Abstract
The human parasternal intercostal muscles are obligatory inspiratory muscles with a diminishing mechanical advantage from cranial to caudal interspaces. This study determined whether inspiratory neural drive to these muscles is graded, and whether this distribution matches regional differences in inspiratory mechanical advantage. To determine the neural drive, intramuscular EMG was recorded from the first to the fifth parasternal intercostals during resting breathing in six subjects. All interspaces showed phasic inspiratory activity but the onset of activity relative to inspiratory flow in the fourth and fifth spaces was delayed compared with that in cranial interspaces. Activity in the first, second and third interspaces commenced, on average, within the first 10% of inspiratory time, and sometimes preceded inspiratory airflow. In contrast, activity in the fourth and fifth interspaces began after an average 33% of inspiratory time. The peak inspiratory discharge frequency of motor units in the first interspace averaged 13.4 ± 1.0 Hz (mean ± s.e.m.) and was significantly greater than in all other interspaces, in particular in the fifth space (8.0 ± 1.0 Hz). Phasic inspiratory activity was sometimes superimposed on tonic activity. In the first interspace, only 3% of units had tonic firing, but this proportion increased to 34% in the fifth space. In five subjects, recordings were also made from the medial and lateral extent of the second parasternal intercostal. Both portions showed phasic inspiratory activity which began within the first 6% of inspiratory time. Motor units from the lateral and medial portions fired at the same peak discharge rate (10.4 ± 0.7 versus 10.7 ± 0.6 Hz). These observations indicate that the distribution of neural drive to the parasternal intercostals in humans has a rostrocaudal gradient, but that the drive is uniform along the mediolateral extent of the second interspace. The distribution of inspiratory neural drive to the parasternal intercostals parallels the spatial distribution of inspiratory mechanical advantage, while tonic activity was higher where mechanical advantage was lower.
Many muscles have an inspiratory action on the rib cage, and during quiet breathing, the timing and amount of their activity varies. In dogs and cats, electromyographic activity during inspiration is not distributed equally among the parasternal (De Troyer & Legrand, 1995; Legrand et al. 1996a) and external intercostal muscles (Greer & Martin, 1990; Legrand & De Troyer, 1999). In both groups of muscles there are mediolateral and rostrocaudal gradients of neural drive. In the dog, the mechanical advantage of the intercostal muscles is not uniform and the topographic distribution of mechanical advantage parallels the distribution of neural drive during breathing. Thus, muscles with a greater mechanical advantage are activated to a greater extent, and they are often activated earlier during inspiration (De Troyer et al. 1996b, 1999; Legrand et al. 1996b; for review, see De Troyer et al. 2005).
In humans, the inspiratory drive to the external intercostal muscles was recently assessed (De Troyer et al. 2003). The third dorsal external intercostal, which has a high mechanical advantage (Wilson et al. 2001), was active earlier in inspiration, and its motor units discharged at a faster rate than for lower external intercostals. As for the dog, the degree of activation of the external intercostals in humans during quiet breathing correlated with the magnitude of their mechanical advantages (De Troyer et al. 2003).
Human parasternal intercostal muscles are active during inspiration (e.g. Tokizane et al. 1952; Taylor, 1960; De Troyer & Sampson, 1982; Gandevia et al. 1996) and have an inspiratory mechanical advantage (De Troyer et al. 1998). Their mechanical advantage diminishes fourfold from the second to the fifth interspace, but it is not known if neural drive to the human parasternal intercostal muscles is graded across the interspaces. The neural drive along the mediolateral extent of the muscles is also not known. Therefore, in the present study, we have measured the timing of activation and the discharge behaviour of single motor units activated during inspiration in the parasternal intercostals of the first to fifth interspaces, as well as along the mediolateral extent of the second space. We have also assessed the tonic activity of these units and correlated the level of both inspiratory neural drive and tonic activity with the known mechanical advantage of the muscles.
Methods
The studies were carried out in six healthy men of 44 ± 4.2 (mean ± s.e.m., range 29–54 years) years of age. The subjects gave informed consent to the procedures, which conformed with the Declaration of Helsinki and were approved by the Human Research Ethics Committee of the University of New South Wales. Three subjects had previously participated in many respiratory experiments, but the other three had little or no prior experience as respiratory subjects. Before the study these subjects were just told that the purpose was to obtain electrical recordings from several muscles of the rib cage compartment of the chest wall. Two experiments were performed: the first investigated the gradient of neural drive in the first to fifth parasternal intercostal muscle at sites close to the sternum, and the second investigated the neural drive at the medial and lateral extent of the parasternal intercostal in the second interspace. There were no adverse consequences of the procedures.
Experiment 1
The medial portions of the parasternal intercostal muscles in the first to fifth interspaces were about 1–2 cm lateral from the sternal edge, and these sites were located by palpitation. The potential recording sites were evaluated on the right side in all six subjects with a 5 MHz ultrasound linear probe (Acuson 128 X/P, Computed Sonography System; Acuson Inc., Mountain View, CA, USA). While the probe was placed perpendicular to the skin, the parasternal intercostal was clearly visualized in each subject, so its thickness and depth from the skin surface could be measured with a built-in digital caliper. Depending on the subject's height and muscularity, the thickness of the parasternal intercostal ranged between 2.5 and 6.8 mm, and the depth of its external layer relative to the skin surface was between 13.5 and 40.0 mm. A topical anaesthetic (Emla cream 5%) was then applied to the area of skin marked for electrode insertion for ∼15 min.
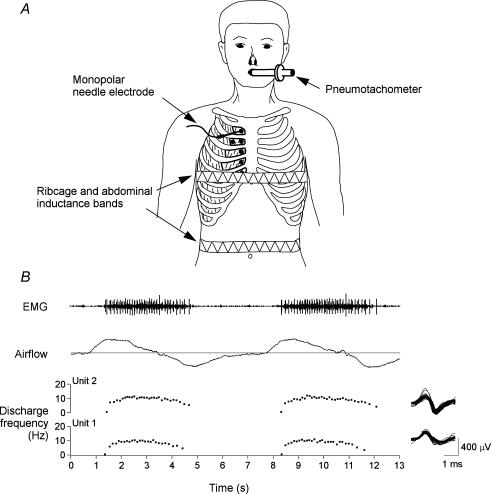
For the experiment, each subject was seated comfortably with all restrictive garments removed (see Fig. 1A). The respiratory displacements of the chest wall were monitored by inductance plethysmography (Respitrace; Ambulatory Monitoring, Ardsley, NY, USA). The inductance bands were positioned around the ribcage at the level of the nipples and around the abdomen at the level of the umbilicus, respectively, and the gains on the two signals were adjusted by using the conventional isovolume manoeuvre (Chadha et al. 1982). No extraneous movements were permitted after this calibration procedure. The subject was then grounded with a large flexible strap on the right shoulder, and recordings of parasternal intercostal EMG activity were made.
Figure 1. Experimental set-up for recordings from parasternal intercostal muscles and recording of single motor-unit activity.
A, subjects were comfortably seated and breathed via a mouthpiece. Volume was obtained by integration of airflow signal. Inductance bands were placed around the chest and abdomen. In the first experiment recordings were made using monopolar needle electrodes inserted into the first to fifth parasternal intercostals close to the sternum. In the second, recordings were made in the second interspace at a medial and lateral location. Recording sites are represented by the filled circles. B, a representative record of inspiratory EMG obtained in the parasternal intercostal of the fourth interspace for two consecutive breaths. From top to bottom, panels show multiunit EMG, airflow (inspiration upwards), and the instantaneous frequencies of two single motor units identified in this record (denoted Units 1 and 2). All action potentials from the two units are superimposed, and shown in the right panel. The discharge frequency of both units increases rapidly with the onset of inspiratory airflow, plateaus and then gradually declines in the first part of expiration.
Recordings were made first from the parasternal intercostal muscle in the second interspace using a Teflon-coated monopolar electrode (Medelec DMG50; Surrey, UK). The recordings were referenced to a surface electrode positioned 2–3 cm away, and the EMG signal was amplified and band-pass filtered below 53 Hz and above 3000 Hz. Since this area had been examined by ultrasonography, we could define in each individual subject the maximal possible length of the needle track and marked this on the electrode. The needle was inserted perpendicular to the skin surface and advanced in small steps through the pectoral muscles and into the parasternal intercostal. Electromyographic activity was continuously monitored on a loudspeaker and an oscilloscope throughout the procedure, and once a site in the parasternal intercostal was encountered that contained audible and visible single motor unit activity with an acceptable signal-to-noise ratio (> 3 : 1) during quiet breathing or mild voluntary hyperpnoea, the audio signal was removed and the subject was instructed to breathe quietly for 1–2 min through a mouthpiece. After the run was completed, the needle was repositioned at a slightly different angle or different depth. In each subject, six to nine sites were studied in the second parasternal intercostal muscle.
Recordings were then made from the parasternal intercostal muscle in the remaining first, third, fourth and fifth interspaces. The overall experimental procedure was similar for each interspace except for the location, and the depth of electrode insertion was reassessed in reference to the sternal edge. As for the second interspace, 4–12 sites were recorded from the parasternal intercostals in the remaining spaces. During recordings from the third, fourth and fifth interspaces, the needle occasionally entered the underlying triangularis sterni muscle, which showed phasic expiratory activity. When recording from the fifth interspace (studied last in all subjects), the inductance band positioned around the ribcage was removed to allow for insertion of the electrode, but the band positioned around the abdomen was retained.
The changes in lung volume (obtained by integration of the airflow signal), end-tidal PCO2 (measured via a port on the pneumotachograph), and ribcage and abdomen movements were checked to ensure that the subject was breathing quietly. All signals were stored on computer via a Cambridge Electronic Design 1401 interface (Cambridge, UK) for subsequent analysis.
A mild transient ache was noted occasionally as the needle penetrated the pectoral or parasternal intercostal muscles, but this always subsided rapidly. Therefore, all EMG recordings were obtained while the subjects had minimal discomfort or none at all.
Experiment 2
Five subjects were subsequently studied usually in separate sessions to assess neural inspiratory drive to both the medial and lateral portions of the parasternal intercostal muscle of the second interspace. The site for electrode insertion into the medial portion of the muscle was ∼10 mm lateral to the sternal edge. Posteroanterior radiographs of the chest at resting end-expiration were obtained to define anatomical landmarks for subsequent insertion of the electrode in the lateral portion of muscle. This was necessary to help locate the costochondral junction and thus the most lateral extent of the parasternal intercostal. The distance from the midline to the second costochondral junction ranged from 80 to 96 mm (mean 87.5 mm) and the site for electrode insertion into the lateral portion of the muscle was 10–20 mm medial to the costochondral junction. The muscle in the lateral portion was thinner than the medial portion, and ranged between 2.6 and 3.8 mm, compared to 5.3 and 7.1 mm in the medial portion. The general procedure was similar to that used in Experiment 1. In each subject, 6–11 sites were studied from both the medial and lateral portion of the muscle in each subject.
Data analysis
The magnitude of neural inspiratory drive to a given area of parasternal intercostal muscle was assessed in three ways. First, all periods of resting breathing (corresponding to different recording sites in the muscle) were played into a leaky integrator (decay time constant, 50 ms) and for each period, the presence or absence of phasic inspiratory activity in the integrated EMG (i.e. with a progressive increase in the signal during the inspiratory phase of the breathing cycle and a decrease in the signal during the expiratory phase) was noted. Second, 10 consecutive breaths from each period were examined to measure the time of onset (TO) of the integrated inspiratory multiunit EMG relative to the onset of inspiratory airflow. Inspiratory time (TI) during these breaths was also measured from the airflow signal. To allow comparison between subjects, TI−TO for each breath was expressed as a percentage of TI; this percentage was less than 100 when activity started after the onset of inspiratory airflow and greater than 100 when activity preceded the onset of airflow.
The third method used to quantify neural drive was based on measurement of the firing rates of single motor units and has previously been described in detail (Gandevia et al. 1996; De Troyer et al. 1997, 2003). Thus, the periods of quiet breathing used for the first two analyses were played into a commercial analysis package (Spike 2; Cambridge Electronic Design), and trigger levels were set manually to capture all spikes with an appropriate signal-to-noise ratio. They were subsequently recalled and manually sorted into one, two, three or four types or ‘templates’ based on their size and detailed morphology. The interactive software allowed: (1) updating of the mean shape of the template for each motor unit; (2) review of the frequency plots of each single motor unit, together with inspiratory flow and respiratory movements; and (3) superimposition of all spikes from a particular motor unit. Using this method, we could follow simultaneously the discharge of up to four single motor units over several consecutive breaths (Fig. 1B).
For each motor unit, the mean discharge frequency was measured from the plateau in the last third of the inspiratory phase during several consecutive breaths. Units that discharged sporadically or very late in the breaths with less than three interdischarge intervals were noted but not included in the main analysis. One subject had four units (1 in the first interspace, 2 in the third, and 1 in the fourth) which fired tonically without inspiratory modulation and these units were not included in the main analysis either. Several units, particularly in the fourth and fifth interspaces, discharged tonically with a superimposed inspiratory modulation. As this study aimed to quantify the magnitude of neural inspiratory drive, the discharge frequency of these units was taken as the difference between the frequency in the last third of the inspiratory phase and the tonic frequency during expiration. The tonic discharge frequency was measured from the plateau in the last third of expiration to avoid an overestimation of the discharge frequency because of any preceding post-inspiratory firing in early expiration. To assess the onset of the inspiratory modulation of these tonic units, several consecutive breaths were examined and the time of onset (TO) of inspiratory modulation (i.e. an increase in frequency from the tonic baseline level) was determined. As for the multiunit recordings, TI−TO for each breath was expressed as a percentage of TI.
For each muscle area in each subject, the values of the (TI−TO)/TI of multiunit activity for a given recording site was averaged over the 10 breaths recorded from that site, and the mean values thus obtained were then averaged over all sites. Similarly (TI−TO)/TI of the inspiratory modulation of the tonic units was also averaged over all sites in an interspace. The firing rates of the single motor units in the different recording sites of a given muscle area were also averaged over all units. In Experiment 1, statistical comparisons between the different muscle areas were made in individual subjects by analysis of variance (ANOVA) with repeated measures and multiple comparison testing of the mean values was performed, when appropriate, using the Student-Newman-Keul post hoc procedure. The changes in lung volume, inspiratory time, inspiratory flow, and pattern of ribcage and abdominal displacement were also compared. Comparison between the numbers of recording sites in which multiunit EMG activity preceded inspiratory airflow in different interspaces was made using a Chi-squared analysis. The number of tonic units recorded from each intercostal population was also compared using a Chi-squared analysis. The tonic and inspiratory modulation of firing rates, and onset time of these tonic units were also averaged, and comparisons across parasternal intercostal spaces were made by a Spearman rank correlation. In Experiment 2, statistical comparisons between the two muscle areas, and respiratory variables, were made in individual subjects with Student's unpaired t tests. Statistical comparison of the linear relationship between neural drive and mechanical advantage between intercostal muscles was made by analysis of covariance (ANCOVA) with the number of motor unit spikes as the dependent variable, muscle as the factor, and mechanical advantage as the covariant. The criterion for statistical significance was taken as P < 0.05.
Results
The indices of the pattern of breathing during recordings from the parasternal intercostal muscles in both experimental protocols are summarized in Table 1. The pattern of breathing during each experiment was largely consistent. However, in Experiment 1, slight but significant differences were noted for two respiratory variables. Inspiratory flow was lower during recordings from the fourth interspace and TI was longer during recordings from the fifth space. It is unlikely that these small changes in respiratory pattern affected our main conclusions (see Discussion).
Table 1.
Pattern of breathing during electromyographic recordings
| Tidal volume (l) | Inspiratory time (s) | Inspiratory flow (l s−1) | Ribcage contribution to tidal volume (%) | |
|---|---|---|---|---|
| Experiment 1 | ||||
| Medial parasternal intercostal (1) | 0.66 ± 0.03 | 2.11 ± 0.14 | 0.32 ± 0.02 | 55.6 ± 5.1 |
| Medial parasternal intercostal (2) | 0.66 ± 0.04 | 2.09 ± 0.13 | 0.32 ± 0.02 | 56.7 ± 5.1 |
| Medial parasternal intercostal (3) | 0.62 ± 0.03 | 2.07 ± 0.13 | 0.31 ± 0.02 | 56.8 ± 6.1 |
| Medial parasternal intercostal (4) | 0.59 ± 0.04 | 2.22 ± 0.19 | 0.27 ± 0.01† | 56.8 ± 8.7 |
| Medial parasternal intercostal (5) | 0.70 ± 0.06 | 2.34 ± 0.16* | 0.03 ± 0.03 | — |
| Experiment 2 | ||||
| Medial parasternal intercostal (2) | 0.65 ± 0.03 | 2.01 ± 0.20 | 0.33 ± 0.03 | 62.5 ± 6.9 |
| Lateral parasternal intercostal (2) | 0.65 ± 0.02 | 2.07 ± 0.22 | 0.33 ± 0.03 | 60.3 ± 6.1 |
Values of Experiment 1 are means ± s.e.m. for six subjects; values of Expperiment 2 are means ± s.e.m. for five subjects. Numbers in parentheses indicate the interspace recorded from. Ribcage contribution to tidal volume was not measured during recordings from the fifth parasternal intercostal (see Methods).
Significantly different from first, second and third interspaces
significantly different from the first and second interspaces (P < 0.05).
Medial parasternal intercostal muscles (Experiment 1)
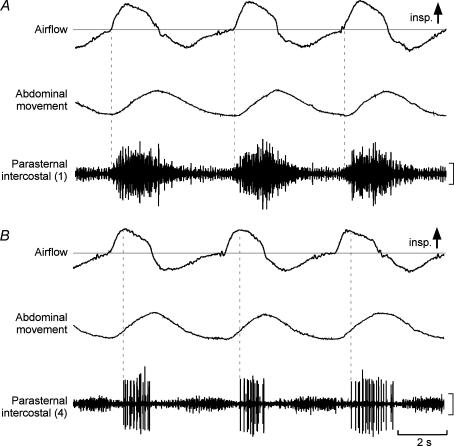
Representative recordings from the medial parasternal intercostal muscle in the first and fourth interspaces from one subject are shown in Fig. 2. Activity in the first parasternal intercostal appeared early relative to the onset of inspiratory flow (vertical dashed lines in Fig. 2A) and persisted after cessation of inspiratory airflow. In 5 of 6 subjects, activity in several sites in this muscle started at or prior to the onset of inspiratory airflow (range, 4–213 ms before flow), such that in 52% of all sites recorded from all subjects, multiunit activity preceded inspiratory airflow. In comparison with the first interspace, the onset of activity in the fourth space was delayed and did not commence until well into inspiration (vertical dashed lines in Fig. 2B), and activity preceded inspiratory airflow in only one site in one subject (by 13 ms). Activity in the fifth interspace also began later, and in one subject it showed no respiratory modulation during quiet breathing. Also, in the fifth interspace, the proportion of sites in which activity preceded inspiratory airflow was zero, significantly less (P < 0.001) than the proportion in the first space.
Figure 2. Data from a single subject for recordings from the first and fourth parasternal intercostal muscles close to the sternum.
Recordings of airflow, abdominal movement, and parasternal intercostal EMG are shown. Inspiration is upwards in the airflow and movement traces. Vertical calibrations: 100 μV. A, recordings from the first parasternal intercostal with the onset of activity shown by vertical dotted lines. Phasic activity commenced close to the onset of inspiratory airflow and there was some tonic activity. B, corresponding data for the fourth parasternal intercostal. Phasic inspiratory activity began well after the onset of inspiratory airflow. Some far-field activity from the triangularis sterni muscle is evident during expiration.
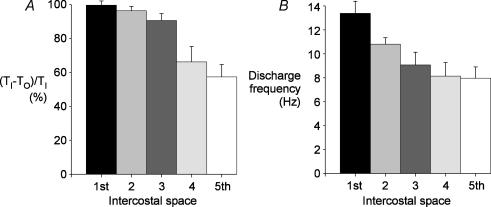
The relative onset time of inspiratory activity ((TI−TO)/TI, see Methods) in the five interspaces for the medial parasternal intercostal muscles is shown in Fig. 3A. Activity commenced earlier in the more cranial interspaces for all subjects, such that (TI−TO)/TI in the first space was 99.6 ± 2.5% (mean ± s.e.m) and only a little less in the second and third spaces. However, (TI−TO)/TI was significantly less in the fourth and fifth interspaces and declined to 57.3 ± 8.0% in the fifth space (P < 0.001).
Figure 3. Rostrocaudal gradient of neural drive to the parasternal intercostal muscles.
Data from recordings in the first to the fifth parasternal intercostal spaces close to the sternum for the six subjects. A, the duration of the phasic inspiratory activity is indicated relative to total inspiratory time ((TI−TO)/TI percentage), shown as the mean ± s.e.m. Inspiratory activity in the parasternal intercostals began close to the onset of airflow in the first and second interspaces but was delayed in the more caudal spaces. B, mean peak discharge rates (± s.e.m.) for the group of subjects derived from the inspiratory firing frequency of 79 units in the first interspace, 81 in the second, 80 in the third, 78 in the fourth, and 47 units in the fifth space. The discharge rate was greatest in the parasternal intercostal in the first interspace, and this rate decreased caudally.
In each parasternal intercostal space, the activity of 47–81 single motor units was recorded and exhibited either purely inspiratory phasic activity (Fig. 1B), or inspiratory modulation of tonic activity (Fig. 4A). However, one subject had no inspiratory modulation in the fifth interspace during quiet breathing, and only tonic activity was noted. The average increase in discharge rates at end-inspiration (i.e. excluding tonic firing during expiration; see Methods) of motor units in the medial portion of the five parasternal intercostal muscles is shown in Fig. 3B. The discharge rate of motor units varied systematically. It was highest in the first interspace, at 13.4 ± 1.0 Hz, and was significantly greater than that of the lower spaces (P < 0.05). The discharge frequency in the second interspace was also greater than in the fifth space, which fired at the lowest frequency, at 8.0 ± 1.0 Hz. The average number of inspiratory motor units identified in each recording site was 2.5 units per site in the first interspace, but only 1.4 in the fifth space. The peak discharge frequency of all units coincided with the last third of inspiration, but across all interspaces, some units exhibited post-inspiratory activity. The large units with a higher threshold (and usually larger amplitude spikes) tended to fire late in inspiration and stop firing at peak inspiration (e.g. Fig. 2B), whereas earlier recruited units continued firing in early expiration (e.g. Fig. 1B).
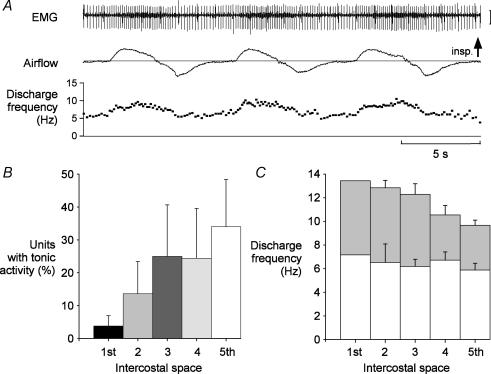
Figure 4. Tonic activity in the parasternal intercostal muscles.
Data from recordings in the first to the fifth parasternal intercostal spaces close to the sternum for six subjects. A, example from a single subject of the discharge of a parasternal intercostal motor unit in the fifth interspace with tonic activity. Parasternal intercostal EMG, inspiratory airflow and the instantaneous frequency of the single motor unit identified in this record are shown. This motor unit exhibited continuous activity throughout the respiratory cycle, but has distinct inspiratory modulation of approximately 5 Hz in each breath. Vertical calibration: 400 μV. B, the percentage of the total number of motor units recorded in each interspace which had tonic activity. The occurrence of tonic activity was more common in the more caudal interspaces. C, mean discharge rates (± s.e.m.) for the group of subjects of the units with tonic activity. The average firing frequency of these units during expiration was similar across interspaces (open bars), but the average inspiratory modulation differed (grey bars). The units in the first interspace had the greatest modulation and the greatest peak firing rate during inspiration, whereas inspiratory modulation was lowest for the units in the fifth space. The average frequencies were derived from the firing frequency of 3 units in the first interspace (from one subject), 11 in the second (two subjects), 20 in the third (four subjects), 19 in the fourth (four subjects), and 16 units in the fifth space (five subjects).
Of the 365 units identified with inspiratory activity, 69 (18.9%) discharged tonically throughout expiration (Fig. 4A), with an average firing rate of 7.1 ± 0.2 Hz (range, 2.9–12.9 Hz). However, as shown in Fig. 4B, the proportion of units which fired tonically in the different interspaces was also different. Few units in the first interspace (3.7 ± 3.1%) had tonic activity. This proportion increased gradually in the caudal direction such that in the fifth interspace, it was 34.0 ± 14.4% (P < 0.001). The average firing rate of these tonic units in expiration did not differ between interspaces and ranged from 6.5 Hz to 7.5 Hz (P > 0.05; Fig. 4C). However, the inspiratory modulation of these tonically firing units did differ. As for the entire population of units (Fig. 3A), the inspiratory modulation of tonic units was greatest in the first, second and third parasternal intercostal spaces (range, 6.1–6.3 Hz) and lower in the fourth and fifth spaces (3.8 Hz; P < 0.05; Fig. 4C). The relative onset time of inspiratory modulation of these tonic units was similar across the five interspaces; it was 105.1 ± 10.6% (range, 85.2–121.2%) for the first space, and 93.3 ± 3.5% (range, 62%– 104%) for the fifth space (P > 0.05).
Medial versus lateral parasternal intercostal muscle (Experiment 2)
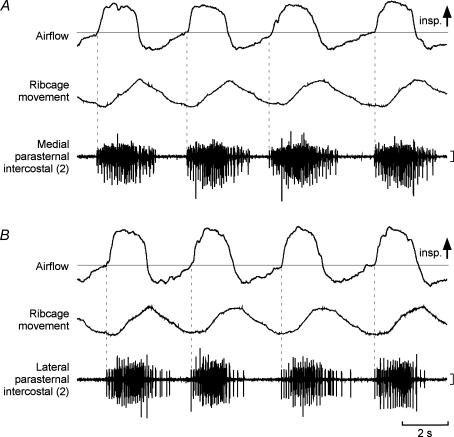
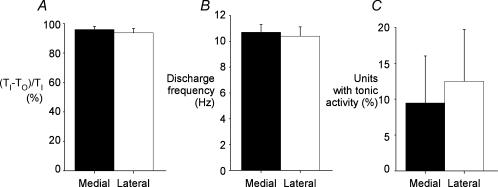
Representative EMG recordings from the medial and lateral portions of the second parasternal intercostal muscle are shown in Fig. 5. Both had activity in phase with inspiration which began with the onset of inspiratory airflow (vertical dashed lines, Fig. 5) and peak activity occurred later during inspiration. Early activation was observed in both portions in all subjects, with activity in some sites preceding inspiratory airflow (range, 9–59 ms before flow). Consequently, as shown in Fig. 6A, (TI−TO)/TI in the medial portion of the second parasternal intercostal was 95.9 ± 2.1%, and did not differ (P > 0.05) from that in the lateral portion (93.7 ± 3.0%).
Figure 5. Recordings in one subject at a medial and lateral location from the second parasternal intercostal muscle.
Inspiratory airflow, ribcage movement, and parasternal intercostal EMG are shown. Inspiration is upwards in the airflow and movement trace. Vertical calibrations: 200 μV. A, recording from a medial site close to the sternum. Vertical dotted lines indicate the onset of EMG activity. B, corresponding data for the lateral recording. Phasic inspiratory activity began close to the onset of airflow for recordings at both the medial and lateral location in the parasternal intercostal space.
Figure 6. Mediolateral gradient of neural drive to the parasternal intercostal muscles.
Data from recordings in medial and lateral portions of the second parasternal intercostal space for five subjects. A, the duration of the phasic inspiratory activity is indicated relative to total inspiratory time ((TI−TO)/TI percentage), shown as mean ± s.e.m.B, data for the mean (± s.e.m.) inspiratory peak firing rate at the medial and lateral locations. There was no difference in the onset of activity, or the discharge frequency of motor units recorded from the two locations in the second parasternal intercostal. C, the percentage of total units with tonic activity, recorded from medial and lateral portions of the second parasternal intercostal space. The occurrence of tonic activity was similar at the two sites.
For the five subjects, 74 single units (13–17 units per subject) were identified in the medial portion of the parasternal intercostal muscle, and 80 units (14–18 units per subject) in the lateral portion. The discharge rates of all units increased with inspiration and the average peak firing frequency of units in the medial portion was 10.7 ± 0.6 Hz. This was similar to the discharge rate in the lateral portion (10.4 ± 0.7 Hz; P > 0.05; Fig. 6B). All units showed phasic inspiratory modulation, but a proportion fired tonically throughout expiration. In the medial portion 9.5 ± 6.6% units fired tonically with an average discharge rate in expiration of 6.9 ± 0.6 Hz (range, 3.5–8.7 Hz), and 12.5 ± 7.2% of units in the lateral portion fired tonically with an average discharge rate of 5.8 ± 0.4 Hz (range, 3.5–7.6 Hz). The proportion of total units recorded which had tonic activity was similar in the medial and lateral portions (P > 0.05; Fig. 6C).
Discussion
We have shown for the first time that there is spatial and temporal distribution of neural drive in the human parasternal intercostal muscles. This is indicated by changes in the timing of the onset of inspiratory activity and in the discharge frequency of populations of single motor units across the interspaces. However, there is no change in these parameters along the mediolateral extent of the second parasternal intercostal space.
Validity of the methods used to assess neural drive
The level of neural drive to inspiratory muscles has been assessed by measurement of the discharge properties of populations of single motor units (Gandevia et al. 1996; Butler et al. 2001; De Troyer et al. 2003). This technique eliminates the difficulties associated with other EMG methods of quantifying neural drive to these muscles such as contamination due to activity in adjacent muscles, recording artifacts due to changes in muscle length, and the need to normalize the level of drive during quiet breathing to that in another respiratory manoeuvre (De Troyer et al. 2003).
The single-unit technique has limitations because we have used rate coding by motoneurones to quantify the magnitude of drive. Increased force production is also achieved by recruitment of new motor units (Adrian & Bronk, 1929; Henneman & Mendell, 1981), but the current method does not quantify the total number of units recruited. Although not studied formally, we found more inspiratory motor units per recording site in the first interspace, compared to the fifth space (2.5 versus 1.4 units per site). Therefore, it is likely that not only was the discharge rate lower in the caudal interspaces (Fig. 3B), but there were relatively fewer units with inspiratory firing. In addition, the rostrocaudal gradient of motor unit discharge activity in the parasternal intercostal muscles has a similar spatial distribution to the timing of the onset of multiunit activity during quiet breathing (Fig. 3A and B). In the intercostal muscles of the dog, greater activation is associated with an earlier onset of inspiratory activity (De Troyer & Legrand, 1995; Legrand et al. 1996a; Leduc et al. 2000).
A second limitation of this technique is that single motor unit recordings from parasternal intercostal muscle of different interspaces were made during different periods of quiet breathing. As neural drive increases with inspiratory flow (Clark & von Euler, 1972), parasternal intercostal activity in the fourth interspace may have been slightly underestimated as inspiratory flow was low during recordings from this space (Table 1). However, across the other interspaces, when inspiratory flow was similar, the greatest activation was in the first and second spaces and lowest in the fifth space. This suggests that neural drive to the parasternal intercostals gradually decreases caudally, and it is unlikely that the neural drive to the fourth interspace was greatly underestimated. A slightly longer TI during recordings from the fifth interspace may have affected the timing of activity onset, with an underestimation of (TI−TO)/TI. However, the discharge rate of motor units in the fifth interspace was also low, confirming the low drive to this space. The absence of phasic inspiratory activity in the fifth interspace in one subject supports this view.
Uneven drive to the parasternal intercostal muscles
A topographic distribution of neural drive to intercostal muscles has been observed in the human external intercostal muscles (De Troyer et al. 2003), as well as in feline and canine intercostal muscles (Greer & Martin, 1990; De Troyer & Legrand, 1995; Legrand et al. 1996a; Legrand & De Troyer, 1999). The mechanism responsible for the graded activation in the intercostal muscles has not been determined. In the anaesthetized dog, afferent inputs do not determine the spatial distribution of activity in the parasternal intercostal muscles as the distribution is preserved when input from the lungs, diaphragm and chest wall is removed (De Troyer & Legrand, 1995; De Troyer et al. 1996a; Legrand et al. 1996a). In human intercostal muscles, local inputs can modulate reflex responses to brief airway occlusions (Butler et al. 1995; Butler et al. 1997), but their role in graded muscle activation during quiet breathing is unclear.
There is limited information on whether drive from inspiratory bulbospinal neurons has a rostrocaudal or mediolateral gradient of intercostal thoracic spaces (for review, see De Troyer et al. 2005). Davies et al. (1985) compared recordings from bulbospinal neurons innervating the dorsal external intercostal muscles of six different interspaces in the cat. The overall strength of connections varied, but there was no graded change along a rostrocaudal axis. In addition, the total average depolarization generated by these monosynaptic connections was estimated to be small relative to the overall depolarization of the external intercostal motoneurones (Merrill & Lipski, 1987).
Muscle recruitment is also determined by the intrinsic properties of the motoneurones. The motoneurones of limb muscles are recruited by their size-related excitability, known as the ‘size principle’ (Henneman, 1957; Henneman & Mendell, 1981). If this holds for intercostal muscles, we would expect a topographic distribution of motoneurone and motor fibre types across the intercostal spaces. Fibre type composition is a good indicator of motoneuronal properties (Henneman & Mendell, 1981), but it is not known if the fibre type composition of human parasternal intercostal muscles differs across interspaces. In the dog, there is no difference in fibre type proportions between intercostal spaces, or between medial and lateral portions of an interspace (De Troyer et al. 1996a; Legrand et al. 1996a). Within the limits of sampling error in humans (Elder et al. 1982), the fibre type composition of the external intercostal muscles from the fifth and eighth interspaces appears similar for the proportion of slow-twitch oxidative fibres (Mizuno & Secher, 1989). If muscle fibre type were the only determinant of firing we would expect that the onset of activation in these two interspaces to be the same. This is not the case. Activity in the seventh external intercostal is lower and delayed relative to that in the fifth (De Troyer et al. 2003). Hence, the distribution of drive and motor unit discharge rate may not solely be determined by an intrinsic, size-related, property of the motoneurones.
A modulatory mechanism which modifies the descending drive to the motoneurones may be responsible for the topographic distribution of drive to intercostal muscles. Respiratory interneurones with inspiratory firing patterns have been identified in the spinal cord of the cat (Lipski & Duffin, 1986; Kirkwood et al. 1988; Bellingham & Lipski, 1990), and these interneurones may contribute to central respiratory drive potentials in respiratory motoneurones. Anatomical and electrophysiological results suggest respiratory interneurones project between spinal segments (Lipski & Duffin, 1986) and to the contralateral ventral horn (Schmid et al. 1993), but the exact role of interneurones in respiratory motor function has not been determined. It has been speculated that there is a propriospinal system for phrenic and intercostal motoneurones similar to the system for forelimb motoneurones (Lipski & Duffin, 1986). In theory, this would allow for common modulation or integration of either involuntary drive from the medulla during quiet breathing, or volitional drive from the motor cortex (Macefield & Gandevia, 1991). However, a more detailed assessment of the respiratory interneurones is required before it is concluded that they mediate the topographic distribution of inspiratory drive between different regions of the thorax. Central inspiratory drive may also be modulated by mechanisms which alter the ‘gain’ of the respiratory motoneurones. Active dendritic mechanisms involving persistent inward currents (Heckman et al. 2003), or ligand-gated channel-mediated synaptic inputs (Berger, 2000; Zhan et al. 2000) may amplify synaptic signals in some motoneurones. Regional differences in the density of serotonergic inputs in the canine parasternal intercostal muscles reflect the topographic distribution of inspiratory activity in this muscle (Zhan et al. 2000).
Neural drive matches the topographic distribution of mechanical advantage
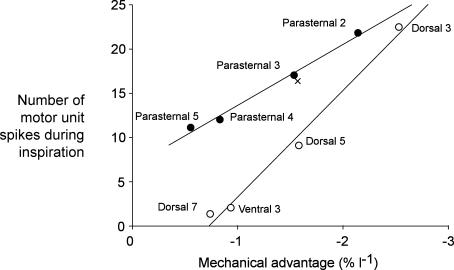
The present study has revealed that the distribution of inspiratory drive to the human parasternal intercostal muscles is closely matched to the distribution of their inspiratory mechanical advantage. Figure 7 shows this relationship for the parasternal intercostals (filled circles) and compares it with that for the external intercostals (open circles). Mechanical advantage was taken from the data of De Troyer et al. (1998), and the number of motor unit discharges during inspiration was calculated by multiplying the average discharge rate (Fig. 3B) by the duration of firing in inspiration (Fig. 3A). Although the two sets of data shown in Fig. 7 were collected from different subjects, each has a strong linear relationship (coefficient of correlation, r2 = 0.99 for both muscle groups). The average number of motor unit discharges during inspiration for the parasternal intercostals in the third interspace has been determined previously (De Troyer et al. 2003), and is similar to that in the present study (Fig. 7, cross versus filled circle). The relationship in Fig. 7 allows estimation of the mechanical advantage of the parasternal intercostal in the first interspace based on its inspiratory neural drive (∼3% per litre). The complex geometry of the fibres in this particular interspace had precluded computation of its mechanical advantage using computed tomography (De Troyer et al. 1998).
Figure 7. Relationship between the inspiratory firing of the parasternal intercostal muscles and their mechanical advantage.
Data for mechanical advantage is derived from De Troyer et al. (1998). Open circles show data for the external intercostals (from De Troyer et al. 2003). Data for the parasternal intercostals are shown as filled circles (current study). The y-axis shows the number of motor unit spikes during inspiration, calculated from the mean group data for each intercostal muscle (see text). The cross shows data for the third parasternal intercostal from the study of De Troyer et al. (2003). Simple linear regressions are shown. There is an increase in inspiratory neural drive to the regions of the intercostal muscles with the higher mechanical advantage.
Further evidence for the link between neural drive and mechanical advantage is observed for the mediolateral extent of the human parasternal intercostal muscle. All the indices of neural drive were identical for the medial and lateral end of the second intercostal space (Fig. 6). In humans, there is minimal change in angulation of fibres along a parasternal intercostal, and the costal cartilages in the first to third interspaces are nearly parallel (De Troyer et al. 1998). Therefore, in these interspaces, the mechanical advantage of the muscle fibres near the costochondral junction is about the same as that of the fibres in the vicinity of the sternum (De Troyer et al. 1998). On the other hand, in the dog, there is a mediolateral gradient of mechanical advantage for the parasternal intercostals in every interspace, and this is associated with a strong gradient of inspiratory neural drive (De Troyer & Legrand, 1995; Legrand et al. 1996b). This species difference strengthens the argument that inspiratory neural drive and mechanical advantage are linked.
Figure 7 also shows that the linear relationship between neural drive and inspiratory mechanical advantage differs in slope for the parasternal and external intercostal muscles (P < 0.05). The relationship is steeper for the external intercostals during quiet breathing. The different relationship between neural drive and mechanical advantage of the parasternal and external intercostals may reflect differences in their operating lengths relative to their optimal length. Overall, neural drive may be set to maximize the efficiency of breathing. The metabolic cost of rib elevation is minimized if the work done by various muscles lifting it is distributed according to the size of their mechanical advantages (De Troyer et al. 2005).
Tonic activity in the parasternal intercostal muscles
Any assessment of tonic and phasic drive to human parasternal and external intercostal muscles should take into account not only their obligatory inspiratory firing (Tokizane et al. 1952; Taylor, 1960; Gandevia et al. 1996; De Troyer et al. 2003), but their contraction in voluntary and postural tasks (e.g. Gandevia et al. 1990; Whitelaw et al. 1992; Rimmer et al. 1995). The present study provides new data about the tonic firing of human parasternal intercostals. Although inspiratory activity decreases caudally, the incidence of tonic firing throughout the respiratory cycle increases from 4% of units in the first interspace to 34% in the fifth space (Fig. 4B). Therefore, during sitting, the parasternal intercostals in caudal interspaces may have greater postural functions compared to the more rostral spaces.
The timing of the increase from tonic firing in the parasternal intercostals was similar across the spaces, but we encountered some units which did not increase their discharge until over 200 ms of inspiratory flow. Such a delay in the inspiratory modulation of tonically firing units has also been observed in the dorsal external intercostal in the fifth interspace (unpublished observations). This delay suggests that the neural mechanisms causing the gradient in the onset of inspiratory firing may occur at a premotoneuronal site, at a spinal or supraspinal level. Alternatively, local factors causing sustained motoneuronal depolarization may be responsible.
There is spatial distribution of neural drive to the human parasternal intercostal muscles along a rostrocaudal gradient during quiet breathing. The inspiratory drive to the muscles mirrors their inspiratory mechanical advantage, and peaks rostrally. In contrast, the occurrence of tonic activity, which may reflect postural drive, is greater in the caudal spaces. Along the mediolateral extent of the second parasternal intercostal space, there is no difference in neural drive, mechanical advantage or occurrence of tonic firing.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council.
References
- Adrian ED, Bronk DW. The discharge of impulses in motor nerve fibres. Part 2. The frequency of discharge in reflex and voluntary contractions. J Physiol. 1929;67:119–151. [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990;533:141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- Berger AJ. Determinants of respiratory motoneuron output. Respir Physiol. 2000;122:259–269. doi: 10.1016/s0034-5687(00)00164-x. [DOI] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Crawford MR, Gandevia SC. Role of airway receptors in the reflex responses of human inspiratory muscles to airway occlusion. J Physiol. 1995;487:273–281. doi: 10.1113/jphysiol.1995.sp020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge frequencies of single motor units in human diaphragm and parasternal muscles in lying and standing. J Appl Physiol. 2001;90:147–154. doi: 10.1152/jappl.2001.90.1.147. [DOI] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Glanville AR, Gandevia SC. Pulmonary afferents are not necessary for the reflex inhibition of human inspiratory muscles produced by airway occlusion. J Neurophysiol. 1997;78:170–176. doi: 10.1152/jn.1997.78.1.170. [DOI] [PubMed] [Google Scholar]
- Chadha TS, Watson H, Birch S, Jenouri GA, Schneider AW, Cohn MA, Sackner MA. Validation of respiratory inductive plethysmography using different calibration procedures. Am Rev Respir Dis. 1982;125:644–649. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Gorman RB, Gandevia SC. Distribution of inspiratory drive to the external intercostal muscles in humans. J Physiol. 2003;546:943–954. doi: 10.1113/jphysiol.2002.028696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev. 2005;85:717–756. doi: 10.1152/physrev.00007.2004. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Respir Crit Care Med. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A. Inhomogenous activation of the parasternal intercostals during breathing. J Appl Physiol. 1995;79:55–62. doi: 10.1152/jappl.1995.79.1.55. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gayan-Ramirez G, Cappello M, Decramer M. On the mechanism of the mediolateral gradient of parasternal activation. J Appl Physiol. 1996a;80:1490–1494. doi: 10.1152/jappl.1996.80.5.1490. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gevenois P-A, Wilson TA. Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. J Physiol. 1998;513:915–925. doi: 10.1111/j.1469-7793.1998.915ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Rostrocaudal gradient of mechanical advantage in the parasternal intercostal muscles of the dog. J Physiol. 1996b;495:239–246. doi: 10.1113/jphysiol.1996.sp021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. J Physiol. 1999;518:283–289. doi: 10.1111/j.1469-7793.1999.0283r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Sampson MG. Activation of the parasternal intercostals during breathing efforts in human subjects. J Appl Physiol. 1982;52:524–529. doi: 10.1152/jappl.1982.52.3.524. [DOI] [PubMed] [Google Scholar]
- Elder GC, Bradbury K, Roberts R. Variability of fiber type distributions within human muscles. J Appl Physiol. 1982;53:1473–1480. doi: 10.1152/jappl.1982.53.6.1473. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Leeper JB, McKenzie DK, De Troyer A. Discharge frequencies of parasternal intercostal and scalene motor units during breathing in normal and COPD subjects. Am J Respir Crit Care Med. 1996;153:622–628. doi: 10.1164/ajrccm.153.2.8564108. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK, Plassman BL. Activation of human respiratory muscles during different voluntary manoeuvres. J Physiol. 1990;428:387–403. doi: 10.1113/jphysiol.1990.sp018218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Martin TP. Distribution of muscle fibre types and EMG activity in cat intercostal muscles. J Appl Physiol. 1990;69:1208–1211. doi: 10.1152/jappl.1990.69.4.1208. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurones and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26:688–695. doi: 10.1016/j.tins.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organisation of motoneuron pool and its inputs. In: Geiger SR, editor. Handbook of Physiology, section 3, The Nervous System. Baltimore: Waverly Press, Inc.; 1981. pp. 423–508. [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, Westgaard RH. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc D, Brunko E, De Troyer A. Response of the canine inspiratory intercostal muscles to chest wall vibration. Am J Respir Crit Care Med. 2000;161:510–516. doi: 10.1164/ajrccm.161.2.9901032. [DOI] [PubMed] [Google Scholar]
- Legrand A, Brancatisano A, Decramer M, De Troyer A. Rostrocaudal gradient of the electrical activation in the parasternal intercostal muscles of the dog. J Physiol. 1996a;495:247–254. doi: 10.1113/jphysiol.1996.sp021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, De Troyer A. Spatial distribution of external and internal intercostal activity in dogs. J Physiol. 1999;518:291–300. doi: 10.1111/j.1469-7793.1999.0291r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, Wilson TA, De Troyer A. Mediolateral gradient of mechanical advantage in the canine parasternal intercostals. J Appl Physiol. 1996b;80:2097–2101. doi: 10.1152/jappl.1996.80.6.2097. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61:625–637. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991;439:545–558. doi: 10.1113/jphysiol.1991.sp018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary repsiratory neurons in the cat. J Neurophysiol. 1987;57:1837–1852. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Secher NH. Histochemical characteristics of human expiratory and inspiratory intercostal muscles. J Appl Physiol. 1989;67:592–598. doi: 10.1152/jappl.1989.67.2.592. [DOI] [PubMed] [Google Scholar]
- Rimmer KP, Ford GT, Whitelaw WA. Interaction between postural and respiratory control of human intercostal muscles. J Appl Physiol. 1995;79:1556–1561. doi: 10.1152/jappl.1995.79.5.1556. [DOI] [PubMed] [Google Scholar]
- Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol. 1993;461:647–665. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol. 1960;151:390–402. doi: 10.1113/jphysiol.1960.sp006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokizane T, Kawamata K, Tokizane H. Electromyographic studies on the human respiratory muscles; studies on the activity pattern of neuromuscular units. Jpn J Physiol. 1952;2:232–247. doi: 10.2170/jjphysiol.2.232. [DOI] [PubMed] [Google Scholar]
- Whitelaw WA, Ford GT, Rimmer KP, De Troyer A. Intercostal muscles are used during rotation of the thorax in humans. J Appl Physiol. 1992;72:1940–1944. doi: 10.1152/jappl.1992.72.5.1940. [DOI] [PubMed] [Google Scholar]
- Wilson TA, Legrand A, Gevenois P-A, De Troyer A. Respiratory effects of the external and internal intercostal muscles in humans. J Physiol. 2001;530:319–330. doi: 10.1111/j.1469-7793.2001.0319l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan WZ, Mantilla CB, Zhan P, Bitton A, Prakash YS, de Troyer A, Sieck GC. Regional differences in serotonergic input to canine parasternal intercostal motoneurons. J Appl Physiol. 2000;88:1581–1589. doi: 10.1152/jappl.2000.88.5.1581. [DOI] [PubMed] [Google Scholar]