm may have been retained within a low-density microsomal compartment that has recently been identified (Chabowski et al. 2005), we have recently found that PPARγ activation via rosiglitazone increases skeletal muscle mitochondrial aspartate aminotransferase (C. Benton and A. Bonen, unpublished data), which is known to be the sequence homologue of FABPpm (Stump et al. 1993; Bradbury & Berk, 2000; Cechetto et al. 2002).
Palmitate transport and plasma membrane abundance of FAT/CD36 and FABPpm in response to low-frequency chronic stimulation, Wy 14,643 and rosiglitazone
In line with our previous work (Bonen et al. 1999; Koonen et al. 2004), chronic stimulation markedly stimulated fatty acid transport into giant sarcolemmal vesicles prepared from skeletal muscle. The observed increase in palmitate transport in the present study was associated with the increase in the expression and plasmalemmal content of FAT/CD36, as has also been shown previously (Bonen et al. 1999, 2000). Based on work demonstrating that FABPpm inhibition reduced the rate of LCFA transport in heart and skeletal muscle (Luiken et al. 1999; Turcotte et al. 2000), our findings of the decreased plasmalemmal FABPpm would have been expected to result in a reduced LCFA transport; however, this was not observed. Previously, we have shown that overexpressing FABPpm in muscle can increase LCFA transport (Clarke et al. 2004). However, we (Clarke et al. 2004) also noted that because the overexpression of plasmalemmal FABPpm (+173%) far exceeded the effects on the rates of palmitate transport (+79%), it seemed that the overexpression of FABPpm alone was not sufficient to induce completely parallel increments in palmitate transport. It is possible that sarcolemmal FABPpm is normally present in excess and that its reduction is therefore of little functional consequence, whereas its overexpression may facilitate interaction with FAT/CD36 to enhance fatty acid transport into the muscle cell. The results of the present study suggest that FAT/CD36 may be more critical than FABPpm in regulating LCFA transport.
Summary
The present studies have shown that in skeletal muscle (i) neither PPARα nor PPARγ activation alters FAT/CD36 protein expression, (ii) activation of PPARγ, but not PPARα, selectively up-regulates FABPpm/mitochondrial aspartate amino transferase expression and (iii) contraction-induced up-regulation of LCFA transport does not appear to occur via PPARα-, or PPARγ-mediated activation of LCFA transporters. We presume that PPARβ, a transcriptional regulator of β-oxidation enzymes in adipose tissue (Wang et al. 2003) and in skeletal muscle (Wang et al. 2004), also does not regulate FAT/CD36, because activation of PPARβ also failed to alter FAT/CD36 expression in C2C12 cells (Dressel et al. 2003).
Acknowledgments
These studies were supported by grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada and the Netherlands Heart Foundation (200.156). C.R.B. was supported by an Ontario Graduate Scholarship. J.F.C.G. is Netherlands Heart Foundation Professor of Cardiac Metabolism. J.J.F.P.L. is a recipient of a VIDI-Innovation Research Grant from the Netherlands Organization for Scientific Research (NOWZonMw Grant 016.036.305). J.J.H. is the Canada Research Chair in Stress Proteins. A.B. is the Canada Research Chair in Metabolism and Health.
References
- Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. doi: 10.1016/s1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Abumrad NA, El-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- Abumrad N, Harmon C, Ibrahimi A. Membrane transport of long-chain fatty acids: evidence for a facilitated process. J Lipid Res. 1998;39:2309–2318. [PubMed] [Google Scholar]
- Abumrad NA, Park JH, Park CR. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem. 1984;259:8945–8953. [PubMed] [Google Scholar]
- Abumrad NA, Perkins RC, Park JH, Park CR. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981;256:9183–9191. [PubMed] [Google Scholar]
- Albrektsen T, Frederiksen KS, Holmes WE, Boel E, Taylor K, Fleckner J. Novel genes regulated by the insulin sensitizer rosiglitazone during adipocyte differentiation. Diabetes. 2002;51:1042–1051. doi: 10.2337/diabetes.51.4.1042. [DOI] [PubMed] [Google Scholar]
- Bonen A, Dyck DJ, Ibrahimi A, Abumrad NA. Muscle contractile activity increases fatty acid metabolism and transport and FAT/CD36. Am J Physiol. 1999;276:E642–E649. doi: 10.1152/ajpendo.1999.276.4.E642. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Arumugam Y, Glatz JF, Tandon NN. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. doi: 10.1074/jbc.275.19.14501. [DOI] [PubMed] [Google Scholar]
- Bonen A, Luiken JJ, Liu S, Dyck DJ, Kiens B, Kristiansen S, Turcotte LP, Van Der Vusse GJ, Glatz JF. Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol. 1998;275:E471–E478. doi: 10.1152/ajpendo.1998.275.3.E471. [DOI] [PubMed] [Google Scholar]
- Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Berk PD. Mitochondrial aspartate aminotransferase: direction of a single protein with two distinct functions to two subcellular sites does not require alternative splicing of the mRNA. Biochem J. 2000;345:423–427. [PMC free article] [PubMed] [Google Scholar]
- Brun S, Carmona MC, Mampel T, Vinas O, Giralt M, Iglesias R, Villarroya F. Activators of peroxisome proliferator-activated receptor-alpha induce the expression of the uncoupling protein-3 gene in skeletal muscle: a potential mechanism for the lipid intake-dependent activation of uncoupling protein-3 gene expression at birth. Diabetes. 1999;48:1217–1222. doi: 10.2337/diabetes.48.6.1217. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT) /CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- Cechetto JD, Sadacharan SK, Berk PD, Gupta RS. Immunogold localization of mitochondrial aspartate aminotransferase in mitochondria and on the cell surface in normal rat tissues. Histol Histopathol. 2002;17:353–364. doi: 10.14670/HH-17.353. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett. 2005;579:2428–2432. doi: 10.1016/j.febslet.2004.11.118. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T, et al. Troglitazone inhibits atherosclerosis in apolipoprotein E-knockout mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–377. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clapham JC, Coulthard VH, Moore GB. Concordant mRNA expression of UCP-3, but not UCP-2, with mitochondrial thioesterase-1 in brown adipose tissue and skeletal muscle in db/db diabetic mice. Biochem Biophys Res Commun. 2001;287:1058–1062. doi: 10.1006/bbrc.2001.5698. [DOI] [PubMed] [Google Scholar]
- Clarke DC, Miskovic D, Han XX, Calles-Escandon J, Glatz JF, Luiken JJ, Heikkila JJ, Bonen A. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism. Physiol Genomics. 2004;17:31–37. doi: 10.1152/physiolgenomics.00190.2003. [DOI] [PubMed] [Google Scholar]
- Coort SL, Coumans WA, Bonen A, Van Der Vusse GJ, Glatz JF, Luiken JJ. Divergent effects of rosiglitazone on protein-mediated fatty acid uptake in adipose and in muscle tissues of Zucker rats. J Lipid Res. 2005;46:1295–1302. doi: 10.1194/jlr.M400426-JLR200. [DOI] [PubMed] [Google Scholar]
- Dhindsa S, Tripathy D, Sanalkumar N, Ravishankar S, Ghanim H, Aljada A, Dandona P. Free fatty acid-induced insulin resistance in the obese is not prevented by rosiglitazone treatment. J Clin Endocrinol Metab. 2005;90:5058–5063. doi: 10.1210/jc.2005-0223. [DOI] [PubMed] [Google Scholar]
- Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Gilde AJ, Van Der Lee KA, Willemsen PH, Chinetti G, Van Der Leij FR, Van Der Vusse GJ, Staels B, Van Bilsen M. Peroxisome proliferator-activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92:518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Kamp F. How are free fatty acids transported in membranes? Is it by proteins or by free diffusion through the lipids? Diabetes. 1999;48:2255–2269. doi: 10.2337/diabetes.48.12.2255. [DOI] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279:E348–E355. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsunoda M, Mochizuki T, Murakami K. Enhancement of insulin signaling through inhibition of tissue lipid accumulation by activation of peroxisome proliferator-activated receptor (PPAR) alpha in obese mice. Med Sci Monit. 2004;10:BR388–395. [PubMed] [Google Scholar]
- Johannsson E, McCullagh KJ, Han XX, Fernando PK, Jensen J, Dahl HA, Bonen A. Effect of overexpressing GLUT-1 and GLUT-4 on insulin- and contraction-stimulated glucose transport in muscle. Am J Physiol. 1996;271:E547–E555. doi: 10.1152/ajpendo.1996.271.3.E547. [DOI] [PubMed] [Google Scholar]
- Kawamura T, Yoshida K, Sugawara A, Nagasaka M, Mori N, Takeuchi K, Kohzuki M. Regulation of skeletal muscle peroxisome proliferator-activated receptor gamma expression by exercise and angiotensin-converting enzyme inhibition in fructose-fed hypertensive rats. Hypertens Res. 2004;27:61–70. doi: 10.1291/hypres.27.61. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Benton CR, Arumugam Y, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A. Different mechanisms can alter fatty acid transport when muscle contractile activity is chronically altered. Am J Physiol Endocrinol Metab. 2004;286:E1042–E1049. doi: 10.1152/ajpendo.00531.2003. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Coumans WA, Arumugam Y, Bonen A, Glatz JF, Luiken JJ. Giant membrane vesicles as a model to study cellular substrate uptake dissected from metabolism. Mol Cell Biochem. 2002;239:121–130. [PubMed] [Google Scholar]
- Luiken JJ, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JF, Bonen A. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab. 2002a;283:E612–E621. doi: 10.1152/ajpendo.00011.2002. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Arumugam Y, Dyck DJ, Bell RC, Pelsers MM, Turcotte LP, Tandon NN, Glatz JF, Bonen A. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276:40567–40573. doi: 10.1074/jbc.M100052200. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Bonen A, Glatz JF. Cellular fatty acid uptake is acutely regulated by membrane-associated fatty acid-binding proteins. Prostaglandins Leukot Essent Fatty Acids. 2002b;67:73–78. doi: 10.1054/plef.2002.0401. [DOI] [PubMed] [Google Scholar]
- Luiken JJ, Turcotte LP, Bonen A. Protein-mediated palmitate uptake and expression of fatty acid transport proteins in heart giant vesicles. J Lipid Res. 1999;40:1007–1016. [PubMed] [Google Scholar]
- McCullagh KJ, Poole RC, Halestrap AP, Tipton KF, O%Brien M, Bonen A. Chronic electrical stimulation increases MCT1 and lactate uptake in red and white skeletal muscle. Am J Physiol. 1997;273:E239–E246. doi: 10.1152/ajpendo.1997.273.2.E239. [DOI] [PubMed] [Google Scholar]
- Mattingly JR, Jr, Rodriguez-Berrocal FJ, Gordon J, Iriarte A, Martinez-Carrion M. Molecular cloning and in vivo expression of a precursor to rat mitochondrial aspartate aminotransferase. Biochem Biophys Res Commun. 1987;149:859–865. doi: 10.1016/0006-291x(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- Muurling M, Mensink RP, Pijl H, Romijn JA, Havekes LM, Voshol PJ. Rosiglitazone improves muscle insulin sensitivity, irrespective of increased triglyceride content, in ob/ob mice. Metabolism. 2003;52:1078–1083. doi: 10.1016/s0026-0495(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning: a Laboratory Manual. Cold Spring Harbour, NY, USA: Cold Spring Harbour Press; 2001. [Google Scholar]
- Schoonjans K, Annicotte JS, Huby T, Botrugno OA, Fayard E, Ueda Y, Chapman J, Auwerx J. Liver receptor homolog 1 controls the expression of the scavenger receptor class B type I. EMBO Rep. 2002;3:1181–1187. doi: 10.1093/embo-reports/kvf238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg GR, Dyck DJ, Calles-Escandon J, Tandon NN, Luiken JJ, Glatz JF, Bonen A. Chronic leptin administration decreases fatty acid uptake and fatty acid transporters in rat skeletal muscle. J Biol Chem. 2002;277:8854–8860. doi: 10.1074/jbc.M107683200. [DOI] [PubMed] [Google Scholar]
- Stump DD, Zhou S-L, Berk PD. Comparison of plasma membrane FABP and mitochondrial isoform of aspartate aminotransferase from rat liver. Am J Physiol. 1993;265:G894–G902. doi: 10.1152/ajpgi.1993.265.5.G894. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, et al. Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci U S A. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte LP, Swenberger JR, Tucker MZ, Trump G, Yee AJ, Luiken JJFP, Bonen A. Muscle palmitate transport and binding are saturable and inhibited by antibodies to FABPpm. Mol Cell Biochem. 2000;210:53–63. doi: 10.1023/a:1007046929776. [DOI] [PubMed] [Google Scholar]
- Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2:e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmsen HM, Ciaraldi TP, Carter L, Reehman N, Mudaliar SR, Henry RR. Thiazolidinediones upregulate impaired fatty acid uptake in skeletal muscle of type 2 diabetic subjects. Am J Physiol Endocrinol Metab. 2003;285:E354–E362. doi: 10.1152/ajpendo.00491.2001. [DOI] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Inskeep K, Bowker-Kinley MM, Popov KM, Harris RA. Mechanism responsible for inactivation of skeletal muscle pyruvate dehydrogenase complex in starvation and diabetes. Diabetes. 1999;48:1593–1599. doi: 10.2337/diabetes.48.8.1593. [DOI] [PubMed] [Google Scholar]
- Zhang J, Phillips DI, Wang C, Byrne CD. Human skeletal muscle PPARalpha expression correlates with fat metabolism gene expression but not BMI or insulin sensitivity. Am J Physiol Endocrinol Metab. 2004;286:E168–E175. doi: 10.1152/ajpendo.00232.2003. [DOI] [PubMed] [Google Scholar]