Abstract
Studies from our laboratory and others show that oestrogen reduces angiotensin II (Ang II)-induced water intake by ovariectomized rats. Elimination of endogenous oestrogen by ovariectomy causes weight gain that can be reversed or prevented by oestrogen replacement. Changes in body weight modify cardiovascular responses to Ang II but whether such changes have similar effects on central and behavioural responses to Ang II is unknown. The goal of this study was to evaluate the contributions of oestrogen and weight loss to isoproterenol (isoprenaline; Iso)-induced Fos immunoreactivity (IR) and to angiotensin type 1 (AT1) receptor mRNA in forebrain regions implicated in the control of fluid balance. Isoproterenol significantly increased Fos IR in the hypothalamic paraventricular and supraoptic nuclei, the subfornical organ (SFO), and the organum vasculosum of the lamina terminalis, but had no effect on AT1 mRNA expression. However, both Iso-induced Fos IR and the AT1 mRNA were attenuated in the SFO of the oestrogen and weight loss groups compared with that of the control group. Consequently, we examined the effect of weight loss on Iso-induced water intake and plasma renin activity (PRA) and found that weight loss decreased water intake after Iso, but had no effect on PRA. Thus, we propose that weight loss decreases Ang II-elicited water intake in the female rat by down-regulating the expression of the AT1 receptor.
The renin–angiotensin system (RAS) is critically involved in the regulation of blood pressure and fluid balance. The effector peptide of the RAS, angiotensin II (Ang II), mediates compensatory responses to blood loss, sodium depletion and hypotension. Ang II influences peripheral and central mechanisms that maintain blood pressure and volume. In the periphery, Ang II causes vasoconstriction by acting on angiotensin type 1 (AT1) receptors on blood vessels. Centrally, Ang II binds to AT1 receptors in circumventricular organs (CVOs) to initiate changes in sympathetic nerve activity and increase water and sodium consumption (McKinley et al. 2003).
Interestingly, the RAS is influenced by sex hormones, and this influence is particularly pronounced in terms of behavioural responses to RAS activation. Studies from our laboratory and others show that oestrogen attenuates RAS-induced water intake by ovariectomized rats (Fregly & Thrasher, 1978; Findlay et al. 1979; Jonklaas & Buggy, 1984; Kisley et al. 1999; Krause et al. 2003; Tanaka et al. 2003). In our previous study, the peripheral synthesis of Ang II, as measured by plasma renin activity (PRA), was similar in oestrogen- and vehicle-treated ovariectomized rats (Krause et al. 2003), suggesting that oestrogen acts centrally to modify responses to circulating Ang II. In fact, peripheral administration of oestrogen decreases neuronal activity in the forebrain CVO, the subfornical organ (SFO), during intracarotid administration of Ang II (Tanaka et al. 2001). The SFO contains AT1 receptors which bind circulating Ang II, thereby activating central pathways that mediate water intake. In addition, oestrogen and AT1 receptors are co-localized within SFO neurons (Rosas-Arellano et al. 1999) and oestrogen treatment reduces Ang II binding to AT1 receptors in the SFO (Kisley et al. 1999). The reduced binding observed in the SFO is probably due to decreased AT1 receptors, as oestrogen down-regulates AT1 receptor expression in vitro (Gragasin et al. 2003; Imanishi et al. 2005). Thus, oestrogen may attenuate the responsiveness of the SFO to circulating Ang II by decreasing AT1 receptor expression, which in turn, modifies behavioural responses to RAS activation.
In addition to sex hormones, changes in body weight influence the RAS. Recent research has demonstrated that several components critical for the biosynthesis of Ang II are present in adipose tissue (Karlsson et al. 1998) and circulating levels of these components are increased in obese rodents and humans (Boustany et al. 2004; Engeli et al. 2005). Specifically, circulating angiotensinogen is elevated in obese rodents, producing hypertension that can be reversed with AT1 receptor antagonists (Boustany et al. 2004; Boustany et al. 2005). Circulating levels of angiotensinogen, renin, aldosterone and angiotensin-converting enzyme were increased in obese post-menopausal women and a 5% reduction in body weight greatly attenuated these increases and the associated hypertension (Engeli et al. 2005). Taken together, these studies suggest that changes in body weight modify RAS control of cardiovascular function. However, whether body weight also influences the central or behavioural responses to RAS activation has yet to be determined.
While there is good evidence that oestrogen influences neural and behavioural responses to RAS activation, an alternative variable that has not been considered is oestrogen effects on body weight. It is well established that elimination of endogenous oestrogen by ovariectomy is associated with a 12–16% increase in body weight that can be reversed or prevented by oestrogen replacement (McElroy & Wade, 1987). In our previous study investigating oestrogen effects on water intake (Krause et al. 2003), oestrogen replacement in ovariectomized rats produced a 3–5% weight loss. Therefore, it is possible that the changes in body weight that accompany ovariectomy and hormone replacement contribute to oestrogen effects on RAS-elicited water intake. At present, few researchers examining oestrogen effects on the central and behavioural responses to RAS activation consider the potential confound of altered body weight.
The goal of the present study was to test the hypothesis that the weight loss that accompanies oestrogen replacement contributes to oestrogen effects on central and behavioural responses to RAS activation. We first investigated the effect of oestrogen or weight loss on neuronal activity by examining Fos immunoreactivity (IR) in forebrain regions implicated in the control of fluid balance after systemic administration of isoproterenol (Iso). Isoproterenol is a β-agonist that increases circulating levels of Ang II by decreasing blood pressure and activating renal β-receptors (Kirby et al. 1994), and commonly is used to examine stimulated water intake (Rettig et al. 1981; Stocker et al. 2000; Krause et al. 2003). Next, we evaluated the effect of oestrogen or weight loss on AT1 receptor mRNA expression in the SFO and the hypothalamic paraventricular nucleus (PVN) using in situ hybridization. Results from these studies indicate that oestrogen and weight loss had similar effects on Fos IR and AT1 receptor mRNA expression. Accordingly, we also examined the effect of weight loss on Iso-induced water intake and PRA and found that weight loss attenuated water intake after Iso but had no effect on PRA. The results of these studies lead to the conclusion that body weight loss contributes to oestrogen attenuation of Ang II-elicited water intake in the female rat.
Methods
Animals
Adult female Sprague Dawley rats weighing between 250 and 350 g were individually housed in plastic cages and given ad libitum access to Purina rodent chow (no. 5001) and water except where noted. Rats were kept in a temperature-controlled room (22–24°C) on a 12: 12 h light–dark cycle with lights on at 07.00 h. Experiments examining water intake used a within subjects design so that each animal served as its own control. All procedures were approved by the Institutional Animal Care and Use Committee at Florida State University.
Ovariectomy and oestrogen replacement
Under sodium pentobarbital anaesthesia (50 mg (kg body weight)−1, i.p.; Abbott Laboratories, Chicago, IL, USA), rats were bilaterally ovariectomized using a ventral approach and given 1 week to recover. Rats then were given 17-β-oestradiol-3-benzoate (OeB; 10 μg (0.1 ml)−1 sesame oil, s.c.; Fisher Scientific, Fair Lawn, NJ, USA) or the oil vehicle (oil; 0.1 ml, s.c.) on a schedule that mimics the pattern of oestrogen fluctuations during the oestrous cycle. Specifically, rats were given injections of OeB or oil on two consecutive days, and tested 48 h following the second injection (i.e. on Day 4). Oestrogen replacement using this schedule reliably elicits lordosis 48 h after the second injection when progesterone also is given (McCarthy et al. 1991; Schumacher et al. 1991) and has been used in other studies examining OeB effects on ingestive behaviours (Kisley et al. 1999; Krause et al. 2003; Curtis et al. 2004; Curtis et al. 2005).
c-Fos immunohistochemistry
Ovariectomized rats were treated with OeB or oil as described and on Day 4 were injected with isoproterenol (30 μg (kg body weight)−1, s.c.; oil, n = 9; OeB, n = 8) or the 0.15 m NaCl vehicle (saline; oil, n = 5; OeB, n = 5). In our previous studies, OeB treatment caused a 3–5% reduction of body weight; therefore, an additional group of ovariectomized rats (weight loss) was treated with oil on Day 1 and 2, and restricted to 8–10 g of chow on Day 3 to produce a 3–5% weight loss. Subsequently, weight loss rats were injected with Iso (n = 9) or saline (n = 5) on Day 4.
Ninety minutes after the injections, rats were deeply anaesthetized with sodium pentobarbital (75 mg (kg body weight)−1, i.p.) and then perfused with 0.15 m NaCl, followed by 4% paraformaldehyde in 0.1 m phosphate buffer. The brains were removed, placed into 30% sucrose for 48 h, and then cut into 40 μm coronal sections using a cryostat.
c-Fos immunocytochemistry was performed as previously described (Curtis et al. 2002). Briefly, free-floating sections were washed in 0.05 m Tris-NaCl, soaked in 0.05 m Tris-NaCl containing 0.5% Triton X-100 and 10% normal goat serum for 1 h, then incubated for 20 h with a rabbit polyclonal anti-c-Fos peptide antisera (Santa Cruz Biotechnology, Inc.) diluted 1: 30 000 in 2% normal goat serum. Sections were washed in 2% normal goat serum and incubated for 2 h at room temperature with a biotinylated goat anti-rabbit antibody (Vector Laboratories) diluted 1: 300 in normal goat serum. Bound secondary antibody was amplified during a 1.5 h incubation in an avidin–biotin complex (ABC Elite Kit, Vector Laboratories). Antibody complexes were visualized using nickel-intensified diaminobenzidine (Kirkegaard and Perry Laboratories, Gaithersberg, MD, USA) and this reaction was terminated by washing the sections in 0.05 m Tris-NaCl. Sections were mounted on microscope slides and coverslipped.
Quantification of Fos immunoreactivity
Fos IR was examined in forebrain regions implicated in fluid balance including the hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei, SFO, and the organum vasculosum of the lamina terminalis (OVLT). Serial sections (40 μm) were taken from the lamina terminalis to the caudal portion of the median eminence. The largest neurons within the areas of interest have diameters of 13–19 μm (Kiss et al. 1991); therefore, every third section was processed for Fos IR to ensure that neurons occurring in consecutive sections were not counted twice. Using NIH Image software, the numbers of Fos-positive nuclei were counted in representative sections from each of these areas, based on anatomical landmarks as described by Paxinos & Watson (1997).
Three sections were taken from the SON, matched between subjects using the optic tracts as a landmark. For the PVN, the three sections that contained the largest lateral magnocellular subnucleus were taken and matched between subjects. In the cases of the SON and PVN, counts were taken from one side. Two sections were taken from the highly vascularized central portion of the SFO, and were matched between subjects. Two sections from the OVLT were matched between subjects using lateral and dorsal boundaries as described by Bisley et al. (1996). The average number of Fos-positive nuclei in each area from each animal was calculated and group means for each area in each experimental condition were determined.
In situ hybridization
The effect of Iso on AT1 mRNA expression was assessed a priori by dividing treatment groups into those treated with saline and those treated with Iso. Using a t test, it was determined that Iso did not affect AT1 mRNA expression in the SFO (P = 0.51) or PVN (P = 0.85). Therefore, results from drug conditions were pooled within groups for subsequent analysis. Ovariectomized rats were treated with OeB (n = 5) or oil (n = 6) on Day 1 and 2 and weight loss rats (n = 6) were food restricted on Day 3 as described. On Day 4, rats were deeply anaesthetized with sodium pentobarbital (75 mg (kg body wt)−1, i.p.) and decapitated. Brains were quickly removed and flash frozen in dry-ice-cooled 2-methylbutane and stored at −80°C. Frozen brains were sectioned at 20 μm using a cryostat. Serial sections were taken from the lamina terminalis to the caudal portion of the median eminence. Every third section was thaw mounted onto a slide and stored at −80°C until processing for in situ hybridization.
In situ hybridization was used to visualize AT1 receptor mRNA expression using a riboprobe transcribed from a cDNA template (nucleotides 1407–2144) from the laboratory of Dr Steven Fluharty (Kisley et al. 1999). This probe is complementary to a specific mRNA sequence and allows visualization of the product of the gene target in specific anatomical locations.
For riboprobe synthesis, 10 μl of [35S]uridine triphosphate and 2.0 μl 5 × transcription buffer, 1.0 μl of 0.1 m dithiothreitol, 1.0 μl each of 10 mm adenosine triphosphate, cytosine triphosphate and guanidine triphosphate, 2.0 μl linearized plasmid (1 μgml−1) DNA 0.5 μl RNAse inhibitor (40 units μl−1), and 1.5 μl SP6 RNA polymerase (15 units μl−1) was combined and incubated for 2 h at 37°C. DNAse (1.0 μl: RNAse-free) was added and the mixture was incubated for 15 min at room temperature. Radiolabelled probe was purified with microspin chromatography columns (Bio-Rad, Hercules, CA, USA).
Slides were removed from −80°C storage and placed in 4% paraformaldehyde at room temperature for 1 h. The slides were then washed in 2 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, three times for 5 min each. Slides were then washed in deionized water for 1 min and placed in 0.1 m triethanolamine (pH 8.0)–acetic anhydride, 400: 1 (v/v) on a stir plate, for 10 min. The final rinse was in 2 × 300 mm NaCl–30 mm sodium citrate, pH 7.2 for 5 min, followed by dehydration through graded alcohols and air-drying for 30 min. A coverslip with 60 μl of 75% formamide hybridization buffer (10% dextrane sulphate, 3 × 300 mm NaCl–30 mm sodium citrate (pH 7.2), 50 mm Na2HPO4 (pH 7.4), 10 mm dithiothreitol, 1 × Denhardt's solution (Sigma-Aldrich, St. Louis, MO, USA), 100 μg (ml)−1 yeast tRNA, and 0.01 m dithiothreitol) was placed on each slide. Strength of probe in 60 μl of hybridization buffer was approximately 1–2 million c.p.m. Slides were placed in a covered tray with filter paper saturated with 75% formamide.
After overnight incubation at 55°C, coverslips were removed and slides were placed at room temperature in 2 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, for 5 min, followed by RNAse (200 μg (ml)−1 in 10 mm Tris-HCl, pH 8.0–0.5 m NaCl) at 37°C for 30 min and then washed as follows: 2 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, at room temperature for 10 min, 1 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, for 10 min at room temperature; 0.5 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, at 55°C for 60 min; and 0.5 × 300 mm NaCl–30 mm sodium citrate, pH 7.2, for 10 min at room temperature. The slides were dehydrated in graded ethanol solutions, air dried, placed in X-ray cassettes, and apposed to Kodak XAR-5 film for 10 days.
In situ hybridization image and data analyses
Film was developed using a Mini-Medical automatic developer (AFP Imaging Corporation Elmsford, NY, USA) and then analysed with Image J 1.24 (NIH, USA). Images were analysed in representative autoradiographs from the SFO and PVN, which were matched between animals based on anatomical landmarks as described by Paxinos & Watson (1997). Tissue background readings were subtracted from grey scale values from these regions. Gray scale values were converted into optical density and subsequently averaged, providing one mean value per region, per animal. Slides contained sections 60 μm apart and counts were taken from the three consecutive sections with the greatest grey scale values, thereby generating the greatest average AT1 mRNA per region per subject. Counts taken for the SFO always contained its highly vascularized central portion and counts taken for the PVN consistently contained the lateral magnocellular subdivision.
Plasma renin activity
A separate group of ovariectomized rats were anaesthetized with sodium pentobarbital (50 mg (kg body weight)−1, i.p.) and implanted with a catheter (MRE-025 tubing; Braintree Scientific, Braintree, MA, USA) into the femoral vein. The catheter was filled with 0.15 m NaCl containing 50 U ml−1 heparin and the free end was guided subcutaneously to exit between the scapulae. Animals were given 2–3 days to recover prior to oil treatment or weight loss as described. On Day 4, water bottles were removed and ovariectomized rats were given subcutaneous injections of saline (weight loss, n = 8; oil, n = 9) or Iso (weight loss, n = 9; oil, n = 12). Thirty minutes later, rats were deeply anaesthetized with sodium pentobarbital (30 mg (kg body weight)−1, i.v.) and decapitated. Trunk blood was collected 8–12 s after anaesthesia was administered, immediately placed into chilled tubes containing EDTA (Vacutainer; Becton Dickinson, Franklin Lakes, NJ, USA), and centrifuged at 1000 g for 15 min at −4°C. The plasma was removed and stored at −80°C prior to radioimmunoassay for PRA using an Automatic Gamma Counter (Titertek Instruments, Inc., Huntsville, AL, USA) and a commercially available kit (DiaSorin, Stillwater, MI, USA).
Water intake
This experiment was designed to complement our previous study investigating the effect of OeB on water intake after Iso (Krause et al. 2003). Another group of ovariectomized rats (n = 8) were treated with weight loss or oil as described and, on Day 4, were given subcutaneous injections of Iso or saline. Rats then were given water in graduated cylinders and intakes were recorded after 2 h. All rats were tested in four conditions with 1 week between conditions. To control for order effects, the rats were randomly assigned to one of two testing sequences: (1) weight loss–Iso, oil–saline, oil–Iso, weight loss–saline; or (2) weight loss–saline, oil–Iso, oil–saline, weight loss–Iso.
Statistics
All data are expressed as means ± s.e.m. Values more than three s.d.s from the mean were considered outliers and were excluded from further analysis (n = 1). A Shapiro-Wilk test of normality showed that the ANOVA residuals for the untransformed Fos IR data were not normally distributed. Consequently, Fos IR data were log transformed; ANOVA residuals from the analysis of the transformed data met the assumption of normality per the Shaprio-Wilk test. Subsequently, differences in Fos IR were assessed with a 2-factor ANOVA with group (OeB, oil, weight loss) and drug (saline and Iso) as the factors. Main effects or interactions (P < 0.05) were analysed with a Scheffe's test for multiple comparisons (SAS 9.1, Cary, NC, USA).
The remaining data were analysed using Statistica (StatSoft, Tulsa, OK, USA). Differences in the percentage change in body weight and AT1 mRNA expression were assessed using a 1-factor analysis of variance (ANOVA) with group (oil, OeB, weight loss) as the factor. Plasma renin activities were assessed with a 2-factor ANOVA with group (oil and weight loss) and drug (saline and Iso) as the factors. Differences in water intake were assessed with a 2-factor repeated measures ANOVA with group (oil and weight loss) and drug (saline and Iso) as the within subject factors. Main effects or interactions (P < 0.05) were analysed with Student-Newman-Keuls tests.
Results
Body weights
Table 1 shows the average body weights of rats on Day 1 and Day 4 of the oestrogen replacement protocol used for Fos IR and in situ hybridization as well as the absolute and percentage change in the body weights of these groups across the 4-day protocol. As expected, there was an effect of group (F (2, 45) = 57.7, P < 0.001) on the percentage change in body weight, with OeB and weight loss groups losing weight, while the oil group gained weight. Post hoc analyses revealed similar reductions in body weight (P = 0.36) in OeB and weight loss rats and both groups weighed significantly less (P < 0.001) than oil-treated rats.
Table 1.
Body weights on Day 1 and Day 4 after oil (n = 14), OeB (n = 13), or wt loss (n = 21)
| Day 1 body wts (g) | Day 4 body wts (g) | Absolute change (g) | % change | |
|---|---|---|---|---|
| Oil | 307 ± 7.8 | 315 ± 7.5 | 7.4 ± 1.4 | 2.5 ± 0.5 |
| OeB | 311 ± 9.7 | 302 ± 9.3 | −9.3 ± 1.6 | −3.0 ± 0.5* |
| Wt loss | 300 ± 6.3 | 289 ± 5.9 | −10 ± 1.1 | −3.5 ± 0.3* |
Significantly different from oil (P < 0.001).
Isoproterenol-induced Fos immunoreactivity
Figure 1 shows the average number of Fos-positive nuclei in four hypothalamic structures after administration of saline or Iso. Fos IR after injection of saline was low in all areas and administration of Iso greatly increased Fos IR in all brain regions examined.
Figure 1. Fos-positive nuclei after injection of 0.15 m NaCl (saline) or Iso in rats treated with oil, OeB, or weight loss.
Oil–saline, n = 5; OeB–saline, n = 5; wt loss–saline, n = 5; oil–Iso, n = 9; OeB–Iso, n = 8, wt loss–Iso, n = 9. Abbreviations: paraventricular nucleus (PVN), subfornical organ (SFO), supraoptic nucleus (SON), organum vasculosum of the lamina terminalis (OVLT). *Significantly different from oil–Iso; †significantly different from oil–saline (data shown as means ± s.e.m.).
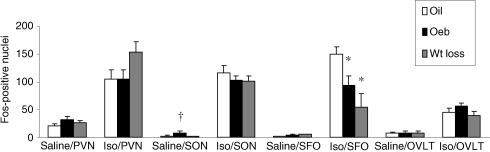
As shown in Fig. 1, the PVN had the highest levels of Fos IR after saline, which were similar in oil, OeB and weight loss groups. Isoproterenol significantly increased Fos IR in the PVN of all three groups (F (1,31) = 113, P < 0.001). Although there was a tendency for Fos IR to be greater in the weight loss group, this difference was not statistically significant (P = 0.10). Figure 2 shows photomicrographs of representative coronal sections through the PVN. The Fos IR observed after Iso in all three groups occurred mostly in the lateral magnocellular subnucleus of the PVN; however, labelling was more extensive in the weight loss group.
Figure 2. Coronal sections of PVN.
Coronal sections (40 μm) at 10 × showing Fos IR in the PVN of rats treated with: 0.15 m NaCl (A), oil and Iso (B), OeB and Iso (C), wt loss and Iso (D). Abbreviation: lateral magnocellular subnucleus (lMG). Scale bar, 250 μm.
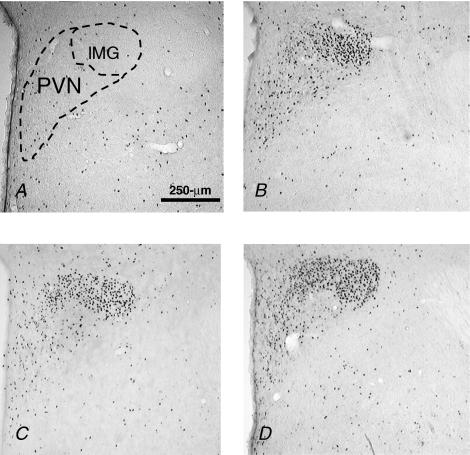
Isoproterenol significantly increased Fos IR in the SON (F (1, 31) = 490, P < 0.001) and this increase was similar in the three groups. Interestingly, there was an interaction between drug and group (F (2, 31) = 6.46, P < 0.05)) and post hoc analyses revealed that OeB rats had significantly greater Fos IR (P < 0.05) after saline than did oil-treated rats (see also Hartley et al. 2004), but were not different (P = 0.06) from weight loss rats. Figure 3 shows photomicrographs of representative coronal sections through the SON, which illustrates that Fos IR after Iso was distributed throughout the SON in all groups.
Figure 3. Coronal sections of SON.
Coronal sections (40 μm) at 10 × showing Fos IR in the SON of rats treated with: 0.15 m NaCl (A), oil and Iso (B), OeB and Iso (C) and wt loss and Iso (D). Scale bar, 250 μm.
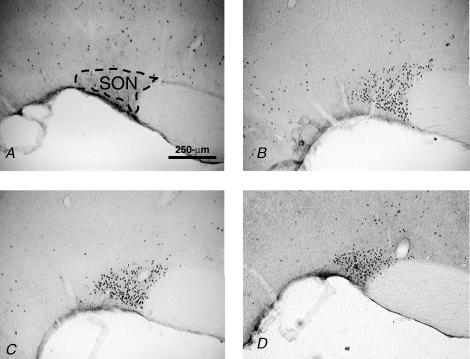
Fos IR in the SFO after saline was similar in all groups (Fig. 1). As expected, Iso significantly increased Fos IR in the SFO (F (1,33) = 114, P < 0.001). There was an interaction between group and drug (F (2,33) = 4.97, P < 0.05) and post hoc analyses revealed that OeB (P < 0.05) and weight loss (P < 0.001) rats had significantly less Fos IR in the SFO after Iso than did oil-treated rats. Wt loss and OeB-treated rats had similar Fos IR in the SFO after Iso (P = 0.097), although Fos labelling tended to be less extensive in the weight loss group, as illustrated in Fig. 4, showing photomicrographs of representative coronal sections through the SFO.
Figure 4. Coronal sections of SFO.
Coronal sections (40 μm) at 10 × showing Fos IR in the SFO of rats treated with: 0.15 m NaCl (A), oil and Iso (B), OeB and Iso (C) and wt loss and Iso (D). Scale bar, 250 μm.
Finally, Fos IR in the OVLT after saline was not different in oil, OeB or weight loss groups (Fig. 1). There was a significant effect of drug on Fos IR in the OVLT (F (1,32) = 84, P < 0.001) but no differences in Fos IR after Iso in the three groups. Figure 5 shows photomicrographs of representative coronal sections through the OVLT, which demonstrate that Fos IR was located in more lateral parts of the OVLT in all groups.
Figure 5. Coronal sections of OVLT.
Coronal sections (40 μm) at 5 × showing Fos IR in the OVLT of rats treated with: 0.15 m NaCl (A), oil and Iso (B), OeB and Iso (C) and wt loss and Iso (D). Scale bar, 250 μm.
In situ hybridization for AT1 receptor mRNA
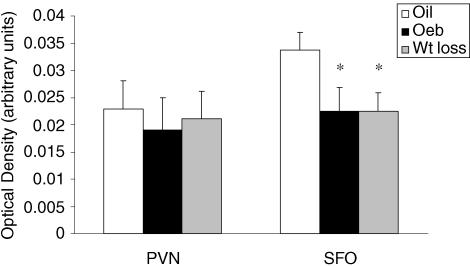
Figure 6 shows the average AT1 mRNA expression, as indicated by optical density, in the PVN and SFO. AT1 mRNA expression was similar in the PVN of oil, OeB and weight loss rats (F (2,14) = 0.1, P = 0.91); however, there was an effect of group on AT1 receptor mRNA expression in the SFO (F (2, 13) = 4.18, P < 0.05). Post hoc analyses revealed that AT1 receptor mRNA expression in the SFO was significantly less in OeB (P < 0.05) and weight loss (P < 0.05) rats than in oil-treated rats, whereas OeB and weight loss rats had similar AT1 receptor mRNA expression in the SFO (P = 0.58).
Figure 6. AT1 mRNA expression.
AT1 mRNA expression in the PVN and SFO of rats treated with oil, n = 6; OeB, n = 5; or wt loss, n = 6 (data shown as means ± s.e.m.). *, significantly different from oil.
Plasma renin activity
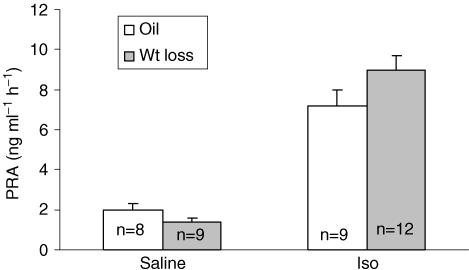
Figure 7 shows the average plasma renin activity (PRA) in oil and weight loss rats after saline or Iso. Plasma renin activity after saline was similar in oil and weight loss rats. There was a significant effect of drug on PRA (F (1,34) = 98.7, P < 0.001, Fig. 7) with greater than a fourfold increase in PRA; however, this increase was similar in the two groups.
Figure 7. Plasma renin activity.
Plasma renin activity (PRA) in oil and wt loss rats after 0.15 m NaCl or Iso (data shown as means ± s.e.m.).
Isoproterenol-induced water intake
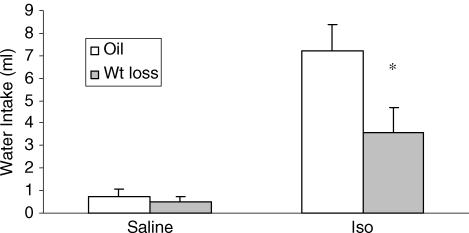
Figure 8 shows the average water intake by oil and weight loss rats after saline or Iso. There was an effect of drug (F (1,7) = 40.8, P < 0.001), with water intake after Iso significantly greater than that after saline. Interestingly, there was a significant interaction between drug and group (F (1,7) = 3.86, P < 0.05) and post hoc analyses revealed that weight loss rats drank significantly less water after Iso than did oil-treated rats (P < 0.01). When intakes were expressed per 100 g body weight, similar differences were found (data not shown).
Figure 8. Water intake.
Water intake of oil and wt loss rats after injection of 0.15 m NaCl (saline) or Iso (n = 8; within subjects design; data shown as means ± s.e.m.). *, significantly different from oil–Iso.
Discussion
The goal of the present study was to evaluate the effects of OeB and weight loss on hypothalamic structures critical for controlling behavioural and physiological responses to increased circulating Ang II. Oestrogen and weight loss rats had decreased levels of Fos IR and of AT1 mRNA in the SFO when compared with the oil-treated rats. In addition, despite similar PRAs, weight loss rats consumed about half the amount of water as did oil-treated rats after Iso, intakes that were very similar to those of OeB and oil-treated rats in our previous study (Krause et al. 2003). Taken together, these results suggest that the reductions in body weight that accompany OeB replacement contribute to OeB effects on RAS-stimulated water intake. To our knowledge, this study demonstrates for the first time that body weight loss affects central responses to endogenously generated Ang II, possibly via the AT1 receptor, and suggests specific behavioural consequences related to that activation.
Angiotensin II is a major influence in body fluid homeostasis and the β-agonist Iso greatly elevates circulating levels of this peptide (Stocker et al. 2000). Hypotension and consequent increases in circulating Ang II activate brain regions implicated in body fluid regulation (Oldfield & McKinley, 1994; Rowland et al. 1994). Therefore, it is not surprising that Iso elicited robust Fos IR in the SON, PVN, SFO and OVLT in the present study. The Fos IR in the SFO and OVLT after Iso is probably the result of circulating Ang II binding to AT1 receptors in these CVOs, as peripheral pre-treatment with AT1 receptor antagonists greatly reduces Iso-elicited Fos IR in these nuclei (Oldfield & Mckinley, 1994). On the other hand, hypotension-induced activation of the SON and PVN is believed to be largely dependent on baroreceptor signals because elimination of baroreceptor input by sinoaortic denervation reduces, but does not eliminate, Fos IR in these nuclei after hypotension (Potts et al. 1997). It has been hypothesized that RAS activation contributes to this residual Fos IR because circulating Ang II activates angiotensin-responsive neurons in the SFO and OVLT and these neurons send excitatory projections to the SON and PVN (Ferguson & Renaud, 1986). Thus, a combination of baroreceptor input and circulating Ang II probably contribute to the Fos IR we observed in the SON and PVN after hypotension.
Although we did not examine the phenotype of Fos-positive neurons in this study, insight can be gained from the pattern of activation. After Iso, Fos IR in the OVLT was confined to the lateral margins, the region that contains the greatest density of AT1 receptors. This pattern of Fos IR in the OVLT is suggestive of Ang II-mediated thirst rather than osmotically driven thirst, which produces Fos IR in the dorsal cap (Oldfield et al. 1994). Similarly, the distribution of Fos IR in the PVN provides information about the function of neurons activated by Iso. In all three groups, Iso elicited robust Fos IR that was most prevalent in the lateral magnocellular subnucleus of the PVN. Many neurons within this region are neurosecretory neurons that release vasopressin into systemic circulation via the posterior pituitary. Circulating vasopressin acts on the kidney to promote water conservation and on peripheral vasculature to produce vasoconstriction, which are compensatory responses to volume loss or hypotension. Vasopressin release is modulated by baroreceptor input (Potts et al. 1997) as well as by Ang II (Lee et al. 1995). Thus, the robust activation we observed in the lateral magnocellular subdivision of the PVN and in the neurosecretory neurons of the SON is consistent with Iso-induced hypotension and stimulation of the RAS.
In the PVN, there was a tendency for Iso-induced Fos IR to be elevated in the weight loss rats, although the difference was not statistically significant (P = 0.10). It is possible that this trend was due to stress caused by food restriction, because experimental procedures that limit food availability elevate basal levels of plasma corticosterone (Kiss et al. 1994). Circulating Ang II activates neurons in the PVN that project to the median eminence (Ferguson, 1988), including neurons that contain corticotropin releasing hormone. Thus, it is possible that food restriction combined with Iso may be sufficiently stressful to activate corticotropin releasing hormone-containing neurons in the PVN of weight loss rats; however, additional experiments will be necessary to address this issue.
In our study, OeB selectively decreased Fos IR in the SFO after Iso. These results conflict with those of another study in which central Ang II administration did not decrease Fos in the SFO of OeB-treated rats but did increase Fos in the PVN (Kisley et al. 2000). It is likely that methodological differences explain why we did not see increased Fos IR in the PVN of our OeB-treated rats but, interestingly, Kisley and colleagues had predicted that OeB would attenuate Fos IR in the SFO after Ang II because their previous work showed that OeB decreased Ang II binding to AT1 receptors in the SFO (Kisley et al. 1999). Thus, our study examining Fos IR elicited by endogenously generated Ang II may have revealed the effect that the authors of this previous study proposed.
It is interesting that OeB and weight loss selectively decreased Iso-elicited Fos IR and AT1 receptor mRNA in the SFO. Earlier studies demonstrated that oestrogen decreases neural activity and AT1 receptor binding in the SFO (Kisley et al. 1999; Tanaka et al. 2001); however, to our knowledge, ours is the first study to demonstrate that weight loss also produces similar effects. This reduction in AT1 receptor mRNA probably underlies the blunted Iso-elicited Fos IR that we observed in both OeB and weight loss rats.
Consistent with this idea, Iso produced similar increases in PRA, an accurate indicator of circulating Ang II (Stocker et al. 2000), in weight loss and oil-treated rats. Moreover, comparisons with our previous study (Krause et al. 2003) indicate percentage increases from basal PRA after Iso in OeB (421%) and oil- (288%) treated rats similar to those in the present study (oil, 260%; weight loss, 543%). PRA commonly is used as an indicator of plasma Ang II levels; however, it is a measurement of the amount of angiotensin I generated during a set period of time, and not the quantity of Ang II produced. Therefore, OeB or weight loss may interfere with other components of the biosynthetic pathway, such as angiotensin converting enzyme, and result in different levels of circulating Ang II. In this regard, increased basal PRAs have been reported in rats (e.g. Katayama & Lee, 1985) and humans (e.g. Krakoff, 1973; Pallas et al. 1977) with oestrogen replacement; however, these studies employed long-term oestrogen replacement or used very high oestrogen doses. Thus, methodological differences may explain the discrepancy between the results of these studies and our previous study in which we did not observe an effect of oestrogen on basal PRA in rats (Krause et al. 2003). In any case, it is clear that the attenuation of Iso-elicited Fos IR we observed in the SFO is not due to decreased PRA after OeB or weight loss. Thus, we suggest that OeB and weight loss inhibit SFO responses to circulating Ang II by down-regulating the expression of the AT1 receptor.
Site-specific injection of AT1 receptor antagonists into the SFO decreases Iso-elicited water intake (Fitts, 1994); therefore, it would be expected that reductions in AT1 receptor mRNA in the SFO would be accompanied by an attenuation of Iso-elicited water intake as was observed in the present study. Weight loss rats, which had decreased AT1 mRNA in the SFO, drank significantly less water after Iso (3.6 ± 1.1 ml) than did oil-treated rats (7.2 ± 1.2 ml) and these intakes were very similar to those observed in our previous study (Krause et al. 2003) that examined the effects of OeB on water intake after Iso (oil, 6.0 ± 0.5 ml; OeB, 2.8 ± 0.8 ml). Thus, central and behavioural responses to RAS activation are very similar in OeB and weight loss rats. We cannot rule out the possibility that the acute nature of the weight loss after food restriction may affect responses to circulating Ang II by different mechanisms than does the more gradual weight loss that accompanies OeB replacement. Nonetheless, taken together, the obtained data suggest that weight loss contributes to OeB attenuation of Ang II-stimulated water intake and central activation.
It is possible that OeB and weight loss have similar effects on the central and behavioural responses to circulating Ang II, albeit through different mechanisms. Although we did not phenotype Fos-positive cells in this study, it is possible that Iso activated different populations of cells in the SFO of OeB and weight loss rats. The SFO contains cholinergic receptors and activation of these receptors by direct injection of carbachol also stimulates drinking (Mangiapane & Simpson, 1983). Moreover, the SFO is responsive to other circulating factors that initiate water consumption such as amylin (Riediger et al. 1999). However, the nature of our stimulus and the fact that both OeB and weight loss rats had attenuated AT1 mRNA in the SFO make these possibilities unlikely.
Although we propose that weight loss is an important contributing factor for the observed effects, an intriguing alternative explanation focuses on weight gain. In the rat, ovariectomy is associated with hyperphagia that results in 12–16% increase in body weight over 5 weeks and much of this weight gain is due to increased adipose tissue (McElroy & Wade, 1987). Obese post-menopausal women have elevated blood pressure that is greatly attenuated by a 5% weight loss (Engeli et al. 2005). Additionally, the hypertension that is observed in obese rats is reversed by administration of AT1 receptor antagonists (Boustany et al. 2005). These studies suggest that weight gain enhances cardiovascular responses to circulating Ang II. In the present study, oil-treated rats, which increased body weight by 2.5%, had greater Fos IR in the SFO and augmented water intakes relative to ovariectomized rats that ate less and lost weight. Moreover, the weight gain that occurs after ovariectomy did not affect basal PRA, which is consistent with studies examining the effect of obesity on PRA (Faloia et al. 2002; Becker et al. 2003). Thus, weight gain also may enhance the central and behavioural responses to circulating Ang II.
Interestingly, Becker et al. (2003) reported that obese rats had a 100% increase in AT1 receptors in the proximal tubule of the kidney and suggested that up-regulation of these receptors may contribute to the observed hypertension by increasing water and sodium reabsorption. In this regard, the onset of hypertension is accelerated in obese rats given access to NaCl solutions, implicating a behavioural component to the high blood pressure that is observed in obese rats (Dobrian et al. 2003). In our study, oil-treated rats had greater AT1 mRNA in the SFO relative to ovariectomized rats that lost weight, suggesting that increased body weight also may up-regulate AT1 receptors in the brain. Given the role of AT1 receptors in the SFO to promote the ingestion of water and sodium (see Fitzsimons, 1998, for review), it seems likely that increased AT1 receptors in the SFO may further exacerbate hypertension in obese subjects by augmenting water and sodium intake. In other words, weight gain may result in receptor-mediated enhancement of renal and behavioural responses to Ang II with deleterious consequences for cardiovascular health.
The mechanism responsible for modulation of AT1 receptors by changes in body weight is unknown. However, several components of the RAS are present in adipose tissue (Karlsson et al. 1998), suggesting a role for adipose RAS in obesity-related hypertension. In fact, angiotensinogen in circulation and in adipose tissue is elevated in obese hypertensive rats (Boustany et al. 2004). Additionally, obese hypertensive post-menopausal women have elevated circulating angiotensinogen (Engeli et al. 2005). Recent work by Kurdi et al. (2005) showed that components of the RAS are mediators of gene expression, an observation that may provide insights into the mechanism underlying body weight effects on the regulation of AT1 receptors.
In summary, the current study examined the effect of OeB or weight loss on central responses to increased circulating Ang II. Both oestrogen and weight loss decreased the responsiveness of SFO neurons to circulating Ang II, as indicated by decreased Fos IR, and selectively reduced the expression of AT1 mRNA in the SFO. Similar to our previous studies of oestrogen effects on RAS-stimulated water intake (Krause et al. 2003), weight loss also reduced water intake elicited by Iso but did not affect stimulated PRAs. Thus, we propose that the weight loss that accompanies OeB replacement in ovariectomized rats contributes to OeB effects on RAS-stimulated water intake via down-regulation of AT1 receptors in the SFO.
Acknowledgments
We thank Lisa Eckel, Frank Johnson and Zuoxin Wang for their valuable advice and assistance. This work was supported by NIH grants DK063754 (E.G.K.), DC006360 (K.S.C.), T32 NS07437 (T.L.S.) and DC04785 (R.J.C.).
References
- Becker M, Umrani D, Lokhandwala MF, Hussain T. Increased renal angiotensin II AT1 receptor function in obese Zucker rat. Clin Exp Hypertens. 2003;25:35–47. doi: 10.1081/ceh-120017739. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Rees SM, McKinley MJ, Hards DK, Oldfield BJ. Identification of osmoresponsive neurons in the forebrain of the rat: a Fos study at the ultrastructural level. Brain Res. 1996;720:25–34. doi: 10.1016/0006-8993(96)00079-0. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;28:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- Boustany CM, Brown DR, Randall DC, Cassis LA. AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R181–R186. doi: 10.1152/ajpregu.00507.2004. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80:657–664. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Krause EG, Contreras RJ. Fos expression in non-catecholaminergic neurons in medullary and pontine nuclei after volume depletion induced by polyethylene glycol. Brain Res. 2002;948:149–154. doi: 10.1016/s0006-8993(02)03051-2. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ. Oestrogen increases the taste threshold for sucrose in rats. Physiol Behav. 2005;86:281–286. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol. 2003;285:F619–F628. doi: 10.1152/ajprenal.00388.2002. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- Faloia E, Gatti C, Camilloni MA, Mariniello B, Sardu C, Garrapa GG, Mantero F, Giacchetti G. Comparison of circulating and local adipose tissue renin-angiotensin system in normotensive and hypertensive obese subjects. J Endocrinol Invest. 2002;25:309–314. doi: 10.1007/BF03344010. [DOI] [PubMed] [Google Scholar]
- Ferguson AV. Systemic angiotensin acts at the subfornical organ to control the activity of paraventricular nucleus neurons with identified projections to the median eminence. Neuroendocrinol. 1988;47:489–497. doi: 10.1159/000124960. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Renaud LP. Systemic angiotensin acts at subfornical organ to facilitate activity of neurohypophysial neurons. Am J Physiol. 1986;251:R712–R717. doi: 10.1152/ajpregu.1986.251.4.R712. [DOI] [PubMed] [Google Scholar]
- Findlay AL, Fitzsimons JT, Kucharczyk J. Dependence of spontaneous and angiotensin-induced drinking in the rat upon the oestrous cycle and ovarian hormones. J Endocrinol. 1979;82:215–225. doi: 10.1677/joe.0.0820215. [DOI] [PubMed] [Google Scholar]
- Fitts DA. Angiotensin II receptors in SFO but not in OVLT mediate isoproterenol-induced thirst. Am J Physiol. 1994;267:R7–R15. doi: 10.1152/ajpregu.1994.267.1.R7. [DOI] [PubMed] [Google Scholar]
- Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- Fregly MJ, Thrasher TN. Attenuation of angiotensin-induced water intake in oestrogen-treated rats. Pharmacol Biochem Behav. 1978;9:509–514. doi: 10.1016/0091-3057(78)90050-3. [DOI] [PubMed] [Google Scholar]
- Gragasin FS, Xu Y, Arenas IA, Kainth N, Davidge ST. Estrogen reduces angiotensin II-induced nitric oxide synthase and NAD(P)H oxidase expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:38–44. doi: 10.1161/01.atv.0000047868.93732.b7. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Dickson SL, Forsling ML. Plasma vasopressin concentrations and Fos protein expression in the supraoptic nucleus following osmotic stimulation or hypovolaemia in the ovariectomized rat: effect of oestradiol replacement. J Neuroendocrinol. 2004;16:191–197. doi: 10.1111/j.0953-8194.2004.01150.x. [DOI] [PubMed] [Google Scholar]
- Imanishi T, Hano T, Nishio I. Estrogen reduces angiotensin II-induced acceleration of senescence in endothelial progenitor cells. Hypertens Res. 2005;28:263–271. doi: 10.1291/hypres.28.263. [DOI] [PubMed] [Google Scholar]
- Jonklaas J, Buggy J. Angiotensin–estrogen interaction in female brain reduces drinking and pressor responses. Am J Physiol. 1984;247:R167–R172. doi: 10.1152/ajpregu.1984.247.1.R167. [DOI] [PubMed] [Google Scholar]
- Karlsson C, Lindell K, Ottosson M, Sjostrom L, Carlsson B, Carlsson LM. Human adipose tissue expresses angiotensinogen and enzymes required for its conversion to angiotensin II. J Clin Endocrinol Metab. 1998;83:3925–3929. doi: 10.1210/jcem.83.11.5276. [DOI] [PubMed] [Google Scholar]
- Katayama S, Lee JB. Estradiol stimulates rat renopapillary prostaglandin E2 (PGE2), but not PGF2 alpha biosynthesis. Endocrinol. 1985;117:656–661. doi: 10.1210/endo-117-2-656. [DOI] [PubMed] [Google Scholar]
- Kirby RF, Novak CM, Thunhorst RL, Johnson AK. The role of beta1 and beta2 adrenoceptors in isoproterenol-induced drinking. Brain Res. 1994;656:79–84. doi: 10.1016/0006-8993(94)91368-4. [DOI] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Flanagan-Cato LM, Fluharty SJ. Estrogen increases angiotensin II-induced c-Fos expression in the vasopressinergic neurons of the paraventricular nucleus in the female rat. Neuroendocrinol. 2000;72:306–317. doi: 10.1159/000054599. [DOI] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999;844:34–42. doi: 10.1016/s0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol. 1999;276:R90–R96. doi: 10.1152/ajpregu.1999.276.1.R90. [DOI] [PubMed] [Google Scholar]
- Kiss A, Jezova D, Aguilera G. Activity of the hypothalamic pituitary adrenal axis and sympathoadrenal system during food and water deprivation in the rat. Brain Res. 1994;663:84–92. doi: 10.1016/0006-8993(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Martos J, Palkovits M. Hypothalamic paraventricular nucleus: a quantitative analysis of cytoarchitectonic subdivisions in the rat. J Comp Neurol. 1991;313:563–573. doi: 10.1002/cne.903130403. [DOI] [PubMed] [Google Scholar]
- Krakoff LR. Measurement of plasma renin substrate by radioimmunoassay of angiotensin I. Concentration in syndromes associated with steroid excess. J Clin Endocrinol Metab. 1973;37:110–117. doi: 10.1210/jcem-37-1-110. [DOI] [PubMed] [Google Scholar]
- Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;79:267–274. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- Kurdi M, De Mello WC, Booz GW. Working outside the system: an update on the unconventional behavior of the renin-angiotensin system components. Int J Biochem Cell Biol. 2005;37:1357–1367. doi: 10.1016/j.biocel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Yang EK, Ahn DK, Park YY, Park JS, Kim HJ. Central ANG II-receptor antagonists impair cardiovascular and vasopressin response to hemorrhage in rats. Am J Physiol. 1995;268:R1500–R1506. doi: 10.1152/ajpregu.1995.268.6.R1500. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Coirini H, Schumaker M, Pfaff DW, McEwen BS, Schwartz-Giblin S. Ovarian steroid modulation of [3H] muscimol binding in the spinal cord of the rat. Brain Res. 1991;556:321–323. doi: 10.1016/0006-8993(91)90323-n. [DOI] [PubMed] [Google Scholar]
- McElroy JF, Wade GN. Short- and long-term effects of ovariectomy on food intake, body weight, carcass composition, and brown adipose tissue in rats. Physiol Behav. 1987;39:361–365. doi: 10.1016/0031-9384(87)90235-6. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:1–122. doi: 10.1007/978-3-642-55532-9. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Simpson JB. Drinking and pressor responses after acetylcholine injection into subfornical organ. Am J Physiol. 1983;244:R508–R513. doi: 10.1152/ajpregu.1983.244.4.R508. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neurosci. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, McKinley MJ. Distribution of Fos in rat brain resulting from endogenously-generated angiotensin II. Kidney Int. 1994;46:1567–1569. doi: 10.1038/ki.1994.448. [DOI] [PubMed] [Google Scholar]
- Pallas KG, Holwwarth GJ, Stern MP, Lucas CP. The effect of conjugated estrogens on the renin-angiotensin system. J Clin Endocrinol Metab. 1977;44:1061–1068. doi: 10.1210/jcem-44-6-1061. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereo-Taxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Potts PD, Polson JW, Hirooka Y, Dampney RA. Effects of sinoaortic denervation on Fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neurosci. 1997;77:503–520. doi: 10.1016/s0306-4522(96)00459-9. [DOI] [PubMed] [Google Scholar]
- Rettig R, Ganten D, Johnson AK. Isoproterenol-induced thirst: renal and extrarenal mechanisms. Am J Physiol. 1981;241:R152–R157. doi: 10.1152/ajpregu.1981.241.3.R152. [DOI] [PubMed] [Google Scholar]
- Riediger T, Rauch M, Schmid HA. Actions of amylin on subfornical organ neurons and on drinking behavior in rats. Am J Physiol. 1999;276:R514–R521. doi: 10.1152/ajpregu.1999.276.2.R514. [DOI] [PubMed] [Google Scholar]
- Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–262. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Fregly MJ, Li BH, Smith GC. Action of angiotensin converting enzyme inhibitors in rat brain: interaction with isoproterenol assessed by Fos immunocytochemistry. Brain Res. 1994;654:34–40. doi: 10.1016/0006-8993(94)91568-7. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–494. doi: 10.1037//0735-7044.105.3.487. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Sved AF, Stricker EM. Role of renin-angiotensin system in hypotension-evoked thirst: studies with hydralazine. Am J Physiol Regul Integr Comp Physiol. 2000;279:R576–R585. doi: 10.1152/ajpregu.2000.279.2.R576. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Miyakubo H, Fujisawa S, Nomura M. Reduced dipsogenic response induced by angiotensin II activation of subfornical organ projections to the median preoptic nucleus in estrogen-treated rats. Exp Neurol. 2003;179:83–89. doi: 10.1006/exnr.2002.8054. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Miyakubo H, Okumura T, Sakamaki K, Hayashi Y. Estrogen decreases the responsiveness of subfornical organ neurons projecting to the hypothalamic paraventricular nucleus to angiotensin II in female rats. Neurosci Lett. 2001;307:155–158. doi: 10.1016/s0304-3940(01)01940-1. [DOI] [PubMed] [Google Scholar]