Abstract
Slow-twitch mechanically skinned fibres from rat soleus muscle were bathed in solutions mimicking the myoplasmic environment but containing different [ADP] (0.1 μm to 1.0 mm). The effect of ADP on sarcoplasmic reticulum (SR) Ca2+-content was determined from the magnitude of caffeine-induced force responses, while temporal changes in SR Ca2+-content allowed determination of the effective rates of the SR Ca2+-pump and of the SR Ca2+-leak. The SR Ca2+-pump rate, estimated at pCa (−log10[Ca2+]) 7.8, was reduced by 20% as the [ADP] was increased from 0.1 to 40 μm, with no further alteration when the [ADP] was increased to 1.0 mm. The SR Ca2+-leak rate constant was not altered by increasing [ADP] from 0.1 to 40 μm, but was increased by 26% when the [ADP] was elevated to 1.0 mm. This ADP-induced SR Ca2+-leak was insensitive to ruthenium red but was abolished by 2,5-di(tert-butyl)-1,4-hydroquinone (TBQ), indicating that the leak pathway is via the SR Ca2+-pump and not the SR Ca2+-release channel. The decrease in SR Ca2+-pump rate and SR Ca2+-leak rate when [ADP] was increased led to a 40% decrease in SR Ca2+-loading capacity. Elevation of [ADP] had only minor direct effects on the contractile apparatus of slow-twitch fibres. These results suggest that ADP has only limited depressing effects on the contractility of slow-twitch muscle fibres. This is in contrast to the marked effects of ADP on force responses in fast-twitch muscle fibres and may contribute to the fatigue-resistant nature of slow-twitch muscle fibres.
The duration of the twitch force response in slow-twitch muscle is approximately four times longer than that of fast-twitch muscle (Close, 1967). This is due partially to differences in contractile properties of the fibre types (Stephenson & Williams, 1981), and also to differences in the myoplasmic Ca2+ transient that is responsible for the activation and relaxation of the contractile apparatus (Baylor & Hollingworth, 2003). The contractile apparatus of slow-twitch muscle is more sensitive to Ca2+ than fast-twitch muscle, and as a result produces force at lower free Ca2+ concentration than fast-twitch muscle (Stephenson & Williams, 1985; Stephenson et al. 1994).
At the level of the SR, there are a number of marked differences between the Ca2+-handling properties of slow-twitch and fast-twitch muscle. The overall volume of the SR is two to three times smaller in slow-twitch muscle than in fast-twitch muscle (Eisenberg et al. 1974), whereas the endogenous SR Ca2+-content of rat slow-twitch muscle is only slightly larger than that of fast-twitch muscle (Fryer & Stephenson, 1996). Furthermore, the SR Ca2+-content in slow-twitch muscle cannot be increased beyond the endogenous level (Fryer & Stephenson, 1996), suggesting that under endogenous conditions the SR is maximally loaded with Ca2+. In contrast, in fast-twitch muscle, the SR is endogenously loaded to only 20–30% of the SR capacity (Fryer & Stephenson, 1996).
Expression of SR Ca2+-pump isoforms also varies between the two muscle fibre types, with the SERCA 2A isoform expressed in slow-twitch muscle, and the SERCA 1 isoform expressed in fast-twitch muscle (van der Linden et al. 1996). The actual SR Ca2+-pump density in slow-twitch muscle is only 30–50% of that in fast-twitch muscle (Ferguson & Franzini-Armstrong, 1988). The content of SR Ca2+-release channels is also different between the two muscle fibre types, with slow-twitch muscle containing only 60% of that in fast-twitch muscle (Franzini-Armstrong et al. 1988), although the SR Ca2+-release channels are of the same isoform (Meissner, 1994). Furthermore, the charge movement associated with the dihydropyridine receptor is also three- to fourfold smaller in slow-twitch than in fast-twitch muscle fibres (Dulhunty & Gage, 1983), indicating that fewer voltage sensors are activated during excitation. Together, these properties contribute greatly to the smaller and slower Ca2+ transients observed between slow-twitch and fast-twitch muscle fibres (Baylor & Hollingworth, 2003) and consequently, to the specific force characteristics of each muscle fibre type.
Slow-twitch muscle is considered to be more fatigue resistant than fast-twitch muscle (Fitts, 1994). However, little is known regarding the possible differences in Ca2+-handling properties between slow-twitch and fast-twitch muscles in conditions occurring during fatiguing muscle contractions. Under normal resting conditions, the [ADP] within a muscle is tightly maintained at <10 μm (Dawson et al. 1978), primarily due to the activity of the creatine kinase reaction (ADP + CP ⇌ ATP + creatine, equilibrium constant = 260; Chase & Kushmerick, 1995). However, during fatiguing muscle contraction, creatine phosphate (CP) is consumed and, together with an elevated creatine concentration, displaces the equilibrium of the creatine kinase reaction towards a higher [ADP]. In situations of near complete CP depletion, the [ADP] is thought to reach up to millimolar levels (Nagesser et al. 1993; Westerblad & Lännergren, 1994). Despite the lower resting CP concentration in slow-twitch muscle (Meyer et al. 1985), the increase in [ADP] during fatigue is smaller in slow-twitch muscle than that in fast-twitch muscle. Presumably, this is due to slower myosin ATPase activity (Schiaffino & Reggiani, 1996) and lower SR Ca2+-pump ATP utilization (Szentesi et al. 2001), along with the lower creatine concentrations (Meyer et al. 1985).
We have previously shown that an elevation in [ADP] to levels occurring during fatiguing stimulation markedly alters the SR Ca2+-handling ability in fast-twitch muscle (Macdonald & Stephenson, 2001). However, the effects of an elevation in [ADP] on the SR Ca2+-handling ability in slow-twitch muscle are not known. In this study we used the freshly dissected mechanically skinned muscle fibre preparation, in conjunction with solutions of varied [ADP] to examine the effects of ADP on SR function. In this preparation, the SR is intact and physiological conditions can be accurately mimicked. The results show that an elevation in [ADP] within the physiological range reduces the ability of the SR to store Ca2+ by both decreasing the SR Ca2+-pump rate, and by increasing the passive SR Ca2+-leak. Importantly, the extent of this reduction in SR Ca2+-handling ability in slow-twitch muscle is much smaller than that in fast-twitch muscle (Macdonald & Stephenson, 2001), which may contribute to the more fatigue resistant nature of slow-twitch muscle.
Methods
Dissection, preparation of fibres and apparatus
Male rats (Long-Evans; 3 months old) were killed by halothane overdose (2% v/v) in accordance with permits issued by La Trobe University Animal Ethics Committee. Soleus muscles were quickly removed, blotted on filter paper, and then transferred to a dish containing paraffin oil (Macdonald & Stephenson, 2001). Mechanically skinned fibres were then prepared and mounted on a force transducer (AME875; SensoNor, Horton, Norway) whilst under oil, as previously described (Fink et al. 1986). The length and diameter were measured and the preparation then stretched to 120% slack resting length to facilitate measurement of force production (Lamb & Stephenson, 1990). The preparation was finally placed into a 2 ml Perspex bath containing low relaxing1 solution (Table 1). The apparatus used in these experiments and the procedure of changing solutions has elsewhere been described in detail (Stephenson & Williams, 1981).
Table 1.
Composition of solutions
| Solution | EGTAtotal (mm) | HDTA (mm) | CP (mm) | Ca2+ (μm) | Mg2+free (mm) |
|---|---|---|---|---|---|
| Max activating1 | 50 | — | 10 | 30 | 1.0 |
| Max activating2 | 50 | 10 | — | 30 | 1.0 |
| High relaxing1 | 50 | — | 10 | <0.001 | 1.0 |
| High relaxing2 | 50 | 10 | — | <0.001 | 1.0 |
| Low relaxing1 | 0.05 | 50 | 10 | 0.05 | 1.0 |
| Low relaxing2 | 0.05 | 60 | — | 0.05 | 1.0 |
| Load1 | 1.0 | 50 | 10 | 0.015 | 1.0 |
| Load2 | 1.0 | 60 | — | 0.015 | 1.0 |
| Release | 1.0 | 50 | 10 | 0.005 | 0.05 |
| Wash1 | 1.0 | 50 | 10 | 0.002 | 1.0 |
| Wash2 | 1.0 | 60 | — | 0.002 | 1.0 |
| Leak1 | 1.0 | 50 | 10 | 0.002 | 1.0 |
| Leak2 | 1.0 | 60 | — | 0.002 | 1.0 |
| pSr 5.4 solution | 50 | — | 10 | <0.001 | 1.0 |
| ADP stock | 0.05 | 30 | — | 0.05 | 1.0 |
All solutions were adjusted to pH 7.10 ± 0.01 at room temperature (22 ± 2°C), with KOH and contained (mm): K+, 126; Na+, 36; total ATP, 8; Hepes, 90; NaN3 1.0. ADP stock solution also contained 20 mm ADP. Total Ca2+ and Mg2+ in solutions was varied to provide the indicated Ca2+free and Mg2+free concentrations. pSr 5.4 solution contained 4 μm Sr. Note that under our conditions EGTA, CaEGTA, HDTA and CP exist almost exclusively as divalent anions. Release solution also contains 30 mm caffeine. CP, creatine phosphate, HDTA, hexamethylene diamine tetraacetate.
Solutions
Solutions were prepared as described by Stephenson & Williams (1981), with their composition shown in Table 1. The osmolarity of all solutions was 290 ± 10 mosmol kg−1 and unless otherwise stated contained (mm): K+, 126; Na+, 37; total ATP, 8.0; MgATP, 7.0; Hepes, 90 (pH 7.10 ± 0.01); free Mg2+, 1.0; NaN3, 1.0. To determine the Ca2+ sensitivity of the contractile apparatus, solutions were prepared by mixing high relaxing1 solution and max activating1 solution to give strongly Ca2+-buffered solutions of a Ca2+ concentration in the pCa (−log10[Ca2+]) range of ∼4.5 to >9. The release solution contained 30 mm caffeine and low ionized Mg2+ concentration (0.02 mm) to facilitate the rapid and thorough release of SR Ca2+ (Fryer & Stephenson, 1996). The free Ca2+ concentration in load solutions was determined with a Ca2+ electrode (Orion, Boston, USA). Hexamethylene diamine tetraacetate (HDTA) was obtained from Fluka, Buchs (Switzerland) and all other chemicals were obtained from Sigma (St Louis, MO, USA). All experiments were conducted at room temperature (22 ± 2°C).
[ADP] in solutions
Freshly mechanically skinned fibres retain a high creatine kinase activity (Saks et al. 1978; Ventura-Clapier et al. 1994; Walliman et al. 1997) and therefore, in the presence of CP, exogenously added ADP is rapidly converted to ATP within the myoplasmic space. [ADP] was maintained in solution using two methods: (i) buffering the [ADP] close to equilibrium using the preparations endogenous creatine kinase activity, and (ii) by the concomitant removal of CP and addition of ADP to solutions, as previously described (Macdonald & Stephenson, 2001, 2004).
Measurement of Ca2+-activated force
Contractile activation parameters were determined by initially exposing the preparation to high relaxing1 solution containing 2% v/v Triton X-100 (5 min) to destroy all membrane compartments (Stephenson, 1981), before washing in a separate high relaxing1 solution (approximately 2 min, two separate washes). The force–pCa relationship and maximum Ca2+-activated force were then determined by exposing the fibre to solutions of a sequence of solutions in which the Ca2+ concentration was heavily buffered at progressively higher levels as previously described (Stephenson & Williams 1982). To compensate for the small deterioration in force between the initial and final response in max activating1 solution, responses at each pCa were expressed as a percentage of the interpolated values for the maximum Ca2+-activated force (van der Poel & Stephenson, 2002). Force was expressed as a percentage of the corresponding maximum Ca2+-activated force for that condition, plotted as a function of pCa and fitted with a Hill equation (described by eqn (1), using the analysis program GraphPad Prism; GraphPad Software, Inc., CA, USA):
| (1) |
where [Ca2+]50 (pCa50) represents the [Ca2+] (pCa) at which 50% of maximum Ca2+-activated force is produced, and nH is the Hill coefficient. The oscillatory force response characteristic in mammalian slow-twitch muscle at submaximal Ca2+ concentrations (Stephenson & Williams, 1981) was observed in all fibres. The force measurement for oscillatory responses at constant Ca2+ concentration was taken as the midpoint between the peak and trough of the oscillation. Increasing the [ADP] did not alter either the amplitude or frequency of the oscillatory response.
Caffeine-induced responses
Caffeine-induced force responses were used to estimate the relative amount of Ca2+ in the SR by referring to the relative area under the caffeine-induced force response (Endo & Iino, 1980; Macdonald & Stephenson, 2001). The preparation was initially equilibrated in low relaxing1 solution (2 min), where the SR remained endogenously loaded, and the endogenous SR Ca2+-content determined by placing the preparation in wash1 solution for 30 s before being transferred to release solution to trigger SR Ca2+-release. The fibre remained in the release solution for 2 min to ensure complete SR Ca2+-depletion (Fryer & Stephenson, 1996), before washing for 30 s in wash1 solution. Thereafter, the preparation was placed in load1 solution to reload the SR with Ca2+ before the SR Ca2+ was again released in release solution, and the cycle then repeated. Force was continuously recorded on a chart recorder and the relative area under the caffeine-induced force response was measured using the gravimetric method (Fink & Stephenson, 1987).
The areas under the caffeine-induced force responses could be used to estimate SR Ca2+-content providing that the areas and loading times were directly proportional (Launikonis & Stephenson, 1997). Under the SR Ca2+-loading conditions used in this study, there was a linear relationship between the area under the caffeine-induced force responses and the loading time (R2 > 0.95), that intersected the y-intercept at +8.2 ± 8.3% endogenous SR Ca2+-content. The relative areas under the caffeine-induced responses were corrected by subtracting the y-intercept for individual fibres as previously described (Launikonis & Stephenson, 1997; Macdonald & Stephenson, 2001), which conferred direct proportionality between the corrected areas and the SR Ca2+-content.
Load experiments
The SR Ca2+-loading ability was investigated by loading the SR with Ca2+, in the load1 solution or load2 solution with or without ADP, then washing for 30 s in either wash1 solution or wash2 solution before releasing SR Ca2+ in release solution. The standard caffeine-induced force response (60 s, in load1 solution) was monitored throughout, to allow for correction of any small deterioration in SR function, and corrected for, as previously described (Macdonald & Stephenson, 2001).
Leak experiments
To estimate Ca2+ lost from the SR due to passive leak of Ca2+, the SR was loaded for 60 s in load1 solution, washed in wash1 solution for 30 s and the SR Ca2+-content released in release solution (control). Thereafter, the preparation was washed in wash1 solution prior to re-loading for 60 s in load1 solution, before being transferred to leak1 solution or leak2 solution (with or without added ADP) for 60 s. The fibre was then washed in wash1 solution for 30 s and the remaining SR Ca2+ was released in release solution. The control was then repeated and the area under the leak response was divided by the average areas under the control responses before and after the leak response, giving an estimate of the fraction of SR Ca2+ leaked over the 60 s in leak1 or leak2 solution, and the SR Ca2+-leak rate constant β was calculated, assuming that the SR Ca2+-content decreased exponentially over the 60 s.
Further experiments were performed to determine the role of the SR Ca2+-release channels, and the SR Ca2+-pump in the passive SR Ca2+-leak. The SR Ca2+-release channel was blocked during the leak period with 6 μm ruthenium red (RR) (added from 1.2 mm stock dissolved in low relaxing1 solution), which is expected to markedly block the SR Ca2+-release channels during the 60 s exposure (Lamb & Stephenson, 1991). The preparation was then washed in wash1 solution for 30 s and then SR Ca2+ released with release solution in the absence of RR. Under these conditions the area under the caffeine-induced force response decreases marginally from that corresponding to the release of the same amount of Ca2+ from the SR but without exposure to RR (see also Lamb & Cellini, 1999). Alternatively, the SR Ca2+-pump was blocked with 20 μm 2,5-di(tert-butyl)-1,4-hydroquinone (TBQ) (Inesi & Sagara, 1994), added from a 20 mm stock solution dissolved in pure dimethyl-d6sulfoxide (DMSO). After exposure to TBQ, the fibre was dipped in paraffin oil for 30 s to remove TBQ from the skinned fibre without markedly changing the SR Ca2+-content (Bakker et al. 1996), washed in wash1 solution for 30 s, and depleted of Ca2+ in release solution. Controls for the TBQ experiments contained an equivalent amount of DMSO, and underwent the same procedure, with no significant alteration to normal control responses.
In all load and leak experiments, the release solution was identical to ensure that any difference in caffeine-induced force response, under the different conditions, was due to differences in SR Ca2+-content and not altered SR Ca2+-release properties (Herrmann-Frank et al. 1999) or alterations to Ca2+ sensitivity of the contractile apparatus.
Muscle fibre typing
All muscles fibres used in this study were confirmed as slow-twitch fibre type based on their greater sensitivity to Sr2+ compared to fast-twitch fibres (O'Connell et al. 2004). Briefly, pSr 5.4 solution (Table 1) was used to differentiate between slow-twitch and fast-twitch fibres, in which slow-twitch fibres produce 80–90% maximum Ca2+-activated force, while fast-twitch fibres produce zero force, as this Sr2+ concentration (pSr (−log10[Sr2+] 5.4 corresponds to 4 μm[Sr2+]) is below the activation threshold (Bortolotto et al. 2000; O'Connell et al. 2004).
Data analysis
Results are expressed as means ± s.e.m. and curve fitting and statistical analyses were performed using the scientific analysis program GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). The statistical significance of any difference between groups was accepted at P < 0.05, as determined using Student's two-tailed t test for non-paired observations or ANOVA, where appropriate.
Results
Effects of elevated [ADP] on contractile properties of slow-twitch fibres
Increasing the [ADP] from the control level (<0.1 μm) to 40 μm did not significantly alter the maximum Ca2+-activated force in Triton X-100 treated fibres (Table 2). To allow elevation of ADP to high concentrations, CP was removed from solutions, which itself significantly increased the maximum Ca2+-activated force by 4.6 ± 0.8% (P < 0.05). Addition of 1.0 mm ADP to this solution without CP, resulted in a significant reduction in the maximum Ca2+-activated force, when compared to the 0 mm CP solution (P < 0.05), but was not significantly different from controls containing CP. The effect of elevating the [ADP] on the force–pCa relationship is also shown in Table 2. Increasing the [ADP] in solution from <0.1–40 μm did not significantly alter either the pCa50 or the nH of the force–pCa curves. The removal of CP from solutions significantly increased the pCa50 (P < 0.05) but not the nH. The addition of 1.0 mm ADP to this 0 mm CP solution further significantly increased pCa50 (P < 0.05) but not nH. Thus, elevation of [ADP] from <0.1 μm to 1.0 mm causes an increase in the Ca2+ sensitivity of the contractile apparatus without altering the maximum Ca2+-activated force.
Table 2.
Effect of ADP and CP on the activation characteristics of mechanically skinned slow-twitch muscle fibres
| Control | 40 μm ADP | 0 mm CP | 1.0 mm ADP | |
|---|---|---|---|---|
| P/Pmax | 1.000 | 1.015 ± 0.008 | 1.046 ± 0.008* | 1.003 ± 0.017† |
| pCa50 | 6.08 ± 0.04 | 6.09 ± 0.05 | 6.15 ± 0.05* | 6.25 ± 0.04*† |
| nH | 3.57 ± 0.66 | 4.19 ± 0.92 | 3.39 ± 0.60 | 2.66 ± 0.57 |
P/Pmax is the maximum Ca2+-activated force under test conditions normalized to the maximum Ca2+-activated force under control conditions. pCa50 corresponds to the Ca2+ concentration giving half-maximum force under specified conditions. nH (Hill coefficient) corresponds to the steepness of the force–pCa activation curve. Data are means ± s.e.m. from nine individual fibres. Significant difference from control
0 mm CP
P < 0.05, Student's t test).
Effects of elevated [ADP] on SR Ca2+-loading properties of slow-twitch fibres
Preliminary experiments were initially performed where the SR was loaded at pCa 7.1, which is close to the resting physiological pCa in slow-twitch fibres (Baylor & Hollingworth, 2003). Under these loading conditions the SR was loaded to 70 ± 11% (n = 4) of the endogenous level after 30 s, and could not be loaded more than the endogenous level even after 10 min in the loading solution. The inability of the SR to load more than endogenous Ca2+ is consistent with the observation of Fryer & Stephenson (1996) that the SR of slow-twitch muscle fibres is maximally loaded under endogenous conditions. Increasing [ADP] from <0.1 μm (control) to 40 μm, did not significantly alter the SR Ca2+-content of the SR (after 30 s loading) (67.3 ± 11% of the endogenous SR Ca2+-content, P > 0.05). However, further increasing the [ADP] to 1.0 mm did significantly reduce the SR Ca2+-content after 30 s loading at pCa 7.1 by about 25% to 52.0 ± 2.5% of the endogenous SR Ca2+-content (P < 0.05).
In order to slow down the rate of Ca2+ loading in the SR, the pCa of the SR loading solution was increased from 7.1 to 7.8 (load1 solution). This allowed a similar analysis of [ADP] effects on the SR Ca2+-handling properties to those in fast-twitch fibres (Macdonald & Stephenson, 2001), and consequently a comparison of these effects between slow-twitch and fast-twitch fibres. At pCa 7.8, increasing the SR Ca2+-loading time resulted in an increase in the area under the caffeine-induced force response, indicating increased loading of Ca2+ into the SR (Fig. 1). Following a 60 s load in load1 solution, the ensuing SR Ca2+-release was only about 30% of the response corresponding to the endogenous (maximal) level. This was equivalent to the SR Ca2+-level under standard loading conditions used previously by our laboratory for fast-twitch muscle (Macdonald & Stephenson, 2001), and therefore allows for appropriate comparison between ADP-dependent SR properties in slow-twitch and fast-twitch muscle. For all subsequent experiments in the present study, a 60 s load in load1 solution was considered to be the standard loading condition.
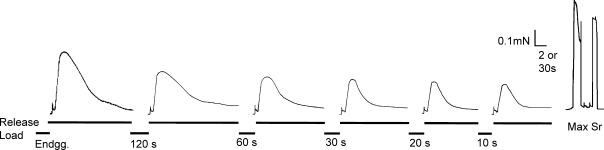
Figure 1. Representative trace showing 30 mm caffeine-induced force responses following SR Ca2+-loading at pCa 7.8 in a slow-twitch soleus fibre for different periods of time.
The first response corresponds to the endogenous SR Ca2+-level for this individual fibre, with smaller responses as the loading time decreased at 120, 60, 30, 20 and 10 s. Between responses the preparation was washed for 30 s in wash1 solution, then reloaded in loads2 solution (pCa 7.8, time indicated in trace) and then washed again in wash1 solution before the next SR Ca2+-release in release1 solution. The two responses to the right of the panel are the maximum Ca2+-activated force response (Max) and the pSr 5.4 force response (Sr) confirming the slow-twitch fibre type, respectively.
Figure 2 indicates that as [ADP] increased, the SR was able to load, and subsequently release, significantly less Ca2+ over the entire range of loading times (P < 0.05), indicating that elevated [ADP] reduces the SR Ca2+-loading ability in slow-twitch muscle. After a 60 s loading period, there was a 20% (P < 0.05) and 35% (P < 0.05) decrease in SR Ca2+-content, when the [ADP] was increased from 0.1 μm to 40 μm and 1.0 mm, respectively.
Figure 2. The effect of elevated ADP on the SR Ca2+-loading ability in slow-twitch fibres.
All fibres were loaded under similar conditions for Ca2+ (pCa 7.8), pH ionic strength, cationic and anionic composition, except for CP, which was substituted with hexamethylene diamine tetraacetate (HDTA), and ADP, which were <0.1 μm (control, black square), 40 μm (blue triangle) and 1.0 mm (red circle). The SR Ca2+ was always released in the same release solution after the preparation had been washed in wash solution to ensure an accurate comparison between results. The data points for individual fibres were normalized to the area obtained under the standard loading condition (60 s) for that fibre. Data points are means ± s.e.m., n = 6, and fitted eqn (2), with an R2 > 0.99.
Effects of elevated [ADP] on SR Ca2+-pump rate and SR Ca2+-leak rate
The results in Fig. 2 and can be accurately described by the following equation
| (2) |
where t is the loading time; [Ca]SR(t) is the SR Ca2+-content at time t; pump rate is the rate of Ca2+ loading into the SR by the SR Ca2+-pump (and in first approximation is considered constant for the period of loading), and β is the SR Ca2+-leak rate constant, which is proportional to the overall SR permeability to Ca2+. Considering that in all experiments the SR was submaximally (<35%) loaded with Ca2+, the free Ca2+ in the SR was assumed to be proportional to the total SR Ca2+, [Ca]SR. (For details of derivation of eqn (2) see Macdonald & Stephenson, 2001.) Therefore, by fitting the data points from Fig. 2 to eqn (2), in principle the SR Ca2+-pump rate and the SR Ca2+-leak rate constant (β) could be estimated for each condition. It is also possible to calculate the SR Ca2+-loading capacity for t = ∞ (pump rate/β) when the Ca2+ uptake equals the Ca2+ leak and no further accumulation of Ca2+ into the SR occurs.
As shown in Fig. 3, under control conditions (<0.1 μm ADP), the maximum SR Ca2+-loading capacity at pCa 7.8 was 36.8 ± 1.4% of the endogenous SR Ca2+-level, while the SR Ca2+-pump rate was 84.0 ± 3.3% endogenous SR Ca2+ min−1, where 100% corresponds to the physiological endogenous Ca2+ level in the SR. The SR Ca2+-leak rate constant β was 2.16 ± 0.12 min−1. Increasing the [ADP] from <0.1 μm to 40 μm significantly reduced the maximum SR Ca2+-capacity by about 12% (32.2 ± 3.0% endogenous SR Ca2+, P < 0.05), by significantly decreasing the SR Ca2+-pump rate to 71.4 ± 3.6% endogenous SR Ca2+ min−1 (P < 0.05), but did not alter the SR Ca2+-leak rate constant (β) (2.37 ± 0.16 min−1, P > 0.05). Increasing the [ADP] to 1.0 mm, further significantly decreased the maximum SR Ca2+-capacity by another 25% to 23.0 ± 5.0% endogenous SR Ca2+ (P < 0.05) but did not further significantly alter either the SR Ca2+-leak rate constant (β) (2.89 ± 0.45 min−1, P > 0.05), or the SR Ca2+-pump rate (67.0 ± 8.0% endogenous SR Ca2+ min−1, P > 0.05). However, compared with controls (<0.1 μm ADP), the SR Ca2+-leak rate constant (β) was significantly increased (P < 0.05), while the SR Ca2+-pump rate was significantly reduced (P < 0.05).
Figure 3. Effects of ADP on key functional parameters of the SR from mechanically skinned slow-twitch fibres.
A, maximum SR Ca2+-capacity; B, SR Ca2+-pump rate; and C, SR Ca2+-leak rate constant (β). The data points are obtained from eqn (2). Data points are means ± s.e.m., n = 6.
Effects of elevated [ADP] on SR Ca2+-leak rate constant (β) at pCa > 8.5
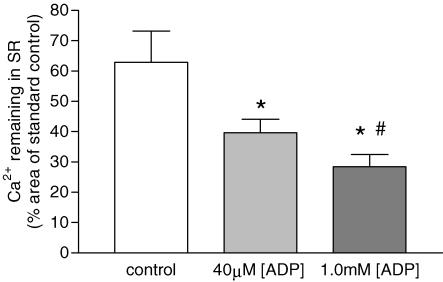
For further comparison of the ADP-induced SR Ca2+-leak rate with fast-twitch muscle (Macdonald & Stephenson, 2001), experiments were performed to assess the SR Ca2+-leak rate constant (β) at pCa > 8.5. Figure 4 shows that the Ca2+ remaining in the SR after exposure to leak1 solution for 60 s was markedly smaller than that for control responses without exposure to the leak1 solution (62.9 ± 10.2% of control), indicating that a significant (P < 0.05) amount of Ca2+ had been lost from the SR during the leak period, with a SR Ca2+-leak rate constant (β) of 0.46 ± 0.08 min−1. Increasing the [ADP] in leak1 solution to 40 μm, significantly increased (P < 0.05) the SR Ca2+-leak rate constant (β) to 0.93 ± 0.10 min−1, with only 39.6 ± 4.4% of initial Ca2+ remaining in the SR after 60 s leak. Elevating the [ADP] to 1.0 mm in leak2 solution, further significantly increased (P < 0.05) the SR Ca2+-leak rate constant (β) to 1.26 ± 0.17 min−1, with only 28.4 ± 4.0% of initial Ca2+ remaining in the SR. Note that in absolute terms, the values of the SR Ca2+-leak rate constant (β) at pCa > 8.5 were smaller than those derived from the SR Ca2+-loading curves in Fig. 3 at pCa 7.8. However, the ADP-dependent relative increase in passive SR Ca2+-leak rate constant at pCa > 8.5 was markedly greater than that estimated from the SR Ca2+-loading curves in Fig. 3 at pCa 7.8.
Figure 4. The effect of elevated ADP on SR Ca2+-leak.
The amount of Ca2+ remaining in the SR of slow-twitch fibres loaded under standard control conditions (60 s load1 solution) after a 60-s leak under control (<0.1 μm), 40 μm and 1.0 mm[ADP]. The amount of Ca2+ remaining in the SR was determined by normalizing the leak response to a standard control response performed before and after the leak response. Data points are means ± s.e.m., n = 7 and 4 for controls, and both 40 μm and 1.0 mm[ADP], respectively. Significant difference from control (*) and 40 μm ADP (#) (P < 0.05, ANOVA).
Effects of blocking SR Ca2+-release channel and SR Ca2+-pump on SR Ca2+-leak rate
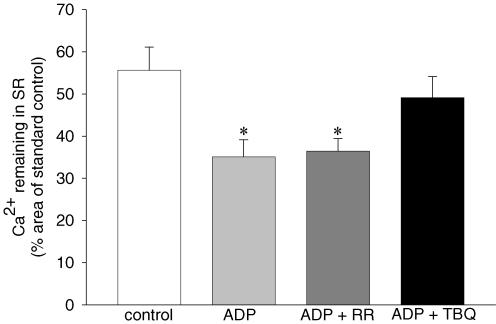
In order to determine whether the ADP-induced increase in the SR Ca2+-leak in slow-twitch muscle was through the SR Ca2+-release channel or through the SR Ca2+-pump, the SR Ca2+-leak was measured in the presence of 1.0 mm[ADP] with and without either 6 μm RR to block the SR Ca2+-release channels, or 20 μm TBQ to block the SR Ca2+-pump. The results (Fig. 5) show that following the leak period performed under control conditions (leak1 solution), the SR Ca2+-content was significantly reduced to 55.6 ± 9.5% of initial SR Ca2+-content (P < 0.05). Increasing the [ADP] to 1.0 mm during the leak period (leak2 solution), reduced the SR Ca2+-content to 35.1 ± 6.5% of initial SR Ca2+ content (P < 0.05), which was not altered by adding RR to the 1.0 mm ADP leak solution (leak2 solution) (37.4 ± 5.6% of initial SR Ca2+-content, P > 0.05). However, when 20 μm TBQ was added to the 1.0 mm ADP leak solution (leak2 solution), the 1.0 mm[ADP]-induced SR Ca2+-leak was abolished, with SR Ca2+ restored back to control levels (49.1 ± 7.7% of initial SR Ca2+-content; P < 0.05). Together these results demonstrate that the SR Ca2+-pump, and not the SR Ca2+-release channel, was responsible for the elevated ADP-induced increase in SR Ca2+-leak in slow-twitch muscle.
Figure 5. The pathway of the ADP-induced increase in SR Ca2+-leak.
The amount of Ca2+ remaining in the SR of slow-twitch fibres loaded under standard control conditions (60 s load1 solution) after a 60 s leak in leak solutions under control (<0.1 μm ADP), 1.0 mm[ADP] (ADP), 1.0 mm ADP + 6 μm ruthenium red (ADP + RR) and 1.0 mm ADP + 20 μm 2,5-di(tert-butyl)-1,4-hydroquinone (ADP + TBQ). The amount of Ca2+ remaining in the SR was determined by normalizing the leak response to a standard control response performed before and after the leak response. Data points are means ± s.e.m., n = 4. *Significant difference (P < 0.05, ANOVA) from control.
Discussion
The present results indicate that in rat slow-twitch muscle, the elevation of [ADP] to levels expected to occur during skeletal muscle fatigue (Karatzaferi et al. 2003; Hancock et al. 2005) had only a minor effect directly on the contractile apparatus, but produced a marked inhibition in the Ca2+-handling ability of the SR, by both decreasing the SR Ca2+-pump rate and by increasing the leak of Ca2+ from the SR.
Effects of elevated [ADP] on the contractile apparatus of slow-twitch fibres
An elevation in the [ADP] to 40 μm, in the presence of 8 mm ATP, had no effect on the steady-state contractile activation characteristics (maximum Ca2+-activated force, Ca2+ sensitivity or Hill coefficient) of slow-twitch muscle fibres in the present study. This is in agreement with Chase & Kushmerick (1995), who used similar conditions to show no effect of elevated [ADP] up to 50 μm ADP. Removing CP from solutions resulted in a small increase in maximum Ca2+-activated force and in Ca2+ sensitivity of the contractile apparatus, as previously shown by Fryer et al. (1995). Addition of 1.0 mm ADP to this solution, in the presence of 8 mm ATP, only slightly altered the maximum Ca2+-activated force and the Ca2+ sensitivity of the contractile apparatus. Small or no changes in maximum Ca2+-activated force and Ca2+ sensitivity in the presence of ADP were also reported in previous investigations on fast-twitch muscle fibres when ATP was 3–8 mm (Cooke & Pate, 1985; Karatzaferi et al. 2003; Macdonald & Stephenson, 2004). However, at low ATP concentrations there is a marked ADP-dependent increase in maximum Ca2+-activated force and Ca2+ sensitivity arising from ADP inhibiting the dissociation of (i) ADP from the actomyosin–ADP complex (Cooke & Pate, 1985; Dantzig et al. 1991) and (ii) actomyosin to actin and myosin in the presence of ATP to form myosinATP. This would lead to crossbridges being attached for longer, thereby causing an increase in the average force produced per crossbridge and in the level of activation at given Ca2+. The increase in Ca2+ sensitivity of the contractile apparatus at elevated [ADP], may in fact help to compensate for the lower SR Ca2+-release that occurs in metabolically fatigued skeletal muscle (Fryer et al. 1995; Westerblad et al. 1998). However, it must be noted that in terms of dynamic contractions, the longer crossbridge attachment due to elevated [ADP] also reduces shortening velocity (Godt & Nosek, 1989; Chase & Kushmerick, 1995) and therefore power output.
Effects of elevated [ADP] on SR Ca2+-handling ability of slow-twitch fibres
In slow-twitch muscle, elevation of [ADP] to 1.0 mm, resulted in a marked reduction in the ability of the SR to load, retain and release Ca2+. At pCa 7.1, the SR Ca2+-content was reduced by approximately 20%. At pCa 7.8, the SR Ca2+-content was reduced by approximately 40%, and was the result of a combination of a 20% decrease in the SR Ca2+-pump rate and a 30% increase in the leak of Ca2+ from the SR (Fig. 3). The small reduction in the SR Ca2+-pump rate was despite a 1000-fold increase in [ADP], which would reduce the ATP free energy available to drive the SR Ca2+-pump by 22.5 kJ mol−1 ATP (RTln10000 = 8.3 × 295 × 9.2 J mol−1). This is a sizeable fraction of the total ATP free energy under our conditions, suggesting that the reduction in ATP free energy is unlikely to play a significant role in causing this effect, because then a much larger effect would have been expected. Interestingly, at pCa > 8.5, the rate of Ca2+ loss from the SR increased threefold as the [ADP] was increased from <0.1 μm to 1.0 mm, i.e. by a factor that was 10-fold greater than that at pCa 7.8. However, the increase was from a smaller base (0.46 min−1 at pCa > 8.5 and [ADP] < 0.1 μmversus 2.37 min−1 at pCa 7.8 and [ADP] < 0.1 μm). The markedly larger SR Ca2+-leak rates from the SR at pCa 7.8 than at pCa > 8.5 at all [ADP], indicates that the SR Ca2+-leak rate is sensitive to myoplasmic [Ca2+]. For example, at very low [ADP] (<0.1 μm), the SR Ca2+-leak rate was fivefold greater at pCa 7.8 than at pCa > 8.5, suggesting that at pCa 7.8 some Ca2+ loss is likely to occur through Ca2+-release channels which display strong Ca2+ dependence. This was not the case for fast-twitch fibres, where the SR Ca2+-leak rate was greater at pCa > 8 than at pCa 6.7 (Macdonald & Stephenson, 2001) and where Ca2+-induced Ca2+ release does not play an important role in the excitation–contraction (E–C) coupling (Launikonis & Stephenson, 2000). In view of these differences between the SR in slow- and fast-twitch fibres, it is possible that Ca2+-induced Ca2+ release may play a role in the E–C coupling of slow-twitch fibres.
The ADP-induced leak of Ca2+ from the SR at pCa > 8.5 does not appear to occur via the SR Ca2+-release channel, as it is insensitive to RR (Fig. 5). However, the SR Ca2+-pump blocker TBQ abolished the ADP-induced increase in SR Ca2+-leak, indicating that at pCa > 8.5 the SR Ca2+-pump is the site of the markedly augmented leak in the presence of high [ADP]. A SR Ca2+-pump mediated SR Ca2+-leak, has been observed in vesicle studies (Inesi & de Meis, 1989) and in fast-twitch muscle fibres (Duke & Steele, 2000; Macdonald & Stephenson, 2001, 2004). As argued previously (Macdonald & Stephenson, 2001), the mechanism of the ADP-induced SR Ca2+-leak is unlikely to be via reversal of the SR Ca2+-pump, as under conditions using similar ATP, ADP, inorganic phosphate (Pi) and Ca2+ concentrations, it is thermodynamically not possible to drive the pump into reverse mode. It is therefore most likely that the ADP-induced SR Ca2+-leak mechanism in slow-twitch muscle is via the ‘slippage’ mechanism described by Inesi & de Meis (1989) and proposed to exist in mechanically skinned fast-twitch muscle, under similar conditions to those used in the current study (Macdonald & Stephenson, 2001, 2004). Essentially, the Ca2+-binding sites of the ADP-sensitive state of the SR Ca2+-pump can face either the luminal or myoplasmic side of the SR membrane. Thus, the ADP-sensitive state may transport Ca2+ in either direction by slipping or flicking from one side of the SR membrane to the other. With an elevation in [ADP], the proportion of SR Ca2+-pumps in the ADP-sensitive state would be greater, therefore increasing the amount of Ca2+ that can leak from the SR via this slippage mechanism.
Interestingly, the rate constants of SR Ca2+-leak at pCa > 8.5 were very similar in slow-twitch and fast-twitch fibres and increased by a similar factor when [ADP] was elevated from 0.1 μm to 1.0 mm. This suggests that an elevated [ADP] affects the two major isoforms of the SR Ca2+-pump, SERCA 1 in fast-twitch fibres and SERCA 2A in slow-twitch fibres in a similar manner, with respect to the slippage mechanism. Nevertheless, there are major functional differences between the two SR Ca2+-pump isoforms with respect to the pCa values where the pump becomes activated, with the SERCA 2A isoform activated at pCa values that are at least one logarithmic unit higher (lower [Ca2+]) than that for SERCA 1. This observation is contrary to that of Lytton et al. (1992) who found these two SERCA isoforms expressed in COS cells, displayed no differences in Ca2+, ATP or pH dependency; or in peak Ca2-ATPase and Ca2+-uptake activity. The results clearly show that when expressed in slow- and fast-twitch fibres, respectively, the two isoforms display major differences with respect to the level of Ca2+ at which they become activated. It is noteworthy that in slow-twitch rat fibres, no phospholamban is present that can modulate SERCA 2A (see Bortolotto et al. 2001). The much lower ADP effect on the rate constant of Ca2+ loss from the SR at pCa < 8 than at pCa > 8.5 can be directly explained by the presence of a Ca2+-induced Ca2+-release mechanism operating in slow-twitch fibres which provides a high background for the Ca2+ leakage from the SR at [ADP] < 0.1 μm.
Slow-twitch versus fast-twitch fibres
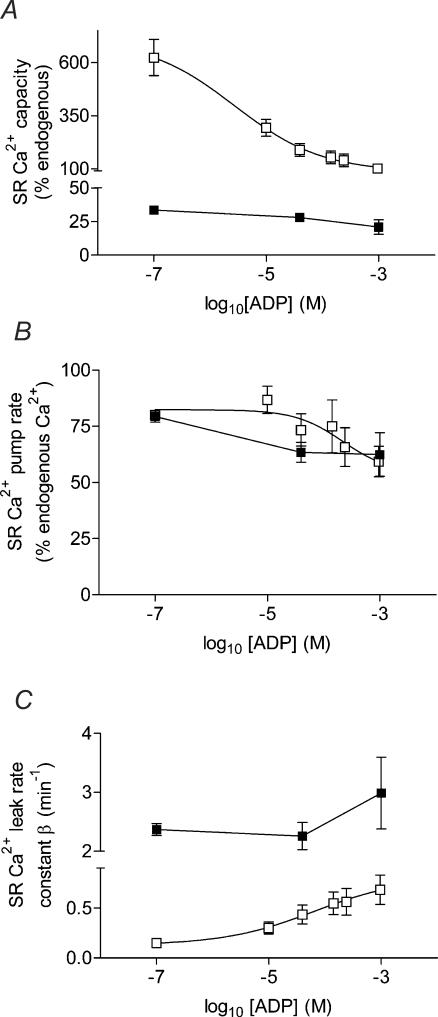
Slow-twitch muscle has long been considered to be more fatigue resistant in nature than fast-twitch muscle. The lower ATP utilization during contraction (Szentesi et al. 2001) and the greater mitochondrial content of slow-twitch muscle result in a smaller build up of ADP, Pi, and H+ and on a slower time scale than in fast-twitch muscle. At physiological pCa, an elevation in [ADP] affects slow-twitch SR Ca2+-handling ability to a smaller degree but by a similar mechanism to that of fast-twitch muscle fibres (Macdonald & Stephenson, 2001). To allow a clearer presentation of this comparison, Fig. 6 summarizes the effects of elevated [ADP] on the SR Ca2+-capacity, SR Ca2+-pump rate and SR Ca2+-leak rate (β) for both slow-twitch and fast-twitch muscle. The data for fast-twitch muscle was taken from Macdonald & Stephenson (2001), where experiments were performed under the similar conditions. In slow-twitch muscle, increasing the [ADP] from <0.1 μm to 40 μm and 1.0 mm decreased the maximum SR Ca2+-capacity by 20 and 55%, respectively, whereas in fast-twitch muscle, the same range of [ADP] reduced maximum SR Ca2+-capacity by four and six times, respectively. In both muscle fibre types, the reduction in SR Ca2+-capacity was due to a concomitant moderate slowing of the SR Ca2+-pump rate, and an increase in the leak of Ca2+ from the SR. The SR Ca2+-leak rate was not altered in slow-twitch muscle until the [ADP] had risen to 1.0 mm, and then only by 25%, while in fast-twitch muscle, the SR Ca2+-leak rate was extremely sensitive to [ADP], increasing by 3 and 4.5 times as the [ADP] was increased to 40 μm and 1.0 mm, respectively.
Figure 6. Comparison of the effects of elevated [ADP] on SR Ca2+-handling parameters of mechanically skinned slow-twitch and fast-twitch muscle fibres.
A, maximum SR Ca2+-capacity; B, SR Ca2+-pump rate; and C, SR Ca2+-leak rate constant (β), for fast-twitch (□) and slow-twitch (▪) muscle fibres, respectively. The data for fast-twitch muscle is taken from Macdonald & Stephenson (2001).
Thus, the effects of elevated [ADP] on SR Ca2+ handling in slow-twitch muscle are much less marked than in fast-twitch muscle (Macdonald & Stephenson, 2001), suggesting that slow-twitch muscle is less sensitive to changes in [ADP]. Indeed, others have shown that slow-twitch SR is less sensitive to elevations in intracellular Mg2+ than fast-twitch muscle (Stephenson et al. 1998), allowing normal SR Ca2+-release to occur at Mg2+ concentrations that would abolish such events in fast-twitch muscle.
Relevance to skeletal muscle fatigue
The role of altered SR Ca2+-handling is considered a significant contributor in skeletal muscle fatigue. Indeed, a decrease in SR Ca2+-release has been implicated in skeletal muscle fatigue (Fitts, 1994; Westerblad et al. 1998), with elevated Mg2+ (Stephenson et al. 1998) and low ATP (Dutka & Lamb, 2004) inhibiting the SR Ca2+-release channel. A depressed SR Ca2+-release may also be due to a reduced SR Ca2+-content, either via precipitation with Pi to form calcium phosphate (Fryer et al. 1995) or by an inability of the SR Ca2+-pump to re-sequester Ca2+ back into the SR (Tupling, 2004). A reduced SR Ca2+-pump would contribute to the elevated myoplasmic [Ca2+] and prolonged time to relaxation of force observed during skeletal muscle fatigue (Allen et al. 1995).
An elevation in [ADP] may explain the mechanisms responsible for each of these processes. The ADP-induced decrease in SR Ca2+-pump rate and increase in SR Ca2+-leak rate would contribute to the elevated resting myoplasmic [Ca2+]. This would also slow the time to relaxation of force, as Ca2+ would be bound to troponin C for a longer period of time. Furthermore, the reduced SR Ca2+-capacity at elevated [ADP] would result in a smaller pool of Ca2+ in the SR available for release, contributing to the depressed SR Ca2+-release. It therefore appears that elevated [ADP] may explain many of the observations associated with skeletal muscle fatigue, however, it must be emphasized that skeletal muscle fatigue is a multifactorial phenomenon (Stephenson et al. 1998).
The smaller ADP-induced reduction in SR Ca2+-pump rate and increase in SR Ca2+-leak rate in slow-twitch compared to fast-twitch muscle, together with the lower dynamic range of [ADP] in slow-twitch muscle, would lead to a greater stability of SR Ca2+-handling in slow-twitch muscle, and may significantly contribute to the fatigue resistant nature of slow-twitch muscle.
Acknowledgments
The authors gratefully acknowledge the financial support of the National Health and Medical Research Council of Australia, and the Australian Research Council.
References
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: Cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J Musc Res Cell Motil. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol. 2003;551:125–138. doi: 10.1113/jphysiol.2003.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GMM. MHC isoform composition and Ca2+- or Sr2+-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–C1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Stephenson DG, Stephenson GMM. Caffeine thresholds for contraction in electrophoretically typed, mechanically skinned muscle fibres from SHR and WKY rats. Pflugers Arch. 2001;441:692–700. doi: 10.1007/s004240000469. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effect of physiological [ADP]s on contraction of skinned fibres from rabbit fast and slow muscles. Am J Physiol. 1995;268:C480–C489. doi: 10.1152/ajpcell.1995.268.2.C480. [DOI] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967;193:45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzig JA, Hibberd MG, Trentham DR, Goldman YE. Cross-bridge kinetics in the presence of MgADP investigated by photolysis of caged ATP in rabbit psoas muscle fibres. J Physiol. 1991;432:639–680. doi: 10.1113/jphysiol.1991.sp018405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978;274:861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Characteristics of phosphate-induced Ca2+ efflux from the SR in mechanically skinned rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;278:C126–C135. doi: 10.1152/ajpcell.2000.278.1.C126. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Gage PW. Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J Physiol. 1983;341:213–231. doi: 10.1113/jphysiol.1983.sp014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Effect of low cytoplasmic [ATP] on excitation-contraction coupling in fast-twitch muscle fibres of the rat. J Physiol. 2004;560:451–468. doi: 10.1113/jphysiol.2004.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg BR, Kuda AM, Peter JB. Stereological analysis of mammalian skeletal muscle. I. Soleus muscle of the adult guinea pig. J Cell Biol. 1974;60:732–754. doi: 10.1083/jcb.60.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Iino M. Specific perforation of muscle cell membrane with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980;1:89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Ferguson DG, Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibers of guinea pig. Muscle Nerve. 1988;11:561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- Fink RHA, Stephenson DG. Ca2+ movements in muscle modulated by the state of K+ channels in the sarcoplasmic reticulum membranes. Pflugers Arch. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- Fink RHA, Stephenson DG, Williams DA. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Ferguson DG, Champ C. Discrimination between fast- and slow-twitch fibres of guinea pig skeletal muscle using the relative surface density of junctional transverse tubule membrane. J Mus Res Cell Motil. 1988;9:403–414. doi: 10.1007/BF01774067. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol. 1989;412:155–180. doi: 10.1113/jphysiol.1989.sp017609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock CR, Brault JJ, Wisemann RW, Terjung RL, Meyer RA. 31P-NMR observation of free ADP during fatiguing, repetitive contractions of murine skeletal muscle lacking AK1. Am J Physiol Cell Physiol. 2005;288:C1298–C1304. doi: 10.1152/ajpcell.00621.2004. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A, Lüttgau HC, Stephenson DG. Caffeine and excitation–contraction coupling in skeletal muscle: a stimulating story. J Mus Res Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum: roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Inesi G, Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPases. J Membr Biol. 1994;141:1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Karatzaferi C, Myburgh KH, Chinn MK, Franks-Skiba K, Cooke R. Effect of an ADP analog on isometric force and ATPase activity of active muscle fibers. Am J Physiol Cell Physiol. 2003;284:C816–C825. doi: 10.1152/ajpcell.00291.2002. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA. High intracellular [Ca2+] alters sarcoplasmic reticulum function in skinned skeletal muscle fibres of the rat. J Physiol. 1999;519:815–827. doi: 10.1111/j.1469-7793.1999.0815n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. J Physiol. 1990;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Excitation–contraction coupling in skeletal muscle fibres of rat and toad in the presence of GTP gamma S. J Physiol. 1991;444:65–84. doi: 10.1113/jphysiol.1991.sp018866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. J Physiol. 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effects of Mg2+ on Ca2+ release from sarcoplasmic reticulum of skeletal muscle fibres from yabby (crustacean) and rat. J Physiol. 2000;526:299–312. doi: 10.1111/j.1469-7793.2000.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden CG, Simonides WS, Muller A, van der Laarse WJ, Vermeulen JL, Zuidwijk MJ, Moorman AF, van Hardeveld C. Fiber-specific regulation of Ca2+-ATPase isoform expression by thyroid hormone in rat skeletal muscle. Am J Physiol. 1996;271:C1908–C1919. doi: 10.1152/ajpcell.1996.271.6.C1908. [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on action potential-induced force responses in mechanically skinned rat fast-twitch fibres. J Physiol. 2004;559:431–445. doi: 10.1113/jphysiol.2004.067603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Brown TR, Kushmerick MJ. Phosphorus nuclear magnetic resonance of fast- and slow-twitch muscle. Am J Physiol. 1985;248:C1239–C1245. doi: 10.1152/ajpcell.1985.248.3.C279. [DOI] [PubMed] [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. J Muscle Res Cell Motil. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- O'Connell B, Stephenson DG, Blazev R, Stephenson GMM. Troponin C isoform composition determines differences in Sr2+-activation characteristics between rat diaphragm fibers. Am J Physiol Cell Physiol. 2004;287:C79–C87. doi: 10.1152/ajpcell.00555.2003. [DOI] [PubMed] [Google Scholar]
- van der Poel C, Stephenson DG. Reversible changes in Ca2+-activation properties of rat skeletal muscle exposed to elevated physiological temperatures. J Physiol. 2002;544:765–776. doi: 10.1113/jphysiol.2002.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EI. Role of creatine phosphokinase in cellular function and metabolism. Can J Physiol Pharm. 1978;56:691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Stephenson EW. Activation of fast skeletal muscle: contribution of studies on skinned fibres. Am J Physiol. 1981;240:C1–C19. doi: 10.1152/ajpcell.1981.240.1.C1. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD, Stephenson GM. Events of the excitation–contraction–relaxation (E–C–R) cycle in fast-twitch muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Stephenson GM, O'Callaghan A, Stephenson DG. Single-fibre study of contractile and biochemical properties of skeletal muscles. Diabetes. 1994;43:622–628. doi: 10.2337/diab.43.5.622. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Temperature-dependent calcium sensitivity changes in skinned muscle fibres of rat and toad. J Physiol. 1985;360:1–12. doi: 10.1113/jphysiol.1985.sp015600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Zaremba R, van Mechelen W, Steinen GJM. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531:393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupling AR. The sarcoplasmic reticulum in muscle fatigue and disease: Role of the sarc (endo) plasmic reticulum Ca2+-ATPase. Can J Appl Physiol. 2004;229:308–329. doi: 10.1139/h04-021. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Veksler V, Hoerter JA. Myofibrillar creatine kinase and cardiac contraction. Mol Cell Biochem. 1994;133/134:125–144. doi: 10.1007/BF01267952. [DOI] [PubMed] [Google Scholar]
- Walliman G, Turner DC, Eppenberger HM. Localization of creatine kinase isoenzymes in myofibrils. I. Chicken skeletal muscle. J Cell Biol. 1997;75:297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Bruton JD, Andrade FH, Lännergren J. Mechanisms underlying the reduction in isometric force in skeletal muscle fatigue. Acta Physiol Scand. 1998;162:253–260. doi: 10.1046/j.1365-201X.1998.0301f.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Changes of the force-velocity relation, isometric tension and relaxation rate during fatigue in intact, single fibres of Xenopus skeletal muscle. J Mus Res Cell Motil. 1994;15:287–298. doi: 10.1007/BF00123481. [DOI] [PubMed] [Google Scholar]