Abstract
Human muscles paralysed chronically by spinal cord injury (SCI) fatigue excessively. Whether these reductions in force reflect a decrease in the fatigue resistance of the motor units is unknown. Our aim was to determine the fatigability of thenar motor units paralysed chronically (10 ± 2 years) by cervical SCI. Surface electromyographic activity (EMG) and force were recorded from 17 paralysed motor units (n = 7 subjects) in response to intraneural motor axon stimulation (13 pulses at 40 Hz, 1 s−1 for 2 min). Unit force decreased progressively, reaching 8–60% of initial after 2 min, whereas both the amplitude and area of the first EMG potentials in the trains increased significantly (both P < 0.05). Thus, transmission of neural signals to the sarcolemma was effective and the reduction in force must reflect impaired processes in the muscle fibres. The median fatigue index for paralysed units (0.31), the ratio of the force at 2 min compared to the initial force, was significantly lower than that for units from control subjects (0.85, P < 0.05), but the distribution of fatigue indices for each population had a similar shape (ranges: 0.08–0.60 and 0.41–0.95, respectively). Hence, chronic paralysis did not limit the range of fatigability typically found for thenar units, only its magnitude. These findings suggest that all paralysed units underwent similar reductions in fatigue resistance. After fatigue, paralysed unit forces were reduced at all frequencies (1–100 Hz, P < 0.05). Twitch contraction and half-relaxation times were increased, as was the frequency needed to produce half maximal force (P < 0.05). Thus, stimulation protocols used to produce functional movements in paralysed muscles need to accommodate the significant and rapid fatigue of the motor units.
Human skeletal muscles that have been paralysed chronically by spinal cord injury (SCI) are more fatigable than muscles of able-bodied individuals when they are stimulated electrically (Stein et al. 1992; Rochester et al. 1995a; Shields, 1995; Thomas, 1997a; Thomas et al. 2003; Olive et al. 2003). This excessive fatigability may reflect chronic changes in muscle use, resulting in alterations in fibre type composition, reductions in the oxidative capacity of the fibres, and impaired blood flow (Grimby et al. 1976; Martin et al. 1992; Stein et al. 1992; Olive et al. 2003; Butler et al. 2004). Long-term EMG recordings show that paralysed human tibialis anterior muscles and paralysed cat hindlimb muscles are indeed less active than muscles of able-bodied subjects (Alaimo et al. 1984; Stein et al. 1992). Leg muscles of persons with chronic SCI have greater proportions of type II fibres, or fibres with fast myosin isoforms than control muscles (Grimby et al. 1976; Martin et al. 1992; Shields, 1995; Andersen et al. 1996). Succinate dehydrogenase, a marker for oxidative metabolism, and the capillary-to-fibre ratio are lower in paralysed tibialis anterior muscles than control muscles (Martin et al. 1992; Rochester et al. 1995b). All these findings imply that there has been a reduction in the fatigue resistance of the paralysed motor units. However, contractions greater than 10% maximal restrict blood flow and accelerate fatigue (Petrofsky & Hendershot, 1984; Sjogaard et al. 1988) so ischaemia complicates interpretation of these whole muscle data (Gardiner & Olha, 1987). One way to examine the intrinsic fatigability of paralysed human muscle fibres without the influences of ischaemia is to measure the fatigability of single motor units, an unexplored issue.
The fatigability of cat medial gastrocnemius motor units is increased several months after transection of the thoracic or lumbar cord (Mayer et al. 1984; Munson et al. 1986) whereas less marked changes in fatigability occur in cat soleus motor units (Cope et al. 1986). Human motor units may adapt to different extents with chronic paralysis especially when the injury involves contusion and compression (Bunge et al. 1993). Units may also show divergent electrical and mechanical responses during fatigue, but this response variability is likely to be masked at the whole muscle level (Mayer et al. 1984; Sandercock et al. 1985; Munson et al. 1986; Dubose et al. 1987; Carpentier et al. 2001). For example, the magnitude of muscle fatigue is likely to be dominated by the reductions in force seen in the most fatigable units. Some paralysed thenar motor units are tonically active, discharging spontaneously at low rates (Zijdewind & Thomas, 2001). This activity may alter the chronic fatigability of only a portion of the motor unit pool (i.e. low threshold units), changes that may be concealed in whole muscle data. Thus, in order to determine how paralysis induced by chronic SCI affects the fatigue responses of different motor units, it is imperative to record individual motor unit behaviour.
In this study, our aim was to examine the fatigability of single motor units in human thenar muscles paralysed chronically by cervical SCI. Each motor axon was stimulated intraneurally, a technique that allows the EMG and tetanic force to be recorded over time (Westling et al. 1990). Other studies of paralysed human units have only recorded twitch forces using percutaneous nerve stimulation (Godfrey et al. 2002; Thomas et al. 2002), intramuscular microstimulation (Yang et al. 1990) or spike-triggered averaging (Thomas, 1997b). Because twitch forces are easily altered by potentiation and fatigue, they are not the most reliable measures of unit strength. Our findings show that paralysed human thenar muscles contain a population of fatigable motor units, whereas thenar units in able-bodied subjects are predominantly fatigue resistant (Thomas et al. 1991b). The exaggerated fatigability of paralysed units reflects impairments within the muscle fibres, not failure of neuromuscular transmission or membrane excitability, because both the amplitude and the area of the first EMG potentials in the trains potentiated as the motor unit force decreased.
Methods
Subjects
Seven subjects (5 men and 2 women, 34 ± 4 years old, mean ± s.e.m., range 20–47 years) with chronic (> 1 year) cervical spinal cord injury (injury duration: 10 ± 2 years, range 1.5–19 years) participated in this study. Current injury level, defined according to American Spinal Injury Association criteria (Maynard et al. 1997), was C4 (n = 4), C5 (n = 1) or C6 (n = 2). Injury occurred in a motor vehicle accident (n = 3), a fall, a diving accident, while horseback riding or from a gunshot. The thenar muscles were completely paralysed based upon a manual muscle examination. The University of Miami Institutional Review Board approved this study. Informed written consent was obtained from each subject prior to the experiment. The study conformed to the standards set by the Declaration of Helsinki.
Experimental setup
The procedures for recording thenar motor unit contractile properties in response to intraneural motor axon stimulation were as described by Westling et al. (1990). Subjects reclined on an adjustable bed. The right arm was abducted about 25 deg and the hand was supinated (complete supination could not be attained in two subjects so data were recorded with the hand pronated). The forearm rested on a support, was immobilized in a vacuum cast, and was secured in place with a velcro strap. The hand was stabilized in modelling clay (Theraputty, North Coast Medical Inc., Morgan Hill, CA, USA). The fingers were kept extended by placing U-shaped aluminium clamps over the joints and pressing the clamps into the clay. The thumb was extended and positioned against a custom made transducer that registered abduction and flexion forces at right angles. In this position, supramaximal stimulation of the median nerve results in maximal tetanic force of the thenar muscles (Thomas, 1997b). Skin temperature was monitored by a thermistor taped to the forearm (52 K/J, Fluke Corporation, USA). An infrared pulse detector was attached to the middle finger of the right hand so that stimuli could be delivered according to the pulse pressure wave.
Distal and proximal electromyographic (EMG) signals were recorded with three electrodes, each made from braided strands of silver-coated copper wire (5 cm long), covered in gel and taped to the skin. The common electrode was placed transversely over the mid-belly of the thenar muscles. The proximal electrode was positioned over the base of the thenar eminence, and the distal electrode was placed over the metacarpophalangeal joint of the thumb. A ground electrode was taped over the wrist crease.
Activation of a single motor unit
The path of the median nerve above the elbow was mapped by applying single pulses (0.2 ms duration, up to 20 mA) to the arm with a surface probe while watching for contractions of median innervated muscles. To locate the median nerve, an uninsulated tungsten electrode (0.2 mm diameter, FHC Inc., Bowdoinham, ME, USA) was inserted through the skin about 10 cm proximal to the elbow. Single stimuli were applied through the electrode while adjusting its depth and angle until weak current pulses (0.2 ms duration, 0.1–0.3 mA) caused contractions of median innervated muscles, an indication of median nerve proximity. The uninsulated electrode was then retracted about 1 mm and this final electrode position was used as a reference for the insertion of an insulated tungsten electrode into the median nerve (0.2 mm diameter, up to 1 MΩ impedance, FHC Inc., Bowdoinham, ME, USA). Once within the median nerve, the position of this insulated electrode was adjusted minutely until single negative current pulses (0.2 ms duration) excited a single thenar motor unit.
The criteria for activation of a single thenar unit were as previously described (Westling et al. 1990). Briefly, the initial stimuli were below threshold for activation of a single axon as reflected by an absence of evoked EMG and force. As the current intensity was progressively increased there was the simultaneous appearance of unit EMG (proximal and distal) and force. These signals were of consistent size and shape with repeated stimuli, reflecting the excitation of one motor unit (i.e. all-or-none behaviour). Further increases in current eventually resulted in the activation of additional unit(s) reflected by larger EMG and force responses. When the current was reduced sufficiently, only the original unit remained active as shown by the return of the EMG and force to their same initial magnitudes. With further decreases in current to below threshold, this unit failed to respond. This cycle of increasing and decreasing the stimulus current was repeated several times to establish the range of current that could be used to selectively activate the unit under study. All subsequent stimuli to the unit were delivered using a current in the middle of this range. As a further check of the stability of unit activation, the abduction and flexion forces were displayed against each other as an x–y plot on a second oscilloscope. The force vector was of consistent magnitude and direction for any one unit.
Minimizing baseline artifacts
Single human motor unit forces are weak and therefore prone to artifacts. Baseline fluctuations due to respiration and the pulse pressure wave were minimized by electronically resetting the baseline to a predetermined level before the delivery of single stimuli and before the first pulse of the trains delivered at different tetanic frequencies. Stimuli were also delivered just after the peak of the pulse pressure wave so that forces were recorded when the pulse pressure was relatively low. Baseline resetting procedures were not applied during the fatigue protocol since it entailed applying stimulus trains at a standard rate (1 s−1). Any resetting would also disrupt fatigue-induced slowing of relaxation and force fusion.
Stimulation protocol
The twitch force of each unit and the forces evoked by different stimulus frequencies were recorded before and after the fatigue protocol. Twenty single pulses were applied to record the initial twitch responses. Force was recorded in response to trains of pulses at 5, 8 and 10 Hz (each for 2 s), 15, 20, 30, 40 and 50 Hz (1 s each) and 100 Hz (0.5 s), followed by another 20 single pulses to record the post-tetanic twitch responses, as for control data (Thomas et al. 1991a). The unit was then fatigued with a protocol of 13 pulses at 40 Hz repeated once per second for 2 min (Burke et al. 1973). The initial EMG and forces of 48 paralysed thenar units have been recorded (Häger-Ross et al. 2006). Here, we characterize the fatigue-induced changes in unit EMG potentials, twitch forces and tetanic forces.
Data collection and analysis
All signals were displayed on a monitor and stored on-line using a SC/Zoom system (Physiology Section, Umeå University, Sweden). The EMG signals were amplified, filtered from 30 Hz to 1 kHz (Astro-Medical, Model P511, West Warwick, RI, USA), and sampled at 3 kHz. Abduction and flexion forces and the pulse-pressure wave were sampled at 375 Hz whereas stimulus current was sampled at 94 Hz. Stimulus pulses and trigger events were sampled at 3 kHz.
EMG and force during fatigue
The EMG potentials and the abduction and flexion forces of five trains of pulses at 40 Hz were averaged at the beginning of the test (i.e. 1–5 s) and every 20 s thereafter to the end (i.e. 116–120 s). The first and last EMG potentials of these averaged trains were each analysed by measuring the following parameters for the proximal and distal EMG: (1) latency, the time from the stimulus pulse to the onset of the EMG; (2) duration, the time between the onset of the EMG potential and the end of the second phase, defined by isoelectric crossings; (3) peak-to-peak amplitude; and (4) area of the first two phases of the EMG potential. The EMG latency, duration, amplitude, and area of the first and last EMG potentials in the trains measured every 20 s were normalized to the corresponding values for the first potential at the start of the test (1–5 s) to provide ‘first EMG potential indices’ and ‘last EMG potential indices’, respectively. Hence, the last EMG potential index reflects the changes that occur after the delivery of 1560 pulses at 40 Hz. Here we report all these EMG parameters because there is no consensus in the literature as to whether EMG amplitude or area is the more optimal parameter for examining the effectiveness of neuromuscular transmission during fatigue (Hamm et al. 1989; Dimitrova & Dimitrov, 2003).
The magnitude of the resultant force was calculated off-line from the abduction and flexion force components. Peak force (the highest resultant force minus any baseline force) and half-relaxation time (hRT, the time from peak force to half force decline) were measured at the beginning of the test and every 20 s thereafter. The fatigue indices for force (and hRT) were calculated as the ratio of peak force (and hRT) every 20 s compared to the corresponding parameter at the start of the test.
Pre-fatigue and post-fatigue measures
Five twitches were averaged from the initial twitches, those recorded after stimulation at different frequencies (prior to fatigue and termed post-tetanic twitches) and immediately post-fatigue. The following parameters were measured: (1) peak force; (2) contraction time (CT, the time between force onset and force peak); and (3) hRT. These same parameters, excluding CT, were measured for the forces evoked at each stimulation frequency. The stimulus frequency that produced 50% of maximal tetanic force (F50) was estimated based on the linear regression equation that best fitted three consecutive data points, which spanned half maximal force. That is, regressions were fitted to the force at 5, 8 and 10 Hz, or to 8, 10 and 15 Hz data (Thomas et al. 1991a). Axon conduction velocity was calculated from conduction distance (stimulus site to common EMG electrode) and EMG latency after correcting for the typical terminal neuromuscular latency and conduction distance between the wrist crease and common electrode (3.4 ms, 7 cm), as done by Westling et al. (1990).
Statistics
The changes in the EMG parameters, force and hRT during fatigue were analysed with one-way repeated measures ANOVA on ranked data. Post hoc comparisons were completed using Dunnett's method to determine when during fatigue the values were significantly different from data at the beginning of the test. Comparisons of pre-fatigue to post-fatigue twitch force, CT and hRT, F50, forces at different frequencies, and ratios of submaximal forces to the maximal tetanic force were assessed with Wilcoxon's signed rank test. Spearman's rank order correlations were applied to examine the relationships between the EMG indices and the force fatigue indices, as well as the associations between these indices, pre-fatigue twitch properties (initial and post-tetanic), and tetanic force. Values are expressed as medians and ranges. Differences were considered significant at P < 0.05. The pre-fatigue values of the EMG recorded from the proximal muscle surfaces (between the base of the thenar eminence and mid-belly) and the fatigue induced changes were not significantly different from the EMG data recorded from the distal muscle surfaces (between mid-belly and the metacarpophalangeal joint of the thumb). Thus, only distal EMG data are presented.
Results
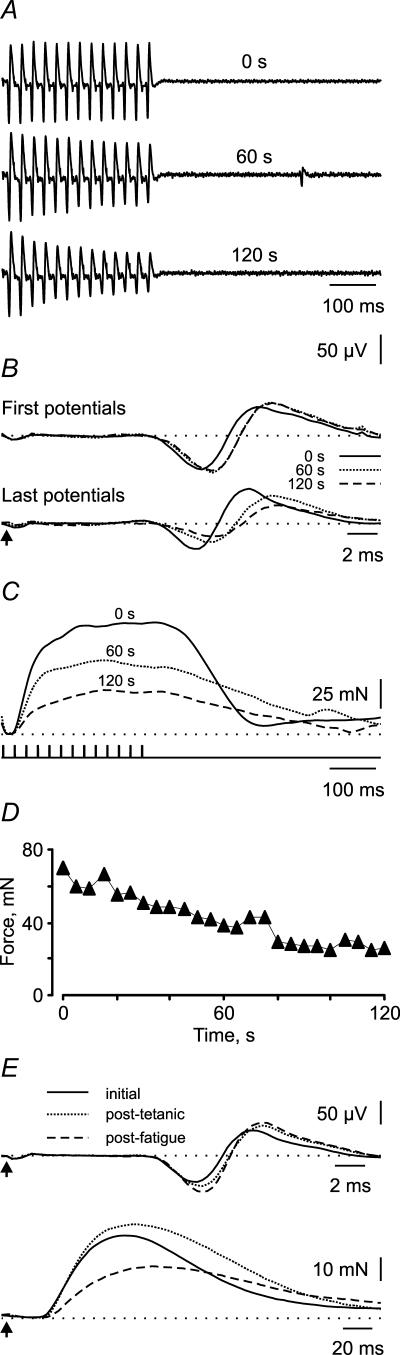
Seventeen paralysed thenar units were fatigued for 2 min. Fatigue-induced changes in the EMG, 40 Hz force and twitch force of a single unit are shown in Fig. 1. The latency, duration, amplitude and area of the first EMG potential of the train all increased during the fatigue protocol. By 120 s the corresponding EMG indices were 1.04, 1.08, 1.11 and 1.22 (Fig. 1A). Overlays of these first EMG potentials show that the increases in amplitude, duration and area were evident at 60 s but there was little change thereafter (Fig. 1B). In contrast, there were progressive changes in the last EMG potentials in the trains. The indices for the latency (1.08) and duration (1.04) of the last EMG potential were prolonged at 120 s compared to the first potential at 0 s, but the amplitude (0.52) and area (0.60) indices were both reduced (Fig. 1B) despite no within-train decrement in force (Fig. 1C). The 40 Hz force decreased progressively to reach 44% of its initial value after 2 min of stimulation (force fatigue index: 120 s force/0 s force = 0.44, Fig. 1D), whereas the hRT was prolonged by 53% (hRT index = 1.53).
Figure 1. Fatigue of a paralysed motor unit.
A, trains of EMG potentials evoked by 13 pulses at 40 Hz at the start of the fatigue test (0 s) and after 60 s and 120 s of stimulation. B, overlays of the first and last potentials of each train at 0 s, 60 s and 120 s. The arrow indicates the time of stimulation. C, corresponding 40 Hz forces. D, reduction in force during fatigue measured every 5 s to show that gradual changes occurred. E, initial, post-tetanic, and post-fatigue EMG potentials and twitch force of the same unit.
After fatigue, the amplitude and area of the EMG for the twitch of this same unit increased but EMG duration was unchanged (post-fatigue twitch/post-tetanic twitch indices of 1.13, 1.15, 1.00, respectively; Fig. 1E). The twitch force decreased (twitch force index = 0.54), while the twitch CT and hRT increased (post-fatigue twitch/post-tetanic twitch indices of 1.17 and 1.29, respectively).
First and last EMG potentials
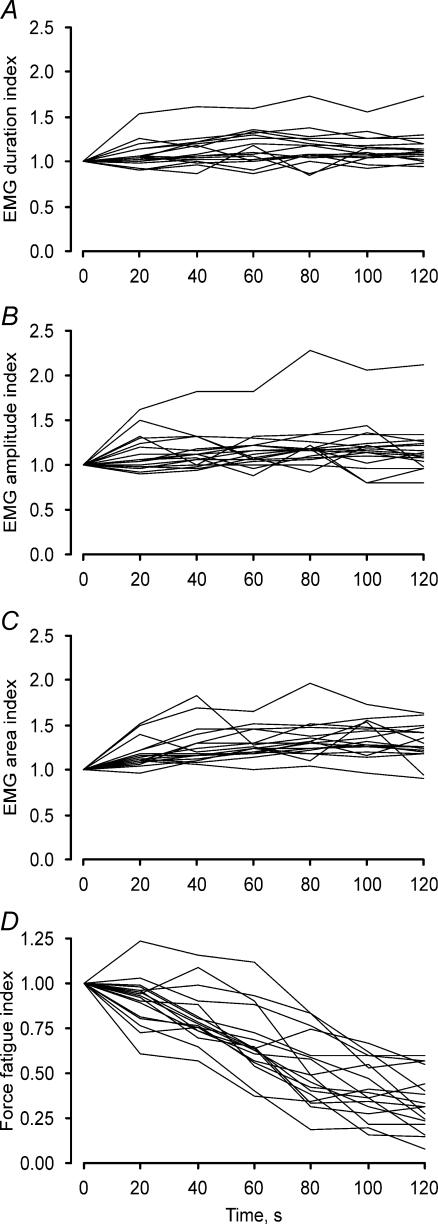
The progressive changes in the first EMG potentials of the trains are shown every 20 s for each unit in Fig. 2A–C. There were increases in the median latency, duration, amplitude and area from 0 s to 120 s (from 10.7 to 11.0 ms, from 9.8 to 11.8 ms, from 61.4 to 67.4 μV, and from 0.17 to 0.22 μV.s, respectively) despite a reduction in the force of every unit (Fig. 2D). The increase in the median for each parameter was significant from 20 s to 120 s (all P < 0.001).
Figure 2. Changes in EMG (first potential in a train) and force with repeated stimulation.
Fatigue indices for EMG duration (A), amplitude (B), area (C) and force (D, n = 17 units). Each line represents data for a single unit, measured every 20 s. Data are expressed relative to the respective parameters at the beginning of the fatigue test.
The median latency (11.1 ms) and duration (11.8 ms) for the last EMG potential at 120 s were both prolonged significantly, relative to the values for the first potential at 0 s (P < 0.05). The median EMG amplitude of the last potential was reduced at 100 s and 120 s (38.7 μV at 120 s, P < 0.05) compared to the first potential at 0 s even though there was no reduction in force in response to a train of stimuli (Fig. 1C), whereas EMG area was unchanged (0.13 μV.s, P > 0.05).
40 Hz force
There were progressive reductions in 40 Hz force and force fatigue indices for each unit (Fig. 2D). Force decreased from a median of 42.7 mN at 0 s to 13.4 mN at 120 s (P < 0.05), the decrease significant from 20 s to 120 s (P < 0.05). The median fatigue indices at 20, 40, 60, 80, 100 and 120 s were 0.94, 0.78, 0.64, 0.49, 0.39 and 0.31, respectively. However, the extent of fatigue varied for different units as revealed by the range of fatigue indices at 120 s (0.08–0.60).
There was an increase in hRT over the course of fatigue, from a median of 160.5 ms at 0 s (range 105.5–295.0 ms) to 189.3 ms at 120 s (96.5–381.0 ms). The increase in median hRT was significant from 20 s to 120 s (n = 8 units, P < 0.05). The hRT could not be measured in the other nine units because the relaxation phase of the force was periodically contaminated by the pulse-pressure, spontaneous unit activity, or muscle spasms. An example of a spontaneously active unit (Fig. 1A) and its effects on force is shown after the train of pulses delivered at 60 s (Fig. 1C).
EMG and force fatigue indices in paralysed and control units
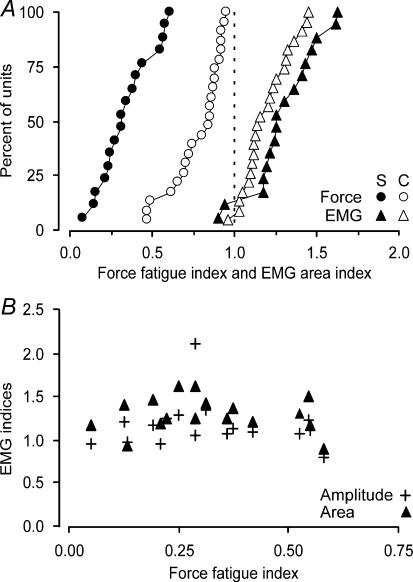
Figure 3A compares cumulative distributions of indices (120 s/0 s) for the area of the first EMG potentials and force for the 17 paralysed units (filled symbols) and 23 thenar units recorded in able-bodied subjects (open symbols, Thomas et al. 1991b, 2006). In both groups, the area of the first EMG potential increased significantly after 2 min of stimulation as reflected by EMG indices greater than 1.0 (no group difference, Mann-Whitney rank sum test, P > 0.05). The paralysed units were significantly more fatigable than the control units, demonstrated by a uniform shift in the force fatigue index distribution towards lower values (Fig. 3A; median force fatigue index for control data = 0.85, range = 0.41–0.95, P < 0.001). Thus, the reductions in paralysed unit forces were accompanied by potentiation of the area (Fig. 2C), amplitude (Fig. 2B) and duration (Fig. 2A) of the first EMG potentials, as found for control data (Thomas et al. 2006).
Figure 3. Cumulative distributions of fatigue indices for paralysed and control units.
A, EMG indices (120 s value/0 s value) for area of the first potentials of the trains and the 40 Hz force fatigue indices for 17 paralysed units (S, filled symbols) and 23 units from able-bodied subjects (C, open symbols; Thomas et al. 1991b, 2006). B, relationship between fatigue indices for force and the amplitude and area of the first EMG potentials for all paralysed units.
Correlations
There were no significant correlations between the final indices for first (Fig. 3B) or last EMG potential duration, amplitude or area and the force fatigue index, or the indices for postfatigue twitch force. Similarly, the indices for EMG duration, amplitude and area (first or last potential) at 120 s, were not significantly correlated with any of the initial or post-tetanic twitch properties or initial maximal tetanic force.
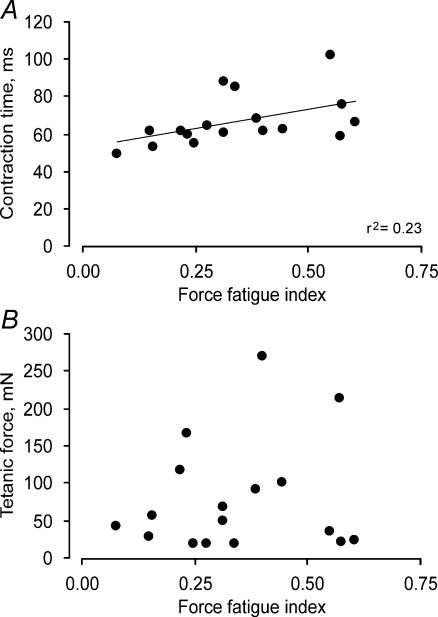
The force fatigue index was positively correlated with the post-tetanic twitch CT (r2 = 0.23; Fig. 4A) and hRT (r2 = 0.21), as well as the initial twitch CT (r2 = 0.35) and hRT (r2 = 0.22), indicating that units with a shorter twitch CT and hRT were more fatigable (both P < 0.05). Similar relationships have been found in some (e.g. Reinking et al. 1975; Kernell et al. 1983) but not all unit studies in animals (e.g. Goslow et al. 1977) or in control human thenar or digit units (Thomas et al. 1991b; Fuglevand et al. 1999). The force fatigue index was not significantly related to tetanic force (Fig. 4B), F50, axon conduction velocity, or twitch force. In control subjects, stronger units were more fatigable than weaker units) (Thomas et al. 1991b). The lack of correlation between strength and fatigue in these paralysed units may reflect partial muscle reinnervation (Luff et al. 1988; Rafuse & Gordon, 1998) due to death of some motoneurons after human cervical SCI (Yang et al. 1990; Thomas, 1997b; Thomas et al. 2002; Thomas & Zijdewind, 2006).
Figure 4. Correlations between unit speed, force and fatigue.
Relationships between paralysed unit force fatigue indices and twitch CT (A) and maximal tetanic force recorded prior to the fatigue test (B).
Post-fatigue responses
Forces evoked by applying trains of stimuli at 5–100 Hz could be measured in 10 of the 17 units after fatigue. In the other seven units, the forces were contaminated by spontaneous discharges of different units or the pulse pressure wave. There was a significant decrease in force at each stimulus frequency (5–100 Hz; P < 0.05), and in the twitch force (Fig. 5C, P < 0.05, n = 16 units). Consequently, higher stimulus rates were needed to produce any given absolute force in paralysed units after fatigue. However, the EMG latency, duration, amplitude and area of the post-fatigue twitches increased significantly compared to the initial twitch data (P < 0.05, Table 1), providing more evidence that fatigue occurred distal to the neuromuscular junction.
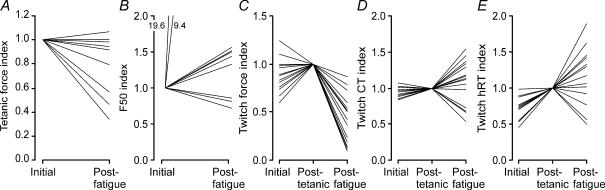
Figure 5. Changes in unit twitch and tetanic parameters with fatigue.
Initial and post-fatigue maximal tetanic force (A), F50 (B, n = 10 units) and initial, post-tetanic and post-fatigue twitch force (C), CT (D) and hRT (E, n = 16 units). Data are expressed relative to the respective parameters recorded initially (A and B) or after trains of stimuli between 5 and 100 Hz (C, D and E). Each line represents data from 1 unit. In 2 units half-maximal force was attained at 1 Hz before fatigue, and increased substantially to 9.4 Hz and 19.6 Hz post-fatigue (B).
Table 1.
Initial, post-tetanic, and postfatigue EMG and contractile properties
| Parameter | Initial | Post-tetanic | Post-fatigue |
|---|---|---|---|
| EMG latency (ms) | 10.3 | 10.8 | 10.7* |
| 8.6–15.3 | 8.6–15.3 | 8.9–15.3 | |
| EMG duration (ms) | 10.9 | 12.1 | 12.6* |
| 5.0–17.0 | 5.0–17.7 | 5.9–16.7 | |
| EMG amplitude (μV) | 48.2 | 58.6 | 67.4* |
| 8.1–327.0 | 8.5–362.0 | 11.0–351.5 | |
| EMG area (μV s) | 0.10 | 0.11 | 0.18* |
| 0.01–1.10 | 0.02–1.23 | 0.02–1.32 | |
| Twitch force (mN) | 13.5 | 14.4 | 6.4*† |
| 4.5–69.7 | 7.5–86.6 | 1.0–36.2 | |
| Twitch contraction time (ms) | 58.3 | 61.8 | 71.5* |
| 46.7–102.0 | 49.6–101.8 | 33.1–120.7 | |
| Twitch half-relaxation time (ms) | 56.2 | 78.9* | 104.9* |
| 41.0–150.0 | 44.7–275.0 | 25.5–211.7 | |
| Maximum tetanic force (mN) | 45.2 | — | 30.4* |
| 18.2–269.9 | 14.3–254.3 | ||
| Twitch/max tetanic force ratio | 0.30 | 0.41* | 0.21*† |
| 0.11–0.58 | 0.17–0.59 | 0.02–0.37 | |
| F50 (Hz) | 11.3 | — | 12.3* |
| 1.0–18.3 | 8.0–20.4 |
Values are medians and ranges for 16 units, except for twitch half-relaxation time (n = 13), maximum tetanic force (n = 10) and F50 (n = 10). EMG properties correspond to unit twitch responses. Initial twitch EMG and contractile properties were recorded prior to delivery of any stimuli at tetanic frequencies. Post-tetanic twitch data were recorded after a series of pulse trains at frequencies of 5–100 Hz. Post-fatigue data were recorded after the 2 min fatigue test.
P < 0.05 compared to initial value
P < 0.05 compared to post-tetanic value.
Post-fatigue maximal tetanic force, which occurred at 30–50 Hz (similar or lower forces were evoked at higher stimulus frequencies), was reduced in six units and had recovered to initial values in four units by the time it was recorded (Fig. 5A, median recovery time, 77 s, did not relate to force recovery). However, the ratios of forces evoked at 5, 8 and 10 Hz compared to the maximal tetanic force were significantly lower post-fatigue (0.22, 0.32 and 0.35, respectively) compared to initial (0.32, 0.41 and 0.47, respectively, P < 0.05), implying that low frequency fatigue developed during recovery, as found for whole paralysed muscles (Thomas, 1997a) and for more fatigable control units (Thomas et al. 1991b).
The post-fatigue F50, twitch CT and hRT increased for some units but decreased for others (Fig. 5B, D and E), as found for control human or cat units (Dubose et al. 1987; Thomas et al. 1991a; Fuglevand et al. 1999). Overall, the median F50 increased significantly post-fatigue (P < 0.05, Table 1) despite significant prolongation of the median post-fatigue twitch CT and hRT (P < 0.05 compared to initial twitches only). Since F50 is usually attained in the steep section of the force–frequency curve, small changes in frequency after fatigue can make large differences in evoked force, an important issue for the frequency control of muscle using functional electrical stimulation.
Discussion
The present data show that thenar motor units are highly fatigable after they have been paralysed chronically by SCI. Unit force declined to 8–60% of initial after just 2 min of stimulation, despite increases in the amplitude and area of the first EMG potentials in the trains. Furthermore, the uniform shift in the force fatigue index distribution to lower values compared to the distribution for motor units in able-bodied controls (Thomas et al. 1991b) suggests that all motor units in paralysed thenar muscles were influenced similarly as a result of chronic SCI. After fatigue, force deficits were evident at all stimulation frequencies. Thus, higher frequencies were needed to evoke any given absolute force in fatigued motor units.
EMG changes with fatigue
During evoked contractions, reductions in force may stem from impairment of any process that occurs between neuromuscular transmission and engagement of the cross-bridges (Gandevia, 2001). In paralysed units, significant prolongation of EMG duration, and potentiation of amplitude and area, occurred early for the first EMG potentials in the trains (from 20 s to 120 s), and at a time when the force of every unit was decreasing (Fig. 2). The broadened motor unit potentials may result from a reduction in action potential conduction velocity, whereas potentiation of amplitude and area could reflect increased membrane Na+–K+ pump activity (Hicks & McComas, 1989). These changes in EMG parameters did not correlate with the reductions in unit force (Fig. 3B). These dissociations between changes in EMG parameters and force, together with the significant potentiation of both EMG amplitude and area, suggest that paralysed units retain a safety margin for effective neuromuscular transmission and that fatigue reflects impaired processes in the muscle fibres. These data also demonstrate that changes in unit EMG parameters do not accurately reflect the fatigability of paralysed units. Similar results have been found with this same fatigue protocol for units in control thenar muscles (Chan et al. 1998; Thomas et al. 2006) and for units in muscles of rats and cats (Burke et al. 1973; Sandercock et al. 1985; Enoka et al. 1992).
Whether the amplitude or area of a motor unit potential is better to detect neuromuscular transmission failure is controversial (Sandercock et al. 1985; Hamm et al. 1989; Enoka et al. 1992; Fuglevand, 1995; Gardiner & Olha, 1987). Both the amplitude and the area of a motor unit potential are influenced by a number of factors including fatigue-induced slowing of fibre conduction velocity (i.e. increased EMG duration; Dimitrova & Dimitrov, 2003). Hence, the decline in the amplitude of the last potentials at 100 s and 120 s may reflect desynchronization of potentials, possibly due to differential slowing of conduction velocity for different muscle fibres, as opposed to activation of fewer fibres (Hamm et al. 1989). Moreover, given that there was no within-train reduction in force for any of the 17 units (Fig. 1C), fibre drop-out is less likely. Alternative explanations for the decline in EMG amplitude include decreases in Na+–K+ pump activity, a reduction in the amplitude of the muscle fibre potentials, or signal cancellation due to the positive phase of one action potential overlapping with the negative phase of an adjacent action potential (Stalberg, 1966; Sandercock et al. 1985; Fuglevand, 1995). The initial EMG latency and duration were longer in paralysed units than in control units (Thomas et al. 2006; Häger-Ross et al. 2006), changes that could exacerbate signal cancellation.
Force changes during fatigue
In paralysed motor units, progressive and significant reductions in force were accompanied by increases in hRT (Fig. 1C). Data from control single fibre studies suggest that this amount of fatigue probably reflects impaired Ca2+ handling (i.e. reduced Ca2+ release from the sarcoplasmic reticulum) and altered cross-bridge kinetics, both of which may have a metabolic origin (Sandercock et al. 1985; Westerblad & Allen, 1991; Edman, 1995). The paralysed thenar units were more fatigable than control units (Fig. 3B, Thomas et al. 1991b), as found after spinal transection in cats (Mayer et al. 1984; Munson et al. 1986). Most paralysed units had intermediate fatigability (70%, fatigue index ≥ 0.25 < 0.75, Burke et al. 1973). The other 30% were fatigable (fatigue index < 0.25). In contrast, 70% of the control units were fatigue resistant (fatigue index ≥ 0.75), similar to the type I fibre predominance of these muscles (63%; Johnson et al. 1973), while the remaining units had intermediate fatigability (Thomas et al. 1991b). These findings suggest that chronic paralysis resulted in profound increases in the intrinsic fatigability of the muscle fibres since ischaemia is unlikely when only one motor unit is stimulated. The strongest paralysed unit in this study (270 mN) produced about 1% of the mean maximal tetanic force of paralysed thenar muscles (Thomas et al. 2003), well below the threshold (about 10% maximal voluntary strength) at which blood flow becomes compromised during whole muscle contractions (Sjogaard et al. 1988). The predominance of paralysed units with intermediate fatigability may also reflect the consequences of motoneuron death after cervical SCI (Yang et al. 1990; Thomas, 1997b; Thomas et al. 1997, 2002; Thomas & Zijdewind, 2006). Intact axons that sprout may not completely respecify the type of fibres they reinnervate (Gordon et al. 1988; Rafuse & Gordon, 1998).
The uniform shift in the distribution of paralysed unit fatigue indices to lower values suggests that chronic paralysis increased the fatigability of all units similarly (Fig. 3A) despite possible muscle reinnervation. Thus, the excessive fatigability of the paralysed units probably relates to a combination of factors that may include reduced use, leading to the conversion of type I fibres to type II, and to a decline in oxidative enzyme activity (Grimby et al. 1976; Alaimo et al. 1984; Mayer et al. 1984; Munson et al. 1986), since chronic electrical stimulation can improve endurance (Peckham et al. 1976; Kernell et al. 1987b; Martin et al. 1992; Stein et al. 1992; Rochester et al. 1995a, b; Gordon et al. 1997). Paralysed thenar motor units are often active spontaneously at low rates (Zijdewind & Thomas, 2001) but whether this reflects a reduction in the total daily amount of activity is unknown. Although the average EMG amplitude recorded over 24 h was reduced in paralysed tibialis anterior muscles (Stein et al. 1992) this may reflect fibre atrophy. Thus, the relationship between the amount of involuntary muscle activity and fatigability remains unclear. Nevertheless, involuntary activity seems insufficient to maintain the fatigue resistance of paralysed thenar motor units and muscles. However, the range of fatigue indices for the paralysed and control unit populations were similar (0.08–0.60 versus 0.41–0.95, respectively; Fig. 3A), consistent with studies that show that the removal or addition of activity modulates the contractile properties of muscle and motor units but does not alter the range of parameters (Kernell et al. 1987a, b; Pierotti et al. 1991; Gordon et al. 1997). Hence, activity-independent factors, including the basic genetic programme of the muscle fibre, also probably contribute to the regulation of fatigue resistance (Talmadge et al. 2002).
The median fatigue index for paralysed units (0.31) was similar to the low fatigue indices reported for whole paralysed muscles (Rochester et al. 1995a; Shields, 1995; Thomas et al. 2003). Hence, our findings imply that the diminished endurance of chronically paralysed muscles stems largely from the excessive fatigability of the motor units. Impairment of blood flow and effects that arise from asynchronous compared to synchronous activation of fibres with electrical stimulation are likely to make smaller contributions to the reductions in force (Godfrey et al. 2002; Thomas et al. 2002; Olive et al. 2003; Butler et al. 2004; Thomas & Zijdewind, 2006).
Methodological considerations
The location and intraneural stimulation of a single motor axon to a target muscle (Westling et al. 1990) is challenging in SCI subjects with impaired sensation, particularly when muscle spasms and involuntary motor unit activity contaminate the EMG and force. Even though these issues limit data yield, three lines of evidence suggest that our results are representative of the unit population in paralysed thenar muscles. First, the initial EMG and contractile properties of these units were similar to the data obtained from a larger sample (Häger-Ross et al. 2006). Second, the range of conduction velocities for the 17 units (25–65 m s−1) suggests that we recorded from both small and large diameter axons (Westling et al. 1990). Finally, a similar range of force fatigue indices was found for both paralysed and control units even though these units differed in absolute fatigability (Thomas et al. 1991b).
Conclusions
In chronically paralysed thenar motor units, the significant potentiation of both EMG amplitude and area that accompanied reductions in force suggest that this fatigue reflects failure of muscle processes, probably impaired excitation–contraction coupling and reduced force generation of the contractile proteins, but not ineffective transmission of electrical signals. The use of single motor axon stimulation is likely to have avoided muscle ischaemia, revealing that the intrinsic fatigability of paralysed units was greater than that of control thenar units (Thomas et al. 1991b). However, paralysis does not restrict the range over which fatigability is modulated for the population of units in paralysed thenar muscles.
Acknowledgments
The authors greatly appreciate the excellent technical support of Göran Westling and Lars Bäckstrom, and thank Ms. Bette Mas for help with the figures. This research was funded by the Research Council of Sweden, Umeå University, USPHS grant NS-30226 and The Miami Project to Cure Paralysis.
References
- Alaimo MA, Smith JL, Roy RR, Edgerton VR. EMG activity of slow and fast ankle extensors following spinal cord transection. J Appl Physiol. 1984;56:1608–1613. doi: 10.1152/jappl.1984.56.6.1608. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Mohr T, Biering-Sørensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibers from m. vastus lateralis in spinal cord injured individuals: effects of long term functional electrical stimulation (FES) Pflugers Arch. 1996;431:513–518. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., III Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Ribot-Ciscar E, Zijdewind I, Thomas CK. Increased blood pressure can reduce fatigue of thenar muscles paralyzed after spinal cord injury. Muscle Nerve. 2004;29:575–584. doi: 10.1002/mus.20002. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534:903–912. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KM, Andres LP, Polykovskaya Y, Brown WF. Dissociation of the electrical and contractile properties in single human motor units during fatigue. Muscle Nerve. 1998;21:1786–1789. doi: 10.1002/(sici)1097-4598(199812)21:12<1786::aid-mus25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cope TC, Bodine SC, Fournier M, Edgerton VR. Soleus motor units in chronic spinal transected cats: physiological and morphological alterations. J Neurophysiol. 1986;55:1202–1220. doi: 10.1152/jn.1986.55.6.1202. [DOI] [PubMed] [Google Scholar]
- Dimitrova NA, Dimitrov GV. Interpretation of EMG changes with fatigue: facts, pitfalls, and fallacies. J Electromyogr Kinesiol. 2003;13:13–36. doi: 10.1016/s1050-6411(02)00083-4. [DOI] [PubMed] [Google Scholar]
- Dubose L, Schelhorn TB, Clamann HP. Changes in contractile speed of cat motor units during activity. Muscle Nerve. 1987;10:744–752. doi: 10.1002/mus.880100811. [DOI] [PubMed] [Google Scholar]
- Edman KA. Myofibrillar fatigue versus failure of activation. Adv Exp Med Biol. 1995;384:29–43. doi: 10.1007/978-1-4899-1016-5_3. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Trayanova N, Laouris Y, Bevan L, Reinking RM, Stuart DG. Fatigue-related changes in motor unit action potentials of adult cats. Muscle Nerve. 1992;15:138–150. doi: 10.1002/mus.880150204. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ. The role of the sarcolemma action potential in fatigue. Adv Exp Med Biol. 1995;384:101–108. doi: 10.1007/978-1-4899-1016-5_8. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force-frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol. 1999;81:1718–1729. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gardiner PF, Olha AE. Contractile and electromyographic characteristics of rat plantaris motor unit types during fatigue in situ. J Physiol. 1987;385:13–34. doi: 10.1113/jphysiol.1987.sp016481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey S, Butler JE, Griffin L, Thomas CK. Differential fatigue of paralyzed thenar muscles by stimuli of different intensities. Muscle Nerve. 2002;26:122–131. doi: 10.1002/mus.10173. [DOI] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Stein RB, Erdebil S. Comparison of physiological and histochemical properties of motor units after cross-reinnervation of antagonistic muscles in the cat hindlimb. J Neurophysiol. 1988;60:365–378. doi: 10.1152/jn.1988.60.1.365. [DOI] [PubMed] [Google Scholar]
- Gordon T, Tyreman N, Rafuse VF, Munson JB. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. I. Muscle and motor unit properties. J Neurophysiol. 1997;77:2585–2604. doi: 10.1152/jn.1997.77.5.2585. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Jr, Cameron WE, Stuart DG. The fast twitch motor units of cat ankle flexors. 1. Tripartite classification on basis of fatigability. Brain Res. 1977;134:35–46. doi: 10.1016/0006-8993(77)90923-4. [DOI] [PubMed] [Google Scholar]
- Grimby G, Broberg C, Krotkiewska I, Krotkiewski M. Muscle fiber composition in patients with traumatic cord lesion. Scand J Rehabil Med. 1976;8:37–42. [PubMed] [Google Scholar]
- Häger-Ross CK, Klein CS, Thomas CK. Twitch and tetanic properties of human thenar motor units paralyzed by chronic spinal cord injury. J Neuroophysiol. 2006 doi: 10.1152/jn.01339.2005. in press. [DOI] [PubMed] [Google Scholar]
- Hamm TM, Reinking RM, Stuart DG. Electromyographic responses of mammalian motor units to a fatigue test. Electromyogr Clin Neurophysiol. 1989;29:485–494. [PubMed] [Google Scholar]
- Hicks A, McComas AJ. Increased sodium pump activity following repetitive stimulation of rat soleus muscles. J Physiol. 1989;414:337–349. doi: 10.1113/jphysiol.1989.sp017691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kernell D, Donselaar Y, Eerbeek O. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. II. Endurance-related properties. J Neurophysiol. 1987b;58:614–627. doi: 10.1152/jn.1987.58.3.614. [DOI] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Exp Brain Res. 1983;50:211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kernell D, Eerbeek O, Verhey BA, Donselaar Y. Effects of physiological amounts of high- and low-rate chronic stimulation on fast-twitch muscle of the cat hindlimb. I. Speed- and force-related properties. J Neurophysiol. 1987a;58:598–613. doi: 10.1152/jn.1987.58.3.598. [DOI] [PubMed] [Google Scholar]
- Luff AR, Hatcher DD, Torkko K. Enlarged motor units resulting from partial denervation of cat hindlimb muscles. J Neurophysiol. 1988;59:1377–1394. doi: 10.1152/jn.1988.59.5.1377. [DOI] [PubMed] [Google Scholar]
- Martin TP, Stein RB, Hoeppner PH, Reid DC. Influence of electrical stimulation on the morphological and metabolic properties of paralyzed muscle. J Appl Physiol. 1992;72:1401–1406. doi: 10.1152/jappl.1992.72.4.1401. [DOI] [PubMed] [Google Scholar]
- Mayer RF, Burke RE, Toop J, Walmsley B, Hodgson JA. The effect of spinal cord transection on motor units in cat medial gastrocnemius muscles. Muscle Nerve. 1984;7:23–31. doi: 10.1002/mus.880070105. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Munson JB, Foehring RC, Lofton SA, Zengel JE, Sypert GW. Plasticity of medial gastrocnemius motor units following cordotomy in the cat. J Neurophysiol. 1986;55:619–634. doi: 10.1152/jn.1986.55.4.619. [DOI] [PubMed] [Google Scholar]
- Olive JL, Slade JM, Dudley GA, McCully KK. Blood flow and muscle fatigue in SCI individuals during electrical stimulation. J Appl Physiol. 2003;94:701–708. doi: 10.1152/japplphysiol.00736.2002. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Mortimer JT, Marsolais EB. Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin Orthop Relat Res. 1976;114:326–333. [PubMed] [Google Scholar]
- Petrofsky JS, Hendershot DM. The interrelationship between blood pressure, intramuscular pressure, and isometric endurance in fast and slow twitch skeletal muscle in the cat. Eur J Appl Physiol Occup Physiol. 1984;53:106–111. doi: 10.1007/BF00422571. [DOI] [PubMed] [Google Scholar]
- Pierotti DJ, Roy RR, Bodine-Fowler SC, Hodgson JA, Edgerton VR. Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J Physiol. 1991;444:175–192. doi: 10.1113/jphysiol.1991.sp018872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafuse VF, Gordon T. Incomplete rematching of nerve and muscle properties in motor units after extensive nerve injuries in cat hindlimb muscle. J Physiol. 1998;509:909–926. doi: 10.1111/j.1469-7793.1998.909bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking RM, Stephens JA, Stuart DG. The motor units of cat medial gastrocnemius: problem of their categorisation on the basis of mechanical properties. Exp Brain Res. 1975;23:301–313. doi: 10.1007/BF00239742. [DOI] [PubMed] [Google Scholar]
- Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia. 1995b;33:514–522. doi: 10.1038/sc.1995.112. [DOI] [PubMed] [Google Scholar]
- Rochester L, Chandler CS, Johnson MA, Sutton RA, Miller S. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 1. Contractile properties. Paraplegia. 1995a;33:437–449. doi: 10.1038/sc.1995.97. [DOI] [PubMed] [Google Scholar]
- Sandercock TG, Faulkner JA, Albers JW, Abbrecht PH. Single motor unit and fiber action potentials during fatigue. J Appl Physiol. 1985;58:1073–1079. doi: 10.1152/jappl.1985.58.4.1073. [DOI] [PubMed] [Google Scholar]
- Shields RK. Fatigability, relaxation properties, and electromyographic responses of the human paralyzed soleus muscle. J Neurophysiol. 1995;73:2195–2206. doi: 10.1152/jn.1995.73.6.2195. [DOI] [PubMed] [Google Scholar]
- Sjogaard G, Savard G, Juel C. Muscle blood flow during isometric activity and its relation to muscle fatigue. Eur J Appl Physiol. 1988;57:327–335. doi: 10.1007/BF00635992. [DOI] [PubMed] [Google Scholar]
- Stalberg E. Propagation velocity in human muscle fibers in situ. Acta Physiol Scand Suppl. 1966;287:1–112. [PubMed] [Google Scholar]
- Stein RB, Gordon T, Jefferson J, Sharfenberger A, Yang JF, Tötösy de Zepetnek J, Belanger M. Optimal stimulation of paralyzed muscle after human spinal cord injury. J Appl Physiol. 1992;72:1393–1400. doi: 10.1152/jappl.1992.72.4.1393. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR. Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol. 2002;93:1487–1497. doi: 10.1152/japplphysiol.00053.2002. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Fatigue in human thenar muscle paralysed by spinal cord injury. J Electromyogr Kinesiol. 1997a;7:15–26. doi: 10.1016/s1050-6411(96)00020-x. [DOI] [PubMed] [Google Scholar]
- Thomas CK. Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve. 1997b;20:788–799. doi: 10.1002/(sici)1097-4598(199707)20:7<788::aid-mus2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Ritchie B, Johansson RS. Force-frequency relationships of human thenar motor units. J Neurophysiol. 1991a;65:1509–1516. doi: 10.1152/jn.1991.65.6.1509. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Broton JG, Calancie B. Motor unit forces and recruitment patterns after cervical spinal cord injury. Muscle Nerve. 1997;20:212–220. doi: 10.1002/(sici)1097-4598(199702)20:2<212::aid-mus12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Griffin L, Godfrey S, Ribot-Ciscar E, Butler JE. Fatigue of paralyzed and control thenar muscles induced by variable or constant frequency stimulation. J Neurophysiol. 2003;89:2055–2064. doi: 10.1152/jn.01002.2002. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. Attempts to physiologically classify human thenar motor units. J Neurophysiol. 1991b;65:1501–1508. doi: 10.1152/jn.1991.65.6.1501. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. EMG changes in human thenar motor units with force potentiation and fatigue. J Neurophysiol. 2006;95:1518–1526. doi: 10.1152/jn.00924.2005. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Nelson G, Than L, Zijdewind I. Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles. Muscle Nerve. 2002;25:797–804. doi: 10.1002/mus.10111. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Zijdewind I. Fatigue of muscles weakened by death of motoneurons. Muscle Nerve. 2006;33:21–41. doi: 10.1002/mus.20400. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J General Physiol. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling G, Johansson RS, Thomas CK, Bigland-Ritchie B. Measurement of contractile and electrical properties of single human thenar motor units in response to intraneural motor-axon stimulation. J Neurophysiol. 1990;64:1331–1338. doi: 10.1152/jn.1990.64.4.1331. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stein RB, Jhamandas J, Gordon T. Motor unit numbers and contractile properties after spinal cord injury. Ann Neurol. 1990;28:496–502. doi: 10.1002/ana.410280405. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve. 2001;24:952–962. doi: 10.1002/mus.1094. [DOI] [PubMed] [Google Scholar]