Abstract
Ang II directly activates neurones in sympathetic ganglia. Our goal was to define the electrophysiological basis of this activation. Neurones from mouse aortic–renal and coeliac ganglia were identified as either ‘tonic’ or ‘phasic’. With injections of depolarizing currents, action potentials (APs) were abundant and sustained in tonic neurones (TNs) and scarce or absent in phasic neurones (PNs). Resting membrane potentials were equivalent in TNs (−48 ± 2 mV, n = 18) and PNs (−48 ± 1 mV, n = 23) while membrane resistance was significantly higher in TNs. Ang II depolarized and increased membrane resistance equally in both TNs (n = 8) and PNs (n = 8) but it induced APs only in TNs, and enhanced current-evoked APs much more markedly in TNs (P < 0.05). The AT1 receptor antagonist losartan (2 μm, n = 6) abolished all responses to Ang II, whereas the AT2 receptor blocker PD123,319 had no effect. The transient K+ current (IA), which was more than twice as large in TNs as in PNs, was significantly inhibited by Ang II in TNs only whereas the delayed sustained K+ current (IK), which was comparable in both TNs and PNs, was not inhibited. M currents were more prominent in PNs and were inhibited by Ang II. The IA channel blocker 4-aminopyridine triggered AP generation in TNs and prevented the Ang II-induced APs but not the depolarization. Blockade of M currents by oxotremorine M or linopirdine prevented the depolarizing action of Ang II. The protein kinase C (PKC) inhibitor H7 (10 μm, n = 9) also prevented the Ang II-induced inhibition of IA and the generation APs but not the depolarization nor the inhibition of M currents. Conversely, the PKC agonist phorbol 12-myristate 13-acetate mimicked the Ang II effects by triggering APs. The results indicate that Ang II may increase AP generation in sympathetic neurones by inducing a PKC-dependent inhibition of IA currents, and a PKC-independent depolarization through inhibition of M currents. The differential expression of various K+ channels and their sensitivity to phosphorylation by PKC may determine the degree of activation of sympathetic neurones and hence may influence the severity of the hypertensive response.
Angiotensin (Ang II) has a powerful influence on the circulation by acting at multiple sites in the peripheral and central nervous systems to increase sympathetic nerve activity (Aiken & Reit, 1968; Reid, 1992; Ferguson & Bains, 1997; Ma et al. 2001a; Dendorfer et al. 2002). We recently demonstrated that intravenous administration of Ang II in mice evokes low-amplitude, continuous action potential (AP) firing in postganglionic renal sympathetic nerves that persists after ganglionic blockade with hexamethonium suggesting a direct action of Ang II on neurones in sympathetic ganglia (Ma et al. 2001a). Such an action is supported by earlier findings that sympathetic neurones contain Ang II AT1 receptors (Castren et al. 1987; Stromberg et al. 1991) and are depolarized by Ang II (Dun et al. 1978; Brown et al. 1980).
Our understanding of the electrophysiological basis for excitatory effects of Ang II on sympathetic neurones is incomplete. Ang II-induced depolarization may be caused by either increased sodium (Na+) conductance (Dun et al. 1978) or decreased potassium (K+) conductance (Brown et al. 1980). Although inhibition of the K+ channel M-current is a likely mechanism of Ang II-induced depolarization (Brown et al. 1980; Shapiro et al. 1994), other types of K+ channels may also play a role. Ang II could also increase AP firing by reducing the threshold for AP generation through effects on voltage-dependent channels (i.e. by increasing excitability). In the nervous system, the transient K+ currents play an important role in the regulation of membrane excitability (Cull-Candy et al. 1989; Sheng et al. 1993; Mei et al. 1995). To our knowledge there are no reports of the effects of Ang II on the excitability of sympathetic neurones.
We recently found that Ang II increases cytosolic calcium (Ca2+) concentration in a subpopulation of postganglionic sympathetic neurones isolated from mouse aortic–renal and coeliac ganglia (Ma et al. 2001b). The failure of many neurones to respond to Ang II suggested that subtypes of sympathetic neurones may respond differentially to Ang II. Two major subtypes of sympathetic postganglionic neurones have been described based on distinct differences in AP firing patterns during injection of depolarizing current (Weems & Szurszewski, 1978; Decktor & Weems, 1981, 1983; Cassell et al. 1986; Zhao et al. 1995; Wang & McKinnon, 1995; Jobling & Gibbins, 1999; Malin & Nerbonne, 2000, 2001). ‘Tonic’ sympathetic neurones generate repetitive APs that are sustained throughout the period of depolarizing current injection. In contrast, ‘phasic’ sympathetic neurones respond with only a single spike or a few APs at the beginning of the period of depolarization, even during high levels of current injection.
The goals of the present study were to test the hypothesis that tonic and phasic sympathetic neurones respond differently to Ang II and to characterize the electrophysiological basis for any differential responses.
Methods
Cell culture
Primary cultures of sympathetic neurones were prepared from aortic–renal and coeliac ganglia of adult C57BL/6J mice (25–30 g) (Harlan, Madison, WI, USA). Animal handling followed the guidelines of the American Physiological Society and the experimental protocol was approved by the Animal Care and Use Committee of the University of Iowa.
Mice were anaesthetized with pentobarbital sodium (60 mg kg−1, i.p.). The left kidney was exposed retroperitoneally through a left flank incision and the left aortic–renal and coeliac ganglia were isolated from the connective tissue and excised for dissociation and cell culture (Li et al. 1998; Ma et al. 2001b). After removal of the ganglia, the mice were killed by an intraperitoneal injection of pentobarbital sodium (∼260 mg kg−1). Because of their larger size, coeliac ganglia were first placed in ice-cold L-15 medium and then transferred to an enzymatic medium composed of modified Leibowitz L-15 medium containing collagenase (type 4, 1 mg ml−1), trypsin (type 2X, 1 mg ml−1), and DNase (type 1, 0.1 mg ml−1) (Worthington, NJ, USA) where they were minced with a surgical blade. Both aortic–renal ganglia and the minced coeliac ganglia were incubated in this enzyme medium for 55 min at 37°C. The enzymatic digestion was terminated by adding soybean trypsin inhibitor (2 mg ml−1), 3 mm CaCl2, and bovine serum albumin (1 mg ml−1). After gentle trituration, the tissue fragments were centrifuged at 800 r.p.m. for 5 min and then resuspended in modified L-15 medium with 5% rat serum and 2% chick embryo extract (Life Technologies, NY, USA). The cells were plated on poly l-lysine coated glass coverslips and incubated overnight at 37°C prior to the studies.
Intracellular recordings
The membrane potential of isolated neurones was measured with sharp microelectrodes as previously described (Snitsarev et al. 2002). The coverslip was placed in a recording chamber, maintained at 37°C, and superfused at 2 ml min−1 with physiological solution containing (mm): NaCl 116, KCl 5.4, NaH2PO4 1.0, MgSO4 0.8, MgCl2 1.0, CaCl2 1.8, glucose 10, NaHCO3 24. The solution was gassed with 95% oxygen and 5% carbon dioxide (300 mosmol l−1, pH 7.4). The neurone under study was impaled with a glass microelectrode (resistance, 100–200 MΩ) filled with KCl (1 m). The microelectrode was connected to an Axoprobe-1A amplifier (Axon Instruments, CA, USA) to allow compensation for microelectrode resistance and passage of hyperpolarizing and depolarizing currents. A neurone was considered to be successfully impaled if the recorded potential showed an abrupt deflection more negative than −35 mV, remained stable, and exhibited AP discharge upon injection of depolarizing current. Action potentials were defined as rapid upward deflections of the intracellular voltage exceeding 0 mV.
After a stabilization period of at least 5 min, membrane properties and classification of neurones were determined as follows: (1) membrane potential was measured and hyperpolarizing current pulses (−100 pA, 100 ms) were applied at 10 s intervals using a Grass S88 stimulator (Grass Medical Instruments, Quincy, MA, USA) to enable calculation of membrane resistance; (2) the threshold for generation of a single AP was determined by brief injections of variable levels of depolarizing currents (20–500 pA, 5 ms), (3) tonic and phasic neurones were classified on the basis of the pattern of AP discharge during sustained depolarizing current injections of 100–500 pA for ∼800 ms. Neurones generating a continuous, repetitive discharge of APs throughout the period of injection of a suprathreshold depolarizing current (3–5 × threshold) were classified as tonic neurones. Neurones that generated only one to five APs at the beginning of the period of depolarization despite the sustained current injection were defined as phasic neurones. Pharmacological agents were administered into the recording chamber as described in the experimental protocol section. Data were processed and recorded (sampling frequency 10 kHz) using an Axoprobe-1A amplifier interfaced with a personal computer and pCLAMP 9.0 (Axon Instruments). All experiments were performed at 37°C.
Patch clamp recordings
K+-currents
Voltage-gated K+ currents were recorded using the whole-cell patch clamp technique (Li et al. 1998; Bielefeldt et al. 1999) with an Axopatch 200A amplifier (Axon Instruments) interfaced with a personal computer. The cells plated onto coverslips were transferred into a recording chamber with bath solution containing (mm): NaCl 145, KCl 5.4, MgCl2 1, CaCl2 2, glucose 5.6, Hepes 10, adjusted with NaOH to pH 7.35. Cells were maintained at room temperature, and continuously superfused at a rate of ∼2 ml min−1. The patch pipettes were pulled from borosilicate glass (PG52151-4, World Precision Instruments, FL, USA) and fire-polished. Pipette tips had resistances of 1–3 MΩ after filling with pipette solution containing (mm): KCl 140, MgCl2 5, Mg-ATP 3, Na-GTP 0.1, Hepes 5, BAPTA 0.1, adjusted with KOH to pH 7.2. In some of the recordings, perforated patch clamp recording was performed by adding nystatin (150 μg ml−1) into the pipette solution. Osmolarity of the solutions was measured with a freezing point depression osmometer (Osmette, Precision System, Natick, MA, USA) and adjusted to 300 ± 5 mosmol l−1 with mannitol. The Ag–AgCl pellet was used as a reference electrode. The offset potential between pipette and bath solution was adjusted to zero prior to seal formation. After establishment of whole-cell recording configuration, the current-clamp recordings were performed to identify the cell firing patterns. Once the firing pattern was identified, TTX (0.5 μm) and CdCl2 (100 μm) were added into the bath solution. The membrane capacitance and resistance were compensated and the whole-cell currents were recorded in voltage-clamp mode. Current recordings were filtered at 2 kHz with a 4 pole Bessel filter and digitized at 10 kHz using a Digidata 1200 interface (Axon Instruments). The pCLAMP 9 was used for voltage protocols, data acquisition, and data analysis. The P/N protocol was used for online subtraction of leak currents and capacitance artifacts.
A delayed sustained potassium (K+) current (IK) was elicited by depolarizing voltage pulses for 200 ms between −50 mV and 40 mV in 10 mV increment from a holding potential of −50 mV. An additional transient K+ current (IA) was elicited when the depolarizing steps were preceded by a 1 s hyperpolarizing prepulse of −100 mV. Currents digitally subtracted at holding potential of −50 mV from −100 mV were analysed as IA (Carrier, 1995) and confirmed by inhibition with 4-aminopyridine (4-AP, 5 mm). IK was averaged for 20 ms at the end of the depolarizing pulses from holding potential of −50 mV and was confirmed by inhibition with tetraethylammonium (TEA, 10 mm).
M current was activated by depolarization to −30 mV and deactivated by membrane hyperpolarization to −60 mV (Shapiro et al. 1994). Membrane potential was held at −30 mV, stepped every 10 s to −60 mV for 1 s, prior to stepping back to −30 mV. M current was analysed as a slowly developing inward deactivation relaxation. The amplitude of M currents was measured as the difference between current amplitudes 20 ms after the onset of hyperpolarization and 20 ms before re-depolarization.
Membrane potential
In another group of experiments, membrane potential and AP discharge were recorded in current clamp configuration with the whole-cell patch clamp technique. The bath solution contained (mm): NaCl 140, KCl 5, MgCl2 2, CaCl2 2, glucose 5, Hepes 20, buffered with NaOH to a pH of 7.35. The pipette solution contained (mm): KCl 140, MgCl2 1, CaCl2 1, Na-ATP 1, Hepes 10, EDTA 10, buffered with KOH to a pH of 7.2. The general information was described as above.
Experimental protocols
Effects of Ang II on sympathetic neurones under resting conditions
Ang II (20–200 nm) was administered into the recording chamber for 3–5 min followed by washout of Ang II to investigate its effects on membrane potential, membrane resistance and AP discharge. The peak frequency and duration of AP discharge evoked by Ang II were measured. Since responses to Ang II decreased rapidly upon repeated administrations we applied Ang II only once per coverslip in the majority of the protocols.
Effects of Ang II on membrane excitability of sympathetic neurones
Membrane excitability was determined by measuring the number and the duration of repetitive AP firing during depolarizing current injections. Neurones were held at −60 mV and APs were recorded during injection of increasing levels of depolarizing currents ranging from 100 to 500 pA (up to ∼3–5 × threshold) and maintained for ∼800 ms. The protocol was repeated in the presence of Ang II (200 nm) and after washout of Ang II.
Roles of AT1 and AT2 receptors
To determine the roles of AT1 and AT2 receptors in mediating the electrophysiological effects of Ang II, the selective AT1 receptor antagonist losartan (2 μm) or the AT2 receptor antagonist PD123,319 (4 μm) was added to the bath 3 min prior to administration of 200 nm Ang II.
Effects of Ang II on voltage-gated K+ currents in sympathetic neurones
To define the ionic mechanisms of Ang II-induced activation of sympathetic neurones, IA, IK and M currents were measured before and after application of Ang II (200 nm).
Role of K+ channels
To confirm the role of IA and M currents in Ang II-induced effects, neurones were pretreated for 5–10 min with IA channel blocker 4-AP (5 mm) and M channel inhibitor oxotremorine M (10 μm) or its blocker linopirdine (10 μm) before application of Ang II (200 nm). Membrane potential and AP discharge were recorded with the patch clamp technique.
Role of protein kinase C
To determine whether activation of protein kinase C (PKC) mediates electrophysiological responses of sympathetic neurones to Ang II, neurones were pretreated with PKC inhibitors (H7, 10 μm or bisindolylmaleimide, 1 μm) for 3–5 min before the addition of 200 nm Ang II. The effect of PKC agonist phorbol 12-myristate 13-acetate (PMA, 2.5 μm) was also tested.
Data analysis
Group data are expressed as means ± s.e.m. Student's paired and unpaired t test was used to analyse responses to a single intervention and differences between two groups, respectively. For protocols involving multiple comparisons, the data were analysed by ANOVA followed by Newman-Keuls post hoc test. The statistical analysis was performed using GB-STAT 9.0 software (Dynamic Microsystems, Inc., MD, USA). Statistical significance of differences was defined at P < 0.05.
Chemicals
All chemicals were analysis grade and were purchased from Sigma (St Louis, MO, USA). Losartan was provided by Merck & Co., Inc. (Rahway, NJ, USA).
Results
Membrane properties of tonic and phasic sympathetic neurones
After overnight culture, neurones could easily be identified as round cells (20–30 μm diameter) with a distinct nucleus and nucleolus. Eighteen of 44 neurones (41%) were classified as tonic neurones and 23 (52%) were classified as phasic neurones based on their response to current injections as described earlier (Fig. 1A). The remaining three neurones (7%) exhibited firing patterns between phasic and tonic and were excluded from further data analysis.
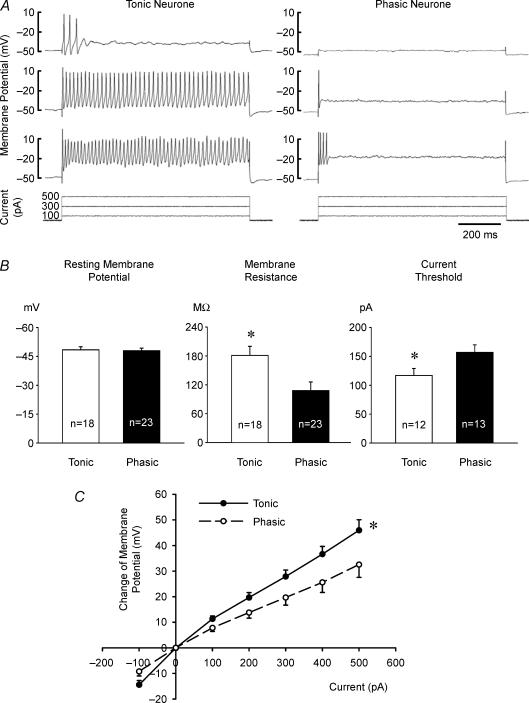
Figure 1. Electrophysiological characteristics of sympathetic neurones.
A, action potential responses of a tonic (left panel) and a phasic neurone (right panel) to three levels of depolarizing current injections (100, 300 and 500 pA). The tonic neurone was able to produce a sustained train of action potentials throughout the period of suprathreshold current injection. In contrast, the phasic neurone fired action potentials very transiently and only in response to a large current injection (500 pA). B, group data showing the electrophysiological properties of tonic (open bars) and phasic (filled bars) neurones. The resting membrane potential of tonic and phasic neurones was not significantly different. However, tonic neurones had higher membrane resistance and lower current threshold needed to evoke one action potential than phasic neurones (*P < 0.05 tonic versus phasic neurones). C, the relationship between the amount of hyperpolarizing and depolarizing current injected and the change in membrane potential. Tonic neurones (n = 15) depolarized to a greater extent than phasic neurones (n = 20) for equivalent current injections (*P < 0.05 tonic versus phasic neurones). To minimize the influence of action potentials (Fig. 1A) on measuring membrane potential, action potentials were filtered out and the membrane potential was then measured and averaged over the entire ∼800 ms period of current injection. Data are expressed as means ± s.e.m. Note that higher depolarizations seen in phasic neurones with 500 pA than in tonic neurones with 300 pA (C) still did not evoke the sustained high frequency action potentials seen in the latter (A).
The resting membrane potentials of tonic (−48.4 ± 1.6 mV, n = 18) and phasic (−48.0 ± 1.3 mV, n = 23) neurones were equivalent (Fig. 1B). Compared with phasic neurones, tonic neurones had significantly higher resting membrane resistance (181 ± 19 MΩversus 108 ± 18 MΩ), and consequently required less depolarizing current to evoke significant depolarization and generated APs at lower current thresholds (Fig. 1B and C). The membrane potential threshold for AP generation, however, was not significantly different in tonic (−31.1 ± 1.3 mV, n = 12) and phasic (−34.5 ± 1.5 mV, n = 8) neurones. Action potential discharge of phasic neurones remained very transient even when greater depolarization than that induced in tonic neurones was evoked by higher levels of depolarizing current (Fig. 1A).
Effects of Ang II on sympathetic neurones under resting conditions
Depolarization
The depolarizations with Ang II were dose related averaging 6.2 ± 1.4 mV (n = 4) and 12.0 ± 1.0 mV (n = 16) at concentrations of 100 nm and 200 nm, respectively (P < 0.05). Lower concentrations of Ang II (20–50 nm) caused more variable smaller depolarizations (data not shown). The depolarizations paralleled the time course of increases in membrane resistance reaching a maximum within 1–2 min and declining gradually thereafter (Fig. 2C).
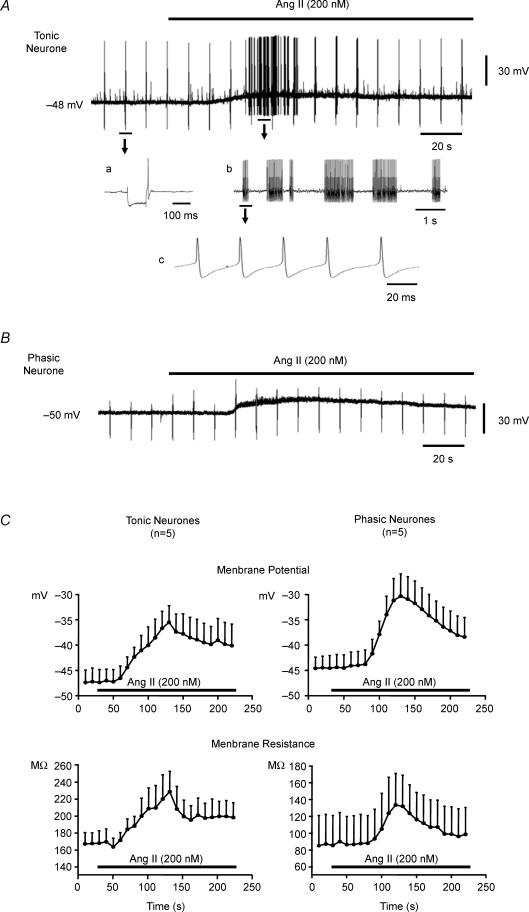
Figure 2. Effects of Ang II on membrane potential and generation of action potentials in tonic and phasic sympathetic neurones.
The resting membrane potential of each neurone is provided to the left of the traces. A, Ang II (200 nm) depolarized and evoked action potential discharge in a tonic neurone. Faster recordings are portrayed in a, b and c. Note that the regularly spaced single spikes present throughout the recording are the result of intermittent brief injections of hyperpolarizing current pulses used to calculate membrane resistance. a, the change in membrane potential induced by a single hyperpolarizing current injection applied to measure membrane resistance. The single action potential following the current injection reflects anodal break and not resting baseline activity. b, the oscillatory nature of Ang II-induced action potential discharge, a section of the trace from A is reproduced at an expanded time scale in the inset. c, to clarify that Ang II-triggered action potentials are of uniform amplitude, a section of the trace from b is reproduced at a further expanded time scale. B, Ang II (200 nm) failed to evoke action potential discharge in the phasic neurone despite a greater depolarization. Note also the absence of anodal break in the phasic neurone compared to the tonic neurone. C, effects of Ang II on membrane potential (top graphs) and membrane resistance (bottom graphs) of tonic and phasic neurones. Superfusion of the neurones with 200 nm Ang II (horizontal bar) depolarized and increased membrane resistance of both tonic (n = 5) and phasic (n = 5) sympathetic neurones. Parallel increases in membrane resistance and decreases in membrane potential (depolarization) were noted peaking at approximately 2 min of infusion and decaying over the subsequent 2 min. Data are expressed as means ± s.e.m.
In tonic neurones (n = 8), Ang II (200 nm) induced a peak depolarization of 10.4 ± 1.4 mV with an increase in resistance of 66 ± 16 MΩ, whereas in phasic neurones (n = 8), Ang II (200 nm) induced a peak depolarization of 13.7 ± 1.4 mV and an increase in resistance of 44 ± 10 MΩ (Fig. 2A–C).
Action potential discharge
In tonic neurones, Ang II triggered AP discharge lasting 43 ± 10.5 s (Fig. 2A) at an average peak frequency of 32 ± 4.6 spikes s−1 (n = 8). The first AP evoked by Ang II occurred at a membrane potential threshold of −41.0 ± 1.2 mV (n = 8) which is significantly more negative than the threshold potential with current injection in the absence of Ang II (−31.1 ± 1.3 mV, n = 12). In phasic neurones (n = 8), Ang II induced depolarizations equivalent to those in tonic neurones, but without evoking any APs (Fig. 2B and C).
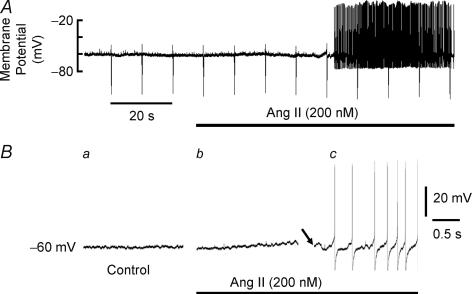
When the resting membrane potential was held at − 60 mV using hyperpolarizing current injections as needed before and during Ang II, APs were still evoked by Ang II in tonic but not in phasic neurones indicating that generation of APs may be uncoupled from the depolarizing effect of Ang II. A more detailed analysis showed very small oscillations of the resting membrane potential with Ang II despite the attempt to maintain it at the −60 mV level (Fig. 3).
Figure 3. Ang II-induced action potential discharge in a tonic sympathetic neurone in the absence of depolarization.
A, Ang II (200 nm) still triggered action potentials at membrane potential of −60 mV held by injection of hyperpolarizing current. B, three sections of the top trace before and during Ang II are reproduced at an expanded time scale to show Ang II-induced depolarization (b). Although Ang II-induced depolarization was reversed by increasing slightly the hyperpolarizing current injection, action potentials still fired (c). The arrow shows where an increase in the hyperpolarizing current brought back the membrane potential to −60 mV yet action potential firing was still induced during continued perfusion of Ang II.
Tachyphylaxis to Ang II
In five neurones (3 tonic, 2 phasic), Ang II (200 nm) was applied a second time 30 min after washout of the initial application. Compared with the initial response, the second application of Ang II triggered a smaller depolarization (6.6 ± 1.9 versus 10.7 ± 1.3 mV, n = 5, P < 0.05). Moreover, the frequency and duration of AP discharge in tonic neurones were reduced during the second compared with the first exposure to Ang II. Because of the desensitization, Ang II was applied only once per coverslip in the majority of protocols.
Effects of Ang II on responses to current injection (membrane excitability)
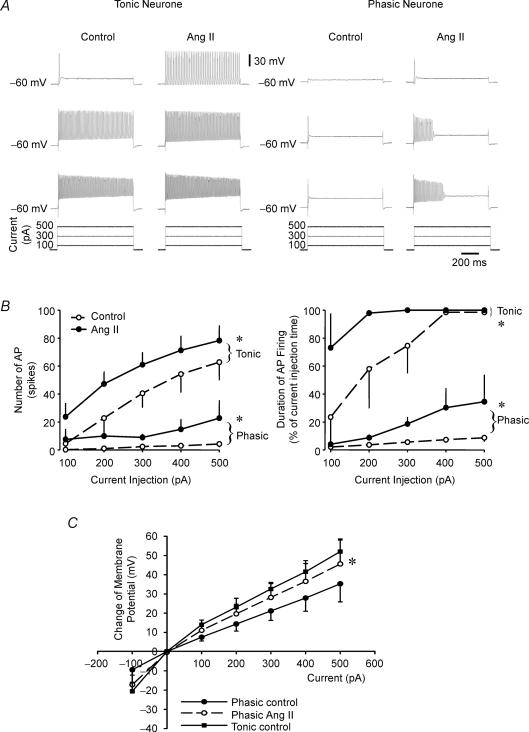
Under control conditions at −60 mV, both the number of APs generated and the duration of repetitive spike firing increased with increasing levels of current injection in tonic but not in phasic neurones (Fig. 4A and B). By definition, the phasic neurones generated only one to five spikes at the onset of the depolarizations even at sustained higher levels of current injections and higher depolarizations than in tonic neurones (Fig. 4). Ang II (200 nm) significantly increased both the number of APs and the duration of spike firing during depolarizing current injections (Fig. 4A and B) in both tonic and phasic neurones but the increases were much more pronounced and sustained in tonic neurones. Although Ang II shifted the I–V relation of phasic neurones closer to that of tonic neurones (Fig. 4C), the AP discharge of phasic neurones was seen only at the beginning of current injection and did not persist for the duration of the current injection as in tonic neurones.
Figure 4. Effects of Ang II on current-evoked action potential discharge at holding potential of −60 mV in both tonic and phasic neurones.
A, representative recordings showing action potential responses of a tonic (left) and a phasic sympathetic neurone (right) to three levels of depolarizing current injection (100, 300 and 500 pA), under control conditions and in the presence of Ang II (200 nm). B, group data showing the number of evoked action potentials (AP) (left) and the duration of AP firing expressed as a percentage of the duration of current injection (right) in both tonic (n = 4) and phasic (n = 5) sympathetic neurones during a series of depolarizing current injections (100–500 pA for ∼800 ms). Ang II significantly increased both the number of APs generated and the duration of repetitive AP firing in both tonic and phasic neurones (*P < 0.05 Ang II versus control). The increases in firing of phasic neurones required greater current injections and more depolarization and remained transient and ephemeral. C, effects of Ang II on current–voltage (I–V) relation in tonic and phasic neurones. Although the I–V relation of phasic neurones with Ang II became closer to that of ‘control’ tonic neurones (*P < 0.05 Ang II versus Control in phasic neurones), phasic neurones still failed to fire as many or as sustained APs as tonic neurones did (A and B). Data are expressed as means ± s.e.m.
Roles of AT1 and AT2 receptors
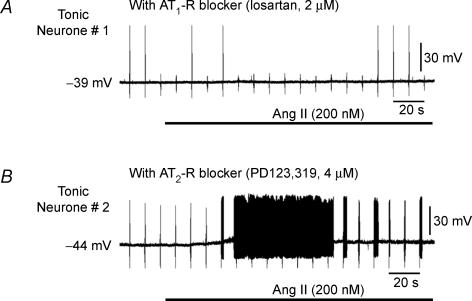
In the presence of the AT1 receptor antagonist losartan, Ang II-induced depolarization was abolished in all six neurones tested (4 phasic and 2 tonic, 0.9 ± 0.7 mV) as were the APs generated in the two tonic neurones (Fig. 5A). The AT2 receptor blocker PD123,319 did not prevent Ang II-induced depolarization (10.6 ± 3.4 mV, n = 6), nor did it prevent AP discharge (Fig. 5B).
Figure 5. Effects of AT1 and AT2 receptor blockers on Ang II evoked responses.
Representative recordings obtained from two tonic neurones show the lack of effect of 200 nm Ang II (horizontal bar) on membrane potential and failure to trigger action potentials after pretreatment with the AT1 receptor blocker losartan (2 μm) (A) and the preservation of the responses to Ang II in the presence of the AT2 receptor blocker PD123,319 (4 μm) (B). Note that the regularly spaced single spikes present throughout the recordings are the result of intermittent brief injections of hyperpolarizing current pulses used to calculate membrane resistance.
Effects of Ang II on voltage-gated K+ currents in sympathetic neurones
Effect of Ang II on transient IA
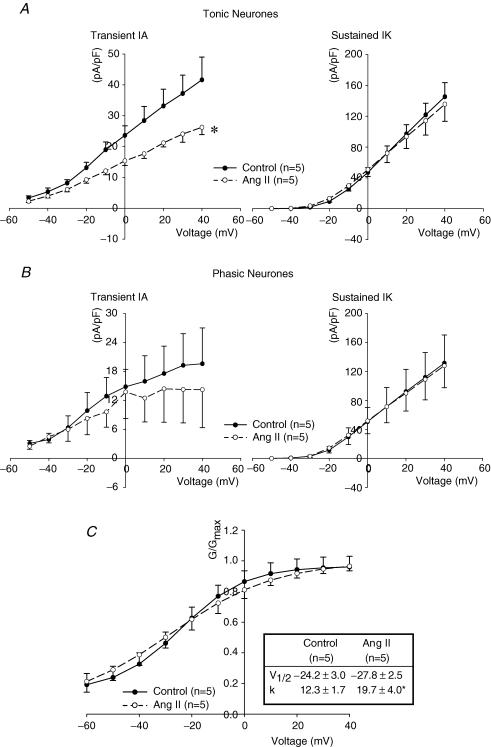
IA currents were activated when depolarizations were initiated from a holding potential of −100 mV as shown in Fig. 6A and B. The magnitude of IA during depolarizations from −100 to −50, −40 and −30 mV averaged 87 ± 21, 147 ± 35, and 222 ± 35 pA, respectively (n = 5). Corresponding values after Ang II were reduced significantly to 64 ± 17, 107 ± 22 and 172 ± 40 pA (n = 5). The characteristics of IA in both tonic and phasic neurones as well as the effects of Ang II are shown in Table 1 and Fig. 7. Peak current was significantly larger in tonic than in phasic neurones and Ang II (200 nm) inhibited IA and decreased the slope of the I–V relationship significantly in tonic but not in phasic neurones.
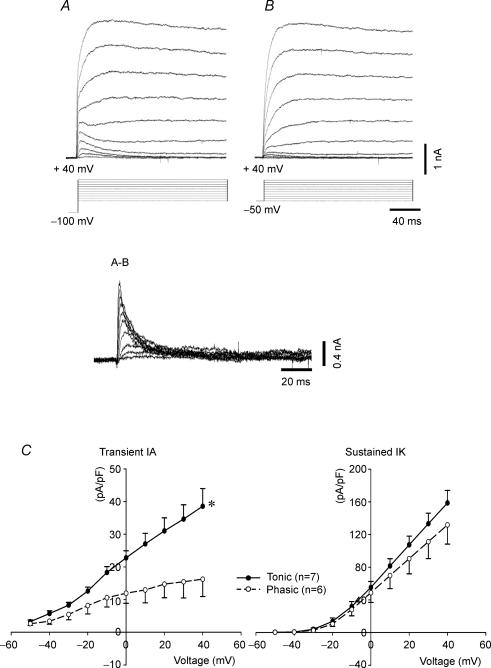
Figure 6. Representative recordings of voltage-gated outward potassium currents obtained by stepwise depolarization from a holding potential of −100 mV (A) and −50 mV (B).
Transient currents (IA) were obtained by subtraction of currents (A−B). C, I–V relation showing that IA currents were more than 2 times as large in tonic (n = 7) than in phasic neurones (n = 6) (*P < 0.05 tonic versus phasic neurones), but IK currents were not significantly different. Data are expressed as means ± s.e.m.
Table 1.
Peak IA currents, cell capacitance, time to peak IA (TTP), and time constant of IA current decay before and during Ang II in both tonic and phasic neurones
| Control | Ang II | |||
|---|---|---|---|---|
| Tonic (n = 7) | Phasic (n = 6) | Tonic (n = 5) | Phasic (n = 5) | |
| Peak Current (pA) | 1012 ± 63 | 466 ± 91* | 756 ± 162† | 370 ± 128 |
| Capacitance (pF) | 29 ± 6 | 31 ± 5 | 29 ± 6 | 31 ± 5 |
| TTP (ms) | 2.4 ± 0.11 | 2.4 ± 0.07 | 2.5 ± 0.04 | 2.6 ± 0.2 |
| τ (ms) | 1.2 ± 0.07 | 1.3 ± 0.03 | 1.2 ± 0.04 | 1.2 ± 0.05 |
Significant difference between phasic and tonic neurones (P < 0.05).
Significant difference between Ang II and control responses (P < 0.05). Data are expressed as means ± s.e.m.
Figure 7. Effects of Ang II on transient (IA) and sustained (IK) potassium currents in both tonic and phasic sympathetic neurones.
A, Ang II (200 nm) selectively inhibited transient IA in tonic neurones (*P < 0.05 Ang II versus control). In contrast, Ang II did not significantly affect sustained IK. B, Ang II (200 nm) did not significantly inhibit either IA or IK (n = 5) in phasic neurones. C, Effect of Ang II on the voltage-dependent activation of IA currents in tonic neurones. Ang II increased the slope factor (k) (*P < 0.05 Ang II versus control), but did not alter the voltage at half activation (V½). Data are expressed as means ± s.e.m.
Blockade of IA currents
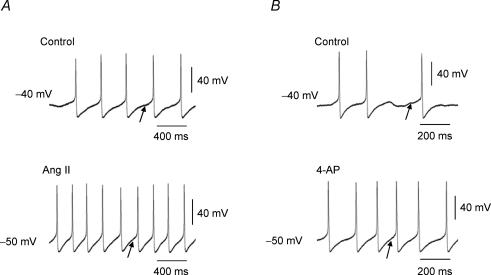
Five millimolar 4-AP (a known blocker of IA) triggered significant increases in AP discharge (Fig. 8B) that were comparable to those with Ang II (9 ± 1.2 spikes s−1, n = 5) (Fig. 8A). The increases in APs with both Ang II and 4-AP are associated with faster rates of diastolic depolarizations following the afterhyperpolarization (Fig. 9). The voltage range of this slow depolarization (−60 to −30 mV) is optimal for activation of IA so that blockade of IA with either Ang II or 4-AP accelerates this depolarization allowing the threshold potential to be reached faster, and hence the increased firing frequency.
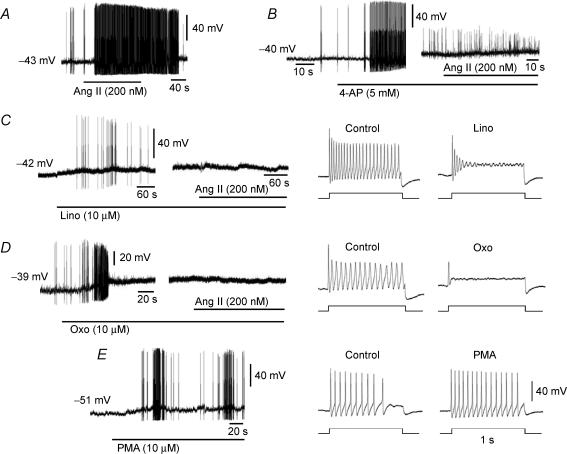
Figure 8. Representative patch-clamp recordings showing effects of Ang II, K+ channel blockers and PKC agonist on membrane potential and generation of action potentials (APs) in tonic sympathetic neurones.
The resting membrane potential of each neurone is provided to the left of the traces. A, Ang II (200 nm) depolarized and increased AP firing in a tonic neurone. B, 4-AP (5 mm), an IA channel blocker, depolarized and increased APs in a tonic neurone. The addition of Ang II depolarized the cell without triggering additional APs. C, linopirdine (lino, 10 μm), an M channel blocker, depolarized and triggered APs in a tonic neurone. The addition of Ang II had no significant effect on membrane potential and did not trigger APs. Current injection-induced APs recorded before and during administration of linopirdine are shown in the right panels. Linopirdine markedly suppressed AP firing during current injection (100 pA for 1 s). D, oxotremorine (Oxo, 10 μm), a muscarinic receptor agonist known to inhibit M current, depolarized and triggered APs in a tonic neurone. The addition of Ang II had no significant effect on membrane potential and did not trigger APs. Oxotremorine M markedly suppressed AP firing during current injection (100 pA for 1 s) (right panels). E, PKC agonist phorbol 12-myristate 13-acetate (PMA, 10 μm) mimicked Ang II effect by depolarizing and triggering APs and increased APs during current injection (right panels) in a tonic neurone.
Figure 9. Representative recordings showing action potential discharge in the absence and presence of Ang II or 4-AP.
Both Ang II (A) and 4-AP (B) accelerated the slow depolarization (indicated by arrows) following the afterhyperpolarization and increased the frequency of action potential discharge.
In the presence of 4-AP, Ang II did not increase the frequency of AP discharge but it did cause some depolarization (3.1 ± 0.5 mV, n = 3).
Effect of Ang II on sustained IK
IK measured at the end of the depolarizing pulses from a holding potential of −50 mV was not significantly different between tonic and phasic neurones (Fig. 6C), and was not affected by Ang II (Fig. 7A and B).
Effect of Ang II on M currents
Five out of seven phasic and six out of 13 tonic neurones showed evidence of M currents. The amplitude of M currents was significantly less (P < 0.05) in tonic (9.0 ± 1.3 pA) than in phasic neurones (15.3 ± 3.0 pA). In five neurones (2 phasic and 3 tonic), M currents were inhibited by Ang II from an average of 10.7 ± 1.5 to 3.9 ± 1.8 pA, P < 0.05) and recovered after wash-out (10.0 ± 2.5 pA) (Fig. 10).
Figure 10. Effect of Ang II on M current in a tonic neurone.
A, top trace: representative current recording showing the ‘relaxation’ (indicated by arrow) during deactivation of M current elicited by a hyperpolarizing voltage step from −30 mV to −60 mV for 1 s. This was inhibited by 200 nm Ang II (middle trace). B, the M current amplitude was reduced progressively over 3 min following application of Ang II.
Blockade of M currents
The M current inhibitor oxotremorine M (10 μm) and its blocker linopirdine (10 μm) depolarized the sympathetic neurones by 4.7 ± 0.9 mV (n = 4) and 4.4 ± 1.0 mV (n = 4), respectively. The Ang II-induced depolarization (5.7 ± 0.6 mV, n = 7) was abrogated by oxotremorine M to 1.4 ± 2.1 mV (n = 4) and by linopirdine to 0.3 ± 1.1 mV (n = 4). Both oxotremorine M and linopirdine also evoked transient increases in AP firing followed by suppression of excitability in response to current injections (Fig. 8C and D).
Role of protein kinase C in Ang II-induced activation of sympathetic neurones
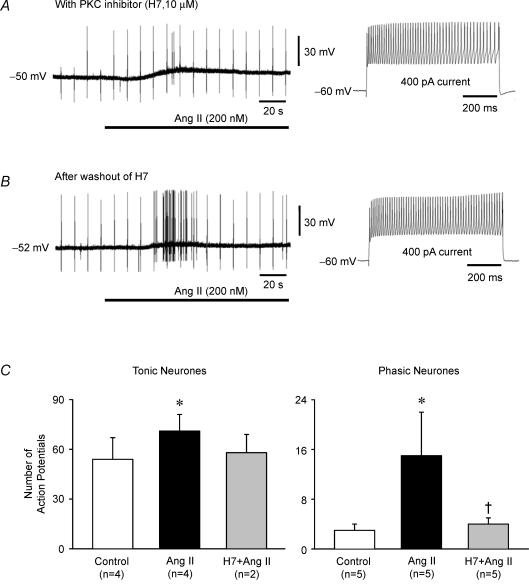
The role of PKC activation by Ang II was tested with the use of the PKC inhibitor H7 (10 μm). H7 essentially abolished Ang II-induced AP firing in each of four tonic neurones (Fig. 11A), and the responses were restored after washout of H7 (Fig. 11B). This inhibitory effect of H7 was selective to Ang II because current-evoked APs were preserved in the presence of H7 (Fig. 11A and B) but the enhancement of current-evoked APs by Ang II was abolished in both tonic and phasic neurones (Fig. 11C). Of interest is that in the presence of H7, Ang II no longer inhibited IA. The reduction of IA current by Ang II, which averaged 28.1 ± 6.6% at 30 mV (n = 5) was prevented by H7 in three experiments (3.4 ± 3.5%). H7 by itself did not alter IA (0.6 ± 5.8%). This supports the concept that AP generation by Ang II is mediated by PKC activation and subsequent inhibition of IA. This is further supported by the effect of PKC agonist PMA (2.5 μm), which mimicked the effect of Ang II by triggering AP in tonic neurones (n = 7) and enhancing the AP discharge during depolarizing current injections (Fig. 8E). Surprisingly, PMA also depolarized the cells (4.8 ± 0.7 mV) like Ang II (5.7 ± 0.6 mV).
Figure 11. Effects of PKC inhibitor H7 on Ang II-induced changes in membrane potential and action potential discharge and on responses to depolarizing current injections.
The resting membrane potential of the neurones is provided to the left of the traces. A, representative recording from a tonic neurone illustrates the relative failure of Ang II (200 nm, horizontal bar) to evoke action potential discharge (only 2 action potentials) after pretreatment with the PKC inhibitor H7 (10 μm). H7 alone did not inhibit current-evoked action potentials (right panel). B, 30 min after washout of H7, the same neurone fired numerous action potentials in response to Ang II. Note that the regularly spaced single spikes present throughout the recordings in A and B are the result of intermittent brief injections of hyperpolarizing current pulses used to calculate membrane resistance. C, Ang II enhancement of current-evoked action potential firing was also prevented by H7 in both tonic and phasic neurones. Shown is the number of action potentials evoked by 400 pA current injection (∼800 ms) before and during exposure to Ang II (200 nm). Ang II significantly increased action potential firing in 4 tonic and 5 phasic untreated neurones but failed to increase activity in 2 other tonic and 5 other phasic neurones pretreated with the PKC inhibitor H7 (10 μm) (*P < 0.05 Ang II versus control; †P < 0.05 H7 + Ang II versus Ang II). Data are expressed as means ± s.e.m.
In contrast to its inhibiting effect on Ang II-induced APs, H7 did not alter resting membrane potential (−47 ± 2 versus−46 ± 2 mV before and during H7, n = 9), membrane resistance (113 ± 25 versus 111 ± 23 MΩ before and during H7, n = 9), or the depolarizing effect of Ang II in either tonic (12.4 ± 4.5 mV, n = 4) or phasic (12.0 ± 4.3 mV, n = 5) neurones. Bisindolylmaleimide (1 μm), a different PKC inhibitor, also did not block the Ang II-evoked depolarization in two phasic neurones (data not shown).
As mentioned above, the Ang II-induced APs as well as the inhibition of IA were abrogated by H7. In contrast, Ang II-induced inhibition of M currents persisted in the presence of H7 (from 11.2 ± 2.1 to 4.4 ± 1.6 pA, n = 4, P < 0.05). H7 alone did not alter M current (9.0 ± 2.6 versus 11.2 ± 2.1 pA, n = 4, P > 0.05).
Discussion
Ang II influences neural control of cardiovascular function by its actions at multiple sites in the peripheral and central nervous systems (Aiken & Reit, 1968; Reid, 1992; Ferguson & Bains, 1997; Ma et al. 2001a; Dendorfer et al. 2002). The majority of studies have focused on central nervous system actions of Ang II. The results of the present study provide new insights into actions of Ang II on sympathetic ganglia that may significantly modulate sympathetic outflow and target organ function.
The main findings of this study are: (1) Ang II depolarizes tonic and phasic sympathetic neurones to a similar extent but evokes APs only in tonic neurones; (2) depolarizing current injections trigger abundant and sustained APs in tonic neurones but only scarce and transient ones in phasic neurones; Ang II enhances the AP discharge markedly in tonic neurones but only minimally and transiently in phasic neurones; (3) Ang II-induced depolarization and AP discharge are both mediated through AT1 receptors; activation of PKC is required for the AP response but not for the depolarization; (4) IA currents are significantly larger in tonic than in phasic neurones, but IK currents are similar; Ang II significantly inhibits IA but not IK; (5) the inhibition of IA by Ang II is significant in tonic neurones, triggers APs, and is PKC dependent; 4-AP, which inhibits IA also triggers APs like Ang II; and (6) M currents are more prominent in phasic neurones, and Ang II inhibits them in both tonic and phasic neurones causing depolarization via a PKC-independent pathway.
Thus, two distinct mechanisms contribute to Ang II-induced activation of sympathetic neurones. First is a PKC-independent inhibition of ionic conductance(s) which includes inhibition of M current resulting in depolarization and an increase in membrane resistance. The second is a PKC-dependent increase in membrane excitability via inhibition of IA resulting in excessive AP discharge. The presence of dual signalling pathways and differences in expression/regulation of voltage-dependent ion channels can explain differential responses of tonic and phasic sympathetic neurones to Ang II.
In the discussion, we address: (1) characteristics of tonic and phasic sympathetic neurones that may explain differential responses to Ang II, (2) the ion channel and second messenger mechanisms responsible for Ang II-induced depolarization and AP firing, and (3) the physiological implications of the results.
Subtypes of sympathetic neurones
Sympathetic neurones have been classified into subtypes based on differences in electrophysiological properties, expression of neuropeptides, and morphology (Weems & Szurszewski, 1978; Decktor & Weems, 1981, 1983; Cassell et al. 1986; Macrae et al. 1986; Gibbins, 1992; Keast et al. 1993; Zhao et al. 1995; Wang & McKinnon, 1995; Boyd et al. 1996; Jobling & Gibbins, 1999; Morris et al. 1999; Malin & Nerbonne, 2000, 2001). Our results confirm the presence of tonic and phasic neurones in sympathetic ganglia of mice (Jobling & Gibbins, 1999). In agreement with previous studies (Decktor & Weems, 1981; Decktor & Weems, 1983; Cassell et al. 1986; Jobling & Gibbins, 1999), our findings indicate that differences in passive (resting) membrane properties, e.g. membrane resistance, cannot explain the distinct differences in AP firing pattern in these neuronal subtypes. For example, AP discharge of phasic neurones during sustained current injection remained very transient even when depolarizations equal to or greater than those observed in tonic neurones were evoked by higher levels of depolarizing currents (see Fig. 1A and C). Therefore, the different firing patterns in tonic and phasic neurones likely reflect differences in expression or regulation of voltage-dependent ion channels. Indeed, previous studies suggest that a higher expression of M current in phasic neurones and a differential expression of Kv1 and Kv4 α-subunits encoding transiently activated K+ currents preferentially in tonic neurones account for the enhanced AP firing patterns in tonic versus phasic neurones (Cassell et al. 1986; Wang & McKinnon, 1995; Malin & Nerbonne, 2000, 2001).
Our studies are consistent with these results. We found that IK expression was similar in tonic and phasic neurones, but IA expression was significantly larger in tonic than in phasic neurones. We also found that M currents, on the other hand, were more prominent in phasic than in tonic neurones.
Recently, we demonstrated that Ang II increases cytosolic Ca2+ concentration in a subpopulation of sympathetic neurones isolated from aortic–renal and coeliac ganglia of mice (Ma et al. 2001b). In the present study, all sympathetic neurones depolarized in response to Ang II acting on AT1 receptors, while only tonic neurones generated APs. We propose therefore that the Ca2+ transient we reported previously was the result of Ca2+ influx during APs evoked in tonic neurones by Ang II. This proposal is supported by the fact that both Ang II-induced increases in cytosolic Ca2+ and AP firing in tonic neurones were blocked by the PKC inhibitor H7 (Ma et al. 2001b; present results). The membrane potential threshold for AP generation determined by brief current injection was the same in tonic (−31.1 ± 1.3 mV, n = 12) and phasic (−34.5 ± 1.5 mV, n = 8) neurones. Therefore, the failure of phasic neurones to generate APs in response to Ang II cannot be explained by a higher intrinsic membrane potential threshold. The differential expression of IA and M currents may account for the differential AP responses to Ang II in phasic versus tonic neurones.
Mechanisms of Ang II-induced depolarization and action potential firing
Our results indicate that activation of sympathetic neurones by Ang II involves two independent mechanisms: membrane depolarization and increased membrane excitability. Depolarization was accompanied by a decrease in membrane conductance that was also observed when membrane potential was held constant at −60 mV, indicating that Ang II-induced depolarization may be due to an inhibition of K+ conductance. To understand the ionic mechanisms, the outward K+ currents including IA, IK and M currents were studied before and after Ang II with whole cell and perforated patch clamp recordings. Ang II significantly inhibited IA currents, which were prominent in tonic but not in phasic neurones. However, Ang II did not affect IK in either tonic or phasic neurones. Furthermore, we show that the PKC inhibitor H7 prevented Ang II-induced inhibition of IA and since the Ang II-induced increase in membrane excitability is also PKC dependent it is likely that inhibition of IA plays a role in the Ang II-induced increase in membrane excitability. As expected, blockade of IA current with 4-AP triggered APs and prevented further Ang II-induced AP discharge. Ang II caused additional depolarization in the presence of 4-AP.
Depolarization is clearly not the only, or even the major, cause of Ang II-induced AP discharge. Action potential firing was dissociated from depolarization in several situations. For example, phasic neurones failed to fire APs during Ang II-induced depolarizations that were equivalent to those that evoked APs in tonic neurones (Fig. 2). The same degree of depolarization of tonic neurones by Ang II failed to evoke APs after inhibition of PKC (Fig. 11A and C). Action potential discharge in tonic neurones was actually triggered by Ang II at more negative membrane potentials (−41.0 ± 1.2 mV) than the potential required to evoke APs during current injection under control conditions (−31.1 ± 1.3 mV). In fact, Ang II was still able to evoke AP discharge when depolarization of the resting potential was prevented by hyperpolarizing current injection (Fig. 3). These results demonstrate that Ang II may initiate AP discharge by reducing the threshold for AP generation. The greater increase in membrane resistance per mV depolarization seen in tonic neurones during Ang II infusion (Fig. 2C) may reflect the greater inhibition of the outward current (IA) which is disproportionately more expressed in tonic neurones.
Our results showing that Ang II increased membrane excitability via activation of the PKC pathway, along with previous work by others, suggest that the mechanism of the striking increase in membrane excitability in the presence of Ang II involves PKC-dependent modulation of voltage-gated Na+ and K+ channels. Previous studies have shown that (1) Ang II increases Na+ permeability in superior cervical sympathetic neurones (Dun et al. 1978); (2) activation of PKC enhances neuronal excitability in somatosensory cortical neurones by increasing persistent Na+ current (Astman et al. 1998); and (3) Ang II inhibits several neuronal voltage-dependent K+ currents in the central nervous system including IA (Nagatomo et al. 1995; Li & Ferguson, 1996; Sumners et al. 1996; Wang et al. 1997; Gelband et al. 1999; Zhu et al. 1999). We also studied Na+ current in our preparation. Na+ current in sympathetic neurones was TTX sensitive, but not affected by Ang II (data not shown). The PKC agonist PMA mimics the effect of Ang II by triggering AP discharge in tonic neurones and enhancing the discharge during depolarizing current injections. PMA also depolarized the cells like Ang II. A similar depolarization by PMA was reported in bull-frog sympathetic neurones and was determined to be independent of PKC (Chen et al. 1994) just as the Ang II-induced depolarization we report here is PKC independent. As described above, the PKC inhibitor H7 blocked Ang II-induced inhibition of IA and prevented Ang II-induced AP discharge but did not prevent the depolarization. Therefore, we conclude that the Ang II-induced increase in membrane excitability is due to a PKC dependent inhibition of IA in sympathetic neurones. IA is activated during the period of slow depolarization following the spike afterhyperpolarization (Fig. 9). Blockade of IA with Ang II or 4-AP during this phase will accelerate the depolarization attaining the threshold potential faster, and hence increasing firing rate.
M current was equally inhibited by Ang II in the absence or presence of the PKC inhibitor. Blockade of M currents with oxotremorine M and linopirdine depolarized the membrane and prevented Ang II-induced depolarization, which indicates that inhibition of M currents was involved in Ang II-induced depolarization via a PKC-independent pathway. This finding is consistent with prior reports that attributed Ang II-induced depolarization to inhibition of K+ conductance, specifically M-current (Brown et al. 1980; Shapiro et al. 1994). An unanticipated result was that in the presence of oxotremorine M or linopirdine Ang II no longer triggered AP discharge. However it is very difficult to suggest that Ang II-mediated inhibition of M currents contributes to the increased membrane excitability in any specific way. Although both oxotremorine M and linopirdine depolarized the cells and triggered AP discharges, these effects were rapidly followed by dramatic suppression of membrane excitability. Oxotremorine M and linopirdine-induced suppression of membrane excitability has been reported in cerebral cortex and chromaffin cells (Lin & Phillis, 1991; Wallace et al. 2002). In cerebral cortex, oxotremorine M abolished glutamate-triggered neuronal activity by activation of M2 receptors (Lin & Phillis, 1991). In chromaffin cells, prolonged exposure to linopirdine resulted in a decline of AP discharge both in frequency and amplitude, which may have been due to inhibition of voltage-gated calcium currents (Wallace et al. 2002).
Analogous dual signalling pathways in cultured sympathetic neurones were recently reported by Scholze et al. (2002). Bradykinin inhibited M current in sympathetic neurones through a PKC-independent mechanism and enhanced noradrenaline release through a PKC-dependent mechanism. Blockade of inositol triphosphate-dependent signalling abolished the bradykinin-induced inhibition of M current without influencing the PKC-dependent release of noradrenaline (Scholze et al. 2002).
Physiological implications
Ang II, produced in sympathetic ganglia or delivered to ganglia via the circulation, may enhance synaptic transmission and/or generate APs selectively in tonic sympathetic neurones even when central or reflex-driven sympathetic drive is low or absent. Kushiku et al. (2001) have shown that high-frequency stimulation of preganglionic nerves to cardiac sympathetic ganglia generates immunoreactive Ang II and that Ang II antagonists reduce the heart rate response to preganglionic, but not postganglionic, nerve stimulation. Angiotensinogen mRNA is expressed in stellate ganglia and is up-regulated by sustained high-frequency preganglionic stimulation (Kushiku et al. 2001). Ang II binding sites and AT1 receptors are present in sympathetic ganglia (Castren et al. 1987; Stromberg et al. 1991). Thus, a functional renin–angiotensin system exists in sympathetic ganglia. Our results define mechanisms of activation of sympathetic ganglion neurones by Ang II.
Do ganglionic actions of Ang II significantly increase postganglionic sympathetic nerve activity in vivo? Ang II administered after ganglionic blockade increases postganglionic sympathetic nerve activity, plasma noradrenaline concentration, heart rate and cardiac contractility (Aiken & Reit, 1968; Farr & Grupp, 1971; Knape & van Zwieten, 1988; Ma et al. 2001a; Tokunaga et al. 2001; Dendorfer et al. 2002). Our earlier work defined the direct ganglionic actions of Ang II as evoking low-amplitude, continuous discharge of renal sympathetic nerves (Ma et al. 2001a). This pattern of activity is distinctly different from the intermittent, high-amplitude bursts of resting sympathetic nerve activity that can be regulated centrally or reflexly. Riedel & Peter (1977) observed that intravenous Ang II selectively increases the activity of postganglionic renal sympathetic nerve fibres with low-amplitude spikes while reflexly inhibiting the activity of fibres with high-amplitude spikes. Thus, Ang II may alter both the pattern and the type of postganglionic sympathetic nerve activity through direct and indirect effects on sympathetic ganglion neurones with possible functional consequences.
Function-specific subtypes of single postganglionic sympathetic nerve fibres subserving vasoconstrictor, vasodilator, pilomotor, sudomotor, secretomotor, and motility- or transport- regulating functions in vivo are well established (Riedel & Peter, 1977; Janig & McLachlan, 1992; DiBona et al. 1996). Differences in AP firing patterns (tonic versus phasic), along with differences in neurochemical markers and morphology, may identify corresponding function-specific subtypes of sympathetic neurones in ganglia (Cassell et al. 1986; Macrae et al. 1986; Gibbins, 1992; Janig & McLachlan, 1992; Keast et al. 1993; Boyd et al. 1996; Morris et al. 1999). For example, phasic neurones expressing tyrosine hydroxylase and neuropeptide Y may preferentially innervate blood vessels and serve a vasoconstrictor function (Cassell et al. 1986; Gibbins, 1992; Morris et al. 1999). In contrast, tonic neurones primarily in prevertebral sympathetic ganglia often project to non-vascular targets (Cassell et al. 1986; Macrae et al. 1986). Phasic and tonic neurones also differ in regard to the number, strength and source of synaptic input they receive (Macrae et al. 1986; Meckler & McLachlan, 1988; McLachlan & Meckler, 1989).
We speculate that selective effects of Ang II on tonic and phasic sympathetic neurones may differentially modulate sympathetic outflow and target organ function in vivo. The dissociated neurone preparation does not allow assessment of effects of Ang II on synaptic transmission or identification of the target organ innervated by individual neurones. Understanding the physiological significance of ganglionic actions of Ang II will require studies of neurones retrogradely labelled from their target tissues and recordings of postganglionic sympathetic activity and target organ function in vivo.
Acknowledgments
We would like to thank Merck & Co., Inc. for providing the selective AT1 receptor blocker losartan. This publication was made possible by grant number HL14388 from the National Institutes of Health, a VA Merit Review Award to MWC from the Department of Veterans Affairs, and a Research Fellowship awarded to XM from the Iowa Affiliate of the American Heart Association (No. 0020423Z). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the AHA.
References
- Aiken JW, Reit E. Stimulation of the cat stellate ganglion by angiotensin. J Pharmacol Exp Ther. 1968;159:107–114. [PubMed] [Google Scholar]
- Astman N, Gutnick MJ, Fleidervish IA. Activation of protein kinase C increases neuronal excitability by regulating persistent Na+ current in mouse neocortical slices. J Neurophysiol. 1998;80:1547–1551. doi: 10.1152/jn.1998.80.3.1547. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Whiteis CA, Chapleau MW, Abboud FM. Nitric oxide enhances slow inactivation of voltage-dependent sodium currents in rat nodose neurons. Neurosci Lett. 1999;271:159–162. doi: 10.1016/s0304-3940(99)00553-4. [DOI] [PubMed] [Google Scholar]
- Boyd HD, McLachlan EM, Keast JR, Inokuchi H. Three electrophysiological classes of guinea pig sympathetic postganglionic neurone have distinct morphologies. J Comp Neurol. 1996;369:372–387. doi: 10.1002/(SICI)1096-9861(19960603)369:3<372::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brown DA, Constanti A, Marsh S. Angiotensin mimics the action of muscarinic agonists on rat sympathetic neurones. Brain Res. 1980;193:614–619. doi: 10.1016/0006-8993(80)90200-0. [DOI] [PubMed] [Google Scholar]
- Carrier GO. Whole-cell and perforated patch recordings of four distinct K+ currents in acutely dispersed coeliac-superior mesenteric ganglia neurons of adult rats. Brain Res. 1995;701:1–12. doi: 10.1016/0006-8993(95)00756-6. [DOI] [PubMed] [Google Scholar]
- Cassell JF, Clark AL, McLachlan EM. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J Physiol. 1986;372:457–483. doi: 10.1113/jphysiol.1986.sp016020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Kurihara M, Gutkind JS, Saavedra JM. Specific angiotensin II binding sites in the rat stellate and superior cervical ganglia. Brain Res. 1987;422:347–351. doi: 10.1016/0006-8993(87)90942-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Jassar BS, Kurenny DE, Smith PA. Phorbol ester-induced M-current suppression in bull-frog sympathetic ganglion cells: insensitivity to kinase inhibitors. Br J Pharmacol. 1994;113:55–62. doi: 10.1111/j.1476-5381.1994.tb16173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Marshall CG, Ogden D. Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol. 1989;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decktor DL, Weems WA. A study of renal-efferent neurones and their neural connexions within cat renal ganglia using intracellular electrodes. J Physiol. 1981;321:611–626. doi: 10.1113/jphysiol.1981.sp014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decktor DL, Weems WA. An intracellular characterization of neurones and neural connexions within the left coeliac ganglion of cats. J Physiol. 1983;341:197–211. doi: 10.1113/jphysiol.1983.sp014801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendorfer A, Thornagel A, Raasch W, Grisk O, Tempel K, Dominiak P. Angiotensin II induces catecholamine release by direct ganglionic excitation. Hypertension. 2002;40:348–354. doi: 10.1161/01.hyp.0000028001.65341.aa. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Sawin LL, Jones SY. Differentiated sympathetic neural control of the kidney. Am J Physiol. 1996;271:R84–R90. doi: 10.1152/ajpregu.1996.271.1.R84. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Nishi S, Karczmar AG. An analysis of the effect of angiotensin II on mammalian ganglion cells. J Pharmacol Exp Ther. 1978;204:669–675. [PubMed] [Google Scholar]
- Farr WC, Grupp G. Ganglionic stimulation: mechanism of the positive inotropic and chronotropic effects of angiotensin. J Pharmacol Exp Ther. 1971;177:48–55. [PubMed] [Google Scholar]
- Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Gelband CH, Warth JD, Mason HS, Zhu M, Moore JM, Kenyon JL, Horowitz B, Sumners C. Angiotensin II type 1 receptor-mediated inhibition of K+ channel subunit Kv2.2 in brain stem and hypothalamic neurons. Circ Res. 1999;84:352–359. doi: 10.1161/01.res.84.3.352. [DOI] [PubMed] [Google Scholar]
- Gibbins IL. Vasoconstrictor, vasodilator and pilomotor pathways in sympathetic ganglia of guinea-pigs. Neuroscience. 1992;47:657–672. doi: 10.1016/0306-4522(92)90174-z. [DOI] [PubMed] [Google Scholar]
- Janig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992;15:475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- Jobling P, Gibbins IL. Electrophysiological and morphological diversity of mouse sympathetic neurons. J Neurophysiol. 1999;82:2747–2764. doi: 10.1152/jn.1999.82.5.2747. [DOI] [PubMed] [Google Scholar]
- Keast JR, McLachlan EM, Meckler RL. Relation between electrophysiological class and neuropeptide content of guinea pig sympathetic prevertebral neurons. J Neurophysiol. 1993;69:384–394. doi: 10.1152/jn.1993.69.2.384. [DOI] [PubMed] [Google Scholar]
- Knape JT, van Zwieten PA. Positive chronotropic activity of angiotensin II in the pithed normotensive rat is primarily due to activation of cardiac β1-adrenoceptors. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;338:185–190. doi: 10.1007/BF00174868. [DOI] [PubMed] [Google Scholar]
- Kushiku K, Yamada H, Shibata K, Tokunaga R, Katsuragi T, Furukawa T. Upregulation of immunoreactive angiotensin II release and angiotensinogen mRNA expression by high-frequency preganglionic stimulation at the canine cardiac sympathetic ganglia. Circ Res. 2001;88:110–116. doi: 10.1161/01.res.88.1.110. [DOI] [PubMed] [Google Scholar]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee HC, Abboud FM. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron. 1998;20:1039–1049. doi: 10.1016/s0896-6273(00)80484-5. [DOI] [PubMed] [Google Scholar]
- Li Z, Ferguson AV. Electrophysiological properties of paraventricular magnocellular neurons in rat brain slices: modulation of IA by angiotensin II. Neuroscience. 1996;71:133–145. doi: 10.1016/0306-4522(95)00434-3. [DOI] [PubMed] [Google Scholar]
- Lin Y, Phillis JW. Muscarinic agonist oxotremorine-M-induced long-term depression in rat cerebral cortex. Brain Res Bull. 1991;27:115–117. doi: 10.1016/0361-9230(91)90291-q. [DOI] [PubMed] [Google Scholar]
- Ma X, Abboud FM, Chapleau MW. A novel effect of angiotensin on renal sympathetic nerve activity in mice. J Hypertens. 2001a;19:609–618. doi: 10.1097/00004872-200103001-00014. [DOI] [PubMed] [Google Scholar]
- Ma X, Chapleau MW, Whiteis CA, Abboud FM, Bielefeldt K. Angiotensin selectively activates a subpopulation of postganglionic sympathetic neurons in mice. Circ Res. 2001b;88:787–793. doi: 10.1161/hh0801.089542. [DOI] [PubMed] [Google Scholar]
- Macrae IM, Furness JB, Costa M. Distribution of subgroups of noradrenaline neurons in the coeliac ganglion of the guinea-pig. Cell Tissue Res. 1986;244:173–180. doi: 10.1007/BF00218395. [DOI] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of Kv4.2W362F: molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Molecular heterogeneity of the voltage-gated fast transient outward K+ current, IAf, in mammalian neurons. J Neurosci. 2001;21:8004–8014. doi: 10.1523/JNEUROSCI.21-20-08004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Meckler RL. Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea-pig. J Physiol. 1989;415:109–129. doi: 10.1113/jphysiol.1989.sp017714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckler RL, McLachlan EM. Axons of peripheral origin preferentially synapse with tonic neurones in the guinea pig coeliac ganglion. Neurosci Lett. 1988;86:189–194. doi: 10.1016/0304-3940(88)90569-1. [DOI] [PubMed] [Google Scholar]
- Mei YA, Louiset E, Vaudry H, Cazin L. A-type potassium current modulated by A1 adenosine receptor in frog melanotrophs. J Physiol. 1995;489:431–442. doi: 10.1113/jphysiol.1995.sp021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Zhu BS, Gibbins IL, Blessing WW. Subpopulations of sympathetic neurons project to specific vascular targets in the pinna of the rabbit ear. J Comp Neurol. 1999;412:147–160. [PubMed] [Google Scholar]
- Nagatomo T, Inenaga K, Yamashita H. Transient outward current in adult rat supraoptic neurones with slice patch-clamp technique: inhibition by angiotensin II. J Physiol. 1995;485:87–96. doi: 10.1113/jphysiol.1995.sp020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–E778. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Riedel W, Peter W. Non-uniformity of regional vasomotor activity indicating the existence of 2 different systems in the sympathetic cardiovascular outflow. Experientia. 1977;33:337–338. doi: 10.1007/BF02002814. [DOI] [PubMed] [Google Scholar]
- Scholze T, Moskvina E, Mayer M, Just H, Kubista H, Boehm S. Sympathoexcitation by bradykinin involves Ca2+-independent protein kinase C. J Neurosci. 2002;22:5823–5832. doi: 10.1523/JNEUROSCI.22-14-05823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B. Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G proteins. Neuron. 1994;12:1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Sheng M, Liao YJ, Jan YN, Jan LY. Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature. 1993;365:72–75. doi: 10.1038/365072a0. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Whiteis CA, Abboud FM, Chapleau MW. Mechanosensory transduction of vagal and baroreceptor afferents revealed by study of isolated nodose neurons in culture. Auton Neurosci. 2002;98:59–63. doi: 10.1016/s1566-0702(02)00033-4. [DOI] [PubMed] [Google Scholar]
- Stromberg C, Tsutsumi K, Viswanathan M, Saavedra JM. Angiotensin II AT1 receptors in rat superior cervical ganglia: characterization and stimulation of phosphoinositide hydrolysis. Eur J Pharmacol. 1991;208:331–336. doi: 10.1016/0922-4106(91)90079-w. [DOI] [PubMed] [Google Scholar]
- Sumners C, Zhu M, Gelband CH, Posner P. Angiotensin II type 1 receptor modulation of neuronal K+ and Ca2+ currents: intracellular mechanisms. Am J Physiol. 1996;271:C154–C163. doi: 10.1152/ajpcell.1996.271.1.C154. [DOI] [PubMed] [Google Scholar]
- Tokunaga R, Kushiku K, Yamada K, Yamada H, Furukawa T. Possible involvement of calcium-calmodulin pathways in the positive chronotropic response to angiotensin II on the canine cardiac sympathetic ganglia. Jpn J Pharmacol. 2001;86:381–389. doi: 10.1254/jjp.86.381. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Chen C, Marley PD. Histamine promotes excitability in bovine adrenal chromaffin cells by inhibiting an M-current. J Physiol. 2002;540:921–939. doi: 10.1113/jphysiol.2001.013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, McKinnon D. Potassium currents in rat prevertebral and paravertebral sympathetic neurones: control of firing properties. J Physiol. 1995;485:319–335. doi: 10.1113/jphysiol.1995.sp020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sumners C, Posner P, Gelband CH. A-type K+ current in neurons cultured from neonatal hypothalamus and brain stem: modulation by angiotensin II. J Neurophysiol. 1997;78:1021–1029. doi: 10.1152/jn.1997.78.2.1021. [DOI] [PubMed] [Google Scholar]
- Weems WA, Szurszewski JH. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978;41:305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Zhao FY, Saito K, Yoshioka K, Guo JZ, Murakoshi T, Konishi S, Otsuka M. Subtypes of tachykinin receptors on tonic and phasic neurones in coeliac ganglion of the guineapig. Br J Pharmacol. 1995;115:25–30. doi: 10.1111/j.1476-5381.1995.tb16315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Gelband CH, Posner P, Sumners C. Angiotensin II decreases neuronal delayed rectifier potassium current: role of calcium/calmodulin-dependent protein kinase II. J Neurophysiol. 1999;82:1560–1568. doi: 10.1152/jn.1999.82.3.1560. [DOI] [PubMed] [Google Scholar]