Abstract
The efficacy of sensory input to the spinal cord can be modulated presynaptically during voluntary movement by mechanisms that depolarize afferent terminals and reduce transmitter release. It remains unclear whether similar influences are exerted on the terminals of descending fibres in the corticospinal pathway of Old World primates and man. We investigated two signatures of presynaptic inhibition of the macaque corticospinal pathway following stimulation of the peripheral nerves of the arm (median, radial and ulnar) and the pyramidal tract: (1) increased excitability of corticospinal axon terminals as revealed by changes in antidromically evoked cortical potentials, and (2) changes in the size of the corticospinal monosynaptic field potential in the spinal cord. Conditioning stimulation of the pyramidal tract increased both the terminal excitability and monosynaptic fields with similar time courses. Excitability was maximal between 7.5 and 10 ms following stimulation and returned to baseline within 40 ms. Conditioning stimulation of peripheral nerves produced no statistically significant effect in either measure. We conclude that peripheral afferents do not exert a presynaptic influence on the corticospinal pathway, and that descending volleys may produce autogenic terminal depolarization that is correlated with enhanced transmitter release. Presynaptic inhibition of afferent terminals by descending pathways and the absence of a reciprocal influence of peripheral input on corticospinal efficacy would help to preserve the fidelity of motor commands during centrally initiated movement.
Considerable evidence indicates that afferent input to the spinal cord of vertebrates can be suppressed by presynaptic control of transmitter release during movement (Ghez & Pisa, 1972; Hultborn et al. 1987; Duenas & Rudomin, 1988; Rudomin et al. 1998; Seki et al. 2003). This mechanism may allow the nervous system to gate peripheral reflexive input to motoneurons during centrally initiated movement, thereby protecting the fidelity of descending commands. Presynaptic inhibition of peripheral input is correlated with primary afferent depolarization (PAD), mediated by presynaptic GABAA receptors (reviewed in Rudomin & Schmidt, 1999). Although the precise role of PAD in reducing synaptic efficacy remains uncertain, changes in membrane conductance associated with depolarization may act to shunt the action potential as it propagates into the afferent terminals (Segev, 1990; Cattaert & El Manira, 1999). In the cat, PAD can be evoked in many afferents by stimulation of other classes of afferent fibres, or descending pathways (Eccles et al. 1961; Carpenter et al. 1963; Schmidt, 1973; Rudomin et al. 1981). Excitability changes at the terminals of premotor interneurons which could reflect PAD-like depolarization have been demonstrated following afferent stimulation (Aggelopoulos et al. 1997), and presynaptic control of transmission at Ia inhibitory interneuron terminals has been implicated in modulating disynaptic reciprocal inhibitory pathways (Enríquez-Denton et al. 2000). By contrast, extensive work in the cat has shown that there is no GABAA influence on descending reticulo-, vestibuleo- or rubrospinal pathways (Rudomin et al. 1975, 1981; Curtis et al. 1984; Curtis & Malik, 1984), although rubrospinal terminals are depolarized following peripheral stimulation, possibly due to accumulation of extracellular potassium ions (Rudomin et al. 1981; Jiménez et al. 1984). These observations have led to the hypothesis that presynaptic GABAA receptors gate only peripheral inputs to the mammalian spinal cord while preserving the efficacy of descending commands (Rudomin et al. 1975, 1981; Nielsen & Petersen, 1994). In addition to GABAergic PAD, a number of other presynaptic mechanisms are believed to modulate synaptic transmission either by reducing calcium influx at the terminals or regulating vesicle release (for review see Miller, 1998), and evidence from a number of vertebrate species suggests descending pathways may be influenced by presynaptic GABAB and glutamate receptors (Jiménez et al. 1991; Krieger et al. 1996; Delgado-Lezama et al. 2004; Ovsepian & Vesselkin, 2004).
In humans, unlike the cat, direct monosynaptic projections from the cortex to motoneurons are an important mechanism of descending control (Porter & Lemon, 1993). It is therefore of interest to determine whether these corticospinal projections are subject to presynaptic modulation. To test this in humans, Nielsen & Petersen (1994) used transcranial magnetic stimulation (TMS) of motor cortex to facilitate the soleus H-reflex response. This facilitation was unaffected by a preceding tendon tap, suggesting that Ia afferent activation does not influence the size of corticospinal EPSPs.
The motor system of Old World primates includes a prominent monosynaptic corticomotoneuronal projection. Techniques to implant chronic recording chambers over the cervical spinal cord of monkeys (Perlmutter et al. 1998) allow more direct tests of presynaptic modulation of corticospinal terminals. We investigated two signatures of presynaptic changes following stimulation of peripheral nerves: (1) increased excitability of corticospinal axon terminals arising from terminal depolarization (Wall's excitability test, Wall, 1958), and (2) an altered monosynaptic field potential recorded in the spinal cord in response to stimulation of the pyramidal tract (PT). Changes in extracellular field potentials following peripheral stimulation have been shown to reflect presynaptic inhibition of afferent input (Sypert et al. 1980; Riddell et al. 1995). In addition, we delivered conditioning stimuli to the PT and spinal cord in order to investigate the effect of autogenic corticospinal terminal depolarization. Stimulation of PT and spinal cord sites produced a clear excitability increase with a time course closely matching that of the of paired-pulse facilitation of the monosynaptic field. In contrast, we observed no significant conditioning effect of peripheral nerve stimulation. We conclude that presynaptic inhibition of the primate corticospinal pathway by peripheral input is either weak or non-existent, consistent with studies of other pathways in the cat, and in support of the hypothesis that descending inputs to the spinal cord are free from PAD-like presynaptic inhibition by peripheral inputs (Rudomin et al. 1975, 1981). This absence of peripheral influence may serve to protect descending motor commands from unpredictable modulation by sensory signals. Furthermore, the finding of a close correlation between paired-pulse facilitation and increased antidromic excitability suggests that terminal depolarization may be involved in the mechanism of short-term synaptic enhancement in the corticospinal pathway, which is thought to amplify the otherwise weak action of individual corticospinal synapses (Phillips & Porter, 1964; Porter & Lemon, 1993).
Methods
Data were obtained from a purpose-bred male Macaca nemestrina (4.5 kg, 3.5 years old). Three separate surgeries were performed under inhalational anaesthesia (isoflurane 2–2.5% in 50:50 O2:N2O) and aseptic conditions to implant the following: (1) PT electrodes and cortical microwires, (2) nerve cuff electrodes, and (3) a spinal recording chamber. All surgeries were followed by a full programme of analgesic (buprenorphine 0.15 mg kg−1i.m. and ketoprofen 5 mg kg−1p.o.) and antibiotic (cephalexin 25 mg kg−1p.o.) treatment.
Two parylene-insulated tungsten PT electrodes (impedance 100 kΩ at 1 kHz; Microprobe, Inc., Fremont, CA, USA; part no. LF501G) were advanced under stereotaxic guidance into the medullary PT above the decussation (co-ordinates: A2 and P3). The penetration was made at a 4.5 deg angle in the coronal plane to avoid midline structures. The optimum depth was found during surgery by recording an antidromic volley over motor cortex, and the correct location within the tract was confirmed by post-mortem histology. The cortical microwires were inserted with fine forceps through an opening in the dura anterior to the central sulcus at 18 mm lateral to the mid-line, corresponding to hand area of primary motor cortex. Bipolar nerve cuff electrodes (Haugland, 1996) were implanted around the median and ulnar nerve for stimulation and connected by subcutaneous wires to terminals fixed to the skull. The radial nerve was stimulated using surface electrodes pressed against the spiral groove of the humerus. The spinal chamber was anchored with dental acrylic to bone screws inserted into the lateral mass of the vertebrae bilaterally (Perlmutter et al. 1998) and covered a laminectomy extending from vertebrae C5 to C7.
Experiments were performed under light sedation with a tiletamine/zolazepam mixture (Tylezol 5 mg kg−1i.m., initial dose), maintained with ketamine (∼5 mg kg−1 h−1i.m.). The animal's body temperature was maintained with a heating blanket. Experimental sessions typically lasted 4–6 h. At the start of each session, a tungsten electrode (impedance 0.5–1.5 MΩ) was inserted into the spinal cord using a microdrive (EPS; Alpha-Omega, Alpharetta, GA, USA) to search for stimulation sites that elicited a cortical response. Cortical potentials were recorded differentially between pairs of cortical microwires to reduce stimulus artefact. These signals were amplified ×10 000 (MCP; Alpha-Omega) and band-pass filtered (300 Hz–10 kHz). In some sessions we subsequently used the spinal electrode to record field potentials, in a single-ended configuration with the same gain and filter settings.
At the end of the recording period, electrolytic lesions were made at several stimulation sites in the spinal cord. A surgical level of anaesthesia was induced with sodium pentobarbitone (25 mg kg−1i.v.) prior to perfusion through the heart with neutral-buffered formalin. Post-mortem, the correct location of both PT electrodes was verified and spinal lesion sites were located.
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
Results
Antidromic M1 fields evoked by intraspinal stimulation
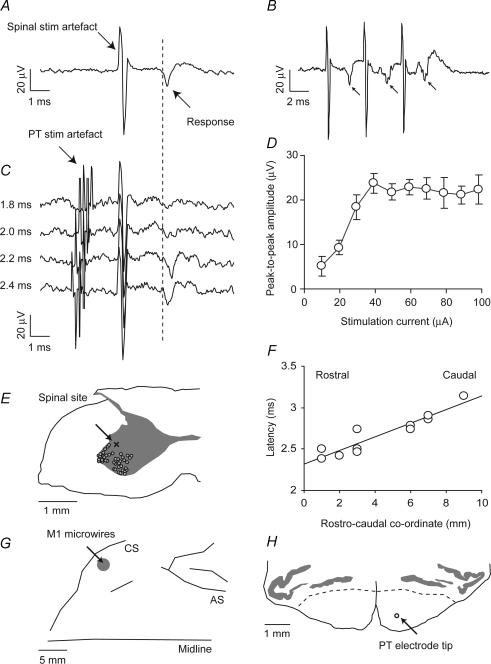
These results are based on 20 experimental sessions and 45 penetrations made over a 7 week period. With the animal sedated, we first positioned a stimulating electrode in the spinal cord grey matter at a location that evoked an antidromic field potential in the motor cortex. As the microelectrode was advanced, the first cells encountered responded to tactile stimulation of the hand and arm and were presumably located in superficial laminae of the dorsal horn. Deeper cells responded to manipulation of the joints, indicative of proprioceptive input to intermediate laminae. At this point, we looked for cortical responses to single-pulse intraspinal stimulation (biphasic, 0.2 ms each phase, 60 μA maximum). This stimulus was usually sufficient to generate a muscle twitch in the hand or arm, beginning at depths of up to 1 mm below the first cells. Cortical field responses were first evoked by spinal stimulation at depths of 0.4–1.9 mm below the first cells, probably corresponding to Rexed's lamina VII. The muscle twitch often disappeared just prior to the appearance of a cortical response. Figure 1A shows an averaged cortical response to spinal stimulation (60 μA, average of 120 responses). A cortical evoked potential was identified as antidromic if (a) the response followed each of a train of three spinal stimuli at 250 Hz (Fig. 1B), and (b) a preceding stimulus delivered to the PT could completely abolish the response to spinal stimulation (due to collision between antidromic and orthodromic volleys). Figure 1C shows a successful collision test. For clarity, the averaged response to PT stimulation alone, which exhibits a large antidromic field, has been subtracted from each trace. A small artefact remains due to incomplete cancellation of the rising and falling phases of the stimulation artefact. Spinal stimuli which follow PT stimulation by 1.8 or 2.0 ms elicit no antidromic cortical response (upper traces) while at intervals of 2.2 and 2.4 ms the response reappears. Not all cortical responses could be eliminated by collision, but 12 fields were identified as antidromic (27% of penetrations) and accepted for further analysis. Figure 1D plots the peak-to-peak amplitude of antidromic cortical responses for different intensities of spinal stimulation. For subsequent conditioning experiments we chose a test stimulus intensity that was above threshold but below the level of saturation (25 μA for the example illustrated here).
Figure 1. Identification of antidromic cortical potential evoked by intraspinal stimulation.
A, field potential recorded differentially between two microwire electrodes in primary motor cortex following a spinal stimulus of 60 μA (average of 120 sweeps). B, cortical field (indicated by arrows) followed each of three stimuli at 250 Hz (average of 60 sweeps). C, a collision test established that the field is antidromic. When pyramidal tract (PT) stimulation at 500 μA preceded the spinal stimulus by 2.0 ms or less, the cortical response was abolished. With an interval of 2.2 ms or more, the field appeared (40 sweeps per interval). For clarity, the averaged response to PT stimulation alone, which exhibits a large antidromic field, has been subtracted from each trace. A small artefact remains due to incomplete cancellation of the rising and falling phases of the stimulation artefact. D, peak-to-peak amplitude of antidromic cortical response to different intensities of spinal stimulation. E, post-mortem localization of lesion made at the C7 level from which an antidromic cortical response was elicited. The lesion site (marked x) was within the grey matter on the mediodorsal edge of the motoneuron territory (large cell bodies, marked ^). F, latency of antidromic response onset as a function of rostrocaudal location of stimulus sites in the recording chamber. G, location of cortical microwire implant relative to the central sulcus (CS) and arcuate sulcus (AS) based on post-mortem photographs. H, transverse section through the brainstem at A2, showing location of anterior PT electrode and grey matter of the olive. Dashed lines indicate the approximate border of the PT. The tip of the second electrode was 5 mm posterior.
Upon advancing the electrode further into the cord about 0.2–0.5 mm, stimuli failed to evoke the cortical potential but did evoke a low-threshold muscle twitch (often in a muscle different from that activated more superficially). At this depth, recordings from the spinal electrode sometimes showed large action potentials with firing rates characteristic of motoneurons. We interpret the order of these stimulation effects from dorsal to ventral to reflect: muscle responses due to activation of intermediate spinal circuitry, a cortical potential evoked by stimulation of corticospinal terminal branches, and a muscle response produced by excitation of motoneurons. Post-mortem localization of electrolytic lesions made at several sites from which cortical responses could be elicited was consistent with this interpretation. Figure 1E shows the location of one site in the ventral horn in Rexed's lamina VII on the dorsomedial border of the motoneuron pool (lamina IX), consistent with the known anatomy of corticospinal projections (Asanuma et al. 1979; Shinoda et al. 1981).
Antidromic responses in the cortex were evoked from an 8 mm rostrocaudal extent of segments C6 and C7. The latency of response ranged from 2.4 to 3.2 ms, and this latency was strongly correlated with the caudal co-ordinate (Fig. 1F; R2 = 0.87). The slope of this correlation gives a conduction velocity within the cervical cord of 12.1 m s−1 (s.e. 1.6 m s−1), significantly lower than estimates derived from orthodromic stimulation over the whole length of the tract (24–90 m s−1; Edgley et al. 1997). The maximum interval for collision with a preceding PT stimulus ranged between 1.9 and 3 ms. This suggests that a substantial part of the response latency comprises conduction time along axonal arbors that follow circuitous paths and have lower conduction velocities than stem axons (Shinoda et al. 1986).
Conditioning of antidromic responses
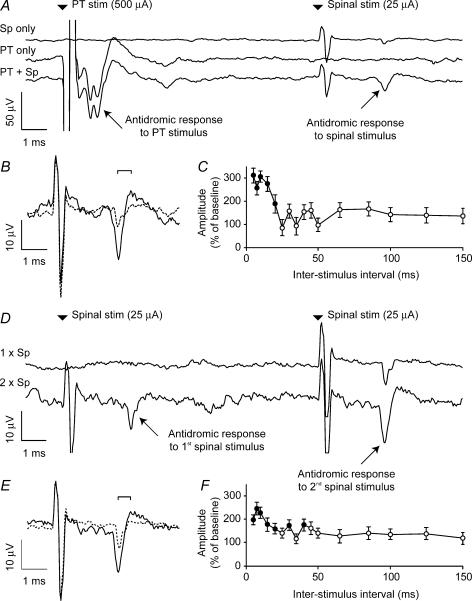
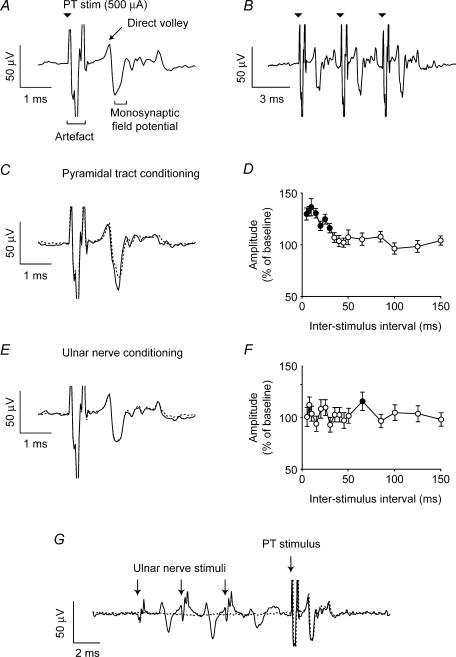
After identifying an antidromic cortical response to spinal cord stimulation, we investigated the effect of conditioning stimuli delivered to five different sites: the PT, the spinal cord (using the same electrode as for the test stimulus) and three peripheral nerves (median, radial and ulnar). In each case the modulation was examined for a range of interstimulus intervals between 5 and 150 ms, delivered at a rate of 2 s−1 in pseudo-random order. The average of 40 sweeps was compiled for each interstimulus interval and 120 sweeps of spinal stimulation alone were compiled for the unconditioned response. Figure 2A shows the result of PT conditioning on the evoked potential documented in Fig. 1. A pronounced facilitation of the cortical response (expanded in Fig. 2B) was obtained when a PT stimulus (500 μA) preceded spinal stimulation (25 μA) by 10 ms. The mean peak-to-peak amplitude of the antidromic field with and without conditioning stimulus was measured and the ratio of these amplitudes (expressed as a percentage) is plotted in Fig. 2C for the entire range of interstimulus intervals. The largest response was around three times bigger than the unconditioned response. For the shortest intervals (<10 ms) we subtracted the response to PT stimulation alone before calculating the amplitude of the antidromic field. Significant facilitatory effects of PT stimulation (P < 0.05, two-tailed t test, filled circles) were obtained for interstimulus intervals up to 20 ms. For short conditioning intervals, the maximum amplitude of response (22 μV) is also the amplitude at which the response to unconditioned stimuli saturates at high current (Fig. 1C). Figure 2D and E shows that a smaller facilitation of around 200% of the unconditioned response resulted from a preceding spinal stimulus at the same intensity and delivered through the same electrode as the test stimulus (i.e. paired-pulse facilitation). The time course for this effect shown in Fig. 2F is similar to that for PT stimulation.
Figure 2. Conditioning of antidromic cortical potential evoked by spinal stimulation.
A, the three traces show averaged responses to spinal stimulation at 25 μA, PT stimulation at 500 μA, and spinal stimulation preceded by PT stimulation with an interstimulus interval of 10 ms. B, expanded plot comparing the response to spinal stimulation alone (dashed line) with the facilitated response following PT conditioning (continuous line). C, peak-to-peak amplitude of response (measured for times bracketed in B) following conditioning stimuli at different interstimulus intervals expressed as a percentage of the unconditioned response. Filled circles indicate intervals at which the conditioned response was significantly modulated (P < 0.05, two-tailed t test). D, average response to single and paired spinal stimulation at 25 μA. E, expanded plot of the enhanced response to the second of a pair of spinal stimuli separated by 10 ms (continuous line) relative to the response to a single stimulus (dashed line). Unconditioned response shows the average of 120 sweeps, conditioned response is the average of 40 sweeps per interstimulus interval throughout.
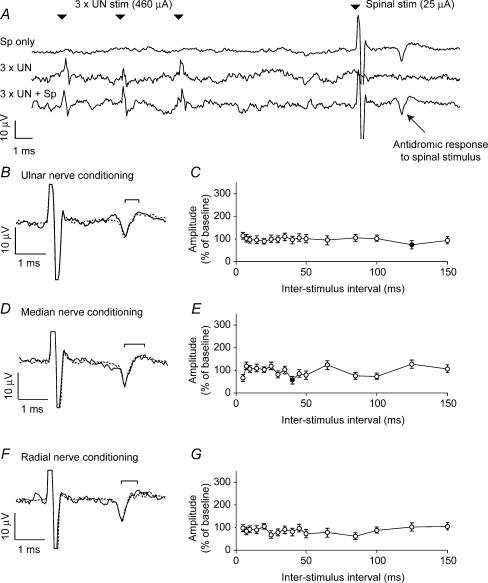
If afferent input to the spinal cord were to exert a presynaptic inhibitory influence on corticospinal terminals via a mechanism equivalent to PAD, stimulation of these pathways should depolarize corticospinal terminal branches and lead to a facilitated antidromic response to stimulation (Wall, 1958). However, Fig. 3A and B shows that no such facilitation was observed following a conditioning stimulation of the ulnar nerve. In these experiments the stimulating current (in this case 460 μA) was above threshold for eliciting a characteristic muscle response from a single stimulus, and to increase the likelihood of observing a conditioning effect we used trains of three stimuli delivered at 300 Hz. Nevertheless we observed no significant facilitation of the cortical evoked potential at any interstimulus interval (Fig. 3C). Similar null results were obtained for stimulation of the median and radial nerves (Fig. 3D–G). In this session, conditioning nerve stimuli alone did not produce a measurable cortical response. In some sessions we saw evidence of small somatosensory evoked potentials (SEPs). These were subtracted from the conditioned responses before measuring the peak-to-peak amplitude of the antidromic field response using a method similar to the PT conditioning analysis. SEPs consisting of a P10 and N20 component can be recorded within motor cortex following nerve stimulation, but these are thought to be generated predominantly in the posterior bank of the central sulcus (McCarthy et al. 1991). These potentials would be largely attenuated by our low-pass filter. Furthermore the use of differential recording within M1 may explain the absence of a clear SEP in most of our data.
Figure 3. Absence of conditioning effects from peripheral nerve stimulation.
A, the three traces show averaged responses to spinal stimulation (25 μA), a train of stimuli (3 × 460 μA at 300 Hz) delivered to the ulnar nerve, and spinal stimulation preceded by ulnar nerve stimulation with an interstimulus interval of 10 ms. B, expanded plot comparing the response to spinal stimulation alone (dashed line) with the response following ulnar nerve conditioning (continuous line). No facilitation of the response was seen. C, modulation of spinally conditioned response for different interstimulus intervals. D and E, comparable plots for median nerve stimulation (3 × 520 μA at 300 Hz). F and G, comparable plots for radial nerve stimulation (3 × 5.2 mA at 300 Hz).
In some sessions we increased the nerve stimulating current to 10 times threshold for a motor response, which should recruit the smaller Group II as well as some Group III fibres (Jack, 1978), but even such strong stimuli failed to produce a significant facilitation of the antidromic field (data not shown).
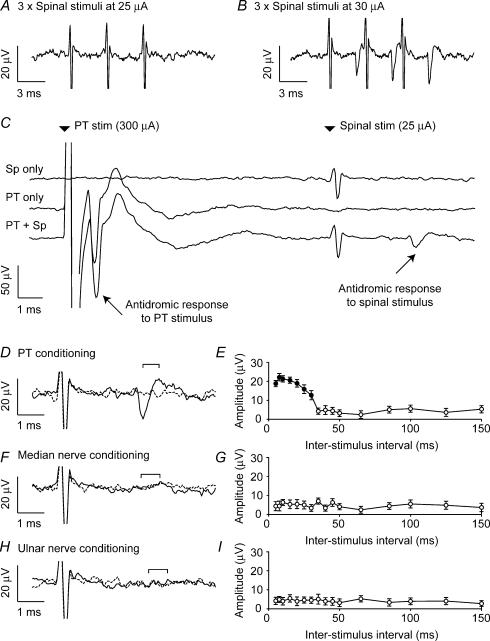
We also examined conditioning of spinal stimuli which were below the threshold for evoking a cortical response. Figure 4 shows results from one session in which an antidromic field potential followed each of three spinal stimuli at 30 μA (Fig. 4B; this field also collided with a PT stimulus at 300 μA up to 2.4 ms before spinal stimulation). A stimulus of 25 μA was below threshold and even triple stimulation elicited no response (Fig. 4A). However, a single spinal stimulus of 25 μA preceded by a PT stimulus of 300 μA did evoke an antidromic response (Fig. 4C; 10 ms interstimulus interval). Figure 4D replots the same data on an expanded scale to show the conditioned and unconditioned response to the spinal stimulus more clearly. The time course of facilitation of subthreshold stimuli was similar to that for supra-threshold stimuli (Fig. 4E). However we were unable to elicit the response when the subthreshold spinal stimulus was preceded by a train of three stimuli delivered to either the median (Fig. 4F and G) or ulnar (Fig. 4H and I) nerves. (The radial nerve was not tested in this case.)
Figure 4. Conditioning of subthreshold spinal stimulation.
A, in this session, a train of three spinal stimuli at 25 μA was below the threshold for evoking a cortical response. B, a clear response followed each of three spinal stimuli when the current was increased to 30 μA. C, field response to the subthreshold stimulus of 25 μA appeared when spinal stimulation was preceded by 10 ms with a PT stimulus at 300 μA. D, magnified trace of PT-conditioned response to spinal stimulation (dashed line, unconditioned response; continuous line, conditioned response). E, peak-to-peak amplitude of cortical field for different interspike intervals. F and G, equivalent plots for conditioning stimulation of the median nerve (3 × 500 μA at 300 Hz). The cortical response could not be evoked with any conditioning interval. H and I, equivalent plots for conditioning stimulation of the ulnar nerve (3 × 440 μA at 300 Hz). 40 sweeps per interstimulus interval throughout.
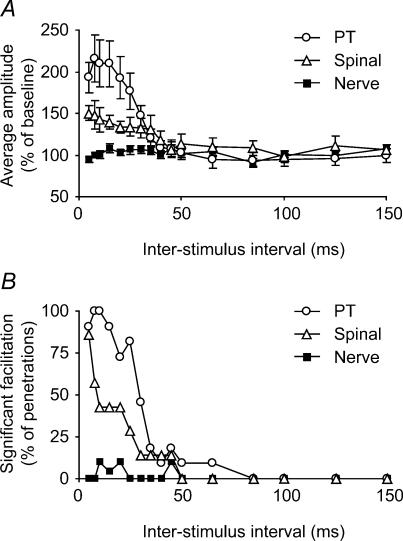
Figure 5 summarizes the results from all spinal penetrations which evoked an antidromic cortical potential. All conditioning experiments were performed with a spinal cord stimulus that was above the threshold for evoking the cortical potential, but below the level of saturation in order to optimize the likelihood of detecting excitability changes. The results from different penetrations were combined in two ways. Figure 5A shows the average percentage modulation resulting from conditioning stimuli at each interstimulus interval. Figure 5B shows the percentage of penetrations in which a significant (P < 0.05, t test) facilitation was observed for each interval. These plots combine the data from all three peripheral nerves (median, radial and ulnar). Pyramidal tract and spinal conditioning stimuli produced significant facilitation with interstimulus intervals up to 30–40 ms. By contrast, within the interstimulus range of 5–30 ms following a conditioning nerve stimulus there were only five (3.6% of total) occurrences of significant facilitation at the P < 0.05 level. However there were also four occurrences of significant suppression, which is well within the expected variability of the data. Across this same range the average facilitation over all nerves and sessions was 0.7%, well within the 4%s.e. on each point. Expressed in another way, any peripherally evoked facilitation which does exist must be smaller than 9%, which is the upper bound of the 95% confidence limits on the size of any effect from nerve stimulation. This is smaller than one-tenth of the conditioning effect of PT stimulation which was clearly demonstrated with this technique.
Figure 5. Summary of conditioning effects on antidromic cortical field potentials.
A, size of antidromic field (percentage of the unconditioned amplitude, as a function of interstimulus interval), averaged across datasets. PT and spinal cord conditioning stimuli produced marked facilitation of the antidromic response for interstimulus intervals up to 40 ms. No facilitation was seen following conditioning stimuli delivered to the peripheral nerves. B, percentage occurrence of facilitation effects which were individually significant at the P < 0.05 level. (PT, 11 datasets; spinal, 7 datasets; nerve, 20 datasets).
PT conditioning affects terminal but not stem axon excitability
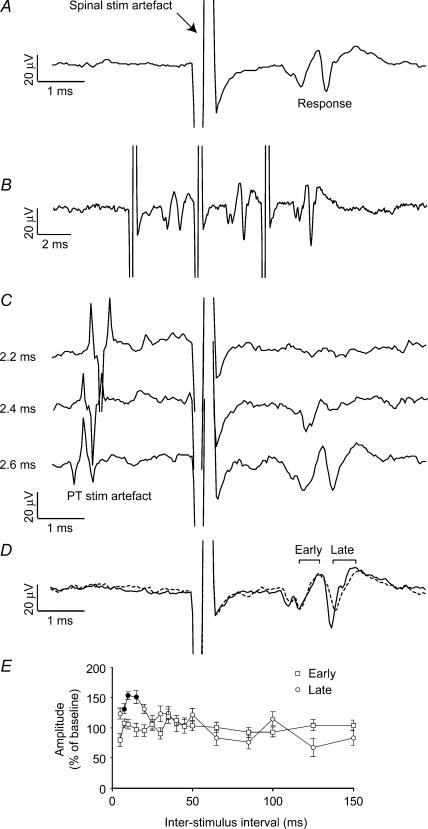
In two sessions spinal cord stimulation evoked an antidromic cortical field consisting of an early and a late component. Figure 6A shows one such example which followed each of a train of three stimuli (Fig. 6B). A PT stimulus delivered 2.2 ms after the spinal stimulus completely abolished both responses (Fig. 6C, top trace). Both early and late fields were observed when the PT stimulus was delayed by 2.6 ms (lower trace). However, an intermediate delay of 2.4 ms abolished only the late field (middle trace) indicating that the two fields arose from distinct antidromic volleys with different latencies (as before, the response to PT stimulation alone, including the antidromic field, has been subtracted from each trace). In addition they had different thresholds: the late field was first observed at a current of 10 μA while the shorter-latency field appeared at 40 μA. This suggests that the early volley may be evoked by direct stimulation of the lateral corticospinal tract (LCST), while the later volley results from stimulation of slower conducting axon terminal branches in the vicinity of the electrode. This interpretation is consistent with the observation that the late cortical field can be significantly facilitated by a conditioning PT stimulus delivered up to 20 ms before spinal stimulation, while the early field is unaffected (Fig. 6D and E). In this case the observed facilitation of the late field is less pronounced than usual, probably because the spinal stimulation current used here (40 μA, sufficient to elicit the early field) was well above threshold for the late field and therefore probably produced some saturation. The ineffectiveness of PT conditioning on the volley evoked from the LCST suggests that the conditioning effects described earlier reflect changes at corticospinal terminals rather than in the cortex.
Figure 6. Early and late antidromic responses to spinal cord stimulation.
A, a cortical response comprising two components was seen following spinal cord stimulation at 40 μA (average of 120 sweeps). B, both responses followed each of a train of stimuli delivered at 250 Hz (average of 50 sweeps). C, both responses collided completely with a PT stimulus at 2.2 ms, but only the late one collided at 2.4 ms (40 sweeps per interstimulus interval). For clarity, the response to PT stimulation alone, including a large antidromic volley, has been subtracted from each trace. The waveform that can be seen preceding spinal stimulation reflects the incomplete cancellation of large PT stimulation artefacts. Although this residual is slightly different in each trace, the actual PT response (before subtraction) was consistent across all conditions. D, the long latency component was facilitated by a PT stimulus preceding spinal stimulation by 15 ms, whilst the short latency component was unaffected. E, modulation of the early and late responses by a conditioning PT stimulus at different interstimulus intervals (40 sweeps per interstimulus interval).
Conditioning of monosynaptic spinal cord field potentials
Our second test for modulation of corticospinal transmission relied on extracellular recording of the monosynaptic field potential evoked in the spinal cord by PT stimulation. If afferent or descending volleys can modulate corticospinal terminals presynaptically, then a conditioning stimulus should change the amplitude of postsynaptic EPSPs. Therefore, we used the spinal cord electrode to record the field potential evoked by PT stimulation. The total recorded response to PT stimulation may include a direct volley and various polysynaptic responses in addition to the monosynaptic field. However, the monosynaptic field can be identified from its short latency, and the fact that it exhibits facilitation following a train of multiple stimuli (Phillips & Porter, 1964). Such an effect was observed in three sessions. The example displayed in Fig. 7A has a short-latency positive deflection that may be due to the direct corticospinal volley, followed by negative potential with a latency of 1.6 ms poststimulus. This later wave follows and is facilitated by each successive PT stimulus delivered at 300 Hz (Fig. 7B) and is hence probably monosynaptic in origin. Large action potentials with low firing rates characteristic of motoneurons were also recorded at this site. Figure 7C illustrates the paired-pulse facilitation of the monosynaptic field with an interstimulus interval of 10 ms, and Fig. 7D shows the time course of this effect.
Figure 7. Conditioning of monosynaptic spinal field potential evoked by PT stimulation.
A, spinal cord recording of the field evoked by PT stimulation at 500 μA (average of 120 sweeps). B, monosynaptic field was enhanced following each of a train of three PT stimuli at 300 Hz (average of 70 sweeps). C, monosynaptic field was facilitated by paired PT stimulation with an interstimulus interval of 15 ms (continuous line) relative to a single stimulus (dashed line). D, percentage modulation of monosynaptic field following paired-pulse PT stimulation with different interstimulus intervals. E, monosynaptic field was unaffected by preceding ulnar nerve stimulation (3 × 400 μA at 300 Hz). F, modulation of monosynaptic field following a conditioning stimulus to the ulnar nerve with different interstimulus intervals. G, spinal field potentials evoked by stimulation of PT, without (dashed) and with (continuous) the ulnar nerve (3 × 400 μA at 300 Hz). Stimulus artefacts are indicated by arrows. Average of 40 sweeps.
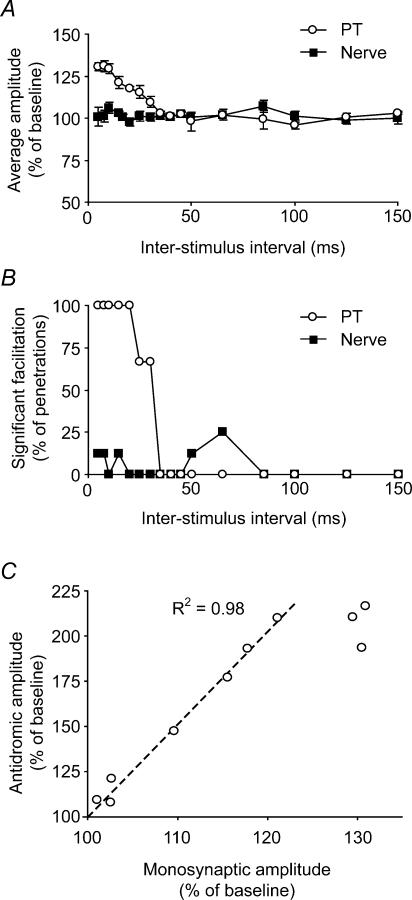
As with the antidromic cortical potentials, we found it was not possible to modulate the size of this monosynaptic spinal field with a preceding train of three stimuli delivered to the ulnar nerve (Fig. 7E and F). This was despite the fact that peripheral nerve stimulation alone evoked a clear field response at this site, which just preceded the spinal stimulation artefact at the shortest conditioning interval (5 ms; Fig. 7G). Similar null results were obtained with the median and radial nerves. Figure 8A and B summarizes the three datasets, using displays similar to those of Fig. 5. A clear facilitation was seen up to 40 ms following a conditioning PT stimulus. Once again there was no effect of a conditioning stimulus to any nerve. Over the interstimulus range of 5–30 ms, there were only five (9% of total) occurrences of significant facilitation at the P < 0.05 level from nerve. Across penetrations there was a mean facilitation of 1.5% in this interval range, but this was non-significant. There were no occurrences of significant suppression of the field.
Figure 8. Summary of conditioning effects on monosynaptic spinal cord field potentials.
A, modulation of monosynaptic field averaged across all datasets following conditioning stimulation of the PT or peripheral nerve. B, percentage occurrence of individual facilitation effects which were individually significant at the P < 0.05 level. No significant suppression events were observed following stimulation of any peripheral nerve. (PT, three datasets; nerve, eight datasets). C, modulation of monosynaptic field potential plotted against modulation of antidromic cortical field by preceding PT stimuli for interstimulus intervals between 5 and 50 ms. The magnitudes of the conditioning effects are proportional over the range 15–50 ms.
The time course of facilitation of the monosynaptic field by a preceding PT stimulus closely matched the time course of presynaptic excitability changes obtained in our first experiment. In Fig. 8C, the magnitude of paired-pulse facilitation (open circles in Fig. 8A) is plotted against the effect of PT conditioning on the antidromic cortical field from our first experiment (open circles in Fig. 5A). Each point represents one interstimulus interval between 5 and 50 ms. In the range 15–50 ms these effects are proportional, as indicated by the linear relationship shown on the graph. The proportionality does not hold for the shortest intervals (5, 7.5 and 10 ms), probably due to saturation of the antidromic effect.
These data therefore indicate that peripheral input does not presynaptically influence corticospinal EPSPs, consistent with the absence of effects on cortical antidromic potentials. However PT stimulation is followed by presynaptic excitability changes and short-term synaptic enhancement with identical time course, suggesting that a common mechanism may be responsible for both.
Discussion
We have used two tests to search for evidence of presynaptic modulation of corticospinal projections in an Old World primate. The first experiment tested for presynaptic depolarization of corticospinal terminals (equivalent to the PAD correlate of presynaptic inhibition of afferent fibres). Such a depolarization should reduce the threshold for direct intraspinal stimulation of terminals, leading to an increased antidromic evoked potential in the cortex. In the second experiment we examined changes in the monosynaptic fields in the spinal cord evoked from the PT following conditioning stimuli delivered to the PT and peripheral nerves.
Conditioning stimulation of the PT and intraspinal sites produced significant increases in the antidromic cortical potential. Several mechanisms could explain this effect. A synaptic effect at the cortex is unlikely to be the cause, since the predominant influence on pyramidal tract neurons (PTNs) from 5 to 30 ms following stimulation of the PT is inhibitory (Jackson et al. 2002). In principle such inhibition could restrict antidromic invasion into the PTN dendrites, but this would have the opposite effect of reducing the evoked potential. Furthermore, we demonstrated a conditioning effect on spinal stimuli that alone were below threshold for evoking any cortical effect, with a similar time course. Finally, the cortical field evoked by direct stimulation of the LCST was unaffected by a conditioning PT stimulus. These observations suggest that the facilitation documented here is due to increased excitability of the corticospinal terminals. This may be caused by the accumulation of extracellular potassium ions around the terminal branches following the initial activation by the conditioning stimulus (Bruggencate et al. 1974; Swadlow et al. 1980; Schmied & Fetz, 1987). An alternative possibility is that corticospinal activity produces autogenic presynaptic depolarization via axo-axonic synapses from GABAergic interneurons. An electron microscopy study of corticospinal terminals in the rat revealed no axo-axonic contacts with corticospinal terminals (Valtschanoff et al. 1993) and to our knowledge there is no evidence for these synapses in the primate. Furthermore, the facilitation we observed reached peak level around 7.5–10 ms after the PT stimulus, whereas GABAergic PAD is maximal at 15–20 ms following conditioning stimulation (Rudomin et al. 1981). However, more specific tests should be used to resolve these possibilities, for example iontophoretic application of pharmacological agents or direct measurement of extracellular potassium concentrations, as has been done to distinguish intrinsic and extrinsic presynaptic mechanisms in other pathways in the cat (Jiménez et al. 1984, 1991; Curtis et al. 1984; Curtis & Malik, 1984).
For descending volleys, the time course of terminal excitability changes closely matched the time course of paired-pulse facilitation of the monosynaptic field, suggesting that these effects may relate to a common mechanism. Short-term synaptic enhancement is observed throughout the nervous system and is often attributed to residual calcium, either bound or close to exocytosis sites at the presynaptic terminals (Katz & Miledi, 1968; Fisher et al. 1997). This mechanism has a time course that is broadly consistent with our data (Fisher et al. 1997) but would not be expected to affect antidromic excitability. Our results suggest that the paired-pulse facilitation observed at corticomotoneuronal synapses (Phillips & Porter, 1964) may be related to presynaptic depolarization following the initial volley, an effect opposite to the presynaptic inhibition correlated with PAD. The different effects on transmitter release could be related to the amount of terminal depolarization. Afferent inhibition involves large depolarizations that activate voltage-gated channels, possibly resulting in action potential shunting (Segev, 1990; Cattaert & El Manira, 1999). However, there is evidence that smaller membrane depolarizations may enhance transmitter release (Awatramani et al. 2005; see also Matyushkin et al. 1995). This effect could also underlie the increased excitation of motoneurons following weak GABA-mediated PAD (Duchen, 1986).
In neither experiment did we observe significant conditioning effects following a train of stimuli delivered to any of three peripheral nerves. This result is in agreement with the study of Nielsen & Petersen (1994) using human subjects, and further supports experiments in the cat indicating other descending pathways to the spinal cord are free from presynaptic modulation (Rudomin et al. 1975, 1981; Curtis et al. 1984; Curtis & Malik, 1984). The stimuli we used were well above the threshold for an overt motor response, suggesting that the efficacy of corticospinal synapses should be unaffected by peripheral inputs during normal motor behaviour. We estimate that a facilitation of 9% or more would have been statistically inconsistent with our data. This finding also implies that accumulation of extracellular potassium following peripheral stimulation has only a small effect, if any, on the corticospinal terminals studied here. This is in contrast to the rubrospinal pathway of the cat (Rudomin et al. 1981), and possibly also corticofugal projections to trigeminal brain stem nuclei which show increased excitability following stimulation of the infraorbital nerve (Dubner et al. 1969; Dubner & Sessle, 1971). The lack of an effect reported here is likely to be due to the intermediate and ventral location of the primate corticospinal terminals, while potassium accumulation is most pronounced in the dorsal horn (Jiménez et al. 1984).
In conclusion, the patterns of presynaptic effects between peripheral and descending pathways make functional sense. The well-known presynaptic inhibition of afferent input by descending motor commands probably protects these commands from interference from variable peripheral feedback (Ghez & Pisa, 1972; Seki et al. 2003). Similarly, the absence of a reciprocal mechanism producing modulation of corticospinal terminals by peripheral input reported here, also avoids a source of unpredictable modulation of descending commands. The increased excitability of these terminals following orthodromic or antidromic activation of the corticospinal pathway may modulate the efficacy of corticospinal transmission; it remains to be seen whether this effect is non-specific or organized in a manner similar to patterns seen between different types of afferent fibres (Schmidt, 1973).
Acknowledgments
This work was supported by National Institutes of Health grants NS12542 and RR00166. S.N.B. was supported by a Wellcome Trust Fellowship.
References
- Aggelopoulos NC, Chakrabarty S, Edgely SA. Evoked excitability changes at the terminals of midlumbar premotor interneurons in the cat spinal cord. J Neurosci. 1997;17:1512–1518. doi: 10.1523/JNEUROSCI.17-04-01512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma H, Zarzecki P, Jankowska E, Hongo T, Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res. 1979;34:73–89. doi: 10.1007/BF00238342. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Bruggencate G, Lux HD, Liebl L. Possible relationship between extracellular potassium activity and presynaptic inhibition in the spinal cord of the cat. Pflugers Arch. 1974;349:301–317. doi: 10.1007/BF00588416. [DOI] [PubMed] [Google Scholar]
- Carpenter D, Lundberg A, Norrsell U. Primary afferent depolarization evoked from the sensory-motor cortex. Acta Physiol Scand. 1963;59:126–142. doi: 10.1111/j.1748-1716.1963.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Cattaert D, El Manira A. Shunting versus inactivation: analysis of presynaptic inhibitory mechanisms in primary afferents of the crayfish. J Neurosci. 1999;19:6079–6089. doi: 10.1523/JNEUROSCI.19-14-06079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Malik R. The effect of GABA on lumbar terminations of rubrospinal neurons in the cat spinal cord. Proc R Soc Lond B Biol Biol Sci. 1984;223:165–169. doi: 10.1098/rspb.1984.0080. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Wilson VJ, Malik R. The effect of GABA on the terminations of vestibulospinal neurons in the cat spinal cord. Brain Res. 1984;295:372–375. doi: 10.1016/0006-8993(84)90989-2. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Aguilar J, Cueva-Rolon R. Synaptic strength between motoneurons and terminals of the dorsolateral funiculus is regulated by GABA receptors in the turtle spinal cord. J Neurophysiol. 2004;91:40–47. doi: 10.1152/jn.00569.2003. [DOI] [PubMed] [Google Scholar]
- Dubner R, Sessle BJ. Presynaptic changes of primary afferent and corticofugal fibers projecting to trigeminal brain stem nuclei. Exp Neurol. 1971;30:223–238. doi: 10.1016/s0014-4886(71)80003-1. [DOI] [PubMed] [Google Scholar]
- Dubner R, Sessle BJ, Gobel S. Presynaptic depolarization of corticofugal fibres participating in a feedback loop between trigeminal brain stem nuclei and sensorimotor cortex. Nature. 1969;223:72–73. doi: 10.1038/223072a0. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Excitation of mouse motoneurones by GABA-mediated primary afferent depolarization. Brain Res. 1986;379:182–187. doi: 10.1016/0006-8993(86)90274-x. [DOI] [PubMed] [Google Scholar]
- Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Exp Brain Res. 1988;70:15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Magni F. Central inhibitory action attributable to presynaptic depolarisation produced by muscle afferent volleys. J Physiol. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain. 1997;120:839–853. doi: 10.1093/brain/120.5.839. [DOI] [PubMed] [Google Scholar]
- Enríquez-Denton M, Nielsen J, Perreault MC, Morita H, Petersen N, Hultborn H. Presynaptic control of transmission along the pathway mediating disynaptic reciprocal inhibition in the cat. J Physiol. 2000;526:623–637. doi: 10.1111/j.1469-7793.2000.t01-1-00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TJ. Multiple overlapping processes underlying short-term synaptic enhancement. Trends Neurosci. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Ghez C, Pisa M. Inhibition of afferent transmission in cuneate nucleus during voluntary movement in the cat. Brain Res. 1972;40:145–155. doi: 10.1016/0006-8993(72)90120-5. [DOI] [PubMed] [Google Scholar]
- Haugland MA. A flexible method for fabrication of nerve cuff electrodes. Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IFESS; 31 October–3 November; Amsterdam. 1996. [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shinodo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;446:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. Some methods for selective activation of muscle afferent fibres. In: Porter R, editor. Studies in Neurophysiology. Cambridge: Cambridge University Press; 1978. pp. 155–176. [Google Scholar]
- Jackson A, Spinks RL, Freeman TC, Wolpert DM, Lemon RN. Rhythm generation in monkey motor cortex explored using pyramidal tract stimulation. J Physiol. 2002;541:685–699. doi: 10.1113/jphysiol.2001.015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I, Rudomin P, Enriquez M. Differential effects of (-) -baclofen on Ia and descending monosynaptic EPSPs. Exp Brain Res. 1991;85:103–113. doi: 10.1007/BF00229991. [DOI] [PubMed] [Google Scholar]
- Jiménez I, Rudomin P, Solodkin M, Vyklicky L. Specific and nonspecific mechanisms involved in generation of PAD of group Ia afferents in cat spinal cord. J Neurophysiol. 1984;52:921–939. doi: 10.1152/jn.1984.52.5.921. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger P, Manira A, Grillner S. Activation of pharmacologically distinct metabotropic glutamate receptors depresses reticulospinal-evoked monosynaptic EPSPs in the lamprey spinal cord. J Neurophysiol. 1996;76:3834–3841. doi: 10.1152/jn.1996.76.6.3834. [DOI] [PubMed] [Google Scholar]
- Matyushkin DP, Krivoi II, Drabkina TM. Synaptic feed-backs mediated by potassium ions. General Physiol Biophys. 1995;14:369–381. [PubMed] [Google Scholar]
- McCarthy G, Wood CC, Allison T. Cortical somatosensory evoked potentials I. Recordings in the monkey macaca fasicularis. J Neurophysiol. 1991;66:53–63. doi: 10.1152/jn.1991.66.1.53. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV, Vesselkin NP. Dual effect of GABA on descending monosynaptic excitatory postsynaptic potential in frog lumbar motoneurons. Neuroscience. 2004;129:639–646. doi: 10.1016/j.neuroscience.2004.07.050. [DOI] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol. 1998;80:2475–2494. doi: 10.1152/jn.1998.80.5.2475. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R. The pyramidal projection to motoneurones of some muscle groups of the baboon's forelimb. Prog Brain Res. 1964;12:222–245. doi: 10.1016/s0079-6123(08)60625-1. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford: Clarendon Press; 1993. [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. J Physiol. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Engberg I, Jimenez I. Mechanisms involved in presynaptic depolarization of group I and rubrospinal fibers in cat spinal cord. J Neurophysiol. 1981;46:532–548. doi: 10.1152/jn.1981.46.3.532. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Nunez R, Madrid J. Modulation of synaptic effectiveness of Ia and descending fibers in the spinal cord. J Neurophysiol. 1975;37:413–429. doi: 10.1152/jn.1975.38.5.1181. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Romo R, Mendell LM. Presynaptic Inhibition and Neural Control. Oxford: Oxford University Press; 1998. [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schmidt RF. Control of the access of afferent activity to somatosensory pathways. In: Iaggo A, editor. Handbook of Sensory Physiology. II. Berlin: Springer; 1973. pp. 151–206. [Google Scholar]
- Schmied A, Fetz EE. Activity-related changes in electrical thresholds of pyramidal tract axons in the behaving monkey. Exp Brain Res. 1987;65:352–360. doi: 10.1007/BF00236308. [DOI] [PubMed] [Google Scholar]
- Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol. 1990;63:987–998. doi: 10.1152/jn.1990.63.5.987. [DOI] [PubMed] [Google Scholar]
- Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci. 2003;6:1309–1316. doi: 10.1038/nn1154. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yamaguchi T, Futami T. Multiple axon collaterals of single corticospinal axons in the cat spinal cord. J Neurophysiol. 1986;55:425–448. doi: 10.1152/jn.1986.55.3.425. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Kocsis JD, Waxman SG. Modulation of impulse conduction along the axonal tree. Annu Rev Biophys Bioeng. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- Sypert GW, Munson JB, Fleshman JW. Effect of presynaptic inhibition on axonal potentials, terminal potentials, focal synaptic potentials, and EPSPs in cat spinal cord. J Neurophysiol. 1980;44:792–803. doi: 10.1152/jn.1980.44.4.792. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ, Rustioni A. Amino acid immunoreactivity in corticospinal terminals. Exp Brain Res. 1993;93:95–103. doi: 10.1007/BF00227784. [DOI] [PubMed] [Google Scholar]
- Wall PD. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958;142:1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]