Abstract
Cerebellar neurones show complex and differentiated mechanisms of action potential generation that have been proposed to depend on peculiar properties of their voltage-dependent Na+ currents. In this study we analysed voltage-dependent Na+ currents of rat cerebellar granule cells (GCs) by performing whole-cell, patch-clamp experiments in acute rat cerebellar slices. A transient Na+ current (INaT) was always present and had the properties of a typical fast-activating/inactivating Na+ current. In addition to INaT, robust persistent (INaP) and resurgent (INaR) Na+ currents were observed. INaP peaked at ∼−40 mV, showed half-maximal activation at ∼−55 mV, and its maximal amplitude was about 1.5% of that of INaT. INaR was elicited by repolarizing pulses applied following step depolarizations able to activate/inactivate INaT, and showed voltage- and time-dependent activation and voltage-dependent decay kinetics. The conductance underlying INaR showed a bell-shaped voltage dependence, with peak at −35 mV. A significant correlation was found between GC INaR and INaT peak amplitudes; however, GCs expressing INaT of similar size showed marked variability in terms of INaR amplitude, and in a fraction of cells INaR was undetectable. INaT, INaP and INaR could be accounted for by a 13-state kinetic scheme comprising closed, open, inactivated and blocked states. Current-clamp experiments carried out to identify possible functional correlates of INaP and/or INaR revealed that in GCs single action potentials were followed by depolarizing afterpotentials (DAPs). In a majority of cells, DAPs showed properties consistent with INaR playing a role in their generation. Computer modelling showed that INaR promotes DAP generation and enhances high-frequency firing, whereas INaP boosts near-threshold firing activity. Our findings suggest that special properties of voltage-dependent Na+ currents provides GCs with mechanisms suitable for shaping activity patterns, with potentially important consequences for cerebellar information transfer and computation.
Granule cells (GCs) are small neurones that occupy a key position within the cerebellar-cortex circuitry forming the input layer of the major cerebellar afferent system. Mossy fibres activate GCs, which emit their output to other neurones in the cerebellar cortex through parallel fibres. In turn, GCs receive feed-forward and feed-back inhibition from Golgi cells (Eccles et al. 1967). Understanding the mechanisms that regulate GC action potential (AP) discharge upon excitation is particularly important, since GCs determine the AP code to be relayed into the cerebellar circuitry.
Voltage-dependent Na+ currents appear to play an important role in regulating the intrinsic activity patterns expressed by cerebellar neurones. Current-clamp recordings in acute cerebellar slices (D'Angelo et al. 1995, 1998; Brickley et al. 1996) and in vivo (Chadderton et al. 2004) have shown that GCs behave as regular spiking neurones in response to sustained, intense depolarizing stimuli. In the presence of just-threshold depolarization, however, GCs also show additional, peculiar patterns of activity and AP organization (D'Angelo et al. 1998, 2001). Single APs are preceded by slow, ramp-like depolarizing prepotentials, and are often followed by transient depolarizing afterpotentials (DAPs) that may also recur a few times before the subsequent AP is fired. APs are not regularly spaced, but tend to cluster into low-frequency bursts. It was suggested that prepotentials, DAPs and spike clustering reflect the intervention of a persistent Na+ current (INaP; D'Angelo et al. 1998), similar to INaP that in various neurones subtend sub- and near-threshold sustained depolarizing events (discussed in Magistretti & Alonso, 2002). Indeed, in the presence of K+- and Ca2+-channel block, the steady-state voltage-current (V–I) relationship of GCs shows an inward rectification region at about −55 to −30 mV that is abolished by tetrodotoxin (TTx) (D'Angelo et al. 1998). Modelling data also support the notion that the depolarizing envelope subtending AP bursts depends on INaP (D'Angelo et al. 2001). In addition, a resurgent Na+-current component (INaR; Raman & Bean, 1997) has recently been demonstrated in GCs (Magistretti et al. 2004; Afshari et al. 2004), and has also been suggested to participate in GC AP clustering (D'Angelo et al. 2001). INaR activates upon repolarization after depolarizations able to fully inactivate the transient Na+ current (INaT), and corresponds to Na+ channels transiently dwelling in the open state during return to closed states (Raman & Bean, 2001). In Purkinje cells, where it was first described, INaR has been proposed to promote the discharge of doublets or triplets of APs, instead of single spikes, in response to brief depolarizing pulses (Raman & Bean, 1997), thus contributing to AP conglomeration. In Purkinje cells, INaR also appears to have a role in enhancing firing frequency during tonic discharge (Khaliq et al. 2003). A similar role could also be played by INaR in GCs, which can produce relatively high maximal firing frequencies (120–150 Hz) (D'Angelo et al. 1998; Chadderton et al. 2004).
The above findings underscore the importance of determining the biophysical and functional properties of voltage-dependent Na+ currents (VDNCs) expressed by native GCs, and especially of those active in the sub- and near-threshold range of membrane potentials. Because a detailed analysis of VDNCs in native GCs is still lacking, in the present work we have carried out a thorough characterization of VDNCs in GCs in situ by performing whole-cell patch-clamp experiments in rat cerebellar slices. Besides INaT, persistent and resurgent Na+ currents were observed. We provide a biophysical characterization for each of these Na+-current components. We also present current-clamp and modelling data suggesting that INaR is involved in generating DAPs and enhances high-frequency firing, whereas INaP controls near-threshold firing activity, thus contributing to set the excitable properties of these neurones.
Methods
The animal experiments described in the manuscript conformed with the rules established by the University of Pavia for the use of animals in experimental studies, in compliance with the guidelines of the Italian Ministry of Health, the national laws on animal research (d.l. 116/92), and the EU guidelines on animal research (N. 86/609/CEE).
Slice preparation
Young Wistar rats (16–23 days old) were anaesthetized by inhalation of alothane (Sigma-Aldrich, Milan, Italy) and decapitated. The cerebellum was quickly extracted under hypothermic conditions and submerged in an ice-cold dissection Krebs solution composed of (mmol l−1): 127 NaCl, 1.32 KCl, 26 NaHCO3, 1.18 KH2PO4, 1.19 MgSO4, 0.2 ethyleneglycol-bis-(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid (EGTA), 16 d-glucose (pH 7.4 by saturation with 95% O2, 5% CO2). The cerebellar vermis was isolated from the hemispheres and blocked on the stage of a Microslicer DTK-1000 vibratome (Dosaka, Kyoto, Japan) using cyanoacrylate glue. During the sectioning procedure the tissue was submerged in an ice-cold (∼1–2°C) cutting solution containing (mmol l−1): 130 potassium gluconate, 15 KCl, 20 N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (Hepes), 0.2 EGTA, 11 d-glucose (pH 7.4 with KOH). Using this high-K+ solution was found to improve neurone viability (Stéphane Dieudonné, unpublished results). Sagittal sections (250 μm thick) were cut, then rinsed in maintenance Krebs solution containing (mmol l−1): 127 NaCl, 1.32 KCl, 26 NaHCO3, 1.18 KH2PO4, 1.19 MgSO4, 2 CaCl2, 11 d-glucose (pH 7.4 by saturation with 95% O2, 5% CO2), and transferred to an incubation chamber filled with the same solution (continuously bubbled with 95% O2, 5% CO2). The slices were kept submerged in the incubation chamber at room temperature for at least 1 h before starting the recording.
Patch-clamp experiments: voltage-clamp recordings
The recording chamber was mounted on the stage of an inverted microscope (Axiovert 100; Zeiss, Oberkochen, Germany). Slices were transferred, one at a time, to the chamber and perfused with a solution suitable for isolating Na+ currents, containing (mmol l−1): 100 NaCl, 26 NaHCO3, 19.5 tetraethylammonium chloride (TEA-Cl), 3 KCl, 2 MgCl2, 2 CaCl2, 2 BaCl2, 0.5 CdCl2, 4 4-aminopyridine, 11 d-glucose (pH 7.4 by saturation with 95% O2, 5% CO2). The perfusion rate was about 1 ml min−1. Patch pipettes were fabricated from thick-wall borosilicate glass capillaries (CEI GC 150–7.5; Harvard Apparatus, Edenbridge, UK) by means of a Sutter P-87 horizontal puller (Sutter Instruments, Novato, CA, USA). The pipette solution contained (mmol l−1): 104 CsF, 50 TEA-Cl, 2 MgCl2, 10 Hepes, 10 EGTA, Na2ATP, and 0.2 NaGTP (pH adjusted to 7.2 with CsOH). The patch pipettes had a resistance of 5–8 MΩ when filled with the above solution. The recording electrode was inserted into the granular layer of the slice after application of positive pressure, and approached blindly to neurones in situ as described elsewhere (D'Angelo et al. 1993). Tight seals (>10 GΩ) and the whole-cell configuration were obtained by suction.
Voltage-clamp recordings of Na+ currents were performed at room temperature (21–24°C) by means of an EPC7 patch-clamp amplifier (List Electronics, Darmstadt, Germany). Series resistance (Rs) was continually monitored during the experiment using the amplifier built-in compensation section, and always compensated by 50–70% (average value, 55.5%; n = 128). Recordings in which Rs levels varied with time by more than 2 MΩ were discarded. Voltage protocols were commanded and current signals were acquired with a Pentium personal computer interfaced to a Tl–1 interface (Axon Instruments, Union City, CA, USA) using program Clampex of the pClamp 6.0.5 software package (Axon Instruments). In all recordings the holding potential was −80 mV. Current signals were low-pass filtered and digitized at different frequencies according to the experimental protocol applied: cut-off and sampling frequencies were 5 and 50 kHz, respectively, for INaT activation protocols and INaR protocols; 5 and 20 kHz, respectively, for INaT steady-state inactivation protocols and INaP 500 ms step protocols; 2 and 5–10 kHz, respectively, for ramp protocols. Currents were always on-line leak subtracted via a P/4 routine, except in the case of protocols applied for INaP study (see Fig. 3).
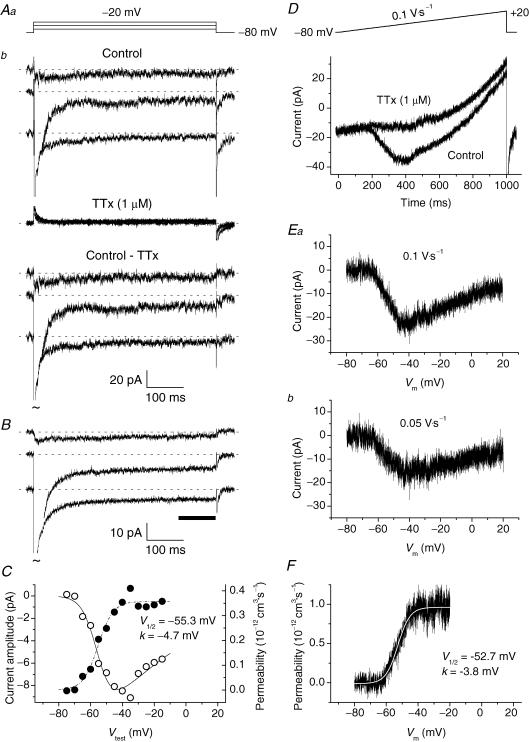
Figure 3. Persistent Na+ current (INaP) GCs.
INaP was activated by long-lasting step depolarizations (A–C) or ramp protocols (D–F). A, selected tracings from a representative GC (cell C3328). The current tracings shown in Ab have been recorded, in control conditions and in the presence of 1 μm TTx, in response to the voltage pulses illustrated in Aa. In this depolarizing-step protocol, the P/4 leakage subtraction routine was disabled to reduce background noise, and Na+ currents were isolated from capacitive and leakage currents and other contaminants via TTx subtraction. TTx-sensitive currents are shown in the lower part of Ab. Tildes indicate that INaT peaks have been truncated. B, average, TTx-subtracted current tracings obtained from 15 different neurones. The voltage protocol applied was the same as illustrated in Aa (test potentials are, from top to bottom, −60, −40 and −20 mV). The horizontal bar indicates the region of each tracing the data points of which were averaged to obtain a measure of current amplitude. C, I–V relationship (^) and plot of permeability as a function of Vtest (•) for the average INaP (see B). Permeability values have been derived from current values as explained in the text. The dashed–dotted line is the best fitting obtained by applying a single Boltzmann function to permeability data points; fitting parameters are also specified. The continuous line has been derived from the same fitting function by converting permeability into current on the basis of the GHK equation. D, the current recorded in a representative GC (cell B3312), in control conditions and in the presence of 1 μm TTx, in response to the ramp depolarization illustrated in the upper part of the panel. E, TTx-sensitive, ramp-evoked currents in the same cell. The current shown in Ea has been obtained by subtraction from the two tracings of D. The current shown in Eb, also TTx-subtracted, was elicited by a voltage ramp twice less steep. Note that in the x-axis time values have been replaced by the corresponding potential values. F, plot of permeability as a function of voltage for the current illustrated in Ea. The ascending branch of the tracing has been best-fitted with a single Boltzmann function (smooth, white curve); fitting parameters are also specified.
Tetrodotoxin (TTx; Alomone Laboratories, Jerusalem, Israel) was applied in the bath with the superfusing solution.
Patch-clamp experiments: current-clamp recordings
Current-clamp recordings were obtained from GCs in cerebellar slices. Slices were perfused with a solution containing (mmol l−1): 120 NaCl, 2 KCl, 1.2 MgSO4, 26 NaHCO3, 1.2 KH2PO4, 2 CaCl2, 11 d-glucose, 1 kynurenic acid, 0.1 picrotoxin (pH 7.4 by saturation with 95% O2, 5% CO2). The pipette solution contained (mmol l−1): 135 or 145 potassium gluconate, 5 KCl, 10 Hepes, 0.2 EGTA, 4.6 MgCl2, 2 Na2ATP, 0.4 NaGTP, pH 7.35. Recordings were performed at room temperature using a Multiclamp amplifier or an Axopatch 200B amplifier (both from Axon Instruments), the latter being used in the Ifast mode. Clampex 8.2 and a Digidata 1322A digitiser were used to inject current steps and acquire voltage signals. Sampling frequency was 50 kHz. No Rs compensation (bridge balance) was used. Voltage measurements were not corrected for errors introduced by Rs (≤1 mV).
Data analysis
Whole-cell current and voltage signals were analysed by means of program Clampfit of pClamp 6.0.5 and pClamp 8.2, respectively. Na+ currents were normally re-filtered off-line at 2–3.5 kHz. Current amplitude was measured at the peak of each tracing, unless otherwise specified. Na+ permeabilities (PNa) were calculated from current amplitudes (INa) by applying the Goldman-Hodgkin-Katz equation in the form:
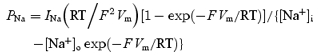 |
(1) |
in which the nominal intra- and extracellular Na+ concentration values (4.2 and 126 mm, respectively) were introduced.
To analyse INaT time course according to a Hodgkin-Huxley (HH) formalism, exponential fittings of INaT inactivation phase were first carried out. Second-order exponential functions were consistently required for proper fitting at test potentials of −35 to +15 mV. Because in the HH model the activation and inactivation processes are independent, current tracings were divided by their inactivation fitting functions, extrapolated to time zero of the depolarizing test pulse, to reconstruct the time course of the HH activation process. The curves thus obtained were then fitted with a HH activation function in the form:
| (2) |
where the power coefficient n was either left free to vary or kept at a fixed value (see Results).
Data fittings with exponential functions, I = ΣAi exp(−t/τi) +C, were carried out using Clampfit. Fittings with Boltzmann functions, y = ymax/{1 + exp[(V−V½)/k}, and HH activation functions (see above) were carried out using Origin 6.0 (OriginLab Corp., Northampton, MA, USA).
Rs was evaluated off-line by analysing the properties of capacitive current transients evoked by −10 mV voltage square pulses from the holding potential of −80 mV, as explained elsewhere (D'Angelo et al. 1993). Average Rs was 27.9 ± 1.2 MΩ (n = 84).
Average values are expressed as means ± s.e.m. Statistical significance was evaluated by means of a two-tail Student's t test for paired or unpaired data.
Computer simulations
Computer simulations of GC electrical activity were performed in the NEURON environment (Hines & Carnevale, 1997) by employing a GC single-compartment model described in detail elsewhere (D'Angelo et al. 2001). Modelling schemes and kinetic parameters were identical to those described in D'Angelo et al. (2001) for all membrane currents except Na+ currents. The HH-type formalism, previously employed to separately model INaT, INaP and INaR, was replaced with the 13-state, allosteric Na+-channel model by Raman & Bean (2001) in the modified form described by Khaliq et al. (2003), which simultaneously generates the three current components, and is shown in Scheme 1.
Scheme 1.
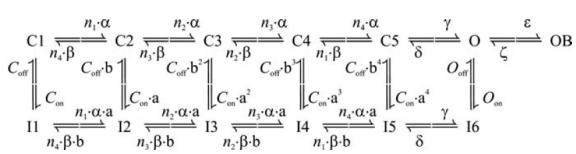
It is worth remarking that the model implicitly assumes the existence of a single Na+ channel accounting for all Na+-current components. The values of the kinetic parameters in Scheme 1 were adjusted to fit the observed properties of all Na+-current components, and were set as follows: α = 353.9 exp(V/kα) ms−1, β = 1.272 exp(−V/kβ) ms−1, kα = kβ = 13.9 mV, n1 = 5.42, n2 = 3.28, n3 = 1.83, n4 = 0.74; γ = 150 ms−1, δ = 40 ms−1, ɛ = 1.75 ms−1, ζ = 0.0201 exp(−V/kζ) ms−1, kζ = 25.0 mV; Con = 0.005 ms−1, Coff = 0.5 ms−1, Oon = 0.75 ms−1, Ooff = 0.005 ms−1, a = (Oon/Con)¼, b = (Ooff/Coff)¼. Na+ current was calculated as: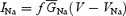 , where f is the fraction of channels in the open (O) state as returned by numerical resolution of Scheme 1 for time and voltage;
, where f is the fraction of channels in the open (O) state as returned by numerical resolution of Scheme 1 for time and voltage; 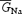 , the maximal Na+ conductance, was 8.0 mS cm−2; and VNa, the Na+ equilibrium potential, was +87.4 mV.
, the maximal Na+ conductance, was 8.0 mS cm−2; and VNa, the Na+ equilibrium potential, was +87.4 mV.
To modify the persistent or the resurgent current components, kinetic constants were modified as follows: (1) INaR was switched off by setting the forward rate constant for the O → OB transition, ɛ, to zero. By itself, this modification (‘0-INaR− condition’, for brevity) also resulted in considerable slowing of INaT decay (see Fig. 8C, violet tracing), due to the lack of the O → OB transition, which is a major exit path from the open state. (2) With ɛ set at zero, the rate constant of the O → I6 transition, Oon, was increased to 2.15 ms−1 to restore the normal INaT decay speed. At the same time, the rate constant of the I6 → O transition, Ooff, was increased to 0.01433 ms−1 to prevent reduction of the persistent component. These changes (‘0-INaR+ condition’, for brevity) allowed us to obtain Na+ currents in which the INaR component was absent while the INaT and INaP components were largely unmodified (see Fig. 8C and D, red tracings). Note that the 0-INaR+ condition can be regarded as the equivalent of endowing the cell with a set of Na+ currents identical to control currents, with the only difference of INaR absence, whereas the 0-INaR− condition can be viewed as the equivalent of removing the mechanism of INaR generation with no additional re-adjustments. (3) To reduce the steady-state INaP in the absence of other changes, the rate constant of the I6 → O transition, Ooff, was decreased to 0.002 or 0.0015 ms−1 (all other kinetic parameters were left unmodified). This resulted in an INaP reduction of 58 or 67%, respectively, at the steady state.
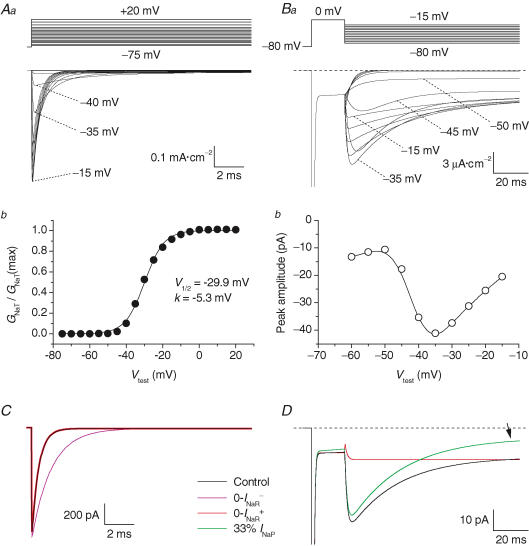
Figure 8. Simulated voltage-clamp activation of GC Na+ currents.
A, simulated INaT. Aa, the currents activated by a voltage-clamp protocol consisting of 15 ms depolarizing voltage steps at −70 to +20 mV in 5 mV increments, from a holding potential of −80 mV. Tracings corresponding to test potentials of −5, +5 and +15 mV have been omitted for clarity. The voltage-clamp protocol is schematized in the upper part of the panel. Ab, plot of normalized peak conductance ( ) as a function of test voltage. The plot has been fitted with a single Boltzmann function (continuous line). Fitting parameters are also specified. B, simulated INaR. Ba, currents activated by repolarizing pulses at −15 to −70 mV in −5 mV increments, after a 19 ms depolarizing step at 0 mV (the voltage-clamp protocol is schematized in the upper part of the panel). Bb, the I–V relationship of the simulated INaR. In all simulations, the integration step size was 0.4 μs. C, effects on INaT of manipulations on the kinetic scheme aimed at modifying INaR. Simulated currents evoked by 15 ms depolarizing steps at 0 mV are shown. The current obtained under control conditions is shown in black. The violet tracing is the current obtained after setting the rate constant of the O → OB reaction, ɛ, to 0 (0-INaR− condition: see kinetic Scheme 1 and the text for details). The red tracing is the current after setting ɛ to 0, the rate constant of the O → I6 reaction, Oon, to 2.15 ms−1, and the rate constant of the I6 → O reaction, Ooff, to 0.01433 ms−1 (0-INaR+ condition). Note that the black and the red tracings are almost completely indistinguishable. D, modifications of INaP and INaR obtained by variously manipulating the kinetic scheme. The black tracing is the simulated control current evoked by the protocol shown in Ba (with a return potential of −35 mV). The red tracing is the current obtained in the 0-INaR+ condition (see above): note that INaR is abolished, whereas INaP is unmodified. The green tracing is the current obtained after setting Ooff to 0.0015 ms−1, and leaving all the other rate constants at their control settings: note that INaP is decreased at the steady state (arrow), whereas INaR is almost unaffected.
) as a function of test voltage. The plot has been fitted with a single Boltzmann function (continuous line). Fitting parameters are also specified. B, simulated INaR. Ba, currents activated by repolarizing pulses at −15 to −70 mV in −5 mV increments, after a 19 ms depolarizing step at 0 mV (the voltage-clamp protocol is schematized in the upper part of the panel). Bb, the I–V relationship of the simulated INaR. In all simulations, the integration step size was 0.4 μs. C, effects on INaT of manipulations on the kinetic scheme aimed at modifying INaR. Simulated currents evoked by 15 ms depolarizing steps at 0 mV are shown. The current obtained under control conditions is shown in black. The violet tracing is the current obtained after setting the rate constant of the O → OB reaction, ɛ, to 0 (0-INaR− condition: see kinetic Scheme 1 and the text for details). The red tracing is the current after setting ɛ to 0, the rate constant of the O → I6 reaction, Oon, to 2.15 ms−1, and the rate constant of the I6 → O reaction, Ooff, to 0.01433 ms−1 (0-INaR+ condition). Note that the black and the red tracings are almost completely indistinguishable. D, modifications of INaP and INaR obtained by variously manipulating the kinetic scheme. The black tracing is the simulated control current evoked by the protocol shown in Ba (with a return potential of −35 mV). The red tracing is the current obtained in the 0-INaR+ condition (see above): note that INaR is abolished, whereas INaP is unmodified. The green tracing is the current obtained after setting Ooff to 0.0015 ms−1, and leaving all the other rate constants at their control settings: note that INaP is decreased at the steady state (arrow), whereas INaR is almost unaffected.
All other membrane parameters of the GC model, including specific membrane conductances, membrane surface area, and ion equilibrium potentials, as well as all parameters related to intracellular-Ca2+ dynamics, were the same as specified in D'Angelo et al. (2001). In both simulated voltage-clamp and current-clamp experiments, temperature was set at 22°C.
Results
Voltage-clamp experiments were performed under ionic conditions suitable to isolate Na+ currents (see Methods). Current tracings recorded in the presence of 1 μm TTx were routinely subtracted from control tracings to better isolate TTx-sensitive Na+ currents from contaminants. Recordings on GCs in situ were obtained from cells located in the external part of cerebellar lobules VI–IX. These experiments allowed us to identify three distinct Na+-current components in GCs.
Transient Na+ current
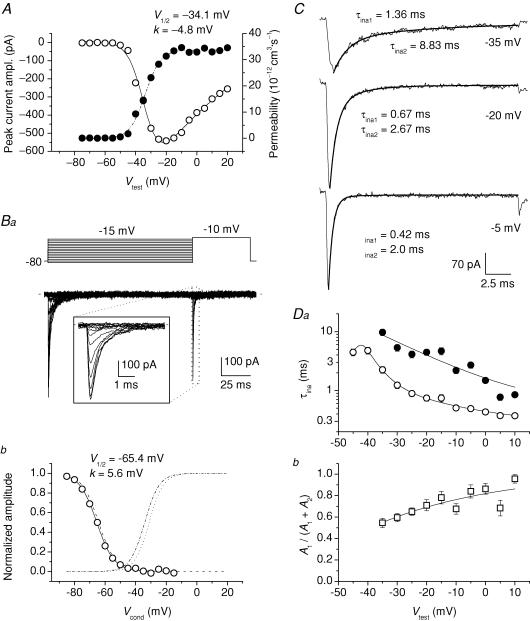
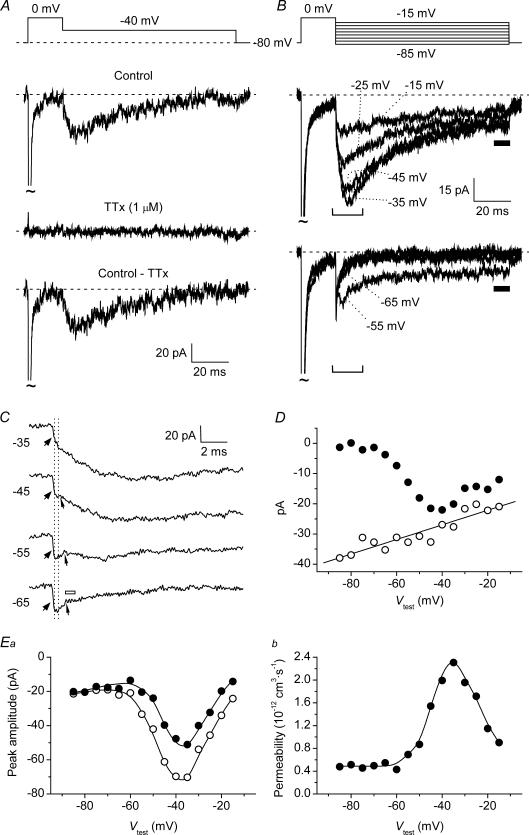
Transient Na+ currents (INaT) were studied by delivering 20 ms voltage square pulses at −75 to +20 mV in 5 mV increments. This depolarizing protocol elicited INaT that were always abolished by 1 μm TTx (Fig. 1A and B). In a large majority of GCs in situ (for brevity, named group 1 cells, n = 60 out of 84) INaTs showed signs of poor space clamp control (Fig. 1A). These currents included an unclamped component that was activated starting from −60 to −45 mV, typically after a more or less pronounced lag, and showed virtually no increase in amplitude in the subsequent one or two depolarizing steps. At more positive voltages a better-clamped component superimposed to the unclamped component, suggesting that in these cells the Na+ current was due to channels located partly in a membrane region electrotonically distant from the soma (presumably the axon), and partly in a more proximal region. In a few other cells (group 2, n = 12), the second, well-clamped Na+-current component was observed in isolation: an example of the currents recorded in these neurones is illustrated in Fig. 1Ba. In the remaining 12 cells, either currents with features intermediate between those of the two other groups (n = 6) or fully unclamped spikes (n = 6) were observed. Only the currents recorded in group 2 cells were used for the study of INaT biophysical properties. In group 2 cells, a measurable INaT was activated starting around −50 mV and the current-voltage (I–V) relationship peaked at −20/−15 mV (Figs 1Bb and 2A, open circles), with INaT amplitude at the I–V peak averaging −446.3 ± 74.9 pA (n = 12) (in group 1 cells, peak INaT amplitude was −640.3 ± 40.3 pA, n = 60). Peak current–amplitude values were used to calculate the permeability underlying INaT (PNaT) as explained in the Methods, and PNaT values were plotted as a function of test potential (Fig. 2A, filled circles). The PNaT–V plots obtained were fitted with a Boltzmann function, which returned an average half-maximal activation potential (V½) of −31.2 ± 1.9 mV and an average activation slope factor (k) of −4.7 ± 0.3 mV (n = 12). A steady-state inactivation protocol (Fig. 2Ba) was also routinely applied, and the results were analysed in group 2 cells (Fig. 2Bb): Boltzmann fitting of steady-state inactivation plots returned average V½ and k values of −64.2 ± 3.6 mV and +5.5 ± 0.2 mV, respectively, (n = 5).
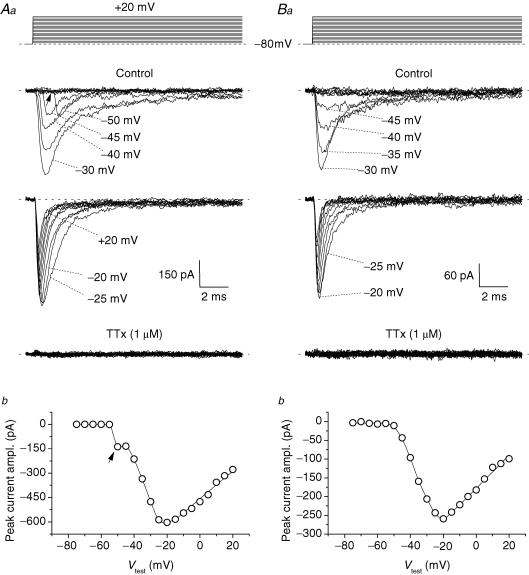
Figure 1. Transient Na+ currents (INaT) in granule cells (GCs) in situ.
A and B, the properties of the INaT recorded in two typical GCs (cell C4120 in A, and cell C4129 in B, representative of 60 and 12 cells, respectively). The voltage-clamp protocol applied to activate INaT is shown in the upper part of Aa and Ba. The middle part of the panels shows the experimental tracings thus obtained in control conditions (the tracings recorded at −75 to −30 mV, and at −25 to +20 mV, have been split in two different subpanels). The lower part of the panels shows the tracings recorded in the presence of 1 μm tetrodotoxin (TTx). Aa, example of the currents recorded in group 1 neurones (see the text), in which an unclamped and a well-clamped INaT component were apparent. The arrow points to the prominent lag preceding the activation of the unclamped component at its threshold. Ba, example of the currents recorded in group 2 neurones (see the text), in which the well-clamped INaT component was observed in isolation. Ab and Bb, current–voltage (I–V) relationships for INaT peak amplitude in the same two cells.
Figure 2. INaT in GCs: voltage dependence of activation, steady-state inactivation, and kinetics.
A, average, normalized (I–V) relationship of INaT in a representative group 2 GC (cell B3512) (^), and the corresponding permeability values (PNaT, calculated as explained in Methods), plotted as a function of test potential, Vtest (•). The PNaT–V plot was best-fitted with a single Boltzmann function (dashed–dotted line): the fitting parameters thus obtained are also specified. The continuous line has been obtained from the same fitting function by converting permeability into current on the basis of the Goldman-Hodgkin-Katz (GHK) equation. Ba, voltage-clamp protocol applied to study INaT steady-state inactivation (top), and the currents thus obtained in the same cell illustrated in A (bottom). The duration of the conditioning prepulse was 125 ms. The inset shows a detail, over an expanded time scale, of the currents elicited by the activating pulse. Bb, plot of voltage dependence of INaT steady-state inactivation for the same cell. The peak amplitude values of the currents elicited by the activating pulse of the protocol illustrated in Ba were normalized for the maximal value observed and plotted as a function of conditioning-prepulse potential, Vcond (^). The continuous line is the best Boltzmann fitting to data points; fitting parameters are also specified. The activation PNaT–V function obtained for this cell, normalized to its asymptotic value, is shown for comparison (dashed–dotted line: same as in A). The dotted line and the dashed line are average activation and steady-state inactivation functions, respectively, constructed as Boltzmann functions in which parameters V½ and k equalled the respective average values (see the text). C, three exemplary INaT tracings recorded at the test potentials of −35, −20 and −5 mV in a representative group 2 GC (cell B4203). The inactivation phase of each current tracing has been best-fitted with a double exponential function (thick lines): the values of the fast and the slow inactivation time constants (τina1 and τina2, respectively) are also specified. D, plots of average INaT inactivation time constants (Da; ^ and •: τina1 and τina2, respectively) and relative amplitude of the fast exponential component (Db) as a function of Vtest (n = 12). Note the logarithmic scale of the y-axis in Da.
INaT kinetics was also analysed in group 2 cells. INaT inactivation was first examined. The current inactivation phase was best described by a second-order exponential function at −35 to +15 mV (Fig. 2C). Both the fast and slow exponential components strongly accelerated with depolarization, as shown by the plot of the two inactivation time constants (τina1 and τina2) as a function of test potential (Vtest) (Fig. 2Da). In addition, the relative weight of the fast exponential component tended to increase with depolarization (Fig. 2Db). INaT activation kinetics was then analysed by either considering rise time from 10 to 90% of peak amplitude (RT10–90) or performing HH fittings of the current waveform (see Methods). RT10–90 decreased from an average value of 1.3 ms at −45 mV to 155 μs at −10 to +10 mV (not shown). The application of the HH fitting procedure revealed that when n, the power coefficient of the HH fitting function (Methods, eqn (2)), was treated as a free fitting parameter, its value varied considerably at different Vtest values, and markedly increased with depolarization (from ∼2 at −45/−40 mV to ∼9 at +5/+10 mV; see Supplemental Fig. A). Fixing the value of n at 4 or, even more, 3, returned a poorer correspondence of fittings with experimental tracings. The time constant of activation, τm, clearly tended to decrease with depolarization in HH fittings performed with free n (from 350 μs at −45/−40 mV to 65 μs at +5/+10 mV; Supplemental Fig. A, panel B1) and also, although to a lesser extent, with fixed n.
To evaluate the influence of series-resistance errors on the above measurements, we calculated the maximal estimated voltage error due to series resistance as Verr(max) = Rs(1 − fcomp)INaT(max), where Rs is series resistance, fcomp is the fraction of Rs compensation, and INaT(max) is the maximal Na+-current amplitude. In group 2 neurones, Verr(max) averaged 4.9 ± 0.9 mV (n = 12).
Persistent Na+ current
The application of long-lasting depolarizing steps and slow depolarizing ramps revealed that GCs in situ express, in addition to INaT, a small non-inactivating Na+-current component. Examples of such a persistent Na+ current (INaP) are illustrated in Fig. 3. Both group 1 and group 2 cells were considered for the study of INaP. The INaP evoked by 500 ms depolarizing pulses at −75 to −15 mV, in 5 mV increments, was first characterized (Fig. 3Ab). The application of this protocol revealed the presence of INaP as a sustained, TTx-sensitive inward-current component (Fig. 3Ab, lower tracings). Because of the very small size of this current, its properties were analysed after averaging experimental tracings obtained from a number of different cells, which substantially increased the signal-to-noise ratio and the reliability of measurements (Fig. 3B). The cells used to obtain the average current (10 group 1 cells plus 5 group 2 cells) were selected on the sole basis of optimal input-resistance and holding-current stability. INaP started to activate at about −65 mV, and its peak was at about −40 mV (Fig. 3C). The absolute amplitude of the average INaP (derived by considering the data points between 400 and 500 ms from the pulse onset) was −9.1 pA at the peak of the I–V relationship, which corresponds to 1.54% of the average maximal INaT amplitude observed in the same cells (−589.3 ± 82.9 pA, n = 15). The voltage dependence of the permeability underlying the average INaP (PNaP) is also shown in Fig. 3C (filled circles). Boltzmann fitting to data points returned V½ and k values of −55.3 mV and −4.7 mV, respectively.
In order to quickly explore the whole voltage range of INaP activation, slow ramp protocols able to inactivate INaT were also used. Ramp depolarizations from −80 to +20 mV at 100 mV s−1 evoked currents that, after TTx subtraction, provided a continuous I–V relationship for INaP (Fig. 3D and E). The I–V relationships thus obtained showed a ‘threshold’ at −70/−60 mV and a peak at about −40 mV (Fig. 3E). PNaP–V plots were also derived from ramp-activated currents and fitted with single Boltzmann functions, which returned V½ and k values very similar to those obtained from the analysis of step protocols (Fig. 3F). We also found that if the ramp slope was decreased to 50 mV s−1, the resulting INaP had a peak amplitude significantly lower than observed with 100 mV s−1 ramps (−15.1 ± 2.4 versus−22.4 ± 3.4 pA, respectively; n = 29; P < 1 × 10−4, t test for paired data) (Fig. 3E), which suggests the existence of slow INaP inactivation during sustained depolarizations (see Discussion).
Resurgent Na+ current
The application of repolarizing pulses after INaT activation and decay led, in most GCs in situ, to activation of a resurgent Na+ current (INaR). Figure 4A shows the currents recorded in a representative cell in response to a voltage-clamp protocol consisting of a 20 ms step pulse at 0 mV that activated INaT, followed by a 100 ms pulse at −40 mV. The repolarizing pulse induced an extra inward current that activated and then decayed in a time-dependent manner, and was abolished by 1 μm TTx. This current was therefore identified as INaR. Both group 1 and group 2 cells were considered for the study of INaR. The presence of space-clamp problems, reported in group 1 cells by poor clamp control of part of INaT, is unlikely to significantly affect INaR, due to the relatively slow time course of this current component.
Figure 4. Resurgent Na+ current (INaR) in GCs.
A, INaR in a representative GC (cell D4206). The experimental tracings are the currents recorded, in control conditions and in the presence of 1 μm TTx, in response to the depolarizing-repolarizing voltage command shown in the top panel. The bottom tracing is the TTx-sensitive current obtained by subtraction. B, INaR tracings obtained by averaging the currents recorded in six different neurones. Eight selected tracings are shown; the corresponding voltage commands are illustrated in the upper part of the panel. C, four tracings from B are shown in an expanded time scale to provide a detail of INaR onset. The tracing region highlighted corresponds to that embraced by the square brackets in B. The wide arrowheads point to the initial phase of current development, characterized by an abrupt and clearly faster rise (‘component (i)’). The narrow arrowheads point to the notch that may be observed between the initial phase and the late, slower phase of current development (‘component (ii)’). The two dotted, vertical lines delimit the tracing region the points of which were averaged to obtain a measure of component-(i) peak amplitude. The open bar next to the lowermost tracing marks the region in which INaR amplitude was measured in those tracings in which, due to the absence of an identifiable INaR peak, component (i) and component (ii) were not clearly separated: note that the region chosen immediately follows an evident reduction of the current's decline speed, indicative of INaP-tail subsiding (see the text). D, I–V relationship of component-(i) peak amplitude (^) and of the sustained inward current observed at the end of the 100 ms repolarizing pulses after INaR decay (Iend; •). Component-(i) peak amplitude was measured as illustrated in C. Iend amplitude was measured by averaging data points in the region marked by the horizontal bar in B. The straight line is the linear regression to component-(i) I–V plot, which returned an x-axis intercept at +71.7 mV, with R (regression coefficient) = 0.92, and P (the probability of R to be 0) < 1 × 10−4. E, voltage dependence of INaR peak amplitude and of the underlying permeability. Ea, the I–V relationship of raw INaR peak amplitude (^), and of ‘net’INaR peak amplitude (•), obtained by subtracting Iend amplitude from raw INaR peak amplitude. Eb, the voltage dependence of the permeability underlying the ‘net’INaR. Data points have been derived from those of Ea (•) by applying the GHK equation as described in Methods.
Although the majority of GCs expressed a measurable INaR, the current size was usually small, making its analysis problematic in individual cells. Therefore, average tracings from recordings made in different cells were constructed and used to perform an accurate analysis of INaR biophysical properties. A set of average tracings derived from six different GCs (4 group 1 cells, 2 group 2 cells) is shown in Fig. 4B. INaR was routinely elicited by commanding repolarizing pulses at −15 to −85 mV in 5 mV steps. Figure 4B (bottom) illustrates the currents evoked at 10 mV intervals. Several lines of evidence indicate that these currents were not merely tail currents due to current flow through non-inactivated Na+ channels (responsible for INaP): (1) peak current amplitude increased and then decreased as the level of repolarization was made more negative (Fig. 4Ea, open symbols), instead of increasing steadily; (2) at −15 to −55 mV, the current onset was not instantaneous but included a clearly gradual, time-dependent rising phase (Fig. 4C); and (3) the current decay was remarkably slow in a wide voltage window (see below, and Fig. 5). This notwithstanding, a close inspection of the current onset (Fig. 4C) clearly revealed the existence of two components: (i) an initial component that developed in an abrupt, nearly instantaneous fashion (Fig. 4C, wide arrowheads), followed by (ii) the gradually activating current. The distinction of the two components was particularly evident in a limited voltage window (−45 to −60 mV) in which component (ii) decreased in amplitude while maintaining relatively slow activation kinetics; in these cases, the two components were separated by a clear notch (Fig. 4C, narrow arrowheads), which indicates that component (i) is decaying as component (ii) is growing. Because the activating pulse at 0 mV also elicited a persistent Na+-current component (Fig. 4B), the above observations suggest that component (i) represents the INaP tail current. To verify this possibility, it was necessary to directly compare the amplitude of component (i) with that of INaP at various voltages. To this aim, the maximal amplitude of component (i) was estimated by measuring, at each voltage, the average current amplitude over the time interval delimited by the two dotted vertical lines in Fig. 4C: this time interval corresponds to a sharp change in the current rising-phase slope, which converts into a relative minimum when the notch appears at lower voltages. The resulting I–V plot showed a linear behaviour over the whole voltage range explored (Fig. 4D, open symbols), with an extrapolated x-axis intercept at +72.0 mV. This value is not far from the predicted Na+ equilibrium potential in our experiments (+86.7 mV). We also compared the voltage dependence of component (i) with that of INaP. As an estimate of INaP amplitude, we measured the sustained inward current at the end of the 100 ms repolarizing pulses following INaR decay (Iend) (Fig. 4B, horizontal bars). It can be seen in Fig. 4D that component (i) and Iend closely paralleled at −15 to −40 mV, a voltage window in which INaP activation reaches a plateau (see above, Fig. 3C and D). The slightly lower amplitude of Iend with respect to component (i) in this voltage range could be justified by INaP inactivation developing during the 100 ms depolarizing pulses (see the second section of Results, and Magistretti & Alonso, 1999). These data are consistent with component (i) of repolarization-elicited current onset corresponding to an INaP tail current.
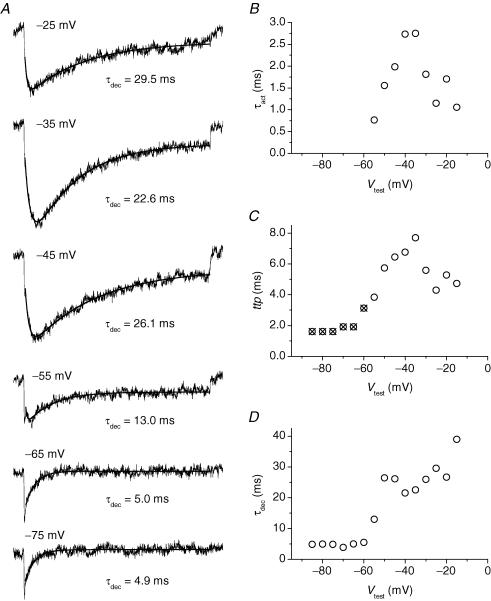
Figure 5. Kinetics of INaR activation and decay in GCs.
A, average INaR tracings at six selected potentials. These tracings are taken from those shown in Fig. 4B (note that here only the tracing region corresponding to the repolarizing pulses is shown; the preceding part of the protocol has been omitted). The repolarization level is specified next to each tracing. Smooth lines are second- (tracings 1–4) or first- (tracings 5 and 6) order exponential fittings to the currents' time course. The time constant of the exponential component describing the current's decay (τdec) is also specified next to each tracing. B, plot of INaR activation time constant (τact) as a function of Vtest. τact values were returned by the fittings illustrated in A, in those cases in which INaR development was separated and distinguishable from INaP tails. C, plot of INaR time-to-peak (ttp) as a function of Vtest. ttp was calculated taking the repolarizing pulse's starting point as time zero. Open circles and crossed circles correspond to ttp values obtained from measurements made in the presence and in the absence, respectively, of a true INaR peak region. In the latter case, the ‘peak’ region to be considered was chosen as illustrated in Fig. 4C (lowermost tracing). D, plot of INaR decay time constant (τdec) as a function of Vtest.
Clearly, it would be desirable to determine the amplitude of the current specifically identifiable as INaR in isolation from the underlying INaP. At −15 to −40 mV, where INaP activation is saturating, this could be conveniently done by subtracting Iend (as an approximate estimate of the steady INaP level) from peak current amplitude; indeed, at these potentials the establishment of a new, steady INaP level upon repolarization will be nearly instantaneous. At more negative voltages, in which a step repolarization will result in a new INaP level after INaP-tail relaxation, this can also be done under the assumption that INaR activation is slow enough as compared to INaP-tail decay. The ‘net’INaR amplitude thus determined is plotted as a function of voltage in Fig. 4Ea (filled symbols). Current amplitude increased from −15 to −35 mV, where it showed a peak. At potentials negative to −35 mV, the current decreased back to reach, at about −60 mV, a non-zero level which then appeared to increase somewhat for further repolarizations. Note that at potentials negative to −55 mV, where INaP tail decay and INaR rising phase appeared to merge, INaR amplitude was obtained by considering the points that immediately followed a clear decrease in current–decay slope (see Fig. 4C, open bar), which presumably indicated INaP-tail subsiding. Permeability values were also calculated from ‘net’INaR amplitude values and plotted as a function of voltage (Fig. 4Eb): the plot was bell-shaped with a maximum at −35 mV and a pedestal level at −60 to −85 mV corresponding to about 20% of the peak.
INaR kinetics was then analysed (Fig. 5). At −15 to −55 mV, INaR activation and decay could be described by a double exponential fitting function (Fig. 5A). Component (i) of the current's rising phase (see above) was excluded from fitting. At potentials negative to −55 mV, a single exponential function was used to describe INaR decay. The activation time constant (τact) showed a bell-shaped dependence on voltage, with a peak of about 2.75 ms at −35/−40 mV (Fig. 5B). The plot of time to peak (ttp) versus voltage also showed a similar bell-shaped behaviour (Fig. 5C). The decay time constant (τdec; Fig. 5D) ranged from 35 to 20 ms at −15 to −40 mV, and appeared to decrease steadily with increasingly negative potentials in this voltage window. τdec also fell abruptly at −35 mV (34 ms) to −60 mV (5 ms), whereas at −60 to −85 mV it maintained a rather constant value of about 5 ms.
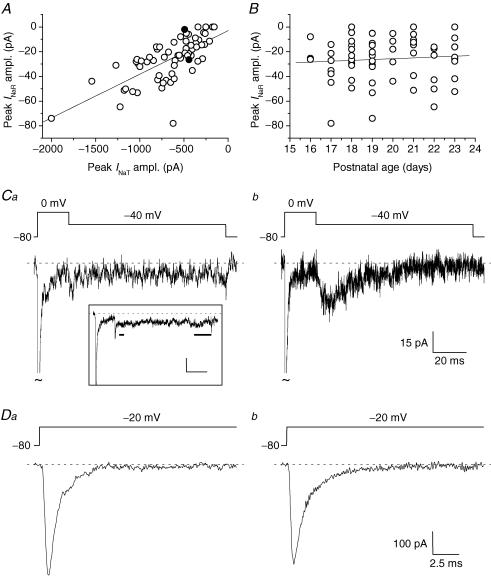
INaR amplitude varied considerably among cells. A strong correlation was found between INaR maximal amplitude and the size of INaT (measured at the peak of the I–V relationship) (Fig. 6A), whereas no significant correlation was observed with the animal's age in the time window considered (16–23 postnatal days) (Fig. 6B). A subset of cells displayed no measurable, or extremely little, INaR component (Fig. 6Ca). Because these cells also showed relatively small INaT (<500 pA), and because of the prominent correlation between INaR and INaT sizes, it could be suspected that in these instances INaR amplitude simply fell below the threshold of detection. This was not the case, however, since clear INaR components could be unequivocally detected in other cells that expressed INaT of similarly low amplitude (Fig. 6C and D). The above findings suggest the existence of heterogeneity in GC expression of INaR. Such diversity in INaR expression did not correlate with significant differences in INaT voltage dependence and kinetics. The half-activation potential (V½) and slope factor (k) obtained from PNaT–V plots averaged −30.9 ± 2.6 and −5.0 ± 0.6 mV, respectively, in INaR-lacking group 2 cells (n = 3), and −34.5 ± 3.1 and −4.5 0.6 mV, respectively, in group 2 cells in which INaR was present and greater than 15 pA in peak amplitude (n = 4; P > 0.4 in both cases). Moreover, in both groups, INaT kinetics was described by two exponential components with similar time constants. For instance, at −20 mV, decay time constants were 781.5 ± 115.2 μs and 6.46 ± 1.62 ms, and the relative A1 coefficient was 0.83 ± 0.05 in the former group (n = 3); and the same parameters averaged 691.4 ± 45.5 μs, 3.94 ± 1.13 ms, and 0.76 ± 0.02, respectively, in the latter group (n = 4; P > 0.16 in all cases). INaP peak amplitude relative to INaT peak amplitude was also not significantly different in INaR-lacking GCs (1.75 ± 0.71%, n = 5) and INaR-expressing GCs (1.87 ± 0.27%, n = 14; P = 0.86) (for this measure, only a subset of cells in which INaT peak amplitude was homogeneous and lower than 500 pA was considered for consistency).
Figure 6. INaR amplitude strongly correlates with INaT amplitude, but INaR expression and amplitude also show intrinsic variability in GCs.
A, scatter plot of INaR amplitude as a function of INaT amplitude. INaR and INaT amplitudes were measured at the peak of the respective I–V relationships (normally −40/−35 mV and −20 mV, respectively). The straight line is the linear regression to data points (slope factor = 35 pA nA−1; R = 0.69, P < 1 × 10−4). B, scatter plot of INaR amplitude as a function of the postnatal age of the animal (in days after birth). The straight line is the linear regression to data points (slope factor = 0.51 pA day−1; R = 0.062, P = 0.62). Data in A and B are from 67 cells. In both cases, INaR amplitude values are ‘net’ values, calculated as peak current minus Iend (see the text). C and D, INaR and INaT, respectively, recorded, at the peak of the I–V relationships, in two exemplary neurones (Ca and Da, cell B3428, representative of 7 cells; Cb and Db, cell B3327; both cells were classified as group 1 cells). Note the similar size of INaT, the presence of a clear INaR in Cb, and its almost complete absence in Ca. The two cells illustrated here are identified by the two filled symbols in A. Ca, inset, average tracing obtained from 7 cells (four group 1 cells, three group 2 cells) that were judged as devoid of a measurable INaR (same voltage protocol as in the main panel). Note that, at the time point at which an INaR peak would be expected (short horizontal bar), current amplitude was not different from that of the late INaP component (long horizontal bar). In the same 7 cells, the average INaT amplitude at the peak of the I–V relationship was −301.0 ± 47.0 pA. Calibration bars in the inset, 20 ms, 15 pA.
Current-clamp recordings: depolarizing afterpotentials
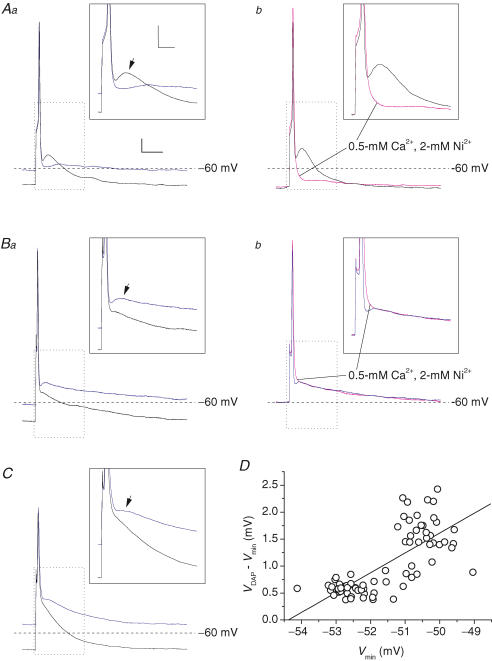
To investigate the presence of functional correlates of INaP and/or INaR in GC intrinsic excitability, we carried out current-clamp experiments in which single APs were evoked with short (0.5 ms) depolarizing square current pulses. Baseline membrane potential (Vbas) was held at stationary levels in the −55 to −75 mV range by steady current injection, and 0.5 ms current steps were repeatedly applied at 1 Hz frequency. Step amplitude was regulated to obtain just above-threshold stimulation. Firing responses consisted in single spikes, evoked with a 1–5 ms delay from stimulus. In 4 out of 16 GCs, the AP terminated with a profound fast afterhyperpolarization (AHP) (n = 2) or with a quick return directly to Vbas (n = 2). In the remaining 12 cells, the AP fast repolarization phase was followed by an evident depolarizing afterpotential (DAP), making a hump 5–10 ms after spike peak. The hump consisted, in different cells, in either a true peak following a postspike relative minimum (Fig. 7A and B; n = 10), or an inflection in the membrane potential (Vm) trajectory after fast spike repolarization (Fig. 7C; n = 2).
Figure 7. Depolarizing afterpotentials (DAPs) in GCs.
A–C, APs evoked in three typical GCs (A, cell D5518, representative of 2 cells; B, cell A5516, representative of 8 cells; C, cell G100305a, representative of 2 cells) by 500 μs depolarizing square current pulses. Aa, Ba and C show APs recorded in each cell starting from two different baseline voltage levels (Vbas) (−70 mV, black tracings; and −55/−60 mV, blue tracings). Ab and Bb, APs recorded, starting from the same Vbas, under control conditions (black or blue tracings) and during perfusion with a solution containing 0.5 mm Ca2+ and 2 mm Ni2+ (tracings in magenta). Calibration bars: 25 ms, 8 mV. The insets show a detail of the tracings, corresponding to the region delimited by the dotted-line box, of each main panel. The arrows point to the DAPs following single APs. Calibration bars in insets: 10 ms, 10 mV. The amplitude of depolarizing current pulses varied from 0.28 (Aa) to 1.3 nA (C). D, plot of the difference between the absolute voltage level at DAP peak (VDAP) and the level of the relative minimum between the AP and the DAP (Vmin; see the text for details) as a function of Vmin for the same cell illustrated in B. Each point corresponds to the measure made in an individual AP. The straight line is the linear regression to data points (slope coefficient = 0.368 mV mV−1; R = 0.74, P < 1 × 10−4).
We next examined whether the above-described DAPs could be underlain by INaR and/or INaP, or, alternatively, by other mechanisms, such as voltage-dependent Ca2+ currents. In two of the 12 cells, the DAP was prominent when resting potential was negative to −65 mV, but was markedly reduced by background depolarization (Fig. 7Aa). Moreover, in the same two cells the DAP was abolished by perfusion with a solution in which Ca2+ concentration was lowered to 0.5 mm and which contained 2 mm Ni2+ (Fig. 7Ab). These findings indicate that in a minority of GCs DAP generation depends on a Ca2+ current that requires hyperpolarization for repriming. In the remaining 10 GCs the DAP showed different properties. In all of these cells the DAP, either consisting in a true peak (n = 8) or in an inflection (n = 2), was reduced, rather that enhanced, by background hyperpolarization (Fig. 7Ba and C). In addition, in all cases tested, perfusion with the 0.5 mm Ca2+, 2 mm Ni2+ solution reduced AP repolarization speed and largely abolished the relative minimum between the AP and the DAP, consistent with a role for Ca2+-activated K+ channels in AP repolarization (D'Angelo et al. 1998), but did not decrease the depolarization level reached during the DAP (Fig. 7Bb; n = 5 out of 5). Hence, in a majority of GCs the DAP is not generated by Ca2+ current(s) reprimed upon hyperpolarization. Other possible candidates that could account for DAP generation in GCs are INaP and INaR. In particular, due to its property of activating upon repolarization in a time-dependent manner, INaR appears particularly suitable to sustain a depolarization following AP repolarization. Slow perfusion with 200 nM TTx resulted in gradual, parallel reduction, and eventually suppression, of both the AP and the DAP (n = 4). Therefore, this approach did not allow us to establish whether the DAP depended on TTx-sensitive Na+ channels or, rather, on the AP in itself. However, an indication about the nature of the DAP was provided by further analysis of its voltage dependence. In 6 out of 10 cells it was possible to regulate the level of the relative minimum between the AP and the DAP (Vmin) by modifying the resting potential with steady current injection. In these cells, Vmin varied in a voltage window comprised between −45 and −65 mV. If the DAP depends on INaR activation, its amplitude is expected to decrease as Vmin is made increasingly negative, for two reasons: (i) with increasing hyperpolarization, INaR amplitude either decreases (at −45 to −60 mV) or remains relatively stable (negative to −60 mV), while its decay becomes progressively faster; and, simultaneously (ii) the negative current injected to hyperpolarize the cell counteracts positively going voltage deflections. Therefore, for the above six cells we plotted the difference between the absolute voltage level at DAP peak (VDAP) and Vmin as a function of Vmin. In all cases, the difference VDAP−Vmin, which is an index of DAP amplitude, positively correlated with Vmin (Fig. 7D). The slope coefficient returned by linear regression of these plots averaged 0.30 ± 0.06 mV mV−1, and the correlation was statistically significant (P < 0.003 in all cases). Hence, in these cells the DAP was enhanced as the voltage relative minimum that preceded it was made more positive in the −65 to −45 mV voltage range. This behaviour is compatible with the properties of INaR.
Roles of INaR and INaP: computer modelling
To obtain further insight into the consequences of INaR and INaP expression on GC excitability, we resorted to computer modelling. Simulations were based on a previous single-compartment GC model (D'Angelo et al. 2001) implemented with the 13-state, allosteric Na+-channel model by Raman & Bean (2001). This model, which generates INaT, INaP and INaR simultaneously, was re-parameterized to adequately reproduce the specific properties of GC VDNCs (see the Methods). Figure 8 illustrates the main properties of INaT and INaR returned, in simulated voltage-clamp experiments, by the model. The main properties of Na+ currents were reproduced with good approximation. The voltage dependence of simulated INaT was very similar to that observed, on average, in experimental currents: the conductance-voltage plot showed V½ and k values of −28.9 mV and −5.4 mV, respectively (Fig. 8Ab; cf. Fig. 2Bb). The decay kinetics of simulated INaT closely approximated the fast decay component of experimental INaT (for instance, at 0 mV simulated INaT decayed monoexponentially with a time constant of 476.6 μs, to be compared with an average value of 431.1 μs for τina1 in experimental currents: cf. Fig. 2Da). Also, the properties of simulated INaR and INaP were reasonably close to those of experimental currents. Simulated INaR showed a peak in the I–V relationship at −35 mV, a monoexponential decay (with τdec = 29.3 ms at −35 mV), and an abrupt acceleration of decay at −55 mV (Fig. 8B; cf. Figs 4Ea and 5D). Simulated INaP peaked at −35 mV, with a half-maximal activation potential of the underlying conductance of −48 mV (not shown). It should be noted that the model did not generate the slow component of INaT decay, suggesting that further kinetic details would be needed to fully reproduce the behaviours of real Na+ channels.
The maximal specific Na+ conductance 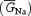 chosen for current-clamp simulations was 8 mS cm−2, which yielded a peak Na+-current density of −541.6 μA cm−2. If one assumes Na+ channels to be uniformly distributed on the somatic membrane, the latter value would correspond to a peak INaT amplitude of −957.1 pA (with a soma diameter of 7.5 μm), a value somewhat greater than those typically observed in real GCs (cf. Fig. 6A). However, Na+-channel expression may be confined to more limited, highly excitable membrane subcompartments in GCs, including the axon initial segment (see Schaller & Caldwell, 2003). The ratios of INaP and INaR peak amplitudes to INaT peak amplitude were within the range of values observed in GCs (0.0078 and 0.026, respectively). With these settings, the model GC responded to injection of depolarizing current steps by firing regular trains of APs (Fig. 9A and B). AP frequency showed an approximately linear relationship with injected current amplitude, and the slope of the frequency–current plot (constructed for a stimulating-current range of 3.5–8.0 pA) was 11.2 Hz pA−1 (Fig. 9Ca). The apparent threshold for AP firing was at about −50 mV. All these properties are consistent with those experimentally observed for real GCs (D'Angelo et al. 1998).
chosen for current-clamp simulations was 8 mS cm−2, which yielded a peak Na+-current density of −541.6 μA cm−2. If one assumes Na+ channels to be uniformly distributed on the somatic membrane, the latter value would correspond to a peak INaT amplitude of −957.1 pA (with a soma diameter of 7.5 μm), a value somewhat greater than those typically observed in real GCs (cf. Fig. 6A). However, Na+-channel expression may be confined to more limited, highly excitable membrane subcompartments in GCs, including the axon initial segment (see Schaller & Caldwell, 2003). The ratios of INaP and INaR peak amplitudes to INaT peak amplitude were within the range of values observed in GCs (0.0078 and 0.026, respectively). With these settings, the model GC responded to injection of depolarizing current steps by firing regular trains of APs (Fig. 9A and B). AP frequency showed an approximately linear relationship with injected current amplitude, and the slope of the frequency–current plot (constructed for a stimulating-current range of 3.5–8.0 pA) was 11.2 Hz pA−1 (Fig. 9Ca). The apparent threshold for AP firing was at about −50 mV. All these properties are consistent with those experimentally observed for real GCs (D'Angelo et al. 1998).
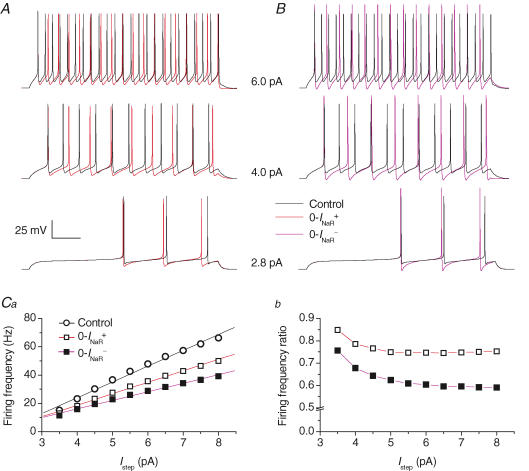
Figure 9. Effects of INaR on repetitive firing in a model of GC electrical activity.
A and B, repetitive AP firing elicited by injection of 1 s (lower tracings) and 500 ms (middle and upper tracings) depolarizing current steps. Step current amplitude was 2.8 pA (lower tracings), 4 pA (middle tracings) and 6 pA (upper tracings). Black tracings are simulated voltage signals obtained under basal conditions. Red tracings (A) and violet tracings (B) were obtained after abolishing INaR by adopting the 0-INaR+ condition settings, and the 0-INaR− condition settings, respectively (see the text for details). The x-axis calibration bar is 150 ms for lower tracings, and 75 ms for middle and upper tracings. Ca, plot of firing frequency as a function of injected step current amplitude (Istep) (^, basal conditions; □, 0-INaR+ condition; ▪, 0-INaR− condition). Straight lines are linear regression fittings to data points. Slope coefficients are 11.2 Hz pA−1 (^), 8.1 Hz pA−1 (□), and 6.0 Hz pA−1 (▪). Cb, plot of the ratio of firing frequency in the 0-INaR+ condition (□) and in the 0-INaR− condition (▪) to control firing frequency, as a function of stimulation intensity.
We next examined the specific effects of INaR and INaP on AP firing. To do this, the two current components were manipulated separately as explained in Methods. In the 0-INaR+ condition, in which INaR is switched off with no changes in INaT kinetics, the frequency of tonic firing was reduced (Fig. 9A, red tracings), and the slope of the frequency–current plot decreased to 8.1 Hz pA−1 (Fig. 9Ca). The decrease in firing frequency was more marked at high stimulation levels (Fig. 9Cb), and maximal decrease was of about 25%. At just-threshold stimulation levels, AP firing was not impaired by the 0-INaR+ condition, and, instead, firing frequency was slightly increased (Fig. 9A, lower tracings), probably due to an enhancement of postspike AHP and, consequently, an acceleration of Na+-channel recovery from activation. In the 0-INaR− condition, in which INaR is suppressed by abolishing the O → OB transition with no compensation for INaT inactivation slowdown (see Methods), the changes in AP repetitive firing were similar to those observed in the 0-INaR+ condition, but more pronounced. The slope of the frequency–current plot further decreased to 6.0 Hz pA−1 (Fig. 9Ca). The maximal decrease in firing frequency, here again observed at high stimulation levels (Fig. 9Cb), was about 40%. The more prominent effects of the 0-INaR− condition, as compared with the 0-INaR+ condition, on tonic firing, are probably the consequence of a markedly enhanced postspike AHP (Fig. 9B; see also below), which more effectively opposes the stimulating current in the interspike period.
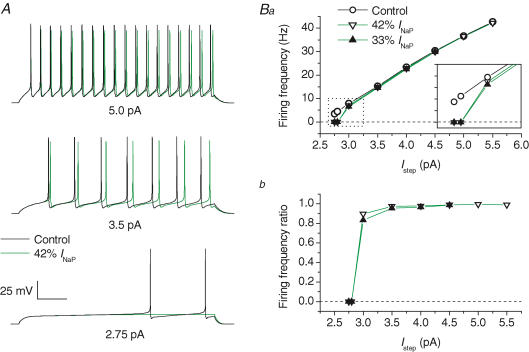
INaP was modified by decreasing the rate constant of the I6 → O transition, Ooff (see Methods). This change reduced the steady-state INaP component while negligibly affecting INaT and INaR (Fig. 8D). Decreasing the steady-state INaP by 58 to 67% impaired AP generation at just-threshold stimulation levels (Fig. 10A, lower tracings). The frequency of repetitive firing was moderately affected at slightly above-threshold stimulation levels, and very little at still higher stimulation levels (Fig. 10A, middle and upper tracings; Fig. 10Ba and Bb). It should be noted that in the 13-state Na+-channel model, the INaP reduction obtained by lowering Ooff is less prominent at early times of sustained depolarizations than at the steady state (see Fig. 8D). This is a consequence of slow release of channels from the OB state to the O state during sustained depolarization. Therefore, lowering Ooff could have little effect on the amplitude of the INaP fraction activated during short interspike periods, and this could limit the significance of the above observation that reducing INaP by lowering Ooff has little influence on tonic high-frequency firing. However, we also verified the effect of lowering Ooff after abolishing the transitions to the OB state (0-INaR+ condition above), a situation in which INaP amplitude is equally decreased at the steady state and at earlier times (Supplemental Fig. B, panel A). Again, we found that INaP reduction markedly affects AP firing at just-threshold stimulation levels, but has no major effects on high-frequency tonic firing (Supplemental Fig. B, panels B and C).
Figure 10. Effects of INaP on repetitive firing in model GCs.
A, AP firing elicited by 1 s (lower tracings) and 500 ms (middle and upper tracings) depolarizing current steps. Step current amplitude was 2.75 pA (lower tracings), 3.5 pA (middle tracings) and 5 pA (upper tracings). Black tracings correspond to basal conditions, green tracings to a 58% reduction of the steady-state INaP component obtained by lowering the rate constant, Ooff (see Scheme 1 and the text for details) to 40% of its control value. The x-axis calibration bar is 150 ms for lower tracings and 75 ms for middle and upper tracings. Ba, plot of firing frequency as a function of Istep for basal conditions (^), for 58% reduction of INaP (▿), and for 67% reduction of INaP (▾). A detail of the plot at low stimulation intensities (dotted-line box) is highlighted in the inset (each y-axis division is 2 Hz). Bb, plot of the ratio of firing frequency after 58% (▿) or 67% (▾) INaP reduction to control firing frequency, as a function of stimulation intensity.
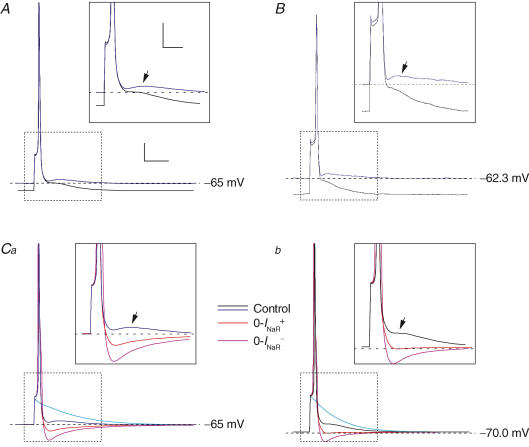
Finally, we tested the ability of the model to reproduce DAPs. Single APs triggered by simulated injection of 0.5 ms depolarizing current pulses under basal conditions were followed by small but clear DAPs (Fig. 11). Starting from a Vbas of −65 mV, the DAP consisted in a true peak following a postspike relative minimum (Fig. 11A, blue tracing). Starting from a more negative Vbas (−70 mV), the DAP consisted in a simple inflection (Fig. 11A, black tracing). The maximal, absolute level reached during the DAP was more positive starting from −65 mV than from −70 mV (−63 versus−65 mV) (Fig. 11A). These properties of the DAP closely resemble those of DAPs experimentally recorded in GCs (compare A with B in Fig. 11). The 0-INaR+ condition abolished the DAP (Fig. 11C, red tracings), which was replaced by a prompt return to baseline when Vbas was −70 mV (Fig. 11Cb), or a fast AHP at more positive Vbas (Fig. 11Ca). These findings indicate that INaR is necessary for DAP generation. In the 0-INaR− condition, AP amplitude and duration were increased due to slowed Na+-channel inactivation. As a consequence, the postspike AHP was further augmented, due to enhanced activation of repolarizing K+ currents (Fig. 11C, violet tracings).
Figure 11. INaR determines DAP generation in model GCs.
Each panel shows single APs evoked by injection of 0.5 ms, above-threshold square current pulses. In all panels, black and blue tracings were obtained, under basal (control) conditions, from a Vbas of −70/−71 mV and −65/−62 mV, respectively. Insets show a detail of the tracings corresponding to the region delimited by the dotted-line box in each main panel, and arrows point to the DAPs that follow APs. A, simulated APs obtained from Vbas of either −70 or −65 mV under basal conditions are shown superimposed. B, single APs and DAPs evoked in a real GC (cell C5518) from Vbas of either ∼−62 or ∼−71 mV: note the similarity with the results of computer simulations (A). C, effect of INaR suppression obtained by adopting the 0-INaR+ condition settings (red tracings) or the 0-INaR− condition settings (violet tracings) at Vbas of −65 (Ca) and −70 mV (Cb). Light blue tracings represent passive responses to just-subthreshold stimuli. The amplitude of depolarizing current pulses was 106–108 pA (Vbas, −65 mV) or 135–137 pA (Vbas, −70 mV) in all simulated experiments. Calibration bars for main panels, 15 ms, 10 mV. Calibration bars for insets, 8 ms, 8 mV.
Discussion
The present study provides a detailed biophysical and functional characterization of voltage-dependent Na+ currents, comprising INaT, INaP and INaR, in cerebellar GCs in situ. The electrophysiological measurements were favoured by the unusually compact electrotonic structure of GCs, which allowed for a precise kinetic analysis of Na+ currents. GCs, like other cerebellar neurones, present complex near-threshold behaviours, to which Na+ currents have been proposed to give a critical contribution (D'Angelo et al. 1998, 2001). Our experiments and simulations support a significant role for INaP in priming AP initiation during near-threshold stimulation, and for INaR in boosting the efficiency of repetitive firing and in generating DAPs. The latter effect may be important to explain the tendency of GCs to discharge in bursts and repetitive AP sequences in vivo (Chadderton et al. 2004).
Properties of INaT
The major problem affecting Na+ current recordings in neurones in situ is the voltage-clamp escape caused by remote localization of Na+ channels in the dendrites and axon. Although GCs are among the most electrotonically compact neurones (Silver et al. 1992; D'Angelo et al. 1993, 1995), in most cells we could not fully prevent clamp problems possibly arising from incomplete space-clamp control and Na+-channel expression in neuronal processes. In some GCs, however, INaT consisted of a well-clamped component only, suggesting that in these cells only channels located in a membrane region electrotonically proximal to the soma were contributing to the recorded current. In such cases, INaT showed the voltage dependence and fast kinetics typical of classical neuronal Na+ currents. In particular, INaT voltage dependence was similar to that observed in cultured GCs (Osorio et al. 2005) in the presence of reverted Na+ gradient to render Na+ currents non-regenerative and prevent local escape from clamp. To describe the kinetic properties of GC INaT, we tentatively applied a classical HH formalism. However, this approach was limited by the fact that proper fitting of INaT kinetics required the n exponent to increase with membrane potential (from ∼2 at −45 mV to ∼9 at +10 mV). This finding is anomalous but not surprising, since it is well known that the HH model does not reflect the real mechanisms of Na+-channel state transitions. INaT inactivation was biexponential, with one time constant in the 4–0.4 ms range and the other in the 10 ms–1 ms range. Biexponential INaT decay has also been observed in other central neurones (Huguenard et al. 1988; Sah et al. 1988; Gähwiler & Llano, 1989; Park & Ahmed, 1991; Cummins et al. 1994).
Properties of INaP
The persistent Na+ current, INaP, is widely present in central neurones, but its biophysical properties show some variability in activation threshold, relative amplitude compared to INaT, and slow inactivation. In GCs, the permeability underlying INaP was half-maximally activated at −55 mV, i.e. 25 mV more negative than INaT, and a maximal, steady level was reached at −40 to −15 mV. The voltage range of GC INaP activation (−60 to −30 mV) overlapped with the region of Na+-dependent inward rectification in the steady-state V–I plot obtained from current-clamp experiments (D'Angelo et al. 1998; see below). The peak amplitude of the INaP evoked with long-lasting depolarizing step pulses averaged about −9 pA and represented 1.5–2% of INaT peak amplitude. It should be noted that these values could be slightly overestimated, because channel expression in neuronal processes may cause spurious increases in the amplitude of steady-state inward currents under voltage-clamp conditions (White et al. 1995). However, no marked distortion in current voltage dependence is expected (White et al. 1995). INaP expression has also been reported for cultured GCs (Osorio et al. 2005). In that case, channels responsible for INaP were located in neuronal processes, and INaP peak amplitude was greater than in GCs in situ (∼20 pA), which could reflect the prominent process development typical of GC cultures. We also observed that when INaP was evoked with slow depolarizing ramps, its amplitude decreased as depolarization rate was reduced. In other neurones (Fleidervish & Gutnick, 1996; Magistretti & Alonso, 1999; Agrawal et al. 2001) this behaviour has been related to the existence of a slow voltage-dependent INaP inactivation that proceeds on a time scale of seconds.
Biophysical properties and possible molecular correlates of INaR
Perhaps the most intriguing property of GC Na+ currents is the expression of a resurgent component, INaR (Magistretti et al. 2004; Afshari et al. 2004). The biophysical properties of GC INaR were similar to those observed in Purkinje cells and other neurones (Raman & Bean, 1997; Do & Bean, 2003; Cummins et al. 2005), and included a bell-shaped voltage dependence with a peak at −35 mV, and a marked voltage dependence of activation and decay kinetics, both of which showed an abrupt acceleration at potentials negative to −40/−45 mV.
To account for INaT, INaP and INaR in Purkinje cells, Raman & Bean (2001) developed a 13-state allosteric model with five closed states, one open state, six inactivated states, and a ‘blocked’ state accessed from the open state (see Scheme 1). This model implicitly assumes that a single Na+ channel is endowed with states explaining a multiplicity of kinetic behaviours. In the model, INaP is determined by the equilibrium between open, inactivated, and blocked states. INaR is generated by release of channels from the blocked to the open state. Blocking is determined by an intracellular ‘blocking particle’ competing with the classical ‘inactivating ball’ for binding to the open channel. Binding of the blocking particle is voltage independent, whereas unbinding is strongly voltage dependent, probably as an indirect consequence of inwardly directed Na+ flow inside the channel upon repolarization (through a mechanism of displacement due to electrostatic repulsion, or mechanical momentum, or both). By preventing the inactivating ball from binding to open channels, the blocking particle would therefore create, upon depolarization, a ‘reservoir’ of non-inactivated channels that would be exploited in repolarization to produce a transient inward current. The relative proportions, voltage dependence, and kinetics of INaT, INaP and INaR in GCs could all be accounted for by the Raman & Bean (2001) model. A more precise correspondence of modelling with experimental currents may require parameterization of the kinetic scheme through the application of specific fitting algorithms (e.g. Vanier & Bower, 1999), and additional data from single-channel recordings.
In Purkinje cells, the presence of INaR has been related to the expression of Nav 1.6 Na+ channels (Raman et al. 1997) as a necessary, although not sufficient (Pan & Beam, 1999), requirement. Na+ channel α subunits different from Nav 1.6 (Do & Bean, 2003), including Nav 1.2 (Rush et al. 2005), have then been shown to also provide a possible molecular substrate for INaR in other neurones. GCs do express Nav 1.6 and Nav 1.2 mRNA and protein at high levels (Schaller & Caldwell, 2003). More recently, a tight correlation between INaR expression and the Na+-channel auxiliary β4 subunit (Grieco et al. 2005) has been established. Indeed, a portion of the β4 subunit cytoplasmic tail can act as the ‘blocking particle’ which, according to the Raman-Bean model, competes with the classical ‘inactivating ball’ for binding to the channel's open state. Unlike Purkinje cells, however, GCs do not show high expression levels of β4 subunit mRNA and protein in in situ hybridization and immunohistochemical tests (Yu et al. 2003). Although this does not rule out the presence of functional β4 subunits, it may indicate that molecules other than the β4 subunit determine INaR in GCs.
Roles of INaP and INaR
The impact of a given membrane current on neuronal electrogenesis depends on the specific electrotonic structure and the channel complement of the neurone in which the current is expressed. In some neurones, INaP is relatively large (hundreds of pA) and determines processes like oscillations and spike priming (discussed in Magistretti & Alonso, 2002). In GCs, INaP has small amplitude, yet it can have a major impact on membrane excitability due to the small size of these neurones and their high input resistance. Indeed, an inward current of 9 pA (the average, peak INaP amplitude we observed in GCs) would produce an 18 mV depolarization on a resistance of 2 GΩ (the average GC input resistance at −70 mV, under physiological ionic conditions: D'Angelo et al. 1995, 1998; Brickley et al. 1996). The fact that the voltage range of GC INaP activation (−60 to −30 mV) overlapped with the region of Na+-dependent steady-state inward rectification observed under current-clamp conditions supports the prediction that a persistent Na+ current critically influences GC near-threshold excitability and the rate at which GC membrane potential approaches firing threshold (D'Angelo et al. 1998). Accordingly, our computer simulations indicated that INaP has a critical influence in promoting AP firing at low, just-threshold levels of stimulation, whereas the impact of INaP on high-frequency firing was much smaller. INaP has also been shown to sustain subthreshold membrane potential oscillations and to amplify GC resonance in the theta-frequency range (D'Angelo, 2001).
Because of its property of activating in repolarization after depolarizations able to activate and inactivate INaT, INaR is more suitable to provide a depolarizing drive following firing of single APs, or during repetitive discharge. The response of Purkinje cells to brief, just-threshold depolarizing pulses, which in most other neurones would trigger single APs, consists in the discharge of doublets or triplets of APs (Raman & Bean, 1997). INaR has been proposed to underlie this firing pattern (Raman & Bean, 1997). In GCs, we found that single APs are most frequently followed by evident DAPs that, in ∼80% of cases, show features compatible with a role of INaR in their generation. Computer modelling confirmed that INaR expression at levels similar to those frequently observed experimentally results in generation of DAPs closely resembling real DAPs. DAPs produced by GCs did not trigger discharge of AP doublets or triplets (except in a single case, data not shown). However, during injection of just-threshold depolarizing steady currents, GC firing is frequently characterized by spike clustering (D'Angelo et al. 1998, 2001). INaR-dependent DAPs could contribute to this behaviour, as well as to oscillatory burst firing occurring during partial inhibition of K+ currents (D'Angelo et al. 1998). It was recently reported that antidromic stimulation of parallel fibres by brief electrical shocks applied to the molecular layer results in doublets or triplets of APs at the GC soma (Isope et al. 2004), and it was concluded that this behaviour is a consequence of intrinsic excitable properties of GCs. Although the mechanism of spike repetition during antidromic stimulation remains to be established, it is possible that INaR contributes to its generation.
Our results also indicate the existence of marked variability in INaR amplitude among GCs expressing transient Na+ currents of similar size. At the same time, a fraction of GCs did not produce DAPs, and in another cell subset DAPs were generated by a Ca2+-, hyperpolarization-dependent mechanism not involving INaR. It could thus be hypothesized that heterogeneity in INaR expression is a critical factor in determining the variable ability of GCs to generate DAPs at resting potential.
How general the role of INaR could be in sustaining DAPs in central neurones is still unexplored. Hippocampal CA1 pyramidal neurones have been found to generate DAPs sensitive to TTx (Jensen et al. 1996; Azouz et al. 1996), that were attributed to INaP action. At the time of those reports, however, the existence of INaR was not known. In principle, therefore, INaR may conceivably contribute to DAP generation also in CA1 neurones. On the other hand, preliminary data suggest that CA1 pyramidal neurones in situ do not express INaR (J. Magistretti and L. Castelli, unpublished results), making a role of INaP still possible. It is worth remarking that, although the results of our modelling strongly suggest that INaR is necessary for DAP generation in cerebellar GCs, they do not rule out a possible contribution of INaP to the overall depolarization. Indeed, in the kinetic model we adopted for Na+-current simulation, INaP is but an aspect of a global Na+ current, and it is not possible to suppress its early phases unless INaR is also abolished. In fact, INaP generation could imply more specialized mechanisms (including modal gating and long-lived burst openings: Alzheimer et al. 1993; Magistretti & Alonso, 2002; Magistretti et al. 2003) that would make it less strictly interconnected with other Na+ current components.
The results of our computer simulations also indicated that an additional functional consequence of INaR expression in GCs is an increase in firing frequency during sustained depolarization and in the gain of the frequency–stimulation relationship. This is in agreement with what has been found for Purkinje cells (Khaliq et al. 2003). The influence of INaR on firing frequency was maximal in the presence of high stimulation levels, and minimal or absent at near-threshold stimulation levels. This behaviour is opposite to that found for INaP. Therefore, INaP and INaR appear to provide cerebellar GCs with two complementary mechanisms for promoting AP firing at different levels of activity.
Distribution of INaR and implications for cerebellar function
INaR is a Na+ current of recent identification. In addition to GCs (this study; Afshari et al. 2004), INaR is expressed in other important cerebellar neurones, including Purkinje cells, where it has been originally described (Raman & Bean, 1997), cerebellar unipolar brush cells (Mossadeghi & Slater, 1998; Afshari et al. 2004), and neurones of deep cerebellar nuclei (Raman et al. 2000; Afshari et al. 2004). Preliminary results indicate that INaR is also present in Golgi cells (J. Magistretti and E. D'Angelo, unpublished data). Being present in five major cerebellar neuronal types, INaR may thus be particularly important for cerebellar function. Subthreshold, persistent Na+ currents also have a well-characterized role in setting the excitable properties of Purkinje cells (Llinás & Sugimori, 1980; Raman & Bean, 1999), as well as in GC and Golgi cell functions (D'Angelo et al. 1998, 2001; Forti et al. 2004). What is the reason for expressing such specific Na+-current components in so many cerebellar neuronal types? A possible interpretation, based on the role attributed to INaR and/or INaP in AP conglomeration and bursting (Franceschetti et al. 1995; Raman & Bean, 1997; Parri & Crunelli, 1998; Brumberg et al. 2000), is that the cerebellum privileges spike bursts to communicate, and that this coding property must be maintained throughout the circuit by distributed mechanisms like those provided by INaR and INaP. GCs are known to produce spike bursts following punctate tactile stimulation (Chadderton et al. 2004), and Purkinje and deep cerebellar nuclei cells also have the ability of conglomerating spikes by exploiting INaR (Raman & Bean, 1997; Afshari et al. 2004). Spike bursts are also important for eliciting parallel fibre–Purkinje cell long-term depression (LTD) (Casado et al. 2002). Unfortunately, there is currently no means to inhibit INaR or INaP selectively in order to directly test their role in cerebellar physiology. Specific network models may help understanding the emergence of INaR and INaP effects on cerebellar network computation.
Acknowledgments
We thank Dr Thierry Nieus for helpful assistance in implementation of Na+-current model. This study was supported by FIRB grant no. RBAU01JRCF from the Italian Ministry of Education, University and Research (MIUR) to J.M., by EU grants IST-2001–35271 and QLG3-CT-2001-02256 to E.D., and by FIRB grant no. RBNE01AAS7 to E.D. L.C. was supported by a fellowship from MIUR (PRIN grant no. 2003060538 to J.M.).
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.106682
http://jp.physoc.org/cgi/content/full/jphysiol.2006.106682/DC1 and contains supplemental material consisting of two figures:
Hodgkin-Huxley (HH) fitting of INaT activation
Effects of manipulations aimed at reducing INaP before and after abolishment of INaR in computer simulations.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR, Raman IM. Resurgent Na+ currents in four classes of neurons of the cerebellum. J Neurophysiol. 2004;92:2831–2843. doi: 10.1152/jn.00261.2004. [DOI] [PubMed] [Google Scholar]
- Agrawal N, Hamam BM, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. doi: 10.1016/s0306-4522(00)00455-3. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y. Ionic basis of spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492:211–223. doi: 10.1113/jphysiol.1996.sp021302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg JC, Nowak LG, McCormick DA. Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. J Neurosci. 2000;20:4829–4843. doi: 10.1523/JNEUROSCI.20-13-04829.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-d-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579:2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Xia Y, Haddad GG. Functional properties of rat and human neocortical voltage-sensitive sodium currents. J Neurophysiol. 1994;71:1052–1064. doi: 10.1152/jn.1994.71.3.1052. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, De Filippi G, Rossi P, Taglietti V. Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J Physiol. 1995;484:397–413. doi: 10.1113/jphysiol.1995.sp020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, De Filippi G, Rossi P, Taglietti V. Ionic mechanism of electroresponsiveness in cerebellar granule cells implicates the action of a persistent sodium current. J Neurophysiol. 1998;80:493–503. doi: 10.1152/jn.1998.80.2.493. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Nieus T, Maffei A, Armano S, Rossi P, Taglietti V, Fontana A, Naldi G. Theta-frequency bursting and resonance in cerebellar granule cells: experimental evidence and modeling of a slow K+-dependent mechanism. J Neurosci. 2001;21:759–770. doi: 10.1523/JNEUROSCI.21-03-00759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, Rossi P, Taglietti V. Different proportions of N-methyl-d-aspartate and non-N-methyl-d-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience. 1993;53:121–130. doi: 10.1016/0306-4522(93)90290-v. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron. 2003;39:109–120. doi: 10.1016/s0896-6273(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer; 1967. [Google Scholar]
- Fleidervish IA, Gutnick MJ. Kinetics of slow inactivation of persistent sodium current in layer V neurons of mouse neocortical slices. J Neurophysiol. 1996;76:2125–2130. doi: 10.1152/jn.1996.76.3.2125. [DOI] [PubMed] [Google Scholar]
- Forti L, Mapelli J, Cesana E, D'Angelo E. Ionic mechanisms of autorhythmic firing and intrinsic electroresponsiveness in rat cerebellar Golgi cells. FENS Abstracts. 2004;2:492, A222.8. [Google Scholar]
- Franceschetti S, Guatteo E, Panzica F, Sancini G, Wanke E, Avanzini G. Ionic mechanisms underlying burst firing in pyramidal neurons: intracellular study in rat sensorimotor cortex. Brain Res. 1995;696:127–139. doi: 10.1016/0006-8993(95)00807-3. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Llano I. Sodium and potassium conductances in somatic membranes of rat Purkinje cells from organotypic cerebellar cultures. J Physiol. 1989;417:105–122. doi: 10.1113/jphysiol.1989.sp017793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel 4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Hamill OP, Prince DA. Developmental changes in Na+ conductances in rat neocortical neurons: appearance of a slowly inactivating component. J Neurophysiol. 1988;59:778–795. doi: 10.1152/jn.1988.59.3.778. [DOI] [PubMed] [Google Scholar]
- Isope P, Franconville R, Barbour B, Ascher P. Repetitive firing of rat cerebellar parallel fibres after a single stimulation. J Physiol. 2004;554:829–839. doi: 10.1113/jphysiol.2003.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Azouz R, Yaari Y. Spike after-depolarization and burst generation in adult rat hippocampal CA1 pyramidal cells. J Physiol. 1996;492:199–210. doi: 10.1113/jphysiol.1996.sp021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci. 2003;23:4899–4912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Biophysical properties and slow-voltage dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study. J Gen Physiol. 1999;114:491–509. doi: 10.1085/jgp.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Fine gating properties of channels responsible for persistent sodium current generation in entorhinal cortex neurons. J Gen Physiol. 2002;120:855–873. doi: 10.1085/jgp.20028676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Castelli L, D'Angelo E. Three functionally different types of voltage-dependent Na+ currents in rat cerebellar granule cells in situ. FENS Abstracts. 2004;2:412, A187.7. [Google Scholar]
- Magistretti J, Ragsdale DS, Alonso A. Kinetic diversity of single-channel burst openings underlying persistent Na+ current in entorhinal cortex neurons. Biophys J. 2003;85:3019–3034. doi: 10.1016/S0006-3495(03)74721-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossadeghi B, Slater NT. Persistent and resurgent sodium currents in cerebellar unipolar brush cells. Society for Neuroscience Abstracts. 1998;24:1078. [Google Scholar]
- Osorio N, Alcaraz G, Padilla F, Couraud F, Delmas P, Crest M. Differential targeting and functional specialization of sodium channels in cultured cerebellar granule cells. J Physiol. 2005;569:801–816. doi: 10.1113/jphysiol.2005.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Beam KG. The absence of resurgent sodium current in mouse spinal neurons. Brain Res. 1999;849:162–168. doi: 10.1016/s0006-8993(99)02060-0. [DOI] [PubMed] [Google Scholar]
- Park CC, Ahmed Z. Characterization of sodium current in developing rat diencephalic neurons in serum-free culture. J Neurophysiol. 1991;65:1011–1021. doi: 10.1152/jn.1991.65.5.1011. [DOI] [PubMed] [Google Scholar]
- Parri HR, Crunelli V. Sodium current in rat and cat thalamocortical neurons: role of a non-inactivating component in tonic and burst firing. J Neurosci. 1998;18:854–867. doi: 10.1523/JNEUROSCI.18-03-00854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J. 2001;80:729–737. doi: 10.1016/S0006-3495(01)76052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Gibb AJ, Gage PW. The sodium current underlying action potentials in guinea pig hippocampal CA1 neurons. J Gen Physiol. 1988;91:373–398. doi: 10.1085/jgp.91.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller KL, Caldwell JH. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2003;2:2–9. doi: 10.1080/14734220309424. [DOI] [PubMed] [Google Scholar]
- Silver RA, Traynelis SF, Cull-Candy SG. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992;355:163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Vanier MC, Bower JM. A comparative survey of automated parameter-search methods for compartmental neural models. J Comput Neurosci. 1999;7:149–171. doi: 10.1023/a:1008972005316. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel 4, a new disulfide-linked auxiliary subunit with similarity to 2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Sekar NS, Kay AR. Errors in persistent inward currents generated by space-clamp errors: a modeling study. J Neurophysiol. 1995;73:2369–2377. doi: 10.1152/jn.1995.73.6.2369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hodgkin-Huxley (HH) fitting of INaT activation
Effects of manipulations aimed at reducing INaP before and after abolishment of INaR in computer simulations.