Abstract
High concentrations of pituitary adenylate cyclase-activating polypeptide (PACAP) receptors are present in the external granule cell layer of the rat cerebellum during postnatal development. In vitro studies have shown that PACAP promotes cell survival and neurite outgrowth on immature cerebellar granule cells in primary culture. In the present study, we have investigated the effect of PACAP on the development of the cerebellar cortex of 8-day-old rats. Incubation of cultured granule cells for 12 or 18 h with PACAP provoked a significant increase in the rate of incorporation of [3H]thymidine in cultured granule cells, suggesting that PACAP could stimulate the proliferation of granule cells. After 96 h of treatment, in vivo administration of PACAP provoked a transient increase in the number of granule cells in the molecular layer and in the internal granule cell layer. In contrast, PACAP did not affect the number of Purkinje cells. The augmentation of the number of granule cells evoked by PACAP was significantly inhibited by the PACAP receptor antagonist PACAP(6–38). Administration of PACAP also caused a significant increase in the volume of the cerebellar cortex. The present study provides evidence that PACAP can act in vivo as a trophic factor during rat brain development. Our data indicate that PACAP increases proliferation and/or inhibits programmed cell death of granule cells, as well as stimulating neuronal migration from the external granule cell layer toward the internal granule cell layer.
Keywords: ontogenesis, cell proliferation, neurogenesis, neuropeptides
The cerebellum is one of the few regions of the rat brain that undergo profound morphogenetic transformations after birth. Postnatal development of the cerebellum mainly consists of proliferation, migration, and differentiation of granule cells. Developing cerebellar granule cells thus provide a suitable model in which to study the molecular basis of neuronal histogenesis. During the first weeks of postnatal life, immature neurons generated in the external granule cell layer (EGL) migrate along radial processes of glial cells through the molecular layer to reach their final destination within the internal granule cell layer (IGL; refs. 1–4). Only half of the number of granule cells will give rise to mature neurons, as massive cell loss occurs in the EGL and, later on, in the IGL (5, 6). Although considerable progress has been made toward identifying the cell and guidance systems that regulate multiplication, differentiation, migration, and death of granule cells (6–12), the molecular signals involved in the control of these processes remain largely unknown.
The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors are actively expressed in the rat cerebellum during postnatal development (13–16). In particular, high concentrations of PACAP receptors are found in the EGL between postnatal day 4 (P4) and P20 (13), a period that corresponds to intense neurogenesis (17). Characterization of these binding sites indicated that they correspond to functional receptors positively coupled to adenylyl cyclase and phospholipase C (18–20). In vitro experiments conducted on cerebellar granule cells cultured in conditions promoting apoptosis have shown that PACAP is capable of retarding cell death (20–23) and stimulating neurite outgrowth (23). Recent studies have shown that the effect of PACAP on cell survival is mediated through activation of the adenylyl cyclase pathway, leading to phosphorylation of the ERK-type mitogen-activated protein kinases (20) and to an increase in c-fos gene expression (24).
The occurrence of both PACAP and its receptors in the immature cerebellum, together with the ability of PACAP to enhance survival of cultured granule cells, strongly suggests that PACAP may act as a trophic factor in the rat cerebellum during development. However, the neurotrophic action of PACAP on the developing rat brain has never been studied in vivo. In the present report, we have investigated the effect of local administration of PACAP on the proliferation and migration of granule cells during histogenesis of the rat cerebellum.
MATERIALS AND METHODS
Chemicals.
The 38-aa form of PACAP (PACAP38; ref. 25) was synthesized by the solid-phase methodology as described (26). PACAP27 and PACAP(6–38) were obtained from American Peptide (Sunnyvale, CA). PACAP27 was radiolabeled by means of the lactoperoxidase technique and purified by reversed-phase HPLC analysis as described (13).
Animals.
A total of 324 young Wistar rats (Dépré, Saint Doulchard, France) were used for this study. After parturition, homogenous litters of 12 pups were left with their mothers. The animals were kept in a ventilated room, at a temperature of 21 ± 1°C, under a 12-h light/12-h dark cycle (light on between 7: 00 a.m. and 7: 00 p.m.). The young rats were lightly anesthetized with ether and injected over 1 min under the skull by using a 50-μl microsyringe (Hamilton) connected to a needle (0.5-mm diameter) equipped with a guard limiting its penetration to 6 mm from the insertion point. The injection (15 μl) was made free-hand at a point 4 mm behind the interaural line (27); the syringe was held horizontally so that the tip of the needle was positioned in the subarachnoid space between the skull and the surface of the cerebellum. Control experiments performed with trypan blue have shown that the needle did not penetrate into the neuroepithelium and that the dorsal part of the cerebellar hemispheres and vermis were completely bathed with the dye. After injection, the needle was left in situ for 1 min to avoid reflux on withdrawal. Animal manipulations were performed according to the recommendations of the French Ethical Committee and under the supervision of authorized investigators.
Cell Culture.
Granule cell suspensions were prepared from cerebella of P8 Wistar rats as described (28). Dispersed cells were seeded in multiwell plates (Costar) coated with poly-l-lysine (5 × 10−3 M) at a density of 5 × 10−5 cells per well. The cells were cultured in a chemically defined medium consisting of 75% (vol/vol) DMEM and 25% (vol/vol) Ham’s F-12 medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 25 mM KCl, the N-2 supplement (×100; Life Technologies, Grand Island, NY), and 1% of an antibiotic–antimicotic solution (10,000 units/ml penicillin/10 mg/ml streptomycin/25 μg/ml fungizone; BioWhittaker). These culture conditions did not favor replication of nonneuronal cells (29). Cells were grown at 37°C in a humidified incubator with an atmosphere of 5% CO2.
[3H]Thymidine Incorporation.
At 2 h after being seeded in the plates, cerebellar granule cells were incubated with [3H]thymidine (2 μCi) in the presence or absence of various concentrations of PACAP (10−10 to 10−6 M) over 6, 12, 18, or 24 h. Cells were washed three times in PBS and dissolved in Ultima Flo (Packard), and the radioactivity was counted in a liquid scintillation counter (LKB 1217 RackBeta, EG & G Wallac, Evry, France).
Morphometric Studies.
P8 rats were injected with 15 μl of vehicle alone or PACAP38 (0.01 μg or 1 μg) and/or PACAP(6–38) dissolved in vehicle. The injections were repeated every 48 h, and P8, P10, P12, P14, or P16 animals were killed by decapitation. Frozen cerebella were cut in the frontal plane into 10-μm-thick serial sections. One section from every 100 μm of tissue was stained by Cresyl Violet, and the areas of the cerebellar cortex and medulla were measured by means of a computer-assisted image analysis station bio500 (Biocom, Les Ulis, France). The volumes of the cortex and medulla were calculated by integrating the areas of each structure.
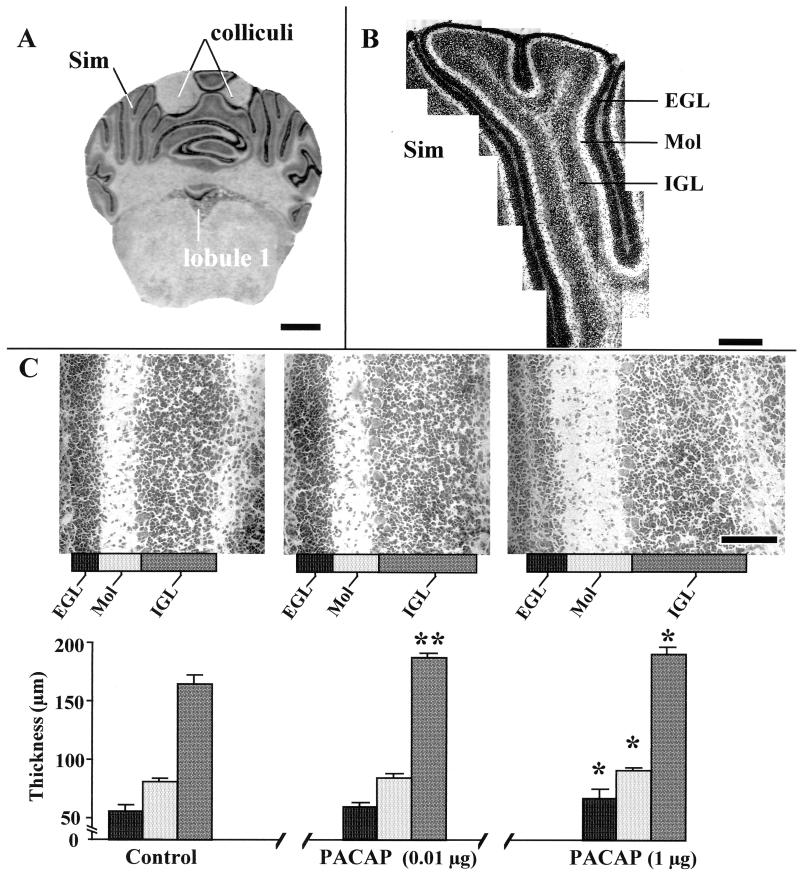
The thickness of the cerebellar cortical layers and the densities of granule and Purkinje cells were measured in the lobulus simplex (Sim) by using a confocal laser-scanning microscope (Noran Instruments, Middleton, WI). Quantification was systematically performed at the level of the caudal extremity of the colliculi and the rostral extremity of lobule 1 (see Fig. 2A). Morphological analysis was carried out by using intervision software (Noran Instruments) on over 3,500 confocal laser-scanning microscope acquisitions.
Figure 2.
Effects of graded doses of PACAP on the histogenesis of the Sim in P12 rat cerebella. (A) Typical Cresyl Violet staining of a rat cerebellum slice at the caudal extremity of the colliculi and the rostral extremity of lobule 1, showing the type of section used for measurements with the confocal laser-scanning microscope. (Bar = 1.4 mm.) (B) Higher magnification of a Sim showing the series of fields that were used for the measurement of the thickness of the EGL, the molecular layer (Mol), and the IGL, as well as for the measurement of the density of granule cells in the molecular layer and IGL. (Bar = 400 μm.) (C) Quantification of the thickness of the EGL, molecular layer, and IGL from the Sim of P12 rats. (Bar = 100 μm.) Each value represents the mean ± SEM of a representative experiment performed in triplicate. ∗, P < 0.05 vs. control; ∗∗, P < 0.01 vs. control.
Autoradiographic Studies.
Cryostat-cut (10-μm thick) sections of cerebella from control or PACAP-treated animals were incubated with 40 pM [125I]PACAP27 in the absence (total binding) or in the presence of 10−12 to 10−5 M unlabeled PACAP38 as described (13). Autoradiograms were quantified by means of the computer-assisted image analysis system bio500. IC50 values were determined in the EGL by using the sigmaplot program (Jandel, Corte Madera, CA).
RESULTS
Effect of PACAP on [3H]Thymidine Incorporation by Cerebellar Granule Cells.
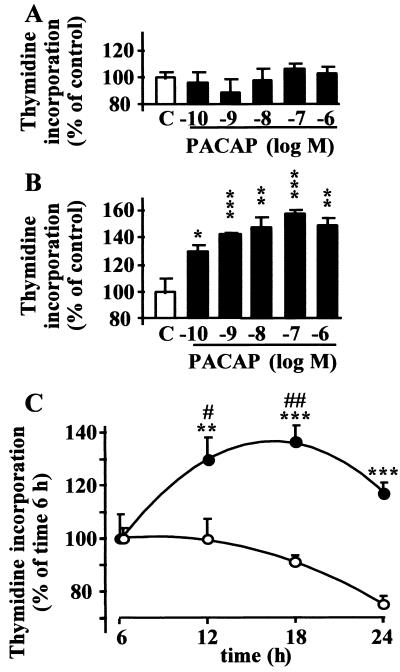
Incubation of cultured granule cells for 6 h with PACAP (10−10 to 10−6 M) did not affect the rate of incorporation of [3H]thymidine (Fig. 1A). In contrast, after 18 h of incubation, PACAP induced a concentration-dependent stimulation of [3H]thymidine incorporation (Fig. 1B). Indeed, exposure of granule cells to PACAP (10−9 M) for 6–24 h induced a time-dependent increase in [3H]thymidine incorporation with a maximum effect after an 18-h incubation (P < 0.01; Fig. 1C).
Figure 1.
Effect of PACAP on [3H]thymidine incorporation by cultured cerebellar granule cells. (A and B) Effect of graded concentrations of PACAP on [3H]thymidine incorporation. Cerebellar granule cells were exposed to PACAP for 6 h (A) or 18 h (B). Data are expressed as percentage of control. (C) Time-course of the effect of PACAP on [3H]thymidine incorporation. Cerebellar granule cells were incubated in control conditions (○) or in the presence of 10−9 M PACAP (●) for durations ranging from 6 to 24 h. Each value represents the mean ± SEM of at least three independent experiments performed in triplicate. ∗, P < 0.05 vs. control; ∗∗, P < 0.01 vs. control; ∗∗∗, P < 0.001 vs. control; #, P < 0.05 vs. time 6 h; ##, P < 0.01 vs. time 6 h.
Morphogenetic Effects of PACAP on the Cerebellar Cortex.
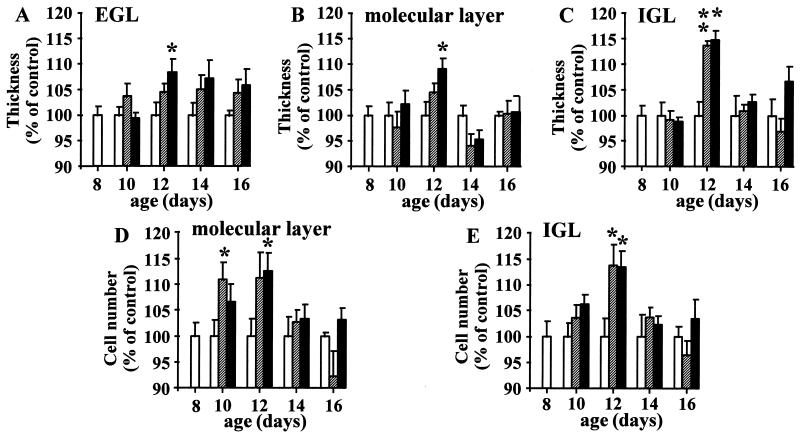
To study the effect of PACAP on cerebellar development, P8 rats were injected with 0.01 or 1 μg PACAP. The injection was repeated every 48 h, and the animals were killed at P8, P10, P12, P14, or P16. The thickness of the molecular layer and IGL and the number of cells in these two layers were measured in the Sim with a confocal laser-scanning microscope as described by Paxinos and Watson (ref. 27; Fig. 2 A and B). The thickness of the EGL was also measured; however, the cell number was not quantified, because the cell density in this layer is too high to allow cell segmentation (Fig. 2 B and C). A 4-day treatment (from P8 to P12) with 1 μg of PACAP induced a significant increase (P < 0.05) in the thickness of the EGL (Figs. 2C and 3A), the molecular layer (Figs. 2C and 3B), and the IGL (Figs. 2C and 3C). Both a 2-day treatment with 0.01 μg of PACAP and a 4-day treatment with 1 μg of PACAP induced a significant augmentation (P < 0.05) of the number of cells in the molecular layer (Fig. 3D). Administration of 0.01 μg or 1 μg of PACAP for 4 days also significantly increased (P < 0.05) the number of cells in the IGL (Fig. 3E). The effect of PACAP on the thickness of the EGL, molecular layer, and IGL, as well as on the number of cells in the molecular layer and IGL was transient, and the responses vanished 6 to 8 days after treatment (Fig. 3 A–E).
Figure 3.
Time-course of the effect of PACAP on the thickness of the EGL (A), the thickness of the molecular layer (B), the thickness of the IGL (C), the number of granule cells crossing the molecular layer (D), and the number of granule cells in the IGL (E). P8 rats were treated with saline (open bars), 0.01 μg of PACAP (hatched bars), or 1 μg of PACAP (black bars) up to P16. Each value represents the mean ± SEM of at least three experiments performed in triplicate. ∗, P < 0.05 vs. control; ∗∗, P < 0.01 vs. control.
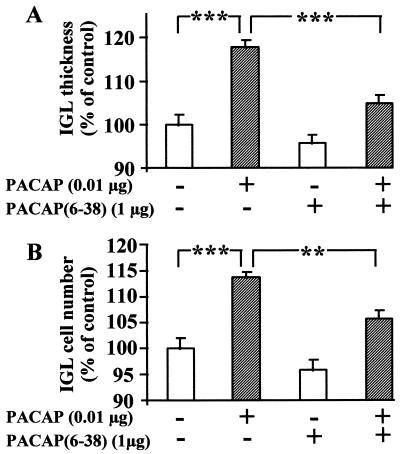
To test the specificity of the action of PACAP, P8 rats were treated with 0.01 μg of PACAP in the absence or presence of 1 μg of the specific PACAP antagonist PACAP(6–38). As already shown in Figs. 2C and 3C, a 4-day treatment with 0.01 μg of PACAP induced a marked increase (P < 0.001) in the thickness of the IGL (Fig. 4A). Injection of 1 μg of PACAP(6–38) alone did not significantly modify the thickness of the EGL, while concomitant administration of 0.01 μg of PACAP and 1 μg of PACAP(6–38) strongly attenuated (P < 0.001) the effect of PACAP (Fig. 4A). Similarly, PACAP(6–38) had no significant effect on the number of cells in the IGL but markedly reduced (P < 0.01) the stimulatory effect of PACAP on the number of cells in the IGL (Fig. 4B).
Figure 4.
Effects of the PACAP antagonist PACAP(6–38) on the PACAP-induced increase of the thickness (A) and number (B) of cells in the IGL. P8 rats were treated with saline, 0.01 μg of PACAP, and/or 1 μg of PACAP(6–38). Injections were renewed at P10, and animals were killed at P12. Data are expressed as percentage of control. Each value represents the mean ± SEM of three experiments performed in triplicate. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
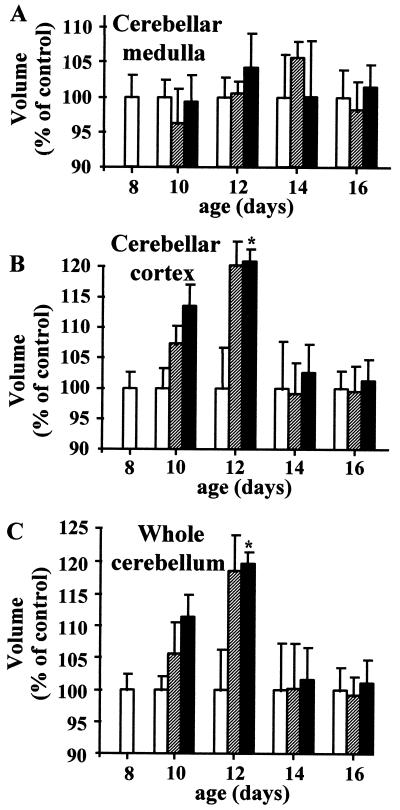
Because PACAP receptors are expressed by Purkinje cells (30) and occur in the medulla (31), we have investigated the ability of PACAP to regulate the number of Purkinje cells as well as the volume of the cortex and medulla. After 4 days of treatment, no significant difference in the number of Purkinje cells was found between control and animals treated with 1 μg of PACAP (28.9 ± 0.9 and 30.2 ± 2 cells per mm, respectively). In addition, treatment with 0.01 or 1 μg of PACAP did not significantly affect the volume of the cerebellar medulla (Fig. 5A). In contrast, a 4-day treatment with 1 μg of PACAP induced a significant increase in the volume of the cerebellar cortex (Fig. 5B) and, as a result, in the volume of the whole cerebellum (Fig. 5C).
Figure 5.
Time-course of the effect of PACAP on the volume of the cerebellar medulla (A), the cerebellar cortex (B), and the whole cerebellum (C). P8 rats were treated with saline (open bars), 0.01 μg of PACAP (hatched bars), or 1 μg of PACAP (black bars) up to P16. Each value represents the mean ± SEM of at least three experiments performed in triplicate. ∗, P < 0.05 vs. control.
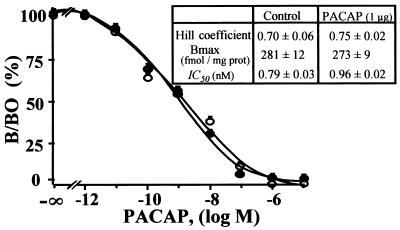
Binding studies on cerebellar slices were performed to investigate whether administration of PACAP could affect the density and/or affinity of PACAP receptors. Quantification of the autoradiograms indicated that a 4-day treatment with 1 μg of PACAP did not modify the maximum binding of [125I]PACAP27 and did not change the IC50 values of PACAP for its receptors (Fig. 6).
Figure 6.
Displacement of [125I]PACAP27 binding by synthetic PACAP on cerebellar slices from P12 rats treated with vehicle (○) or 1 μg of PACAP (●). Each competition curve was established by quantifying autoradiograms of cerebellar sections incubated with increasing concentrations of PACAP. (Inset) Hill coefficient, Bmax, and IC50 values in control and PACAP-treated animals. Each value is the mean ± SEM of at least three experiments performed in triplicate.
DISCUSSION
It has been reported that PACAP promotes proliferation and/or differentiation of nerve cells in vitro (23, 32–37). In particular, PACAP prevents programmed cell death and stimulates neurite outgrowth in cultured cerebellar granule cells (20–23). Our data show that PACAP exerts neurotrophic effects in the rat cerebellum during postnatal development.
In cultured cortical cells, PACAP has been shown to inhibit cell proliferation (37), whereas, in peripheral sympathetic neuroblasts, PACAP exerts a mitogenic effect (38). The present study shows that, in cultured cerebellar granule cells from P8 rats, PACAP induces a dose-dependent stimulation of [3H]thymidine incorporation, the maximum effect (+ 46%) of which is observed after an 18-h incubation with 10−7 M PACAP. This observation prompted us to investigate in vivo the effect of PACAP on the development of the cerebellar cortex of P8 rats.
Local injections of PACAP were performed at the surface of the cerebellum of P8 rats. At this stage, the meninges are not developed fully (39), and the EGL is only about 50 μm thick. As such, the peptide can easily reach cells of the EGL. In fact, it is well established that the fluid of the subarachnoid space where PACAP was injected flows rather freely through the brain parenchyma (40). Our data showed that treatment with PACAP caused a significant increase in the thickness of the EGL, the molecular layer, and the IGL. In contrast, PACAP had no effect on the medulla so that the overall increase in the volume of the cerebellum resulted only from an increase in the volume of the cortical layers.
The EGL is a secondary neuroepithelium composed of a matrix where mitosis occurs and a premigratory zone where granule cells pause momentarily before migration (17). During the first 2 postnatal weeks, granule cells of the EGL undergo apoptosis (6). Therefore, the effect of PACAP on the number of cells in the EGL may result either from stimulation of granule cell multiplication or from inhibition of programmed cell death. The fact that PACAP promotes survival of cerebellar granule cells in vitro (20–23) strongly suggests that the effect of PACAP on the number of cells on the EGL can be accounted for by inhibition of apoptosis.
In the molecular layer, granule neurons migrate along Bergmann glial fibers at an average speed of 11 μm/h and reach their final destination in the IGL within 24 h (4, 41). The transient increase in the density of granule cells in the molecular layer induced by PACAP can be ascribed to an augmentation of the number of migrating immature granule cells. In support of this hypothesis, we observed an increase in the number of granule cells in the IGL at P12. Concurrently, PACAP caused an enlargement of the molecular layer that is attributable to an increase in the number of ascending T-shaped axons originating from granule cell bodies in the IGL. Consistent with these data, PACAP has been shown to promote neurite outgrowth on cultured granule cells (23).
In the IGL, migrating granule cells move radially toward the bottom of the layer (4), leading to an increase in the thickness of the IGL without substantial modification of the cell density. The fact that local injections of PACAP increased the thickness of the IGL but did not affect the density of granule cells indicates that the peptide causes acceleration of the physiological process of granule cell migration. Although Purkinje cells express PACAP receptors (30, 42), the number of Purkinje cells was not modified by PACAP treatment, indicating that, during postnatal development of the cerebellum, PACAP acts specifically on granule cells. Taken together, these results suggest that in vivo administration of PACAP causes an increase in the number of granule cells migrating from the EGL toward the IGL and stimulates neuritogenesis.
The effect of PACAP on the number of granule cells was abrogated by concomitant administration of the selective antagonist PACAP(6–38). Consistent with this finding, we have shown previously that PACAP(6–38) inhibits in vitro the survival-promoting action of PACAP on cultured cerebellar granule cells (23). In the developing rat cerebellum, PACAP is expressed by Purkinje cells (16), which are known to regulate the number of granule cells. The observation that PACAP(6–38) alone provoked a modest decrease in the number of granule cells in the IGL strongly suggests that endogenous PACAP can actually stimulate granule cell survival.
Because the effect of PACAP reached a maximum within 96 h after the first injection and vanished during prolonged treatment, autoradiographic studies were conducted to investigate whether administration of PACAP could modulate the expression and/or affinity of PACAP receptors. We found that PACAP had no effect on the binding capacity and affinity of its own receptors. This observation indicates that the transient effect of PACAP on the number of granule cells cannot be accounted for by a desensitization process. In fact, the normalization of the number of granule neurons that occurred after 6 days of treatment likely results from massive cell death that takes place in the IGL before synaptogenesis (6). During this second period of apoptosis, the regulation of the granule cell number is clearly under the control of Purkinje cells (43–45). In particular, previous experiments with transgenic mice in which Purkinje cells have been ablated selectively have shown that these cells regulate the survival of cerebellar granule cells during the first postnatal week (46). Thus, the transitory nature of the effect of PACAP on the number of granule cells in the IGL is likely mediated by Purkinje cells, which produce various factors regulating the number of granule neurons that must survive. For example, transforming growth factor-β, which is expressed by Purkinje cells and which induces apoptosis of cultured granule cells, is potentially one of the factors that may regulate the number of cells in the IGL (47).
The present report has established that administration of PACAP to P8 rats increases the number of surviving granule cells in the EGL and IGL and stimulates neuronal migration from the EGL to the IGL. These data, together with the observation that PACAP ligand and PACAP receptors are actively expressed in the cerebellar cortex of young rats, indicate that PACAP may exert a physiological role as a trophic factor promoting granule cell proliferation and/or inhibiting programmed cell death during postnatal development.
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale (U-413), funds from an Institut National de la Santé et de la Recherche Médicale–Fonds de la Recherche Scientifique Québécois exchange program (to A.F. and H.V.), and the Conseil Régional de Haute Normandie. H.V. is Affiliated Professor at the Institut National de la Recherche Scientifique-Santé.
ABBREVIATIONS
- EGL
external granule cell layer
- IGL
internal granule cell layer
- PACAP
pituitary adenylate cyclase-activating polypeptide
- Pn
postnatal day n
- Sim
lobulus simplex
References
- 1.Le Douarin N M, Hallonet M E R, Pourquié O. Prog Brain Res. 1994;100:3–18. doi: 10.1016/s0079-6123(08)60762-1. [DOI] [PubMed] [Google Scholar]
- 2.Ryder E F, Cepko C L. Neuron. 1994;12:1011–1029. doi: 10.1016/0896-6273(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 3.Hager G, Dodt H U, Zieglgänsberger W, Liesi P. J Neurosci Res. 1995;40:207–219. doi: 10.1002/jnr.490400209. [DOI] [PubMed] [Google Scholar]
- 4.Komuro H, Rakic P. J Neurosci. 1998;18:1478–1490. doi: 10.1523/JNEUROSCI.18-04-01478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 6.Wood K A, Dipasquale B, Youle R J. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 7.Rakic P. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- 8.Gao W Q, Heintz N, Hatten M E. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke N A, Dailey M E, Smith S J, McConnel S K. Science. 1992;258:299–301. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- 10.Otero R A, Sotelo C, Alvarado-Mallart R M. J Comp Neurol. 1993;333:597–615. doi: 10.1002/cne.903330411. [DOI] [PubMed] [Google Scholar]
- 11.Rivas R J, Hatten M E. J Neurosci. 1995;15:981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono K, Shokunbi T, Nagata I, Tokunaga A, Yasui Y, Nakatsuji N. Exp Brain Res. 1997;117:17–29. doi: 10.1007/pl00005787. [DOI] [PubMed] [Google Scholar]
- 13.Basille M, Gonzalez B J, Leroux P, Jeandel L, Fournier A, Vaudry H. Neuroscience. 1993;57:329–338. doi: 10.1016/0306-4522(93)90066-o. [DOI] [PubMed] [Google Scholar]
- 14.Masuo Y, Tokito F, Matsumoto Y, Shimamoto N, Fujino M. Neurosci Lett. 1994;170:43–46. doi: 10.1016/0304-3940(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 15.Arimura A, Shioda S. Front Neuroendocrinol. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen H S, Hannibal J, Fahrenkrug J. NeuroReport. 1998;9:2639–2642. doi: 10.1097/00001756-199808030-00039. [DOI] [PubMed] [Google Scholar]
- 17.Altman J. J Comp Neurol. 1972;145:465–514. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- 18.Basille M, Gonzalez B J, Desrues L, Demas M, Fournier A, Vaudry H. J Neurochem. 1995;65:1318–1324. doi: 10.1046/j.1471-4159.1995.65031318.x. [DOI] [PubMed] [Google Scholar]
- 19.Favit A, Scapagnini U, Canonico P L. Neuroendocrinology. 1995;61:377–382. doi: 10.1159/000126859. [DOI] [PubMed] [Google Scholar]
- 20.Villalba M, Bockaert J, Journot L. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallaro S, Copani A, D’Agata V, Musco S, Petralia S, Ventra C, Stivala F, Travali S, Canonico P L. Mol Pharmacol. 1996;50:60–66. [PubMed] [Google Scholar]
- 22.Campard P K, Crochemore C, René F, Monnier D, Koch B, Loeffler J P. DNA Cell Biol. 1997;16:323–333. doi: 10.1089/dna.1997.16.323. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez B J, Basille M, Vaudry D, Fournier A, Vaudry H. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- 24.Vaudry D, Gonzalez B J, Basille M, Anouar Y, Fournier A, Vaudry H. Neuroscience. 1998;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- 25.Miyata A, Arimura A, Dahl R R, Minamino N, Uehara A, Jiang L, Culler M D, Coy D H. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 26.Chartrel N, Conlon J M, Danger J M, Fournier A, Tonon M C, Vaudry H. Proc Natl Acad Sci USA. 1991;88:3862–3866. doi: 10.1073/pnas.88.9.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez B J, Leroux P, Lamacz M, Bodenant C, Balazs R, Vaudry H. Proc Natl Acad Sci USA. 1992;89:9627–9631. doi: 10.1073/pnas.89.20.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo V, Kingsbury A, Balazs R, Jorgensen O S. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 31.Basille M, Gonzalez B J, Fournier A, Vaudry H. Dev Brain Res. 1994;82:81–89. doi: 10.1016/0165-3806(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch P J, Sun Y. J Biol Chem. 1992;267:5108–5113. [PubMed] [Google Scholar]
- 33.Hoshino M, Li M, Zheng L Q, Suzuki M, Mochizuki T, Yanaihara N. Neurosci Lett. 1993;159:35–38. doi: 10.1016/0304-3940(93)90792-j. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez A, Kimball B, Romanchuk G, Mulholland M W. Peptides. 1995;5:927–932. doi: 10.1016/0196-9781(95)00059-s. [DOI] [PubMed] [Google Scholar]
- 35.Wolf N, Krieglstein K. Neurosci Lett. 1995;200:207–210. doi: 10.1016/0304-3940(95)12116-l. [DOI] [PubMed] [Google Scholar]
- 36.Sheward W J, Lutz E M, Harmar A J. Neurosci Lett. 1996;216:45–48. doi: 10.1016/0304-3940(96)13002-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu N, DiCicco-Bloom E. Proc Natl Acad Sci USA. 1997;94:3357–3362. doi: 10.1073/pnas.94.7.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiCicco-Bloom E, Deutsch P J. Regul Pept. 1992;37:319–325. [Google Scholar]
- 39.Jacobson M. Developmental Neurobiology. London: Plenum; 1991. [Google Scholar]
- 40.Nicholson C. Trends Neurosci. 1999;22:143–145. doi: 10.1016/s0166-2236(98)01388-5. [DOI] [PubMed] [Google Scholar]
- 41.Gao W Q, Hatten M E. Science. 1993;260:367–369. doi: 10.1126/science.8469990. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Shioda S, Somogyvari-Vigh A, Onda H, Arimura A. Endocrine. 1997;7:183–190. doi: 10.1007/BF02778140. [DOI] [PubMed] [Google Scholar]
- 43.Sidman R L, Lane P W, Dickie M M. Science. 1962;137:610–612. doi: 10.1126/science.137.3530.610. [DOI] [PubMed] [Google Scholar]
- 44.Herrup K, Sunter K. J Neurosci. 1987;7:829–836. doi: 10.1523/JNEUROSCI.07-03-00829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wetts R, Herrup K. Brain Res. 1982;250:358–362. doi: 10.1016/0006-8993(82)90431-0. [DOI] [PubMed] [Google Scholar]
- 46.Smeyne R J, Chu T, Lewin A, Bian F, Crisman S S, Kunsch C, Lira S A, Oberdick J. Mol Cell Neurosci. 1995;6:230–251. doi: 10.1006/mcne.1995.1019. [DOI] [PubMed] [Google Scholar]
- 47.Luca A, Weller M, Fontana A. J Neurosci. 1996;16:4174–4185. doi: 10.1523/JNEUROSCI.16-13-04174.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]