Abstract
The present study investigated the influence of creatine and protein supplementation on satellite cell frequency and number of myonuclei in human skeletal muscle during 16 weeks of heavy-resistance training. In a double-blinded design 32 healthy, male subjects (19–26 years) were assigned to strength training (STR) while receiving a timed intake of creatine (STR-CRE) (n = 9), protein (STR-PRO) (n = 8) or placebo (STR-CON) (n = 8), or serving as a non-training control group (CON) (n = 7). Supplementation was given daily (STR-CRE: 6–24 g creatine monohydrate, STR-PRO: 20 g protein, STR-CON: placebo). Furthermore, timed protein/placebo intake were administered at all training sessions. Muscle biopsies were obtained at week 0, 4, 8 (week 8 not CON) and 16 of resistance training (3 days per week). Satellite cells were identified by immunohistochemistry. Muscle mean fibre (MFA) area was determined after histochemical analysis. All training regimes were found to increase the proportion of satellite cells, but significantly greater enhancements were observed with creatine supplementation at week 4 (compared to STR-CON) and at week 8 (compared to STR-PRO and STR-CON) (P < 0.01–0.05). At week 16, satellite cell number was no longer elevated in STR-CRE, while it remained elevated in STR-PRO and STR-CON. Furthermore, creatine supplementation resulted in an increased number of myonuclei per fibre and increases of 14–17% in MFA at week 4, 8 and 16 (P < 0.01). In contrast, STR-PRO showed increase in MFA only in the later (16 week, +8%) and STR-CON only in the early (week 4, +14%) phases of training, respectively (P < 0.05). In STR-CRE a positive relationship was found between the percentage increases in MFA and myonuclei from baseline to week 16, respectively (r = 0.67, P < 0.05). No changes were observed in the control group (CON). In conclusion, the present study demonstrates for the first time that creatine supplementation in combination with strength training amplifies the training-induced increase in satellite cell number and myonuclei concentration in human skeletal muscle fibres, thereby allowing an enhanced muscle fibre growth in response to strength training.
In skeletal muscle cells, satellite cells (SC) are located between the sarcolemma and the basal lamina of the muscle fibre (Mauro, 1961). Postnatal muscle growth occurs through myofibre hypertrophy, and concurrent with the increase in myofibre size muscle cells demonstrate an increased number of myonuclei. The ratio between number of myonuclei and fibre cross-sectional area has been defined as the myonuclear domain, whereby each nucleus regulates a particular volume of cytoplasm (Allen et al. 1999). The source of new myonuclei is satellite cells. These are normally in a non-proliferative quiescent state, but when stimulated during exercise, they can proliferate and provide additional myonuclei to the enlarging muscle fibres, hence playing an important role in the growth of adult skeletal muscle (Grounds, 1998, 1999; Vierck et al. 2000; Yan, 2000). In fact, several findings indicate a governing role for SC in the growth of adult skeletal muscle. When gamma irradiation is used to inactivate SC (in mice and rats), the muscle demonstrates only a limited capacity for growth (Rosenblatt & Parry, 1993; Rosenblatt et al. 1994; Barton-Davis et al. 1999). In human studies it has been shown that resistance training can increase the proportion of SC and the number of myonuclei in trained muscles (Kadi & Thornell, 2000; Roth et al. 2001; Kadi et al. 2004b), which suggests that training-induced activation of SC represents an important adaptive mechanism during muscular hypertrophy. The activation of SC in response to strength training ensures that the myonuclear domain remains constant despite the increase in fibre size as myonuclei are added during the process of cellular hypertrophy, while conversely being lost during atrophy (Allen et al. 1999).
It was recently shown that creatine affects satellite cell proliferation and differentiation in cell culture (Vierck et al. 2003), and that creatine supplementation in combination with an increased functional load induced increased satellite cell mitotic activity in rat skeletal muscles (Dangott et al. 2000). However, it has not previously been investigated whether creatine supplementation in association with strength training causes satellite cell content and myonucleus number to be up-regulated in human skeletal muscle fibres. Further, timed protein intake with strength training results in acutely elevated myofibrillar protein synthesis (Tipton et al. 2001) and longitudinal gains in muscle fibre size (Esmarck et al. 2001; Andersen et al. 2005). It is not known, however, whether this effect is mediated via enhanced activation of satelitte cells. Therefore, the aim of the present study was to investigate the influence of timed creatine and protein supplementation on myogenic satellite cell proportion and myonucleus number in human skeletal muscle fibres during 16 weeks of heavy resistance training.
Methods
Subjects
Forty-one male subjects (ranging from 19 to 28 years) gave written informed consent to participate in the study, which was approved by the Copenhagen Ethics Committee (KF 01-212/00), and performed in accordance with the Declaration of Helsinki. Three subjects dropped out of the study during the training period for reasons unrelated to the study and six subjects were not included in the present study due to problems with muscle analyses, giving a total of 32 subjects completing the study. Subjects were assigned in double blinded–randomized fashion to one of three iso-caloric supplementation regimes: creatine (STR-CRE) (n = 9), protein (STR-PRO) (n = 8), or placebo (STR-CON) (n = 8). The remaining subjects were assigned to an unsupplemented control group (CON) (n = 7) that did not train. Baseline subject characteristics are presented in Table 1. Subjects trained for 16 weeks and muscle biopsies were obtained before the start of the training period, and after 4, 8 and 16 weeks of heavy resistance strength training.
Table 1.
Physical characteristics of subjects at baseline
| Age (year) | Height (cm) | Body mass (kg) | Lean body mass (kg) | BMI (kg m−2) | |
|---|---|---|---|---|---|
| STR-CRE (n = 11) | 24.1 ± 2.0 | 183.9 ± 5.0 | 76.7 ± 7.0 | 59.4 ± 5.0 | 22.7 ± 3.0 |
| STR-PRO (n = 10) | 23.8 ± 2.2 | 182.1 ± 6.6 | 75.2 ± 9.8 | 58.4 ± 7.6 | 22.1 ± 3.2 |
| STR-CON (n = 9) | 23.4 ± 2.4 | 183.9 ± 4.5 | 74.2 ± 4.2 | 59.7 ± 3.6 | 22.0 ± 2.1 |
| CON (n = 8) | 23.8 ± 1.7 | 189.4 ± 5.7* | 80.5 ± 7.9 | 64.0 ± 4.2† | 24.0 ± 2.3 |
Values are means ± s.d. BMI: body mass index.
Significantly different from STR-CRE (P < 0.05).
Significantly different from STR-PRO (P < 0.05).
Supplementation
An overview of the supplementation regimes is presented in Table 2. Creatine monohydrate (Promax Kreatin, Promax, Denmark) mixed in water was administered four times a day during the first 7 days of the study period. After this loading phase creatine supplements were taken once a day during the remaining 15 weeks of the study (STR-CRE). Creatine supplements were given in doses of 6 g creatine and 14 g carbohydrate, mainly containing glucose (Tropical Energi Drink, Matas, Denmark). STR-PRO and STR-CON training groups received carbohydrate mixtures that were similar in taste and appearance to the creatine drink. On training days subjects received a supplement mixed in water half of which they ingested immediately prior to training and the other half after the last set of the training session. Protein supplementation consisted of 20 g protein (95% cow milk hydrolysate) (Protein – plus 95, Promax, Denmark) and 60 g carbohydrate (mainly glucose) (Tropical Energi Drink, Matas, Denmark) (STR-PRO). Likewise, on training days STR-CRE and STR-CON groups received carbohydrate supplementation consisting of 80 g carbohydrate (Tropical Energi Drink, Matas, Denmark). Consequently, carbohydrate was administered in an isocaloric manner to all three training groups to ensure that the energy intake in conjunction with training did not differ among the groups.
Table 2.
Supplementation regimes
| All day supplement | Supplement at training sessions | |
|---|---|---|
| STR-CRE | 6 g creatine monohydrate + 14 g carbohydrate | 80 g carbohydrate |
| STR-PRO | 14 g carbohydrate | 20 protein + 80 g carbohydrate |
| STR-CON | 14 g carbohydrate | 80 g carbohydrate |
| CON | No supplement | No training |
All day supplements were ingested mixed in water by all persons every day throughout the study period. Supplements at training sessions were ingested mixed in water pre (½) and post (½) each training session. Supplementation regimes were isocaloric. See Methods for more details.
Subjects were not allowed to ingest anything else besides water and the supplementation drink for 1.5 h prior to and 1.5 h following training. Supplements were iso-caloric between groups and corresponded to an energy intake of 252–306 kJ and 1440 kJ for the daily and training supplements, respectively.
Resistance training protocol
Resistance training was conducted three times per week for 16 weeks. Three different resistance-training exercises were performed for the legs: incline leg press, knee extension and hamstring curl. The resistance exercises were conducted in 3–5 sets of 6–12 repetitions (corresponding to a 6–12 RM loading). Training was periodized according to procedures previously used in our lab (Andersen & Aagaard, 2000). In brief, in the early weeks exercises involved 10–12 RM loads, followed by heavier loads of 8–10 RM in the later weeks, and very heavy loads of 6–8 RM in the final week. Upper body exercises were included primarily for motivational purposes, but were obligatory in order to keep a similar level of activity across the groups.
Total load lifted by each subject during the entire period of training did not differ among the three experimental subject groups (STR-CRE: 424181 ± 62767 kg (± s.d.), STR-PRO: 428828 ± 51789 kg, STR-CON: 403737 ± 40493 kg). Likewise, total training load in the quadriceps exercises (leg press + knee extension) did not differ among the experimental groups (STR-CRE: 348208 ± 53712 kg, STR-PRO: 362767 ± 48719 kg, STR-CON: 345028 ± 33943 kg).
Muscle biopsies
Muscle biopsies of the vastus lateralis muscle were obtained at week 0, 4, 8 (not the control group) and 16 using the needle biopsy technique. Incisions were made through the skin and muscle fascia following the administration of local anaesthesia (2–3 ml of 1% lidocaine). Following removal a piece of each muscle biopsy sample was immediately freed from blood and visible connective tissue, rapidly frozen in liquid N2, and stored at −80°C for subsequent analysis. The remaining muscle was mounted in embedding medium, frozen in isopentane, cooled to just above its freezing point in liquid N2, and stored at −80°C until analyses were performed at a later date.
Immunohistochemistry and histochemistry
Serial transverse sections, 10 μm thick, were cut using a microtome at −20°C and mounted on glass slides. For identification of fibre types the sections were mounted on glass slides and stained for ATPase activity after reincubation at pH 4.37, 4.6 and 10.3 (Brooke & Kaiser, 1970). The serial sections were visualized and analysed using an image-analysing computer program (Tema, Scanbeam, Hadsund, Denmark). Four fibre types were distinguished from the staining pattern (I, I/IIA, IIA and IIA/IIX). Muscle mean fibre cross-sectional area was calculated as [(type I fibre area ×% type I fibres) + (type II fibre area ×% type II fibres)]× 100−1. Fibres determined as type I/IIA were divided equally into the two categories. For determination of muscle fibre area, 259 ± 21 fibres (mean ± s.e.m.) were analysed per biopsy. Satellite cells were analysed using a monoclonal antibody directed against the neural cell adhesion molecule (NCAM/CD56) (Becton Dickinson, San Jose, CA, USA) (Schubert et al. 1989; Kadi, 2000; Charifi et al. 2003; Kadi et al. 2004a, b). Muscle biopsies were air dried, rinsed for 20 min in phosphate-buffered saline (PBS), and incubated for 20 min with diluted normal blocking goat serum. Sections were incubated for 2 h at 37°C with the primary mouse antibody diluted in bovine serum albumin. Slides were washed in PBS for 15 min and incubated for 30 min with the diluted biotinylated goat anti-mouse secondary antibody (Vector BA-9200, Burlingame, CA, USA). Subsequently, slides were washed for 20 min in PBS and incubated for 30 min with Vectastain ABC reagent. For the visualization of the primary antibody binding, the diaminobenzidine (DAB) substrate kit for peroxidase (Vector, SK-4100) was used. For the visualization of myonuclei, cross-sections were counterstained with Mayer's haematoxylin. Myonuclei were blue and satellite cells were stained brown. Images were acquired with an Olympus BX40 microscope (Olympus Optical, Tokyo, Japan), a Sanyo high-resolution colour charge-coupled device camera (Sanyo Electronic) and an 8-bit Matrox Meteor Framegrabber (Matrox Electronic Systems, Quebec, Canada), combined with an image-analysing computer program (Tema, Scanbeam, Hadsund, Denmark). Visualization of satellite cells and myonuclei was performed at high magnification (objective, × 40 or × 60). The use of such high magnification allowed a clear distinction between myonuclei inside the fibres and nuclei outside the fibres. Moreover, the immunohistochemical staining using DAB gave the fibre cytoplasm a slight tint that enhanced the distinction between muscle fibres and the surrounding connective tissue.
Morphometric muscle fibre analysis
In each section, the numbers of muscle fibres, satellite cells (SC), and myonuclei were counted. One person (S.O.) performed all counting and was blinded to the subject's identity until all counting was completed. From these data the following morphological variables were derived: the number of satellite cells per muscle fibre (SC/fibre), the number of myonuclei per muscle fibre (myonuclei/fibre), the total number of nuclei (SC + myonuclei), and the relative number of satellite cells as a fraction of the total number of nuclei [SC/(SC + myonuclei) × 100%] (Kadi et al. 2004a). For the satellite cell analysis 241 ± 20 fibres (mean ± s.e.m.) were analysed per biopsy, and for determination of myonuclei 66 ± 6 fibres were analysed.
Maximal muscle strength (MVC)
Maximal isometric muscle strength was measured for the knee extensors (quadriceps femoris) of the right leg as described in detail previously (Aagaard et al. 2002). In brief, maximal unilateral isometric knee extension was performed in an isokinetic dynamometer (KinCom, Chattecx Corp., Chattanooga, TN, USA). Subjects were seated 10 deg reclined in a rigid chair and firmly strapped at the distal thigh and hip. The rotational axis of the dynamometer was visually aligned to the lateral femoral epicondyle, and the lower leg was attached to the dynamometer lever arm 2 cm above the medial malleolus, with no static fixation of the ankle joint. Knee joint angle was 70 deg (0 deg = full knee extension) and hip joint angle was 80 deg (0 deg = neutral standing position). Three knee extension repetitions at maximal voluntary effort consisting of 3 s continuous maximal isometric tension were performed (60 s pause) after a standardized warm-up procedure that included a number of submaximal and maximal dynamic and isometric contractions. The highest knee extensor moment achieved during the three repetitions was chosen to represent the maximal voluntary contraction. On-line visual feedback of the instantaneous dynamometer force was provided to the subjects on a computer screen. All recorded moments were corrected for the effect of gravitation (Aagaard et al. 2002). All subjects were familiarized with the dynamometer and the procedures of the experiment on a separate occasion.
Statistics
All data are presented as means ± standard deviation (s.d.) unless otherwise stated. Group by time interactions were evaluated using two-way ANOVA, with subsequent Bonferroni corrected post hoc test. Correlation analysis was performed using the Pearson product-moment method.
Results
Number of satellite cells per fibre
SC/fibre showed a significant group by time effect (P < 0.001), increasing in STR-CRE from baseline to week 4 (+111%) and week 8 (+93%) (P < 0.01); however, at week 16 no difference could be observed compared to baseline (Fig. 1A). Likewise, time effects were observed in STR-PRO and STR-CON at week 4 (+58% and +22%, respectively), week 8 (+ 41% and +40%), and week 16 (+56% and +27%) (Table 3). A significant treatment effect was observed for STR-CRE, which was higher than STR-CON and CON at week 4 (P < 0.01), and higher than STR-PRO and STR-CON at week 8 (P < 0.01) (Fig. 1A). Likewise, STR-PRO was higher than STR-CON and CON at week 4 (P < 0.01), and higher than CON at week 4, 8 and 16 (P < 0.05). No changes were observed for CON.
Figure 1.
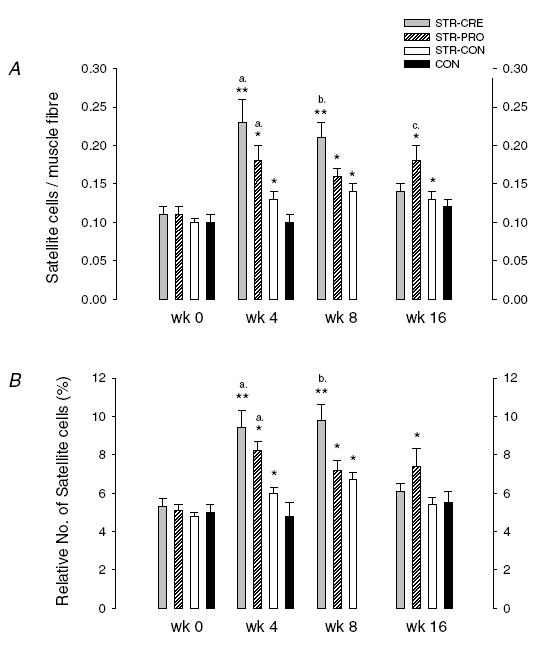
A, number of satellite cells per muscle fibre obtained prior to (week 0) and after 4, 8 and 16 weeks of strength training combined with intake of creatine (STR-CRE), protein (STR-PRO) or placebo (STR-CON). CON denotes untrained controls (no data at week 8). B, relative number of satellite cells [SC/(myonuclei + SC)] before and after 4, 8 and 16 weeks of strength training. Pre- < post-training (*P < 0.05, **P < 0.01); a, STR-CRE, STR-PRO > STR-CON, CON (P < 0.01); b, STR-CRE > STR-PRO, STR-CON, CON (P < 0.05); c, STR-PRO > STR-CON, CON (P < 0.05).
Table 3.
Satellite cells per fibre, relative number of satellite cells, myonuclei per fibre, and mean muscle fibre area (MFA) during the 16- week study period
| Week 0 | Week 4 | Week 8 | Week 16 | |
|---|---|---|---|---|
| No. of satellite cells/fibre | ||||
| STR-CRE | 0.11 ± 0.03 | 0.23 ± 0.10**a | 0.21 ± 0.07**b | 0.14 ± 0.03 |
| STR-PRO | 0.11 ± 0.03 | 0.18 ± 0.06*a | 0.16 ± 0.04* | 0.18 ± 0.06*c |
| STR-CON | 0.10 ± 0.01 | 0.13 ± 0.03* | 0.14 ± 0.03* | 0.13 ± 0.03* |
| CON | 0.10 ± 0.02 | 0.10 ± 0.03 | ND | 0.12 ± 0.04 |
| Relative no. of satellite cells (%) | ||||
| STR-CRE | 5.3 ± 1.3 | 9.4 ± 3.0**a | 9.8 ± 2.7**b | 6.1 ± 1.3 |
| STR-PRO | 5.1 ± 1.0 | 8.2 ± 1.6*a | 7.2 ± 1.6* | 7.4 ± 2.9* |
| STR-CON | 4.8 ± 0.6 | 6.0 ± 0.9* | 6.7 ± 1.2* | 5.4 ± 1.2 |
| CON | 5.0 ± 1.1 | 4.8 ± 1.9 | ND | 5.5 ± 1.7 |
| No. of myonuclei/fibre | ||||
| STR-CRE | 1.90 ± 0.23 | 2.21 ± 0.13**d | 2.13 ± 0.24* | 2.13 ± 0.17* |
| STR-PRO | 1.98 ± 0.28 | 2.05 ± 0.44 | 1.96 ± 0.27 | 2.18 ± 0.22* |
| STR-CON | 1.98 ± 0.18 | 1.94 ± 0.21 | 2.06 ± 0.35 | 2.16 ± 0.30 |
| CON | 2.01 ± 0.24 | 1.93 ± 0.22 | ND | 1.92 ± 0.29 |
| Mean muscle fibre area (μm2) | ||||
| STR-CRE | 5268 ± 646 | 5983 ± 849* | 6003 ± 1002* | 6148 ± 969* |
| STR-PRO | 5065 ± 702 | 5252 ± 1183 | 5296 ± 946 | 5461 ± 873* |
| STR-CON | 5052 ± 450 | 5752 ± 765* | 5567 ± 591 | 5635 ± 648 |
| CON | 5971 ± 690 | ND | ND | 5725 ± 404 |
STR-CRE, STR-PRO, STR-CON denotes strength training with creatine, protein or placebo supplementation, respectively. CON denotes untrained, unsupplemented controls. Values are means ± s.d. ND: no data at specific time point. Rel. no. of satellite cells: [sc(myonuclei + sc)−1× 100] (%).
Significantly different from pre (P < 0.05).
Significantly different from pre (P < 0.01).
Significantly different from STR-CON and CON (P < 0.01).
Significantly different from STR-CON and CON (P < 0.05).
Significantly different from STR-CON, CON (P < 0.05).
Significantly different from STR-PRO, STR-CON and CON (P < 0.05).
Relative number of satellite cells
The relative number of satellite cells showed a significant group by time effect, increasing in STR-CRE at week 4 (+84%) and week 8 (+99%) (P < 0.01) (Fig. 1B). Similar time effects were observed in STR-PRO and STR-CON at week 4 (+61% and +27%), and week 8 (+42% and +44%), respectively (P < 0.05), and at week 16 for STR-PRO (+50%) (P < 0.05) (Table 3). A significant treatment effect was observed for STR-CRE, which was elevated compared to STR-CON and CON at week 4 (P < 0.01) and compared to STR-PRO, STR-CON and CON at week 8 (P < 0.01). Likewise, STR-PRO was higher than STR-CON and CON at week 4 (P < 0.01) (Fig. 1B). No changes were observed for CON.
Number of myonuclei per fibre
Myonuclei/fibre showed a significant time effect, incerasing in STR-CRE at week 4 (+17%), week 8 (+13%), and week 16 (+13%) (P < 0.05) (Table 3, Fig. 2). Likewise, a significant time effect was observed from baseline in STR-PRO at week 16 (+11%) (P < 0.05) (Fig. 2). A significant treatment effect was observed for STR-CRE, which was elevated compared to STR-CON and CON at week 4 (P < 0.05) (Fig. 2). No changes were observed for STR-CON and CON.
Figure 2. Number of myonuclei per muscle fibre obtained prior to (week 0) and after 4, 8 and 16 weeks of strength training combined with intake of creatine (STR-CRE), protein (STR-PRO) or placebo (STR-CON).
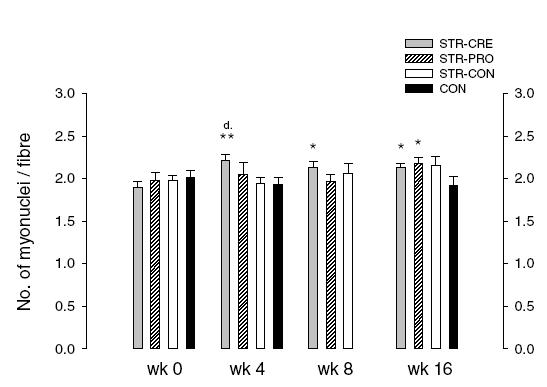
CON denotes untrained controls (no data at week 8). Pre < post training (*P < 0.05, **P < 0.01); d, STR-CRE > STR-CON, CON (P < 0.05).
Muscle mean fibre cross-sectional area (MFA)
MFA showed significant time (P < 0.05) and group (P < 0.01) effects, increasing in STR-CRE (+14.4%, +14.6% and +16.8% at week 4, 8 and 16, respectively), for STR-PRO at week 16 (+7.9%), and for STR-CON at week 4 (+13.8%) (P < 0.01) (Table 3, Fig. 3). No changes were observed for CON. In STR-CRE there was a positive correlation between the relative increases in MFA and myonucleus number, respectively, from baseline to week 16 (r = 0.73, P < 0.05).
Figure 3. Mean muscle fibre area measured prior to (week 0) and after 4, 8 and 16 weeks of strength training combined with intake of creatine (STR-CRE), protein (STR-PRO) or placebo (STR-CON).
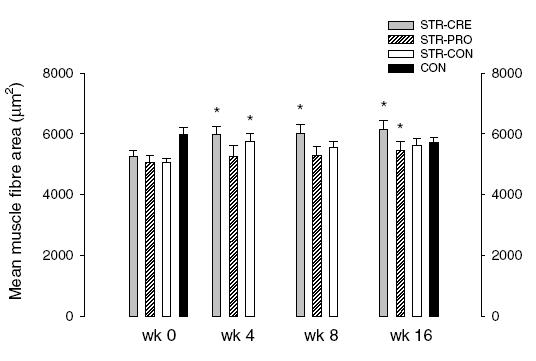
CON denotes untrained controls (no data at week 8). Pre < post training (*P < 0.05, **P < 0.01); e, CON > STR-CRE, STR-PRO, STR-CON, CON at week 0 (P < 0.05).
Maximal muscle strength (MVC)
Maximal isometric muscle strength increased (P < 0.05) 15, 18 and 22% for STR-CON, STR-PRO and STR-CRE, respectively, while remaining unaltered in CON (Table 4). Following the period of training MVC was greater in STR-CRE compared to STR-PRO, STR-CON and CON (P < 0.05). Likewise, post-training MVC was greater in STR-PRO and STR-CON than CON (P < 0.05).
Table 4.
Maximal isometric quadriceps contraction strength (MVC) before (Pre) and after (Post) training
| MVC Pre (N m) | MVC Post (N m) | |
|---|---|---|
| STR-CRE | 307.2 ± 25.9 | 371.8 ± 69.7**† |
| STR-PRO | 277.4 ± 49.3 | 326.3 ± 66.4* |
| STR-CON | 294.0 ± 26.7 | 339.9 ± 63.0* |
| CON | 330.0 ± 67.9 | 315.3 ± 59.4 |
Values are means ± s.d.
Significantly different from pre (P < 0.05).
Significantly different from pre (P < 0.01).
STR-CRE > STR-PRO, STR-CON, CON (P < 0.05).
Discussion
The present study is the first to demonstrate that creatine supplementation in association with strength training amplifies the training-induced increase in the number of satellite cells (SC) and myonuclei in human skeletal muscle fibres. In response to strength training, both creatine (STR-CRE) and protein (STR-PRO) supplementation as well as unsupplemented training (STR-CON) were found to increase SC per fibre and relative number of satellite cells (Fig. 1). However, greater increases occurred with creatine supplementation at week 4 (compared to STR-CON) and at week 8 (compared to STR-PRO and STR-CON). Furthermore, creatine supplementation resulted in an increased number of myonuclei per fibre (Fig. 2) and in an amplified hypertrophy response to training as indicated by increased in MFA at week 4, 8 and 16 (Fig. 3), while protein supplementation caused MFA to increase at week 16 and unsupplemented training increased MFA at week 4 only.
Only few data exist on the change in SC number and activity in response to training in humans. In the present study, the unsupplemented strength training group (STR-CON) was comparable to the training groups used in previous studies (i.e. Kadi et al. 2004b), and from values around 5% at baseline (for all groups) the relative number of SC increased 27% and 44% in STR-CON at week 4 and 8, respectively. Similar increases were reported by Kadi et al. (2004b) where SC content increased by 19–31% in response to unsupplemented strength training. Further, in the present study substantially larger gains in the relative number of SC were observed when strength training was combined with protein supplementation (+61% at week 4) and even more so with creatine supplementation (+84% and +99% at week 4 and 8, respectively), which yielded a peak SC content of 10% at week 8 (Fig. 1). Evidence exists to suggest that creatine supplementation can stimulate satellite cell proliferation in vitro (Vierck et al. 2003) and increase satellite cell mitotic activity (Dangott et al. 2000) (cellular effects are discussed in detail below), which could explain the present finding that relative SC content increased most markedly when strength training was combined with creatine supplementation. This effect may have been mediated, at least in part, via creatine-induced facilitation of myogenic regulatory factor (MRF) pathways (effects on MRFs are discussed in detail below). In previous training studies the relative proportion of SC increased 46% (from 3.7% to 5.4%) in the trapezius muscle following 10 weeks of resistance training in women (Kadi & Thornell, 2000). Likewise, Roth et al. (2001) found an increases of 18% (from 2.8% to 3.3%) in young men in response to 9 weeks of resistance training of the knee extensors. More recently, a 29% increased SC content (from 2.4% to 3.1%) was reported in elderly men in response to endurance training for 14 weeks (Charifi et al. 2003). These study differences in the relative proportion of SC both at baseline and in response to training may be explained by several factors. Firstly, age and training status of the subjects can affect baseline values of SC. It has been shown that SC proportion decrease with age, and possibly is further diminished by reduced physical activity with ageing (Kadi et al. 2004a). Secondly, differences in the magnitude and type of training may affect the magnitude of adaptive change in SC proportion. Based on its documented effect on muscle protein accretion, it is likely that strength training increases SC proportion more than endurance training. In the present study, both the intensity and the number of training sets were reduced in the initial phase of the strength training programme, while being progressively increased in the later weeks of training. Since the largest increases in SC content were observed already at week 4 in the present study it seems therefore that the duration and intensity of training are not the governing factors responsible for the increase in the relative proportion of SC, at least when training is combined with timed creatine or protein intake. Thirdly, SC content may differ between various muscles due to differences in fibre type composition and/or functional demand. And finally, different methods have been utilized, with immuno-histochemistry and light microscopy allowing analysis of a much larger number of fibres compared to electron microscopy.
As demonstrated for the first time, creatine supplementation induced superior gains in the number of SC and myonuclei with strength training in the present study (Fig. 1). This suggests an increased contribution of SC-derived myonuclei to the muscle fibres, which is expected to increase the capacity for mRNA transcription and thereby lead to elevated rates of myofibrillar protein synthesis (Kadi, 2000). In turn, this is likely to have contributed to the accelerated hypertrophy response presently observed in the creatine supplemented training group. In the present study, strength training without creatine or protein supplementation (i.e. STR-CON) did not lead to increases in number of myonuclei, in accordance with previous reports (Kadi et al. 2004b). Nevertheless, transient muscle fibre hypertrophy was observed in STR-CON despite the absence of elevated myonucleus number, as also reported previously (Kadi et al. 2004b). In contrast, creatine supplementation combined with training led to an elevated myonucleus number (+14–17% at week 4–16), which was likely to be responsible for the accelerated time course and more marked muscle fibre hypertrophy observed in this training group (cf. Fig. 3). These amplified training responses were accompanied by a corresponding change in mechanical muscle function, since post-training maximum isometric muscle strength (MVC) was found to be greater when strength training was combined with creatine supplementation.
Previous studies have reported amplified muscle accretion and elevated fibre size gains in response to long-term strength training with creatine or protein supplementation. Thus, following 6 weeks of strength training lean tissue mass increased to a greater extent with combined creatine–protein compared to protein or carbohydrate supplementation, respectively, and for protein compared to carbohydrate supplementation (Burke et al. 2001). Similarly, amplified gains in muscle fibre size have been reported both in young (Andersen et al. 2005) and old individuals (Esmarck et al. 2001) when strength training was combined with timed intake of protein. In support of these findings, ingestion of amino acids results in a more positive net protein balance compared to carbohydrates when ingested acutely after exercise (Borsheim et al. 2004). Creatine supplementation in conjunction with strength training also appears to lead to greater gains in lean body mass (Vandenberghe et al. 1997; Kreider et al. 1998; Steenge, 1999), cross-sectional muscle area (Hespel et al. 2001) and single muscle fibre area (Volek et al. 1999; Becque et al. 2000) compared to carbohydrate supplementation alone.
In the present study a positive correlation between the training-induced increases in MFA and myonucleus number from baseline to week 16 was demonstrated with creatine supplementation (r = 0.67, P < 0.05). This finding supports that the ratio between myonucleus number and fibre cross-sectional area (myonuclear domain) remained constant during the process of myofibre hypertrophy when training was supplemented by creatine. The finding that SC content was no longer elevated at week 16 in STR-CRE (Fig. 1) suggests that creatine supplementation accelerated the incorporation of SC-derived myonuclei to the growing muscle fibres, establishing the appropriate myonuclear domain earlier than the other training groups. Interestingly, strength training with carbohydrate supplementation alone (STR-CON) transiently increased MFA at week 4 despite the lack of increased myonucleus number. As mentioned above, MFA has previously been found to increase along with no change in myonucleus number in response to unsupplemented training (Kadi et al. 2004b). Collectively therefore the findings of the present study indicate that while an increase in myonucleus number is not a permissive factor to achieve muscle fibre hypertrophy, it does seem to set the limit for fibre hypertrophy – likely by regulating the nuclear domain of the muscle cell. Notably, the substantial increase in myonucleus number in the STR-CRE group appeared to be the result of training-mediated creatine action on myonucleus number that occurred independently of the change in fibre area.
Cellular effects of creatine supplementation recently have been documented, in which creatine was found to affect satellite cell proliferation and differentiation in cell cultures (Vierck et al. 2003). Furthermore it has been shown in rats that creatine supplementation during increased functional loading and compensatory hypertrophy (synergist ablation) induced increased satellite cell mitotic activity (Dangott et al. 2000). The increase in SC number, myonuclei and MFA in the present study supports a role for creatine in activating myogenic satellite cells, thereby adding nuclei and augmenting the training-induced accretion of muscle mass, especially in the early part of the time course of training. As an osmotically active substance creatine can cause water retention in the muscle fibres (Ziegenfuss et al. 1998), and increased osmotic pressure and resultant cell swelling due to increased creatine concentration and muscle glycogen content (Op't Eijnde et al. 2001a, b) may represent an anabolic stimulus on cellular protein synthesis (Haussinger, 1993), and further it may stimulate satellite cells to proliferate and fuse with the enlarging myofibres (Dangott et al. 2000). In the present study, muscle creatine concentration increased significantly in STR-CRE and was higher compared to STR-PRO and STR-CON at week 8 (data not shown). The myogenic effect of elevated muscle creatine concentration probably is linked to the activity of training, since creatine supplementation without training does not seem to lead to increases in satellite cell mitotic activity (Dangott et al. 2000) or muscle fibre area (Steenge, 1999). Recently, it was reported that creatine per se did not increase myofibrillar and sarcoplasmatic protein synthesis at rest or after an acute bout of exercise at a fixed absolute intensity (Louis et al. 2003a, b). However, these results do not exclude the possibility of increased transcriptional changes or enhanced activation of satellite cells when creatine intake and physical activity are combined (Rennie et al. 2004). Recent findings have supported the idea of a facilitating effect of creatine on skeletal muscle growth with training. Myogenin and MRF-4 mRNA and protein expression increased more after creatine supplementation compared to training alone after 12 weeks of resistance training (Willoughby & Rosene, 2003). These myogenic regulatory factors (MRFs) are thought to regulate muscle heavy chain (MHC) expression at the transcriptional level, and therefore up-regulation of MRF may lead to muscle accretion, which was supported by correlations between increase in myofibrillar protein and increased mRNA expression of Myo-D and myogenin (Willoughby & Nelson, 2002), although data on muscle size were not reported (Willoughby & Rosene, 2001, 2003).
It has been suggested that the enhanced muscle size gain observed when strength training is combined with creatine supplementation could be caused by a rise in training quality and/or greater total training load (Volek et al. 1999), which was supported by a higher total resistance load lifted by creatine supplemented subjects as reported by Steenge (1999). Such effect of increased work output during creatine supplementation could cause a greater than normal stimulus to muscle anabolism (Louis et al. 2003a). However, it is not obvious how increased hypertrophy should result merely from a marginally greater training intensity or volume in a progressive training programme, as the acute stimulation of muscle protein synthesis does not seem affected by the intensity of the preceding contractile activity (M. Rennie, personal communication; manuscript in preparation). Accordingly, in the present study total training load was not greater in subjects supplemented by creatine compared to the subjects supplemented by protein and placebo.
In conclusion, the present study is the first to demonstrate that creatine supplementation and to a lesser extent protein supplementation in combination with strength training augment the training-induced increase in the number of satellite cells and myonuclei in human skeletal muscle, resulting in enhanced muscle fibre growth. Furthermore, creatine supplementation appears to induce an early accelerated adaptation of satellite cells and myonuclei, which peaked at week 4 and 8 followed by a return of satellite cells to baseline levels at week 16 of training, while myonucleus number and myofibre area remained elevated.
Acknowledgments
This work was supported by Team Danmark Elite Sports Association and Idraettens Forskningsraad.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, Suetta C, Magnusson P, Aagaard P. The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metabolism. 2005;54:151–156. doi: 10.1016/j.metabol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Becque MD, Lochmann JD, Melrose DR. Effects of oral creatine supplementation on muscular strength and body composition. Med Sci Sports Exerc. 2000;32:654–658. doi: 10.1097/00005768-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol. 2004;96:674–678. doi: 10.1152/japplphysiol.00333.2003. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Burke DG, Chilibeck PD, Davidson KS, Candow DG, Farthing J, Smith-Palmer T. The effect of whey protein supplementation with and without creatine monohydrate combined with resistance training on lean tissue mass and muscle strength. Int J Sport Nutr Exerc Metab. 2001;11:349–364. doi: 10.1123/ijsnem.11.3.349. [DOI] [PubMed] [Google Scholar]
- Charifi N, Kadi F, Feasson L, Denis C. Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve. 2003;28:87–92. doi: 10.1002/mus.10394. [DOI] [PubMed] [Google Scholar]
- Dangott B, Schultz E, Mozdziak PE. Dietary creatine monohydrate supplementation increases satellite cell mitotic activity during compensatory hypertrophy. Int J Sports Med. 2000;21:13–16. doi: 10.1055/s-2000-8848. [DOI] [PubMed] [Google Scholar]
- Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535:301–311. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann NY Acad Sc. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Grounds MD. Muscle regeneration: molecular aspects and therapeutic implications. Curr Opin Neurol. 1999;12:535–543. doi: 10.1097/00019052-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Haussinger D. Control of protein turnover by the cellular hydratation state. Ital J Gastroenterol. 1993;25:42–48. [PubMed] [Google Scholar]
- Hespel P, Eijnde BO, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA. Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol. 2001;536:625–633. doi: 10.1111/j.1469-7793.2001.0625c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F. Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand Suppl. 2000;646:1–52. [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004a;29:120–127. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004b;558:1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113:99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Kreider RB, Ferreira M, Wilson M, Grindstaff P, Plisk S, Reinardy J, Cantler E, Almada AL. Effects of creatine supplementation on body composition, strength, and sprint performance. Med Sci Sports Exerc. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003a;285:E1089–E1094. doi: 10.1152/ajpendo.00195.2003. [DOI] [PubMed] [Google Scholar]
- Louis M, Poortmans JR, Francaux M, Hultman E, Berre J, Boisseau N, Young VR, Smith K, Meier-Augenstein W, Babraj JA, Waddell T, Rennie MJ. Creatine supplementation has no effect on human muscle protein turnover at rest in the postabsorptive or fed states. Am J Physiol Endocrinol Metab. 2003b;284:E764–E770. doi: 10.1152/ajpendo.00338.2002. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op't Eijnde B, Richter EA, Henquin JC, Kiens B, Hespel P. Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol Scand. 2001a;171:169–176. doi: 10.1046/j.1365-201x.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- Op't Eijnde B, Urso B, Richter EA, Greenhaff PL, Hespel P. Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes. 2001b;50:18–23. doi: 10.2337/diabetes.50.1.18. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch. 1993;423:255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Metter EJ, Hurley BF, Rogers MA. Skeletal muscle satellite cell characteristics in young and older men and women after heavy resistance strength training. J Gerontol a Biol Sci Med Sci. 2001;56:B240–B247. doi: 10.1093/gerona/56.6.b240. [DOI] [PubMed] [Google Scholar]
- Schubert W, Zimmermann K, Cramer M, Starzinski-Powitz A. Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci U S A. 1989;86:307–311. doi: 10.1073/pnas.86.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenge G. UK: University of Nottingham; 1999. Factors affecting creatine accumulation in human skeletal muscle. Phd Thesis. [Google Scholar]
- Tipton KD, Rasmussen BB, Miller SL, Wolf SE, Owens-Stovall SK, Petrini BE, Wolfe RR. Timing of amino acid carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–E206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- Vandenberghe K, Goris M, Van Hecke P, Van Leemputte M, Vangerven L, Hespel P. Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol. 1997;83:2055–2063. doi: 10.1152/jappl.1997.83.6.2055. [DOI] [PubMed] [Google Scholar]
- Vierck JL, Icenoggle DL, Bucci L, Dodson MV. The effects of ergogenic compounds on myogenic satellite cells. Med Sci Sports Exerc. 2003;35:769–776. doi: 10.1249/01.MSS.0000065005.96298.01. [DOI] [PubMed] [Google Scholar]
- Vierck J, O'Reilly B, Hossner K, Antonio J, Byrne K, Bucci L, Dodson M. Satellite cell regulation following myotrauma caused by resistance exercise. Cell Biol Int. 2000;24:263–272. doi: 10.1006/cbir.2000.0499. [DOI] [PubMed] [Google Scholar]
- Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomez AL, Pearson DR, Fink WJ, Kraemer WJ. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc. 1999;31:1147–1156. doi: 10.1097/00005768-199908000-00011. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Nelson MJ. Myosin heavy-chain mRNA expression after a single session of heavy-resistance exercise. Med Sci Sports Exerc. 2002;34:1262–1269. doi: 10.1097/00005768-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Rosene J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc. 2001;33:1674–1681. doi: 10.1097/00005768-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Willoughby DS, Rosene JM. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc. 2003;35:923–929. doi: 10.1249/01.MSS.0000069746.05241.F0. [DOI] [PubMed] [Google Scholar]
- Yan Z. Skeletal muscle adaptation and cell cycle regulation. Exerc Sport Sci Rev. 2000;28:24–26. [PubMed] [Google Scholar]
- Ziegenfuss TN, Lowery LM, Lemon PWR. Acute fluid volume changes in men during three days of creatine supplementation. J Exerc Physiol online. 1998;1(3) http://faculty.css.edu/tboone2/asep/jan13d.htm. [Google Scholar]


