Abstract
The dental pulp consists of loose connective tissue encased in rigid dentinal walls. Because of its topography the tissue has low interstitial compliance and limited capacity to expand during fluid volume changes. Due to limitations regarding access to interstitial fluid, basic knowledge on transcapillary fluid transport parameters is lacking for this organ. The scope of this project was dual: first we aimed at establishing a method for isolation of pulp interstitial fluid (IF), and second we applied the method in rats subjected to lipopolysaccharide (LPS)-induced endotoxaemia. The aim was to measure colloid osmotic pressure (COP) and pro-inflammatory cytokines in the pulp IF during acute inflammation. Fluid volumes and pulpal blood flow (PBF) were measured to obtain more information about microcirculatory changes that take place in this pulpitis model. By centrifugation of incisor pulp at 239 g we were able to extract fluid representative for IF. Pulp IF had a relative high control COP (∼83% of plasma COP) and was similar to plasma COP 3 h after LPS challenge. The pulp exhibited a high content of IF (0.60 ± 0.03 ml (g wet weight)−1) and a vascular volume of 0.03 ± 0.01 ml (g w.w.)−1 No differences were observed in the distribution of fluid volumes after 1.5 and 3 h LPS exposure. PBF and systemic blood pressure dropped significantly after LPS administration. PBF remained low whereas systemic blood pressure was re-established during the 3-h period, implying organ dysfunction. There was a differential pattern of cytokine expression in pulp IF and serum with cytokines such as IL-1α, IL-1β and TNF-α locally produced, whereas others such as IFN-γ and IL-6 were produced systemically and probably spilled over to the pulp IF after LPS exposure. Our findings show that pulp IF can be isolated by centrifugation and that this method is useful when studying fluid balance and extracellular signalling mechanisms in the dental pulp in normal and pathological conditions.
The dental pulp is a highly vascular connective tissue enclosed in the rigid mineralized dentin. It shares many similarities with other connective tissues of the body but it also has circulatory characteristics with physiological implications. The pulp is a microcirculatory system lacking collateral circulation, and is situated within a low-compliance environment similar to, e.g. the brain. The limited ability to expand may severely compromise the circulation under conditions with increased fluid volume. The above features render the pulp vulnerable to circulatory changes occurring in inflammation such as hyperaemia and increased fluid filtration.
Pulpitis may be painful and is a very common inflammatory condition in man, usually caused by carious bacteria (Sindet-Pedersen et al. 1985; Khabbaz et al. 2001; Morgan et al. 2005). The first vascular reactions during pulpitis are vasodilatation and increased vascular permeability (Kerezoudis et al. 1993; Heyeraas et al. 1994). The observed increase in interstitial fluid pressure (Heyeraas & Berggreen, 1999) suggests that there is an increased interstitial fluid volume in this situation that will counteract further fluid filtration, but there are, however, no data available concerning intra- and extra-vascular fluid volumes either in normal or inflamed pulp. Furthermore, increased vascular permeability may again induce changes in colloid osmotic pressure of the interstitial fluid (COPi), another key factor in transcapillary flow according to Starling's equation. Up to now, COPi in normal as well as in inflamed pulp is unknown.
Since the dental pulp is enclosed in hard dentinal walls, direct access to the pulp tissue is difficult without exposing the pulp tissue and thereby creating inflammation. Using a recent technique applied in tumours and skin (Wiig et al. 2003) we tested if this method could be used for isolation of interstitial fluid (IF) from tooth pulp. We centrifuged pulp tissue at 239 g and isolated pulp fluid to determine local levels of pro-inflammatory cytokines during the development of acute pulpitis in order to assess the role of extracellular signalling in the microenvironment surrounding the pulpal cells. We chose a model of sepsis by administration of lipopolysaccharide (LPS) through the vascular system to achieve widespread pulpitis in the rats, as LPS has been implicated in the pathogenesis of pulpitis by entering the pulp via dentinal tubules (Warfvinge et al. 1985). The concentrations of six among the most investigated pro-inflammatory cytokines (interleukin-1α (IL-1α), IL-1β, IL-2, IL-6, interferon-γ (IFN-γ) and tumour necrosis factor α (TNF-α)) were measured in both pulp IF and serum, to test the hypothesis that LPS can cause local production and release of cytokines from pulpal cells. Furthermore, COPi and fluid volume measurements were performed after LPS challenge to obtain more information about transcapillary fluid balance during pulpitis. In addition, pulpal blood flow (PBF) was measured continuously during LPS exposure to observe the microcirculatory changes that take place in this model of acute inflammation.
Methods
Experimental animals
The experiments were performed in intraperitoneally anaesthetized (50 mg kg−1 sodium pentobarbital, Svaneapoteket, Bergen, Norway) female Wistar rats (n = 69, 190–220 g body weight). A femoral vein was catheterized (polyethylene PE-50 catheter) for injection of supplemental anaesthetic (2–3 mg kg−1i.v.) and substances, and a femoral artery for continuous systemic blood pressure (PA) recordings with a Gould pressure transducer and recorder (RS 3400; Cleveland, OH, USA). Body temperature was kept at 37–38°C with a servo-controlled heating pad. The depth of anaesthesia was assessed by the absence of spontaneous eye movements and foot or tail withdrawal in response to pinch and supplemental anaesthesia was given when necessary. At the end of the experiments the animals were killed with 0.5 ml saturated potassium chloride (KCl) i.v. All experiments were performed in accordance with recommendations given by the Norwegian State Commission for Laboratory Animals and were approved by the local ethical committee.
Measurements of fluid volumes
Twenty rats were used for fluid volume measurements. Total extracellular fluid volume (Vx) and intravascular volume (Vv) were measured as the distribution volumes of 51Cr-labelled EDTA and 125I-labelled human serum albumin (HSA), respectively. Following anaesthesia and placement of catheters, both kidney pedicles were ligated via flank incisions, and 30 μCi of 51Cr-EDTA was injected i.v. After a 120 min equilibration period, 3–4 μCi of 125I-HSA was given i.v. and allowed to circulate for 5 min. A blood sample of 0.5–0.7 ml was obtained from the arterial catheter and the rat was killed. A small area at the back of the rat was shaved and a 2 cm × 2 cm piece of skin was cut with scissors and placed in a preweighed airtight tube. The rat was transferred to an incubator kept at room temperature (20–24°C) and 100% relative humidity. The four incisor teeth were removed and cracked and the pulp was taken out in one piece, and transferred to preweighed airtight tubes in order to avoid evaporation of fluid from the tissue. All tubes with samples were reweighed to obtain wet weight (w.w.) of the tissues. The blood samples were centrifuged at 11000 r.p.m. (12839 g) for 15 min. Known volumes of plasma were removed and used for further analysis. Samples (plasma, pulp and skin) were counted in an LKB γ-counter (Wallac 1282 Compugamma, Turku, Finland) using window settings of 15–75 keV for 125I and 290–350 keV for 51Cr. Standards were counted in every experiment to obtain spillover corrections and counts were corrected for background and spillover.
Fluid volumes were calculated as the plasma equivalent distribution volumes of the tracers, assuming that labelled EDTA (51Cr-EDTA) will distribute in the extracellular fluid phase and labelled HSA (125I-HSA) will distribute only in plasma. Intravascular plasma volume in a tissue sample (Vv) was calculated as the 5 min distribution volume of 125I-HSA:
| (1) |
Since 125I-HSA was circulating in the animal for only 5 min, extravasation was assumed to be negligible. Tissue extracellular fluid volume (Vx) was calculated as the 2 h distribution volume of 51Cr-EDTA:
| (2) |
The tissue IF volume was calculated as the difference between extracellular fluid and plasma volume:
| (3) |
Isolation of interstitial fluid
To isolate pulp IF we tested whether an approach described by Wiig et al. (2003) was applicable for this organ. The rats (n = 12) were anaesthetized and nephrectomized as described above. Sixty to seventy microcuries of 51Cr-EDTA was injected i.v. After a 120 min equilibration period, 3–4 μCi of 125I-HSA was injected and allowed to circulate for 5 min. Blood samples and pulp tissues from the incisors were obtained the same way as previously described. The pulp was transferred to centrifuge tubes provided with a basket of nylon mesh with pore size ∼15 μm × 20 μm designed to keep the sample away from the bottom of the tube (Aukland, 1991), and spun in an Eppendorff 5417 R centrifuge for 10 min at 239 g. Thereafter the tubes were transferred back in the incubator and the fluid accumulated at the bottom of the tubes was collected in graded glass microcapillaries for volume measurements. Samples of 0.5–5 μl of pulp IF could then be collected.
To examine if fluid was derived from the intracellular compartment, the ratio (R) of the extracellular tracer 51Cr-labelled EDTA between isolated fluid and plasma was measured:
| (4) |
R < 1 would indicate that fluid not containing the tracer is added to the centrifugate and opposed, R > 1 would indicate that the extracellular fluid is concentrated, e.g. by cell swelling. The intravascular tracer 125I-HSA was used for determining contribution of fluid from the vascular compartment.
The distribution of macromolecules in isolated pulp fluid, pulp eluate, pulp tissue extract (homogenate) and plasma was determined by high performance liquid chromatography (HPLC). The pulp eluate was prepared by soaking intact pulp in phosphate buffer saline (PBS) for 6 h at 4°C. A whole pulp extract was obtained by cutting with micro-scissors, freeze-drying and crushing the pulp and soaking the minced tissue in PBS overnight at 4°C. After centrifugation the supernatant was collected for further analysis. For the HPLC we used two 4.6 mm (i.d.) × 30.0 cm TSK-gel size-exclusion columns coupled in series (Super SW2000 and 3000, Tosoh Biosciences, Stuttgart, Germany) with an optimal separation range for globular proteins of 5–100 kDa and 10–500 kDa, respectively. The protein concentration in the elution fluid was measured by UV detection at 220 nm on an Ettan™ LC System (GE Healthcare) and the buffer/mobile phase was 0.1 m Na2SO4 in 0.1 m PBS, pH 7.0.
Blood-flow recordings
A Periflux Model 4001 Master laser-Doppler flow meter (Perimed KB; Järafälla, Sweden) equipped with a needle-probe PF 415 : 10 (fibre diameter 125 μm with separation of 500 μm) was used to measure pulp blood flux proportional to local changes in blood flow in the left maxillary incisor tooth of representative animals that were treated 3 h with LPS. The head of the rat was immobilized and fixed to the operating table by a stereotaxic frame with the rat lying on its right side. The lips were pulled gently with thread in order to increase accessibility to the incisor tooth. The laser probe was positioned 3–5 mm above the level of the gingiva on the distal aspect of the tooth which gave the largest resting blood flow signal. Zero blood flow was determined as the value recorded from a stationary white card with the same intensity of reflected light as was measured when recording from the tooth. The flow meter's time constant was set at 0.03 s with an upper bandwidth of 20 kHz.
Experimental protocol of LPS-induced acute inflammation
LPS from Escherichia coli 0127:B8 (Sigma-Aldrich Chemie, Schnelldorf, Germany) was dissolved in 0.9% NaCl containing 0.1% bovine serum albumin (BSA, Fraction V; Sigma) to a final concentration of 2.5 mg ml−1.
The rats received a dose of 4.0 mg kg−1 LPS i.v. and were observed for 1.5 h (1.5 h LPS group, n = 6) or 3 h (3 h LPS group, n = 6). Control rats (n = 8) received the equivalent volume of vehicle (0.9% NaCl with 0.1% BSA) and were kept for 1.5 h (n = 4) or 3 h (n = 4). In order to quantify cytokines in the pulp microenvironment, cytokine levels in pulp IF and serum from the above animals were determined with the multiplex assay. In addition, two more rats treated with LPS or vehicle for 3 h were used for immunohistochemical analysis of the pulp tissue.
For fluid volume measurements, the rats received 4 mg kg−1 LPS after the 120 min equilibration period of 51Cr-EDTA. One and a half hours (n = 6) or 3 h (n = 5) after LPS administration, the intravascular marker 125I-HSA was given and the rats were killed 5 min later. The control group (n = 9) received only the extracellular and the intravascular isotopes as previously described (measurement of fluid volumes).
Measurements of colloid osmotic pressure were performed in samples from rats treated with LPS for 3 h (n = 8) and in seven controls. When possible, measurements of COP (from two incisors) were combined with measurements of fluid volumes (the other two incisors).
Measurements of colloid osmotic pressure
After isolation of pulp IF and plasma, the COP (n = 15) was measured in a colloid osmometer designed for submicrolitre samples, using membranes with a cut-off size of 30 kDa (Wiig et al. 1988).
Analysis of cytokines in IF and serum
Pulp IF and serum from 20 rats not given isotopes were isolated following the procedures described earlier. The graded microcapillaries with IF and isolated serum were stored at −80°C until multiplex cytokine assay was performed.
IL-1α, IL-1β, IL-2, IL-6, TNF-α and IFN-γ were measured simultaneously in pulp fluid or serum using a Lincoplex kit (Linco Research, St Charles, MO, USA) according to the manufacturer's instructions. This kit offers a multiplexed microsphere-based flow cytometric immunoassay using the Luminex technology (Luminex Corporation Austin, TX, USA). A broad range of standards (4.8–20 000 pg ml−1) was provided in the multiplex kit and the multiplexed assay was analysed on a flow cytometer (Luminex100, Luminex Corporation).
Immunohistochemistry
Incisor teeth from rats exposed to LPS for 3 h and from control rats (3 h vehicle) were removed and split, and the pulp tissue was fixed in 4% paraformaldehyde with 0.2% picric acid. The pulp tissue was then rinsed in 0.1 m phosphate buffer, soaked in 30% sucrose overnight and stored at −80°C until sectioning. The frozen specimens were then embedded in Tissue-Tek OCT compound (Sakura, Zoeterwoude, Netherlands), and 25 μm thick saggital sections were cut in a freezing (−20°C) slide microtome. The immunoreactions were performed on precoated glass slides (SuperFrost Plus, MenzelGlaser, Braunschweig, Germany). Alternate serial sections were incubated for 72 h in rat IL-1α (dilution 1 : 400, Endogen, MA, USA) or TNF-α (dilution 1 : 300, Endogen) polyclonal antibodies raised in rabbit, at 4°C. The specificity of the immune reaction was tested by omission of the primary antibody. Antigen–antibody complexes were detected by the avidin–biotin peroxidase (ABC) method, using a commercially available kit (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA) and visualized by 3,3′-diaminobenzidine (DAB, Sigma) in the presence of 0.2% (NH4)2Ni(SO4)26H2O to enhance the immunostaining. Finally, the sections were counterstained with methylene blue/azure II in 1% sodium borate and distilled water. The sections were then dehydrated in graded alcohol series, cleared in xylene and coverslipped with Eukitt (O. Kindler, Freiburg, Germany). The sections were evaluated in a Nikon photomicroscope (Nikon Eclipse E600, Nikon Instruments Inc., Japan).
Statistical analysis
All values are means ± s.e.m. unless otherwise stated. The data were analysed with one-way analysis of variance (ANOVA) or one-way repeated measures analysis of variance (RM-ANOVA) followed by Bonferroni's t test or Dunn's method (if the normality test failed). Student's paired t test was used for comparison of the COP measurements and unpaired t test for the distribution of fluid volumes. P < 0.05 was considered statistically significant.
Results
Isolation of pulp fluid, validation of the method
In pilot studies, pulp tissue was exposed to consecutive centrifugations from 106 to 424 g and the extracellular tracer ratio (R) in pulp centrifugate and plasma was calculated. Centrifugation at 424 g for 10 min averaged a fluid to plasma ratio of 51Cr-EDTA tracer 0.88 ± 0.02, significantly different from 1. By modifications of the centrifugation procedure (change of G-force and centrifugation time) we found that centrifugation at 239 g for 10 min was optimal since the extracellular tracer ratio was 1.05 ± 0.05 (n = 12 rats), not significantly different from 1.0. The corresponding ratio for the intravascular marker 125I-HSA was 0.08 ± 0.01 (n = 12 rats).
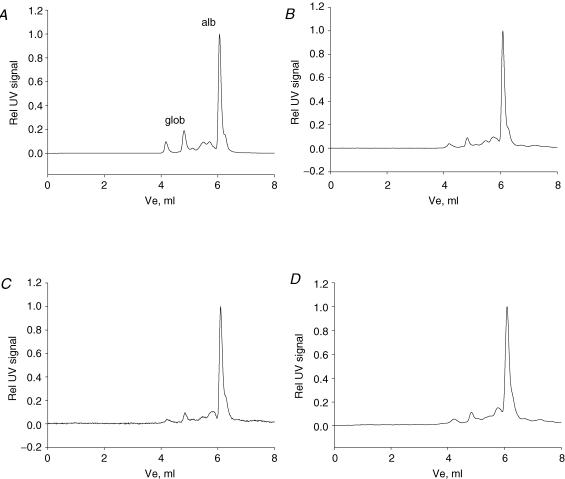
The HPLC pattern of pulp fluid isolated by centrifugation is shown in Fig. 1B, which also shows the elution pattern of plasma (Fig. 1A), pulp eluate (Fig. 1C) and pulp homogenate (Fig. 1D) for comparison. The elution pattern of pulp fluid is quite similar to plasma except for a relatively large albumin fraction in the former. We compared the fluid isolated from pulp tissue by centrifugation with the fluid eluted into a buffer (pulp eluate) to see whether the centrifugation process per se affected the fluid composition. In addition, we isolated fluid from pulp tissue homogenate. The pulp eluate displayed an HPLC elution pattern similar to pulp centrifugate. The elution pattern of pulp homogenate resembled that of plasma and pulp centrifugate for proteins with hydrodynamic radius larger or similar to albumin, while this pattern differed for smaller molecules having several peaks in the area with lower molecular weight. This area constituted 0.1, 8.7, 11.6 and 13.8% of the total area under the UV220 elution curve of plasma, pulp centrifugate, eluate and homogenate, respectively.
Figure 1. High performance liquid chromatography patterns.
Representative patterns for plasma and tissue fluid samples eluted in two TSK-gel size-exclusion columns coupled in series with an optimal separation range for globular proteins of 5–100 kDa and 10–500 kDa. The panels represent plasma (A), fluid isolated from the pulp of incisor teeth by centrifugation at 239 g for 10 min (B), pulp tissue eluate (C) and pulp tissue extract (D). alb, albumin; glob, globulins.
Fluid distribution volumes in pulp
Measurements of fluid distribution volumes in skin were performed in order to determine normal distribution of the isotopes at the extracellular and intravascular compartment. Total extracellular fluid volume (Vx) averaged 0.44 ± 0.01 ml (g w.w.)−1 and vascular volume Vv = 0.005 ± 0.0004 ml (g w.w.)−1, in agreement with previous data (Gyenge et al. 2003). The incisor pulp exhibited a high content of IF compared to skin, as Vi averaged 0.60 ± 0.03 ml (g w.w.)−1 and vascular volume Vv = 0.03 ± 0.01 ml (g w.w.)−1 (Table 1). No differences were observed in the fluid distribution volumes in the pulp after 1.5 and 3 h LPS exposure (Table 1).
Table 1.
Interstitial fluid volume (Vi) and vascular fluid volume (Vv) in rat pulp after i.v. administration of LPS (4.0 mg kg−1) or vehicle (0.1% BSA in 0.9% NaCl)
| Group | n | Vi (ml (g wet weight)−1) | Vv (ml (g wet weight)−1) |
|---|---|---|---|
| Control | 9 | 0.60 ± 0.03 | 0.03 ± 0.01 |
| 1.5 h LPS | 6 | 0.60 ± 0.02 (ns) | 0.03 ± 0.002 (ns) |
| 3 h LPS | 5 | 0.55 ± 0.05 (ns) | 0.04 ± 0.02 (ns) |
Values are means ± s.e.m., n = number of rats, ns, not significant from control values, unpaired t test, P < 0.05.
Pulpal blood flow and systemic blood pressure measurements during LPS exposure
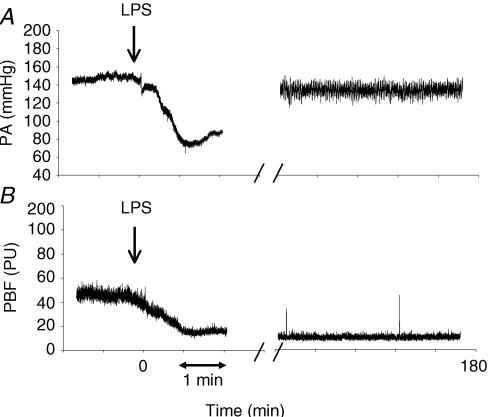
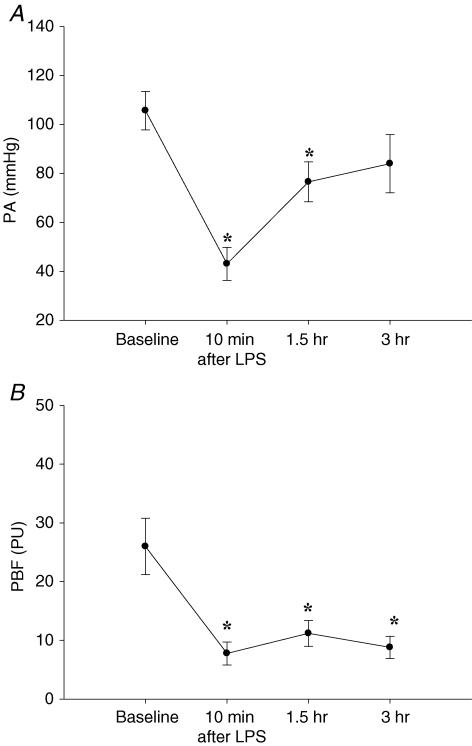
In 12 rats that received LPS, PBF was continuously recorded for 3 h. A significant drop in both PA and PBF within 10 min after LPS administration was observed. PA increased towards the end of the 3 h experimental period, whereas PBF remained low throughout the experimental period as exemplified in Fig. 2. PBF averaged 26.0 ± 4.8 perfusion units (PU) before LPS administration with corresponding PA 105.7 ± 7.9 mmHg (n = 12) (Fig. 3). PA dropped significantly within 10 min after LPS administration to 43.0 ± 6.8 mmHg (P < 0.001 compared to baseline values). PBF followed PA and dropped simultaneously to 7.8 ± 2.0 PU after LPS administration (P < 0.001) (Fig. 3). PA and PBF remained low for the first 1.5 h after LPS exposure with PA of 76.5 ± 8.2 mmHg (P < 0.003) and PBF of 11.2 ± 2.2 PU (P < 0.001) respectively (Fig. 3). At the end of the 3 h-observation period, PA increased to 84.0 ± 11.9 mmHg, whereas PBF remained significantly lower (8.8 ± 1.9 PU) than baseline values (P < 0.001) (Fig. 3).
Figure 2. Original simultaneous measurements of systemic blood pressure and pulpal blood flow before, during and after lipopolysaccharide infusion.
Original traces showing effect of intravenous injection of lipopolysaccharide (LPS), 4 mg kg−1 resulting in an experimental endotoxaemia, on arterial systemic blood pressure (PA) (A) and pulpal blood flow (PBF) (measured in arbitrary perfusion units (PU)) (B). Infusion of LPS (arrow) caused an almost immediate reduction in PA as well as PBF. Note that PA increased almost to levels seen before administration of LPS whereas, PBF remained low at the end of the 3 h-experimental period (right part of both panels).
Figure 3. Effect of lipopolysaccharide on blood pressure and pulpal blood flow.
Effect of intravenous injection of lipopolysaccharide (LPS), 4 mg kg−1, resulting in an experimental endotoxaemia, on arterial systemic blood pressure (PA) (A) and pulpal blood flow (PBF) (B) under control conditions (baseline) and various time points after infusion of LPS. PBF measured in arbitrary perfusion units (PU). Values are means ± s.e.m., n = 12; one-way RM-ANOVA, *P < 0.05 compared with baseline measurements.
Cytokine concentrations in serum and pulp IF
Controls
Serum and pulp IF from control animals exhibited undetectable or low levels of cytokines (Table 2). No differences were found in cytokine levels between serum and pulp IF (Fig. 4).
Table 2.
Cytokine levels (pg ml−1) in pulp interstitial fluid and serum from control rats (n = 8)
| IL-1α | IL-1β | IL-2 | IL-6 | TNFα | IFNγ | |
|---|---|---|---|---|---|---|
| Pulp IF | 31.1 ± 16 | 52.7 ± 35.4 | 68.4 ± 45.5 | 217 ± 140 | 44.3 ± 23.9 | 56.9 ± 36.7 |
| (0–91) | (0–229) | (0–289) | (0–760) | (7.0–154) | (0–199) | |
| Serum | 121.4 ± 109 | 2.0 ± 2.0 | 277.7 ± 201.7 | 1643 ± 1513 | 16.6 ± 7.3 | 513.7 ± 376.4 |
| (0–773) | (0–14) | (0–1440) | (0–10705) | (0–51) | (0–2758) |
Values are means ± s.e.m., range in parentheses.
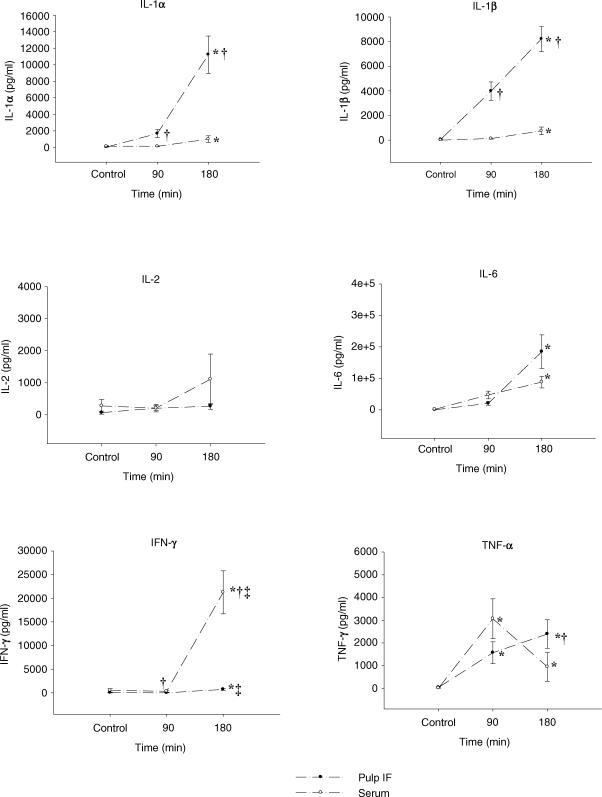
Figure 4. Effect of lipopolysaccharide on cytokines in pulp interstitial fluid and serum.
Concentration of cytokines in pulp interstitial fluid and serum in experimental endotoxaemia induced by intravenous injection of lipopolysaccharide (LPS), 4 mg kg−1. The experiment ended 90 min (n = 6) and 180 min (n = 6) after LPS administration. Controls (n = 8) received vehicle alone and were kept for an equivalent time (90 min, n = 4 and 180 min, n = 4). Values are means ± s.e.m.; ANOVA, *P < 0.05 compared with respective control values, †P < 0.05 compared with serum at the same time interval, ‡P < 0.05 compared with respective values at 90 min.
LPS-treated animals
The levels of the cytokines tested increased compared to control values in both serum and pulp IF, except for IL-2, which remained at constant levels during the experimental period (Fig. 4).
IL-1α level was statistically higher than control values in both serum (991 ± 400 pg ml−1) and IF (11222 ± 2255 pg ml−1) after 3 h. Local production of IL-1α in the pulp was evident as a several-fold higher concentration of this cytokine was found in pulp IF compared to serum after 1.5 and 3 h (Fig. 4). The same pattern was found for IL-1β with increased levels seen 3 h after LPS administration (748 ± 318 and 8211 ± 1013 pg ml−1 for serum and pulp IF, respectively) when compared with controls. Again, pulp IF expressed higher levels of IL-1β than serum throughout the observation period, indicating local production of this cytokine in the pulp tissue (Fig. 4).
IL-6 was increased at 3 h (88194 ± 18401 and 184958 ± 53275 pg ml−1 for serum and pulp IF, respectively) compared with controls. No difference in IL-6 concentration was observed between serum and IF (Fig. 4).
IFN-γ increased during the experimental period and 3 h post-LPS treatment. The concentration of IFN-γ was higher in both serum (21242 ± 4547 pg ml−1) and IF (734 ± 219 pg ml−1) compared with control values and levels exhibited at 1.5 h (336 ± 173 and 10 ± 6.4 pg ml−1 for serum and pulp IF, respectively). There was a differential response in serum and IF as well, but this inflammatory mediator showed higher levels in serum than in IF at both 90 and 180 min (Fig. 4).
TNF-α was also found to be increasing throughout the observation period with a slightly different pattern: the levels of TNF-α were significantly higher at 90 min (3061 ± 878 and 1569 ± 481 pg ml−1 for serum and pulp IF, respectively) and 180 min (946 ± 635 and 2393 ± 623 pg ml−1 for serum and pulp IF, respectively) compared with control values for both serum and IF, but it seemed that serum concentration reached a peak at 90 min, whereas TNF-α in IF continued to rise. There was a difference in TNF-α concentration between serum and pulp IF at 180 min (Fig. 4).
COP measurements
COP in pulp IF of control rats averaged 19.6 ± 1.3 mmHg with a corresponding COP in plasma of 23.6 ± 0.8 mmHg (n = 7) giving a significant difference (P < 0.05). The pulp fluid COP corresponded to 83% of control plasma COP. Since most differences in cytokine levels were found 3 h after LPS challenge, we measured COP in pulp fluid and plasma only in rats exposed to LPS for 3 h (n = 8). In three of these rats, plasma COP at the beginning of the experiments averaged 19.3 ± 0.3 mmHg. Three hours after LPS exposure, COP in pulp IF averaged 15.6 ± 1.1 mmHg (80.5 ± 5.6% of control plasma COP (n = 3)) whereas plasma COP dropped to 14.8 ± 1.0 mmHg (76.3 ± 5.4% of control plasma COP). There was no difference in COP levels between pulp IF and plasma at 3 h after LPS exposure.
Immunostaining
Since local production of IL-1α and TNF-α was found in LPS exposed pulps, we aimed at verifying the results by immunohistochemical staining of the pulp for the above mentioned cytokines.
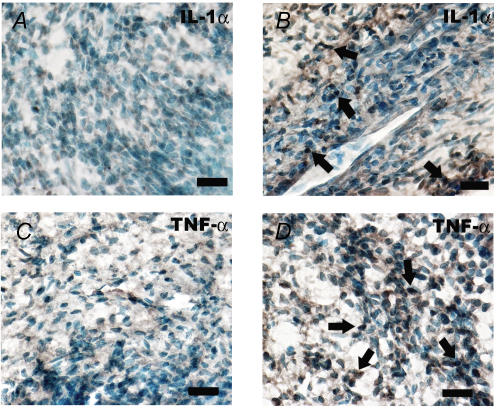
Pulp sections from the control rat exhibited scarce IL-1α-immunoreactive (IR) cells and TNF-α-IR cells in the pulp body. Pulp tissue from the rat exposed to LPS showed an increased number of both IL-1α- and TNF-α-IR cells in the pulp proper. The stained cells had either a round or an elongated appearance indicative of immune cells or fibroblasts, respectively (Fig. 5).
Figure 5. Immunohistochemical microphotographs of dental pulp from untreated and lipopolysaccharide-exposed rats.
A, saggital section of incisor pulp from control rat showing no IL-1α-immunoreactive (IR) cells in the pulp body. B, pulp section of rat challenged with lipopolysaccharide (3 h) showing numerous IL-1α-IR cells (arrows). Note that the stained cells are either round or elongated. C, few TNF-α-IR cells in the pulp tissue proper of control rat, whereas increased number of TNF-α-IR cells appears 3 h after LPS exposure (D) (arrows). Bars, 10 μm.
All the negative controls showed a lack of specific immunostaining.
Discussion
The microenvironment of the pulpal cells has not been thoroughly explored. One reason for this is that the nature of tooth pulp does not permit direct access without harming the tissue. Dentinal fluid represents pulp IF collected from cut dentin surfaces. Data regarding its composition has been published (Haldi et al. 1965; Coffey et al. 1970; Knutsson et al. 1994) but cutting the dentin surface and exposing the dentinal tubules induces inflammatory reactions in the tissue. In this study, we showed that it is possible to obtain pulp IF by centrifugation of pulp tissue at low G-force. By isolation of the pulp after cardiac arrest, we avoided inflammatory changes in the IF composition due to the procedure, and we could measure the levels of inflammatory mediators, as well as COP in the pulp IF. New information concerning intercellular communication and fluid exchange during health and disease could thereby be provided.
The pulp houses a number of tissue elements, namely nerves, vascular tissue, connective tissue fibres, ground substance, IF and cellular components. Cell compression during centrifugation may lead to the extrusion of cellular fluid, resulting in the isolation of a mixture of IF and cell fluid. 51Cr-EDTA was used as an extracellular marker. This probe is not metabolized and is not taken up by cells. A reduced ratio (R) of 51Cr-EDTA concentration (centrifugate relative to plasma) could reveal possible ‘contamination’ with cellular fluid. We evaluated the isolated fluid and concluded that centrifugation of the pulp tissue at 239 g for 10 min was optimal for the isolation of fluid representative for pulp IF since R was not significantly different from 1.0. Increased time and/or G force for centrifugation showed a reduction in R, suggesting dilution of the pulp centrifugate with intracellular fluid. The centrifugate was found to contain 8% of the intravascular tracer (125I-HSA). In addition, the HPLC pattern of isolated pulp fluid was similar to that of the plasma, and not different from the pattern of pulp tissue eluate (Fig. 1), indicating that the centrifugation process per se did not cause serious cell damage. The impression of a lower share of globulins in isolated pulp fluid than in plasma most likely reflects a size selectivity of the capillary membrane. Also the HPLC pattern of the pulp homogenate was similar to that of the pulp fluid. Only a small amount of low molecular weight substances were eluted in the column after albumin, most likely representing intracellular proteins. The dental pulp has a very high total water content (75% of total weight) (Berkovitz et al. 2002) with 63% localized extracellularly (present study), showing relatively low intracellular fluid volume (12%). It seems therefore reasonable to assume that the somewhat unexpectedly small contribution of non-plasma proteins of pulp tissue homogenate can be explained by its relatively low content of cellular components. Taken together, our data suggest that low speed pulp centrifugation is indeed a simple and reliable method for the isolation of pulp IF.
We showed that in normal pulp, the fluid isolated by centrifugation had a COP of ∼83% of that in plasma, showing a relatively high protein concentration in the pulp fluid during physiological condition, i.e. significantly higher than COP found in skin (Wiig et al. 2003) and muscle (Wiig et al. 1991). Three hours after LPS exposure, COP had fallen in plasma suggesting a substantial protein leakage to the interstitium of other tissues and areas (intestines, abdomen) (Aust et al. 1957; Dormehl et al. 1992) whereas the relative pulp fluid COP remained unchanged (80.5% of control plasma COP). It is likely that the permeability of the pulp vessels was increased after the LPS challenge as the COP in pulp IF and plasma was similar after the exposure. Furthermore, cytokines known to cause increased permeability, such as IL-1(Martin et al. 1988; Daffonchio et al. 2002), were up-regulated and LPS as such is known to have a direct effect on the endothelial barrier and to cause increased vascular permeability (Bannerman & Goldblum, 1999, 2003). In addition, the reduced pulp perfusion after LPS exposure most likely resulted in a reduced capillary pressure favouring fluid reabsorption from the interstitium and thereby an increased interstitial fluid colloid osmotic pressure. Such a mechanism may also partly explain our finding of a similar COP in plasma and pulp fluid in this situation. The fact that we found no changes in fluid volumes in the pulp during this model of acute inflammation may indicate that the dental pulp, enclosed in hard dentin, is such a low-compliant tissue that significant changes in fluid volumes are not possible or are too small to be detected with the method used.
However, PBF was significantly lower 3 h after LPS challenge, even though the systemic arterial pressure normalized, indicating impaired pulpal circulation. Organ failure occurs commonly in sepsis even after restoration of haemodynamic status, and can be related to the direct impairment of cellular function or hypoxia and/or redistribution of blood flow between and within the organs at the microcirculatory level (King et al. 1999; De Backer et al. 2002). De Backer et al. (2002) reported a reduced number of continuously perfused small vessels (< 20 μm) in the sublingual area in septic patients compared with controls. The very low perfusion observed in the pulp after LPS application, even after restoration of the systemic blood pressure, indicates that the pulpal tissue was seriously affected by LPS and developed organ dysfunction. In the clinical situation, bacterial infection frequently induces pulpal necrosis after periods with insufficient perfusion. The vulnerable pulp, due to its lack of collateral circulation, is prone to necrotize, resulting in the need for root canal treatment.
To the best of our knowledge, this is the first report quantifying cytokines in IF of normal and inflamed pulps. The IF has traditionally been considered a transport medium for nutrients and waste products, but it also represents a communication medium between cells. There are several reports regarding elevated levels of cytokines in pulpal inflammation (Tani-Ishii et al. 1995; Rauschenberger et al. 1997; Barkhordar et al. 1999; Kim & Lim, 2002; Pezelj-Ribaric et al. 2002; Zehnder et al. 2003), but these were done in tissue homogenates including both cellular and blood-derived cytokines in the samples. In addition, measurements of cytokines in both serum and IF could give more information on inflammatory processes occurring locally at tissue level.
We investigated six of the most common pro-inflammatory cytokines and a main finding was the differential cytokine response in serum and pulp IF. Low levels of cytokines were detected in the control rats in agreement with previously reported data from normal pulp (Bletsa et al. 2004; Kawashima et al. 2005). Elevated levels of cytokines were found after LPS challenge but the main finding was the differential cytokine response in serum and pulp IF. Specifically, IL-1α and IL-1β showed up to 11 times higher concentrations in pulp IF compared with serum after 3 h LPS challenge. This indicates that these cytokines are locally produced in the pulp and released into the interstitium. This finding may be explained by the abundance of various types of macrophages in the normal dental pulp (Jontell et al. 1987; Okiji et al. 1992) and their role in defence mechanisms following exogenous invasion of pathogenic stimuli. Activated macrophages represent the major source of IL-1, and are likely to be responsible for the high local production of the pro-inflammatory cytokines IL-1α and IL-1β in inflamed pulp since the producing cells already reside in the tissue. In addition, cells at the odontoblastic layer of normal rat pulp stain positively for IL-1α (Bletsa et al. 2004) and they might play a role in this prompt cytokine production during pulpitis. Immunohistochemical analysis of pulp tissue confirmed the presence of numerous IL-1α-IR cells 3 h after LPS exposure.
IFN-γ concentration in the serum was significantly higher than pulp IF, suggesting cytokine transport across the microvasculature from plasma to pulp IF. Although local production of this mediator cannot be excluded, it is more reasonable to conclude that it is blood derived since it was the only cytokine found at higher concentration in serum compared to pulp IF throughout the whole experimental period. Natural killer cells (NK) are the predominant IFN-γ-producing cells after LPS challenge (Varma et al. 2002) and they are scarcely found in normal pulp. The diverse source of IFN-γ compared to the locally produced IL-1 could clarify the difference in levels of those cytokines in serum and IF.
A surprising result was that there were minimal changes in the IL-2 levels after endotoxin administration. There are contradictory results in the literature regarding IL-2 levels in dental pulps. Elevated levels of IL-2 have been related to pulpal inflammation (Rauschenberger et al. 1997). However, later studies found no differences in IL-2 levels in healthy and diseased pulps, suggesting a favourable Th2 cytokine production in inflamed pulp tissue (Anderson et al. 2002). A shift in cell responses from Th1 to Th2 subtype appears to be a common finding in sepsis, with the ultimate goal of protecting the organism from systemic ‘overshooting’ with Th1 pro-inflammatory cytokines (Ayala et al. 1994) and IL-2 belongs to the latter category of cytokines. It should be kept in mind that this study utilized a model of sepsis in order to achieve pulpitis.
IL-6 was the cytokine that expressed the highest levels in both IF and serum. No differences were seen between pulp IF and serum. It cannot therefore be concluded that this cytokine is systemically and/or locally produced under the experimental conditions. IL-6 represents the main mediator of the acute phase reaction that takes place shortly after the onset of infections (Akira et al. 1990; Kishimoto et al. 1992), and it and IL-1 and TNF-α are the major pro-inflammatory cytokines involved in the septic condition (Ertel et al. 1991). Physiological stimuli for its synthesis are LPS, IL-1 and TNF-α, all present in high levels in the current model of acute inflammation. Production of IL-6 after bacterial challenge of cultured human pulp cells (Matsushima et al. 1998; Tokuda et al. 2001), as well as detection of increased levels of IL-6 in inflamed human pulp and periapical lesions (Barkhordar et al. 1999), strongly suggests the involvement of this cytokine in pulpal inflammation.
TNF-α reached a peak in serum at 90 min after LPS challenge. By contrast, TNF-α continued to rise in the pulp IF throughout the 3 h experimental period. TNF-α is secreted by macrophages, monocytes, neutrophils, T-cells and NK cells following stimulation by bacterial LPS, justifying both local and systemic production of this cytokine. Locally, in the pulp, up-regulation of TNF-α-IR cells was observed 3 h after LPS challenge.
In a recent study exploring the role of cytokines in pulpal and periapical inflammation, the kinetics of pro-inflammatory cytokine expression were evaluated in a rat pulpitis model (Kawashima et al. 2005). Slight expression of IL-1α and IL-1β was observed at the onset of the experiment. LPS applied directly in contact with the pulp tissue after pulp exposure induced the expression of IL-6 and TNF-α and also enhanced the expression of IL-1α and IL-1β at 3 h with a peak for all the above cytokines at 6 h. Although a different quantification method was used (RT-PCR) on the entire pulp tissue extract, our results can be related to this study since IL-1α, IL-1β, TNF-α and IL-6 had significantly higher levels in the pulp IF at 3 h post LPS-treatment.
Few studies exist on cytokines in IF but recently, levels of IL-1β and TNF-α in skin IF and serum from rats with endotoxaemia were measured (Nedrebø et al. 2004) and a differential cytokine response in serum and skin IF was shown, with local production of IL-1β and systemic production of TNF-α. Our results are in line with the results from skin regarding local production of IL-1. However, also TNF-α was found to be locally produced in the pulp, suggesting a different tissue response to LPS challenge between skin and dental pulp. IL-1 and TNF-α have been shown to induce lowering of interstitial fluid pressure thereby facilitating oedema in skin and oral mucosa (Nedrebø et al. 1999; Bletsa et al. 2006). We can only speculate about the detrimental effect of this local IL-1 and TNF-α production during inflammation in the pulp that has extremely limited ability to expand.
There is growing evidence that cytokines are involved in the generation of pain and hyperalgesia (Sommer & Kress, 2004. The pathways by which cytokines affect brain function have also been under investigation. Cytokine-to-brain communication via blood is one route, but it cannot explain the fact that illness responses are often observed under situations in which blood levels of cytokines are very low (Kluger, 1991). Therefore a neural route rather than a humoral one has been suggested (Watkins et al. 1995). By such a mechanism, cytokines do not need to rise systemically because increases in local tissue concentrations would be sufficient to induce illness responses and pain. In this context, our findings of local production of IL-1 and TNF-α in the nerve-rich dental pulp may have a direct clinical significance in pain development during pulpitis.
In summary, this study established a reliable method for isolating pulp IF. We applied this method for measuring cytokine levels and colloid osmotic pressure in pulp IF in order to study the pathophysiology of pulpitis. We have shown that certain pro-inflammatory mediators were markedly up-regulated in the pulp interstitium during acute pulpitis. Moreover, we obtained novel data regarding fluid exchange in dental pulp during health and disease. Analysis of pulp IF offers a better understanding of the mechanisms involved in inflammatory reactions.
Acknowledgments
We would like to acknowledge Karl Brokstad, Odd Kolmannskog, Matthias Hofmann, A^se Eriksen and Siren Østvold for expert technical assistance. Financial support was provided from the Medical Faculty, Locus no. 230624; Faculty of Dentistry, Project no. 101330, University of Bergen and from the Research Council of Norway.
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- Anderson LM, Dumsha TC, McDonald NJ, Spitznagel JK., Jr Evaluating IL-2 levels in human pulp tissue. J Endod. 2002;28:651–655. doi: 10.1097/00004770-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Aukland K. Distribution volumes and macromolecular mobility in rat tail tendon interstitium. Am J Physiol. 1991;260:H409–H419. doi: 10.1152/ajpheart.1991.260.2.H409. [DOI] [PubMed] [Google Scholar]
- Aust JB, Johnson JA, Visscher MB. Plasma sequestration in endotoxin shock. Surg Forum. 1957;8:8–10. [PubMed] [Google Scholar]
- Ayala A, Deol ZK, Lehman DL, Herdon CD, Chaudry IH. Polymicrobial sepsis but not low-dose endotoxin infusion causes decreased splenocyte IL-2/IFN-γ release while increasing IL-4/IL-10 production. J Surg Res. 1994;56:579–585. doi: 10.1006/jsre.1994.1092. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium: barrier function and injury. Laboratory Invest. 1999;79:1181–1199. [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- Barkhordar RA, Hayashi C, Hussain MZ. Detection of interleukin-6 in human dental pulp and periapical lesions. Endod Dent Traumatol. 1999;15:26–27. doi: 10.1111/j.1600-9657.1999.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Berkovitz BKB, Holland GR, Moxham BJ. Oral Anatomy, Histology and Embryology. 3. Edinburgh: Harcourt Publishers Limited; 2002. Dental pulp; pp. 149–167. [Google Scholar]
- Bletsa A, Heyeraas KJ, Haug SR, Berggreen E. IL-1α and TNF-α expression in rat periapical lesions and dental pulp after unilateral sympathectomy. Neuroimmunomodulation. 2004;11:376–384. doi: 10.1159/000080148. [DOI] [PubMed] [Google Scholar]
- Bletsa A, Nedrebø T, Heyeraas K, Berggreen E. Edema in oral mucosa after LPS or cytokine exposure. J Dent Res. 2006 doi: 10.1177/154405910608500509. in press. [DOI] [PubMed] [Google Scholar]
- Coffey CT, Ingram MJ, Bjorndal AM. Analysis of human dentinal fluid. Oral Surg Oral Med Oral Pathol. 1970;30:835–837. doi: 10.1016/0030-4220(70)90348-8. [DOI] [PubMed] [Google Scholar]
- Daffonchio L, Novellini R, Bertuglia S. Protective effect of ketoprofen lysine salt on interleukin-1β and bradykinin induced inflammatory changes in hamster cheek pouch microcirculation. Inflamm Res. 2002;51:223–228. doi: 10.1007/pl00000297. [DOI] [PubMed] [Google Scholar]
- De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- Dormehl IC, Hugo N, Pretorius JP, Redelinghuys IF. In vivo assessment of regional microvascular albumin leakage during E. coli septic shock in the baboon model. Circ Shock. 1992;38:9–13. [PubMed] [Google Scholar]
- Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214:141–148. doi: 10.1097/00000658-199108000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyenge CC, Tenstad O, Wiig H. In vivo determination of steric and electrostatic exclusion of albumin in rat skin and skeletal muscle. J Physiol. 2003;552:907–916. doi: 10.1113/jphysiol.2003.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldi J, Law ML, John K. Comparative concentration of various constituents of blood plasma and dental-pulp fluid. J Dent Res. 1965;44:427–430. doi: 10.1177/00220345650440022001. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Berggreen E. Interstitial fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Medical. 1999;10:328–336. doi: 10.1177/10454411990100030501. [DOI] [PubMed] [Google Scholar]
- Heyeraas KJ, Kim S, Raab WH, Byers MR, Liu M. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc Res. 1994;47:329–343. doi: 10.1006/mvre.1994.1026. [DOI] [PubMed] [Google Scholar]
- Jontell M, Gunraj MN, Bergenholtz G. Immunocompetent cells in the normal dental pulp. J Dent Res. 1987;66:1149–1153. doi: 10.1177/00220345870660061101. [DOI] [PubMed] [Google Scholar]
- Kawashima N, Nakano-Kawanishi H, Suzuki N, Takagi M, Suda H. Effect of NOS inhibitor on cytokine and COX2 expression in rat pulpitis. J Dent Res. 2005;84:762–767. doi: 10.1177/154405910508400815. [DOI] [PubMed] [Google Scholar]
- Kerezoudis NP, Olgart L, Edwall L. Evans blue extravasation in rat dental pulp and oral tissues induced by electrical stimulation of the inferior alveolar nerve. Arch Oral Biol. 1993;38:893–901. doi: 10.1016/0003-9969(93)90099-8. [DOI] [PubMed] [Google Scholar]
- Khabbaz MG, Anastasiadis PL, Sykaras SN. Determination of endotoxins in the vital pulp of human carious teeth: association with pulpal pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:587–593. doi: 10.1067/moe.2001.113831. [DOI] [PubMed] [Google Scholar]
- Kim SA, Lim SS. T lymphocyte subpopulations and interleukin-2, interferon-γ, and interleukin-4 in rat pulpitis experimentally induced by specific bacteria. J Endod. 2002;28:202–205. doi: 10.1097/00004770-200203000-00014. [DOI] [PubMed] [Google Scholar]
- King CJ, Tytgat S, Delude RL, Fink MP. Ileal mucosal oxygen consumption is decreased in endotoxemic rats but is restored toward normal by treatment with aminoguanidine. Crit Care Med. 1999;27:2518–2524. doi: 10.1097/00003246-199911000-00032. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- Kluger MJ. Fever: Role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson G, Jontell M, Bergenholtz G. Determination of plasma proteins in dentinal fluid from cavities prepared in healthy young human teeth. Arch Oral Biol. 1994;39:185–190. doi: 10.1016/0003-9969(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Martin S, Maruta K, Burkart V, Gillis S, Kolb H. IL-1 and IFN-γ increase vascular permeability. Immunology. 1988;64:301–305. [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Ohbayashi E, Takeuchi H, Hosoya S, Abiko Y, Yamazaki M. Stimulation of interleukin-6 production in human dental pulp cells by peptidoglycans from Lactobacillus casei. J Endod. 1998;24:252–255. doi: 10.1016/S0099-2399(98)80107-6. [DOI] [PubMed] [Google Scholar]
- Morgan CR, Rodd HD, Clayton N, Davis JB, Boissonade FM. Vanilloid receptor 1 expression in human tooth pulp in relation to caries and pain. J Orofac Pain. 2005;19:248–260. [PubMed] [Google Scholar]
- Nedrebø T, Berg A, Reed RK. Effect of tumor necrosis factor-alpha, IL-1β, and IL-6 on interstitial fluid pressure in rat skin. Am J Physiol. 1999;277:H1857–H1862. doi: 10.1152/ajpheart.1999.277.5.H1857. [DOI] [PubMed] [Google Scholar]
- Nedrebø T, Reed RK, Jonsson R, Berg A, Wiig H. Differential cytokine response in interstitial fluid in skin and serum during experimental inflammation in rats. J Physiol. 2004;556:193–202. doi: 10.1113/jphysiol.2003.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiji T, Kawashima N, Kosaka T, Matsumoto A, Kobayashi C, Suda H. An immunohistochemical study of the distribution of immunocompetent cells, especially macrophages and Ia antigen-expressing cells of heterogeneous populations, in normal rat molar pulp. J Dent Res. 1992;71:1196–1202. doi: 10.1177/00220345920710051201. [DOI] [PubMed] [Google Scholar]
- Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. Detection of tumor necrosis factor α in normal and inflamed human dental pulps. Arch Med Res. 2002;33:482–484. doi: 10.1016/s0188-4409(02)00396-x. [DOI] [PubMed] [Google Scholar]
- Rauschenberger CR, Bailey JC, Cootauco CJ. Detection of human IL-2 in normal and inflamed dental pulps. J Endod. 1997;23:366–370. doi: 10.1016/S0099-2399(97)80184-7. [DOI] [PubMed] [Google Scholar]
- Sindet-Pedersen S, Petersen JK, Gotzsche PC. Incidence of pain conditions in dental practice in a Danish county. Community Dent Oral Epidemiol. 1985;13:244–246. doi: 10.1111/j.1600-0528.1985.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Tani-Ishii N, Wang CY, Stashenko P. Immunolocalization of bone-resorptive cytokines in rat pulp and periapical lesions following surgical pulp exposure. Oral Microbiol Immunol. 1995;10:213–219. doi: 10.1111/j.1399-302x.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Tokuda M, Sakuta T, Fushuku A, Torii M, Nagaoka S. Regulation of interleukin-6 expression in human dental pulp cell cultures stimulated with Prevotella intermedia lipopolysaccharide. J Endod. 2001;27:273–277. doi: 10.1097/00004770-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin Diagn Laboratory Immunol. 2002;9:530–543. doi: 10.1128/CDLI.9.3.530-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfvinge J, Dahlen G, Bergenholtz G. Dental pulp response to bacterial cell wall material. J Dent Res. 1985;64:1046–1050. doi: 10.1177/00220345850640080401. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Goehler LE, Relton J, Brewer MT, Maier SF. Mechanisms of tumor necrosis factor-α (TNF-α) hyperalgesia. Brain Res. 1995;692:244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol Heart Circ Physiol. 2003;284:H416–H424. doi: 10.1152/ajpheart.00327.2002. [DOI] [PubMed] [Google Scholar]
- Wiig H, Halleland EG, Fjaertoft M, Aukland K. Measurement of colloid osmotic pressure in submicrolitre samples. Acta Physiol Scand. 1988;132:445–452. doi: 10.1111/j.1748-1716.1988.tb08351.x. [DOI] [PubMed] [Google Scholar]
- Wiig H, Sibley L, DeCarlo M, Renkin EM. Sampling interstitial fluid from rat skeletal muscles by intermuscular wicks. Am J Physiol. 1991;261:H155–H165. doi: 10.1152/ajpheart.1991.261.1.H155. [DOI] [PubMed] [Google Scholar]
- Zehnder M, Du Delaleu N, Y Bickel M. Cytokine gene expression – part of host defence in pulpitis. Cytokine. 2003;22:84–88. doi: 10.1016/s1043-4666(03)00116-9. [DOI] [PubMed] [Google Scholar]