Abstract
Idiopathic constipation is higher in women of reproductive age than postmenopausal women or men, suggesting that female steroid hormones influence gastrointestinal motility. How female hormones affect motility is unclear. Colonic motility is regulated by ion channels in colonic myocytes. Voltage-dependent K+ channels serve to set the excitability of colonic muscles. We investigated regulation of Kv4.3 channel expression in response to acute or chronic changes in female hormones. Patch clamp experiments and quantitative PCR were used to compare outward currents and transcript expression in colonic myocytes from male, non-pregnant, pregnant and ovariectomized mice. Groups of ovariectomized mice received injections of oestrogen or progesterone to investigate the effects of hormone replacement. The capacitance of colonic myocytes from non-pregnant females was larger than in males. Net outward current density in male and ovariectomized mice was higher than in non-pregnant females and oestrogen-treated ovariectomized mice. Current densities in late pregnancy were lower than in female controls. Progesterone had no effect on outward currents. A-type currents were decreased in non-pregnant females compared with ovariectomized mice, and were further decreased by pregnancy or oestrogen replacement. Kv4.3 transcripts did not differ significantly between groups; however, expression of the potassium channel interacting protein KChIP1 was elevated in ovariectomized mice compared with female controls and oestrogen-treated ovariectomized mice. Delayed rectifier currents were not affected by oestrogen. In the mouse colon, oestrogen suppresses A-type currents, which are important for regulating excitability. These observations suggest a possible link between female hormones and altered colonic motility associated with menses, pregnancy and menopause.
Idiopathic constipation is a disorder which predominantly affects females of reproductive age, and many of these patients have coexistent disorders of menstruation (Preston & Lennard-Jones, 1986; Turnbull et al. 1989; Kamm et al. 1991). A recent clinical study revealed that colonic transit time is significantly longer in the luteal phase of the menstrual cycle, when oestrogen levels are elevated, compared with the follicular phase, when oestrogen levels are relatively low (Jung et al. 2003). Additionally, during pregnancy (when oestrogen levels are elevated) there is a higher incidence of constipation (Baron et al. 1993). Thus, there appears to be a strong correlation between female hormone levels and colonic motility. These clinical observations are supported by in vivo experiments, in which oestrogen and progesterone have been shown to influence gastrointestinal transit time (Ryan & Bhojwani, 1986). Rats in a high oestrogen–progesterone cycle stage had significantly slower colon transit times compared with animals in a low hormonal stage. Ovariectomized rats pretreated with oestrogen and progesterone had significantly slower transit times than untreated ovariectomized animals. Colonic transit time in pregnant rats was essentially identical to those of animals pretreated with oestradiol (Ryan & Bhojwani, 1986). However, the mechanisms by which female steroid hormones affect gastrointestinal motility are unclear.
Oestrogen affects transcriptional regulation by binding to specific response elements and regulating target gene expression. Oestrogen also exerts ‘non-genomic’ effects which involve steroid-induced modulation of cytoplasmic or of cell membrane-bound regulatory proteins such as mitogen-activated protein kinases, phosphatidylinositol 3-OH kinase (PI3K), ion channels, and G-protein-coupled receptors. Genomic effects of oestrogen include the regulation of expression of a variety of ion channels, including: down-regulation of Cav1.2 mRNA expression and L-type Ca2+ channel density (Johnson et al. 1997; Patterson et al. 1998) and up-regulation of large conductance Ca2+-activated K+ channels (Jamali et al. 2003), ATP-sensitive K+ channels (Ranki et al. 2002), small conductance K+ channels (Jacobson et al. 2003) and Kv1.5 channels (Tsang et al. 2004). Recent studies have made an important link between oestrogen and down-regulation of A-type currents and Kv4.3 channels in myometrial smooth muscle (Wang et al. 1998; Song et al. 2001; Suzuki & Takimoto, 2005). It is possible that loss of this conductance, which is important in regulating excitability in smooth muscles, might affect excitability activity. We have previously shown that A-type currents in colonic myocytes are mainly encoded by Kv4.3 channels based on pharmacology, kinetics and molecular expression. (Amberg et al. 2002). A-type currents are inhibited by 4-aminopyridine (4-AP) and flecainide (Vogalis et al. 1993; Amberg et al. 2002) and activate at negative potentials. Currents via Kv4.3 channels influence the resting membrane potentials and excitability of colonic smooth muscle and inhibition of A-type current by 4-AP-induced depolarization and increased contractility of colonic muscles (Koh et al. 1999). Here we investigated the effects of female steroid hormones on Kv4.3 channel expression in colonic smooth muscle cells to study the link between female hormones and altered colonic excitability.
Methods
Animals
BALB/c mice between 30 and 60 days old were used for the described studies. Animals were obtained from the Jackson Laboratory (Bar Harbour, MN, USA). Mice were anaesthetized with isoflurane drop method (Baxter, Deerfield, IL, USA) to effect prior to cervical dislocation and decapitation. The animals were maintained and the experiments performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Institutional Animal Use and Care Committee at the University of Nevada approved all procedures used.
Ovariectomized mice (OVX; 20–30 days old) were purchased from Charles River Laboratories (Wilmington, MA, USA) 10 days following ovariectomy. A group of OVX mice received injections of 17β-oestradiol (5 ng (g body wt)−1) or progesterone (5 ng (g body wt)−1) for 7 days prior to killing and tissue collection.
Immunohistochemistry
For preparation of whole-mount colon tissue specimens for immunohistochemistry, entire colons (from cecum to rectum) were removed through a midline abdominal incision. Colons were cut open along the longitudinal axis close to the mesenteric border and washed clean with a calcium-free solution containing (mm): 125 NaCl, 5.36 KCl, 15.5 NaOH, 0.336 Na2HPO4, 0.44 KH2PO4, 10 glucose, 2.9 sucrose and 11 Hepes, adjusted to pH 7.4 with tris (hydroxymethyl)aminomethane (Tris). After pinning flat to a Sylgard-lined dissecting dish, the mucosa and submucosa were carefully removed, leaving the underlying tunica muscularis intact. Tissue segments were then fixed in acetone for 10 min at 4°C and washed thoroughly (5 × 15 min or overnight) with phosphate-buffered saline (PBS). To reduce non-specific binding, tissues were incubated in bovine serum albumin (1% v/v in PBS) for 1 h prior to incubation of tissues in primary antisera. Primary rabbit polyclonal antibodies specific for α- and β-isoforms of the oestrogen receptor (obtained from Abcam Inc., Cambridge, MA, USA) were diluted to 2 μg ml−1 in PBS containing 0.3% Triton X-100 (Calbiochem, San Diego, CA, USA). Tissues were incubated in primary antibody solution for 48 h at 4°C. Following 3–4 h of washing with PBS, tissues were incubated in corresponding secondary antibody conjugates (Alexa Fluor 488 goat anti-rabbit IgG for both α- and β-receptor isoforms; purchased from Molecular Probes Inc, CA, USA) diluted to 1: 500 in PBS for 1 h at room temperature. After 3–5 × 15 min washes with PBS, tissue specimens were mounted and viewed using a Zeiss LSM 510 confocal microscope (Carl Zeiss Inc.). Confocal micrographs of whole-mount preparations of colon tissue are digital reconstructions of 15 optical sections, each with a depth of 1.0 μm. Final images were constructed using Zeiss LSM 5 Image Examiner Software.
Preparation of isolated myocytes
To prepare isolated colonic myocytes, colons were cut open along the longitudinal axis, pinned in a Sylgard-lined dish and washed with Ca2+-free solution as described above. Mucosa and submucosal layers were removed and the remaining tunica muscularis cut into segments. Tissue segments were incubated in 1 ml of Ca2+-free solution supplemented with 2 mg fatty acid-free bovine serum albumin, 1.3 mg collagenase and 2 mg trypsin inhibitor for 8–12 min at 37°C. Following incubation in the enzyme solution, tissues were washed repeatedly (3–5 times) with Ca2+-free solution and gently agitated to create a cell suspension. Dispersed cells from both circular and longitudinal muscle layers were stored at 4°C in Ca2+-free solution. Experiments were performed at room temperature within 6 h of dispersing cells.
Voltage-clamp patch experiments
The whole-cell patch clamp technique was used to record membrane currents from dissociated murine colonic smooth muscle cells. Currents were amplified with a List EPC-7 (List Electronic, Darmstadt, Germany) or Axopatch 200B (Axon Instruments, Union City, CA, USA). Data were digitized with 16-bit analog-to-digital converter (Digidata 1322A, Axon Instruments). Data were stored directly and digitized on-line using pCLAMP software (version 9.2, Axon Instruments). Data were sampled at 4 kHz and filtered at 2 kHz using an eight-pole Bessel filter, and analysed using pCLAMP (version 9.2; Axon Instruments), Graphpad Prism (version 3.0, Graphpad Software Inc., San Diego, CA, USA) and Origin software (version 5.0, OriginLab Corp., Northampton, MA).
Solutions for patch clamp experiments
In order to measure outward currents, smooth muscle cells were bathed in a Ca2+-free physiological salt solution (MnPSS) containing (mm): 5 KCl, 135 NaCl, 2 MnCl2, 10 glucose, 1.2 MgCl2 and 10 Hepes adjusted to pH 7·4 with Tris. The pipette solution contained (mm): 110 potassium gluconate, 20 KCl, 5 MgCl2, 2.7 K2ATP, 0.1 Na2GTP, 2.5 creatine phosphate disodium, 5 Hepes and 10 BAPTA, adjusted to pH 7·2 with Tris. 4-Aminopyridine (4-AP, Sigma) or tetraethylammonium (TEA, Sigma) were added as described in the Results section. Patch clamp experiments were performed at room temperature (22°C).
Analysis of electrophysiological data
Data are expressed as means ± standard errors of the mean (s.e.m.). Student's t test was used where appropriate to evaluate differences in the data. P values less than 0.05 were taken as statistically significant. For patch clamp experiments, we analysed the peak currents before and after drug treatments.
Total RNA isolation and quantitative PCR
Total RNA was isolated from the tunica muscularis of mouse colon (following removal of the mucosa and submucosa) using the SNAP Total RNA isolation kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. First-strand cDNA was prepared from the total RNA using the Superscript reverse transcriptase kit (Gibco, Gaithersburg, MD, USA). One microgram of total RNA was reverse transcribed with 200 units reverse transcriptase in a 20 μl reaction mixture containing 25 ng oligo-dT primer, 500 μm each dNTP, 75 mm KCl, 3 mm MgCl2, 10 mm dithiothreitol and 50 mm Tris-HCl (pH 8.3). As a control, PCR primers specific for GAPDH (see Table 1 for details) were used to establish that the prepared cDNA was non-genomic. In qualitative experiments GAPDH primers amplified only the non-intron amplification product (170 bp band) from all cDNA samples, indicating that preparations were free of genomic DNA contamination (270 bp band was not observed; data not shown). cDNA reverse transcription products were amplified with Kv4.3, KChIP1, KChIP4 and GAPDH-specific primers by reverse transcriptase polymerase chain reaction (RT-PCR) using AmpliTaq Gold reagents (PE Applied Biosystems, Foster City, CA, USA). The primer pairs used are listed in Table 1. The amplification protocol for these primer pairs was as follows: 95°C for 10 min to activate the AmpliTaq polymerase, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. Aliquots of the PCR reactions were analysed by 2% agarose gel electrophoresis and visualized by ethidium bromide fluorescence. The identity of PCR amplification products obtained were confirmed by DNA sequencing.
Table 1.
PCR primer pairs
| mRNA (Genebank accession no.) | Position | Primer sequence (5′-to 3′) |
|---|---|---|
| Kv4.3 (AF107781) | 1398–1418 | CAAGACCACCTCACTCATCGA |
| 1553–1573 | TCGAGCTCTCCATGCAGTTCT | |
| KChIP1 (AB075041) | 126–145 | ACCGGCCTGAGGGACTGGAG |
| 270–289 | GCTGGCATCTCCGTGAGGGA | |
| KChIP4 (AF305071) | 229–250 | GAGGCCCAGAGCAAATTCACCA |
| 394–414 | TCCATTGTGGTCCGTGTCGAA | |
| GAPDH (BI851476) | 353–371 | GTCTTCACCACCATGGAG |
| 505–522 | AAGCAGTTGGTGGTGCAG |
Real-time RT-PCR was used to quantify the expression levels of Kv4.3, KChIP1 and KChIP4 relative to the GAPDH between animal groups. SYBR Green I was used as the fluorescent probe on an ABI 5700 sequence detector (PE Applied Biosystems). Real-time PCR was performed in triplicate using the same amplification protocol described above. Reaction mixtures lacking cDNA (non-template controls) were included during each session to assess contamination and non-specific amplification. To examine Kv4.3 and KChIP primer efficiencies, standard curves were generated for each primer pair by regression analysis of PCR amplifications on log10 serial dilutions of cDNA. Standard curves were used to normalize the abundance of each transcript relative to the amount of GAPDH transcript present within the same sample. Data are reported as the mean ± s.e.m.; n refers to the number of animals from which tissues were collected. Statistical significance was evaluated by one-way analysis of variance with Tukey's multiple comparison test. P values less than 0.05 were considered significant.
Results
Oestrogen receptors are expressed by colonic smooth muscle cells
Immunohistochemical staining was performed on whole-mount preparations with antibodies targeted to the α- and β-isoforms of oestrogen receptors to determine whether these receptors are expressed in colonic smooth muscle cells. α- and β-oestrogen receptor-like immunoreactivity was distributed throughout both the circular and longitudinal muscle cells of the murine colon (Fig. 1A and B).
Figure 1. Oestrogen receptors are expressed in murine colonic myocytes.
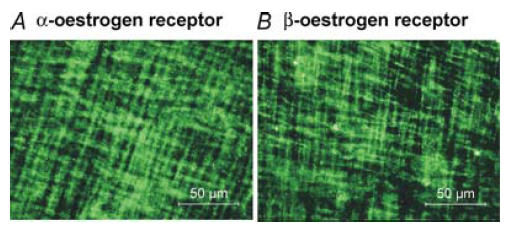
Immunohistochemistry using an antibody against the α-isoform of the oestrogen receptor revealed α-oestrogen receptor-like immunoreactivity within smooth muscle cells of the circular and longitudinal muscle layers of the murine colon (A). β-oestrogen-like immunoreactivity was also ubiquitously expressed within both muscle layers (B). Images are digital reconstructions of 15 confocal optical sections (each with depth of 1.0 μm) within the tunica muscularis of the murine colon.
Acute application of oestrogen or progesterone did not alter outward currents
Control experiments were performed to test the acute application of steroid hormones on voltage-dependent K+ currents in colonic myocytes. Recordings of outward currents were made from freshly dispersed colonic myocytes using conventional, dialysed whole-cell patch clamp techniques. Contamination from large conductance Ca2+-activated K+ channels was minimized by bathing cells in MnPSS (see Methods) and including BAPTA (10 mm) in the pipette solutions. Under these conditions, 17β-oestradiol (5 ng ml−1) or progesterone (5 ng ml−1) had no effect on outward current density or kinetics (data not shown).
Outward current density was higher in male and ovariectomized mice compared with non-pregnant females
Colonic myocytes were isolated from male, female and ovariectomized (OVX) mice to test the effects of chronic differences in female steroid hormone levels on outward currents. Cell capacitance of female colonic myocytes was significantly greater than for male myocytes (i.e. 60.4 ± 5.7 pF, n = 10 females versus 40.3 ± 2.3 pF, n = 13 males, P < 0.01). This difference was not observed between male myocytes and cells isolated from OVX females (i.e. 38.5 ± 3.3 pF, n = 8, OVX). Due to the differences in cell capacitance, we used current density and voltage dependence of conductances for comparative analysis. Colonic myocytes were held at −80 mV and stepped to test potentials ranging from −80 to +50 mV in 10 mV intervals (Fig. 2A–C). Outward current density at 0 mV and +40 mV were 26.8 ± 3.1 and 62.5 ± 5.4 pA pF−1, respectively, in male myocytes (n = 13, Fig. 2D and E), 18.3 ± 2.6 and 46.7 ± 4.4 pA pF−1, respectively, in female myocytes (n = 10), and 22.3 ± 1.6 and 59.0 ± 2.9 pA pF−1, respectively, in female myocytes from OVX mice (n = 8, Fig. 2E and F). The current density in male mice was significantly higher than that of female mice (Fig. 2D, P < 0.05). Current densities in female and OVX myocytes were also significantly different (Fig. 2E, P < 0.05). However, current densities in myocytes from OVX mice and males were not significantly different (Fig. 2F). We also examined the voltage dependence of activation and inactivation among the three groups. Half-activation voltage (Va1/2) in male, female and OVX myocytes were −5 ± 1 mV (n = 13), −1 ± 1 mV (n = 10) and 1 ± 1 mV (n = 8), respectively. Half-inactivation voltage (Vi1/2) in male, female and OVX mice were −50 ± 1, −47 ± 2 and −49 ± 2 mV, respectively. There were no significant differences in the voltage dependence of the outward current among the three groups.
Figure 2. Outward currents are higher density in male and ovariectomized (OVX) mice compared with females.
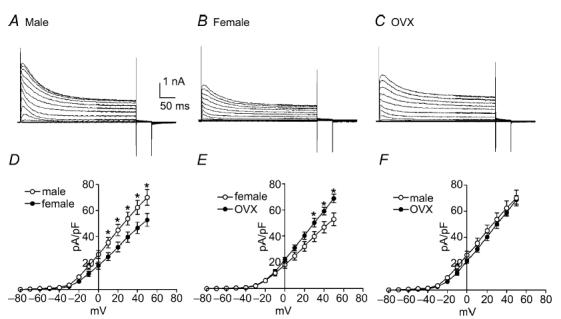
Typical recordings of outward currents elicited from colonic myocytes isolated from male (A), female (B) and OVX female (C) mice. To elicit outward currents, colonic myocytes were held at −80 mV and stepped to test potentials ranging from −80 to +50 mV in 10 mV increments (A–C). Comparison of outward current density revealed that current density in males and OVX females was significantly higher than that of females (D and E). Current density in male and OVX mice were not different at any of the tested potentials (F).
Treatment of ovariectomized mice with oestrogen suppressed outward current density
In order to examine oestrogen effects on outward currents, 17β-oestradiol (OES, 5 ng (g body wt)−1) was injected subcutaneously into OVX mice for 7 days. We compared the current densities in myocytes from OVX mice that received saline injections (OVX), OVX mice that had received OES injections (OES), and non-pregnant females. Typical recordings from colonic myocytes isolated from each group are shown in Fig. 3A–C. Peak outward current density at 0 mV and +40 mV were 22.3 ± 1.6 and 59.0 ± 2.9 pA pF−1, respectively, in OVX (n = 8, Fig. 3D), 16.4 ± 2.6 and 44.1 ± 4.0 pA pF−1, respectively, in OES (n = 17, Fig. 3D and E), and 18.3 ± 2.6 and 46.7 ± 4.4 pA pF−1, respectively, in non-pregnant females (n = 10, Fig. 3E and F). The current density in OES and non-pregnant females was significantly lower than in myocytes from OVX mice receiving saline injections only (Fig. 3D and F, P < 0.05). Comparison of current densities in non-pregnant female myocytes and in myocytes from OVX females receiving OES showed no significant difference (Fig. 3E). Voltage dependence of activation and inactivation were not affected by oestrogen injection (i.e. Va1/2 = 1 ± 1 mV in OVX and 1 ± 2 mV in OES; Vi1/2 = −49 ± 2 mV in OVX and −49 ± 1 mV in OES).
Figure 3. Net outward current density in OVX mice by chronic injection of 17β-oestradiol and progesterone.
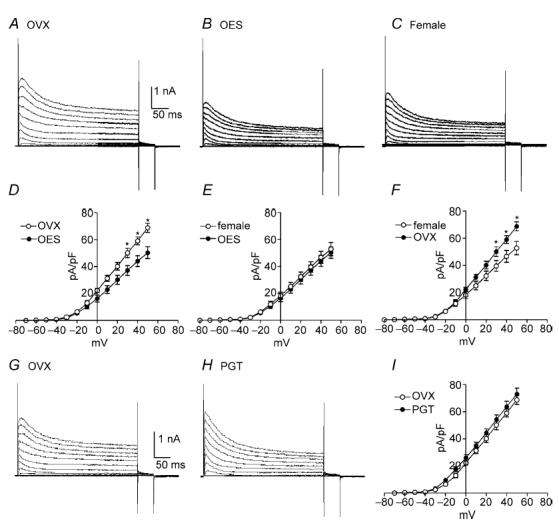
To examine the effect of elevating circulating levels of oestrogen in OVX mice, 17β-oestradiol (OES) was injected into OVX mice for 7 days. Outward currents were recorded from colonic myocytes from three animal groups: OVX (A), OVX with OES (B) and non-pregnant females (C). Outward current density in OVX with OES and non-pregnant females was lower than OVX mice (D and F, P < 0.05 at test potentials > +30 mV). Current densities between non-pregnant female and OVX with OES treatment were not significantly different at any test potentials tested (E). To examine chronic effects of progesterone on outward currents, current density was compared between three animal groups: OVX (G) and OVX which had received progesterone (PGT) injections for 7 days (H). Current density in OVX with PGT treatment was not significantly different from OVX mice (I).
Treatment of ovariectomized mice with progesterone did not alter outward current density
In order to examine the chronic effects of progesterone on outward currents from colonic myocytes, we compared the current density between three groups; OVX that had received saline only injections (OVX), OVX that had received progesterone injections, and non-pregnant females that had received saline injections (Fig. 3G–I). Peak outward current density at 0 mV and +40 mV, respectively, were 22.3 ± 1.6 and 59.0 ± 2.9 pA pF−1 for OVX (n = 8, Fig. 3I), and 26.1 ± 2.3 and 63.7 ± 4.1 pA pF−1 for progesterone (n = 12). The current density in OVX with treatment of progesterone was not significantly different from OVX mice (Fig. 3I), but significantly larger compared with non-pregnant females (data not shown). These data suggest that progesterone did not significantly alter the expression of ion channels generating outward currents in colonic myocytes.
Outward current density decreases during pregnancy
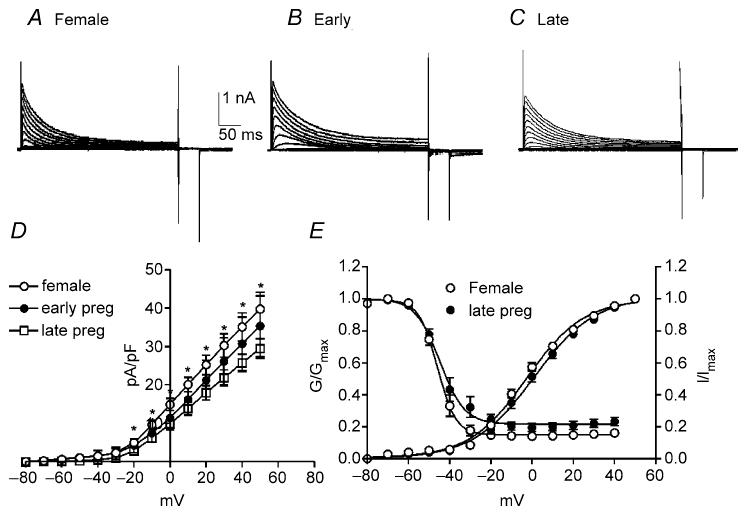
Oestrogen levels climb during pregnancy and pregnancy is often associated with decreased colonic transit and constipation (Lawson et al. 1985; Ryan & Bhojwani, 1986; Baron et al. 1993). Therefore, we examined and compared outward current density in colonic myocytes isolated from non-pregnant mice and those within the first and final trimesters of pregnancy (Fig. 4A–C). The capacitance of colonic myocytes did not differ significantly between non-pregnant and pregnant females (i.e. non-pregnant, 60.4 ± 5.7 pF; first trimester, 57.3 ± 6.8 pF; last trimester, 67.8 ± 3.8 pF). Peak outward current density at 0 mV and +40 mV were 18.3 ± 2.6 and 46.7 ± 4.4 pA pF−1, respectively, in myocytes from non-pregnant mice (n = 10, Fig. 4D), 17.7 ± 4.0 and 45.7 ± 7.1 pA pF−1 in myocytes isolated during the first trimester of pregnancy (n = 7, Fig. 4D), and 11.4 ± 1.5 and 31.9 ± 1.2 pA pF−1 in myocytes isolated during the last trimester of pregnancy (n = 10, Fig. 4D). Outward current densities in myocytes isolated during the last trimester were significantly decreased compared with myocytes from non-pregnant females and those within the first trimester of pregnancy (Fig. 4D, P < 0.05 at test potential more than +10 mV). Voltage dependence of activation and inactivation were not affected by pregnancy (i.e. Va1/2 = 1 ± 1 mV in non-pregnant and −0.5 ± 4 mV in the last trimester; Vi1/2 = −47 ± 2 mV in non-pregnant and −46 ± 2 mV in the last trimester, Fig. 4E).
Figure 4. Net outward currents are reduced during the last trimester of pregnancy.
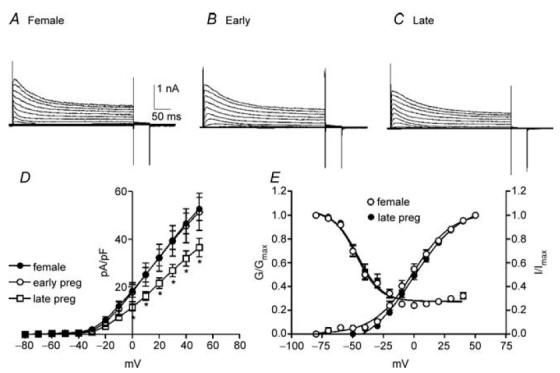
To assess the effects of pregnancy on outward currents in colonic myocytes comparisons were made between non-pregnant females (A) and pregnant mice in the first trimester (Early; B) and last trimester (Late; C) of pregnancy. Current density in late pregnancy was significantly reduced compared with non-pregnant females and those in the early stage of pregnancy (D). Voltage dependence of activation and inactivation were not affected by pregnancy (E).
A-type K+ currents in colonic myocytes are increased by ovariectomy
In order to determine the effects of female steroid hormones on A-type currents, we added tetraethylammonium chloride (TEA, 10 mm) into the bathing solution (MnPSS; see Koh et al. 1999). As previously reported, remaining currents after TEA have fast activation and inactivation kinetics (Koh et al. 1999). We compared the density of TEA-insensitive A-type currents in colonic myocytes isolated from male, female and OVX mice (Fig. 5A–C). The peak current density at 0 mV and +40 mV were 25.5 ± 3.2 and 55.0 ± 6.2 pA pF−1, respectively, in male mice (n = 13, Fig. 5D and E), 14.9 ± 1.5 and 35.1 ± 3.7 pA pF−1, respectively, in non-pregnant female mice (n = 10), and 26.8 ± 6.2 and 60.6 ± 12.6 pA pF−1, respectively, in OVX mice (n = 8, Fig. 5E and F). A-type current density in male mice was significantly higher than that of non-pregnant female mice (Fig. 5D, P < 0.05 at test potentials more positive than −20 mV). Current density measurements also revealed that A-type current density was significantly higher in OVX females compared with non-pregnant females (P < 0.05 at test potentials more positive than −20 mV, Fig. 5E). Interestingly, A-type current density in male and OVX mice were not different at all tested potentials (Fig. 5F), suggesting that ovariectomy leads to a significant increase in A-type currents. We also examined the voltage dependence of activation (Va1/2) and inactivation (Vi1/2) among the three groups. Va1/2 in male, female and OVX mice were −9 ± 1 mV (n = 13), −3 ± 1 mV (n = 10) and −6 ± 1 mV (n = 8), respectively (Fig. 5G and H). Vi1/2 in male, female and OVX mice were −51 ± 1, −46 ± 1 and −51 ± 1 mV, respectively (Fig. 5G and H). There was no significant difference between Va1/2 in male, female and OVX groups. Interestingly the differences between Vi1/2 in males and females, and between female and OVX were statistically significant (P < 0.05 for each comparison).
Figure 5. A-type K+ current density is significantly higher in male and OVX females compared with females.
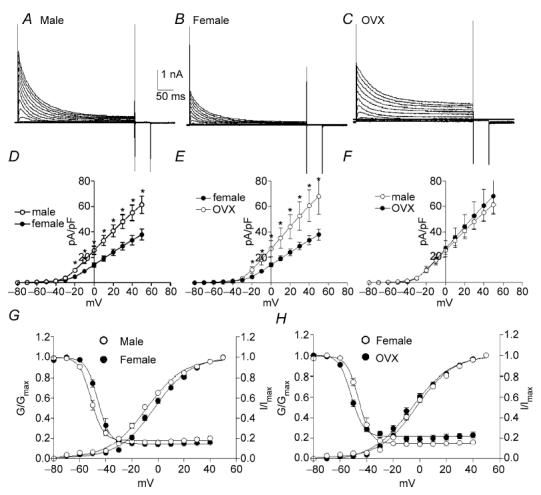
A-type currents were isolated in colonic myocytes by application of TEA (10 mm) to the bathing solution. Typical A-type currents recorded from male, non-pregnant female and OVX female mice are shown in A–C, respectively. Statistical analysis revealed that the density of A-type currents was significantly higher for males compared with non-pregnant females (D). A-type currents were also of significantly greater density for the OVX group compared with non-pregnant females (E). There was no difference in A-type current density between male and OVX mice (F). Voltage dependence of activation and inactivation were shifted to slightly more negative potentials for both male and OVX mice (G and H).
Oestrogen replacement in OVX mice reduces A-type current density
In order to evaluate the effects of oestrogen on A-type currents, we compared the current density in the presence of TEA among three groups; OVX, OVX that had received oestrogen (OES) injections for 7 days (OES), and non-pregnant female mice (Fig. 6A–C). In these conditions the peak current density at 0 mV and +40 mV were 26.8 ± 6.2 and 60.6 ± 12.6 pA pF−1, respectively, in OVX (n = 8, Fig. 6D), 13.8 ± 2.2 and 34.8 ± 3.5 pA pF−1, respectively, in OES (n = 19, Fig. 6D and E), and 14.9 ± 1.5 and 35.1 ± 3.7 pA pF−1, respectively, in non-pregnant females (n = 10, Fig. 6E). The current density in OVX with treatment of OES was significantly lower than that of OVX mice (P < 0.05 at test potentials more positive than −20 mV, Fig. 6D). Comparison of A-type current density between female and OES showed no significant difference (Fig. 6E). There was no significant shift in the voltage dependence of activation and inactivation in OES cells compared with OVX cells (i.e. Va1/2 = −6 ± 1 mV for OVX and −1 ± 1 mV for OES; Vi1/2 = −51 ± 1 mV for OVX and −49 ± 1 mV for OES, Fig. 6F).
Figure 6. 17β-oestradiol (OES) reduces A-type current density in OVX mice.
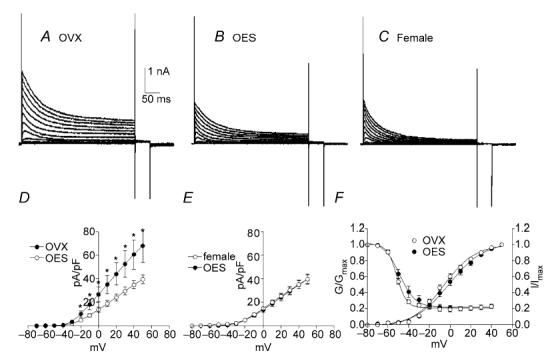
Typical A-type currents from untreated OVX, OVX with 7 days OES treatment and non-pregnant females are shown in A–C, respectively. A-type current density in OVX with OES treatment (OES) was significantly lower than OVX mice (D). Current densities between non-pregnant female and OVX with OES treatment were not different at any test potentials tested (E). Voltage dependence of activation and inactivation were not shifted in OVX mice by OES injection (OES) compared with OVX mice which had received saline injections (OVX) (F).
Inactivation time constants of A-type currents are altered by ovariectomy
A-type current in male colonic myocytes declined in two phases that were well described by the sum of two exponentials (Fig. 7A). The magnitude and rate of current decline was too small to be measured accurately at potentials negative to −20 mV. The voltage dependence of the rate of decline of the A-type currents is summarized in Fig. 7E where the averaged fast and slow time constants (τf and τs) from eight cells are plotted as a function of test potentials. τf and τs were 32 ± 6 ms and 132 ± 25 ms, respectively, at +30 mV and were insensitive to potential over the range of voltages tested (n = 9). Interestingly, inactivation of A-type currents in OVX colonic myocytes were also well fitted with two exponentials (τf = 34 ± 5 ms and τs = 98 ± 19 ms at +30 mV, n = 6, Fig. 7B and E). There was no statistical difference between inactivation time constants for males and non-OES-treated OVX females over the range of voltages tested. Inactivation of A-type currents in female and OES groups were better fitted with a single exponential function than with two exponentials (Fig. 7C and D). Time constants of inactivation at +30 mV in non-pregnant female and OES groups were 69 ± 8 ms (n = 10) and 74 ± 7 ms (n = 17), respectively. For both female and OES groups, inactivation time constants were not significantly altered by voltage. Summarized data are shown in Fig. 7F. There was no statistical difference in time constants of A-type current inactivation between female and OES groups over the range of voltages tested.
Figure 7. Inactivation time constants of A-type currents in male, female, OVX and OES groups.
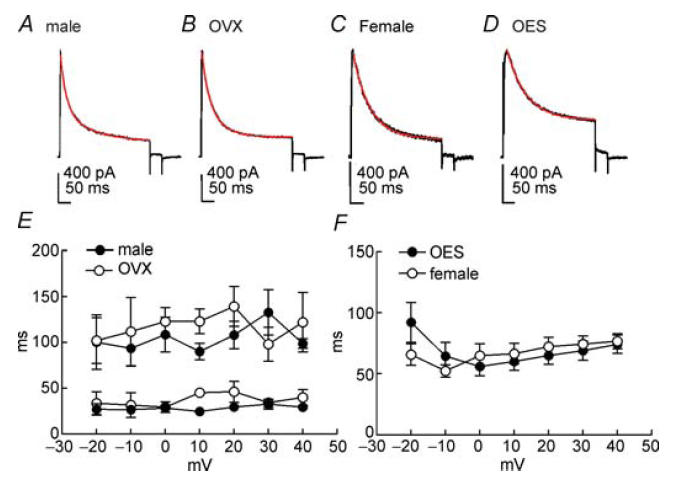
Typical A-type currents from male, OVX, female and OVX with 7 days OES treatment are shown in A–D, respectively. Membrane potential was stepped from −80 mV to +30 mV for 380 ms. Inactivation of A-type currents were fitted with double exponential time constants (male and OVX) and single exponential time constants (female and OES). Fits (red lines) are shown superimposed upon current traces (A–D). Plot of the fast and slow time constants describing inactivation of A-type currents during the 380 ms steps as a function of test potential in male (•; n = 9) and OVX (○; n = 6) groups (E). Plot of the single time constants describing inactivation of A-type currents during the 380 ms steps as a function of test potential in female (n = 10) and OES (n = 17) groups (F). Error bars denote s.e.m.; where error bars cannot be seen, they are smaller than the symbol.
A-type current density is reduced during pregnancy
We also compared A-type current density (in the presence of TEA) between colonic myocytes isolated from non-pregnant mice and those in the first and last trimesters of pregnancy (Fig. 8A–C). Peak A-type current density at 0 mV and +40 mV was 14.9 ± 1.5 and 35.1 ± 3.7 pA pF−1, respectively, in non-pregnant mice (n = 10, Fig. 8D), 11.4 ± 3.1 and 30.7 ± 6.9 pA pF−1 in the first trimester (n = 7, Fig. 8D), and 9.9 ± 1.4 and 25.7 ± 2.3 pA pF−1 in the final trimester (n = 10, Fig. 8D). Statistical analysis revealed that peak A-type current density in the final trimester of pregnancy was significantly lower than non-pregnant and first trimester groups (Fig. 8D, P < 0.05 at test potentials more positive than −20 mV). There was no significant difference in the voltage dependence of activation and inactivation with pregnancy (i.e. Va1/2 = −3 ± 1 mV in cells of non-pregnant mice and 1 ± 1 mV in the last trimester of pregnancy (P > 0.05); Vi1/2 = −46 ± 1 mV in non-pregnant and −44 ± 1 mV in the last trimester, Fig. 8E; P > 0.05).
Figure 8. A-type current density is reduced in late stages of pregnancy.
Typical A-type currents from non-pregnant, the first and last trimester of pregnancy are shown in A–C. Peak outward current density in the presence of TEA was significantly reduced in the last trimester of pregnancy compared with non-pregnant females and those in the first trimester (D). However, voltage dependence of activation and inactivation were not shifted significantly in the last trimester of pregnancy (E).
Delayed rectifier K+ currents are increased by ovariectomy
In murine colonic myocytes, outward currents remaining after the application of 4-AP are classical delayed rectifier K+ currents (Koh et al. 1999). These currents exhibit slow activating and inactivating kinetics which are different from currents that remain after TEA. Therefore, in order to determine the effects of female steroid hormones on delayed rectifier K+ currents, we added 4-AP (5 mm) to the bathing solution. We compared the density of delayed rectifier K+ currents in male, female and OVX mice in the presence of 4-AP (Fig. 9A–C). The current density at 0 mV and +40 mV was 9.5 ± 1.3 and 28.4 ± 3.3 pA pF−1, respectively, in male mice (n = 13, Fig. 9D and E), 5.5 ± 0.6 and 18.8 ± 1.7 pA pF−1, respectively, in female mice (n = 10) and 11.3 ± 1.8 and 35.7 ± 3.6 pA pF−1, respectively, in OVX mice (n = 8, Fig. 9E and F). The current density in male mice was significantly higher than that of female mice (P < 0.05 at test potentials more positive than −30 mV, Fig. 9D). Comparison of OVX and non-pregnant female data demonstrated that delayed rectifier current densities were significantly higher in OVX than non-pregnant females (P < 0.05 at test potential more than −20 mV, Fig. 9E). Density of delayed rectifier currents in OVX mice and males were not different at all tested potentials (Fig. 9F). We also compared the voltage dependence of activation and inactivation between the three groups. Va1/2 in male, female and OVX mice were 4 ± 1 mV (n = 13), 6 ± 1 mV (n = 10) and 5 ± 1 mV (n = 8), respectively (Fig. 9G and H). Vi1/2 in male, female and OVX mice were −46 ± 2, −40 ± 2 and −43 ± 2 mV, respectively (Fig. 9G and H). Average voltage dependence of activation and inactivation values for male and OVX mice were shifted toward more negative potentials (∼5 mV) than female mice. However, statistical analysis determined that this shift was not significant.
Figure 9. Density of delayed rectifier currents is higher in male and OVX females compared with females.
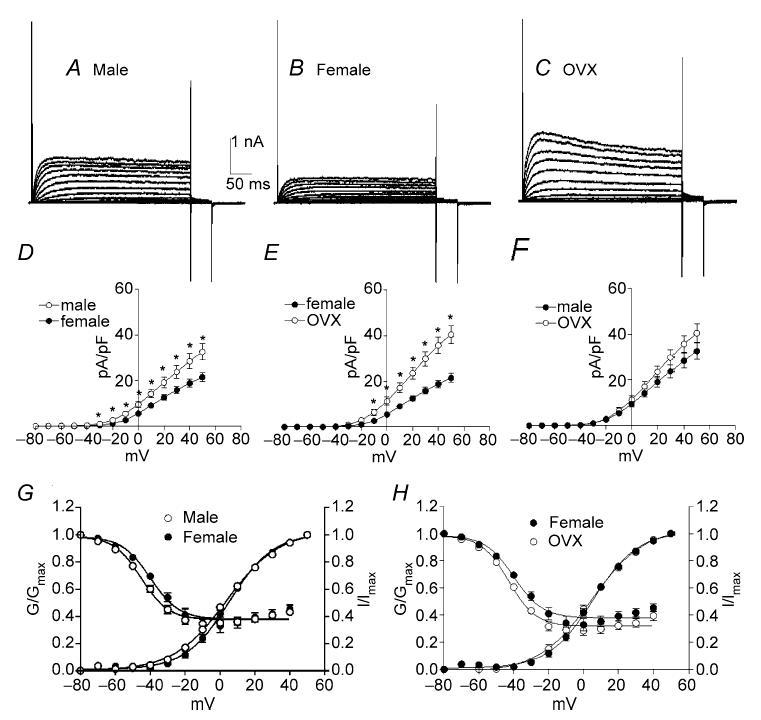
4-AP (5 mm) applied to the bathing solution revealed slowly activating and inactivating delayed rectifier currents in colonic myocytes from male (A), female (B) and OVX (C) mice. Current density in male and OVX mice was significantly higher than for females (D and E). Current density for OVX and male mice were not significantly different at any tested potential (F). There were no significant differences in the voltage dependence of the outward current among the three groups (G and H).
Oestrogen replacement in OVX mice did not significantly change density or voltage dependence of delayed rectifier currents
The effect of oestrogen on delayed rectifier K+ currents was examined by comparing current density in the presence of 4-AP in three groups: OVX, OVX injected daily with 17β-oestradiol for 7 days (OES) and non-pregnant females (Fig. 10A–C). The current densities at 0 mV and +40 mV were 11.3 ± 1.8 and 35.7 ± 3.6 pA pF−1, respectively, in OVX mice (n = 8, Fig. 10D), 9.1 ± 1.0 and 30.9 ± 2.0 pA pF−1, respectively, in OES (n = 19, Fig. 10D and E), and 5.5 ± 0.6 and 18.8 ± 1.7 pA pF−1, respectively, in the non-pregnant female group (n = 10, Fig. 10E). Statistical analysis revealed that although mean current density values were lowered in OVX animals by OES injections, differences between OVX and OES were not statistically significant (Fig. 10D). Delayed rectifier current densities for untreated OVX mice and those receiving and oestrogen (OES) were significantly higher than non-pregnant females (P < 0.05 at test potentials more positive than −20 mV, Fig. 10E). Voltage dependence of activation and inactivation was not shifted by oestrogen injection to OVX mice (i.e. Va1/2 = 5 ± 1 mV in OVX and 9 ± 1 mV in OES, Vi1/2 = −43 ± 2 mV in OVX and −44 ± 1 mV in OES, Fig. 10F). These data suggest that 17β-oestradiol injections did not affect the transcription or translation of channels generating delayed rectifier K+ currents.
Figure 10. Density of delayed rectifier currents is not significantly reduced by chronic administration of 17β-oestradiol.
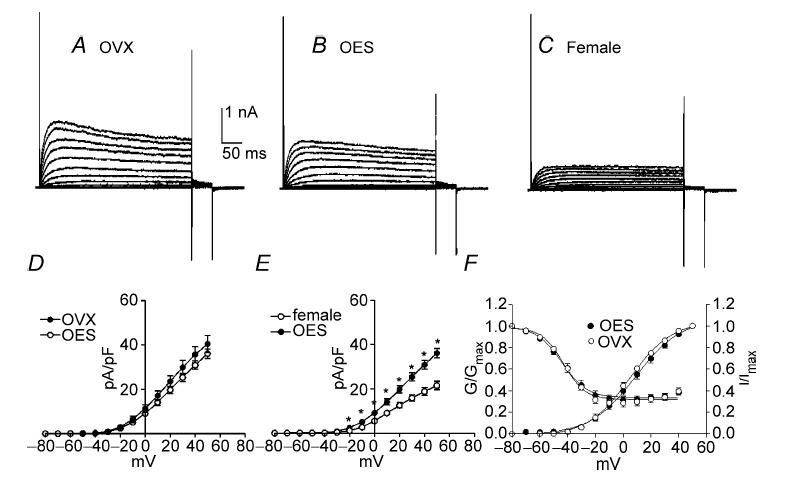
Delayed rectifier currents (isolated by application of 4-AP; 5 mm) were recorded from colonic myocytes of ovariectomized females which had received injections of either saline (OVX; A) or 17β-oestradiol (OES; B). 4-AP-insensitive currents were also recorded from non-pregnant females (Female; C). In these conditions, current densities from saline or 17b-oestradiol-treated OVX mice were not significantly different at any voltage tested (D) and there was no change in either the voltage dependence of activation or inactivation with oestradiol administration (F). The density of delayed rectifier currents was significantly lower for non-pregnant females compared with oestradiol-treated OVX females through a wide range of voltages tested (E).
Delayed rectifier current density was not changed by pregnancy
We also examined and compared the density of delayed rectifier K+ currents in non-pregnant mice, and those in the first and last trimesters of pregnancy (Fig. 11A–C). In the presence of 4-AP peak outward current density at 0 mV and +40 mV was 5.5 ± 0.6 and 18.8 ± 1.7 pA pF−1, respectively, in non-pregnant mice (n = 10, Fig. 11D), 6.4 ± 0.9 and 22.3 ± 2.5 pA pF−1, respectively, in the first trimester (n = 7, Fig. 11D) and 4.9 ± 0.9 and 19.7 ± 4.1 pA pF−1, respectively, in the last trimester (n = 10, Fig. 11D). There was no significant difference between delayed rectifier current density in non-pregnant, first trimester or last trimester pregnant mice (Fig. 11D). Additionally, the voltage dependence of activation and inactivation were not affected by pregnancy (i.e. Va1/2 = 6 ± 1 mV in non-pregnant and 9 ± 1 mV in the final trimester of pregnancy; Vi1/2 = −40 ± 2 mV in non-pregnant and −39 ± 2 mV in the final trimester, Fig. 11E). These data further suggest that oestrogen does not influence the expression of delayed rectifier K+ currents in murine colonic myocytes.
Figure 11. Density of delayed rectifier currents is not altered by pregnancy.
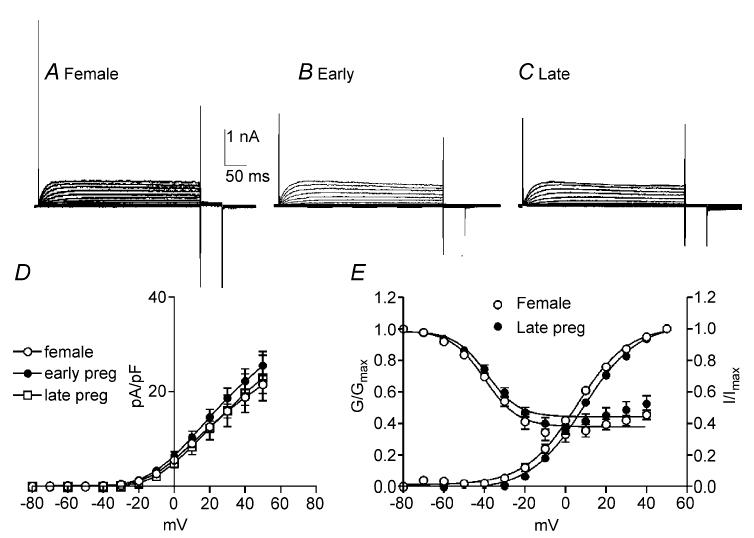
Delayed rectifier currents (isolated by application of 4-AP; 5 mm) were recorded from colonic myocytes of non-pregnant females (A) and females within the first (B) and last (C) trimesters of pregnancy. Neither current density (D) nor voltage dependence (E)were significantly affected by pregnancy.
Quantitative PCR analysis revealed an increase in KChIP1 expression in colons of ovariectomized mice
Results from electrophysiological experiments suggested that oestrogen suppresses A-type current expression in colonic myocytes. Previous studies suggest that A-type currents in colonic smooth muscle cells are primarily encoded by Kv4.3 (Amberg et al. 2002). Therefore, using quantitative PCR, we compared the relative expression of transcripts encoding Kv4.3 in female, OVX and OES mice. Statistical analysis revealed that there was no significant difference in transcriptional expression of Kv4.3 between the three groups of female mice (i.e. intact females, 6.78 × 10−2 ± 3.55 × 10−2 (n = 5); OVX, 12.66 × 10−2 ± 9.41 × 10−2 (n = 6); and OES, 4.10 × 10−2 ± 1.20 × 10−2 (n = 4), values normalized to GAPDH expression; Fig. 12A. Interestingly, quantitative PCR demonstrated that levels of KChIP1 (K+ channel interacting protein type 1) transcripts were significantly different between non-pregnant females (2.58 × 10−2 ± 0.24 × 10−2), OVX (7.75 × 10−2 ± 2.24 × 10−2) and OES (1.59 × 10−2 ± 0.24 × 10−2) groups (P = 0.03, by analysis of variance followed by multiple comparisons Tukey's test, Fig. 12B). No significant difference was found between KChIP4 transcript expression between intact, OVX and OES females (i.e. intact females, 4.23 × 10−2 ± 1.72 × 10−2; OVX, 3.43 × 10−2 ± 1.06 × 10−2; and OES, 2.24 × 10−2 ± 0.05 × 10−2; values normalized to GAPDH expression, Fig. 12C).
Figure 12. Relative expression of mRNA transcripts encoding Kv4.3, KChIP1 and KChIP4 in murine colon tissues.
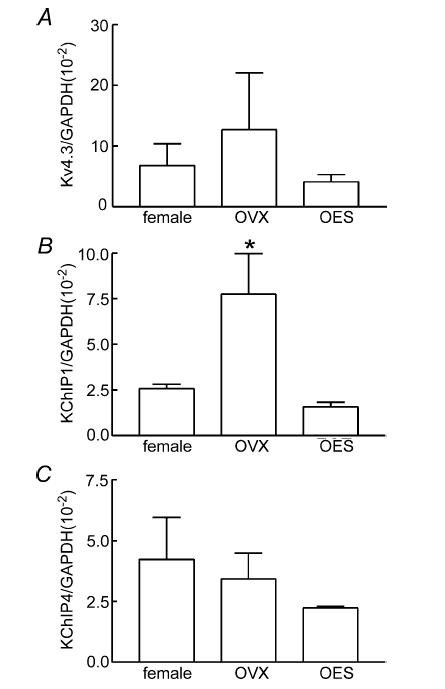
Quantitative PCR analysis failed to detect a significant difference in Kv4.3 transcript expression between non-pregnant female (n = 5), OVX (n = 6) or OES (n = 4) groups (A). Expression of transcripts encoding KChIP1 were statistically different between groups, with the highest expression level in the OVX group (B; *statistical significance; P = 0.03 by analysis of variance followed by multiple comparisons; Tukey's test). No significant difference in expression levels of KChIP4 transcripts was detected between the three groups (C).
Discussion
Previous clinical studies (Lawson et al. 1985; Hutson et al. 1989; Baron et al. 1993; Meier et al. 1995; Bond et al. 1996; Jung et al. 2003; Sadik et al. 2003) and in vivo and in vitro animal experiments (Ryan & Bhojwani, 1986; Chen et al. 1995; Coskun et al. 1995) have suggested that female steroid hormones can significantly influence gastrointestinal function and motility. To address the hypothesis that ovarian hormones influence the activity and expression of ionic conductances involved in colonic smooth muscle excitability we investigated outward current density following acute and chronic manipulation of oestrogen and progesterone levels.
There are a number of studies which suggest that acute application of oestrogen can modulate K+ channels through multiple intracellular signalling pathways (Kelly et al. 2003; Tsang et al. 2003; Coiret et al. 2005; Fatehi et al. 2005). We found that acute application of oestrogen or progesterone did not affect outward current amplitude or voltage dependence in colonic myocytes. We have not yet carried out experiments to test the effects of acute oestrogen and progesterone on cells with permeabilized patches where intracellular signalling pathways are left intact. So at present we can conclude there is no direct blocking or facilitating effects of oestrogen and progesterone, but this does not rule out non-genomic effects on outward currents that are mediated by intracellular signalling pathways activated or suppressed by female hormones.
Elevation of female steroid hormones that accompanies pregnancy produces electrical remodelling of some smooth muscle tissues, such as the myometrium (Thorburn & Challis, 1979; Verhoeff et al. 1985a,b). Consistent with the hypertrophy that occurs during pregnancy in the uterus, the capacitance of uterine myocytes increases as pregnancy progresses (Yoshino et al. 1997). In the present study we examined the capacitance of colonic myocytes from male, intact non-pregnant and OVX female mice. Colonic myocytes from intact females had significantly larger capacitance than cells from males or OVX females, supporting the hypothesis that ovarian hormones can influence cell size.
A major finding of this study was that the density of net outward currents for non-pregnant females (a model for females of reproductive age) was significantly lower than for males or OVX females (a model for postmenopausal females), suggesting female steroid hormones may modulate the expression of outward currents in colonic myocytes and therefore influence the excitability of colonic muscles. We found that treatment of OVX mice with 17β-oestradiol for 7 days suppressed outward current density to levels similar to non-pregnant females. In contrast, injection of progesterone did not alter outward current density, suggesting that oestrogen, but not progesterone, regulates K+ channel expression. Cell capacitance and outward current density was also compared in non-pregnant and pregnant animals. No statistical difference was detected in capacitances of colonic myocytes from non-pregnant and pregnant females, suggesting that pregnancy did not produce significant hypertrophy of colon smooth muscle cells. However, colonic myocytes isolated from animals within the final trimester of gestation (when blood oestrogen concentrations are highest) exhibited decreased outward current density compared with those within the first trimester or non-pregnant females. Together, these data suggest that oestrogen is an important determinant of K+ channel expression in colonic myocytes. Changes in the expression of K+ channels which are critically involved in setting the resting membrane potential probably has important implications for colonic motility. For example, if increased oestrogen levels suppress the expression of a K+ channel necessary to maintain a hyperpolarized resting membrane potential, this is likely to promote depolarization and tonic contraction. To determine which types of K+ channels are affected by oestrogen, we isolated and examined A-type currents and classical delayed rectifier K+ currents in each of our animal models.
A-type currents are voltage-gated, Ca2+-independent K+ currents that are distinguished from typical delayed rectifier K+ currents by rapid inactivation. A-type currents activate at negative membrane potentials, with measurable thresholds typically between −45 and −60 mV and display strong steady-state voltage-dependent inactivation (Amberg et al. 2003). Repolarization to potentials negative to −50 mV is typically required for restoration of channel availability with rapid recovery from inactivation (Beech & Bolton, 1989; Smirnov et al. 1992; Vogalis et al. 1993; Serodio et al. 1994; Koh et al. 1999; Hatano et al. 2002). Inhibition by 4-AP and insensitivity to extracellular TEA ions are considered to be pharmacological hallmarks of A-type currents (Thompson, 1977). Previous studies have deduced that the most likely molecular candidate for the A-type current in murine colonic myocytes is Kv4.3 (Amberg et al. 2002).
In the current study, A-type currents were isolated in colonic myocytes of male, female and OVX mice by application of TEA. The current density of A-type currents was significantly decreased in non-pregnant females compared with males or OVX females, with a positive shift in voltage dependence of activation and inactivation. Females in the final trimester of gestation and OES mice (which had received injections of oestradiol) exhibited a reduced density of A-type currents compared with non-pregnant and OVX groups, respectively. Our results are consistent with a previous study which demonstrated that A-type K+ current densities decline in myometrial smooth muscle cells during pregnancy (Wang et al. 1998). Changes in myometrial transient A-type currents were also mimicked by administration of 17β-oestradiol to sexually immature female rats whilst progesterone was without effect (Erulkar et al. 1994). However, in contrast to our study on colonic smooth muscle, myometrial Kv4.3 mRNA and protein levels were reduced by pregnancy or oestradiol replacement to OVX rats (Song et al. 2001). We did not observe a significant change in Kv4.3 transcript expression between intact female, OVX or OES mice suggesting that the increase in A-type current density in colonic myocytes that accompanies ovariectomy may not be a direct consequence of up-regulated Kv4.3 expression. It has been suggested that functional expression of Kv4.3 channels is not only controlled by the levels of pore-forming subunits, but requires their association with auxiliary subunits such as K+ channel interacting proteins or ‘KChIPs’ (Suzuki & Takimoto, 2005). Therefore, it is most noteworthy that we detected a significant elevation of transcripts encoding the auxillary subunit KChIP1 (K+ channel interacting protein, type 1), but not KChIP4, in OVX females compared with intact females. KChIP1 is thought to bind to the cytoplasmic domain of Kv4.3 and serve to modulate the inactivation kinetics and rate of recovery from inactivation (An et al. 2000). Expression studies have also demonstrated that A-type current density is markedly enhanced by the coexpression of Kv4.3 with KChIP1 as compared with when Kv4.3 is expressed alone (Hatano et al. 2002). Thus, increased expression of KChIP1 may provide a possible explanation for the elevated A-type current density and altered kinetics of inactivation observed in colonic myocytes isolated from OVX mice.
Delayed rectifier currents are encoded by Kv1 family channels in the murine colon (Koh et al. 1999). In the current study we found that the density of delayed rectifier K+ currents (isolated by adding 4-AP to the external solution) was lower in colonic myocytes from non-pregnant females compared with males and OVX females. However, interestingly delayed rectifier currents were not reduced by oestrogen to OVX mice, suggesting that Kv1 expression is not regulated by oestrogen. There appears to be an unknown consequence of OVX that regulates Kv1 family channels in the colon.
In conclusion, these data suggest that chronic changes of oestrogen concentration can influence A-type current density in murine colonic smooth muscle. Differences in A-type current density and inactivation kinetics observed in males, control and OVX females may be partially due to oestrogen-mediated changes in the transcriptional expression of the auxiliary subunit KChIP1. As A-type currents have been implicated in setting the resting membrane potential of colonic smooth muscle, changes in current density are likely to affect colonic excitability and motility. Thus, these findings may provide a possible link between changes in colonic motility and the altered hormonal status associated with menstrual cycle stage, pregnancy and menopause in females.
Acknowledgments
This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Program Project Grant DK-41315 and Pilot Grant of Sanford Center for Aging.
References
- Amberg GC, Koh SD, Hatton WJ, Murray KJ, Monaghan K, Horowitz B, Sanders KM. Contribution of Kv4 channels toward the A-type potassium current in murine colonic myocytes. J Physiol. 2002;544:403–415. doi: 10.1113/jphysiol.2002.025163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993;118:366–375. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Bolton TB. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond EF, Heitkemper MM, Perigo R. Gastric emptying and gastric-intestinal transit in rats with varying ovarian hormone status. Nurs Res. 1996;45:218–224. doi: 10.1097/00006199-199607000-00005. [DOI] [PubMed] [Google Scholar]
- Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268:G171–G176. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- Coiret G, Matifat F, Hague F, Ouadid-Ahidouch H. 17-β-estradiol activates maxi-K channels through a non-genomic pathway in human breast cancer cells. FEBS Lett. 2005;579:2995–3000. doi: 10.1016/j.febslet.2005.02.085. [DOI] [PubMed] [Google Scholar]
- Coskun T, Sevinc A, Tevetoglu I, Alican I, Kurtel H, Yegen BC. Delayed gastric emptying in conscious male rats following chronic estrogen and progesterone treatment. Res Exp Med (Berl) 1995;195:49–54. doi: 10.1007/BF02576773. [DOI] [PubMed] [Google Scholar]
- Erulkar SD, Rendt J, Nori RD, Ger B. The influence of 17β-oestradiol on K+ currents in smooth muscle cells isolated from immature rat uterus. Proc Biol Sci. 1994;256:59–65. doi: 10.1098/rspb.1994.0049. [DOI] [PubMed] [Google Scholar]
- Fatehi M, Kombian SB, Saleh TM. 17β-estradiol inhibits outward potassium currents recorded in rat parabrachial nucleus cells in vitro. Neuroscience. 2005;135:1075–1086. doi: 10.1016/j.neuroscience.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Hatano N, Ohya S, Imaizumi Y. Functional interaction between KChIP1 and GFP-fused Kv4.3L co-expressed in HEK293 cells. Pflugers Arch. 2002;444:80–88. doi: 10.1007/s00424-002-0798-9. [DOI] [PubMed] [Google Scholar]
- Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- Jamali K, Naylor BR, Kelly MJ, Ronnekleiv OK. Effect of 17β-estradiol on mRNA expression of large-conductance, voltage-dependent, and calcium-activated potassium channel α and β subunits in guinea pig. Endocrine. 2003;20:227–237. doi: 10.1385/ENDO:20:3:227. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Zheng W, Korach KS, Scheuer T, Catterall WA, Rubanyi GM. Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J General Physiol. 1997;110:135–140. doi: 10.1085/jgp.110.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HK, Kim DY, Moon IH. Effects of gender and menstrual cycle on colonic transit time in healthy subjects. Korean J Intern Med. 2003;18:181–186. doi: 10.3904/kjim.2003.18.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm MA, Farthing MJ, Lennard-Jones JE, Perry LA, Chard T. Steroid hormone abnormalities in women with severe idiopathic constipation. Gut. 1991;32:80–84. doi: 10.1136/gut.32.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- Koh SD, Ward SM, Dick GM, Epperson A, Bonner HP, Sanders KM, Horowitz B, Kenyon JL. Contribution of delayed rectifier potassium currents to the electrical activity of murine colonic smooth muscle. J Physiol. 1999;515:475–487. doi: 10.1111/j.1469-7793.1999.475ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M, Kern F, Jr, Everson GT. Gastrointestinal transit time in human pregnancy: prolongation in the second and third trimesters followed by postpartum normalization. Gastroenterology. 1985;89:996–999. doi: 10.1016/0016-5085(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Meier R, Beglinger C, Dederding JP, Meyer-Wyss B, Fumagalli M, Rowedder A, Turberg Y, Brignoli R. Influence of age, gender, hormonal status and smoking habits on colonic transit time. Neurogastroenterol Motil. 1995;7:235–238. doi: 10.1111/j.1365-2982.1995.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Patterson E, Ma L, Szabo B, Robinson CP, Thadani U. Ovariectomy and estrogen-induced alterations in myocardial contractility in female rabbits: role of the L-type calcium channel. J Pharmacol Exp Ther. 1998;284:586–591. [PubMed] [Google Scholar]
- Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: ‘idiopathic slow transit constipation’. Gut. 1986;27:41–48. doi: 10.1136/gut.27.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranki HJ, Budas GR, Crawford RM, Davies AM, Jovanovic A. 17β-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–374. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Ryan JP, Bhojwani A. Colonic transit in rats: effect of ovariectomy, sex steroid hormones, and pregnancy. Am J Physiol. 1986;251:G46–G50. doi: 10.1152/ajpgi.1986.251.1.G46. [DOI] [PubMed] [Google Scholar]
- Sadik R, Abrahamsson H, Stotzer PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol. 2003;38:36–42. doi: 10.1080/00365520310000410. [DOI] [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Zholos AV, Shuba MF. A potential-dependent fast outward current in single smooth muscle cells isolated from the newborn rat ileum. J Physiol. 1992;454:573–589. doi: 10.1113/jphysiol.1992.sp019280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Takimoto K. Differential expression of Kv4 pore-forming and KChIP auxiliary subunits in rat uterus during pregnancy. Am J Physiol Endocrinol Metab. 2005;288:E335–E341. doi: 10.1152/ajpendo.00250.2004. [DOI] [PubMed] [Google Scholar]
- Thompson SH. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977;265:465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn GD, Challis JR. Endocrine control of parturition. Physiol Rev. 1979;59:863–918. doi: 10.1152/physrev.1979.59.4.863. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Chan HY, Wong CM, Chen ZY, Au CL, Huang Y. Contribution of K+ channels to relaxation induced by 17β-estradiol but not by progesterone in isolated rat mesenteric artery rings. J Cardiovasc Pharmacol. 2003;41:4–13. doi: 10.1097/00005344-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Tsang SY, Yao X, Wong CM, Chan FL, Chen ZY, Huang Y. Differential regulation of K+ and Ca2+ channel gene expression by chronic treatment with estrogen and tamoxifen in rat aorta. Eur J Pharmacol. 2004;483:155–162. doi: 10.1016/j.ejphar.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Turnbull GK, Thompson DG, Day S, Martin J, Walker E, Lennard-Jones JE. Relationships between symptoms, menstrual cycle and orocaecal transit in normal and constipated women. Gut. 1989;30:30–34. doi: 10.1136/gut.30.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeff A, Garfield RE, Ramondt J, Wallenburg HC. Electrical and mechanical uterine activity and gap junctions in peripartal sheep. Am J Obstet Gynecol. 1985a;153:447–454. doi: 10.1016/0002-9378(85)90085-7. [DOI] [PubMed] [Google Scholar]
- Verhoeff A, Ramondt J, Garfield RE, Wallenburg HC. Modulation of spontaneous myometrial activity in chronically instrumented ovariectomized sheep. Eur J Obstet Gynecol Reprod Biol. 1985b;19:113–124. doi: 10.1016/0028-2243(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Lang RJ, Bywater RA, Taylor GS. Voltage-gated ionic currents in smooth muscle cells of guinea pig proximal colon. Am J Physiol. 1993;264:C527–C536. doi: 10.1152/ajpcell.1993.264.3.C527. [DOI] [PubMed] [Google Scholar]
- Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from nonpregnant and late-pregnant rats. J General Physiol. 1998;112:737–756. doi: 10.1085/jgp.112.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino M, Wang SY, Kao CY. Sodium and calcium inward currents in freshly dissociated smooth myocytes of rat uterus. J General Physiol. 1997;110:565–577. doi: 10.1085/jgp.110.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]


