Abstract
Sympathetic nerves innervate the airways of most species but their reflex regulation has been essentially unstudied. Here we demonstrate sympathetic nerve-mediated reflex relaxation of airway smooth muscle measured in situ in the guinea-pig trachea. Retrograde tracing, immunohistochemistry and electrophysiological analysis identified a population of substance P-containing capsaicin-sensitive spinal afferent neurones in the upper thoracic (T1–T4) dorsal root ganglia (DRG) that innervate the airways and lung. After bilateral vagotomy, atropine pretreatment and precontraction of the trachealis with histamine, nebulized capsaicin (10–60 μm) evoked a 63 ± 7% reversal of the histamine-induced contraction of the trachealis. Either the β-adrenoceptor antagonist propranolol (2 μm, administered directly to the trachea) or bilateral sympathetic nerve denervation of the trachea essentially abolished these reflexes (10 ± 9% and 6 ± 4% relaxations, respectively), suggesting that they were mediated primarily, if not exclusively, by sympathetic adrenergic nerve activation. Cutting the upper thoracic dorsal roots carrying the central processes of airway spinal afferents also markedly blocked the relaxations (9 ± 5% relaxation). Comparable inhibitory effects were observed following intravenous pretreatment with neurokinin receptor antagonists (3 ± 7% relaxations). These reflexes were not accompanied by consistent changes in heart rate or blood pressure. By contrast, stimulating the rostral cut ends of the cervical vagus nerves also evoked a sympathetic adrenergic nerve-mediated relaxation that were accompanied by marked alterations in blood pressure. The results indicate that the capsaicin-induced reflex-mediated relaxation of airway smooth muscle following vagotomy is mediated by sequential activation of tachykinin-containing spinal afferent and sympathetic efferent nerves innervating airways. This sympathetic nerve-mediated response may serve to oppose airway contraction induced by parasympathetic nerve activation in the airways.
Sympathetic adrenergic nerves innervate the airways of all vertebrate species studied (McLean & Burnstock, 1967; Cabezas et al. 1971; O'Donnell & Saar, 1973; Diamond & O'Donnell, 1980; Mustafa et al. 1982; Sheller & Brigham, 1982; Matsumoto et al. 1985; Pack et al. 1988; Mitchell et al. 1990; Broadstone et al. 1991; Kummer et al. 1992; Baker & McDonald, 1992; Zaccone et al. 2004). When activated, sympathetic nerves initiate dilatation of the airways through the actions of noradrenaline acting on airway smooth muscle β-adrenoceptors. In humans, airway sympathetic nerves may play a critical role in disease or under extreme physiological conditions (Molho et al. 1977; Larson, 1985; Sands et al. 1985; Noppen & Vincken, 1996; Heindl et al. 2001; Schilero et al. 2005). In asthmatics, for example, propranolol can evoke a profound constriction of the airways that is prevented by muscarinic receptor antagonists (Sly et al. 1967; Grieco & Pierson, 1971; Ind et al. 1989). Similarly, in animals, airways responsiveness and bronchospasm are markedly potentiated by propranolol (Diamond, 1972; Colebatch & Engel, 1974). These β-adrenoceptor-dependent effects, either neuronally or hormonally regulated, may be recruited to compensate for dysfunction or dysregulation of the airway parasympathetic non-cholinergic nerves or to oppose the constricting effects of airway cholinergic nerves (Grieco & Pierson, 1971; Baker & Don, 1987; Ind et al. 1989; Wechsler et al. 2000; Canning & Fischer, 2001).
Action potentials in pre- and post-ganglionic sympathetic nerves potentially regulating airway smooth muscle tone have been recorded at rest and during airway stimulation (Widdicombe, 1966; Bachoo & Polosa, 1987; Habler et al. 1994; Shirai et al. 1995a, b). Reflex-mediated alterations in their activity have been attributed mostly to activation of vagal afferent nerves, primarily pulmonary stretch receptors (Barman & Gebber, 1976; Bachoo & Polosa, 1987; Yu et al. 1990; Seals et al. 1993; Habler et al. 1994; St Croix et al. 1999; Huang et al. 2000; Zhou et al. 2002). Spinal afferent nerves emanating from thoracic dorsal root ganglia (T1–T4) also innervate the airways and lungs (Kostreva et al. 1975, 1978; Saria et al. 1985; Kummer et al. 1992; Wang et al. 2003; Soukhova et al. 2003; Plato et al. 2006). Activation of these spinal afferent nerves can alter respiration and/or renal sympathetic nerve activity, but their role in regulating airway sympathetic nerves is unknown. In fact, no study has directly studied and quantified reflex regulation of airway smooth muscle by sympathetic nerves. Rather, the role of sympathetic nerves in regulating airway smooth muscle tone has been inferred from the effects of propranolol on bronchospasm. Mostly, however, the effects of β-adrenoceptor antagonists on responsiveness of airways can be attributed to preventing the effects of hormonal catecholamines (Diamond, 1972; Colebatch & Engel, 1974; Underwood et al. 1997). It is likely that the difficulty with which the effects of neuronal catecholamines can be differentiated from those mediated by hormonal catecholamines in measures of whole lung mechanics has contributed to this gap in our understanding of airway neuronal control.
We have developed a preparation of the guinea-pig in which reflex responses of an isolated segment of the extrathoracic trachea can be monitored in situ (Mazzone & Canning, 2002c). Monitoring reflex effects in the trachea accurately predicts neuronal regulation of the airways as a whole but provide the added advantage that the pharmacology and specific neuronal pathways regulating these effects can be studied selectively and systematically. In the present study we describe reflex regulation of airway smooth muscle by sympathetic nerves.
Methods
The institutional animal care and use committees at either the Johns Hopkins University or the University of Maryland approved all methods described below.
Immunohistochemistry of airway-identified dorsal root ganglia DRG neurones
Male Hartley guinea-pigs (200–250 g, Charles River, Wilmington, MA, USA) were anaesthetized with ketamine and xylazine (60 mg kg−1 and 10 mg kg−1, respectively, i.p.) and treated with atropine (0.05 mg kg−1, i.p.) to decrease bronchial mucus secretion and/or bronchospasm. The animal was placed head up on a 45 deg incline. The midcervical trachea was exposed and 400 μl of the tracer DiI (dissolved in 100% ethanol and diluted in sterile saline to a final concentration of 0.5 mg ml−1 in 1% ethanol) was instilled into the lumen using a 28.5-gauge needle. Animals were maintained in this position after tracer instillation and suture of the cut until they woke up. Ten to fourteen days later the guinea-pigs were deeply anaesthesized (pentobarbital, 100 mg kg−1, i.p.). When no heart beat or respiratory efforts were apparent, the chest was opened and the animal was transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 m phosphate-buffered saline (PBS, 4°C) containing 6 U ml−1 heparin and 0.1% procaine. DRG were dissected and placed in 4% PFA for 2 h. After rinsing in PBS, DRG were cryoprotected in an 18% sucrose solution overnight at 4°C, subsequently frozen in O.C.T. medium (VWR, Bridgeport, NJ, USA) and sectioned (20 μm). DiI-labelled neurones were visualized by fluorescence microscopy (Olympus BX60), photographed using a digital camera and localized by their x–y coordinates on the slides. Tissue sections were then treated with blocking solution (10% goat serum, 1% bovine serum albumin and 0.5% Tween 20 in 0.1 m PBS) for 1 h at room temperature followed by incubation with primary antibodies against substance P (SP; 1: 100 dilution, Chemicon, Temecula, CA, USA) or neurofilament (NF; 1: 100 dilution, Sigma) at 4°C overnight. Thereafter the slides were incubated with fluorescence-labelled anti-primary antibodies (goat anti-mouse or rat IgG antibodies (1: 100 dilutions, Molecular Probes) for 2 h at room temperature. Antibodies were diluted in 0.1 m PBS containing 0.3% Triton and 1% bovine serum albumin. After secondary labelling the slides were coverslipped with antifade buffer (Molecular Probes). The airway-identified neurones were re-localized and examined for immunoreactivity for SP and NF as previously described (Mazzone & Canning, 2002b).
Electrophysiology
Dorsal root ganglia (DRG) neurones were retrogradely labelled with DiI as described above. Ten to fourteen days later, the animals were killed by asphyxiation in a chamber filled with 100% CO2 and exsanguinated. DRG (T1–T4) were quickly extracted from the animals, placed in 4°C Locke solution (composition (mm): 136 NaCl, 5·6 KCl, 14·3 NaHCO3, 1·2 NaH3PO4, 2·2 CaCl2, 1·2 MgCl2 and 10 dextrose, equilibrated continuously with 95% O2–5% CO2; pH 7·2–7·4), de-sheathed, cut into pieces and placed in Ca2+ and Mg2+-free Hanks' balanced salt solution (CMFH) (composition (mm): 138 NaCl, 5·0 KCl, 4·0 NaHCO3, 0·3 Na2HPO4, 0·3 KH2PO4, 5 dextrose and 0·03 Phenol Red). The ganglia were then incubated for 7 min in 10 ml CMFH containing 1 μg ml−1 papain (Boehringer Mannheim), which was activated by 0.2 mg ml−1l-cysteine. After two washes in CMFH the tissue was incubated for 10 min in CMFH containing 2 mg ml−1 dispase (grade II, Boehringer Mannheim) and 1 mg ml−1 type 1A collagenase. Ganglia neurones were dissociated during this last incubation by trituration with a fire-polished Pasteur pipette. After two more washes in Leibovitz L-15 medium (Gibco) containing 10% (v/v) fetal bovine serum (FBS; JRH Biosciences, Lexena, KS, USA), the cells were resuspended in L-15–10% FBS and applied in 150 μl aliquots to poly d-lysine-coated coverslips in 2.5 mm culture plates. Neurones were allowed to settle and attach overnight at 37°C before use.
Within 9 h of plating on the coverslips, DiI-labelled DRG neurones were visualized using fluorescence microscopy for whole-cell patch-clamp recordings using an Axopatch 200B amplifier and pCLAMP8 software (Axon Instruments, Foster City, CA, USA). The resistance of the patch pipettes was 1–3 MΩ when they were filled with the following solution (mm): 140 KCl, 2 MgCl2, 1 CaCl2, 10 N-[2-hydroxyethyl] piperazine-N′-[2-ethanesulphonic acid] (Hepes), 11 EGTA, 2 Mg-ATP, and 1 Li-GTP; pH 7.3 adjusted with KOH, 314 mosmol l−1. Coverslips were continuously superfused (6–7 ml min−1) during recording with a Locke solution (mm): 136 NaCl, 5.6 KCl, 1.2 NaH2PO4, 14.3 NaHCO3, 1.2 MgCl2, 2.2 CaCl2, and 10 dextrose, equilibrated with 95% O2–5%CO2 pH 7.3–7.5. The Locke solution was maintained at 33°C. Pipette voltage offset was neutralized prior to the formation of a gigaseal. Membrane resistance (Rm), series resistance (Rs), and membrane capacitance (Cm) were determined from current transients elicited by a 5 mV depolarizing step from a holding potential −60 mV, using the ‘Membrane Test’ application of pCLAMP8. Capacitance and 80%Rs were compensated electronically. Criteria for cell inclusion in the study were as follows: Rs≤ 10 MΩ, Rm > 100 MΩ, and stable recording with 80%Rs compensation throughout the experiment. For testing the chemosensitivity of neurones to bath-applied capsaicin, cells were voltage-clamped to −60 mV and ionic currents were measured.
In vivo measurement of airway smooth muscle tone
Male Hartley guinea-pigs (300–400 g) were anaesthetized with urethane (1.48 ± 0.03 g kg−1, i.p.) and placed supine on a heated pad (see Fig. 1). A midline incision in the neck exposed the extrathoracic trachea, which was cannulated at its caudal-most end with a bent 15-gauge leur stub adaptor. After neuromuscular blockade with succinylcholine (2 mg kg−1s.c.), the animal was mechanically ventilated (60 breaths min−1, 6 ml kg−1, 3 cmH2O positive end expiratory pressure). Depth of anaesthesia was repeatedly assessed prior and subsequent to blockade and supplemental anaesthetic administered as needed based on responses (withdrawal, cardiovascular) to sharp pinches to the limbs and skin. The ventilator was attached in series to an ultrasonic nebulizer and pulmonary inflation pressure was monitored using a pressure transducer attached to a side port of the tracheal cannula. Stainless steel dry fly hooks (Mustad, Auburn, NY, USA; size 14, 4.9 mm in width, 12 mm long) were placed between two to three cartilage rings in the cervical trachea on the lateral aspects of the trachea. One hook was tied to a fixed bar while the other was connected to an isometric force transducer (Grass Instruments, Quincy, MA, USA) to monitor tracheal tension (TT; grams force (mN) per tissue width (4.9 mm)). Baseline tension was set at 3–4 mN mm−1. The tracheal lumen was continuously superfused (20 ml min−1) with warmed (37°C) oxygenated Krebs buffer, introduced to the tracheal lumen via a small slit, two cartilage rings below the hooks. The buffer was collected at the larynx via gentle suction. The buffer (composition (mm): 118 NaCl, 5.4 KCl, 1 NaHPO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, 11.1 dextrose, pH 7.4) contained 3 μm indomethacin to prevent formation of neuromodulatory prostanoids and a mixture of neurokinin (NK) receptor antagonists (0.1 μm ZD6021 or 0.1 μm each CP99994, SR48968 and SB223412) to block the effects of peripherally released tachykinins. The α-adrenoceptor antagonist phentolamine (1 μm) was also added to the superfusate to minimize prejunctional adrenoceptor-dependent effects and effects on the tracheal vasculature and/or airway smooth muscle.
Figure 1. Schematic representation of afferent and efferent innervation of the airways and in vivo experimental set-up for studying sympathetic airway reflexes.
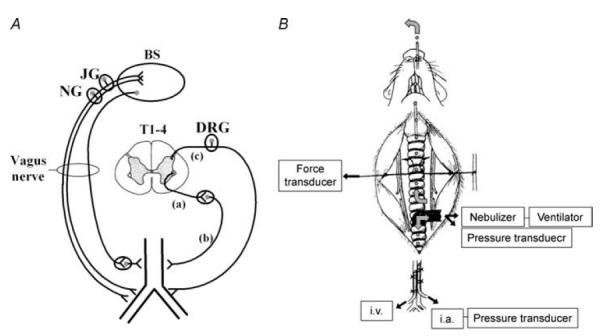
A, the airway is innervated by both parasympathetic and sympathetic efferent and vagal and spinal afferent neurones. To study sympathetic reflex effects, the vagus nerves are cut bilaterally to disrupt all afferent and efferent vagal pathways. Activating the peripheral terminals of spinal afferent neurones or electrically stimulating the rostral cut ends of the vagus nerves evokes sympathetic reflexes in the airways. Spinal afferent nerves innervating the airways have their somata in thoracic (T1–T4) dorsal root ganglia (DRG). Preganglionic sympathetic neurones regulating airway smooth muscle tone arise from the thoracic spinal cord and project to thoracic sympathetic ganglia or to the superior cervical ganglia via the cervical sympathetic trunks. Some preganglionic sympathetic neurones dually innervate postganglionic neurones in both the superior cervical and thoracic sympathetic ganglia. To modulate sympathetic reflexes, the pre- (a) or post-ganglionic (b) axons regulating airway tone can be severed, or (c), dorsal rhizotomy can be performed to disrupt the spinal afferent nerves innervating the lungs. B, to study airway sympathetic reflexes, anaesthetized animals are artificially ventilated through a tube inserted into the caudal trachea. A nebulizer connected in series with the ventilator and tracheal tube facilitates selective delivery of chemical stimuli such as capsaicin to the lower airways. A pressure transducer connected to a side port of the tracheal tube monitors pulmonary inflation pressure. Isometric tension of the extrathoracic trachealis is measured using metal hooks, with one hook tied to a fixed bar and the other tied to a force transducer. The tracheal lumen is perfused with Krebs buffer, allowing selective delivery of drugs to the segment of the trachea in which isometric tension is monitored. To study reflex-mediated relaxations, the trachealis is first precontracted to 30–50% of the maximum attainable contraction by adding histamine (10–50 μm) to the tracheal perfusate. The abdominal aorta and the inferior vena cava are cannulated to monitor blood pressure and for intravenous drug delivery, respectively. See text for more details (also see Mazzone & Canning, 2002c).
The abdominal aorta and inferior vena cava were cannulated with PE-60 tubing filled with heparinized saline for monitoring arterial blood pressure (ABP) and for intravenous drug delivery, respectively. Physiological parameters were displayed on a Grass polygraph and digitized to computer using a Biopac data collection system (Goleta, CA, USA). At the end of each experiment, animals were killed by inhalation of 100% CO2 followed by exsanguination.
In initial experiments the optimal conditions for monitoring sympathetic nerve-mediated relaxations of the trachealis were determined. After a 10 min equilibration period, 1 μm atropine was added to the tracheal perfusate followed by bilateral vagotomy to eliminate any parasympathetic influences over tracheal tone. Thereafter, the trachealis was precontracted to ∼50% of the maximum contraction by continuously superfusing 10–50 μm histamine into the tracheal lumen (precontraction is necessary to study any subsequently evoked relaxations). When the histamine contractions had stabilized, the cervical sympathetic trunks were electrically stimulated (5–10 V, 1–32 Hz, 1 ms pulse duration, 10 s trains) bilaterally to evoke relaxations. The adrenergic nature of these responses was confirmed by assessing the antagonistic effects of 2 μm propranolol on the evoked responses. For these pharmacological analyses, the nerves were stimulated at an optimal stimulation frequency (≥ 16 Hz) at 5 min intervals before and during continuous perfusion of the trachea with propranolol (in control preparations, sympathetic nerve-mediated relaxations remain essentially unchanged (± 5%) in five consecutive stimuli at 5 min intervals; n = 5). The effects of propranolol were assessed until two consecutive stimuli evoked identical responses. The results were expressed as a percentage blockade of the evoked relaxations. To determine the relative role of β1- and β2-adrenoceptors, the effects of the β1- and β2-adrenoceptor-selective antagonists practolol and ICI118551, respectively, on the sympathetic nerve-mediated relaxations were assessed. Relaxations were evoked by sympathetic nerve stimulation (16 Hz) before and during continuous perfusion with either 1 nm–0.1 μm ICI118551 or 0.01–3 μm practolol. Cumulatively increasing concentrations of the antagonists were administered until consecutive doses produced no further blockade. When the maximum effect of either compound was attained, the other antagonist was added at its optimal concentration to assess the effects of combined antagonism of β1- and β2-adrenoceptors. These results were expressed as a percentage blockade of the relaxant response.
At the end of each experiment, the H1 histamine receptor antagonist pyrilamine (1 μm) was added to the tracheal perfusate to induce a maximal reversal of the histamine contraction. Neurally evoked relaxations were expressed as a percentage of this maximum relaxation.
Once the optimal conditions for monitoring sympathetic nerve-mediated responses had been established, we studied reflex activation of the sympathetic nerves with aerosolized capsaicin. Capsaicin (0.1 m) was dissolved in 100% ethanol and diluted in sterile saline to 10–60 μm (median 30 μm). This range of concentrations of capsaicin is near threshold and/or submaximal for evoking airway responses (Thompson & Sheppard, 1988; Xiang et al. 1998). Two millilitres of the capsaicin solution was introduced in a nebulizer (Mystique, Airsep, Buffalo, NY, USA; particle size ∼5 μm); the nebulizer was turned on for 2–5 min (median 3.5 min) to induce sympathetically mediated tracheal relaxations. Capsaicin challenges with each concentration continued until pulmonary inflation pressure increased by 50% (secondary to the capsaicin-evoked axon reflex) or for 5 min with 100 μm challenge concentration when modest changes in pulmonary inflation pressure were noted (< 10% of preparations). Responses were monitored for 10 min or until a maximum effect was attained. The antagonistic effects of 3 μm propranolol added to the tracheal perfusate or intravenous administration of the ganglionic blocker hexamethonium (4 mg kg−1) on the evoked responses was assessed. To distinguish the effects of neuronal catecholamines from hormonal catecholamines in the capsaicin-induced reflexes, we assessed the effect of cutting both cervical sympathetic trunks and recurrent laryngeal nerves while leaving the tracheal vasculature intact. We also assessed the effects of adding 1 μm tetrodotoxin (TTX) to the tracheal perfusate on these relaxations (this would abolish transmitter release from sympathetic nerve terminals in the trachea while having no effect on the actions of circulating catecholamines). To evaluate the role of spinal afferents in these responses, bilateral dorsal rhizotomy from T1 to T4 was performed after upper thoracic laminectomy and opening of the dura. Control preparations consisted of a sham operation, with laminectomy and opening of the dura without cutting the dorsal roots. Finally, we assessed the effects of systemically administered tachykinin receptor antagonists on these responses. CP99994, SR48968 and SB223412 were administered simultaneously at 1 mg kg−1 i.v. prior to capsaicin challenge. Vehicle control experiments were carried out in parallel.
In another series of experiments, we assessed the effect of intravenous propranolol treatment on capsaicin and neurokinin A (NKA)-evoked increases in pulmonary inflation pressure. Concentration response curves for capsaicin (0.1–10 μg kg−1, i.v.) and NKA (0.1–10 nmol kg−1, i.v.) were constructed in the absence and presence of propranolol (1 mg kg−1, i.v.), given 10 min prior to capsaicin or NKA. Vehicle control experiments were carried out in parallel.
Drugs
ZD6021 (AstraZeneca, Wilmington, DE, USA), SR48968 and SB223412 (GlaxoSmithKline, King of Prussia, PA, USA), and CP99994 (Schering Plough, New Brunswick, NJ, USA) were gifts. All other drugs were purchased from Sigma (St Louis, MO, USA) unless specified. Stock solutions were made in distilled water and diluted in Krebs buffer except indomethacin and capsaicin (100% ethanol), and ZD6021, CP99994, SR48968 and SB223412 (100% DMSO). Drugs given intravenously or subcutaneously (1–50 mg ml−1) were dissolved in saline except: CP99994 and SR48968 were dissolved in DMSO (10 mg ml−1) and diluted (1 mg ml−1) in saline; SB223412 was dissolved in DMSO (10 mg ml−1) and diluted (1 mg ml−1) with 20% acid in saline.
Data analysis and statistics
All data are presented as mean ± s.e.m. Group means were compared by analysis of variance. When statistically significant differences amongst group means were detected by ANOVA, group means were compared using Scheffe's F test for unplanned comparisons. P values less than 0.05 are considered significant.
Results
Characteristics of spinal airway afferent neurones
DiI delivered to the airways and lungs by intratracheal instillation in three guinea-pigs retrogradely labelled neurones in the right and left thoracic (T1–T4) dorsal root ganglia (DRG). Immunohistochemical analyses revealed that 72% (21 of 29 labelled neurones) of the airway projecting DRG neurones had immunoreactivity for substance P (SP; a marker for nociceptive-type, capsaicin-sensitive C-fibre afferent neurones; Szolcsanyi et al. 1988; Lawson et al. 1993) whereas only 14% (4 out of 29 neurones) were immunoreactive for neurofilament (NF; a marker for myelinated nerves; Lawson et al. 1993). No labelled neurones evaluated were dually labelled for SP and NF, and four labelled neurones were not labelled for either SP or NF (Fig. 2A). Whole-cell patch-clamp recordings revealed that the majority of airway projecting DRG neurones produced large (1.8 ± 0.43 nA, n = 22 neurones from 7 animals) inward currents to bath-applied 10 μm capsaicin (Fig. 2B). Together, these results imply that both A- and C-type DRG neurones innervate the airways (primarily tachykinin-containing C-fibres) and also show that capsaicin stimulates many airway afferent DRG neurones.
Figure 2. Evaluation of substance P and neurofilament immunoreactivity of dorsal root ganglia (DRG) neurones innervating the airways and their responsiveness to capsaicin.
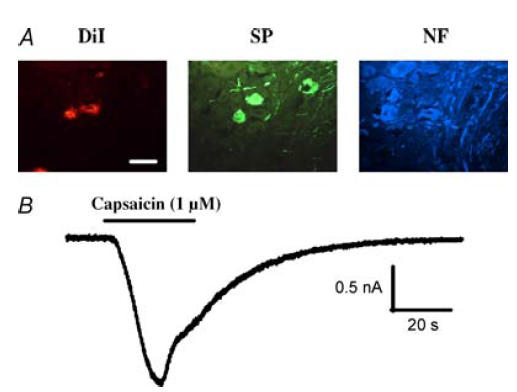
A, DRG neurones projecting to the airways and lungs were retrogradely labelled with DiI instilled into the airway lumen. The neurochemistry of DiI-labelled neurones was determined in 20 μm sections of thoracic DRG. Amongst the DiI-labelled neurones, 72% were immunoreactive for substance P (SP), 14% were immunoreactive to neurofilament (NF), and none were immunoreactive for both SP and NF. Overall, DiI instilled into the airways labelled 2 ± 0.3% of the neurones in the upper thoracic DRG in 3 guinea-pigs. Scale bar indicates 50 μm. B, whole-cell patch-clamp recordings in this study revealed that the majority of (39/64 neurones from 7 animals) of DRG neurones innervating the airways and lungs generated inward currents in response to bath application of capsaicin. Horizontal bars indicate time of capsaicin application. Other stimuli that activate DRG neurones retrogradely labelled from the airways and lungs include serotonin, ATP and bradykinin (E. J. Oh and D. Weinreich, unpublished observations).
Airway relaxation induced by electrical stimulation of sympathetic efferent neurones
Electrical stimulation (10–20 V, 1–32 Hz, 1 ms pulse duration, 10 s trains) of the cervical sympathetic trunks evoked frequency-dependent relaxations of the precontracted guinea-pig trachealis (Fig. 3). These relaxations were fast in onset and reversal, characteristic of adrenergic nerve-mediated relaxations of airway smooth muscle (Diamond & O'Donnell, 1980; Canning & Undem, 1993). At frequencies ≥ 16 Hz, the histamine-induced contractions were nearly completely reversed by the end of the 10 s trains. Coincident with the effects on the airways, cervical sympathetic nerve stimulation (16 Hz, 10 s) transiently increased heart rate and blood pressure by 10 ± 3% and 18 ± 2%, respectively (n = 6). Cutting the cervical sympathetic trunks and stimulating either the rostral or caudal cut ends evoked relaxations that were abolished by the ganglionic blocker hexamethonium (4 mg kg−1 i.v.). Adding the β-adrenoceptor antagonist propranolol to the tracheal perfusate also nearly abolished the relaxations evoked by sympathetic nerve stimulation. By contrast, neither ICI118551 (1 nm–0.1 μm) nor practolol (0.01–3 μm) substantially antagonized the electrically evoked relaxations. When added simultaneously, however, the β1- (3 μm practolol) and β2- (0.1 μm ICI118551) adrenoceptor antagonists mimicked the effects of the non-selective antagonist propranolol (Fig. 4).
Figure 3. Tracheal relaxation induced by electrical stimulation of cervical sympathetic trunk.
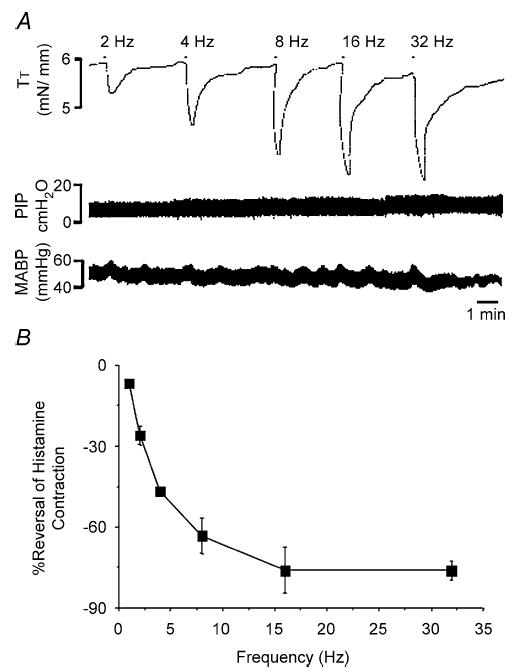
A, representative trace of sympathetic nerve-mediated relaxations of the guinea-pig trachealis. Histamine (10–50 μm) was used to precontract the trachealis. When the histamine-induced contraction had stabilized (30–50% of the maximum attainable contraction), the sympathetic nerves were stimulated in 10 s trains at varying frequencies (1–32 Hz as indicated) and at optimal stimulation intensities (10–20 V, 1 ms pulse duration). B, relaxations of the guinea-pig trachealis evoked by sympathetic nerve stimulation expressed as the mean ± s.e.m. of the maximum relaxation of the histamine-induced contraction (n = 3).
Figure 4. Effects of β-adrenoceptor antagonists on sympathetic nerve-induced relaxations of the guinea-pig trachealis.
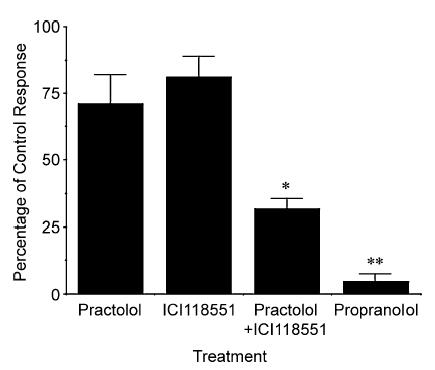
Relaxations were evoked by electrically stimulating the cervical sympathetic trunks (16 Hz, 5–10 V, 1 ms pulse duration, 10 s train). Relaxations were evoked at 5 min intervals and when consecutive stimuli evoked identical relaxations, either a β1- (practolol; 0.1–3 μm; n = 4) or β2- (ICI1118551; 10–100 nm; n = 4) adrenoceptor antagonist was administered to the tracheal perfusate in concentrations selective for antagonizing β1 and β2 receptors, respectively. Subsequent doses of each drug were added when consecutive stimuli produced identical relaxant responses. When the maximal effect of one drug was attained, the maximally effective concentration of the other compound (3 μm practolol and 0.1 μm ICI118551) was added to assess the effect of combined β1- and β2-receptor antagonism on the evoked relaxations (n = 7). The maximum attainable blockade of the evoked relaxations was determined by adding 2 μm propranolol to the tracheal perfusate (n = 7). Results are presented as mean ±s.e.m. *Blockade of sympathetic nerve-mediated relaxations produced by the combination of practolol and ICI118551 is statistically greater than that produced by administering either compound alone (P < 0.01). **Blockade of sympathetic nerve-mediated relaxations produced by propranolol was greater than that produced by practolol and/or ICI118551 (P < 0.05).
Capsaicin-induced sympathetic airway reflex
Based on the results of our electrophysiological analyses, we used capsaicin to evoke sympathetic nerve-mediated reflexes in the airways. As capsaicin also induces parasympathetic relaxant reflexes in the airways (Mazzone & Canning, 2002a, b, c), we cut the vagus nerves bilaterally, caudal to the nodose ganglia. Atropine was also added to the tracheal perfusate to prevent parasympathetic nerve-mediated contractions, and the trachealis was then precontracted with histamine. Under these conditions, capsaicin inhalation still evoked a slowly developing but pronounced fall in tracheal tension (Fig. 5). The average maximal relaxation induced by capsaicin inhalation was 63 ± 7% (n = 13). Relaxations started within 2 min of initiating the capsaicin challenge and reached a maximum in ≤ 6 min (capsaicin challenges in the control preparations lasted 4.6 ± 0.8 min). The relaxations evoked were sustained for several minutes, long after terminating the capsaicin aerosol challenge and were reversed by propranolol. When propranolol was added prior to capsaicin challenge, the relaxations were attenuated (10 ± 9% of the maximum relaxation, n = 7; Fig. 5B). Pretreatment with hexamethonium (4 mg kg−1 i.v.; n = 3) or sympathetic denervation of the trachea (n = 5) nearly abolished the reflex relaxations (−2 ± 4% and 6 ± 4% of the maximum relaxation, respectively; Table 1).
Figure 5. Capsaicin-induced, reflex-mediated relaxations of the trachealis.
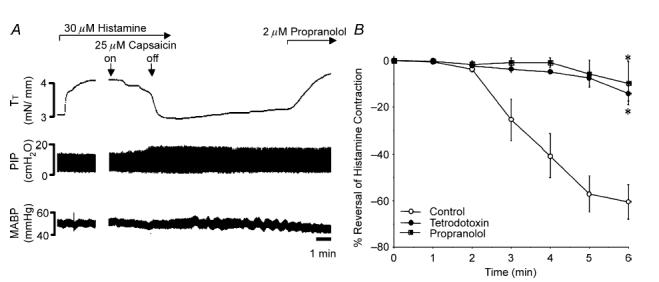
A, representative trace of capsaicin-induced, sympathetic reflex-mediated relaxation of the trachealis. Capsaicin (25 μm) inhalation for ∼4 min evoked a slowly developing but long lasting relaxation of the precontracted trachealis. Adding propranolol to the tracheal perfusate reversed the sustained relaxations of the trachealis (TT). Note the modest effects on pulmonary inflation pressure (PIP) and that little if any change in arterial blood pressure (MABP) was induced by capsaicin. B, time course of capsaicin-induced relaxation evoked in control preparations (n = 10) and in preparations in which 2 μm propranolol (n = 7) or 2 μm TTX (n = 4) were added directly to the tracheal perfusate. Time 0 indicates the onset of capsaicin inhalation. The data are expressed as the mean ± s.e.m. of the maximum relaxation of the trachealis. *Maximum relaxant response produced 6 min after initiating the capsaicin challenge was reduced in animals pretreated with either TTX or propranolol (P < 0.01).
Table 1.
Modulation of nebulized capsaicin induced, reflex-mediated relaxations of the guinea-pig trachealis in situ
| Treatment | n | Capsaicin-induced increase in PIP (%) | Reversal of histamine contraction (%) |
|---|---|---|---|
| Control | 13 | 67 ± 10 | −63 ± 7 |
| 2 μm propranolol | 7 | 71 ± 22 | −10 ± 9* |
| Sympathectomy | 5 | 51 ± 19 | −6 ± 4* |
| T1–T4 dorsal rhizotomy | 4 | 51 ± 17 | −9 ± 5* |
| Hexamethonium (4 mg kg−1i.v.) | 3 | 44 ± 17 | 2 ± 4* |
| Neurokinin receptor antagonists | 5 | 30 ± 6* | −3 ± 6* |
The trachealis was precontracted with histamine (10–50 μm) and relaxations were evoked reflexively by nebulizing capsaicin (10–100 μm) for up to 6 min. The concentration of histamine and capsaicin used and the duration of the capsaicin challenges did not differ amongst the treatment groups (not shown). The vehicle for the neurokinin receptor antagonists and sham dorsal rhizotomy had no effect on the capsaicin induced responses (see Fig. 8). The results are presented as the mean+-sem percentage increase in pulmonary inflation pressure (PIP) and as a mean+-sem percentage reversal of the histamine-induced contractions. For these experiments, propranolol was added directly to the tracheal perfusate while the neurokinin antagonists CP99994, SR48968 and SB223412 were administered intravenously (1 mg kg−1 each). An asterisk
indicates that the treatment resulted in a statistically significant reduction in the response relative to the corresponding control value (P < 0.05). See text for further details.
Capsaicin inhalation in these vagotomized animals evoked no cardiovascular effects during the initial phase of the challenge that could be attributed to adrenergic nerves, as neither mean arterial blood pressure (MABP; 49 ± 2 versus 57 ± 5 mmHg) nor heart rate (HR; 256 ± 7 versus 245 ± 6 beats min−1) increased significantly during the first 5 min after initiating the capsaicin challenge in control preparations (n = 10). The lack of effect of inhaled capsaicin on HR or ABP and the effects of sympathetic denervation of the trachea on the capsaicin-evoked, catecholamine-dependent relaxations of the trachealis argue for a sympathetic nerve-dependent reflex with little contribution of circulating catecholamines to this initial response. Indeed, in one animal in which the adrenal glands were removed at the outset of the experiment, 20 μm capsaicin inhalation for 3 min induced a relaxation of the trachea that peaked at 74% of the maximum relaxation after just 4 min. The effects of adding tetrodotoxin (TTX) to the tracheal perfusate also argues for an airway sympathetic nerve-mediated reflex. TTX substantially reduced the magnitude and delayed the onset of the relaxations evoked by capsaicin inhalation. In time course studies, the peak relaxant effect evoked by capsaicin in control animals averaged 61 ± 8% and was achieved within 6 min of initiating the capsaicin challenge (n = 10) whereas the average peak relaxation evoked by capsaicin following TTX pretreatment was 34 ± 22% of the maximum and was achieved at 9 min. At 6 min, relaxations evoked by capsaicin inhalation in the presence of TTX averaged only 14 ± 3% of the maximum (Fig. 5B).
Capsaicin inhalation invariably increased pulmonary inflation pressure (PIP) secondary to axon reflex-mediated, tachykinin-dependent bronchospasm (Thompson & Sheppard, 1988). In control preparations, the maximum increase in PIP averaged 78 ± 7% over baseline (n = 10). The increases in PIP evoked by capsaicin typically preceded the relaxations measured in the trachealis and the magnitude of the tracheal relaxations was highly correlated (r2 = 0.91; P < 0.01) with the increases in PIP. It is thus possible that the sympathetic-adrenergic nerve-mediated reflex effects initiated by inhaled capsaicin occur secondary to a mechanical effect induced by the bronchospasm (or as a consequence of the bronchospasm). However, when bronchospasm (54 ± 23% increase in PIP) was evoked by inhalation of 10 μm NKA instead of capsaicin, little or no relaxant response was evoked (4 ± 3% of maximum relaxation; n = 3). Second, the potency of NKA as a bronchoconstrictor when administered intravenously was potentiated by only ∼2-fold following pretreatment with propranolol (1 mg kg−1 i.v.), whereas propranolol increased the potency of i.v. capsaicin at evoking bronchospasm 10- to 30-fold (Fig. 6).
Figure 6. Effects of propranolol on bronchospasm evoked by intravenously administered neurokinin A (NKA) and capsaicin in anaesthetized guinea-pigs.
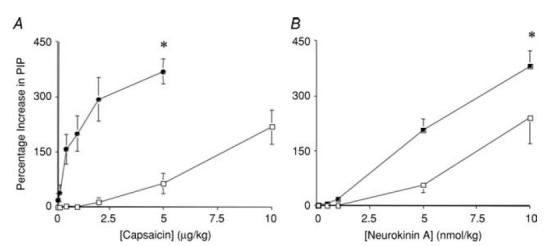
A, capsaicin (1–10 μg kg−1) or B, NKA (1–10 nmol kg−1) were administered intravenously in cumulatively increasing concentrations in the absence (□) and presence (•) of propranolol (2 mg kg−1 i.v.). Pulmonary inflation pressure (PIP) was monitored to assess bronchospasm. The results are presented as the mean ± s.e.m. of 4–8 experiments. *Maximum increase in PIP produced by either capsaicin or NKA was increased in the presence of propranolol relative to that evoked by the same doses of capsaicin or NKA in control animals (P < 0.05). Propranolol increased the potency of capsaicin by more than 10-fold (the dose of capsaicin increasing PIP by 100% (PD100) averaged 6.9 ± 1.0 and 0.6 ± 0.2 μg kg−1 in the absence and presence of propranolol, respectively; P < 0.01). By contrast, propranolol only modestly decreased the PD100 for NKA (6.1 ± 1.3 and 2.9 ± 0.5 nmol kg−1 in the absence and presence of propranolol, respectively; P < 0.05).
Adding propranolol to the tracheal perfusate in control preparations increased the magnitude of the histamine-induced contractions by 14 ± 4% (range, 0–36%). This tonic catecholamine-dependent effect was not unique to the airways, as intravenous administration of propranolol (2 mg kg−1) also slowed heart rate (269 ± 6 versus 201 ± 4 beats min−1 before and after propranolol administration, respectively; n = 14) and decreased MABP (49 ± 2 versus 42 ± 2 mmHg before and after propranolol administration, respectively; n = 14). Evidence that circulating catecholamines contribute to this basal β-adrenoceptor-mediated tone in the airways was apparent in preparations pretreated with TTX (n = 2) or in a preparation in which the sympathetic nerves innervating the trachea were cut (n = 1). In these three preparations, propranolol increased the histamine-induced contractions by 15 ± 9% (range, 0–32%). Circulating catecholamines may also mediate part of the late phase of the response to inhaled capsaicin in control preparations and most if not all of the modest relaxations evoked by capsaicin following addition of TTX directly to the tracheal perfusate. Thus, although blood pressure did not increase significantly during the first 5 min of the capsaicin challenge, by 6 min MABP began to increase significantly (36 ± 2% increase; n = 10). This may have occurred secondary to the progressively increasing airways obstruction evoked by the capsaicin and the coincident effects on arterial blood gases. Arterial PCO2 rose from 37 ± 1 mmHg to 46 ± 3 mmHg, while PO2 and O2 saturation fell from 121 ± 3 mmHg and 98 ± 0% to 43 ± 9 mmHg and 67 ± 13%, respectively, after 3–5 min of 30 μm capsaicin inhalation (which evoked a 109 ± 27% increase in PIP; n = 3). Shutting the ventilator off for 90 s produced profound alterations in blood gases (PCO2, 58 ± 3 mmHg; PO2, 23 ± 4 mmHg; O2 saturation, 29 ± 8%). Coincident with these effects on blood gases, MABP increased by 70 ± 18% over baseline (n = 6) and marked relaxations of the precontracted trachealis were evoked (59 ± 12% of the maximum relaxation; n = 6; Fig. 7). Adding propranolol to the tracheal perfusate blocked the tracheal relaxations (15 ± 8% of the maximum relaxation) but had no effect on the increase in MABP (60 ± 16% increase; n = 6). By contrast, in a preparation pretreated with TTX, 25 μm capsaicin inhalation evoked a 40% increase in PIP but no effect on the trachealis and no effects on MABP, while shutting the ventilator off for 90 s evoked a 78% reversal of the histamine-induced contraction and a 33% increase in MABP.
Figure 7. Representative trace of asphyxia-induced reflex-mediated relaxations of the trachealis.
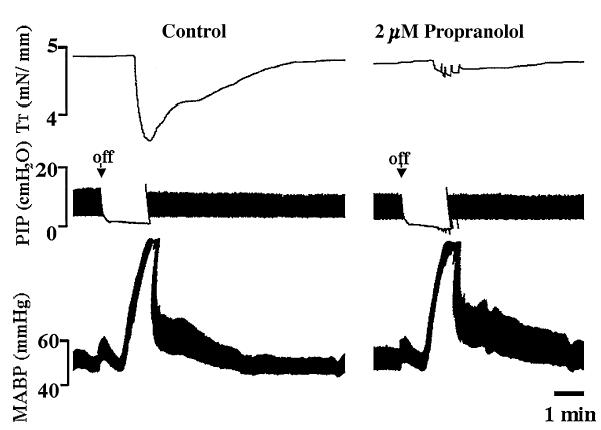
The trachealis was precontracted with histamine. Shutting the ventilator off for 90 s evoked a pronounced but transient reversal of tracheal tone (59 ± 12% of the maximum relaxation; n = 6). Unlike the relaxations evoked by capsaicin inhalation, the relaxations initiated by the asphyxia were accompanied by pronounced increases in MABP (70 ± 18% increase). Adding propranolol to the tracheal perfusate blocked the relaxations (15 ± 8% of the maximum relaxation) but was without effect on the evoked increase in MABP. Trace is reprsentative of 6 similar experiments. See text for further details.
Identification of the afferent nerves regulating the sympathetic nerve-mediated response
Bilateral dorsal rhizotomy (T1–T4) prior to capsaicin inhalation essentially abolished the subsequently evoked reflex relaxation of the trachea (9 ± 5% of the maximum relaxation, n = 4; Fig. 8, Table 1). Sham operation in two animals did not prevent the subsequently evoked relaxation. Based on the effects of the dorsal rhizotomies and our immunohistochemical analyses, we subsequently evaluated the role of tachykinins on the reflex relaxations evoked. As mentioned above, NKA administered by inhalation or intravenously failed to mimic the effects of capsaicin challenge, so we assumed that any role of tachykinins in mediating this reflex are confined to actions in the spinal cord. To address this hypothesis, we treated animals with a mixture of NK1, NK2 and NK3 receptor antagonists (each at 1 mg kg−1 i.v.), prior to capsaicin application (as described in Methods, these antagonists were always present in the tracheal perfusate in all of the experiments carried out in this study). The neurokinin receptor antagonists nearly abolished the reflex relaxation of the trachea (3 ± 7% of the maximum relaxation, n = 5, Fig. 8, Table 1). The vehicles for these antagonists were without effect on the capsaicin-induced reflex relaxations (69 ± 20% of the maximum relaxation, n = 3).
Figure 8. Effects of dorsal rhizotomy or tachykinin receptor antagonism on capsaicin-induced, sympathetic nerve-mediated reflex relaxations of the trachealis.
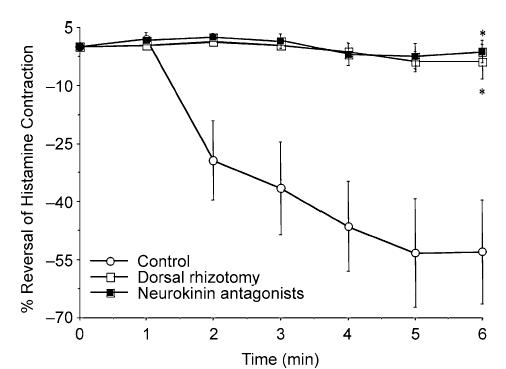
Sympathetic nerve-mediated reflexes were evoked by capsaicin inhalation. Prior to initiating the challenges, animals underwent bilateral dorsal rhizotomy (T1–T4) to disrupt the spinal afferent nerves innervating the airways and lungs (n = 4), sham rhizotomy (n = 2) or were pretreated intravenously with the neurokinin receptor antagonists CP99994, SR48968 and SB223412 (1 mg kg−1 each; n = 5) or the vehicle for these antagonists (n = 3). The responses evoked following sham rhizotomy and vehicle pretreatment were not different and were therefore pooled in the mean data presented. The results are expressed as the mean ± s.e.m. of 4–5 experiments. *Maximum relaxant response produced 6 min after initiating the capsaicin challenge was reduced in animals pretreated with the neurokinin receptor antagonists or undergoing dorsal rhizotomy relative to that obtained in control animals (P < 0.01).
Vagal afferent nerves also regulate airway sympathetic nerve activity. In preparations with both vagus nerves cut, stimulation (10 Hz, 8 V, 10 s train) of the rostral cut ends of the vagus nerves evoked relaxations of the trachealis (46 ± 6% of maximum relaxation; n = 12). The onset of these relaxations was considerably delayed, beginning 12 ± 2 s after initiating the stimulation. Variable, statistically insignificant effects on heart rate but highly reproducible alterations in blood pressure accompanied the vagally mediated relaxations of the trachea (Fig. 9). In 8 of 12 preparations, blood pressure initially fell from 54 ± 4 to 47 ± 3 mmHg, and then rose transiently to 63 ± 5 mmHg, returning to basal levels within 1–2 min of the stimulation. In the remaining four preparations studied, blood pressure either only increased or decreased transiently in response to vagus nerve stimulation (n = 2 each). Severing the vagus nerves again, rostral to the electrodes, abolished all of these responses (n = 2). Adding propranolol to the tracheal perfusate also essentially abolished the relaxations of the trachealis (94 ± 3% reduction) while having no effect on the alterations in blood pressure evoked by stimulation of the rostral cut end of the vagus nerves (n = 5).
Figure 9. Representative trace of reflex-mediated relaxations of the trachealis evoked by electrically stimulating the rostral ends of the cut vagus nerves.
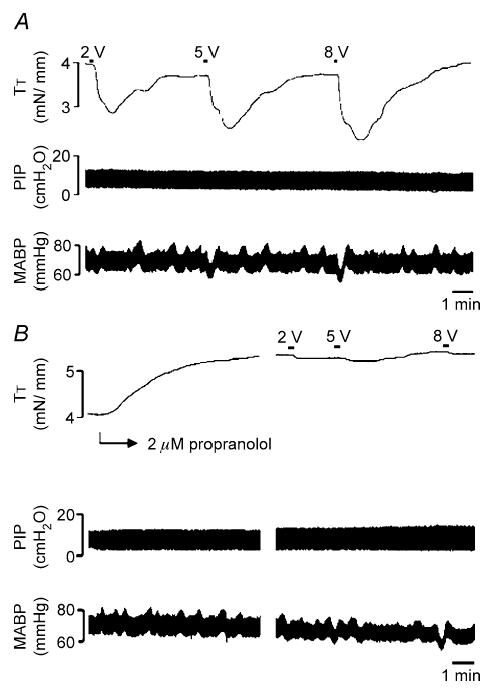
A, the trachealis was precontracted with histamine. When the histamine contraction had stabilized, electrical stimulation (10 Hz, 10 s train, 2–10 V) of the rostral cut ends of the vagus nerves evoked pronounced relaxations of the trachealis. These relaxations were slow in onset, typically starting after the 10 s stimulus train had terminated. Unlike the relaxations evoked by capsaicin inhalation, apnoea or sympathetic nerve stimulation, the relaxations evoked by stimulating the rostral ends of the cut vagus nerve were accompanied by a characteristic fall in blood pressure followed by a rebound increase in pressure that coincided with the onset of the tracheal relaxation. B, adding propranolol to the tracheal perfusate abolished these relaxations. Also note the marked increase in the histamine-induced contractions produced by adding propranolol to the tracheal perfusate. This trace is representative of 12 similar experiments. See text for further details.
Discussion
The results of the present study provide the first detailed description of the reflex pathways regulating airway sympathetic nerves. The afferent nerves regulating this reflex arise from thoracic dorsal root ganglia and are primarily capsaicin sensitive and express the neuropeptide substance P. Activation of vagal afferent nerves may also initiate these sympathetic reflexes. The efferent limb of this reflex includes both thoracic and superior cervical sympathetic ganglia and is mediated by adrenergic nerves. The receptors mediating the airway smooth muscle relaxation are both β1- and β2-adrenoceptors. Finally, the data indicate that reflex-mediated activation of airway sympathetic nerves can be regulated independent of cardiac and vascular sympathetic nerves. We speculate that airway sympathetic efferent nerves and spinal afferent nerves act in concert with as well as independent from the parasympathetic and vagal afferent nerves to control airway smooth muscle tone and thus airway caliber.
Sympathetic innervation of airway smooth muscle
As has been well described in cats, dogs and guinea-pigs (Cabezas et al. 1971; Diamond & O'Donnell, 1980; Russell, 1980; Canning & Undem, 1993), sympathetic nerve stimulation evoked marked relaxations of the guinea-pig trachealis in situ. The frequency dependence and kinetics of the relaxations were identical to that previously described, and their sensitivity to propranolol confirmed the sympathetic and adrenergic nature of these responses. Comparable vasoconstrictor effects in the bronchial circulation have been described upon sympathetic nerve stimulation in cats and pigs (Hyman et al. 1990; Franco-Cereceda et al. 1995). These observations and many in vitro physiological and morphological studies suggest that sympathetic adrenergic innervation of the airways and lungs is common to all vertebrate species.
The sympathetic nerves innervating the trachea are derived from both the cervical as well as the thoracic (stellate) sympathetic ganglia (Kummer et al. 1992; B. J. Canning, unpublished observations). That stimulating either the rostral or caudal cut ends of the cervical sympathetic trunk evoked relaxations of the trachealis that were blocked by hexamethonium is consistent with this hypothesis. Innervation of the intrathoracic airways is primarily derived from the thoracic sympathetic ganglia. Immunohistochemical analyses and circumstantial evidence gathered in functional studies suggests that a subpopulation of the postganglionic thoracic sympathetic nerves innervating the airways are non-adrenergic, utilizing the peptide vasoactive intestinal peptide (VIP and related peptides) and/or the gaseous transmitter nitric oxide (NO, synthesized from arginine by the neuronal isoform of NO synthase) to relax airway smooth muscle (Bowden & Gibbins, 1992; Fischer et al. 1996; Matsumoto et al. 1997). In our pharmacological analyses, we found little evidence for a prominent non-adrenergic component of the sympathetic response in the trachea, as propranolol reduced the nerve-mediated relaxations (evoked electrically or reflexively) by an average of >95%. Comparable results have been reported previously (Diamond & O'Donnell, 1980; Canning & Undem, 1993).
The airway smooth muscle of guinea-pigs expresses both β1- and β2-adrenoceptors. Based on the effects of chemical sympathectomy with 6-hydroxydopamine or reserpine and the resulting supersensitivity to β1- but not β2-adrenoceptor selective agonists, it has been proposed that β1-adrenoceptors on airway smooth muscle are ‘innervated’ and activated by neuronally released catecholamines, while the β2-adrenoceptors are not innervated but activated by circulating catecholamines and inhaled β2-adrenoceptor selective agonists used for treating pulmonary disease (Broadley et al. 1986; Grassby & Broadley, 1986). This hypothesis has not been directly tested, however, and can only be addressed using preparations such as ours in which the sympathetic nerves can be directly and selectively stimulated. We found that neither practolol nor ICI118551, when administered at concentrations selective for antagonizing β1- and β2-adrenoceptors, respectively, substantially blocked the sympathetic nerve-mediated relaxations of the trachealis (20–30% reduction). When administered simultaneously, however, the β1- and β2-adrenoceptor antagonists markedly reduced the sympathetic nerve-evoked relaxations. These data suggest that both β1- and β2-adrenoceptors mediate airway smooth muscle relaxation evoked by sympathetic-adrenergic nerve activation. Comparable results have been reported in studies of the sympathetic innervation of the pulmonary circulation in cats (Hyman et al. 1990).
Afferent nerves reflexively regulating airway sympathetic nerves
Retrograde neuronal tracing studies described here and previously reveal that spinal afferent nerves arising from thoracic DRG innervate the airways and lungs of several species including guinea-pigs (Saria et al. 1985; Kummer et al. 1992; Plato et al. 2006). Functional studies in rabbits, dogs and primates also suggest that spinal afferent nerves innervate the airways and lungs (Kostreva et al. 1975, 1978; Wang et al. 2003; Soukhova et al. 2003). We found that the majority of spinal afferent nerves innervating the airways and lungs express the neuropeptide substance P and were activated by capsaicin. Following vagotomy to prevent airway parasympathetic reflexes in the trachea (both contractions and relaxations; Mazzone & Canning, 2002a), capsaicin inhalation induced a marked relaxation of the trachealis. The effects of propranolol, TTX and sympathetic denervation of the trachea on this reflex provide conclusive evidence that sympathetic adrenergic nerves reflexively mediate these relaxations. The effects of dorsal rhizotomy suggest that the reflex initiated by capsaicin inhalation may be mediated by activation of the capsaicin-sensitive nerves innervating the airways and lungs.
We speculate that reflexes comparable to those we measured in the trachea regulate airway smooth muscle and vascular tone throughout the tracheobronchial tree. The effects of propranolol on the bronchoconstrictor response to intravenously administered capsaicin (10- to 30-fold increase in potency) are consistent with this notion. The data also suggest that the effects of capsaicin are mediated directly and are not dependent upon indirect effects (e.g. bronchospasm). Thus, NKA inhalation did not mimic the effects of inhaled capsaicin, and propranolol only modestly altered the response to intravenously administered NKA.
A small percentage of retrogradely labelled DRG neurones studied were capsaicin insensitive or expressed immunoreactivity for neurofilament, suggesting that a subpopulation of myelinated spinal afferent nerves innervate the airways and lungs. Their role in regulating airway autonomic nerve activity and their sensitivity to chemical and mechanical stimuli has not yet been assessed. We did, however, find evidence for vagal regulation of airway sympathetic nerves. Stimulating the rostral cut ends of the vagus nerves evoked pronounced relaxations of the trachealis. It is unclear what vagal afferent nerves were driving this response but it is likely that bronchopulmonary vagal afferent nerves play some role (Barman & Gebber, 1976; Bachoo & Polosa, 1987; Daly & Kirkman, 1988; Yu et al. 1990; Seals et al. 1993; Habler et al. 1994; St Croix et al. 1999; Huang et al. 2000; Zhou et al. 2002). Activation of bronchopulmonary afferent nerves can also initiate airway parasympathetic reflexes, both cholinergic contractions and non-adrenergic, non-cholinergic relaxations (Mazzone & Canning, 2002a). These coincident sympathetic and parasympathetic reflexes may act in concert or in parallel to maintain a stable airway caliber. Dysfunction or dysregulation of any of these components may lead to the airways obstruction associated with asthma and chronic obstructive pulmonary disease (see below).
Selective reflex regulation of airway sympathetic nerves and role in regulating airway caliber
The pronounced relaxations evoked reflexively by inhaled capsaicin in the vagotomized animals were prevented by propranolol and occurred independent of any marked changes in heart rate or blood pressure. These observations suggest that the spinal afferent nerves innervating the airways can selectively regulate airway sympathetic adrenergic nerve activity. This contrasts with vagal afferent nerves, which tonically influence basal sympathetic nerve activity and can reflexively modulate sympathetic nerves innervating multiple organs (Yu et al. 1990; Foreman, 1999; present study). This selective regulation of airway sympathetic nerves adds further evidence against historical notions regarding regulation of sympathetic nerve activity and its role in both vegetative and defensive reflex responses (Peterson et al. 1983; Morrison, 2001; Jänig & Habler, 2003).
Stimulating either the caudal or rostral cut ends of the cervical sympathetic trunks evoked hexamethonium-sensitive relaxations of the trachealis. This confirms previous studies indicating that at least a subpopulation of the preganglionic sympathetic nerves innervating the superior cervical ganglia also innervate neurones in the stellate ganglia (Lichtman et al. 1980). It is possible (but untested) that a single population of preganglionic neurones selectively innervates postganglionic neurones in both the stellate and superior cervical ganglia that project to the airways. Given that we observed reflex-mediated, sympathetic nerve-dependent airway smooth muscle relaxations either coincident with or independent of changes in heart rate or blood pressure, it is also possible that multiple and differentially regulated subpopulations of preganglionic sympathetic nerves regulate sympathetic outflow to the airways. Alternatively, airway afferent nerves may selectively regulate sympathetic outflow to the airways through modulatory actions in the spinal cord.
We found little evidence for the involvement of circulating catecholamines in mediating the response to inhaled capsaicin. Only when blood gases were compromised secondary to bronchospasm or asphyxia could a marked systemic effect be measured. However, there are many stimuli that initiate a surge in circulating catecholamines (e.g. histamine, allergen, asphyxia), so the role of adrenal regulation of airway caliber cannot be discounted (Diamond, 1972; Colebatch & Engel, 1974; Underwood et al. 1997). Circulating catecholamines may also tonically regulate airway smooth muscle tone and reactivity (Larson, 1985; Weinmann et al. 1985; Knox et al. 1992; Fontana et al. 2002; Mazzone & Canning, 2002c). The driving force behind this basal activation of β-adrenoceptors in the airways is unclear.
The relative importance of sympathetic-adrenergic nerves in regulating airway tone is both species dependent and dependent upon the physiological conditions experienced by the animal (Canning & Fischer, 2001). In dogs, adrenergic nerves are the only functional relaxant nerves innervating the airways (Russell, 1980). However, in most other species including humans, non-cholinergic parasympathetic nerves subserve a primary role in mediating airway smooth muscle relaxation. In fact, although sympathetic-adrenergic innervation has been clearly demonstrated in human pulmonary arteries and circumstantial evidence suggests that adrenergic nerves also innervate the airway smooth muscle and glands of human airways and lungs (Gothert & Hentrich, 1985; Davis & Kannan, 1987; Pack et al. 1988; Martinez et al. 1995), the β-adrenoceptor antagonist propranolol and/or high thoracic epidural anaesthesia has little or no effect on resting airway mechanics and no effect on airways responsiveness to constricting stimuli in healthy human subjects (Laitinen et al. 1976; Habib et al. 1979; Sterk et al. 1985; Groeben et al. 1994, 1995). Propranolol is also without effect on nerve-mediated responses evoked in vitro in human and non-human primate airway preparations (Richardson & Beland, 1976; Middendorf & Russell, 1980; Canning & Fischer, 2001). This is not due to insufficient β-adrenoceptor expression by the airway smooth muscle, as β2-receptor agonists have a profound bronchodilating effect in the airways of all human subjects, and potently and effectively relax isolated preparations of human airway smooth muscle from all patient populations (Goldie et al. 1990). Rather, the data suggest that the airways of healthy human subjects are either sparsely innervated and/or only modestly regulated by adrenergic nerves. Recruitment of these sympathetic reflexes in disease, perhaps to counteract exaggerated parasympathetic cholinergic tone, or to compensate for dysfunctional parasympathetic non-cholinergic nerves, may help regulate airway caliber (Sly et al. 1967; Grieco & Pierson, 1971; Molho et al. 1977; Larson, 1985; Sands et al. 1985; Ind et al. 1989; Noppen & Vincken, 1996; Heindl et al. 2001; Schilero et al. 2005).
Acknowledgments
This research was supported by grants NS 22069 and HL 32273 from the National Institutes of Health (NIH, Bethesda, MA, USA). S.M. is an NH and MRC of Australia CJ Martin fellow (no. 007188). The authors thank Ms Nanako Mori for expert technical assistance.
References
- Bachoo M, Polosa C. Properties of the inspiration-related activity of sympathetic preganglionic neurones of the cervical trunk in the cat. J Physiol. 1987;385:545–564. doi: 10.1113/jphysiol.1987.sp016507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Don H. Catecholamines abolish vagal but not acetylcholine tone in the intact cat trachea. J Appl Physiol. 1987;63:2490–2498. doi: 10.1152/jappl.1987.63.6.2490. [DOI] [PubMed] [Google Scholar]
- Baker DG, McDonald DM. Distribution of catecholamine-containing nerves on blood vessels of the rat trachea. J Comp Neurol. 1992;325:38–46. doi: 10.1002/cne.903250104. [DOI] [PubMed] [Google Scholar]
- Barman SM, Gebber GL. Basis for synchronization of sympathetic and phrenic nerve discharges. Am J Physiol. 1976;231:1601–1607. doi: 10.1152/ajplegacy.1976.231.5.1601. [DOI] [PubMed] [Google Scholar]
- Bowden JJ, Gibbins IL. Vasoactive intestinal peptide and neuropeptide Y coexist in non-noradrenergic sympathetic neurons to guinea pig trachea. J Auton Nerv Syst. 1992;38:1–19. doi: 10.1016/0165-1838(92)90211-x. [DOI] [PubMed] [Google Scholar]
- Broadley KJ, Chess-Williams RG, Grassby PF. A physiological basis for subclassifying β-adrenoceptors examined by chemical sympathectomy of guinea-pigs. J Physiol. 1986;373:367–378. doi: 10.1113/jphysiol.1986.sp016053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadstone RV, LeBlanc PH, Derksen FJ, Robinson NE. In vitro responses of airway smooth muscle from horses with recurrent airway obstruction. Pulm Pharmacol. 1991;4:191–202. doi: 10.1016/0952-0600(91)90011-q. [DOI] [PubMed] [Google Scholar]
- Cabezas GA, Graf PD, Nadel JA. Sympathetic versus parasympathetic nervous regulation of airways in dogs. J Appl Physiol. 1971;31:651–655. doi: 10.1152/jappl.1971.31.5.651. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Fischer A. Neural regulation of airway smooth muscle tone. Respir Physiol. 2001;125:113–127. doi: 10.1016/s0034-5687(00)00208-5. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ. Relaxant innervation of the guinea-pig trachealis: demonstration of capsaicin-sensitive and -insensitive vagal pathways. J Physiol. 1993;460:719–739. doi: 10.1113/jphysiol.1993.sp019496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch HJ, Engel LA. Constriction of the lung by histamine before and after adrenalectomy in cats. J Appl Physiol. 1974;37:798–805. doi: 10.1152/jappl.1974.37.6.798. [DOI] [PubMed] [Google Scholar]
- Daly MD, Kirkman E. Cardiovascular responses to stimulation of pulmonary C fibres in the cat: their modulation by changes in respiration. J Physiol. 1988;402:43–63. doi: 10.1113/jphysiol.1988.sp017193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Kannan MS. Sympathetic innervation of human tracheal and bronchial smooth muscle. Respir Physiol. 1987;68:53–61. doi: 10.1016/0034-5687(87)90076-4. [DOI] [PubMed] [Google Scholar]
- Diamond L. Potentiation of bronchomotor responses by beta adrenergic antagonists. J Pharmacol Exp Ther. 1972;181:434–445. [PubMed] [Google Scholar]
- Diamond L, O'Donnell M. A nonadrenergic vagal inhibitory pathway to feline airways. Science. 1980;208:185–188. doi: 10.1126/science.7361114. [DOI] [PubMed] [Google Scholar]
- Fischer A, Mayer B, Kummer W. Nitric oxide synthase in vagal sensory and sympathetic neurons innervating the guinea-pig trachea. J Auton Nerv Syst. 1996;56:157–160. doi: 10.1016/0165-1838(95)00085-2. [DOI] [PubMed] [Google Scholar]
- Fontana GA, Pantaleo T, Lavorini F, Bongianni F, Mannelli M, Bridge PD, Pistolesi M. Handgrip-induced airway dilation in asthmatic patients with bronchoconstriction induced by MCh, inhalation. J Appl Physiol. 2002;93:1723–1730. doi: 10.1152/japplphysiol.00326.2002. [DOI] [PubMed] [Google Scholar]
- Foreman RD. Mechanisms of cardiac pain. Annu Rev Physiol. 1999;61:143–167. doi: 10.1146/annurev.physiol.61.1.143. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Matran R, Alving K, Lundberg JM. Sympathetic vascular control of the laryngeo-tracheal, bronchial and pulmonary circulation in the pig: evidence for non-adrenergic mechanisms involving neuropeptide Y. Acta Physiol Scand. 1995;155:193–204. doi: 10.1111/j.1748-1716.1995.tb09964.x. [DOI] [PubMed] [Google Scholar]
- Goldie RG, Paterson JW, Lulich KM. Adrenoceptors in airway smooth muscle. Pharmacol Ther. 1990;48:295–322. doi: 10.1016/0163-7258(90)90051-3. [DOI] [PubMed] [Google Scholar]
- Gothert M, Hentrich F. Identification of presynaptic β2-adrenoceptors on the sympathetic nerve fibres of the human pulmonary artery. Br J Pharmacol. 1985;85:933–941. doi: 10.1111/j.1476-5381.1985.tb11094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassby PF, Broadley KJ. Responses mediated via β-1 adrenoceptors but not β-2 adrenoceptors exhibit supersensitivity after chronic reserpine pretreatment. J Pharmacol Exp Ther. 1986;237:950–958. [PubMed] [Google Scholar]
- Grieco MH, Pierson RN., Jr Mechanism of bronchoconstriction due to beta adrenergic blockade. Studies with practolol, propranolol, and atropine. J Allergy Clin Immunol. 1971;48:143–152. doi: 10.1016/0091-6749(71)90009-1. [DOI] [PubMed] [Google Scholar]
- Groeben H, Schwalen A, Irsfeld S, Lipfert P, Hopf HB. Pulmonary sympathetic denervation does not increase airway resistance in patients with chronic obstructive pulmonary disease (COPD) Acta Anaesthesiol Scand. 1995;39:523–526. doi: 10.1111/j.1399-6576.1995.tb04112.x. [DOI] [PubMed] [Google Scholar]
- Groeben H, Schwalen A, Irsfeld S, Tarnow J, Lipfert P, Hopf HB. High thoracic epidural anesthesia does not alter airway resistance and attenuates the response to an inhalational provocation test in patients with bronchial hyperreactivity. Anesthesiology. 1994;81:868–874. doi: 10.1097/00000542-199410000-00014. [DOI] [PubMed] [Google Scholar]
- Habib MP, Pare PD, Engel LA. Variability of airway responses to inhaled histamine in normal subjects. J Appl Physiol. 1979;47:51–58. doi: 10.1152/jappl.1979.47.1.51. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Jänig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994;43:567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Heindl S, Lehnert M, Criee CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Respir Crit Care Med. 2001;164:597–601. doi: 10.1164/ajrccm.164.4.2007085. [DOI] [PubMed] [Google Scholar]
- Huang WX, Yu Q, Cohen MI. Fast (3 Hz and 10 Hz) and slow (respiratory) rhythms in cervical sympathetic nerve and unit discharges of the cat. J Physiol. 2000;523:459–477. doi: 10.1111/j.1469-7793.2000.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AL, Lippton HL, Kadowitz PJ. Analysis of pulmonary vascular responses in cats to sympathetic nerve stimulation under elevated tone conditions. Evidence that neuronally released norepinephrine acts on alpha 1-, alpha 2-, and beta 2-adrenoceptors. Circ Res. 1990;67:862–870. doi: 10.1161/01.res.67.4.862. [DOI] [PubMed] [Google Scholar]
- Ind PW, Dixon CM, Fuller RW, Barnes PJ. Anticholinergic blockade of beta-blocker-induced bronchoconstriction. Am Rev Respir Dis. 1989;139:1390–1394. doi: 10.1164/ajrccm/139.6.1390. [DOI] [PubMed] [Google Scholar]
- Jänig W, Habler HJ. Neurophysiological analysis of target-related sympathetic pathways – from animal to human: similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Knox AJ, Campos-Gongora H, Wisniewski A, MacDonald IA, Tattersfield AE. Modification of bronchial reactivity by physiological concentrations of plasma epinephrine. J Appl Physiol. 1992;73:1004–1007. doi: 10.1152/jappl.1992.73.3.1004. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Hopp FA, Zuperku EJ, Igler FO, Coon RL, Kampine JP. Respiratory inhibition with sympathetic afferent stimulation in the canine and primate. J Appl Physiol. 1978;44:718–724. doi: 10.1152/jappl.1978.44.5.718. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Zuperku EJ, Hess GL, Coon RL, Kampine JP. Pulmonary afferent activity recorded from sympathetic nerves. J Appl Physiol. 1975;39:37–40. doi: 10.1152/jappl.1975.39.1.37. [DOI] [PubMed] [Google Scholar]
- Kummer W, Fischer A, Kurkowski R, Heym C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience. 1992;49:715–737. doi: 10.1016/0306-4522(92)90239-x. [DOI] [PubMed] [Google Scholar]
- Laitinen LA, Empey DW, Poppius H, Lemen RJ, Gold WM, Nadel JA. Effects of intravenous histamine on static lung compliance and airway resistance in normal man. Am Rev Respir Dis. 1976;114:291–295. doi: 10.1164/arrd.1976.114.2.291. [DOI] [PubMed] [Google Scholar]
- Larson K. Studies of sympatho-adrenal reactivity and adrenoceptor function in bronchial asthma. Eur J Respir Dis Supplement. 1985;141:1–52. [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurons: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Purves D, Yip JW. Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol. 1980;298:285–299. doi: 10.1113/jphysiol.1980.sp013081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JR, Burnstock G. Innervation of the lungs of the toad (Bufo marinus) II Fluorescent histochemistry of catecholamines. Comp Biochem Physiol. 1967;22:767–773. doi: 10.1016/0010-406x(67)90769-4. [DOI] [PubMed] [Google Scholar]
- Martinez C, Cases E, Vila JM, Aldasoro M, Medina P, Marco V, Lluch S. Influence of endothelial nitric oxide on neurogenic contraction of human pulmonary arteries. Eur Respir J. 1995;8:1328–1332. doi: 10.1183/09031936.95.08081328. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Aizawa H, Takata S, Inoue H, Takahashi N, Hara N. Nitric oxide derived from sympathetic nerves regulates airway responsiveness to histamine in guinea pigs. J Appl Physiol. 1997;83:1432–1437. doi: 10.1152/jappl.1997.83.5.1432. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Inoue H, Ichinose M, Ishii M, Inoue C, Sasaki H, Takishima T. Effective sites by sympathetic beta-adrenergic and vagal nonadrenergic inhibitory stimulation in constricted airways. Am Rev Respir Dis. 1985;132:1113–1117. doi: 10.1164/arrd.1985.132.5.1113. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Evidence for differential reflex regulation of cholinergic and noncholinergic parasympathetic nerves innervating the airways. Am J Respir Crit Care Med. 2002a;165:1076–1083. doi: 10.1164/ajrccm.165.8.2001121270c. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. Synergistic interactions between airway afferent nerve subtypes mediating reflex bronchospasm in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002b;283:R86–R98. doi: 10.1152/ajpregu.00007.2002. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci. 2002c;99:91–101. doi: 10.1016/s1566-0702(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Middendorf WF, Russell JA. Innervation of airway smooth muscle in the baboon: evidence for a nonadrenergic inhibitory system. J Appl Physiol. 1980;48:947–956. doi: 10.1152/jappl.1980.48.6.947. [DOI] [PubMed] [Google Scholar]
- Mitchell HW, Sparrow MP, Tagliaferri RP. Inhibitory and excitatory responses to field stimulation in fetal and adult pig airway. Pediatr Res. 1990;28:69–74. doi: 10.1203/00006450-199007000-00015. [DOI] [PubMed] [Google Scholar]
- Molho M, Kurchin A, Ohry A, Bass A, Adar R. Pulmonary functional abnormalities after upper dorsal sympathectomy. Am Rev Respir Dis. 1977;116:879–883. doi: 10.1164/arrd.1977.116.5.879. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–R698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Mustafa KY, Elkhawad AO, Bicik V, Mardini IA, Thulesius O. Adrenergic and cholinergic induced contractions of tracheal smooth muscle in the rabbit as demonstrated by a new in vivo method. Acta Physiol Scand. 1982;114:129–134. doi: 10.1111/j.1748-1716.1982.tb06961.x. [DOI] [PubMed] [Google Scholar]
- Noppen M, Vincken W. Thoracoscopic sympathicolysis for essential hyperhidrosis: effects on pulmonary function. Eur Respir J. 1996;9:1660–1664. doi: 10.1183/09031936.96.09081660. [DOI] [PubMed] [Google Scholar]
- O'Donnell SR, Saar N. Histochemical localization of adrenergic nerves in the guinea-pig trachea. Br J Pharmacol. 1973;47:707–710. doi: 10.1111/j.1476-5381.1973.tb08197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack RJ, Richardson PS, Smith IC, Webb SR. The functional significance of the sympathetic innervation of mucous glands in the bronchi of man. J Physiol. 1988;403:211–219. doi: 10.1113/jphysiol.1988.sp017246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DF, Coote JH, Gilbey MP, Futuro-Neto HA. Differential pattern of sympathetic outflow during upper airway stimulation with smoke. Am J Physiol. 1983;245:R433–R437. doi: 10.1152/ajpregu.1983.245.3.R433. [DOI] [PubMed] [Google Scholar]
- Plato M, Kummer W, Haberberger RV. Structural and neurochemical comparison of vagal and spinal afferent neurons projecting to the rat lung. Neurosci Lett. 2006;395:215–219. doi: 10.1016/j.neulet.2005.10.078. [DOI] [PubMed] [Google Scholar]
- Richardson J, Beland J. Nonadrenergic inhibitory nervous system in human airways. J Appl Physiol. 1976;41:764–771. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- Russell JA. Noradrenergic inhibitory innervation of canine airways. J Appl Physiol. 1980;48:16–22. doi: 10.1152/jappl.1980.48.1.16. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.res.85.5.457. [DOI] [PubMed] [Google Scholar]
- Sands MF, Douglas FL, Green J, Banner AS, Robertson GL, Leff AR. Homeostatic regulation of bronchomoter tone by sympathetic activation during bronchoconstriction in normal and asthmatic humans. Am Rev Respir Dis. 1985;132:993–998. doi: 10.1164/arrd.1985.132.5.993. [DOI] [PubMed] [Google Scholar]
- Saria A, Martling CR, Dalsgaard CJ, Lundberg JM. Evidence for substance P-immunoreactive spinal afferents that mediate bronchoconstriction. Acta Physiol Scand. 1985;125:407–414. doi: 10.1111/j.1748-1716.1985.tb07736.x. [DOI] [PubMed] [Google Scholar]
- Schilero GJ, Grimm DR, Bauman WA, Lenner R, Lesser M. Assessment of airway caliber and bronchodilator responsiveness in subjects with spinal cord injury. Chest. 2005;127:149–155. doi: 10.1378/chest.127.1.149. [DOI] [PubMed] [Google Scholar]
- Seals DR, Suwarno NO, Joyner MJ, Iber C, Copeland JG, Dempsey JA. Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circ Res. 1993;72:440–454. doi: 10.1161/01.res.72.2.440. [DOI] [PubMed] [Google Scholar]
- Sheller JR, Brigham KL. Bronchomotor responses of isolated sheep airways to electrical field stimulation. J Appl Physiol. 1982;53:1088–1093. doi: 10.1152/jappl.1982.53.5.1088. [DOI] [PubMed] [Google Scholar]
- Shirai M, Matsukawa K, Nishiura N, Kawaguchi AT, Ninomiya I. Changes in efferent pulmonary sympathetic nerve activity during systemic hypoxia in anesthetized cats. Am J Physiol. 1995a;269:R1404–R1409. doi: 10.1152/ajpregu.1995.269.6.R1404. [DOI] [PubMed] [Google Scholar]
- Shirai M, Matsukawa K, Nishiura N, Ninomiya I. Effects of baroreceptor reflex on efferent pulmonary sympathetic nerve activity in anesthetized cat. Am J Physiol. 1995b;268:R1078–R1083. doi: 10.1152/ajpregu.1995.268.4.R1078. [DOI] [PubMed] [Google Scholar]
- Sly RM, Heimlich EM, Busser RJ, Strick L. Exercise-induced bronchospasm: effect of adrenergic or cholinergic blockade. J Allergy. 1967;40:93–99. doi: 10.1016/0021-8707(67)90102-5. [DOI] [PubMed] [Google Scholar]
- Soukhova G, Wang Y, Ahmed M, Walker JF, Yu J. Bradykinin stimulates respiratory drive by activating pulmonary sympathetic afferents in the rabbit. J Appl Physiol. 2003;95:241–249. doi: 10.1152/japplphysiol.00582.2002. [DOI] [PubMed] [Google Scholar]
- Sterk PJ, Daniel EE, Zamel N, Hargreave FE. Limited maximal airway narrowing in nonasthmatic subjects. Role of neural control and prostaglandin release. Am Rev Respir Dis. 1985;132:865–870. doi: 10.1164/arrd.1985.132.4.865. [DOI] [PubMed] [Google Scholar]
- Szolcsanyi J, Anton F, Reeh PW, Handwerker HO. Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res. 1988;446:262–268. doi: 10.1016/0006-8993(88)90885-2. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Sheppard D. Phosphoramidon potentiates the increase in lung resistance mediated by tachykinins in guinea pigs. Am Rev Respir Dis. 1988;137:337–340. doi: 10.1164/ajrccm/137.2.337. [DOI] [PubMed] [Google Scholar]
- Underwood DC, Matthews JK, Osborn RR, Bochnowicz S, Torphy TJ. The influence of endogenous catecholamines on the inhibitory effects of rolipram against early- and late-phase response to antigen in the guinea pig. J Pharmacol Exp Ther. 1997;280:210–219. [PubMed] [Google Scholar]
- Wang Y, Soukhova G, Proctor M, Walker J, Yu J. Bradykinin causes hypotension by activating pulmonary sympathetic afferents in the rabbit. J Appl Physiol. 2003;95:233–240. doi: 10.1152/japplphysiol.00584.2002. [DOI] [PubMed] [Google Scholar]
- Wechsler ME, Grasemann H, Deykin A, Silverman EK, Yandava CN, Israel E, Wand M, Drazen JM. Exhaled nitric oxide in patients with asthma: association with NOS1 genotype. Am J Respir Crit Care Med. 2000;162:2043–2047. doi: 10.1164/ajrccm.162.6.2003089. [DOI] [PubMed] [Google Scholar]
- Weinmann GG, Spannhake EW, Bromberger-Barnea B, Menkes HA. Tonic beta-sympathetic activity in the lung periphery in anesthetized dogs. J Appl Physiol. 1985;59:979–984. doi: 10.1152/jappl.1985.59.3.979. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Action potentials in parasympathetic and sympathetic efferent fibres to the trachea and lungs of dogs and cats. J Physiol. 1966;186:56–88. doi: 10.1113/jphysiol.1966.sp008020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang A, Uchida Y, Nomura A, Iijima H, Dong F, Zhang MJ, Hasegawa S. Effects of airway inflammation on cough response in the guinea pig. J Appl Physiol. 1998;85:1847–1854. doi: 10.1152/jappl.1998.85.5.1847. [DOI] [PubMed] [Google Scholar]
- Yu J, Roberts AM, Joshua IG. Lung inflation evokes reflex dilation of microvessels in rat skeletal muscle. Am J Physiol. 1990;258:H939–H945. doi: 10.1152/ajpheart.1990.258.4.H939. [DOI] [PubMed] [Google Scholar]
- Zaccone G, Mauceri A, Lo Cascio P, Minniti F, Parrino V, Fasulo S. Immunohistochemical study of the innervation of pulmonary vessels and smooth muscles in the respiratory tract of two frog species. Acta Histochem. 2004;106:179–193. doi: 10.1016/j.acthis.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Gebber GL, Zhong S, Barman SM. Pathways involved in synchronization of sympathetic nerve discharge to lung inflation. Brain Res. 2002;931:107–116. doi: 10.1016/s0006-8993(02)02255-2. [DOI] [PubMed] [Google Scholar]


